Abstract
Many endocytic proteins accumulate in the reserve pool of synaptic vesicles (SVs) in synapses and relocalize to the endocytic periactive zone during neurotransmitter release. Currently little is known about their functions outside the periactive zone. Here we show that in the Drosophila neuromuscular junction (NMJ), the endocytic scaffolding protein Dap160 colocalizes during the SV cycle and forms a functional complex with the SV-associated phosphoprotein synapsin, previously implicated in SV clustering. This direct interaction is strongly enhanced under phosphorylation-promoting conditions and is essential for proper localization of synapsin at NMJs. In a dap160 rescue mutant lacking the interaction between Dap160 and synapsin, perturbed reclustering of SVs during synaptic activity is observed. Our data indicate that in addition to the function in endocytosis, Dap160 is a component of a network of protein–protein interactions that serves for clustering of SVs in conjunction with synapsin. During the SV cycle, Dap160 interacts with synapsin dispersed from SVs and helps direct synapsin back to vesicles. The proteins function in synergy to achieve efficient clustering of SVs in the reserve pool.
SIGNIFICANCE STATEMENT We provide the first evidence for the function of the SH3 domain interaction in synaptic vesicle (SV) organization at the synaptic active zone. Using Drosophila neuromuscular junction as a model synapse, we describe the molecular mechanism that enables the protein implicated in SV clustering, synapsin, to return to the pool of vesicles during neurotransmitter release. We also identify the endocytic scaffolding complex that includes Dap160 as a regulator of the events linking exocytosis and endocytosis in synapses.
Keywords: Drosophila neuromuscular junction, scaffolding proteins, synapse, synapsin, synaptic vesicles, vesicle clustering
Introduction
The synaptic vesicle cycle is a key cellular mechanism that sustains neurotransmitter release in the synapses, the contacts between nerve cells in the brain. Neurotransmitter release occurs at the active zone of synapses by exocytotic fusion of discrete, uniformly sized synaptic vesicles (SVs), which undergo local recycling by endocytosis at the synaptic periactive zone (PAZ; Haucke et al., 2011; Südhof, 2013; Gauthier-Kemper et al., 2015). Numerous studies have examined the mechanisms of exocytosis and endocytosis in synapses. Little is known, however, about the molecular links coordinating these two events (Haucke et al., 2011; Gauthier-Kemper et al., 2015). This coordination is critical to assure proper sorting of the lipids and SV cargo proteins after fusion, to facilitate translocation of these components from the active to the PAZ for endocytosis, and finally to enable clustering of newly formed vesicles back at the site of release.
For decades a family of SV-associated phosphoproteins, the synapsins, have been implicated in SV clustering at the active zone (Greengard et al., 1993; Cesca et al., 2010). Recent studies identified additional proteins in the vesicle pool, many of which appeared to be endocytic proteins (Shupliakov, 2009; Denker and Rizzoli, 2010). Among them is the multidomain scaffolding protein intersectin (ITSN), named Dap160 in Drosophila, in complex with epidermal growth factor receptor substrate 15 (Eps15; Koh et al., 2007; Pechstein and Shupliakov, 2010). The function of these proteins in the SV cluster still remains obscure. Molecular studies have confirmed that this complex interacts both with synaptic proteins involved in exocytosis (e.g., SNAP-25) and with key endocytic proteins, including FCHo (Fer/Cip4 homology domain-only), AP2, stonin 2, epsin, synaptojanin, and dynamin. In addition, its binding partners include several actin-regulating molecules, such as N-Wasp (neuronal Wiskott–Aldrich syndrome protein), Cdc42, and Nwk (nervous wreck; Montesinos et al., 2005; Henne et al., 2010; Pechstein et al., 2010; Gubar et al., 2013). The plethora of interactions, in which the ITSN/Dap160–Eps15 complex is engaged in during the course of the SV cycle infers that switches between binding partners must occur in a spatially and temporally regulated manner.
ITSN and Eps15 both have several genes in the mammalian genome, thus making genetic manipulations of the proteins very complex. Knock-out of one of the genes, ITSN1, in mice displayed only a mild phenotype (Yu et al., 2008; Hunter et al., 2011). Complete genetic deletion of both proteins has been achieved in Drosophila, due to the presence of only one copy of dap160 and one copy of the eps15 gene (Koh et al., 2007). Ablation of the Dap160 and Eps15 proteins in this model organism causes severe developmental defects and results in abnormal synaptic architecture and reduction in vesicle number. Furthermore, larvae do not survive the pupal stage. The inability to sustain neurotransmission at high stimulation rates and profound loss of SVs during stimulation is consistent with defects in SV recycling found in double knock-out synapses and were proposed to be the main cause of the observed defects. It has been noted in addition that the localization of the Dap160–Eps15 complex in Drosophila neuromuscular junctions (NMJs) is dynamic. The proteins are present in the reserve vesicle pool at rest and are then detected at the PAZ during endocytosis. It is thus possible that other steps of the vesicle cycle also could also be disrupted (Koh et al., 2007). Parallel studies have reported similar behavior of the Dap160 ortholog ITSN in vertebrate synapses (Evergren et al., 2006, 2007). All these data suggest that these scaffolding proteins serve as a complex that coordinates effector proteins at multiple steps of the vesicle cycle (Shupliakov, 2009).
Here we show that the Dap160 component of the Dap160–Eps15 scaffolding complex is involved in interaction with the SV-associated protein synapsin in Drosophila NMJs. Genetic perturbation of the Dap160–synapsin complex formation perturbs trafficking of synapsin in the nerve terminal and proper assembly of SV into clusters at release sites during synaptic activity. Our study uncovers a novel molecular mechanism that controls clustering of SVs in synapses.
Materials and Methods
Drosophila melanogaster stocks, dap160 transgenes, and expression constructs. dap160Δ1/Df(2L)bur-K1, referred to as dap160 null in the text, have been generated in other studies and was a kind gift from Dr. H. J. Bellen (Koh et al., 2004). For dap160 rescue experiments, the pan-neural driver line C155-Gal4; Df(2L)bur-K1/CyO, twi-GFP were crossed to UAS-dap160ΔB15C; dap160Δ1/CyO, twi-GFP, or w; dap160Δ1/CyO, twi-GFP; UAS-dap160ΔAB28B or w; dap160Δ1/CyO, twi-GFP; UAS-dap160WT (kind gift from Dr. G. Davies) and F1, non-GFP larvae were selected for experiments. Synapsin-null mutant, P-insertion strain SynCS97 was a kind gift from E. Buchner (Godenschwege et al., 2004). shibirets1 was obtained from the Bloomington Stock Center. w1118 was used as wild-type control with the same genetic background as transgenes and referred to in the text as “control.” Animals of either sex were used in experiments.
Generation of the UAS-rescue constructs with the SH3A and SH3B domains deleted (pUAST-dap160ΔAB) or with the SH3B domain deleted (pUAST-dap160ΔB) has been described previously (Winther et al., 2013). The expression construct of Dap160 SH3 domains (GST-ABCD; Fig. 1) and synapsin constructs (GST-N-prp and GST-C-prp) in the pGEX-6P-2 vector (GE Healthcare) were obtained by PCR using as a template Dap160 cDNA clone IP14822, FlyBase ID FBcl0001466, and synapsin cDNA clone RE44971, FlyBase ID FBcl0194064 (Drosophila Genomics Resource Center, Bloomington, IN). The GST-ABC, GST-ABD, GST-ACD, GST-CD, GST-PP/AA, and GST-R/A constructs were generated by self-ligation of blunt-ended PCR products (Q5 polymerase, New England BioLabs). The primer sequences are as follows with the corresponding template plasmid shown in parentheses: for GST-ABCD (dap160 cDNA clone): ATCGCGGATCCGAGCTCAAGGCCGAGCTC and AGCGTTGCGGCCGCTCATCACTTCTTGGTGGTGCCATTTG; GST-ABC (GST-ABCD): GAGAACCTTCACGTAAGT and ACGTCCGGTAAGCCAGCAAAAG; GST-ABD (GST-ABCD*): GGAAGAGGTGCGTGACATG and GACAAGGTCATTGCTCTCTATC; GST-ACD (GST-ABCD): CTCGACATCGCCGGCGGC and GCTGATGTGGGCACAGCGAG; GST-CD (GST-ABCD): ACTGGAAGAGCCTGTGTCCCA and GCTGATGTGGGCACAGCGAG; GST-A (GST-ABCD): ATCGCGGATCCAAATATCAAGCAGTATACGAGTTCAAC and AGCGTTGCGGCCGCTCACTCGACATCGCCGGCGG; GST-B (GST-ABCD): ATCGCGGATCCCTAGAAGTCGGTGAAGTTG and AGCGTTGCGGCCGCTCACTTTTGGACATAGTTCGAAG; GST-C (GST-ABCD): ATCGCGyGATCCGCTGATGTGGGCACAGC and AGCGTTGCGGCCGCTCAGTCCAAGATCTGTTCAGTCATCTC; GST-D (GST-ABCD): ATCGCGGATCCCTCCAAGGCGGGCGCAAC and AGCGTTGCGGCCGCTCACTTCTTGGTGGTGCCATTTG; GST-N-prp (Synapsin cDNA clone): ATCGCGGATCCGAGCTGTCGTTGAGCTTTGG and AGCGTTGCGGCCGCTCACTTGTCCTTGCTGAATGCCG; GST-C-prp (Synapsin cDNA clone): ATCGCGGATCCTCCGGACGGATGCAGAACGTC and AGCGTTGCGGCCGCTCAGGAAGTCTGCGAATCACGACG; GST-PP/AA (GST-C-prp): TATAGGAGCTGGTCCACCCATGGGC and GCAGAGCGTACCTCACCCGCC; GST-R/A (GST-C-prp): CTCCGGTATCGGTGGTGGTC and GCTACCTCACCCGCCGTGGG.
Figure 1.
