Abstract
Nicotinic acetylcholine receptors (nAChRs) play an important role in the modulation of many cognitive functions but their role in integrated network activity remains unclear. This is at least partly because of the complexity of the cholinergic circuitry and the difficulty in comparing results from in vivo studies obtained under diverse experimental conditions and types of anesthetics. Hence the role of nAChRs in the synchronization of cortical activity during slow-wave sleep is still controversial, with some studies showing they are involved in ACh-dependent EEG desynchronization, and others suggesting that this effect is mediated exclusively by muscarinic receptors. Here we use an in vitro model of endogenous network activity, in the form of recurring self-maintained depolarized states (Up states), which allows us to examine the role of high-affinity nAChRs on network dynamics in a simpler form of the cortical microcircuit. We find that mice lacking nAChRs containing the β2-subunit (β2-nAChRs) have longer and more frequent Up states, and that this difference is eliminated when β2-nAChRs in wild-type mice are blocked. We further show that endogenously released ACh can modulate Up/Down states through the activation of both β2- and α7-containing nAChRs, but through distinct mechanisms: α7-nAChRs affect only the termination of spontaneous Up states, while β2-nAChRs also regulate their generation. Finally we provide evidence that the effects of β2-subunit-containing, but not α7-subunit-containing nAChRs, are mediated through GABAB receptors. To our knowledge this is the first study documenting direct nicotinic modulation of Up/Down state activity.
SIGNIFICANCE STATEMENT Through our experiments we were able to uncover a clear and previously disputed effect of nicotinic signaling in synchronized activity of neuronal networks of the cortex. We show that both high-affinity receptors (containing the β2-subunit, β2-nAChRs) and low-affinity receptors (containing the α7-subunit, α7-nAChRs) can regulate cortical network function exhibited in the form of Up/Down states. We further show that the effects of β2-nAChRs, but not α7-nAChRs, are mediated through the activation of GABAB receptors. These results suggest a possible synthesis of seemingly contradictory results in the literature and could be valuable for informing computational models of cortical function and for guiding the search for therapeutic interventions.
Keywords: β2-nAChR, barrel cortex, cholinergic, network activity, oscillations, persistent activity
Introduction
Nicotinic acetylcholine receptors (nAChRs) play an important role in the modulation of cortical processing during cognitive tasks such as attention, learning, and memory. Impairments of the nicotinic circuitry have been associated with disease states such as dementia, depression, and attention deficit disorder (Picciotto and Zoli, 2002; Potter and Newhouse, 2004; Mansvelder et al., 2006) rendering nAChRs a prime target for therapeutic interventions. Because of the complexity of the multiple cholinergic pathways the mechanisms underlying nicotinic modulation in the cortex are still unclear. Receptors come in different varieties, with distinct kinetics and desensitization properties. They are differentially expressed in presynaptic and postsynaptic compartments, in different cortical layers, neuronal types, and subcellular compartments, and can affect network activity through both synaptic and nonsynaptic mechanisms (Dani and Bertrand, 2007; Zaborszky et al., 2012). In addition, ACh can either depolarize or hyperpolarize neurons leading to diverse and context-specific effects on excitation/inhibition balance. Consequently, although many aspects of nicotinic signaling have been characterized over the years, studies addressing the integration of these different modes of action in functional networks are still scarce. Moreover, it is often difficult to compare and combine results from in vivo studies because of the diversity of experimental conditions and the effects of anesthetics on receptor function (Hara and Harris, 2002). For instance, the role of nAChRs in the synchronization of cortical activity during slow-wave sleep is still controversial. Some studies show that nicotinic receptors are involved in ACh-dependent EEG desynchronization—a signature of aroused brain states associated with wakefulness or REM sleep (Yamamoto and Domino, 1965; Armitage et al., 1969; Salin-Pascual et al., 1999)—while others suggest that the effect is mediated exclusively by muscarinic receptors (Metherate et al., 1992; Steriade et al., 1993; Toth et al., 2012).
One of the most abundant types of nAChRs in the neocortex contains the β2-subunit, which forms high-affinity receptors. Genetic manipulations of this subunit have provided indirect evidence that β2-nAChRs participate in cortical EEG desynchronization. Mice lacking β2-nAChRs exhibit reduced micro-arousals during NREM sleep (Léna et al., 2004), while mutations that cause receptor hyperactivity lead to increased numbers of micro-arousals (Halász et al., 2013). These studies indicate a regulatory role for β2-nAChRs in the transition between synchronized and desynchronized states that has not been explored systematically.
Here we use an in vitro model of cortical network activity in mouse brain slices that spontaneously generate Up/Down states in the absence of pharmacological or electrical stimulation. Several studies have reported that this model approximates slow-wave activity during sleep (Sanchez-Vives and McCormick, 2000; Shu et al., 2003; Compte et al., 2008) and have used it to explore cortical network dynamics (Rigas and Castro-Alamancos, 2007; Mann et al., 2009; Fanselow and Connors, 2010). We use β2 knock-out mice (β2−/−) and pharmacological experiments to investigate whether β2-nAChRs participate in the regulation of Up/Down state activity. In addition, given the accelerated aging phenotype exhibited by these mice (Zoli et al., 1999; Konsolaki and Skaliora, 2014), we examine this network phenomenon in both adult and old animals.
Materials and Methods
Animals.
C57BL/6J WT and β2−/− mice were bred in the animal facility of the Center for Experimental Surgery of the Biomedical Research Foundation of the Academy of Athens. The facility is registered as a breeding and experimental facility according to the Presidential Decree of the Greek Democracy 160/91, which harmonizes the Greek national legislation with the European Council Directive 86/609/EEC on the protection of animals used for experimental and other scientific purposes. Mice were weaned at P21, housed in groups of 5–9, in 267 × 483 × 203 mm cages supplied with bedding material, and kept at a 12 h dark/light schedule. Food was provided ad libitum.
Brain slice preparation.
Coronal brain slices (400 μm) from the primary somatosensory (S1) cortex of the mouse whisker system (i.e., barrel cortex, S1BF; AP from bregma: 0.58–1.58 mm, ML: 2.5–4 mm) were prepared from adult (3–9 months old) and aged (20–27 months old) male mice. After the animal was killed by cervical dislocation the brain was removed and placed in an oxygenated (95% O2–5% CO2), ice-cold dissection solution containing the following (in mm): 2.14 KCl, 1.47 NaH2PO4. H2O, 27.0 NaHCO3, 2.2 MgSO4, 10.0 d- glucose, 200 sucrose, and 2.0 CaCl2. 2H2O, osmolarity (mean ± SD) 298 ± 5 mOsm, pH 7.4. Slices were cut using a vibratome (VT 1000S; Leica), placed in a holding chamber with aCSF [containing the following (in mm): 126 NaCl, 3.53 KCl, 1.25 NaH2PO4. H2O, 26.0 NaHCO3, 1.0 MgSO4, 10.0 d-glucose, and 2.0 CaCl2. 2H2, osmolarity (mean ± SD) 317 ± 4 mOsm, pH 7.4], and left to recover at room temperature (RT; 24–26°C) for at least 1 h before use.
In vitro electrophysiology.
Slices were transferred to a submerged chamber (Luigs & Neumann), where they were constantly perfused at high flow rates (10–15 ml/min) to ensure optimal oxygenation of the cortical tissue (Hájos et al., 2009; Ivanov and Zilberter, 2011). Recordings were performed in “in vivo like” aCSF (composition as above but with 1 mm CaCl2), because this ionic solution is thought to better mimic CSF in vivo (Harris et al., 1984; Jones and Keep, 1988). Recordings were performed at RT, after at least 30 min of incubation in 1 mm [CaCl2] aCSF. Spontaneous network activity was assessed by means of (1) local field potential (LFP) recordings (sampled at 5 kHz or 10 kHz, band-passed filtered at 1–3000 Hz) obtained from cortical layers II/III using low impedance (∼0.5 MΩ) glass pipettes filled with aCSF and (2) visually guided whole-cell patch-clamp recordings from layer II-IV cells with 7–10 MΩ impedance electrodes. Patch electrodes were filled with a solution containing the following (in mm): 135 K-gluconate, 4 KCl, 2 NaCl, 0.2 EGTA, 5 Tris-phosphocreatine, 0.3 Tris-GTP, 10 HEPES, and 4 MgATP (290 mOsm). Intracellular recordings were performed as proof of concept to ensure that the signal we obtained in the LFP recordings corresponded to intracellular Up and Down states and to evaluate the pharmacological response. Only cells with stable membrane voltage and input resistance were used for our assessment. Signals were acquired and amplified (MultiClamp 700B; Molecular Devices), digitized (InstruTech; ITC-18), and viewed on-line with appropriate software (AxoGraph X, version 1.3.5).
Pharmacology.
