Abstract
Active (new and reactivated) memories are considered to be labile and sensitive to treatments disrupting the time-dependent consolidation/reconsolidation processes required for their stabilization. Active memories also allow the integration of new information for updating memories. Here, we investigate the possibility that, when active, the internal state provided by amnesic treatments is represented and integrated within the initial memory and that amnesia results from the absence of this state at testing. We showed in rats that the amnesia resulting from systemic, intracerebroventricular and intrahippocampal injections of the protein synthesis inhibitor cycloheximide, administered after inhibitory avoidance training or reactivation, can be reversed by a reminder, including re-administration of the same drug. Similar results were obtained with lithium chloride (LiCl), which does not affect protein synthesis, when delivered systemically after training or reactivation. However, LiCl can induce memory given that a conditioned taste aversion was obtained for a novel taste, presented just before conditioning or reactivation. These results indicate that memories can be established and maintained without de novo protein synthesis and that experimental amnesia may not result from a disruption of memory consolidation/reconsolidation. The findings more likely support the integration hypothesis: posttraining/postreactivation treatments induce an internal state, which becomes encoded with the memory, and should be present at the time of testing to ensure a successful retrieval. This integration concept includes most of the previous explanations of memory recovery after retrograde amnesia and critically challenges the traditional memory consolidation/reconsolidation hypothesis, providing a more dynamic and flexible view of memory.
SIGNIFICANCE STATEMENT This study provides evidence challenging the traditional consolidation/reconsolidation hypotheses that have dominated the literature over the past 50 years. Based on amnesia studies, that hypothesis states that active (i.e., new and reactivated) memories are similarly labile and (re)established in a time-dependent manner within the brain through processes that require de novo protein synthesis. Our data show that new/reactivated memories can be formed without protein synthesis and that amnesia can be induced by drugs that do not affect protein synthesis. We propose that amnesia results from memory integration of the internal state produced by the drug that is subsequently necessary for retrieval of the memory. This interpretation gives a dynamic view of memory, rapidly stored and easily updated when active.
Keywords: amnesia, consolidation, malleability, memory reactivation, reconsolidation, state dependency
Introduction
For nearly 50 years, the general view concerning memory processing holds that, shortly after acquisition, when a memory is in an active state, the memory is labile and susceptible to disruption by amnesic agents. The memory vulnerability is time dependent, diminishing as a function of time since acquisition. The underlying neuronal processes required to stabilize memory have been conceptualized as a consolidation process (McGaugh, 1966). The finding that a similar amnesic phenomenon occurs after an old memory is reactivated (Misanin et al., 1968; Lewis, 1979) was later described as “reconsolidation” (Przybyslawski and Sara, 1997), involving processes similar to those in consolidation (Nader et al. 2000), including de novo protein synthesis. An important element of both of these hypotheses is that the retrograde amnesia reflects the disruption of storage (or re-storage) of the memory.
Since 2000, several authors (who agree with the basic tenets of the consolidation/reconsolidation hypothesis), have provided evidence suggesting that, in the absence of amnesic treatment, active memories involve an adaptive process, allowing new information to be integrated within preexisting memories to update previous knowledge (Przybyslawski and Sara, 1997; Dudai and Eisenberg, 2004; Hupbach et al., 2007; Lee, 2009). Hence, the literature suggests that, depending on the type of information delivered while the memory is active, two different outcomes may happen. Information with no relationship with the initial memory but that modifies the internal state of the subject such as most amnesic agents, including electroconvulsive shock (Duncan, 1949; Misanin et al., 1968) and protein synthesis inhibitors (Squire and Barondes, 1972; Nader et al., 2000), is presumed to interfere with consolidation/reconsolidation processes. Conversely, information, such as cues providing additional content, can become integrated into and modify the original memory (Tronel et al., 2005; Lee, 2009). Such an idea has been particularly developed in human cognitive studies (Hupbach et al., 2007; Roediger and Butler, 2011; Schlichting and Preston, 2015).
Here, we investigated the possibility that any information (amnesic treatments or additional cues), presented while a memory is active, may have the potential to acquire an internal representation that can be integrated or encoded with the initial memory (Gisquet-Verrier and Riccio, 2012). Because the internal state is an important and salient cue, a difference in internal state at the time of testing could render the initial memory inaccessible. According to this view, memory impairment resulting from amnesic treatments would not be attributable to disruption of the consolidation/reconsolidation process but rather to the absence of a representation of the internal state induced by these treatments. An important implication is that reinstating the modified internal state at the time of testing should reverse amnesia. To investigate this possibility directly, we chose a protein synthesis inhibitor, cycloheximide (Cyclo), because protein synthesis inhibition can induce memory deficits in a time-dependent manner during consolidation or reconsolidation periods (Flood et al., 1972; Squire and Barondes, 1972; Quartermain and Botwinick, 1975; Nader et al., 2000; Alberini, 2008). Based on these previous studies, prevailing neuroscience theories state that the process of learning something novel initiates the synthesis of new proteins that participate in the processes of consolidation and reconsolidation of the memory. Using Cyclo, we investigated the possibility of inducing amnesia for an inhibitory avoidance response in rats and then recovering the memory by reintroducing Cyclo before testing. The possibility that the amnesic effects of a protein synthesis inhibitor could be based on an internal state associated with active memory was tested when the treatment was delivered just after training and again before testing. We then extended this approach to memory reactivation by administering the drug systemically or within the brain (intracerebroventricular or intrahippocampal administration) and again before testing. A second series of experiments investigated the possibility of obtaining similar results, i.e., time-dependent amnesia and recovery, using lithium chloride (LiCl), an agent known to modify the internal state and to induce gastric malaise (Garcia and Koelling, 1966). However, LiCl establishes strong memory in the form of conditioned taste aversion (CTA) while not inducing protein synthesis inhibition (Squire et al., 1975).
Materials and Methods
Experiments using posttraining treatments were conducted in Orsay, France, whereas those using post-reactivation treatments were conducted in Kent, Ohio. Despite some obvious differences between these two sets of experiments, the results are very consistent, indicating that these results are not attributable to some peculiarity of the behavioral protocol.
Subjects
NeuroPSI.
Adult male Sprague Dawley rats (Charles River Laboratories), ∼70 d old and weighing 250–275 g on arrival, were used for all consolidation experiments. The rats were housed in pairs under a 12 h light/dark cycle (lights on at 7:30 A.M.), with food and water available ad libitum during the duration of the experiments, except in LiCl experiments. Animals were habituated to the animal colony room at least 6 d before the start of the experiment. During this habituation, rats were handled, numbered on their tails, and weighed. All experimental sessions took place during the lighted portion of the light/dark cycle and at the same time each day. All experiments were performed in accordance with the European Communities Council Directive (86/609/EEC, November 24, 1986) and the French Directives concerning the use of laboratory animals (Decree 87-848, October 19, 1987).
Kent State University.
Adult male Long–Evans rats, ∼70–90 d old and weighing 315–500 g on experimentation, were used for all reactivation experiments. For all behavioral procedures, animals were housed individually 10 d before the start of the experiments and were maintained on a 14/10 h light/dark cycle. Food and water were available ad libitum throughout the experiments, except in LiCl experiments. All experimental sessions took place during the lighted portion of the light/dark cycle and at the same time each day. All animal procedures were performed in accordance with Kent State University Institutional Animal Care and Use Committee guidelines.
Experiments 1a and 1b: effect of Cyclo after conditioning—NeuroPSI
The inhibitory avoidance conditioning took place in a Plexiglas chamber (49 × 22 × 21 cm) separated by a sliding door dividing it into two equal size compartments: one black and one white. The floor in the black compartment consisted of metal grids spaced 1.2 cm apart, and the grids were connected to a shock source. A 10 W light bulb was suspended above the center of the white compartment.
Conditioning.
After being weighed, rats were transported in an individual cage in the experimental room and placed on a table next to the experimental chamber for 30 s. The rat was then placed gently into the white compartment of the chamber. After 15 s, the sliding door separating the two compartments was opened, and the latency of the rat to cross into the black compartment was recorded. Once the animal entered the black compartment, the sliding door was closed and two footshocks were delivered 5 and 10 s later at 0.3 mA (Experiment 1a) or 0.5 mA (Experiment 1b). Fifteen seconds after shock delivery, subjects were removed from the black side, placed back in their home cage, and returned to the colony room. Pseudotrained rats were treated the same way but did not receive any shock. Immediately after conditioning, rats received an initial intraperitoneal injection of the protein synthesis inhibitor Cyclo (Sigma-Aldrich), dissolved in physiological saline (0.9%) at the dose of 2.8 mg/kg (Gold and Sternberg, 1980; Milekic et al., 2006) or saline (0.9%; Sal). Despite the fact that a lethal dose (3.7 mg/kg) of Cyclo was not used, two rats died during these experiments.
Retention test.