Direct interaction between Dap160 and synapsin. A, Representative IP from DHE using preimmune serum (control) or Dap160 antibodies (anti-Dap160) under nontreated (NT), dephospho-promoting (CIP), and phosphorylation-promoting (ATP) conditions. Samples (n = 3) were analyzed by immunoblotting for Dap160, Eps15, and synapsin, or for actin as a negative control. Arrows show position of the bands corresponding to synapsin isoforms. Coprecipitation of synapsin increases under ATP compared with CIP and NT. B, Schematic representation of amino acid sequences from Dap160 SH3 domain cassette expressed as GST-fusion proteins used in pull-down experiments shown in C–E. C, GST-ABCD cassette binds synapsin under NT, CIP, and ATP conditions. Samples (n = 3) were analyzed by immunoblotting for synapsin and dynamin and for actin as negative control. D, Association of synapsin and dynamin present in detergent-lysed head extract to GST-SH3 domains of Dap160. Samples (n = 4) were analyzed by immunoblotting for synapsin, dynamin, and actin. Bar graph below shows quantification of densitometry measurements (mean + SD) for different GST-SH3 constructs plotted as percentage of association to GST-ABCD. Bars represent mean + SEM; ***p < 0.001, one-way ANOVA with Bonferroni's post-test. E, Pull-down experiment with combinations of GST-Dap160 SH3 domains (n = 4). Note that GST-CD does not show binding to synapsin. F, Two constructs containing N-terminus and C-terminus class II proline-rich motifs (shown in bold) in Drosophila synapsin (N-prp and C-prp) and mutants, in which prolines 445 and 448 or arginine 450 were replaced with alanine (gray), that were fused with GST and used in pull-down experiments shown in G and H. G, H, GST-C-prp directly interacts with Dap160. GST and GST-fused N-prp and C-prp in G, or C-prp peptide binding defective mutants (PP/AA) or (R/A) in H were incubated with DHE. Samples were analyzed by immunoblotting for Dap160 and Eps15 or for actin as a negative control. The pull-down experiments shown in G and H were replicated twice.
All constructs were verified by sequencing. The GST-ABCD* and GST-AB constructs were described previously (Winther et al., 2013).
Immunoprecipitation and GST pull-down.
Drosophila head extract (DHE) was prepared by homogenization of fly heads in a buffer [immunoprecipitation (IP) buffer: 20 mm HEPES, 100 mm KCl, 2 mm MgCl2, 0.5% NP40] plus protease inhibitors (Sigma-Aldrich). Debris was removed by centrifugation. Five micrograms of an affinity purified rabbit anti-Dap160 or control IgGs were bound to Dynabeads (14311D, Life Technologies) or Protein A/G PLUS-Agarose (Santa Cruz Biotechnology), washed with the IP buffer, and then incubated with 1 mg of DHE for 2 h (nontreated conditions). Protein A (GE Healthcare) purified preimmune rabbit serum was used as control. For IP under dephospho-promoting conditions, DHE was supplemented with kinase inhibitor roscovitine (Sigma-Aldrich) and 20 U calf intestinal alkaline phosphatase (CIP) FastAP (ThermoScientific); for phospho-promoting conditions, DHE was supplemented with phosphatase inhibitor mixtures 1 and 2 (P5726 and P2850, Sigma-Aldrich), calcineurin inhibitor FK506 (Abcam), 2 mm ATP, and 1 mm CaCl2 (modified from Slepnev et al., 1998). All extracts were incubated at 30°C for 10 min, transferred into ice, and added to bound antibodies. After incubation for 2 h at 4°C on a rotating wheel and extensive washes in IP buffer, bound complexes were eluted with a NuPAGE lithium dodecyl sulfate sample buffer (Invitrogen) and analyzed by NuPAGE Bis-Tris gel (Invitrogen) and by immunoblotting using specific antibodies (see below). To verify that phospho-promoting conditions used may induce phosphorylation of proteins, they were tested on rat brain extract with dynamin phospho-specific antibodies pSer744 (ThermoFisher Scientific) as earlier described (Slepnev et al., 1998).
For GST pull-down, GST and GST-fusion proteins were expressed in BL21 (DE3) cells and purified according to standard protocols using glutathione Sepharose (GE Healthcare). Twenty-five to forty-five micrograms (taken in equal molar amounts) of corresponding proteins were bound to glutathione Sepharose beads (GE Healthcare) and added to 3 mg of DHE in IP buffer with protease inhibitors (Sigma-Aldrich). The extracts were incubated on a rotation wheel for 4 h at 4°C. Following extensive washes, samples were eluted with a sample buffer and analyzed by SDS/PAGE (Invitrogen) and immunoblotting. A BCA Protein Assay Kit (Pierce) was used to determine total protein concentration of DHE. Densitometry was used to quantify IP data. Measured intensity levels were normalized to the control condition to enable statistical comparison.
Immunohistochemistry, imaging, and quantification of fluorescence images.
Labeling of third-instar larval fillets was performed as previously described (Winther et al., 2013). Briefly, animals were dissected in HL3 without Ca2+ and fixed in 4% paraformaldehyde in PBS for 20 min. Tissue was washed in PBS and permeabilized in PBS with 0.4% Triton X-100 and then incubated with primary antibodies overnight at 4°C. Secondary antibodies conjugated to Alexa 488, 555, or 647 (Invitrogen) were used at 1:500. Samples were mounted in Vectashield mounting medium (Vector Laboratories). Confocal microscopy images were captured with 63×, 1.4 numerical aperture oil-immersion objectives using the LSM 700 system (Carl Zeiss). All measurements were performed in ImageJ or Volocity (PerkinElmer). Volocity was used for evaluating levels of colocalization. Pearson's correlation coefficient (PCC) was determined for individual boutons selected as region of interest. Measurements based on confocal images were from 10 to 15 boutons from ≥3 different animals for each condition and genotype. To reveal bouton outline, third-instar larval fillets were labeled with anti-HRP (Jackson Immunoresearch). Presynaptic T-bars of the active zone were detected by labeling with anti-Brp.
Quantification of immunofluorescence levels was performed as previously described (Koh et al., 2004, 2007). Briefly, labeling of control and mutant fillets was performed in the same Eppendorf tube, and images were captured and processed using identical settings. The ratio between the labeling intensity over the whole presynaptic bouton and the labeling intensity sampled in 0.5 μm2 region of the axon juxtaposed to the bouton was calculated to evaluate dispersion of immunofluorescence into the axonal shaft adjacent to the bouton. To follow dynamic changes in this ratio during recovery after synaptic activity, several preparations were stimulated by immersion into 60 mm K+ for 10 min in the same dish and then fixed after incubation in normal HL3 saline for different periods. Mean ratio values were calculated by averaging data from 15 boutons in ≥3 larvae and plotted at respective time points of recovery to visualize dynamic changes in fluorescence intensity.
Pre-embedding immunocytochemistry and electron microscopy.
Fillets from third-instar larvae were prepared in HL3 without Ca2+ and then transferred to normal HL3 saline. For experiments, fillets were incubated in either Ca2+-free HL3 buffer containing 50 mm EDTA for 10 min (resting conditions) or stimulated by addition of 60 mm K+ for 10 min. The specimens were fixed in 3% glutaraldehyde in 0.1 m cacodylate buffer, pH 7.2, for conventional TEM and 4% paraformaldehyde solution, in 0.1 m phosphate buffer, pH 7.2, for immuno-electron microscopy (immuno-EM). For immunogold experiments, the muscles from the fillets were cut off from the cuticle, washed in PBS, and embedded in Agarose (Sigma-Aldrich). The block was then cut into 100-μm-thick sections with a Vibratome (Leica). Vibratome slices were incubated with primary antibodies followed by secondary antibodies conjugated to 1.4 nm gold particles (Nanoprobes). The immunogold labeling was silver enhanced using an IntenSE Silver Enhancement kit (GE Healthcare) or an HQ Silver kit (Nanoprobes). Samples were postfixed in 0.2% OsO4, dehydrated in alcohol, and embedded in Durcupan ACM (Fluka) for ultrathin sectioning. Serial ultrathin sections were cut with a diamond knife (Diatome) and stained with 1% uranyl acetate and lead citrate on grids. The grids were then examined with a Tecnai 12 electron microscope (FEI).
Antibodies.
A panel of antibodies was used for immunocytochemistry experiments in the following dilutions: polyclonal rabbit and guinea pig Dap160 antisera (rabbit, 1:500; guinea pig, 1:1000; Koh et al., 2007); mouse anti-dynamin (1:300; clone 41; BD Biosciences); mouse anti-Brp (1:100; Wagh et al., 2006); guinea pig anti-Eps15 (1:1000; Koh et al., 2007); rabbit anti-synaptotagmin (1:1000; Littleton et al., 1993); mouse anti-synapsin (1:25; University of Iowa Hybridoma Bank), and goat anti-HRP directly conjugated to DyLigth649 (1:200; Jackson Immunoresearch). Ten times lower concentrations were used for immunoblotting. Affinity-purified rabbit anti-Dap160 antibody was used in IP experiments. Mouse monoclonal anti-actin (#1501, clone C4; Millipore) was used as previously described (Winther et al., 2013).
Electrophysiology.
Third-instar larvae of either sex were dissected in Ca2+-free HL3 solution and physiological recordings performed from muscles 6 and 7 as described previously (Koh et al., 2007; Winther et al., 2013). All recordings were analyzed using Clampfit v10 software and data processed using Graph Pad Prism v5. Recordings were taken from 5 to 11 animals per genotype and condition.
Quantification of EM images.
Images were quantified using National Institutes of Health ImageJ software as earlier described (Winther et al., 2013). Relative distribution of gold particles (percentage) was calculated as the ratio of gold particle density in the proximal SV pool, distal SV pool, or PAZ to the sum of gold particle densities in these areas. Proximal SV pool area was defined as the cytosolic space 70 nm into the lumen from the platform of the T-bar. Distal SV pool area was defined by a trapezoid space on top of the proximal SV pool stretching 200 nm into the lumen with interior angles of 135° (Fig. 2D,E). The PAZ area was defined as the plasma membrane space within 250 nm of the active zone border and the cytosolic space ≤50 nm within the lumen from the plasma membrane (Fig. 2G,H). Gold-particle density in the axoplasmic matrix was calculated as the ratio of gold-particle density outside the SV cluster divided by the sum of gold-particle density outside and inside SV clusters. SV density was calculated as the number of vesicles per square micrometer in the proximal and the distal SV pool.
Figure 2.