Recordings were also obtained in the presence of (1) the β2-nAChR-antagonist, dihydro-β-erythroidine hydrobromide (DHβE; 3 μm; Tocris Bioscience) and (2) both DHβE and the α7-nAChR-selective antagonist, methyllycaconitine citrate (MLA; 10 nm; Tocris Bioscience). Although MLA is known to also block α6β2 nAChRs with ∼30-fold lower affinity (Ki: 33 and 1.4 nm, for α6β2 and α7, respectively), it was added only after β2 blockade with DHβE so that its effect would reveal the α7-mediated component (Klink et al., 2001; Mogg et al., 2002). After recording baseline activity in the absence of any drugs, DHβE was added to the perfusion medium and the LFP signal was monitored for 30 min. Subsequently, MLA was added to the DHβE-containing medium and Up/Down state activity was monitored for another 30 min. In some experiments the same protocol was followed with the exception that the GABAB receptor antagonist, CGP55845 (1 μm; Tocris Bioscience), was applied 30 min before DHβE. The AChR agonist carbachol (500 nm; Sigma) was applied in separate experiments.
Data analysis.
For visualization and analysis of spontaneous LFP events, traces were exported to MATLAB format. The analysis of each recorded trace was performed with MATLAB scripts that automatically detected the deflections in the LFP trace. These data were first low-pass filtered at 200 Hz (third-order Butterworth filter) and the DC offset was subtracted. Detection of individual LFP bursts was performed with the following automated method: (1) the signal was transformed using the Hilbert Transform to estimate its envelope (Oppenheim et al., 1999) and (2) a threshold was applied to detect signal segments with fluctuation values larger than 40% of the SD of the entire signal. This threshold was calculated for each trace (data-driven threshold) to ensure that the detection procedure is adjusted to the corresponding signal-to-noise ratio of each recording and to the specific properties of each time series (e.g., size and frequency of events). Subsequently, the automatically detected LFP events were visually inspected to reject artifacts caused by electrical and/or mechanical noise. Up state duration of each event was calculated as the time interval between the onset and offset of individual events, while Up state occurrence was defined as the number of events divided by the duration of the recording session.
Statistics.
All statistical comparisons are based on LFP traces, unless otherwise stated. Data were initially tested for normality and homoscedasticity to explore if the assumptions for ANOVA are satisfied. Data regarding the occurrence of Up states were transformed using the Box–Cox transformation (Box and Cox, 1964) to become normally distributed and with equal variance, and parametric tests were performed on the transformed data. Since recordings were obtained from one or more slices from each animal, we first tested for the best-fit model, according to the smallest Akaike Information Criterion, with two fixed effects (independent variables: age and genotype) and two random effects (animal and brain slice). For both dependent variables (Up state duration and occurrence) the best-fit model was the one without random effects, leading to p values identical to those of a two-way ANOVA using the same statistical hypothesis testing. For the pharmacological experiments we performed a repeated-measures three-way ANOVA with one within-subjects factor [variable drug with three levels: (1) control, (2) DHβE, and (3) DHβE + MLA] and two between-subjects factors (variables genotype and age), unless otherwise stated. In cases where there was no statistically significant triple interaction, the effect of the drug was estimated with a repeated-measures two-way ANOVA for each of the between-subjects factors (i.e., variables genotype or age). Double interactions were further investigated using post hoc pairwise comparisons of the within-subjects data (variable drug) with the least significant difference test. The p values reported for the repeated-measures test are corrected for departures from sphericity using the Greenhouse–Geisser estimate of sphericity.
Results
Cortical slices maintained in vitro spontaneously generate recurring Up and Down states
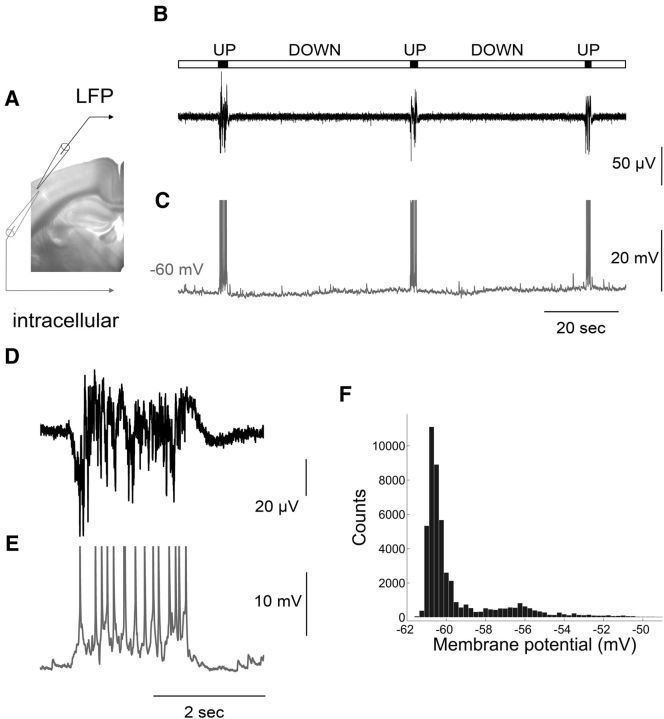
Recordings were performed in coronal brain slices in which direct (from the basal forebrain) or indirect (through glutamatergic thalamocortical axons) cholinergic axons are cut from their cell bodies to examine intracortical circuits in isolation from subcortically mediated cholinergic modulation. Slices from adult mice exhibited spontaneous network activity characterized by short epochs of sustained LFP events interrupted by longer epochs of relative quiescence (Fig. 1B,D). Simultaneous intracellular recordings (Fig. 1C,E) revealed that during the periods of inactivity (the Down state) the cell membrane remained hyperpolarized, while during periods of sustained activity (the Up state) the cells became depolarized with occasional firing of action potentials (average rate: 4.33 ± 3.35 spikes per event, n = 72). This bistability of membrane potential (Vm) is reflected by two peaks in the histogram of Vm distributions (Fig. 1F). In line with previous work (Shu et al., 2003), the interspike interval during the Up state was highly variable compared with the regular spiking activity produced in the same neurons in response to current injection (data not shown), while the variance of membrane potential was significantly larger (5.58 ± 3.18 mV) compared with the Down state (0.3 ± 0.18 mV; n = 72). Up state duration (ranging between 0.5 and 1.98 s) was comparable to in vivo recordings (Sanchez-Vives and McCormick, 2000; Timofeev et al., 2001). In contrast, Up state occurrence (ranging between 0.16 and 4 events/min) was significantly lower, but similar to recordings from isolated cortical slabs in living cats (Timofeev et al., 2001), consistent for preparations that contain a more restricted network.
Figure 1.
Spontaneous synchronized network activity in the form of Up/Down states recorded in a mouse brain slice. A, Picture of a coronal slice used for simultaneous LFP (black electrode) and intracellular (gray electrode) recording from layer 2/3 of the primary somatosensory cortex. B, C, LFP and intracellular recordings, respectively. The bar above the traces indicates Up states (in black) and Down states (in gray). Action potentials are truncated at −30 mV. D, E, Close-up of the LFP and intracellular recording, respectively, showing an Up state at higher resolution. F, Membrane potential distribution with two peaks reflecting the bistable fluctuations of membrane potential during spontaneous network activity.
To establish relations with Up states recorded in vivo we performed pharmacological tests. Blocking AMPA-mediated glutamate transmission (10 μm of the ionotropic receptor antagonist CNQX) completely abolished the Up states, while blocking GABAA-mediated transmission (500 nm gabazine or 1 μm bicuculline) transformed the spontaneous events to seizure-like activity (data not shown). Bath application of the cholinergic agonist carbachol (500 nm) resulted in a complete block of the LFP activity, in line with previous in vivo and in vitro reports (Favero et al., 2012; Toth et al., 2012). These data indicate that adult mouse cortical slices bathed in in vivo-like aCSF generate spontaneous Up states closely resembling (both in properties and in pharmacological profile) those occurring in vivo during NREM sleep or anesthesia (Sanchez-Vives and McCormick, 2000; Shu et al., 2003; Sanchez-Vives et al., 2008).
Spontaneous Up state activity is enhanced in β2−/− mice
To assess if high-affinity nAChRs participate in cortical network function, we first examined whether the chronic absence of β2-nAChRs affects the duration and occurrence of spontaneous Up states. Experiments were performed on both adult (3–9 months old) and old (20–27 months old) animals to test for possible age-dependent effects.