The retention test took place 48 h after conditioning. Thirty minutes before testing, rats received a second Sal or Cyclo injection. Another group of rats was not injected but exposed to a reminder cue known to decrease the disruption of the retention performance induced by an amnesic agent or other sources of forgetting (Spear, 1973; Miller and Springer, 1974; Gordon and Mowrer, 1980). The reminder consisted of placing rats in the white compartment for 15 s with the sliding door closed and then opened for 30 s. Rats were then returned to their home cage for 5 min before receiving the retention test.
During testing, rats were taken from their home cage, weighed, and transported in an individual cage in the experimental room. Testing was identical to training with the following exceptions: no shock was delivered on entry, the door was not closed, and the rat was free to move around in the apparatus for 10 min. Response latency to enter the black compartment was recorded. Rats that did not enter the black compartment were given a 600 s response latency.
Experiments 2a and 2b: effects of Cyclo after reactivation, systemic injections—Kent State University
In the reactivation experiments, behavior was conducted in a black–white inhibitory avoidance chamber (52 × 30 × 35; Passive Avoidance Apparatus 7550; Ugo Basil).
Conditioning.
For training, animals were brought to the experimental room, held on the experimenter's hand for 30 s, then placed on the white side of the shuttle box. The sliding door was opened 20 s after placement on the white side, and the initial latency to cross into the black compartment (all four paws) was recorded. After crossing, the sliding door closed and a 2 s, 1.0 mA scrambled footshock was delivered 5 s after the door closed. Ten seconds after receiving the footshock, the animal was removed from the chamber and returned to the main colony.
Reactivation.
For reactivation, animals were brought back into the experimental room 48 h after training and given a 15 s exposure to the white compartment with the sliding door closed. After reactivation, animals received an intraperitoneal injection of Cyclo (2.8 mg/kg; Sigma-Aldrich) or Sal (0.9%). Injections occurred immediately after reactivation or at a delay (e.g., 6 h).
Retention test.
Testing was conducted 48 h after reactivation. Thirty minutes before the retention test, animals were given an injection of Cyclo or Sal, unless specified otherwise. For testing, rats were brought back into the experimental room 48 h after reactivation. The test procedure was identical to training except that the sliding door remained open for 540 s and no shocks were delivered. The initial latency to cross was recorded as the dependent measure. Any animal that did not cross was given a score of 540 s, the limit of the automated apparatus. After crossing or at 540 s, the animal was removed and returned to the main colony. To determine whether the disruptive effect of Cyclo may be attributable to the novelty of the treatment, in Experiment 2b, a group of rats was injected with Cyclo 1 week before conditioning to familiarize them with the drug effects. At the end of the week, this group received a Cyclo injection immediately after conditioning and was tested 48 h later (see Fig. 2).
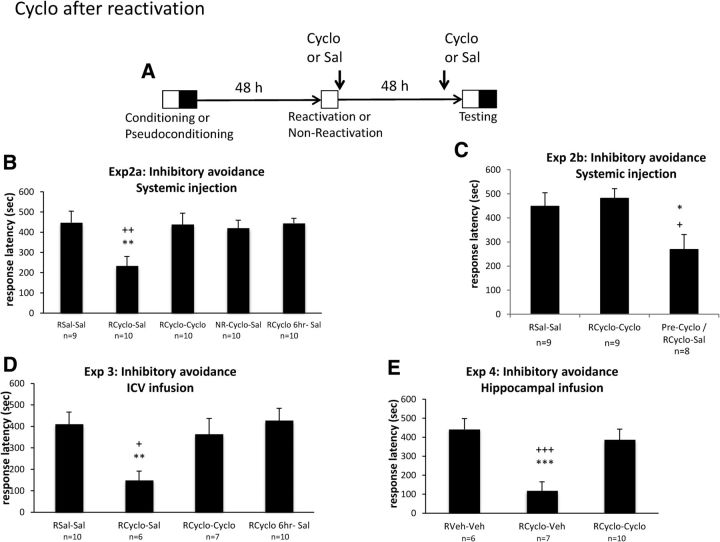
Figure 2.
Peripheral and central administration of Cyclo after reactivation of memory induces amnesia that is reversible with exposure to Cyclo. A, Timeline of the experiment. Reactivation (15 s exposure to the safe white compartment) or no reactivation took placed 48 h after conditioning. All groups of rats (n = 6–10; see B–E) received two injections: one given after reactivation (immediately or 6 h later) and one given 30 min before testing. Rats were injected with Cyclo (2.8 mg/kg) or Sal. The retention test occurred 48 h after the reactivation. B, Experiment 2a, Mean ± SEM response latency obtained during the test, 48 h after inhibitory avoidance conditioning, for rats given intraperitoneal injections. **p < 0.01 versus RSal–Sal; ++p < 0.01 versus RCyclo–Cyclo. C, Experiment 2b, Preexposure to Cyclo 1 week before conditioning did not affect the memory deficits produced by Cyclo treatment after a reactivation. *p < 0.05 versus RSal–Sal; +p < 0.05 versus RCyclo–Cyclo. D, Experiment 3, Mean ± SEM response latency obtained for rats given intraventricular infusion. **p < 0.01 versus RSal–Sal; +p < 0.05 versus RCyclo–Cyclo. E, Experiment 4, Mean ± SEM response latency obtained for rats given intrahippocampal infusion. ***p < 0.001 versus RVeh–Veh; +++p < 0.001 versus RCyclo–Cyclo.
Experiment 3: effects of Cyclo after reactivation, intracerebroventricular administration—Kent State University
Surgical procedure.
Rats were anesthetized with isoflurane and received a 5 mg/kg dose of ketoprofen 5 min before cannulation and 24 h after the completion of the surgery. Rats received cannula implantations (Plastics One) into the lateral ventricle (intracerebroventricularly; dorsoventral, −3.4 mm; anteroposterior, −0.9 mm; mediolateral, 1.6 mm; Paxinos and Watson, 1986). Animals were allowed to recover for 10 d before the start of behavior. Dummy cannulas were changed every day leading up to and during the experiment. Cannula placement was checked using India ink injections, followed by rapid decapitation. Brains were fresh frozen and sliced on a cryostat, and slices were mounted and observed for correct placement in the lateral ventricle using an inverted microscope. Any animal with a misplaced cannula was not included in the final analysis.
Conditioning.
Conditioning was the same as Experiment 2.
Reactivation.
Reactivation was the same as Experiment 2. After reactivation, animals received an infusion of Cyclo (20 μg/2 μl) or Sal (0.9%; 2 μl). Infusions occurred immediately after reactivation or at a delay (e.g., 6 h) as indicated (see Fig. 2).
Retention test.
Retention testing followed the same procedure as Experiment 2 except that animals received an intracerebroventricular infusion of Cyclo or Sal 30 min before testing.
Experiment 4: effects of Cyclo after reactivation, hippocampal administration—Kent State University
Surgical procedure.
The surgical procedure was the same as Experiment 3 except that animals received bilateral cannulation into the dorsal hippocampus (14°; dorsoventral, −3.1 mm; anteroposterior, −4.0 mm; mediolateral, 3.3 mm). Cannula verification was conducted as described in Experiment 3. Any animal with a misplaced cannula was not included in the final analysis (see Fig. 2).
Conditioning.
Conditioning was the same as Experiment 2.
Reactivation.
Reactivation was the same as Experiment 2. After reactivation, animals received an infusion of Cyclo (20 g/0.8 μl) or Sal (0.9%; 0.8 μl). Infusions occurred immediately after reactivation.
Retention test.
Retention testing followed the same procedure as Experiment 2 except that animals received an infusion of Cyclo or Sal into the dorsal hippocampus 5 min before testing (see Fig. 2).
Experiment 5: LiCl after conditioning—NeuroPSI
Rats were first water deprived for 12 h (from 8:00 P.M. until the next morning) before being exposed to a 15% sucrose solution (grams per liter) for 30 min in an individual cage. The quantity of sucrose consumed was recorded. Immediately thereafter, rats were conditioned and received two 0.4 mA electrical footshocks in the inhibitory avoidance apparatus, followed by an intraperitoneal injection of LiCl (Sigma-Aldrich) dissolved in physiological saline (0.9%) or Sal. The LiCl (0.25 m, 300 mg/kg) was delivered immediately or 60 or 120 min after inhibitory conditioning. Despite the fact that a lethal dose (600 mg/kg) of LiCl was not administered, two rats died during these experiments. Twenty-four hours later, animals were given an injection of LiCl (0.15 m, 127 mg/kg) or Sal (0.9%). Thirty minutes later, they were tested for retention of inhibitory avoidance. Another group of rats was exposed to the reminder 5 min before the retention test. Control rats were injected with an equivalent volume of 0.9% physiological saline (see Fig. 3).