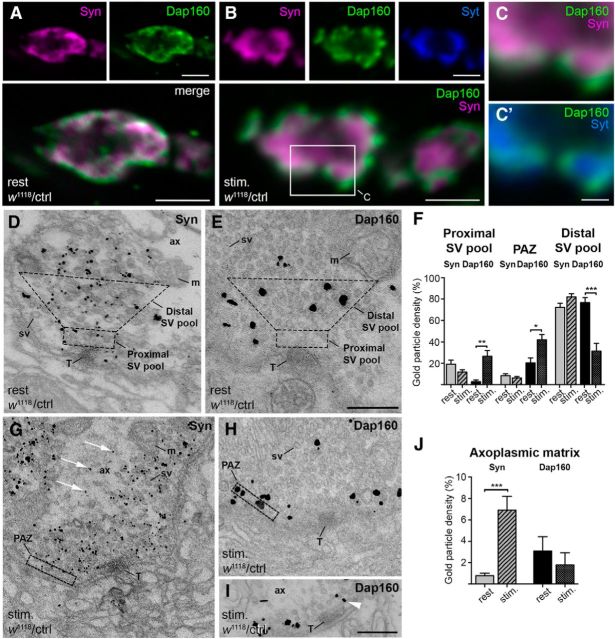
Localization of synapsin (Syn) and Dap160 at NMJs at rest and during synaptic activity. A, B, Confocal images showing middle optical sections of control (w1118/ctrl) NMJs labeled with antibodies against Syn (magenta), Dap160 (green), and synaptotagmin (Syt, blue) at rest (A) and fixed following 10 min exposure to high K+ (B). Boxed area in B is shown enlarged in C and C′ to compare Syn labeling with Syt labeling. D, E, G–I, Electron micrographs of control terminals labeled for Syn and Dap160 using immunogold technique at rest (D, E) and during stimulation (G–I). Masks that outline the areas of the distal SV pool and the proximal SV pool in D and E, and the PAZ in G and H were used to quantify densities of gold particles in F. In stimulated synapses, Syn labeling appears in the axoplasmic matrix (G, white arrows) and Dap160 labeling is observed in the PAZ (H, I) and the proximal SV pool (I, arrowhead). F, Bar graph illustrating changes in Syn and Dap160 immunogold labeling in proximal and distal pools of SVs, and in PAZ in NMJs at rest and during stimulation. Bars represent mean + SEM; *p < 0.05; **p < 0.01; ***p < 0.001, Kruskal–Wallis with Dunn's post-test; n = 15–27 terminals from three animals in each group. J, Bar graph showing relative immunogold particle density in the axoplasmic matrix of NMJs labeled with Syn and Dap160 at rest and during stimulation. Bars represent mean ± SEM; ***p < 0.01, Mann–Whitney U test; n = 10. ax, Axoplasmic matrix; m, mitochondrion; sv, synaptic vesicle; T, T-bar. Scale bars: A, B, 2 μm; C, 0.5 μm; D, E, G–I, 0.2 μm.
SV centroids were localized in Fiji (ImageJ, version 2.0.0-rc-23/11.49m) and the Euclidean distance to the first through the tenth neighbor was calculated in R (The R Foundation for Statistical Computing, version 2.15.3) using algorithm knn.dist (Arya et al., 1998). The average values are presented in box-plot generated in Prism (Prism 6).
Statistics.
Statistical analysis was performed using GraphPad Prism 6.0 (GraphStat Software). Normality of datasets larger than seven was tested with D'Agostino and Pearson omnibus normality test. When data were found normally distributed, statistical analysis of two groups was evaluated using Student's t test. To evaluate the difference between >2 groups of normally distributed data, one-way ANOVA was used, either with Tukey's multiple-comparison test, comparing every mean with every other mean, or Bonferroni's multiple-comparison test, comparing selected pairs of means or comparing every mean with the mean of a control. When a non-Gaussian distribution was observed, the Mann–Whitney U test comparing two groups was used or the Kruskal–Wallis test with Dunn's multiple-comparison test was used when comparing >2 groups. Nonsignificant differences are not indicated in figures.
Results
Dap160 interacts with synapsin
To identify novel interactions of the Dap160–Eps15 complex we performed IP experiments and found that antibodies against Dap160 coimmunoprecipitate synapsin (Fig. 1A). Although synapsin is encoded by a single gene, five splice isoforms have been identified in earlier studies running at 70, 74, and 80 kDa and two at ∼140 kDa in SDS-PAGE (Godenschwege et al., 2004; Nuwal et al., 2011). The 70, 74, and 80 kDa isoforms were the dominant forms observed herein. In IP experiments using DHE under in vitro phosphorylation-promoting or dephosphorylating control conditions, the Dap160 association with synapsins was >3 times increased under phosphorylation-promoting conditions (Fig. 1A). This increase was statistically significant (p < 0.05, Student's t test). Relative level of synapsin found in the immunoprecipitate normalized to the control condition (CIP, 100%) was 335 ± 66% (mean ± SD, n = 3). Association to Eps15 was not significantly changed (p > 0.05, Student's t test). Relative level of Eps15 found in the immunoprecipitate normalized to the control condition (CIP, 100%) was 106 ± 11% (mean ± SD, n = 3). Thus, our experiments show that Dap160 and synapsin form a complex and that Dap160 association with synapsin is under regulatory control. Remarkably, the Dap 160 lacking the first 589 aa from the N terminus that contains four SH3 domains pulls down synapsin both under phosphorylation-promoting and dephosphorylating conditions, thus indicating that the N terminus part of the molecule is essential for the regulation of the interaction between Dap160 and synapsin (Fig. 1B,C).
To further characterize the interaction from the Dap160 site pull-down, experiments from fly head homogenate were performed with single Dap160 GST-SH3 domains and constructs composed of combinations of Dap160 GST-SH3 domains (Fig. 1B,D). Experiments show that the SH3B domain, which also binds dynamin, is the major binding module (Fig. 1D). Although no evident interaction with other single GST-SH3 domains was detected, neighboring domains cooperatively promoted the interaction and all four domains display the strongest binding to synapsin (Fig. 1D). The GST-SH3 domain construct lacking the SH3B is still able to pull down synapsin (Fig. 1D), suggesting that the tandem of the domains creates an interacting surface. Such properties have been earlier reported for proteins containing multiple SH3 domains (Wunderlich et al., 1999). Only deletion of both SH3A and SH3B domains abolishes the binding of synapsin (Fig. 1E). The SH3AB module thus appears to be essential to sustain the interaction with synapsin. Interestingly, SH3A and SH3B domains also bind dynamin in an additive fashion (Fig. 1D; Winther et al., 2013). The binding of synapsin to GST-SH3AB and GST-SH3B were significantly lower than to the entire SH3 cassette (GST-SH3ABCD; 46.2 ± 19.1% and 24,7 ± 14.4% respectively, n = 5, p < 0.001, one-way ANOVA with Bonferroni's post-test). The binding of dynamin to both GST-SH3AB and GST-SH3B compared to GST-SH3ABCD were not significantly different (102 ± 24.8% and 71.4 ± 35.4% respectively, n = 4, p > 0.05, one-way ANOVA with Bonferroni's post-test). Moreover, the binding of dynamin to GST-SH3ABCD was decreased under phosphorylation-promoting conditions, thus outlining differential binding properties of the proteins competing for the same region of the Dap160 SH3 domain cassette (Fig. 1C,D).
To characterize the synapsin binding site, we first identified proline-rich motifs in the sequence that might most likely bind to an SH3 domain. Two such type-II SH3-binding motifs (PxxPxR/K) present in all isoforms were found at the C terminus (C-prp) and the N terminus (N-prp). Both C-prp and N-prp constructs containing these motifs were expressed as GST-fusion proteins (Fig. 1F). Only GST-C-prp was able to pull down Dap160 from fly head extract (Fig. 1G). Mutations of prolines 445 and 448 or arginine 450 to alanine abolished the interaction (Fig. 1H). Thus the C terminus of synapsin is involved in the interaction with Dap160.
Localization of Dap160 and synapsin at NMJs at rest and during synaptic activity
To define the stage of the SV cycle when synapsin and Dap160 interact, we looked for the synaptic compartment where these proteins colocalize. Using specific antibodies, we explored the localization of the proteins at NMJs in control flies at rest and during stimulation. Confocal microscopy shows that synapsin immunoreactivity (synapsin-ir) and Dap160 immunoreactivity (Dap160-ir) largely overlap (Fig. 2A,B) and PCC values did not differ significantly for both conditions (mean PCC ± SD: at rest, 0.77 ± 0.03, n = 3; during stimulation, 0.83 ± 0.02, n = 4, p > 0.05, Student's t test). A redistribution of the proteins within the area of colocalization was evident during stimulation (Fig. 2A,B). Dap160 accumulated in bright spots at the plasma membrane in agreement with our earlier studies (Winther et al., 2013), while synapsin-ir is observed toward the lumen of the presynaptic bouton partially overlapping with synaptotagmin immunoreactivity (Fig. 2A–C). To further investigate this redistribution at higher resolution, we used EM in combination with an immunogold technique (Jiao et al., 2010). We found that at rest immunogold particles signaling for synapsin strictly associate with areas containing SVs (Fig. 2D,F). Given that Dap160 is localized to the distal pool of synaptic vesicles at rest (Fig. 2E,F; Winther et al., 2013), these observations confirm that synapsin colocalizes with Dap160 in the distal pool of SV at rest.
EM studies of stimulated synapses confirmed that the distribution of both proteins changes dramatically (Fig. 2F–J). Gold particles signaling for synapsin in addition to the vesicle pool appear in the axoplasmic matrix outside the pool (Fig. 2G,J) thus indicating that synapsin disperses from the SVs in a way similar to what has been reported for vertebrate synapses during synaptic activity (Chi et al., 2001; Bloom et al., 2003). Meanwhile, an increase in Dap160-ir is observed at the PAZ during stimulation (Fig. 2F,H). Gold particles signaling for Dap160 are observed at the plasma membrane in this compartment; they also associate with SVs close to the plasma membrane, and are present near T-bars (Fig. 2F,I). Immunogold labeling over the distal pool of SVs is significantly reduced (Fig. 2F,H). In contrast with synapsin-ir, there is no significant increase in Dap160-ir in the axoplasmic matrix (Fig. 2J). Thus in intact terminals both proteins change locality during stimulation, but remain colocalized to areas containing SVs.