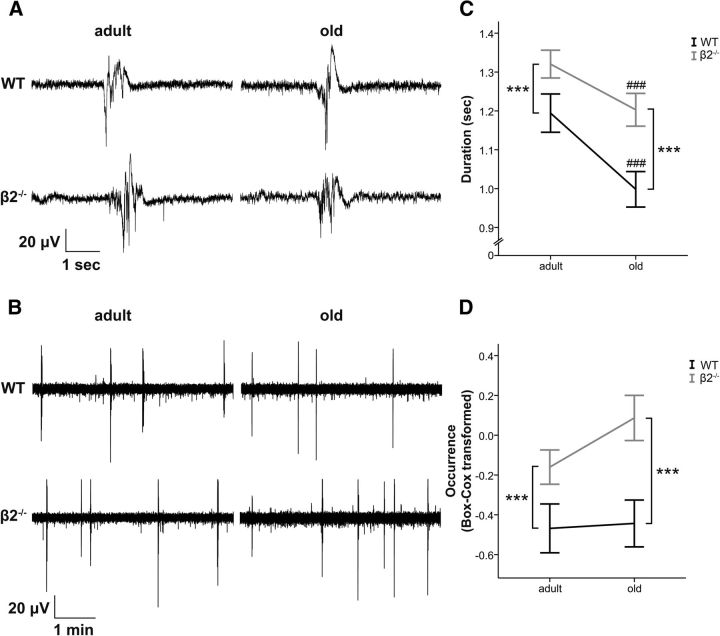
Mice lacking the β2-subunit exhibit Up states of increased duration compared with WT mice (Fig. 2A,C). Statistical analysis did not reveal a significant interaction between genotype and age (F(1,150) = 0.815, p = 0.368), whereas there was a significant main effect of genotype (F(1,150) = 14.472, p < 0.001) indicating that β2−/− animals had longer Up states and that the effect of the mutation was similar for both ages. There was also a significant main effect of age (F(1,150) = 13.007, p < 0.001) indicating that Up states become shorter in old animals, as we have previously shown (Rigas et al., 2011; mean ± SEM, WT adult: 1.19 ± 0.05 s, n = 38; WT old: 1 ± 0.05 s, n = 38; β2−/− adult: 1.32 ± 0.04 s, n = 38; β2−/− old: 1.2 ± 0.04, n = 40).
Figure 2.
Up state activity is enhanced in β2−/− mice. A, B, LFP traces (at higher and lower temporal resolution, respectively) obtained from adult (left) and old (right) animals of both genotypes. C, D, β2−/− mice (gray lines) exhibit increased Up state duration and occurrence (Box–Cox transformed for normality) compared with age-matched WT mice (black lines). Graphs show mean ± SEM. Asterisk indicates genotype differences, while # indicates age differences (two-way ANOVA, ***p < 0.001 or ###p < 0.001).
In addition to the longer duration, there was also an increased occurrence of Up states in β2−/− mice (Fig. 2B,D). Here the distribution of the data showed deviations from normality for most genotype-age groups (Shapiro–Wilk test for each group: PWTadult = 0.001, PWTold < 0.001, Pβ2−/−adult = 0.247, Pβ2−/−old = 0.015) so we used the Box–Cox transformation to obtain a normal distribution (details in Materials and Methods). This enabled us to satisfy the assumptions required to perform statistical tests that estimate the interaction between genotype and age. We found that there was no significant interaction between the two variables (F(1,150) = 1, p = 0.319), whereas there was a significant main effect of genotype (F(1,150) = 14.237, p < 0.001), indicating that β2−/− mice exhibit more frequent Up states compared with WT animals (back-transformed mean ± SEM, WT adult: 0.62 ± 0.15 events/min, n = 38; WT old: 0.63 ± 0.14 events/min, n = 38; β2−/− adult: 0.85 ± 0.1 events/min, n = 38; β2−/− old: 1.1 ± 0.14 events/min, n = 40). As we have previously shown, there was no main effect of age on the occurrence of Up states (F(1,150) = 1.498, p = 0.223; Rigas et al., 2011).
These results indicate that the chronic absence of high-affinity nicotinic receptors has a significant impact on both the generation (increased occurrence) and the termination (increased duration) of spontaneous cortical Up states, and that this effect is not differentially modulated by aging.
Inhibition of endogenously active β2-nAChRs leads to enhanced Up state activity
Since β2−/− mice are conventional knock-out animals where the gene is absent throughout embryonic and postnatal development, it is possible that the observed effect is a result of altered connectivity secondary to the genetic manipulation. Alternatively, the effects could have been a direct consequence of reduced nicotinic transmission. This latter scenario rests on the assumption that endogenous ACh is spontaneously released during network activity in cortical slices, and, hence, pharmacological blockade of β2-nAChRs in WT mice would be expected to cause changes similar to those observed in β2−/− mice. To distinguish between the two possibilities we recorded spontaneous Up states in both genotypes before and after application of the β2-selective antagonist, DHβE. We also tested for possible counterbalancing action from the α7-nAChRs by further adding the α7-nAChR antagonist, MLA, to the DHβE-containing medium (described in more detail below). As before, the experiments were performed in adult and aged mice to examine potential age-related changes in nAChR-mediated activity. The statistical analysis indicated that (1) there was no triple interaction between drug (three levels: control, DHβE, and DHβE + MLA), age, and genotype on either parameter (duration: F(2,62) = 0.452, p = 0.6; occurrence: F(2,62) = 1.628, p = 0.21) and (2) there was also no double interaction between age and drug on either parameter (duration: F(2,62) = 0.052, p = 0.922; occurrence: F(2,62) = 1.183, p = 0.305). Thus, the data from adult and old animals in each group were pooled together for demonstration purposes.
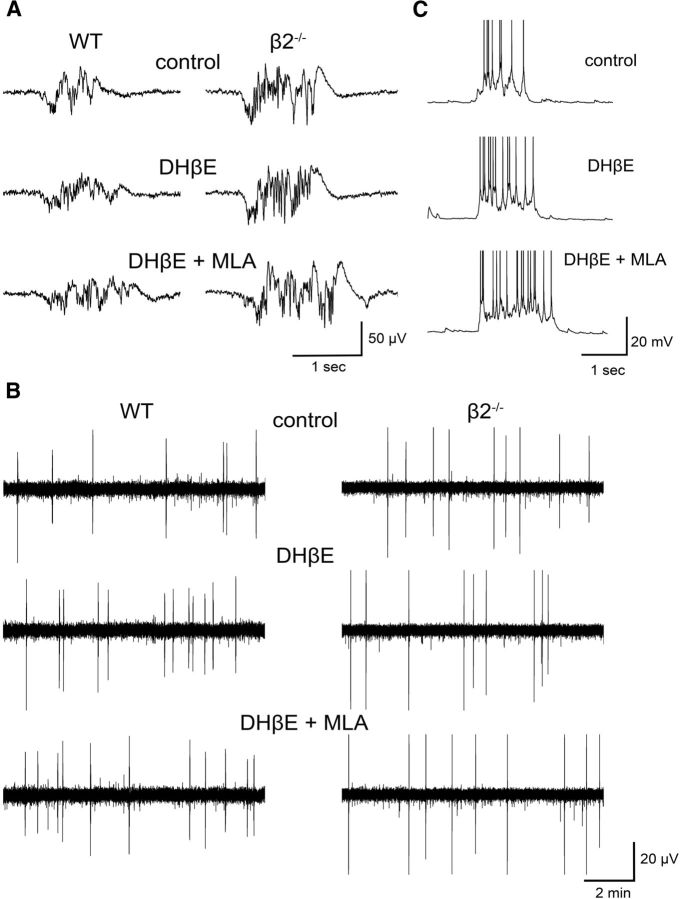
We find that application of the β2 antagonist leads to an increase in Up state activity in WT animals (Fig. 3). There was a significant interaction between drug and genotype for both parameters (duration: F(2,62) = 4.439, p = 0.023; occurrence: F(2,62) = 9.186, p < 0.001), indicating that the drug application has distinct effects on the two genotypes (Fig. 4A,B). Further analysis with post hoc pairwise comparisons for each genotype group demonstrated that DHβE significantly increased both the duration (p = 0.012) and the occurrence (p < 0.001) of Up states in the WT group only. As expected, there was no effect on either Up state duration (p = 0.697) or occurrence (p = 0.813) of β2−/− mice (nWTadult = 11, nβ2−/−adult = 10, nWTold = 6, nβ2−/−old = 8), indicating that DHβE acts exclusively on β2-nAChRs and is not associated with nonspecific effects on Up/Down state activity.
Figure 3.
Application of the β2-nAChR antagonist DHβE increases Up state duration and occurrence, and subsequent addition of the α7-nAChRs antagonist MLA causes further prolongation of Up state duration. A, B, LFP traces of Up state activity at higher and lower temporal resolution, respectively, and (C) intracellular recordings obtained in control conditions in the absence of drugs (top), in the presence of 3 μm DHβE (middle), and in the presence of 3 μm DHβE + 10 nm MLA (bottom). LFP traces in A and B are from WT (left) and β2−/− (right) mice. Traces from intracellular recordings are truncated at −25 mV.
Figure 4.