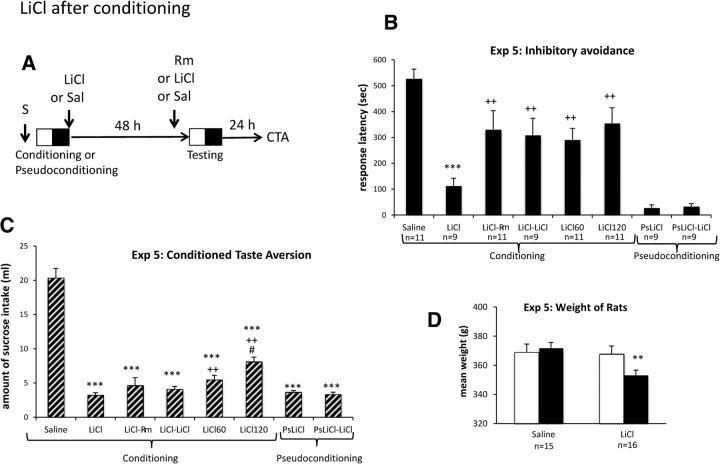
Figure 3.
LiCl after conditioning induces CTA and inhibitory avoidance amnesia that is reversible with exposure to a reminder or LiCl. A, Timeline of experiment. After sucrose (S) exposure, groups of rats (n = 9–11; see B) received one injection of LiCl (0.25 m; 300 mg/kg) or Sal just after conditioning and another injection of Sal or LiCl (0.15 m; 127 mg/kg) 30 min before the retention test. Another group that received an LiCl injection after conditioning and was exposed to the reminder, 5 min before testing. B, Experiment 5, Mean ± SEM response latency obtained during the test, 48 h after inhibitory avoidance conditioning or pseudoconditioning. ***p < 0.001 versus Sal; ++p < 0.01 versus LiCl. C, Experiment 5, Values displayed as mean ± SEM amount of sucrose intake obtained during the CTA occurring 48 h after exposure to the sucrose solution and the inhibitory avoidance conditioning test or pseudoconditioning. ***p < 0.001 versus Sal; ++p < 0.05 versus LiCl; #p < 0.05 versus LiCl60. D, Experiment 5, Mean ± SEM weight obtained in nondeprived rats receiving an injection of Sal or Cyclo, 1 week after testing (white bars) and the following day (black bars). **p < 0.01 versus Sal.
Experiments 6a and 6b: LiCl after reactivation—Kent State University
Rats were trained in inhibitory avoidance and were water deprived 36 h after training for a duration of 12 h (from 10:00 P.M. until the next morning). After the water deprivation, animals were given a 15% sucrose solution (grams per liter) for 30 min and then were exposed to the inhibitory avoidance chamber for 15 s. Injections of LiCl (0.25 m, 300 mg/kg, i.p.) or Sal (0.9%) were given immediately after reactivation or 120 min after reactivation. Thirty-six hours after the reactivation session, rats were water deprived again for 12 h. After water deprivation, animals were given an injection of LiCl (0.15 m, 127 mg/kg) or Sal (0.9%) and then given access to sucrose solution for 30 min. After the CTA test, animals were given an inhibitory avoidance retention test. To determine whether the amnesia was reversible, in Experiment 6b, one group of rats received LiCl after reactivation and again shortly before the test for inhibitory avoidance (see Fig. 4).
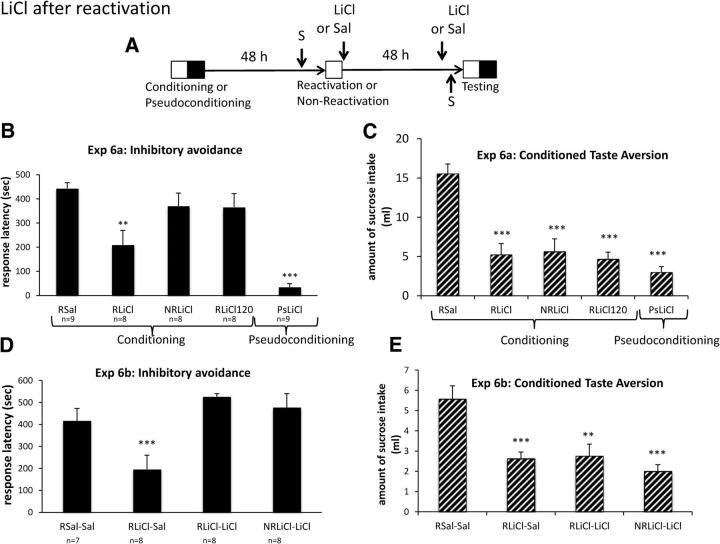
Figure 4.
LiCl after reactivation of memory induces CTA and inhibitory avoidance amnesia that is reversible with exposure to LiCl. A, Timeline of experiment. Reactivation or no reactivation took placed just after sucrose exposure, 48 h after conditioning or pseudoconditioning. All groups of rats (n = 7–9; B, C) received a Sal or LiCl (0.25 m; 300 mg/kg) injection delivered either just after the end of reactivation or 120 min later. The CTA occurred 48 h after memory reactivation. B, Experiment 6a, Values displayed as mean ± SEM response latency obtained during the test, 48 h after reactivation of inhibitory avoidance conditioning. C, Experiment 6a, Values displayed as mean ± SEM amount of sucrose intake obtained during the test for CTA. **p < 0.01 versus RSal; ***p < 0.001 versus RSal. D, Experiment 6b, In this experiment, rats received an additional injection of Sal or LiCl (0.15 m; 127 mg/kg), 30 min before the retention test. Values displayed mean ± SEM response latency obtained during the test, 48 h after reactivation of inhibitory avoidance conditioning. ***p < 0.001 versus RSal versus rSal–Sal. E, Experiment 6b, Values displayed as mean ± SEM amount of sucrose intake obtained during the CTA test. **p < 0.01 versus RSal–Sal; ***p < 0.001 versus RSal–Sal.
Experiment 7: protein synthesis inhibition—NeuroPSI
The level of protein synthesis inhibitors after application of Cyclo or LiCl was measured via incorporation of 35S-labeled methionine (GE Healthcare) into newly synthesized proteins. Rats were injected intraperitoneally with Cyclo (2.8 mg/kg), LiCl (300 mg/kg), or Sal and given an injection of 125 μCi l-[35S]methionine. Rats were then killed 15 or 45 min after l-[35S]methionine injections. After the animals were killed, brain samples were homogenized in 5 vol of cold lysis buffer (1% SDS, 62 mm Tris, pH 7.4, 40 mm DTT, 62 mm imidazol, and 10% glycerol). The homogenates were boiled for 5 min and centrifuged at 18,000 × g for 15 min at 4°C. Protein precipitation was achieved by mixing the supernatant with TCA (25% final concentration), followed by incubation for 60 min (ice cold) and centrifugation (13,000 rpm for 20 min at 4°C). A scintillation counter was used to measure radioactivity. The inhibition of protein synthesis was calculated as follows: [1 − CPMCyclo or LiCl/CPMSal)] × 100.
Statistics
ANOVAs were used for comparisons of the different groups. These analyses were completed by planned comparisons. All parameters were analyzed with contrast ANOVAs, using VAR3 software (Rouanet et al., 1990) or SPSS version 20 (IBM).
Results
In all the experiments, no between-group differences in initial latencies of rats were observed.
Experiments 1a and 1b: effect of Cyclo on new memory
In the first experiment (Fig. 1A), four independent groups of rats were assigned to inhibitory avoidance conditioning using a 0.3 mA electrical footshock and tested for retention 48 h later. Three groups of rats received two injections of Sal or Cyclo (2.8 mg/kg), one delivered immediately after conditioning and the other 30 min before testing (groups Sal–Sal, Cyclo–Sal, and Cyclo–Cyclo). A fourth group received a Sal injection after training and was exposed briefly to a reminder (Rm), the safe white box, 5 min before testing (group Cyclo–Rm). An ANOVA showed a significant effect of group (F(3,30) = 8.05, p < 0.001). As expected, treatment with Cyclo administered after training induced a strong amnesia (Cyclo–Sal vs Sal–Sal, F(1,15) = 28.39, p < 0.001), which was almost complete given that no significant difference was found between initial latencies before conditioning and testing for the Cyclo–Sal group (F(1,8) = 2.44, NS), whereas all the other groups demonstrated a clear conditioning effect (p values <0.01). The performance deficit was removed when Cyclo was re-administered before test (Cyclo–Sal vs Cyclo–Cyclo, F(1,16) = 6.20, p = 0.23; Cyclo–Sal vs Sal–Sal, F(1,15) = 2.94, ns), and reexposing rats to a reminder alleviated the performance disruption (Cyclo–Rm vs Cyclo–Sal, F(1,15) = 8.40, p < 0.01).