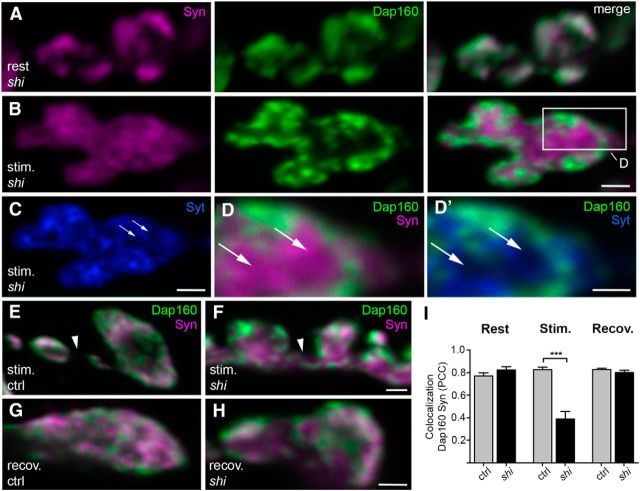
To define whether Dap160 and synapsin take differential paths after onset of the stimulation, we disrupted the vesicle cycle in NMJs in the temperature-sensitive dynamin-mutant shits1. Exposure to high K+ for 10 min at the nonpermissive temperature (29°C) results in a profound depletion of SVs in nerve terminals in the mutant due to a block of SV recycling at the dynamin-dependent membrane fission step (Koenig and Ikeda, 1989). NMJs at rest and NMJs depleted of SVs (i.e., stimulated at nonpermissive temperature) were stained with antibodies against synapsin and Dap160 (Fig. 3A–D). Synapsin is observed through the axoplasmic matrix of the synaptic bouton (Fig. 3A–D) and even spreads into axonal shafts between boutons (Fig. 3E,F). Dap160-ir is confined to spots at the presynaptic membrane and largely colocalizes with the synaptic membrane marker synaptotagmin (Fig. 3B–D). The level of colocalization in confocal images significantly decreases during stimulation (Fig. 3I), thus supporting the assertion that after onset of stimulation, synapsin dissociates from vesicles, while Dap160 moves with the SV membrane and accumulate at sites of endocytosis. Synapsin and Dap160 largely colocalize after 5 min recovery at the permissive temperature (19°C) in shits1 NMJs, thus restoring the pattern of distribution observed in control at resting condition (Fig. 3G–I).
Figure 3.
Localization of synapsin (Syn) and Dap160 in NMJs after disruption of the synaptic vesicle cycle at the dynamin-dependent step in a temperature-sensitive mutant shits1. A–D, G–H, Confocal images of middle sections of NMJs stained for Syn (magenta), Dap160 (green), and synaptotagmin (Syt, blue). shits1 NMJs at rest (A) and fixed following 10 min exposure to high K+ at nonpermissive temperature, 29°C (B, C, stim). Arrows indicate areas where Syn immunoreactivity (Syn-ir) is not overlapping with Syt immunoreactivity (Syt-ir). Boxed area in B is shown enlarged in D and D′ to compare Syn labeling with Syt labeling. E, F, Z-stack projection of confocal images from control (ctrl) and shits1 stained with Syn and Dap160 antibodies following stimulation at nonpermissive temperature. Syn-ir is observed in neurites (arrowhead) connecting boutons in shits1 but not in control. G, H, Control and shits1 NMJs stained with Syn and Dap160 antibodies after 5 min recovery at permissive temperature (19°C) after 10 min stimulation. I, Colocalization between Dap160 and Syn in control and shits1 NMJs at rest, during stimulation at 29°C, and after 5 min recovery at 19°C. Bars represent mean PCC + SEM; ***p < 0.001, Student's t test; n = 4. Scale bars: A–C, E–H, 1 μm; D, 0.5 μm.
Together these data indicate that the proteins colocalize in the vesicle pool and regions containing newly formed vesicles, but are not overlapping at the PAZ and in the axoplasmic matrix.
Dap160 is essential for synapsin localization at NMJs
To determine the site where synapsin and Dap160 form a functional complex, we first investigated whether Dap160 localization at NMJs is altered in synapsin-null mutants. NMJs from third-instar larvae from the syn97CS mutant characterized in earlier studies were used in these experiments (Godenschwege et al., 2004). Surprisingly, no significant alterations in Dap160 level (100.6 ± 3.4% of immunofluorescence level in control; n = 3; p > 0.05; Student's t test) and localization occurs in the mutant synapses at rest compared with control NMJs. Also unchanged is the distribution of Dap160 in relation to the active zone protein, Bruchpilot, which displays identical distribution compared with the control (Fig. 4A–C), and the distribution of Dap160 in relation to its binding partner dynamin (Fig. 4D,E). No detectable alterations in Dap160-ir in syn97C NMJs are evident in stimulated NMJs compared with control boutons (Fig. 4F,G). Thus, interaction with synapsin is not required for proper Dap160 localization at synapses.
Figure 4.
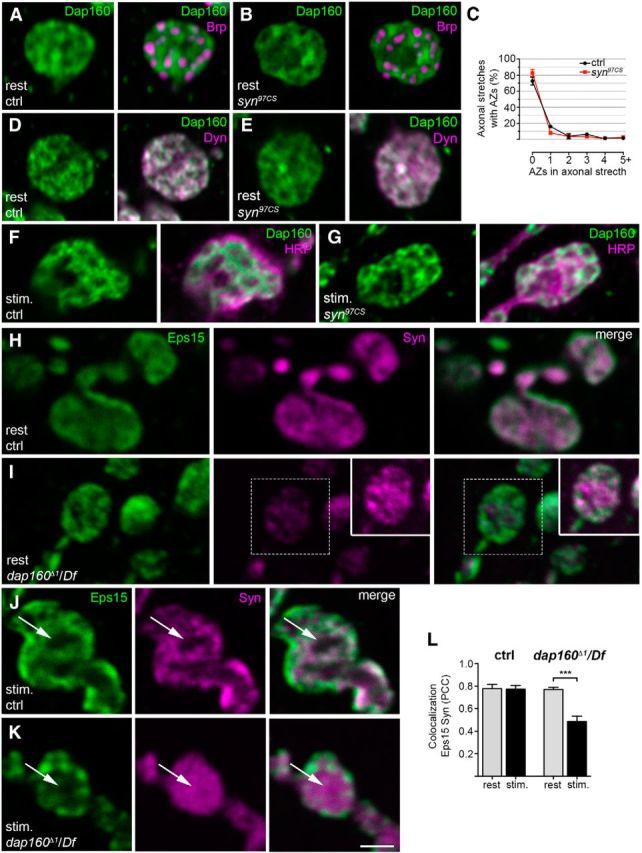
Dap160 is essential for the synaptic localization of synapsin (Syn), but deletion of syn does not alter Dap160 distribution in synapses. A, B, D–G, Confocal images of NMJs from control (ctrl) and synapsin-null mutant syn97CS at rest (A, B, D, E) and after stimulation with high K+ (F, G). A, B, Staining for Dap160 (green) and the T-bar protein Bruchpilot (Brp, magenta). C, Quantification of the percentage of active zones (AZs), as revealed by Brp labeling, in axonal stretches connecting boutons in control and syn97CS NMJs. Data are represented as mean + SEM; p > 0.05, one-way ANOVA with Bonferroni's post-test; n = 3. D, E, Staining for Dap160 (green) and its binding partner, the GTPase dynamin (Dyn, magenta). F, G, Images of NMJs from control and syn97CS stained with antibodies against Dap160 and HRP (magenta) to reveal bouton outline. H, I, Confocal images of middle optical sections of NMJs from the dap160-null mutant dap160Δ1/Df at rest stained with antibodies against the endocytic protein Eps15 (green) and Syn (magenta). The level of Syn immunoreactivity is decreased in NMJs from the dap160-null mutant compared with the control as previously shown (Koh et al., 2004), while the level of Eps15-immunoreactivity is not altered (Koh et al., 2007). Inset in I shows the Syn labeling from the same area at higher gain. J, K, Confocal images of middle optical sections from control and dap160Δ1/Df nerve terminals after high-K+ stimulation stained with the same antibodies. Following stimulation, the center of the bouton (arrows) is devoid of Syn labeling in control NMJs, whereas dap160Δ1/Df NMJs display dispersed Syn labeling. Scanning settings for the Syn channel is optimized as for the inset in I. L, Colocalization between Eps15 and Syn in control and dap160Δ1/Df NMJs at rest and during stimulation. Bars represent mean PCC + SEM; ***p < 0.001, Student's t test; n = 3–8. Scale bar: A, B, D, E–K, 2 μm.
Earlier studies have reported that dap160-null mutants do not develop into viable adults. NMJs in third-instar larvae display reduction in number of SVs, strong defects in synaptic vesicle recycling, and abnormal synaptic architecture with “satellite” boutons present (Koh et al., 2004; Marie et al., 2004). A decrease in synapsin level at synapses and abnormal “spot-like” distribution of synapsin in dap160-null mutant NMJs have been reported (Koh et al., 2004). In agreement with this study, we also observed a reduction in synapsin-ir in NMJs in this mutant (Fig. 4H,I). We found, however, that the overall distribution of synapsin under resting conditions is similar to that observed in the control (Fig. 4H,I,L). Strong dispersion of synapsin to the center of the bouton and appearance of occasional spots of synapsin-ir are observed after exposure of synapses to stimulation (Fig. 4J–L). Thus, two major perturbations are observed in the dap160-null mutant NMJs: decreased levels of synapsin and abnormal redistribution of the protein during synaptic activity.
To avoid potential involvement of other Dap160-interacting proteins during development, we investigated synapsin distribution in transgenics that expressed Dap160 lacking the SH3AB module essential for the interaction with synapsin (Fig. 1) in a dap160Δ1/Df(2L)bur-K1-null background (ΔAB). In contrast to the dap160-null mutant, in which NMJs contain numerous satellite boutons, ΔAB larvae display a normal presynaptic architecture. Transgenic dap160 ΔAB is expressed at similar levels in NMJs. Dap160 ΔAB accumulates in nerve terminals, and displays the distribution pattern at rest and during stimulation as in control NMJs (Winther et al., 2013). Levels of expression of synapsin and key endocytic proteins, such as dynamin and endophilin, are also not altered significantly (Fig. 5A). Immunofluorescence experiments did not reveal substantial differences in synapsin expression in NMJs (Fig. 5B–D), thus confirming that the direct interaction with synapsin is not essential for the normal delivery of the protein to the nerve terminal.
Figure 5.