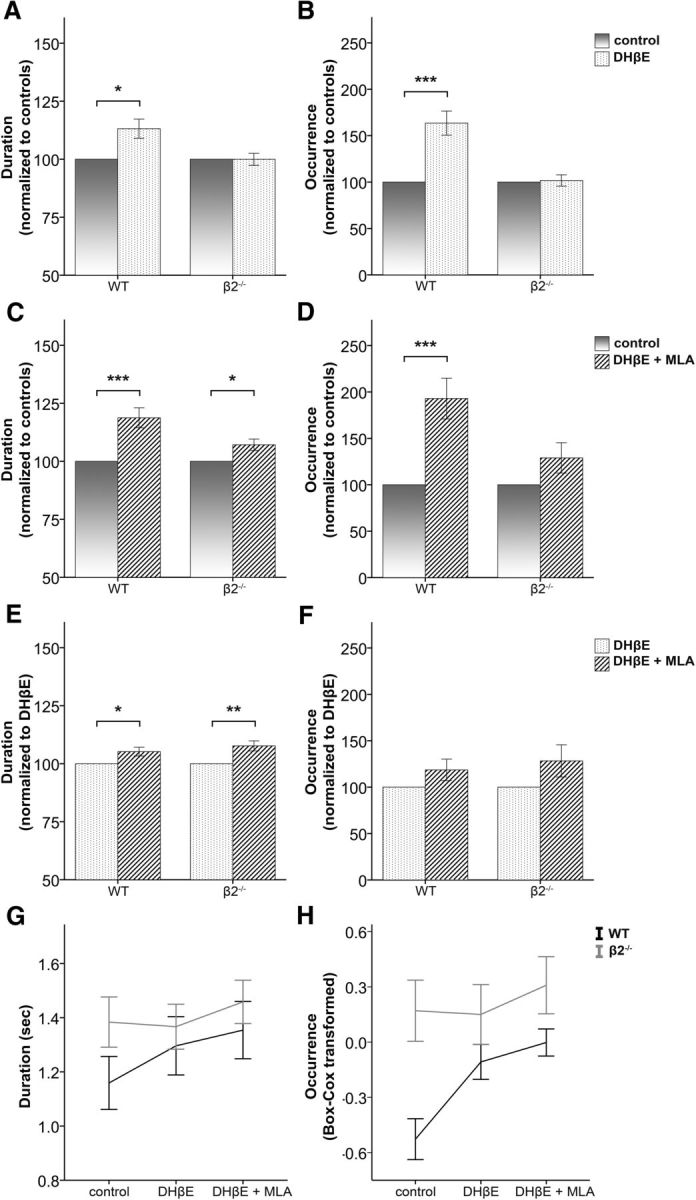
Inhibition of endogenously active β2-nAChRs and α7-nAChRs enhances Up state activity. A, B, Up state duration and occurrence, respectively, are increased in the presence of the β2-nAChR antagonist DHβE (3 μm) in WT mice only (mean ± SEM, data normalized to values obtained in the absence of DHβE). C, D, The combined effect of β2 and α7 inhibition (3 μm DHβE and 10 nm MLA) is an increase of both duration and occurrence in WT animals and an increase in duration in β2 mutants (mean ± SEM, data are normalized to the values obtained in the absence of any drug). E, F, The α7-nAChRs antagonist MLA (10 nm) causes an additional increase of Up state duration in both WT and β2−/− mice, over and above the effect of DHβE, but has no significant effect on Up state occurrence (mean ± SEM, data normalized to values obtained in the presence of DHβE). G, H, Non-normalized values of Up state duration and occurrence, respectively, before and after the addition of DHβE and MLA (mean ± SEM). Black and gray lines indicate WT and β2−/− mice, respectively. Data shown are pooled from both age groups (three-way repeated-measures ANOVA, with pairwise comparisons post hoc tests, *p < 0.05, **p < 0.01, and ***p < 0.001).
Notably, blocking β2-nAChRs in WT animals had similar effects on Up state activity as those observed in mutant mice, suggesting that β2-antagonism can accurately mimic the effect of the genetic deletion and that no significant compensatory effects take place, at least in the context of this network function. These results indicate that ACh is endogenously released in spontaneously active cortical slices and directly modulates both the generation and termination of Up states through activation of β2-nAChRs.
Inhibition of α7-nAChRs can also modify spontaneous network activity
Our findings of a clear modulatory role of the high-affinity β2-nAChRs on Up/Down states appear in conflict with certain in vivo and in vitro studies that have failed to find an effect of nicotine on this type of activity (Favero et al., 2012; Toth et al., 2012). We reasoned that the discrepancy could be from an opposite action of the other major class of nicotinic receptors in the cortex, the lower affinity α7-nAChRs, which could have masked the effect of β2-nAChRs by producing a zero net change in Up state activity after nicotine application. To test for this possibility we compared Up states recorded in the presence of DHβE + MLA (hence blocking both β2-nAChRs and α7-nAChRs) to those recorded under control conditions. If the α7-nAChR-mediated effect was opposing the β2-nAChR-mediated effect then the activity recorded in WT animals under both antagonists should not be significantly different from the control condition. Instead, our data showed that both Up state occurrence and duration increased in the presence of the two antagonists (post hoc pairwise comparisons, WT, duration: p = 0.001, occurrence: p < 0.001; Fig. 4C,D), indicating that the α7 component is not counteracting the effects of β2-nAChRs. In addition, this experiment revealed that blocking α7-nAChRs in β2−/− leads to a modest but significant further increase of Up state duration (post hoc pairwise comparisons, β2−/−, p = 0.035; Fig. 4C) but not occurrence (p = 0.260; Fig. 4D), suggesting that different components of the nicotinic pathway could have distinct effects on spontaneous Up states. This unanticipated finding prompted us to examine whether the same was true in WT mice. We therefore compared Up state characteristics in the presence of DHβE + MLA with those in the presence of DHβE alone. This approach allowed us to estimate in the same slices the effect of α7 inhibition: bath application of both antagonists compared with DHβE increased Up state duration in both genotype groups (post hoc pairwise comparisons, WT: p = 0.022; β2−/−: p = 0.005). In contrast, there was no change on Up state occurrence for either genotype (WT: p = 0.190; β2−/−: p = 0.112; nWTadult = 11, nβ2−/−adult = 10, nWTold = 6, nβ2−/−old = 8). These results revealed that inhibition of α7-nAChRs had a small but significant additional effect on the duration, but not on the occurrence of Up states, in both WT and mutant mice (Fig. 4E,F) and demonstrate that the addition of MLA did not cause a counterbalancing effect on Up state activity.
Finally, to examine whether the magnitude of this response is different between the two genotypes, we performed an additional three-way ANOVA that included only the last two levels of the variable drug (i.e., DHβE and DHβE + MLA). This analysis showed that there were no triple or double interaction effects for Up state duration (drug, genotype, and age interaction: F(1,31) = 0.502, p = 0.484; drug and genotype interaction: F(1,31) = 0.523, p = 0.475), while there was a significant main effect of drug (F(1,31) = 16.947, p < 0.001). Therefore, blocking α7-nAChRs produces similar changes in both genotype groups, suggesting a lack of functional upregulation of α7-nAChRs in mutant animals as a result of the genetic ablation of high-affinity nicotinic receptors, consistent with previously published anatomical findings (Zoli et al., 1998).
Nicotine application decreases spontaneous network activity
Our results documenting the involvement of nAChRs in spontaneous activity are at odds with recently published work examining the same network phenomenon in thalamocortical (TC) slices, according to which exogenous application of nicotine had no effect on Up state activity (Favero et al., 2012). Thus, we next explored whether this discrepancy was because of experimental differences, or, instead, reflected a distinct mechanism of action between endogenous and exogenous activation of nAChRs.
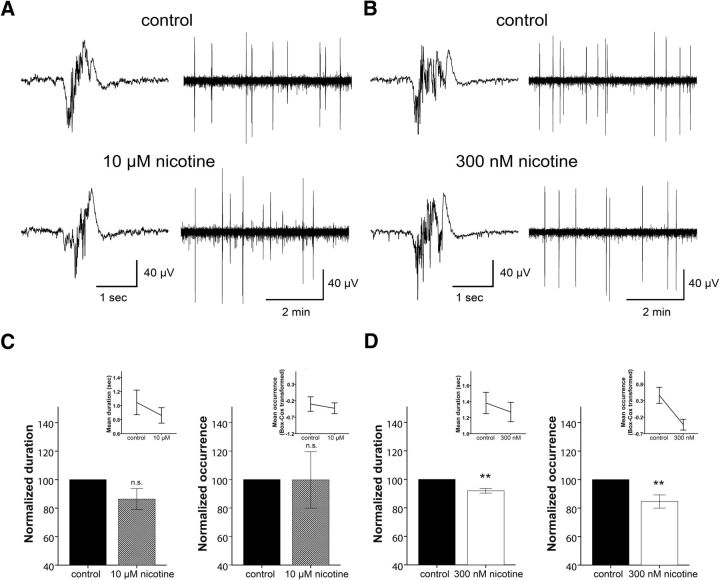
Following a 30 min baseline recording, WT slices were perfused with a solution containing 10 μm nicotine, as in Favero et al. (2012). Similarly to their data on TC slices we found that this dose of nicotine had no effect on Up state duration (t(6) = 1.962, p = 0.097) or occurrence (t(6) = 0.626, p = 0.554; n = 7; paired t test; Fig. 5A,C). This suggests that the modulation of network activity by exogenous stimulation of nAChRs is similar in the two studies, despite experimental differences. However, given the effects of β2 and α7 antagonists described above, we would have expected a decrease in Up state occurrence and duration in response to application of nicotine. We hypothesized that this inconsistency could be from the very high dose of nicotine (10 μm), which could cause rapid desensitization of the nAChRs (Dani and Bertrand, 2007). Hence, we relied on a previous study that demonstrated that intravenously supplied nicotine caused desynchronization of EEG activity in sleeping cats (Yamamoto and Domino, 1965) and estimated that the supplied nicotine ranged between 500 and 600 nm (estimation performed for maximum nicotine concentration in plasma calculated by 0.005 mg/kg nicotine supplied intravenously and assuming cat blood volume ranging between 50 and 66 ml/kg). Indeed, when we repeated the above experiment with a lower dose of nicotine (300 nm) we found that both Up state duration (t(6) = 4.555, p = 0.004) and occurrence (t(6) = 3.296, p = 0.016; n = 7) were significantly reduced in the presence of the drug (Fig. 5B,D). Hence, these results are in line with those obtained by the application of nicotinic antagonists, and provide additional confirmation that nAChRs can directly modulate the synchronized activity of local recurrent networks in the cerebral cortex.