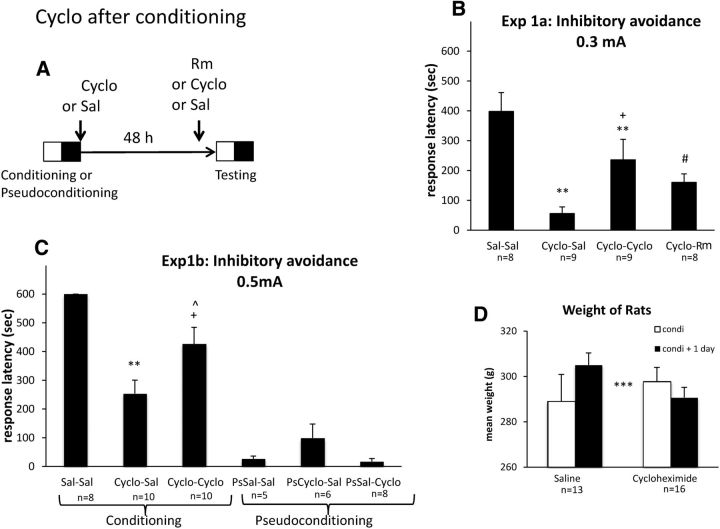
Figure 1.
Cyclo after conditioning induces amnesia that is reversible with exposure to a reminder or Cyclo. A, Timeline of the experiment. During conditioning, rats were placed in the white compartment of a double box and received two electrical footshocks after entering the black compartment. Pseudoconditioned rats were exposed to the conditioning context but not shocked. All groups of rats (n = 5 to 10; see B, C) were injected twice with intraperitoneal injections with either Cyclo (2.8 mg/kg) or Sal, once just after the end of conditioning and the other 30 min before the retention test, except one group that was exposed to a reminder (45 s exposure to the safe white compartment), 5 min before testing. During testing occurring 48 h after conditioning, rats were placed in the white compartment, and their latency to enter the black compartment was registered (cutoff at 600 s). B, Experiment 1a, Mean ± SEM response latency obtained during the test, 48 h after inhibitory avoidance conditioning with a 0.3 mA electrical footshock, or pseudoconditioning. C, Experiment 1b, Mean ± SEM response latency obtained during the test, 48 h after inhibitory avoidance conditioning with a 0.5 mA electrical footshock. **p < 0.05 versus Sal–Sal; ***p < 0.001 versus Sal–Sal; +p < 0.05 versus Cyclo–Sal; ∧p < 0.05 versus Sal–Sal; #p < 0.07 versus Cyclo–Sal. D, Experiment 1b, Mean ± SEM weight obtained during the conditioning day (white bars) and the following day (black bars) in rats receiving a postconditioning injection of Sal or Cyclo. ***p < 0.001 significant groups × treatment interaction.
Experiment 1b was designed to replicate the previous finding and investigate the effects of Cyclo on pseudotrained rats. Also, stronger shock intensity (0.5 mA) was used to induce stronger avoidance of the dark compartment for the control rats. Under those conditions, all control rats avoided the shock compartment and less amnesia was observed (Fig. 1C). A one-way ANOVA performed on the response latency (Fig. 1B) indicated a significant effect of group (F(5,41) = 29.78, p < 0.001). Again, treatment with Cyclo induced a strong amnesia, with shorter response latencies than controls during testing (Cyclo–Sal vs Sal–Sal, F(1,16) = 43.67, p < 0.001). These deficits were partially eliminated when Cyclo was re-administered before test (Cyclo–Cyclo vs Cyclo–Sal, F(1,18) = 5.41, p = 0.03), replicating the basic findings of the previous experiment.
Three pseudoconditioned (Ps) groups received the same exposure to the inhibitory avoidance chamber during conditioning but did not receive any shocks and were also injected twice with either Cyclo or Sal to determine whether the drug had any non-mnemonic effects on performance (PsSal–Sal, PsCyclo–Sal, and PsSal–Cyclo). The response latencies obtained in these groups clearly indicated that Cyclo injections alone did not affect response latencies (PsSal–Sal vs PsCyclo–Sal and PsSal-Cyclo: NS). As illustrated in Figure 1D, Cyclo affected the weight of the rats as revealed by a significant interaction of groups × weight repetition (F(1,32) = 14.80, p < 0.001). This result indicates that Cyclo treatment was strong enough to affect the general state of the treated rats.
Hence, these experiments showed that the performance disruption induced by Cyclo can be alleviated not only by reexposing rats to a reminder but also by reinducing the same drug state thought to interrupt consolidation processes and storage of memory (Miller and Springer, 1972; Millin and Newman, 2008; Briggs and Olson, 2013). These results confirm and extend previous findings that amnesic treatments do not destroy the memory but rather impair its expression (Spear, 1973; Riccio et al., 2006) and question the view that amnesia results from a disruption of consolidation.
Experiments 2a and 2b: effect of Cyclo on reactivated memory—systemic injections
In the next experiment, the amnesic effect of Cyclo for a reactivated memory was tested by bringing rats back into the experimental room 48 h after conditioning and exposing them to the white compartment for 15 s as a reminder that minimizes extinction (Briggs and Olson, 2013). After this reactivation (R), animals were injected with Sal or Cyclo. Animals were also injected 48 h later and then placed in the inhibitory avoidance chamber 30 min later for a retention test (Fig. 2A). To determine whether the drug effect was time dependent, another group received Cyclo 6 h after reactivation. A sixth group was administered Cyclo in the absence of the reactivation (NR). As Figure 2B suggests, a significant effect of group (F(4,44) = 4.17, p < 0.01) was found. Cyclo given after a reactivation produced time-dependent memory deficits that were restored when Cyclo was given again before test: administering Cyclo after reactivation impaired memory if Sal was given before testing (RCyclo–Sal vs RSal–Sal, F(1,17) = 8.78, p < 0.01) but not if Cyclo was re-administered, (RCyclo–Cyclo vs RSal–Sal, F < 1; Cyclo–Cyclo vs Cyclo–Sal, F(1,18) = 7.945, p < 0.01; Fig. 2B). However, administering the drug in the absence of reactivation or 6 h after reactivation had no effect on retention (NRCyclo–Sal and RCyclo6h–Sal vs Sal–Sal, F values < 1; Fig. 2B). Thus, not only is reactivation required but also the state of the reactivated memory is time limited.
To evaluate whether novelty of the Cyclo treatment was the source of the memory deficit, we conducted an additional experiment. One group of rats received an injection of the drug 1 week before training. Two comparison conditions that received either Sal or Cyclo after reactivation and the same agent at testing were included for a partial replication of the initial reactivation experiment. A one-way ANOVA indicated a main effect of group (F(2,23) = 4.5, p < 0.05), as seen in Figure 2C. Preexposure to Cyclo did not prevent the memory deficits produced by Cyclo treatment after a reactivation, because Cyclo was still able to induce significant performance disruption (F(1,15) = 4.65, p < 0.05), suggesting that the novelty of Cyclo at reactivation does not explain the memory deficits produced.
Experiment 3: effect of Cyclo on reactivated memory—intracerebroventricular administration
To determine whether the amnesic effects of Cyclo on a reactivated fear memory could be reproduced with central administrations of the drug, animals were given infusions of Cyclo or Sal into the lateral ventricle immediately after the reactivation and again 30 min before testing 48 h later (Fig. 2). Two of 36 rats had a misplaced cannula and were thus excluded in the final analysis. An ANOVA on test latencies indicated an effect of treatment (F(3,29) = 3.82, p < 0.05). Figure 2D suggests that direct infusions of Cyclo into the lateral ventricle after a reactivation produced time-dependent memory deficits that were reduced by another infusion of Cyclo before test. Rats receiving the infusion of Cyclo showed impaired memory (RSal–Sal vs RCyclo–Sal, F(1,14) = 10.387, p < 0.01), but the Cyclo-induced memory deficit was reversed when the drug was reinfused before testing (Cyclo–Cyclo vs Cyclo–Sal, F(1,11) = 5.86, p < 0.05). Furthermore, the infusion effect was time dependent, because a delay of 6 h produced no observable memory deficit (RSal–Sal vs RCyclo6h–Sal, F < 1).
Experiment 4: effect of Cyclo on reactivated memory—hippocampal administration
Finally, we attempted to determine whether site-specific infusions of Cyclo into the dorsal hippocampus, known to result in memory deficits in inhibitory avoidance retention (Lee et al., 1992), would also produce amnesia that could be reversed. Therefore, we infused Cyclo into the dorsal hippocampus immediately after a reactivation session and again 5 min before a retention test 48 h later (Fig. 2E). A one-way ANOVA revealed a significant effect of treatment (F(2,17) = 7.17, p < 0.01). Site-specific infusions of Cyclo after reactivation resulted in significant memory impairment compared with vehicle-treated animals (F(1,11) = 22.507, p < 0.001). However, infusions of Cyclo, 5 min before the test, resulted in recovery from the memory deficits produced by Cyclo given after a reactivation trial (F(1,12) = 16.34, p < 0.001).
In all, this first series of experiments indicates that amnesia resulting from protein synthesis inhibition is not based on loss of newly formed or reactivated memories, given that the initial representation can be recovered by providing a reminder. Effective reminders involved either the conditioning context or the amnesic treatment itself, regardless of the way in which the amnesic treatment was delivered (systemic, intracerebroventricular, or intrahippocampal administration), suggesting that both types of information (the state induced by Cyclo or contextual cues) were associated with the initial memory. These findings are not predicted by the consolidation/reconsolidation hypothesis but are in agreement with an integration hypothesis proposed previously (Gisquet-Verrier and Riccio, 2012).