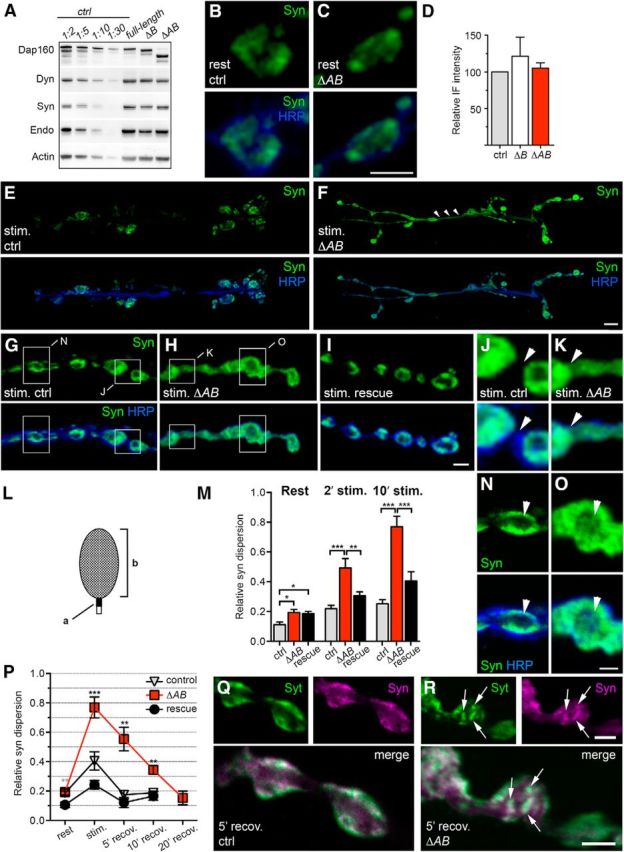
Perturbed distribution of synapsin during stimulation and recovery in a dap160 mutant lacking the synapsin-interacting SH3 domains. A, Protein expression levels in dap160 rescue mutants. Mutant flies expressing different UAS-dap160-rescue constructs (ΔB, lacking SH3B; ΔAB, lacking SH3A and SH3B and full-length rescue) pan-neuronally produce Dap160, dynamin (Dyn), synapsin (Syn), and endophilin (Endo) as revealed by Western blot of head extracts stained with antibodies against Dap160, Dyn, Syn, Endo, and actin. Fifteen micrograms of protein from rescue mutant extracts were loaded; control (ctrl) extract was diluted as indicated above each lane. B, C, Z-stack projections of confocal images of control and ΔAB NMJs at rest stained with antibodies against Syn (green) and HRP (blue) to reveal bouton outline. D, Quantification of Syn-immunofluorescent intensity (IF) in ctrl, ΔB, and ΔAB. Bars represent mean + SEM; p > 0.05, one-way ANOVA with Tukey's post-test; n = 3–8. E–K, Z-stack projections of confocal images of NMJs labeled against Syn and HRP after high-K+ stimulation. E, F, Images of the entire NMJ6/7 show that during stimulation Syn-immunoreactivity (Syn-ir) is observed in axonal connections between boutons in the ΔAB rescue mutant (arrowheads) but not in control. G–I, Images of stimulated NMJs from control, ΔAB, and full-length rescue mutants at higher magnification. Boxed areas in G are shown enlarged in J and N. Boxed areas in H are shown enlarged K and O. Axonal connections between boutons in J and K are indicated by arrowheads. L, Schematic illustrating the method for calculating the relative distribution of Syn-ir as a ratio between the labeling intensity within a 0.7 × 0.7 μm square (a) placed on the axon juxtaposed to the bouton to the labeling intensity over the bouton (b). M, Bar graph showing mean Syn-ir dispersion + SEM in NMJs from control, ΔAB, and full-length rescue after 2 and 10 min of stimulation (*p < 0.05; **p < 0.01; ***p < 0.001, one-way ANOVA with Tukey's post-test; n = 8–14). N, O, Middle optical sections from the Z-stack projections shown in G and H show Syn-ir in the center of the bouton (arrowhead) in ΔAB (K) but not in control (N). P, Recovery of Syn dispersion induced by 10 min exposure to high K+. Graph shows mean Syn-ir dispersion (±SEM) in NMJs from control, ΔAB, and full-length rescue after 5, 10, and 20 min of recovery in normal saline (**p < 0.01; ***p < 0.001, one-way ANOVA with Tukey's post-test within each condition; n = 3–14). Q, R, Confocal images of optical middle sections of boutons from control and ΔAB after 5 min recovery stained for synaptotagmin (Syt, green) and Syn (magenta). Note spots of fluorescence of colocalizing Syn-ir and Syt-immunoreactivity (arrows) in ΔAB boutons. Scale bars: B, C, G–I, Q, R, 2 μm; E, F, 10 μm; J, K, N, O, 1 μm.
We then investigated whether removal of the interaction site affects the distribution of synapsin in ΔAB mutant NMJs under resting conditions and during stimulation. In some experiments a ΔB rescue mutant was also used for comparison. At rest, synapsin predominantly accumulates inside the presynaptic bouton as in control terminals (Fig. 5B,C). However, a careful analysis shows a small but significant increase in synapsin-ir outside bouton boundaries, suggesting an abnormality in synapsin localization (Fig. 5L,M). The level of synapsin-ir outside the bouton boundaries is strongly increased during stimulation with high K+, which is similar to the increase observed in dap160-null NMJs. A significant increase in synapsin dispersion is most evident after 10 min stimulation, but was significant even after 2 min stimulation (Fig. 5E–M). Dispersion of synapsin-ir within the bouton also occurs (Fig. 5N,O). Eps15 and Dap160 distribution in ΔAB as well as in dap160-null synapses (Fig. 4H–L) remains unchanged, compared with the control.
Reclustering of synapsin that followed the dispersion during recovery in normal saline is significantly retarded. A period of 20 min is required to restore the synapsin clustering in ΔAB following 10 min stimulation (Fig. 5P). Local puncta of synapsin-ir are observed in ΔAB synapses at recovery in normal saline (Fig. 5Q,R). After 5 min recovery, these spots of synapsin-ir strongly colocalize with the integral SV protein synaptotagmin. The “spot-like” pattern of synapsin-ir is no longer present after 20 min of recovery and the distribution was indistinguishable from control. Such spots of synapsin-ir are not observed in control NMJs.
Together, these findings lead to the conclusion that the formation of the Dap160–synapsin complex is essential for proper targeting of synapsin to release sites and is essential for the proper recovery of SV membranes during synaptic activity in NMJs.
Synaptic vesicle distribution in the absence of Dap160–synapsin interaction
To further investigate the revealed defect in SV membrane recycling, we compared SV organization in ΔAB, syn97CS and control NMJs at rest at the ultrastructural level. We found that the density of SVs in the distal pool (70 nm from the T-bar) that structurally corresponds to the reserve SV pool is significantly decreased in ΔAB and syn97CS mutants. Full recovery in vesicle density has been reported for NMJs from synapsin full-length rescue mutants (Akbergenova and Bykhovskaia, 2009b, 2010) and a partial recovery is observed in dap160 full-length rescue mutants (Fig. 6A–H). Density of SVs in the pool adjacent to the T-bar is not changed in any of the studied genotypes (Fig. 6A–H). Accordingly a significant increase in the distance between SVs in the distal pool, estimated as the average distance to the first neighbor (Arya et al., 1998), is recorded in ΔAB and syn97CS, thus further confirming a defect in clustering (Fig. 6I). Abnormal organization of the distal pool becomes more apparent when distance between higher-order neighboring vesicles is compared (Fig. 6M). Distances between SVs for both ΔAB and syn97CS were significantly larger than that seen in control. Thus, genetic removal of synapsin and the SH3 domain module of Dap160 involved in the interaction with synapsin under resting condition results in decreased SV clustering in the distal pool.
Figure 6.
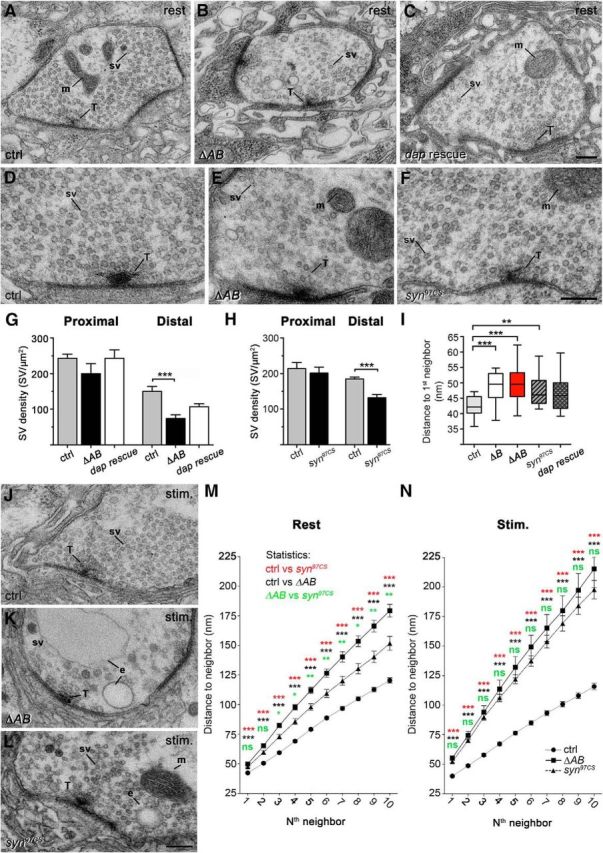
Organization of SVs at the active zone (AZ) in ΔAB and synapsin-null mutants. A–C, Electron micrographs of NMJs from control (ctrl, A), ΔAB (B), and dap160 full-length rescue mutants (dap rescue, C) at rest. D–F, Electron micrographs of middle sections of synapses in control (D), ΔAB (E), and synapsin-null mutant syn97CS (F) at higher magnification. G, H, Bar graphs showing mean SV densities (+SEM) in the proximal and distal SV pools (Fig. 2); *** p < 0.001, Kruskal–Wallis with Dunn's post-test (G); Mann–Whitney U test; n = 10–22 (H). I, Box-and-whisker plot showing the mean distance to the nearest SV neighbor in the distal pool at rest. Height in boxes shows interquartile range; horizontal line corresponds to median; whiskers show minimum–maximum values in the group; *p < 0.05; **p < 0.01, Kruskal–Wallis with Dunn's post-test; n = 21–34. J–L, Electron micrographs of NMJs from control (J), ΔAB (K), and syn97CS (L), after 10 min exposure to high K+ (stim). M, N, Mean distance to the first to 10th SV neighbor in the distal pool of SVs at rest (M; n = 30–34) and after 10 min stimulation (N; n = 29–32, *p < 0.05; **p < 0.01; ***p < 0.001, Kruskal–Wallis with Dunn's post-test within each measurement point). e, Endosome-like profile; m, mitochondrion; sv, synaptic vesicle; T, T-bar. Scale bars: A–C, D–F, J–L, 0.2 μm.