Figure 5.
Dose-dependent effect of nicotine on Up state activity in WT mice. LFP traces of Up state activity at higher (left) and lower (right) temporal resolution in the presence of 10 μm (A) or 300 nm (B) nicotine. C, High concentration of nicotine (10 μm) is ineffective in producing significant changes in Up state duration or occurrence. D, In contrast, a low concentration of nicotine (300 nm) reduces both Up state duration and occurrence. Values in C and D are normalized to controls in the absence of 10 μm and 300 nm nicotine, respectively, while insets demonstrate non-normalized values (mean ± SEM, paired t tests, **p < 0.01).
The effect of β2-nAChRs is mediated through GABABRs
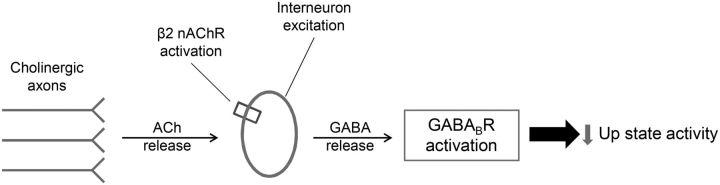
Having established a clear and previously questioned effect of nicotinic regulation of endogenous network activity, we next aimed to explore the possible mechanism of this action. Earlier studies in rats and mice have shown that ACh can directly activate a subset of cortical interneurons expressing nAChRs (Porter et al., 1999; Christophe et al., 2002; Gulledge et al., 2007; Arroyo et al., 2012), suggesting that cholinergic activation may modulate inhibition in the cortex through nAChRs. In addition, another study has demonstrated that blockade of GABABR-mediated inhibition leads to a significant increase in spontaneous Up state duration (Mann et al., 2009), very similar in direction and magnitude to the changes we observed with the β2-nAChR antagonist. Based on the results from these studies and on our present findings, we formulated a working hypothesis for the β2-mediated nicotinic regulation of Up states (Fig. 6). According to this model, the release of endogenous ACh in spontaneously active slices leads to β2-nAChR-mediated excitation of cortical interneurons, release of GABA, and reduction of Up state activity through activation of GABAB receptors. Following this rationale, the application of the β2 antagonist in the presence of GABABR blockade should fail to cause the β2-nAChR-mediated increase in Up state activity.
Figure 6.
Illustration of working hypothesis: spontaneously active cholinergic fibers release ACh leading to the depolarization of β2-nAChR-containing cortical interneurons. The subsequent release of GABA mediates Up/Down state transitions through the activation of GABAB receptors, leading to reduced Up state activity.
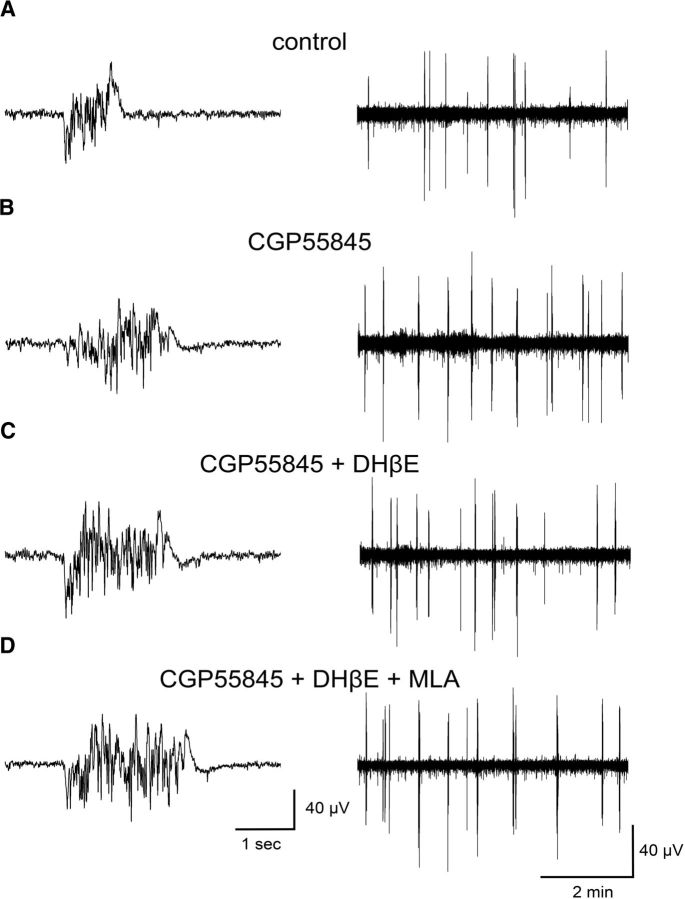
To test this hypothesis, we monitored spontaneous Up states in brain slices from adult WT animals before and after the addition of 1 μm CGP55845 in the bathing solution (Fig. 7). Repeated-measures one-way ANOVA (independent variable drug with four levels: control, CGP55845, DHβE + CGP55845, and MLA + DHβE + CGP55845) indicated that there were significant differences among the four conditions for both parameters (duration: F(3,33) = 59.23, p < 0.001; occurrence: F(3,33) = 5.173, p = 0.025). In agreement with Mann et al. (2009), further analysis with post hoc pairwise comparisons showed that Up state duration was significantly enhanced (p < 0.001, n = 12) in the presence of the GABABR antagonist (Fig. 8A). In addition, we also observed a significant increase in Up state occurrence (p = 0.022; Fig. 8B). When we further applied DHβE in the presence of CGP55845 there was no significant change in either Up state duration (p = 0.536) or occurrence (p = 0.627), compared with CGP55845 alone (Fig. 8C,D). These results suggest that blocking GABAB receptors intercepts the antagonizing effects of DHβE and are in line with our hypothesis that blocking GABAB-mediated inhibition prevents the endogenously released ACh from eliciting its effects on Up state activity.
Figure 7.
Inhibition of GABAB receptors eliminates the DHβE-mediated effects on Up state activity. LFP traces of Up state activity at higher (left) and lower (right) temporal resolution from recordings obtained in control conditions in the absence of drugs (A), and in the presence of 1 μm CGP55845 (B), 1 μm CGP55845 + 3 μm DHβE (C), and 1 μm CGP55845 + 3 μm DHβE + 10 nm MLA (D).
Figure 8.