Experiment 5: effect of LiCl on new memory
According to the view that any information delivered while the memory is active can be acquired as a representation and integrated within the memory, other treatments modifying the internal state without disrupting the protein synthesis should also be able to produce amnesia in a time-dependent manner. We chose LiCl, a toxic agent known to produce gastric malaise and used widely to induce well-retained CTA (Garcia and Koelling, 1966; Kalat and Rozin, 1970). To determine whether LiCl can produce memory impairment while preserving new associations required for CTA, rats were exposed to sucrose (15%) just before inhibitory avoidance conditioning and LiCl injections and then were tested for retention of inhibitory avoidance 2 d later and then for CTA on the next day (Fig. 3A).
First, we investigated the dose of LiCl able to induce an amnesic effect. Preliminary experiments indicated that, when 0.15 m (127 mg/kg) and 0.25 m (50 mg/kg) LiCl injections were delivered immediately after conditioning, no amnesic effect was obtained. At a stronger dose, 0.25 m at 300 mg/kg, LiCl induced significant amnesia. Another preliminary experiment was conducted to demonstrate that LiCl could form a memory for a new taste, even when the consumption of sucrose was followed by electrical footshocks mimicking inhibitory conditioning. In these experiments, water-deprived rats were exposed to sucrose during 30 min before receiving two electrical footshocks (0.4 mA), followed by an LiCl injection. Two days later, rats were tested for CTA. Under those conditions, LiCl injections induced significant CTA, even when animals received the two electrical footshock (data not shown).
In the main experiment, eight groups of rats were used. All rats were water deprived before being conditioned or pseudoconditioned. Immediately thereafter, one group received a Sal injection and two others a LiCl injection (300 mg/kg). Two other groups received a delayed LiCl injection, occurring 60 or 120 min later (LiCl60 and LiCl120). Before the retention test, occurring 48 h later, groups having received an immediate postconditioning LiCl injection were either exposed to the reminder, 5 min before the retention test (LiCl–Rm), or to another LiCl injection (0.15 m, 127 mg/kg; LiCl–LiCl and PsLiCl–LiCl). The second LiCl injection was administered 30 min before testing. An ANOVA performed on the response latencies indicated a significant main effect of group (F(7,72) = 12.65, p < 0.001). An injection of LiCl resulted in significant memory deficits (Fig. 3B) compared with animals injected with Sal (F(1,18) = 72,39, p < 0.001). The effect was significantly reduced by a pretest exposure to the reminder cues (LiCl vs LiCl–Rm, F(1,18) = 6.60, p = 0.018) and by re-administration of the drug at testing (LiCl vs LiCl–LiCl, F(1,16) = 7.43, p = 0.014). The longer latencies after reexposure to the LiCl reflect memory recovery rather than a performance artifact because pseudoconditioned rats that also received the drug at testing had short cross-through latencies. In addition, LiCl induced a time-dependent disruption of performance. Relative to immediate LiCl, the response latency increased when the injection was delayed by 1 h (F(1,18) = 10.61, p = 0.004) or 2 h (F(1,18) = 11.42, p = 0.003). Consistent with numerous CTA experiments, LiCl induced a strong CTA for all the treated groups (Fig. 3C) when compared with the Sal group (p values < 0.001). However, delaying LiCl injection progressively reduced CTA (LiCL vs LiCl60, F(1,18) = 7.62, p < 0.01; LiCl60 vs LiCl120, F(1,20) = 7.52, p = 0.012). A control experiment to assess the effect of LiCl on body weight was performed 1 week later on nondeprived rats that had received a single injection previously. These rats were weighed before receiving either a Sal or LiCl injection (300 mg/kg) and weighed again on the next day. Results indicated a significant weight loss on the second day for the LiCl-injected rats (F(1,29) = 8.50, p < 0.01) and a significant interaction of treatment × day (F(1,29) = 209, p < 0.001; Fig. 3D). This result indicates that LiCl treatment affected the general state of the rats, as did the Cyclo treatment previously. It must be noted that, when weaker doses of LiCl were delivered in the preliminary experiments, neither an amnesic effect nor any weight loss were observed.
Experiments 6a and 6b: effect of LiCl on reactivated memory
Experiment 6a investigated whether LiCl would impair a reactivated memory and produce a CTA within the same paradigm. Before the reactivation exposure, all rats were given 30 min access to a sucrose solution (15%). Two groups of rats were given injections of LiCl or Sal either immediately or 2 h after the reactivation session. To assess the role of reactivation itself, another group received LiCl without reactivation. Another group was pseudotrained and administered LiCl (Fig. 4A). A one-way ANOVA on the test latencies indicated a significant difference among groups (Fig. 4B; F(4,37) = 14.13, p < 0.001). As the figure suggests, LiCl immediately after reactivation impaired retention of the shocked compartment (RSal vs RLiCl, F(1,15) = 8.248, p < 0.01) but had no effect on performance when administered after a 2 h delay (RSal vs RLiCl120, F(1,15) = 1.67, NS) or in the absence of a reactivation (RSal vs NR-LiCl, F(1,15) = 2.95, p = 0.105). Additionally, LiCl alone had no effect on initial cross-latency because pseudoconditioned animals not given shocks during conditioning displayed short latencies with LiCl injections (Sal vs PsLiCl, F(1,16) = 223.33, p < 0.001).
A one-way ANOVA on the consumption of sucrose solution indicated a significant effect of treatment (Fig. 4C; F(4,37) = 19.37, p < 0.001). A single injection of LiCl produced a CTA memory for a sucrose solution because all groups that received an injection of LiCl displayed significant CTA to the sucrose solution, even when injections were delayed 120 min after sucrose exposure (RSal vs RLiCl, F(1,15) = 36.39, p < 0.001; RSal vs NR–LiCl, F(1,15) = 28.69, p < 0.001; RSal vs RLiCl120, F(1,15) = 51.98, p < 0.001; RSal vs PsLiCl, F(1,16) = 78.70, p < 0.001).
To determine whether the memory deficits for a reactivated memory produced by LiCl can be alleviated by re-administering LiCl at testing, animals were given another injection of LiCl before an inhibitory avoidance retention test (Experiment 6b). As in the previous experiment, all animals were given access to a sucrose solution before reactivation. One group given LiCl after reactivation received an additional injection of LiCl (0.15 m, 127 mg/kg) 30 min before testing in inhibitory avoidance, whereas the two other groups were given Sal (Fig. 4A). A fourth condition received LiCl in the absence of reactivation and again before testing. An ANOVA on response latencies indicated a significant effect of treatment (F(3,27) = 7.67, p < 0.001). After a reactivation, LiCl produced memory deficits that were recovered when another injection was given before testing (Fig. 4D). LiCl injections produced memory deficits when Sal was given before test (RSal–Sal vs RCyclo–Sal, F(1,13) = 27.7, p < 0.001) but not when LiCl was re-administered before the retention test (RLiCl–Sal vs RLiCl–LiCl, F(1,14) = 25.63, p < 0.001). Furthermore, the re-administration of the drug produced performance comparable with the retention control given Sal after reactivation (RSal–Sal vs RLiCl–LiCl, F(1,13) = 3.57, NS) or the group receiving the drug without being reactivated (RLiCl–LiCl vs NRLiCl–LiCl, F < 1). A test for CTA was conducted when LiCl was given after reactivation. An ANOVA on the consumption of sucrose solution indicated an effect of treatment (F(3,27) = 10.29, p < 0.001). As can be seen in Figure 4E, LiCl injections produced CTA for a sucrose solution (RSal–Sal vs RLiCl–Sal, F(1,13) = 19.83, p < 0.001; RSal–Sal vs, RLiCl–LiCl, F(1,13) = 12.35, p < 0.01; Sal–Sal vs NRLiCl–LiCl, F(1,13) = 27.73, p < 0.001).
In all, this second series of experiments indicate that a treatment affecting the general internal state of rats can induce a time-dependent amnesia.
Experiment 7: effect of Cyclo and LiCl on protein synthesis
First, we verified that, unlike after an injection of Cyclo, strong doses of LiCl did not inhibit protein synthesis. With LiCl (300 mg/kg), the degree of protein synthesis was increased 3% at 30 min and 5% at 1 h, confirming previous results (Squire et al., 1975). In contrast, after an injection of Cyclo (2.8 mg/kg), the degree of protein synthesis inhibition was 72% at 30 min and 68% at 1 h, similar to the findings of others (Squire et al., 1975; Flood et al., 1977; Milekic et al., 2006; Díaz-Trujillo et al., 2009).