In further experiments, we then compared SV organization in stimulated synapses. In agreement with our earlier studies, a significant reduction in density and in number of SVs in nerve terminals is observed in ΔAB following prolonged stimulation, consistent with an earlier described defect in SV recycling (Fig. 6J,K,N; Winther et al., 2013). Effects of stimulation on synaptic transmission and organization of the distal pool in syn97CS are controversial in the existing literature. Initial studies did not observe any effects on excitatory junction potentials (EJPs) induced in muscles 6/7 by prolonged high-frequency stimulation of a nerve and on subcellular SV organization (Godenschwege et al., 2004). Later studies, however, in which the period of stimulation was increased to 8–10 min, have reported a decrease in the amplitude of the EJPs and a reduction in SV density and numbers (Akbergenova and Bykhovskaia, 2010). In agreement with the observations of Bykhovskaia and coworkers (Akbergenova and Bykhovskaia, 2010), a significant depression of EJPs after prolonged stimulation at 10 Hz was observed in our experiments. After 8 min, the amplitude of EJPs was reduced to 79.7 ± 1.7% (mean ± SD) from the initial value in syn97CS, while an 89.0 ± 0.1% reduction in EPJ amplitude was recorded in control (p < 0.01, Student's t test; n = 6). Moreover, prolonged stimulation of NMJs in syn97CS with high K+ results in a decrease in the number of SVs (Fig. 6J,L,N). Quantitative analysis revealed a significant increase in distance between SVs in the distal pool both for ΔAB and syn97CS as compared with the control (Fig. 6N,M).
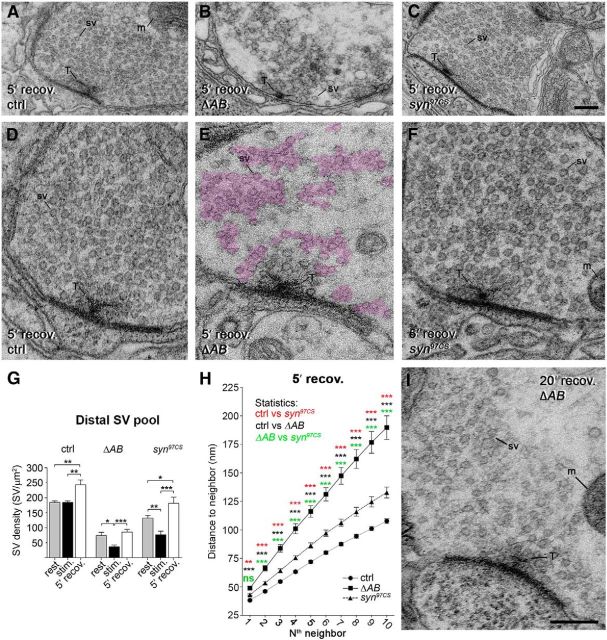
To define the recovery time at which nerve terminals had rebuilt most of their SVs and compare their ability to cluster, we examined mutant and control nerve terminals after 1, 5, and 20 min recovery following 10 min exposure to high K+ at EM level. We found that after 5 min recovery, a slightly higher number of SVs is observed at the distal SV pool for each genotype (Fig. 7A–G), a phenomenon earlier reported for stimulated NMJs (Akbergenova and Bykhovskaia, 2009a). We then compared SV organization in the distal pool after 5 min recovery in ΔAB, syn97CS, and control nerve terminals. Control synapses and synapses lacking synapsin restore their morphology to a significant degree (Fig. 7D,F). ΔAB synapses, however, display multiple dense miniclusters of SVs dispersed in the vicinity of the nerve terminal (Fig. 7B,E). This pattern corroborates with confocal images of ΔAB synapses stained by synapsin and synaptotagmin antibodies that revealed synapsin/synaptotagmin-positive aggregates in NMJs (Fig. 5R,S). Nearest SV neighbor analysis showed that SV organization after 5 min recovery in ΔAB NMJs differs significantly starting from the second SV neighbor compared with the one in control and syn97CS (Fig. 7H). The pattern of SV clustering observed under resting conditions is fully restored after 20 min recovery (Fig. 7I). Thus, the reassembly of vesicles into a single cluster is significantly retarded and perturbed in ΔAB NMJs.
Figure 7.
SV reclustering during recovery after synaptic activity is dependent on the Dap160–synapsin interaction. A–F, Electron micrographs of control (ctrl, A, D), ΔAB (B, E), and syn97CS (C, F) NMJs fixed after 5 min of recovery following 10 min stimulation with high K+. Examples in D–F are shown at higher magnification. Areas containing SVs in the distal pool are outlined (magenta) in E. G, Bar graph showing changes in mean SV density at rest, following 10 min stimulation and 5 min recovery in normal saline; *p < 0.05; **p < 0.01; ***p < 0.001, Kruskal–Wallis with Dunn's post-test; n = 10–27. H, Mean distance to the first to 10th SV neighbor in the distal pool of SVs after 5 min recovery after high-K+ stimulation; n = 32–36; *p < 0.05; **p < 0.01; ***p < 0.001; Kruskal–Wallis with Dunn's post-test within each measurement point. I, EM image of an NMJ from a ΔAB mutant after 20 min recovery in normal saline.
Discussion
Our study shows that the endocytic scaffolding protein Dap160/ITSN in Drosophila NMJs forms a functional complex with the phosphoprotein synapsin. We show that this interaction is essential for reclustering of synapsin and SVs to the reserve pool during the vesicle cycle. We propose a model explaining the involvement of this interaction in the proper functioning of the cycle (Fig. 8).
Figure 8.
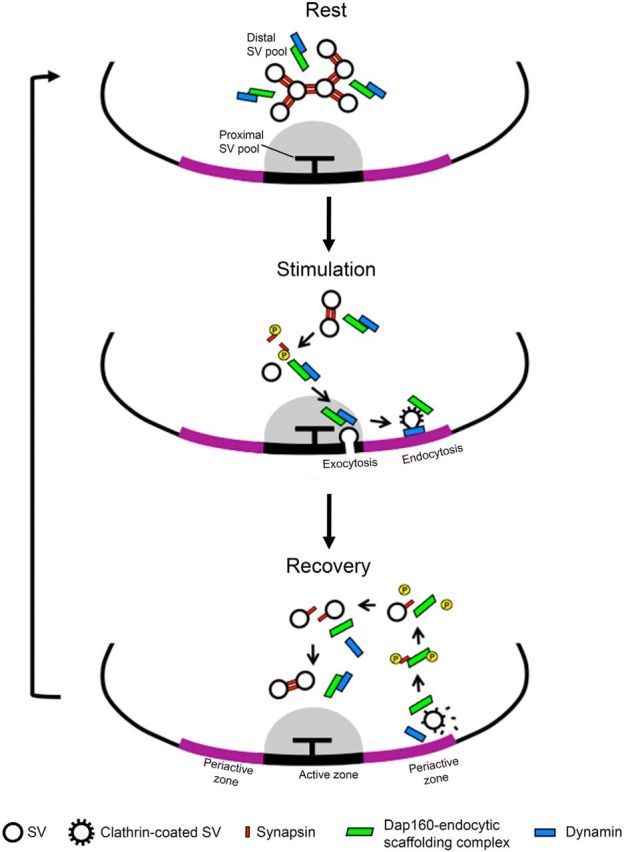
Schematic illustration of the proposed mechanism for SV clustering in the reserve pool during the vesicle cycle. Two systems organize SVs in the distal/reserve pool at rest. Synapsin (red) controls short-distance interaction between SVs, while the scaffolding complex that includes Dap160 (green) as well as other endocytic proteins is interspacing SVs forming an intervesicular matrix. During synaptic activity, phosphorylated synapsin disperses from SVs, while the scaffolding complex accompanies SV membrane during fusion and promotes its recycling. After disassembly of clathrin-coated vesicles, Dap160 becomes phosphorylated, interacts with phosphorylated synapsin, and directs synapsin to SVs. After or during translocation back to the active zone, proteins become dephosphorylated and interaction between synapsin and Dap160 is reduced. Other endocytic proteins, such as dynamin (blue), begin to interact with Dap160. Synapsin interlinks vesicles while Dap160, together with other endocytic proteins, forms a scaffolding complex that interspaces SVs, thus completing the cycle.
Using immunocytochemistry, we demonstrated that both proteins colocalize in the distal pool that structurally corresponds to the reserve SV pool (Cesca et al., 2010; Denker and Rizzoli, 2010; Pechstein and Shupliakov, 2010; Bykhovskaia, 2011). Genetic deletion of synapsin or SH3 domains of Dap160 involved in the interaction with synapsin led to abnormal clustering of SVs in this pool at rest, indicating that both proteins are involved in organization of SVs during this state. The question arises: do they come into interaction at this location? Our biochemical experiments on the interaction of synapsin and Dap160 show that under phospho-promoting conditions, the interaction between the proteins is the strongest. This finding strongly suggests that phosphorylation of the proteins acts as a switch that turns the interaction on and off. Synapsin crosslinks vesicles in the reserve pool, and phosphorylation of the protein induces synapsin dispersion from SVs in mammalian synapses (Chi et al., 2001; Cesca et al., 2010). Dispersion of synapsin during stimulation is also observed in our experiments in Drosophila NMJs. Thus, it is most probable that the phosphorylation switch promoting the interaction may be either on Dap160, implying that dephosphosynapsin comes into interaction with phosphorylated Dap160 in the reserve pool at rest, or alternatively, the proteins simply do not interact at rest at this location. The former possibility implies that during the SV cycle, Dap160 should undergo several phosphorylation–dephosphorylation cycles. The latter possibility further suggests that synapsin and Dap160 belong to two independent systems organizing SVs at the active zone. The first system includes synapsin dimers that tether vesicles to each other (Cesca et al., 2010) and the second one comprises interacting endocytic and exocytic molecules that accumulate around vesicles in which the Dap160/ITSN complex functions as an organizing component (Shupliakov, 2009, 2011; Denker and Rizzoli, 2010). Existence of a protein matrix between vesicles has been observed in cryosubstitution EM studies in mammalian synapses (Siksou et al., 2007). Its organization still remains unclear. Our present study provides potential insight into the functional significance of this intervesicular matrix.