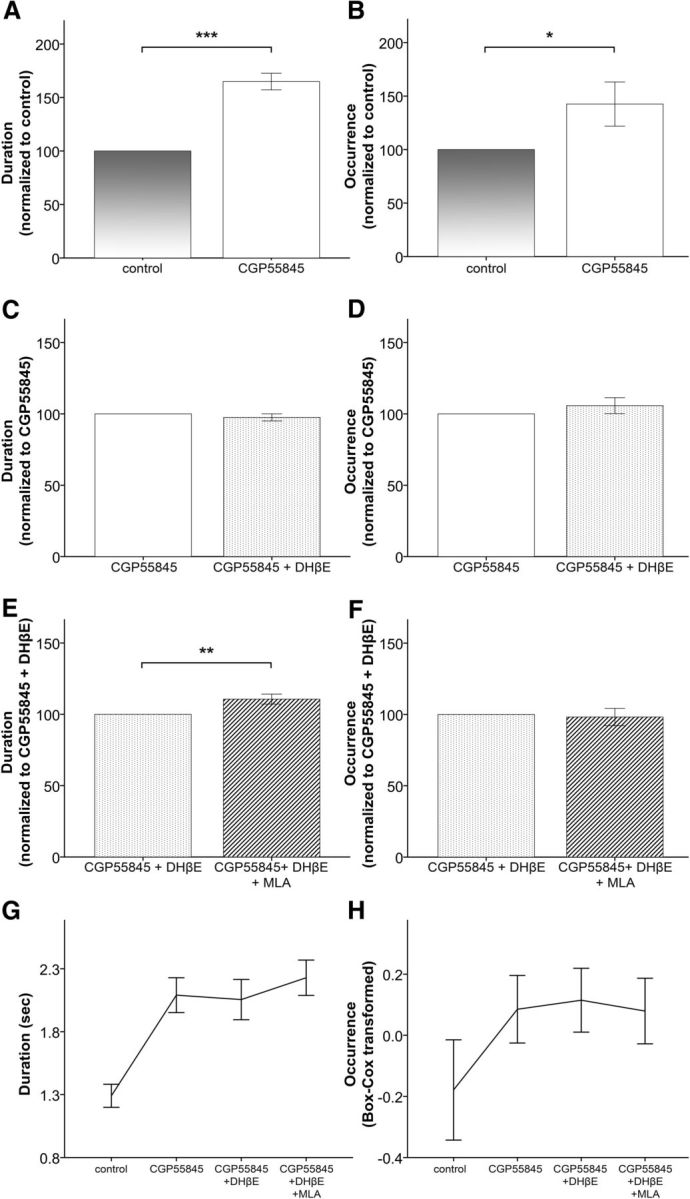
The effects of β2-nAChRs on Up state activity are at least partly mediated through GABAB receptors. A, B, Application of the GABAB receptor antagonist CGP55845 leads to increased Up state duration and occurrence (mean ± SEM, data normalized to control values obtained in the absence of CGP55845). C, D, Subsequent application of DHβE (in the presence of CGP55845) fails to produce the expected changes in Up state activity (mean ± SEM, data normalized to values in the presence of CGP55845 before the addition of DHβE). E, F, Further application of MLA leads to the expected increase in Up state duration (mean ± SEM, data normalized to values obtained in the presence of both CGP55845 and DHβE before the addition of MLA). G, H, Non-normalized values of Up state duration and occurrence, respectively, before and after the addition of CGP55845, DHβE, and MLA (mean ± SEM). These results are obtained in adult WT animals (one-way repeated-measures ANOVA, with pairwise comparisons post hoc tests, *p < 0.05, **p < 0.01, and ***p < 0.001).
Finally, we investigated whether the effects of α7-nAChRs are also mediated through the GABABR pathway, by further adding the α7 blocker to the medium already containing DHβE and CGP55845. In the presence of MLA we observed a significant increase in Up state duration (p = 0.007) but not occurrence (p = 0.536; Fig. 8E,F), similar to the effects obtained in the absence of CGP55845 (Fig. 4C,D). Hence, it seems that, unlike β2-nAChRs, the effects of α7-nAChRs on spontaneous network activity are not mediated by GABAB receptors. Moreover, the finding that MLA caused an additional increase in Up state duration demonstrates that CGP55845 does not lead to a ceiling effect in Up state activity, which could have prevented the appearance of any DHβE effects. These results suggest that blocking the nicotinic effects mediated by β2-nAChRs leads to an increase in both the occurrence and duration of Up states through reduced activation of GABAB signaling. In contrast, blocking the α7-nAChRs affects only the termination of Up state activity through a GABAB-independent mechanism.
Discussion
Previous studies have reported nAChR-mediated responses in specific types of cortical cells (Alkondon et al., 2000; Lambe et al., 2003; Poorthuis et al., 2013), but their effect on integrated network function has remained elusive. Here we examined the involvement of high-affinity nAChRs in an in vitro model of spontaneous cortical network activity. Our data reveal that ACh is endogenously released in spontaneously active slices and can modulate the transition between Up/Down states through the activation of nAChRs. We further show that the effects of β2-subunit-containing nAChRs, but not α7-subunit-containing nAChRs, are mediated through GABAB receptors. To our knowledge this is the first study documenting nicotinic modulation of Up/Down state activity.
Endogenous release of ACh in cortical slices
The effect of cholinergic blockers on Up state activity strongly suggests that ACh is endogenously released from cellular and/or synaptic elements that are preserved in the slice preparation. Possible sources of ACh could be the synaptic terminals originating in the cut fibers of the basal forebrain (Mesulam et al., 1983), which become stimulated by the intrinsic activity of the cortical tissue, and/or the sparse population of cholinergic cortical interneurons (Levey et al., 1984; Houser et al., 1985). Neurotransmitter release from neurons and severed axons in brain slices has been documented before (Zohar, 2001; Bandyopadhyay et al., 2006; von Engelhardt et al., 2007; Threlfell et al., 2012) but always after exogenous stimulation (electrical, pharmacological, or optogenetic). While such results reveal the existence of a release machinery, they do not necessarily reflect the physiological network operation. To our knowledge, our study is the first to report spontaneous release of a neuromodulator that can actively regulate synchronized network activity.
Cholinergic modulation through β2- nAChRs and α7-nAChRs
Although the main focus of the present study was the high-affinity β2-containing nAChRs, our pharmacological analysis suggests distinct roles for low- and high-affinity nicotinic receptors, by revealing that α7-nAChRs affect only the termination of spontaneous Up states, while β2-nAChRs also regulate their generation. The existence of a distinct mechanism of β2-nAChR- and α7-nAChR-mediated signaling is further supported by their different response to GABABR blockade; in the presence of CGP55845 the effect of the former was blocked implying a shared common pathway, while the latter remained unaffected. GABABR-mediated inhibition on spontaneous Down state transitions had been described in entorhinal cortical slices (Mann et al., 2009), but the mechanism that might engage this pathway remained unknown. Here we show that release of ACh can recruit GABABR-mediated inhibition and promote termination of the Up state. We propose a model in which endogenously released ACh activates β2-nAChRs on cortical interneurons (Alkondon and Albuquerque, 2004; Arroyo et al., 2012), leading to GABA release. This in turn activates GABAB receptors, resulting in an attenuation of Up state activity. In contrast, the effect of α7-nAChR-mediated activation occurs through a GABAB-independent mechanism. Further experiments should test the validity of this proposed mechanism.
Spontaneous Up states in the neocortex of β2−/− mice
The differences we observed between WT and β2−/− mice were eliminated in the presence of DHβE, suggesting that the greater Up state activity in mutant animals is directly linked to the lack of β2-nAChR-mediated signaling and not an indirect effect of altered circuitry secondary to the genetic deletion. This was surprising, given that pyramidal cell microanatomy is altered in β2 mutants (Ballesteros-Yáñez et al., 2010; Konsolaki and Skaliora, 2014). One possibility is that the increased dendritic branching and spine numbers observed in S1 neurons do not contribute to this type of network activity. Alternatively, and given the critical importance of these self-maintained depolarized states as a basic operation of cortical networks (Sanchez-Vives and McCormick, 2000; Shu et al., 2003; McCormick, 2005), we could speculate that such activity represents a robust signature of cortical dynamics that is under tight homeostatic regulation. Perhaps this robustness also accounts for the lack of an amplified or differential effect of age in β2−/− mice, despite the accelerated aging phenotype previously reported in morphological and behavioral studies (Zoli et al., 1999; Konsolaki and Skaliora, 2014). This does not contradict the possibility that the structural modifications are reflected in other aspects of network function, but it suggests that spontaneous Up states may be tolerant to alterations in individual features of neuronal microanatomy.
Nicotinic modulation of endogenous cortical activity
Our results document a clear involvement of nicotinic signaling on cortical network dynamics and, thus, seem to contradict certain in vivo studies that report mAChRs as the sole mediators of the cholinergic modulation of Up state activity during EEG desynchronization (Metherate et al., 1992; Steriade et al., 1993). However, these studies have used stimulation of cholinergic inputs that consist of heterogeneous pathways, including reciprocal connections and GABAergic axons (Zaborszky et al., 2012), which may have masked a nicotinic contribution. In addition, they examined anesthetized animals, using urethane as anesthetic, a drug that has been shown to directly affect α4β2-nAChRs (Hara and Harris, 2002). Consistent with this interpretation, and with our results, studies using naturally sleeping animals have shown that nicotine administration causes EEG desynchronization and behavioral arousal (Yamamoto and Domino, 1965; Armitage et al., 1969). Our finding of reduced Up state activity in the presence of nicotine further confirms the involvement of the nicotinic pathway in this type of network dynamics. Interestingly, the higher dose of the drug had no effect, as indeed had been reported by Favero et al. (2012). This may be attributable to a dose effect of desensitization of nAChRs (Dilger and Brett, 1990) and suggests a note of caution when interpreting data obtained with exogenous application of neuromodulators. Our results provide a potential synthesis of previously contradictory findings regarding the effect of nicotine on cortical dynamics and reveal that nAChR-mediated activity significantly modulates both the generation and the termination of cortical Up states.