The findings obtained with LiCl show that a treatment that affects the general state of the rats, but not protein synthesis, is able to induce a time-dependent amnesia that can be reversed by a reminder, including the treatment itself, delivered before the retention test. However, LiCl cannot be considered as a typical amnesic agent, given that rats demonstrated a strong aversive memory for sucrose (CTA) when the drug was delivered after sucrose exposure and just before inhibitory conditioning. Therefore, the results obtained with LiCl injections can be explained by the integration of the state provided by LiCl within the active memory.
Discussion
The aim of the present study was to determine whether experimental amnesia could be the result of a failure of memory retrieval attributable to a change in the internal state rather than the consequence of the disruption of a consolidation/reconsolidation process. The results confirmed that Cyclo, a protein synthesis inhibitor, delivered with peripheral injections after training or reactivation of an old memory induced a time-dependent retrograde amnesia. The same effect was obtained when Cyclo was delivered with intracerebroventricular or intrahippocampal administration after reactivation. In all these conditions, we found that amnesia was alleviated by a pretest exposure to a reminder, including the amnesic treatment itself, suggesting that amnesia did not correspond to a failure to store memory of the learning episode information. Finally, both a time-dependent amnesia and recovery of memory was obtained with LiCl, a treatment that does not affect protein synthesis. At the same time, LiCl established new associations (CTA).
According to the consolidation/reconsolidation hypothesis, recently acquired or reactivated memories are transformed progressively from an initially labile state into a stable form, through a protein-dependent process (Nader, 2003; Dudai and Eisenberg, 2004; Alberini, 2008). Preventing these processes should result in an irreversible impairment. This view is challenged by the present study in several ways.
Amnesia is not a loss of information
The performance disruption induced by Cyclo can be alleviated by providing Cyclo again at the time of retention test, suggesting that amnesia resulting from protein synthesis inhibitors, thought to interrupt consolidation/reconsolidation processes, is not based on loss of newly formed or reactivated memories (Miller and Springer, 1972; Mactutus and Riccio, 1978; Gordon and Mowrer, 1980).
Successful use of amnesic agents as a reminder has been demonstrated repeatedly for several treatments, such as electroconvulsive shock (Thompson and Neely, 1970; Thompson and Grossman, 1972), hypothermia (Hinderliter et al., 1975; Mactutus and Riccio, 1978), hyperthermia (Mactutus et al., 1980), and even protein synthesis inhibition (Bradley and Galal, 1988; Millin and Newman, 2008; Briggs and Olson, 2013). Although in most of these studies the amnesic treatment was delivered after initial learning, similar outcomes have also been obtained after memory reactivation (Briggs and Olson, 2013). Typically, these results showing recovery of memory have been interpreted as revealing cases of state dependency induced by the amnesic agents, although drugs were administered after training (or reactivation), whereas in traditional state-dependent research, the drug is administered before training. Several authors suggested that memory itself was not lost but that amnesia resulted from retrieval difficulties attributable to a different state at training and testing (Quartermain et al., 1970; Miller and Springer, 1973; Millin et al., 2001). However, despite these criticisms and numerous others showing recovery after exposure to reminders, the consolidation hypothesis has remained: (1) because a fully convincing alternative explanation has never been proposed; and (2) because the consolidation was in agreement with results provided by molecular and cellular approaches of memory (Alberini, 2005; Tronson and Taylor, 2007). The current results return to an old and long debated question about whether amnesia results from a disruption of storage (McGaugh, 1966; Hardt et al., 2010) or retrieval processes (Miller and Springer, 1973; Spear, 1978; Judge and Quartermain, 1982; Miller and Matzel, 2006).
A novel strength of the present studies is in demonstrating that reversal of amnesia from protein synthesis inhibition is obtained not only after new learning but also after reactivation of old learning. In the latter case, amnesia and its reversal were seen with intracerebroventricular and intrahippocampal administrations, as well as with systemic injection of the drug. In fact, cases of state dependency with a drug delivered intracerebrally have been reported previously, demonstrating that even a very small amount of drug delivered within the brain can induce a particular internal state that may affect retrieval processes (Nasehi et al., 2009; Jamali-Raeufy et al., 2011; Rossato et al., 2015). These findings, along with the present results, suggest that information provided by an internal state is not limited to peripheral feedback.
New memories can be established and maintained without protein synthesis
The present results further indicate that Cyclo, shown to induce a strong inhibition of protein synthesis (Experiment 7; Squire et al., 1975; Milekic et al., 2006), did not prevent consolidation or reconsolidation.
In addition, we showed that a time-dependent amnesia can be obtained with a treatment (LiCl) that modifies the internal state without affecting protein synthesis (present results; Squire et al., 1975) when given after conditioning or reactivation. Under these conditions, amnesia was also alleviated by re-administering LiCl before the retention test. However, LiCl did not prevent the inhibitory avoidance memory given that (1) amnesia was mostly alleviated by re-administering the treatment (or a reminder) before the retention test (Zarrindast et al., 2007) and (2) LiCl established a new memory, a CTA, for a flavor delivered just before the conditioning or the reactivation. These findings led us to conclude that, under our conditions, establishing new memories or maintaining reactivated memories does not require de novo protein synthesis. They also provide additional evidence supporting the view that inhibition of protein synthesis is not the necessary basis for amnesias. Interestingly, this finding gives support to previous studies questioning the role of the protein synthesis in the stabilization of memories (Biedenkapp and Rudy, 2004; Lattal and Abel, 2004; Alberini, 2008; Briggs and Olson, 2013).
A retrieval hypothesis: memory integration
Although the present results do not support the consolidation/reconsolidation hypotheses, they are in agreement with a retrieval hypothesis based on the integration of new information with the memory. According to Lewis (1979), memory can be distinguished as existing in one of two states: (1) an active but time-limited state, prevailing at the time of acquisition and reactivation; and (2) an inactive phase into which the active state transitions. During reactivation, older memories are brought back online and become labile, so that new information can be acquired, modifying and updating the initial memory (Przybyslawski and Sara, 1997; Lee, 2010). Importantly, labile does not necessarily refer to fragility but rather to malleability. As a consequence, when new information is presented while the memory is malleable, a representation of that information can be integrated within the active memory (Gisquet-Verrier and Riccio, 2012; Schlichting and Preston, 2015) and become a new memory attribute, i.e., a part of the memory (Underwood, 1969; Spear, 1978). Here, we showed that, despite the fact that Cyclo and LiCl are very different treatments, they both affect the general state of the animals. Delivering these treatments while a fear memory is active produced the same effects: a disruption of performance. This effect does not seem to be attributable to a disruption of the consolidation processes and/or of protein synthesis but more likely to a form of state dependency, given that performance disruptions were abolished by reinstating the modified state, allowing retrieval processes to access the initial memory. These results suggest that, because of the malleability of active memory, the new information provided by a treatment delivered just after training or reactivation can become a new memory attribute integrated within the memory. In our conditions, it seems that this attribute replaced or took priority over the one representing the internal state at the time of training, given that retrieval was disrupted when rats were tested in their initial state, as in several studies showing recovery by reinducing the amnesic treatment before testing (Mactutus and Riccio, 1978). Conceptually, integration might be viewed as similar to extinction, a process that does not erase the previous representation but creates another one that competes with the original one to take control of behavior. Interestingly, as we reported, when LiCl was delivered at a lower dose, we did not get any disruption of performance when rats were tested in their initial state, suggesting that the novel information must be salient enough, relative to the initial memory cues, for its absence to disrupt retrieval processes.
Our results indicate that the malleability of active memories permits the integration of new memory attributes provided not only by peripheral but also central administration. Memory integration can account for other cases of memory disruption and not only those attributable to amnesic treatments. For instance, memory integration can also account for interference that may happen when a second acquisition occurs after the reactivation of the former one, a phenomenon observed in animals (Gordon and Feldman, 1978) and humans for motor (Walker et al., 2003) and episodic (Hupbach et al., 2007, 2009) memories, presumed to imply a reconsolidation process. Finally, it may explain recent results showing changes in emotional evaluation of episodic memory (Arminjon et al., 2015).
Time-dependent gradients of amnesic treatments are often considered as the key factor supporting the consolidation hypothesis (Dudai, 2012). The central issue concerns the processes involved in producing this effect. Here, we showed that a time-dependent gradient can be obtained with LiCl that did not affect consolidation processes based on protein synthesis. This result can also be explained by the integration hypothesis, because the degree of integration should be a function of the level of memory activity. As a consequence, the integration of the internal state, and thus a greater state dependency on that state for retrieval, should be expected when new information is presented shortly after training or reactivation.