The potential existence of a second network of protein–protein interactions that controls the clustering of SVs at rest is supported by recent functional studies that conclude that synapsin serves mainly to immobilize synaptic vesicles in the reserve pool and contributes to the number of SVs available for exocytosis, while a synapsin-independent mechanism replenishes the releasable pool during high-frequency stimulation (Orenbuch et al., 2012; Vasileva et al., 2012). The synapsin-independent mechanism is capable of clustering of vesicles at active zones in neurons from synapsin triple-knockout mice treated with tetrodotoxin (Fornasiero et al., 2012). Complete deletion of synapsin in mammalian and Drosophila synapses in current and earlier studies did not result in a complete ablation of SVs at release site at rest (Godenschwege et al., 2004; Akbergenova and Bykhovskaia, 2007, 2010; Siksou et al., 2007; Fornasiero et al., 2012). Moreover, Dap160 localization at synapses in synapsin-null mutants remains unchanged, further supporting the idea that it belongs to an independent system of protein–protein interactions. The defect in SV clustering observed in ΔAB dap160 rescue mutants is, however, very clear, suggesting that the interaction through the module that interacts with synapsin is important. It is possible that interactions with other proteins, such as dynamin, which is interacting with Dap160 through this module, are important for proper organization of the matrix around vesicles and affects clustering of SVs. In agreement with this suggestion, dynamin localization in ΔAB NMJs is also disrupted (Winther et al., 2013).
It is possible that the intervesicular matrix composed of endocytic molecules is directly connected with integral SV proteins. One such link could be via endophilin I, which interacts with the glutamate transporter (Weston et al., 2011), recently identified as a binding partner of ITSN1 in vertebrate synapses (Pechstein et al., 2015). Endophilin also forms a complex with Dap160 in Drosophila. Accordingly, a reduction in number of SVs in the reserve pool in NMJs has been reported in the endophilin-null mutants in Drosophila (Verstreken et al., 2002) and in mammalian synapses lacking endophilin (Milosevic et al., 2011).
Similarly to vertebrate synapses, we observed a profound dispersion of synapsin from active zones in Drosophila synapses during synaptic activity (Chi et al., 2001; Bloom et al., 2003). This dispersion into the interior of the bouton was most evident in shibirets1 mutants after disruption of exocytosis and endocytosis at nonpermissive temperatures. While synapsin dispersed from SVs at the site of release during activity, Dap160 relocated along with the endocytic proteins, including dynamin, to the periactive zone. Thus synapsin and Dap160 at this step have differential locality that makes the interaction between Dap160 and synapsin unlikely. The relocation of Dap160 to the PAZ most likely occurs via the active zone. Accordingly, Dap160-ir was detected in the pool of vesicles attached to the active zone during synaptic activity. SVs directly attached to the T-bar probably have differential mobility since they lack Dap160-ir. This further implies that Dap160 may be involved in modulation of synaptic transmission at the active zone. Supporting this, recent studies have shown that ITSN1 modulates neurotransmitter release in mammalian synapses (Sakaba et al., 2013).
Synapsin–Dap160 interaction appears to be critical for targeting synapsin back to the vesicle cluster, as it is dispersed far outside the active zone in the dap160 rescue ΔAB mutant that lacks this interaction. It appears that the endocytic complexes that include Dap160 after fulfilling their functions in endocytosis at the PAZ limit the diffusion of synapsin outside the bouton boundaries (Fig. 8). We suggest that Dap160 undergoes a postranslational modification during disassembly of the clathrin-coated vesicle that strongly enhances its interaction with synapsin. At this stage both synapsin and Dap160 are phosphorylated, in agreement with our biochemical experiments. Supporting this possibility, both proteins are colocalized during stimulation around the active zone, the site where newly formed vesicles return back to the SV cluster after endocytosis. Profound defects in vesicle reclustering were observed in our experiments in ΔAB mutants. Numerous miniclusters were observed around the active zone in NMJs during recovery after intense stimulation at stages when synapsin was still dispersed. At the light microscopic level, synapsin formed aggregates that colocalized with the integral SV protein synaptotagmin in NMJs and thus corresponded to those SV miniclusters. The assembly of SVs into such miniclusters most likely delays their incorporation into the intervesicular matrix.
Dephosphorylation of both synapsin and Dap160 should lead to weakening of the Dap160–synapsin association and promote binding of synapsin to SVs and incorporation of Dap160 into the intervesicular matrix of the reserve pool, closing the cycle.
Although several steps of the proposed model require further validation, our present experiments demonstrate for the first time the functional significance of the synapsin proline rich domain–endocytic SH3 domain interaction for SV clustering. Several earlier in vitro studies have reported interactions between synapsin and proteins containing SH3 domains (Cesca et al., 2010). In addition to Dap160/ITSN, several endocytic proteins, such as amphiphysin (Onofri et al., 2000), endophilin (Modregger et al., 2003), and syndapin (Qualmann and Kelly, 2000), interact with synapsin I in vertebrate synapses; their functional role still remains obscure. In Drosophila NMJs, amphiphysin and syndapin are expressed postsynaptically (Mathew et al., 2003; Kumar et al., 2009). This limited number of possible interacting partners helped to reveal the functional significance of Dap160–synapsin complex formation. Despite the evident defect in vesicle organization in the reserve pool of the ΔAB, SVs are still targeted to the active zone in NMJs in this mutant. Thus, it is possible that another mechanism may still be involved in reclustering of synapsin and SVs. This mechanism, however, cannot fully compensate for the Dap160 function. The ongoing challenge will be to demonstrate whether a similar mechanism is involved in reclustering of SVs in vertebrate synapses.
Footnotes
This work was supported by the European Union Seventh Framework Programme under Grant Agreement HEALTH-F2-2009-242167 (“SynSys” Project), Parkinsonfonden, Hjärnfonden to O.S. and Swedish Research Council grants to Å.M.E.W. and O.S. K.A.R. received a stipend form the Wenner-Gren Foundation. We thank Dr. A. Pechstein for help and advice with biochemistry experiments; Drs. H.J. Bellen, E. Buchner, M. González-Gaitán, and the University of Iowa Hybridoma Bank for antibodies. Drosophila stocks and clones obtained from the Bloomington Drosophila Stock Center, supported by National Institutes of Health (NIH) Grant P40OD018537, and Drosophila Genomics Resource Center, supported by NIH Grant 2P40OD010949-10A1, were used in this study.
The authors declare no competing financial interests.
References
- Akbergenova Y, Bykhovskaia M. Synapsin maintains the reserve vesicle pool and spatial segregation of the recycling pool in Drosophila presynaptic boutons. Brain Res. 2007;1178:52–64. doi: 10.1016/j.brainres.2007.08.042. [DOI] [PubMed] [Google Scholar]
- Akbergenova Y, Bykhovskaia M. Enhancement of the endosomal endocytic pathway increases quantal size. Mol Cell Neurosci. 2009a;40:199–206. doi: 10.1016/j.mcn.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbergenova Y, Bykhovskaia M. Stimulation-induced formation of the reserve pool of vesicles in Drosophila motor boutons. J Neurophysiol. 2009b;101:2423–2433. doi: 10.1152/jn.91122.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbergenova Y, Bykhovskaia M. Synapsin regulates vesicle organization and activity-dependent recycling at Drosophila motor boutons. Neuroscience. 2010;170:441–452. doi: 10.1016/j.neuroscience.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arya S, Mount DM, Netanyahu NS, Silverman R, Wu AY. An optimal algorithm for approximate nearest neighbor searching fixed dimensions. J ACM. 1998;45:891–923. doi: 10.1145/293347.293348. [DOI] [Google Scholar]
- Bloom O, Evergren E, Tomilin N, Kjaerulff O, Löw P, Brodin L, Pieribone VA, Greengard P, Shupliakov O. Colocalization of synapsin and actin during synaptic vesicle recycling. J Cell Biol. 2003;161:737–747. doi: 10.1083/jcb.200212140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bykhovskaia M. Synapsin regulation of vesicle organization and functional pools. Semin Cell Dev Biol. 2011;22:387–392. doi: 10.1016/j.semcdb.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Cesca F, Baldelli P, Valtorta F, Benfenati F. The synapsins: key actors of synapse function and plasticity. Prog Neurobiol. 2010;91:313–348. doi: 10.1016/j.pneurobio.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Chi P, Greengard P, Ryan TA. Synapsin dispersion and reclustering during synaptic activity. Nat Neurosci. 2001;4:1187–1193. doi: 10.1038/nn756. [DOI] [PubMed] [Google Scholar]
- Denker A, Rizzoli SO. Synaptic vesicle pools: an update. Front Synaptic Neurosci. 2010;2:135. doi: 10.3389/fnsyn.2010.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evergren E, Zotova E, Brodin L, Shupliakov O. Differential efficiency of the endocytic machinery in tonic and phasic synapses. Neuroscience. 2006;141:123–131. doi: 10.1016/j.neuroscience.2006.03.038. [DOI] [PubMed] [Google Scholar]
- Evergren E, Gad H, Walther K, Sundborger A, Tomilin N, Shupliakov O. Intersectin is a negative regulator of dynamin recruitment to the synaptic endocytic zone in the central synapse. J Neurosci. 2007;27:379–390. doi: 10.1523/JNEUROSCI.4683-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornasiero EF, Raimondi A, Guarnieri FC, Orlando M, Fesce R, Benfenati F, Valtorta F. Synapsins contribute to the dynamic spatial organization of synaptic vesicles in an activity-dependent manner. J Neurosci. 2012;32:12214–12227. doi: 10.1523/JNEUROSCI.1554-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier-Kemper A, Kahms M, Klingauf J. Restoring synaptic vesicles during compensatory endocytosis. Essays Biochem. 2015;57:121–134. doi: 10.1042/bse0570121. [DOI] [PubMed] [Google Scholar]