Implications for cortical network function
The motivation for this study was to clarify the role of β2-nAChR-mediated signaling on endogenous cortical dynamics. The coronal slice preparation offers the opportunity to examine the cortical microcircuit isolated from the thalamus, which is also modulated by nAChRs (Léna and Changeux, 1997) and without the confounding factors of anesthetics (Hara and Harris, 2002). The spontaneous release of ACh present in the slice further allowed us to investigate the intrinsic activation of nAChRs and not rely (exclusively) on exogenous application of arbitrarily defined nicotine concentrations, which would have acted nonselectively on all receptors. In this way, we were able to identify a distinct nicotinic contribution to the regulation of Up state activity that had been controversial in the literature and showed that this regulation occurs through both β2-nAChRs and α7-nAChRs, but with different mechanisms. Moreover, we were able to provide a link to an already established regulatory pathway for Up states that is mediated through GABABRs. Our results favor a model according to which endogenous ACh directly stimulates cortical interneurons (containing β2-nAChRs) rather than indirectly through nAChRs on presynaptic glutamatergic fibers (such as those arriving from the thalamus), because blocking β2 receptors in the presence of a GABABR antagonist failed to produce any effects.
Our findings provide a framework for targeted in vivo experiments to examine the significance of these results in the intact brain. Hasselmo and McGaughy (2004) proposed a model in which ACh acting through muscarinic receptors reduces intracortical processing, while simultaneously the activation of nicotinic receptors on glutamatergic thalamocortical neurons enhances the impact of afferent inputs. Based on our results, we speculate that (in addition to mAChRs) nAChRs may also be contributing to the modulation of intracortical processing. For instance, nAChR stimulation during slow-wave activity could affect internal processing by altering the rules for plasticity (Couey et al., 2007). In addition, a basal nicotinic tone may be required to persist even in the absence of active afferent inputs to keep the network in a responsive state, akin to the function of micro-arousals during NREM sleep (for review, see Halász et al., 2013).
Concluding remarks
Local cortical networks exhibit spontaneous network activity in the form of Up/Down states—a prominent feature of cortical activity during slow-wave sleep in vivo. The fact that this type of persistent activity can be sustained in the absence of subcortical or long-range inputs has fueled the proposal that Up/Down state activity in brain slices can serve as a model of the basic operation of the cortex and that the mechanisms that generate or modulate this type of activity may form the substrate for cognitive functions such as short-term memory, memory consolidation, or modulation of neuronal activity during attention (Sanchez-Vives and McCormick, 2000; Shu et al., 2003; Major and Tank, 2004; Yuste et al., 2005). Here we have revealed a direct modulatory role for the high-affinity nAChRs acting through GABABRs in the regulation of this network phenomenon. Our results provide a framework that integrates previously contradictory results and suggests a new role for the nicotinic modulation of intrinsic persistent cortical activity under conditions of low ACh release, which could generate novel insights for therapeutic interventions of disorders related to abnormal patterns of cortical dynamics.
Footnotes
This work was supported by EU-FP7 Transmed 245928 and the Marie Curie Re-integration Grant (INTRICA). We thank Professor G.K. Kostopoulos for critical reading of this manuscript and Dr. E. Konsolaki for her contribution in animal care and valuable input on the statistical methods.
The authors declare no competing financial interests.
References
- Alkondon M, Albuquerque EX. The nicotinic acetylcholine receptor subtypes and their function in the hippocampus and cerebral cortex. Prog Brain Res. 2004;145:109–120. doi: 10.1016/S0079-6123(03)45007-3. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Eisenberg HM, Albuquerque EX. Nicotinic receptor activation in human cerebral cortical interneurons: a mechanism for inhibition and disinhibition of neuronal networks. J Neurosci. 2000;20:66–75. doi: 10.1523/JNEUROSCI.20-01-00066.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage AK, Hall GH, Sellers CM. Effects of nicotine on electrocortical activity and acetylcholine release from the cat cerebral cortex. Br J Pharmacol. 1969;35:152–160. doi: 10.1111/j.1476-5381.1969.tb07976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo S, Bennett C, Aziz D, Brown SP, Hestrin S. Prolonged disynaptic inhibition in the cortex mediated by slow, non-α7 nicotinic excitation of a specific subset of cortical interneurons. J Neurosci. 2012;32:3859–3864. doi: 10.1523/JNEUROSCI.0115-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesteros-Yáñez I, Benavides-Piccione R, Bourgeois JP, Changeux JP, DeFelipe J. Alterations of cortical pyramidal neurons in mice lacking high-affinity nicotinic receptors. Proc Natl Acad Sci U S A. 2010;107:11567–11572. doi: 10.1073/pnas.1006269107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay S, Sutor B, Hablitz JJ. Endogenous acetylcholine enhances synchronized interneuron activity in rat neocortex. J Neurophysiol. 2006;95:1908–1916. doi: 10.1152/jn.00881.2005. [DOI] [PubMed] [Google Scholar]
- Box GEP, Cox DR. An analysis of transformation. J R Stat Soc Ser B Methodol. 1964;26:211–252. [Google Scholar]
- Christophe E, Roebuck A, Staiger JF, Lavery DJ, Charpak S, Audinat E. Two types of nicotinic receptors mediate an excitation of neocortical layer I interneurons. J Neurophysiol. 2002;88:1318–1327. doi: 10.1152/jn.2002.88.3.1318. [DOI] [PubMed] [Google Scholar]
- Compte A, Reig R, Descalzo VF, Harvey MA, Puccini GD, Sanchez-Vives MV. Spontaneous high-frequency (10–80 Hz) oscillations during up states in the cerebral cortex in vitro. J Neurosci. 2008;28:13828–13844. doi: 10.1523/JNEUROSCI.2684-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couey JJ, Meredith RM, Spijker S, Poorthuis RB, Smit AB, Brussaard AB, Mansvelder HD. Distributed network actions by nicotine increase the threshold for spike-timing-dependent plasticity in prefrontal cortex. Neuron. 2007;54:73–87. doi: 10.1016/j.neuron.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- Dilger JP, Brett RS. Direct measurement of the concentration- and time-dependent open probability of the nicotinic acetylcholine receptor channel. Biophys J. 1990;57:723–731. doi: 10.1016/S0006-3495(90)82593-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow EE, Connors BW. The roles of somatostatin-expressing (GIN) and fast-spiking inhibitory interneurons in UP-DOWN states of mouse neocortex. J Neurophysiol. 2010;104:596–606. doi: 10.1152/jn.00206.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favero M, Varghese G, Castro-Alamancos MA. The state of somatosensory cortex during neuromodulation. J Neurophysiol. 2012;108:1010–1024. doi: 10.1152/jn.00256.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulledge AT, Park SB, Kawaguchi Y, Stuart GJ. Heterogeneity of phasic cholinergic signaling in neocortical neurons. J Neurophysiol. 2007;97:2215–2229. doi: 10.1152/jn.00493.2006. [DOI] [PubMed] [Google Scholar]
- Hájos N, Ellender TJ, Zemankovics R, Mann EO, Exley R, Cragg SJ, Freund TF, Paulsen O. Maintaining network activity in submerged hippocampal slices: importance of oxygen supply. Eur J Neurosci. 2009;29:319–327. doi: 10.1111/j.1460-9568.2008.06577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halász P, Kelemen A, Szűcs A. The role of NREM sleep micro-arousals in absence epilepsy and in nocturnal frontal lobe epilepsy. Epilepsy Res. 2013;107:9–19. doi: 10.1016/j.eplepsyres.2013.06.021. [DOI] [PubMed] [Google Scholar]
- Hara K, Harris RA. The anesthetic mechanism of urethane: the effects on neurotransmitter-gated ion channels. Anesth Analg. 2002;94:313–318. doi: 10.1213/00000539-200202000-00015. table of contents. [DOI] [PubMed] [Google Scholar]
- Harris RJ, Wieloch T, Symon L, Siesjö BK. Cerebral extracellular calcium activity in severe hypoglycemia: relation to extracellular potassium and energy state. J Cereb Blood Flow Metab. 1984;4:187–193. doi: 10.1038/jcbfm.1984.27. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, McGaughy J. High acetylcholine levels set circuit dynamics for attention and encoding and low acetylcholine levels set dynamics for consolidation. Prog Brain Res. 2004;145:207–231. doi: 10.1016/S0079-6123(03)45015-2. [DOI] [PubMed] [Google Scholar]