Conclusion
The present findings critically challenge the consolidation/reconsolidation hypotheses and strongly suggest that de novo protein synthesis may not be required to acquire and maintain a memory representation (Ryan et al., 2015). As already pointed out, various alternatives to the consolidation/reconsolidation hypothesis, such as retrieval difficulties, interference, and state dependency, have been proposed previously (Spear, 1973; Miller and Springer, 1974; Riccio et al., 2006). Although memory integration is not demonstrated in the present paper, this hypothesis combines most of the previous hypotheses and provides a more compelling explanation for the results obtained with amnesic treatments, including those that have never been explained convincingly by the consolidation/reconsolidation hypothesis (Spear, 1973; Riccio et al., 2006; Nader and Einarsson, 2010). Integration of new information while memory is in an active state is not a new concept and has been mentioned repeatedly for memory updating (Tronel et al., 2005; Briggs et al., 2007; Hupbach et al., 2007; Briggs and Riccio, 2008; Lee, 2010; De Oliveira Alvares et al., 2013). Hence, information delivered while a memory is active should no longer be divided into two categories: (1) those serving to update memories and (2) those disrupting memories. Memory integration proposes that any new information delivered while the memory is active can become a new attribute of the initial memory. Depending on its relative salience, that attribute may compete or not with the other memory attributes of the initial representation, at the time of retrieval. Depending also on the content of this information, the performance obtained could be either strengthened (additional training trials, arousing drugs) or weakened (amnesic agents, extinction trials; Gisquet-Verrier and Riccio, 2012).
We believe the integration hypothesis has heuristic value in that it seems applicable to a wide range of memory phenomena reported in both human and animal research, as mentioned previously, and suggests much more rapid and specific elaborative and updating processes (Debiec et al., 2006; Tse et al., 2007). Memory integration further suggests that memories cannot be erased but can be durably modified, a characteristic that offers the prospect of promising new therapeutic approaches.
Footnotes
P.C. was supported by a French–Italian Erasmus program; D.T. was supported by a General Directorate for Armament/National Center of Scientific Research PhD fellowship. Partial support was provided by the Farris Family Foundation and Whitehall Foundation (A.M.J.). We thank Patrick Winiecki, Tyler Vanderhoof, Dina Dejanovic, and Dan Burbles for help with data collection. P.G.-V. is particularly grateful to Françoise Schenk for her intellectual help and support. The present paper is dedicated to Bernard Hars who made important contributions in the field of memory and who passed away prematurely at the beginning of the year.
The authors declare no competing financial interests.
References
- Alberini CM. Mechanisms of memory stabilization: are consolidation and reconsolidation similar or distinct processes? Trends Neurosci. 2005;28:51–56. doi: 10.1016/j.tins.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Alberini CM. The role of protein synthesis during the labile phases of memory: revisiting the skepticism. Neurobiol Learn Mem. 2008;89:234–246. doi: 10.1016/j.nlm.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arminjon M, Preissmann D, Chmetz F, Duraku A, Ansermet F, Magistretti PJ. Embodied memory: unconscious smiling modulates emotional evaluation of episodic memories. Front Psychol. 2015;6:650. doi: 10.3389/fpsyg.2015.00650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedenkapp JC, Rudy JW. Context memories and reactivation: constraints on the reconsolidation hypothesis. Behav Neurosci. 2004;118:956–964. doi: 10.1037/0735-7044.118.5.956. [DOI] [PubMed] [Google Scholar]
- Bradley PM, Galal KM. State-dependent recall can be induced by protein synthesis inhibition: behavioural and morphological observations. Brain Res. 1988;468:243–251. doi: 10.1016/0165-3806(88)90136-8. [DOI] [PubMed] [Google Scholar]
- Briggs JF, Olson BP. Reexposure to the amnestic agent alleviates cycloheximide-induced retrograde amnesia for reactivated and extinction memories. Learn Mem. 2013;20:285–288. doi: 10.1101/lm.030270.113. [DOI] [PubMed] [Google Scholar]
- Briggs JF, Riccio DC. Transfer of old “reactivated” memory retrieval cues in rats. Learn Motiv. 2008;39:13–23. doi: 10.1016/j.lmot.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs JF, Fitz KI, Riccio DC. Transfer of memory retrieval cues in rats. Psychon Bull Rev. 2007;14:495–499. doi: 10.3758/BF03194096. [DOI] [PubMed] [Google Scholar]
- De Oliveira Alvares L, Crestani AP, Cassini LF, Haubrich J, Santana F, Quillfeldt JA. Reactivation enables memory updating, precision-keeping and strengthening: exploring the possible biological roles of reconsolidation. Neuroscience. 2013;244:42–48. doi: 10.1016/j.neuroscience.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Debiec J, Doyère V, Nader K, Ledoux JE. Directly reactivated, but not indirectly reactivated, memories undergo reconsolidation in the amygdala. Proc Natl Acad Sci U S A. 2006;103:3428–3433. doi: 10.1073/pnas.0507168103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Trujillo A, Contreras J, Medina AC, Silveyra-Leon GA, Antaramian A, Quirarte GL, Prado-Alcalá RA. Enhanced inhibitory avoidance learning prevents the long-term memory-impairing effects of cycloheximide, a protein synthesis inhibitor. Neurobiol Learn Mem. 2009;91:310–314. doi: 10.1016/j.nlm.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Dudai Y. The restless engram: consolidations never end. Annu Rev Neurosci. 2012;35:227–247. doi: 10.1146/annurev-neuro-062111-150500. [DOI] [PubMed] [Google Scholar]
- Dudai Y, Eisenberg M. Rites of passage of the engram: reconsolidation and the lingering consolidation hypothesis. Neuron. 2004;44:93–100. doi: 10.1016/j.neuron.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Duncan CP. The retroactive effect of electroshock on learning. J Comp Physiol Psychol. 1949;42:32–44. doi: 10.1037/h0058173. [DOI] [PubMed] [Google Scholar]
- Flood JF, Bennett EL, Rosenzweig MR, Orme AE. Influence of training strength on amnesia induced by pretraining injections of cycloheximide. Physiol Behav. 1972;9:589–600. doi: 10.1016/0031-9384(72)90017-0. [DOI] [PubMed] [Google Scholar]
- Flood JF, Jarvik ME, Bennett EL, Orme AE, Rosenzweig MR. The effect of stimulants, depressants, and protein synthesis- inhibition on retention. Behav Biol. 1977;20:168–183. doi: 10.1016/S0091-6773(77)90734-9. [DOI] [PubMed] [Google Scholar]
- Garcia J, Koelling R. Relation of cue to consequence in avoidance learning. Psychon Sci. 1966;4:123–124. doi: 10.3758/BF03342209. [DOI] [Google Scholar]
- Gisquet-Verrier P, Riccio DC. Memory reactivation effects independent of reconsolidation. Learn Mem. 2012;19:401–409. doi: 10.1101/lm.026054.112. [DOI] [PubMed] [Google Scholar]
- Gold PE, Sternberg DB. Neurobiology of amnesia. Science. 1980;209:837. doi: 10.1126/science.209.4458.837-a. [DOI] [PubMed] [Google Scholar]
- Gordon WC, Feldman DT. Reactivation-induced interference in a short-term retention paradigm. Learn Motiv. 1978;9:164–178. doi: 10.1016/0023-9690(78)90018-8. [DOI] [Google Scholar]
- Gordon WC, Mowrer RR. An extinction trial as a reminder treatment following electroconvulsive shock. Anim Learn Behav. 1980;8:363–367. doi: 10.3758/BF03199618. [DOI] [Google Scholar]
- Hardt O, Einarsson EO, Nader K. A bridge over troubled water: reconsolidation as a link between cognitive and neuroscientific memory research traditions. Annu Rev Psychol. 2010;61:141–167. doi: 10.1146/annurev.psych.093008.100455. [DOI] [PubMed] [Google Scholar]
- Hinderliter CF, Webster T, Riccio DC. Amnesia induced by hypothermia as a function of treatment-test interval and recooling in rats. Anim Learn Behav. 1975;3:257–263. doi: 10.3758/BF03213441. [DOI] [Google Scholar]
- Hupbach A, Gomez R, Hardt O, Nadel L. Reconsolidation of episodic memories: a subtle reminder triggers integration of new information. Learn Mem. 2007;14:47–53. doi: 10.1101/lm.365707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hupbach A, Gomez R, Nadel L. Episodic memory reconsolidation: updating or source confusion? Memory. 2009;17:502–510. doi: 10.1080/09658210902882399. [DOI] [PubMed] [Google Scholar]