- Godenschwege TA, Reisch D, Diegelmann S, Eberle K, Funk N, Heisenberg M, Hoppe V, Hoppe J, Klagges BR, Martin JR, Nikitina EA, Putz G, Reifegerste R, Reisch N, Rister J, Schaupp M, Scholz H, Schwärzel M, Werner U, Zars TD, et al. Flies lacking all synapsins are unexpectedly healthy but are impaired in complex behaviour. Eur J Neurosci. 2004;20:611–622. doi: 10.1111/j.1460-9568.2004.03527.x. [DOI] [PubMed] [Google Scholar]
- Greengard P, Valtorta F, Czernik AJ, Benfenati F. Synaptic vesicle phosphoproteins and regulation of synaptic function. Science. 1993;259:780–785. doi: 10.1126/science.8430330. [DOI] [PubMed] [Google Scholar]
- Gubar O, Morderer D, Tsyba L, Croisé P, Houy S, Ory S, Gasman S, Rynditch A. Intersectin: the crossroad between vesicle exocytosis and endocytosis. Front Endocrin (Lausanne) 2013;4:109. doi: 10.3389/fendo.2013.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haucke V, Neher E, Sigrist SJ. Protein scaffolds in the coupling of synaptic exocytosis and endocytosis. Nat Rev Neurosci. 2011;12:127–138. doi: 10.1038/nrn2948. [DOI] [PubMed] [Google Scholar]
- Henne WM, Boucrot E, Meinecke M, Evergren E, Vallis Y, Mittal R, McMahon HT. FCHo proteins are nucleators of clathrin-mediated endocytosis. Science. 2010;328:1281–1284. doi: 10.1126/science.1188462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter MP, Nelson M, Kurzer M, Wang X, Kryscio RJ, Head E, Pinna G, O'Bryan JP. Intersectin 1 contributes to phenotypes in vivo: implications for Down's syndrome. Neuroreport. 2011;22:767–772. doi: 10.1097/WNR.0b013e32834ae348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao W, Shupliakov A, Shupliakov O. A semi-correlative technique for the subcellular localization of proteins in Drosophila synapses. J Neurosci Methods. 2010;185:273–279. doi: 10.1016/j.jneumeth.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Koenig JH, Ikeda K. Disappearance and reformation of synaptic vesicle membrane upon transmitter release observed under reversible blockage of membrane retrieval. J Neurosci. 1989;9:3844–3860. doi: 10.1523/JNEUROSCI.09-11-03844.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh TW, Verstreken P, Bellen HJ. Dap160/intersectin acts as a stabilizing scaffold required for synaptic development and vesicle endocytosis. Neuron. 2004;43:193–205. doi: 10.1016/j.neuron.2004.06.029. [DOI] [PubMed] [Google Scholar]
- Koh TW, Korolchuk VI, Wairkar YP, Jiao W, Evergren E, Pan H, Zhou Y, Venken KJ, Shupliakov O, Robinson IM, O'Kane CJ, Bellen HJ. Eps15 and Dap160 control synaptic vesicle membrane retrieval and synapse development. J Cell Biol. 2007;178:309–322. doi: 10.1083/jcb.200701030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Alla SR, Krishnan KS, Ramaswami M. Syndapin is dispensable for synaptic vesicle endocytosis at the Drosophila larval neuromuscular junction. Mol Cell Neurosci. 2009;40:234–241. doi: 10.1016/j.mcn.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littleton JT, Bellen HJ, Perin MS. Expression of synaptotagmin in Drosophila reveals transport and localization of synaptic vesicles to the synapse. Development. 1993;118:1077–1088. doi: 10.1242/dev.118.4.1077. [DOI] [PubMed] [Google Scholar]
- Marie B, Sweeney ST, Poskanzer KE, Roos J, Kelly RB, Davis GW. Dap160/intersectin scaffolds the periactive zone to achieve high-fidelity endocytosis and normal synaptic growth. Neuron. 2004;43:207–219. doi: 10.1016/j.neuron.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Mathew D, Popescu A, Budnik V. Drosophila amphiphysin functions during synaptic Fasciclin II membrane cycling. J Neurosci. 2003;23:10710–10716. doi: 10.1523/JNEUROSCI.23-33-10710.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milosevic I, Giovedi S, Lou X, Raimondi A, Collesi C, Shen H, Paradise S, O'Toole E, Ferguson S, Cremona O, De Camilli P. Recruitment of endophilin to clathrin-coated pit necks is required for efficient vesicle uncoating after fission. Neuron. 2011;72:587–601. doi: 10.1016/j.neuron.2011.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modregger J, Schmidt AA, Ritter B, Huttner WB, Plomann M. Characterization of endophilin B1b, a brain-specific membrane-associated lysophosphatidic acid acyl transferase with properties distinct from endophilin A1. J Biol Chem. 2003;278:4160–4167. doi: 10.1074/jbc.M208568200. [DOI] [PubMed] [Google Scholar]
- Montesinos ML, Castellano-Muñoz M, García-Junco-Clemente P, Fernández-Chacón R. Recycling and EH domain proteins at the synapse. Brain Res Brain Res Rev. 2005;49:416–428. doi: 10.1016/j.brainresrev.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Nuwal T, Heo S, Lubec G, Buchner E. Mass spectrometric analysis of synapsins in Drosophila melanogaster and identification of novel phosphorylation sites. J Proteome Res. 2011;10:541–550. doi: 10.1021/pr100746s. [DOI] [PubMed] [Google Scholar]
- Onofri F, Giovedi S, Kao HT, Valtorta F, Bongiorno Borbone L, De Camilli P, Greengard P, Benfenati F. Specificity of the binding of synapsin I to Src homology 3 domains. J Biol Chem. 2000;275:29857–29867. doi: 10.1074/jbc.M006018200. [DOI] [PubMed] [Google Scholar]
- Orenbuch A, Shalev L, Marra V, Sinai I, Lavy Y, Kahn J, Burden JJ, Staras K, Gitler D. Synapsin selectively controls the mobility of resting pool vesicles at hippocampal terminals. J Neurosci. 2012;32:3969–3980. doi: 10.1523/JNEUROSCI.5058-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechstein A, Shupliakov O. Taking a back seat: synaptic vesicle clustering in presynaptic terminals. Front Synaptic Neurosci. 2010;2:143. doi: 10.3389/fnsyn.2010.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechstein A, Shupliakov O, Haucke V. Intersectin 1: a versatile actor in the synaptic vesicle cycle. Biochem Soc Trans. 2010;38:181–186. doi: 10.1042/BST0380181. [DOI] [PubMed] [Google Scholar]
- Pechstein A, Gerth F, Milosevic I, Jäpel M, Eichhorn-Grünig M, Vorontsova O, Bacetic J, Maritzen T, Shupliakov O, Freund C, Haucke V. Vesicle uncoating regulated by SH3-SH3 domain-mediated complex formation between endophilin and intersectin at synapses. EMBO Rep. 2015;16:232–239. doi: 10.15252/embr.201439260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qualmann B, Kelly RB. Syndapin isoforms participate in receptor-mediated endocytosis and actin organization. J Cell Biol. 2000;148:1047–1062. doi: 10.1083/jcb.148.5.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaba T, Kononenko NL, Bacetic J, Pechstein A, Schmoranzer J, Yao L, Barth H, Shupliakov O, Kobler O, Aktories K, Haucke V. Fast neurotransmitter release regulated by the endocytic scaffold intersectin. Proc Natl Acad Sci U S A. 2013;110:8266–8271. doi: 10.1073/pnas.1219234110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shupliakov O. The synaptic vesicle cluster: a source of endocytic proteins during neurotransmitter release. Neuroscience. 2009;158:204–210. doi: 10.1016/j.neuroscience.2008.03.035. [DOI] [PubMed] [Google Scholar]
- Shupliakov O, Haucke V, Pechstein A. How synapsin I may cluster synaptic vesicles. Semin Cell Dev Biol. 2011;22:393–399. doi: 10.1016/j.semcdb.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Siksou L, Rostaing P, Lechaire JP, Boudier T, Ohtsuka T, Fejtová A, Kao HT, Greengard P, Gundelfinger ED, Triller A, Marty S. Three-dimensional architecture of presynaptic terminal cytomatrix. J Neurosci. 2007;27:6868–6877. doi: 10.1523/JNEUROSCI.1773-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slepnev VI, Ochoa GC, Butler MH, Grabs D, De Camilli P. Role of phosphorylation in regulation of the assembly of endocytic coat complexes. Science. 1998;281:821–824. doi: 10.1126/science.281.5378.821. [DOI] [PubMed] [Google Scholar]
- Südhof TC. Neurotransmitter release: the last millisecond in the life of a synaptic vesicle. Neuron. 2013;80:675–690. doi: 10.1016/j.neuron.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasileva M, Horstmann H, Geumann C, Gitler D, Kuner T. Synapsin-dependent reserve pool of synaptic vesicles supports replenishment of the readily releasable pool under intense synaptic transmission. Eur J Neurosci. 2012;36:3005–3020. doi: 10.1111/j.1460-9568.2012.08225.x. [DOI] [PubMed] [Google Scholar]
- Verstreken P, Kjaerulff O, Lloyd TE, Atkinson R, Zhou Y, Meinertzhagen IA, Bellen HJ. Endophilin mutations block clathrin-mediated endocytosis but not neurotransmitter release. Cell. 2002;109:101–112. doi: 10.1016/S0092-8674(02)00688-8. [DOI] [PubMed] [Google Scholar]
- Wagh DA, Rasse TM, Asan E, Hofbauer A, Schwenkert I, Dürrbeck H, Buchner S, Dabauvalle MC, Schmidt M, Qin G, Wichmann C, Kittel R, Sigrist SJ, Buchner E. Bruchpilot, a protein with homology to ELKS/CAST, is required for structural integrity and function of synaptic active zones in Drosophila. Neuron. 2006;49:833–844. doi: 10.1016/j.neuron.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Weston MC, Nehring RB, Wojcik SM, Rosenmund C. Interplay between VGLUT isoforms and endophilin A1 regulates neurotransmitter release and short-term plasticity. Neuron. 2011;69:1147–1159. doi: 10.1016/j.neuron.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Winther ÅM, Jiao W, Vorontsova O, Rees KA, Koh TW, Sopova E, Schulze KL, Bellen HJ, Shupliakov O. The dynamin-binding domains of Dap160/intersectin affect bulk membrane retrieval in synapses. J Cell Sci. 2013;126:1021–1031. doi: 10.1242/jcs.118968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunderlich L, Gohér A, Faragó A, Downward J, Buday L. Requirement of multiple SH3 domains of Nck for ligand binding. Cell Signal. 1999;11:253–262. doi: 10.1016/S0898-6568(98)00054-0. [DOI] [PubMed] [Google Scholar]
- Yu Y, Chu PY, Bowser DN, Keating DJ, Dubach D, Harper I, Tkalcevic J, Finkelstein DI, Pritchard MA. Mice deficient for the chromosome 21 ortholog Itsn1 exhibit vesicle-trafficking abnormalities. Hum Mol Genet. 2008;17:3281–3290. doi: 10.1093/hmg/ddn224. [DOI] [PubMed] [Google Scholar]