- Houser CR, Crawford GD, Salvaterra PM, Vaughn JE. Immunocytochemical localization of choline acetyltransferase in rat cerebral cortex: a study of cholinergic neurons and synapses. J Comp Neurol. 1985;234:17–34. doi: 10.1002/cne.902340103. [DOI] [PubMed] [Google Scholar]
- Ivanov A, Zilberter Y. Critical state of energy metabolism in brain slices: the principal role of oxygen delivery and energy substrates in shaping neuronal activity. Front Neuroenergetics. 2011;3:9. doi: 10.3389/fnene.2011.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HC, Keep RF. Brain fluid calcium concentration and response to acute hypercalcaemia during development in the rat. J Physiol. 1988;402:579–593. doi: 10.1113/jphysiol.1988.sp017223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klink R, de Kerchove d' Exaerde A, Zoli M, Changeux JP. Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci. 2001;21:1452–1463. doi: 10.1523/JNEUROSCI.21-05-01452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konsolaki E, Skaliora I. Premature aging phenotype in mice lacking high-affinity nicotinic receptors: region-specific changes in layer V pyramidal cell morphology. Cereb Cortex. 2014 doi: 10.1093/cercor/bhu019. doi: 10.1093/cercor/bhu019. Advance online publication. Retrieved February 18, 2014. [DOI] [PubMed] [Google Scholar]
- Lambe EK, Picciotto MR, Aghajanian GK. Nicotine induces glutamate release from thalamocortical terminals in prefrontal cortex. Neuropsychopharmacology. 2003;28:216–225. doi: 10.1038/sj.npp.1300032. [DOI] [PubMed] [Google Scholar]
- Léna C, Changeux JP. Role of Ca2+ ions in nicotinic facilitation of GABA release in mouse thalamus. J Neurosci. 1997;17:576–585. doi: 10.1523/JNEUROSCI.17-02-00576.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léna C, Popa D, Grailhe R, Escourrou P, Changeux JP, Adrien J. β2-containing nicotinic receptors contribute to the organization of sleep and regulate putative micro-arousals in mice. J Neurosci. 2004;24:5711–5718. doi: 10.1523/JNEUROSCI.3882-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey AI, Wainer BH, Rye DB, Mufson EJ, Mesulam MM. Choline acetyltransferase-immunoreactive neurons intrinsic to rodent cortex and distinction from acetylcholinesterase-positive neurons. Neuroscience. 1984;13:341–353. doi: 10.1016/0306-4522(84)90234-3. [DOI] [PubMed] [Google Scholar]
- Major G, Tank D. Persistent neural activity: prevalence and mechanisms. Curr Opin Neurobiol. 2004;14:675–684. doi: 10.1016/j.conb.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Mann EO, Kohl MM, Paulsen O. Distinct roles of GABA(A) and GABA(B) receptors in balancing and terminating persistent cortical activity. J Neurosci. 2009;29:7513–7518. doi: 10.1523/JNEUROSCI.6162-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansvelder HD, van Aerde KI, Couey JJ, Brussaard AB. Nicotinic modulation of neuronal networks: from receptors to cognition. Psychopharmacology. 2006;184:292–305. doi: 10.1007/s00213-005-0070-z. [DOI] [PubMed] [Google Scholar]
- McCormick DA. Neuronal networks: flip-flops in the brain. Curr Biol. 2005;15:R294–R296. doi: 10.1016/j.cub.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Wainer BH, Levey AI. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1-Ch6) Neuroscience. 1983;10:1185–1201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- Metherate R, Cox CL, Ashe JH. Cellular bases of neocortical activation: modulation of neural oscillations by the nucleus basalis and endogenous acetylcholine. J Neurosci. 1992;12:4701–4711. doi: 10.1523/JNEUROSCI.12-12-04701.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogg AJ, Whiteaker P, McIntosh JM, Marks M, Collins AC, Wonnacott S. Methyllycaconitine is a potent antagonist of α-conotoxin-MII-sensitive presynaptic nicotinic acetylcholine receptors in rat striatum. J Pharmacol Exp Ther. 2002;302:197–204. doi: 10.1124/jpet.302.1.197. [DOI] [PubMed] [Google Scholar]
- Oppenheim AV, Schafer RW, Buck JR. Discrete-time signal processing. ed 2. Upper Saddle River, NJ: Prentice Hall; 1999. [Google Scholar]
- Picciotto MR, Zoli M. Nicotinic receptors in aging and dementia. J Neurobiol. 2002;53:641–655. doi: 10.1002/neu.10102. [DOI] [PubMed] [Google Scholar]
- Poorthuis RB, Bloem B, Schak B, Wester J, de Kock CP, Mansvelder HD. Layer-specific modulation of the prefrontal cortex by nicotinic acetylcholine receptors. Cereb Cortex. 2013;23:148–161. doi: 10.1093/cercor/bhr390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JT, Cauli B, Tsuzuki K, Lambolez B, Rossier J, Audinat E. Selective excitation of subtypes of neocortical interneurons by nicotinic receptors. J Neurosci. 1999;19:5228–5235. doi: 10.1523/JNEUROSCI.19-13-05228.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter AS, Newhouse PA. Effects of acute nicotine administration on behavioral inhibition in adolescents with attention-deficit/hyperactivity disorder. Psychopharmacology. 2004;176:183–194. doi: 10.1007/s00213-004-1874-y. [DOI] [PubMed] [Google Scholar]
- Rigas P, Castro-Alamancos MA. Thalamocortical Up states: differential effects of intrinsic and extrinsic cortical inputs on persistent activity. J Neurosci. 2007;27:4261–4272. doi: 10.1523/JNEUROSCI.0003-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigas P, Sigalas C, Skaliora I. Developmental regulation of spontaneous network activity in mouse cortical slices. Neurosci Lett. 2011:e40. doi: 10.1016/j.neulet.2011.05.184. 500, Supplement. [DOI] [Google Scholar]
- Salin-Pascual RJ, Moro-Lopez ML, Gonzalez-Sanchez H, Blanco-Centurion C. Changes in sleep after acute and repeated administration of nicotine in the rat. Psychopharmacology. 1999;145:133–138. doi: 10.1007/s002130051041. [DOI] [PubMed] [Google Scholar]
- Sanchez-Vives MV, McCormick DA. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat Neurosci. 2000;3:1027–1034. doi: 10.1038/79848. [DOI] [PubMed] [Google Scholar]
- Sanchez-Vives MV, Descalzo VF, Reig R, Figueroa NA, Compte A, Gallego R. Rhythmic spontaneous activity in the piriform cortex. Cereb Cortex. 2008;18:1179–1192. doi: 10.1093/cercor/bhm152. [DOI] [PubMed] [Google Scholar]
- Shu Y, Hasenstaub A, McCormick DA. Turning on and off recurrent balanced cortical activity. Nature. 2003;423:288–293. doi: 10.1038/nature01616. [DOI] [PubMed] [Google Scholar]
- Steriade M, Amzica F, Nuñez A. Cholinergic and noradrenergic modulation of the slow (approximately 0.3 Hz) oscillation in neocortical cells. J Neurophysiol. 1993;70:1385–1400. doi: 10.1152/jn.1993.70.4.1385. [DOI] [PubMed] [Google Scholar]
- Threlfell S, Lalic T, Platt NJ, Jennings KA, Deisseroth K, Cragg SJ. Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron. 2012;75:58–64. doi: 10.1016/j.neuron.2012.04.038. [DOI] [PubMed] [Google Scholar]
- Timofeev I, Grenier F, Steriade M. Disfacilitation and active inhibition in the neocortex during the natural sleep-wake cycle: an intracellular study. Proc Natl Acad Sci U S A. 2001;98:1924–1929. doi: 10.1073/pnas.98.4.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth A, Hajnik T, Detari L. Cholinergic modulation of slow cortical rhythm in urethane-anesthetized rats. Brain Res Bull. 2012;87:117–129. doi: 10.1016/j.brainresbull.2011.10.005. [DOI] [PubMed] [Google Scholar]
- von Engelhardt J, Eliava M, Meyer AH, Rozov A, Monyer H. Functional characterization of intrinsic cholinergic interneurons in the cortex. J Neurosci. 2007;27:5633–5642. doi: 10.1523/JNEUROSCI.4647-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto KI, Domino EF. Nicotine-induced EEG and behavioral arousal. Int J Neuropharmacol. 1965;4:359–373. doi: 10.1016/0028-3908(65)90016-X. [DOI] [PubMed] [Google Scholar]
- Yuste R, MacLean JN, Smith J, Lansner A. The cortex as a central pattern generator. Nat Rev Neurosci. 2005;6:477–483. doi: 10.1038/nrn1686. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, van den Pol A, Gyengesi E. The basal forebrain cholinergic projection system in mice. In: Watson C, Paxinos G, Puelles L, editors. The mouse nervous system. Amsterdam: Elsevier-Academic Press; 2012. pp. 684–718. [Google Scholar]
- Zohar O. Electrophysiological and ultrastructural changes in severed motor axons of the crayfish. Neurosci Res. 2001;41:151–159. doi: 10.1016/S0168-0102(01)00273-5. [DOI] [PubMed] [Google Scholar]
- Zoli M, Léna C, Picciotto MR, Changeux JP. Identification of four classes of brain nicotinic receptors using β2 mutant mice. J Neurosci. 1998;18:4461–4472. doi: 10.1523/JNEUROSCI.18-12-04461.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoli M, Picciotto MR, Ferrari R, Cocchi D, Changeux JP. Increased neurodegeneration during ageing in mice lacking high-affinity nicotine receptors. EMBO J. 1999;18:1235–1244. doi: 10.1093/emboj/18.5.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]