- Jamali-Raeufy N, Nasehi M, Ebrahimi-Ghiri M, Zarrindast MR. Cross state-dependency of learning between WIN55, 212-2 and scopolamine in rat dorsal hippocampus. Neurosci Lett. 2011;491:227–231. doi: 10.1016/j.neulet.2011.01.056. [DOI] [PubMed] [Google Scholar]
- Judge ME, Quartermain D. Characteristics of retrograde amnesia following reactivation of memory in mice. Physiol Behav. 1982;28:585–590. doi: 10.1016/0031-9384(82)90034-8. [DOI] [PubMed] [Google Scholar]
- Kalat J, Rozin P. “Salience”: a factor which can override temporal contiguity in taste-aversion learning. J Comp Physiol Psychol. 1970;71:192–197. doi: 10.1037/h0029158. [DOI] [Google Scholar]
- Lattal KM, Abel T. Behavioral impairments caused by injections of the protein synthesis inhibitor anisomycin after contextual retrieval reverse with time. Proc Natl Acad Sci U S A. 2004;101:4667–4672. doi: 10.1073/pnas.0306546101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EH, Hung HC, Lu KT, Chen WH, Chen HY. Protein synthesis in the hippocampus associated with memory facilitation by corticotropin-releasing factor in rats. Peptides. 1992;13:927–937. doi: 10.1016/0196-9781(92)90051-4. [DOI] [PubMed] [Google Scholar]
- Lee JLC. Reconsolidation: maintaining memory relevance. Trends Neurosci. 2009;32:413–420. doi: 10.1016/j.tins.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JLC. Memory reconsolidation mediates the updating of hippocampal memory content. Front Behav Neurosci. 2010;4:168. doi: 10.3389/fnbeh.2010.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DJ. Psychobiology of active and inactive memory. Psychol Bull. 1979;86:1054–1083. doi: 10.1037/0033-2909.86.5.1054. [DOI] [PubMed] [Google Scholar]
- Mactutus CF, Riccio DC. Hypothermia-induced retrograde amnesia: role of body temperature in memory retrieval. Physiol Psychol. 1978;6:18–22. doi: 10.3758/BF03326685. [DOI] [Google Scholar]
- Mactutus CF, Ferek JM, Riccio DC. Amnesia induced by hyperthermia: an unusually profound, yet reversible, memory loss. Behav Neural Biol. 1980;30:260–277. doi: 10.1016/S0163-1047(80)91150-4. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Time-dependent processes in memory storage. Science. 1966;153:1351–1358. doi: 10.1126/science.153.3742.1351. [DOI] [PubMed] [Google Scholar]
- Milekic MH, Brown SD, Castellini C, Alberini CM. Persistent disruption of an established morphine conditioned place preference. J Neurosci. 2006;26:3010–3020. doi: 10.1523/JNEUROSCI.4818-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RR, Matzel LD. Retrieval failure versus memory loss in experimental amnesia: definitions and processes. Learn Mem. 2006;13:491–497. doi: 10.1101/lm.241006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RR, Springer AD. Induced recovery of memory in rats following electroconvulsive shock. Physiol Behav. 1972;8:645–651. doi: 10.1016/0031-9384(72)90089-3. [DOI] [PubMed] [Google Scholar]
- Miller RR, Springer AD. Amnesia, consolidation, and retrieval. Psychol Rev. 1973;80:69–79. doi: 10.1037/h0033897. [DOI] [PubMed] [Google Scholar]
- Miller RR, Springer AD. Implications of recovery from experimental amnesia. Psychol Rev. 1974;81:470–473. doi: 10.1037/h0036951. [DOI] [PubMed] [Google Scholar]
- Millin P, Newman E. A comparison of the effects of state and non-state reminder treatments on morphine state-dependency and cycloheximide-induced retrograde amnesia. J Behav Neurosci Res. 2008;6:6–14. [Google Scholar]
- Millin PM, Moody EW, Riccio DC. Interpretations of retrograde amnesia: old problems redux. Nat Rev Neurosci. 2001;2:68–70. doi: 10.1038/35049075. [DOI] [PubMed] [Google Scholar]
- Misanin JR, Miller RR, Lewis DJ. Retrograde amnesia produced by electroconvulsive shock after reactivation of a consolidated memory trace. Science. 1968;160:554–555. doi: 10.1126/science.160.3827.554. [DOI] [PubMed] [Google Scholar]
- Nader K. Memory traces unbound. Trends Neurosci. 2003;26:65–72. doi: 10.1016/S0166-2236(02)00042-5. [DOI] [PubMed] [Google Scholar]
- Nader K, Einarsson EO. Memory reconsolidation: an update. Ann N Y Acad Sci. 2010;1191:27–41. doi: 10.1111/j.1749-6632.2010.05443.x. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Nasehi M, Sahebgharani M, Haeri-Rohani A, Zarrindast MR. Effects of cannabinoids infused into the dorsal hippocampus upon memory formation in 3-days apomorphine-treated rats. Neurobiol Learn Mem. 2009;92:391–399. doi: 10.1016/j.nlm.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Ed 2. San Diego: Academic; 1986. [DOI] [PubMed] [Google Scholar]
- Przybyslawski J, Sara SJ. Reconsolidation of memory after its reactivation. Behav Brain Res. 1997;84:241–246. doi: 10.1016/S0166-4328(96)00153-2. [DOI] [PubMed] [Google Scholar]
- Quartermain D, Botwinick CY. Role of the biogenic amines in the reversal of cycloheximide-induced amnesia. J Comp Physiol Psychol. 1975;88:386–401. doi: 10.1037/h0076208. [DOI] [PubMed] [Google Scholar]
- Quartermain D, McEwen BS, Azmitia EC., Jr Amnesia produced by electroconvulsive shock or cycloheximide: conditions for recovery. Science. 1970;169:683–686. doi: 10.1126/science.169.3946.683. [DOI] [PubMed] [Google Scholar]
- Riccio DC, Millin PM, Bogart AR. Reconsolidation: a brief history, a retrieval view, and some recent issues. Learn Mem. 2006;13:536–544. doi: 10.1101/lm.290706. [DOI] [PubMed] [Google Scholar]
- Roediger HL, 3rd, Butler AC. The critical role of retrieval practice in long-term retention. Trends Cogn Sci. 2011;15:20–27. doi: 10.1016/j.tics.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Rossato JI, Köhler CA, Radiske A, Lima RH, Bevilaqua LRM, Cammarota M. State-dependent effect of dopamine D1/D5 receptors inactivation on memory destabilization and reconsolidation. Behav Brain Res. 2015;285:194–199. doi: 10.1016/j.bbr.2014.09.009. [DOI] [PubMed] [Google Scholar]
- Rouanet H, Bernard J, Leroux B. Statistiques en sciences humaines: analyse inductive des donnees. Paris: Dunod; 1990. [Google Scholar]
- Ryan TJ, Roy DS, Pignatelli M, Arons A, Tonegawa S. Memory. Engram cells retain memory under retrograde amnesia. Science. 2015;348:1007–1013. doi: 10.1126/science.aaa5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlichting ML, Preston AR. Memory integration: neural mechanisms and implications for behavior. Curr Opin Behav Sci. 2015;1:1–8. doi: 10.1016/j.cobeha.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear NE. Retrieval of memory in animals. Psychol Rev. 1973;80:163–194. doi: 10.1037/h0034326. [DOI] [Google Scholar]
- Spear NE. The processing of memories: forgetting and retention. Hillsdale, NJ: Erlbaum; 1978. [Google Scholar]
- Squire LR, Barondes SH. Variable decay of memory and its recovery in cycloheximide-treated mice. Proc Natl Acad Sci U S A. 1972;69:1416–1420. doi: 10.1073/pnas.69.6.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, 2nd, Emanuel CA, Davis HP, Deutsch JA. Inhibitors of cerebral protein synthesis: dissociation of aversive and amnesic effects. Behav Biol. 1975;14:335–341. doi: 10.1016/S0091-6773(75)90467-8. [DOI] [PubMed] [Google Scholar]
- Thompson CI, Grossman LB. Loss and recovery of long-term memories after ECS in rats: evidence for state-dependent recall. J Comp Physiol Psychol. 1972;78:248–254. doi: 10.1037/h0032178. [DOI] [PubMed] [Google Scholar]
- Thompson CI, Neely JE. Dissociated learning in rats produced by electroconvulsive shock. Physiol Behav. 1970;5:783–786. doi: 10.1016/0031-9384(70)90279-9. [DOI] [PubMed] [Google Scholar]
- Tronel S, Milekic MH, Alberini CM. Linking new information to a reactivated memory requires consolidation and not reconsolidation mechanisms. PLoS Biol. 2005;3:e293. doi: 10.1371/journal.pbio.0030293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronson NC, Taylor JR. Molecular mechanisms of memory reconsolidation. Nat Rev Neurosci. 2007;8:262–275. doi: 10.1038/nrn2090. [DOI] [PubMed] [Google Scholar]
- Tse D, Langston RF, Kakeyama M, Bethus I, Spooner PA, Wood ER, Witter MP, Morris RGM. Schemas and memory consolidation. Science. 2007;316:76–82. doi: 10.1126/science.1135935. [DOI] [PubMed] [Google Scholar]
- Underwood BJ. Attributes of memory. Psychol Rev. 1969;76:559–573. doi: 10.1037/h0028143. [DOI] [Google Scholar]
- Walker MP, Brakefield T, Hobson JA, Stickgold R. Dissociable stages of human memory consolidation and reconsolidation. Nature. 2003;425:616–620. doi: 10.1038/nature01930. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Shendy MM, Ahmadi S. Nitric oxide modulates state dependency induced by lithium in an inhibitory avoidance task in mice. Behav Pharmacol. 2007;18:289–295. doi: 10.1097/FBP.0b013e3281f520b0. [DOI] [PubMed] [Google Scholar]