Abstract
Acute lung injury (ALI) is a critical syndrome that is associated with a high morbidity and mortality in patients. Sevoflurane has a lung protective effect in ALI as it reportedly has anti-inflammatory and apoptotic-regulating activity. However, the mechanism is still not entirely understood. The aim of the present study was to explore the effects of sevoflurane on lipopolysaccharide (LPS)-induced ALI in mice and the possible mechanisms involved. The results revealed that sevoflurane treatment improved LPS-induced lung injury, as evidenced by the reduction in mortality, lung permeability, lung wet/dry ratio and lung histopathological changes in mice. Total cell counts and the production of pro-inflammatory cytokines [tumor necrosis factor-α, interleukin (IL)-1β and IL-6] in bronchoalveolar fluid were also decreased following treatment with sevoflurane. Additionally, LPS-triggered apoptosis in lung tissues, which was eliminated by sevoflurane. Furthermore, a miRCURY™ LNA array was employed to screen for differentially expressed microRNAs (miRs/miRNAs). Among these miRNAs, 6 were differentially expressed and were involved in the inflammatory response, but only miR-27a-3p (miR-27a) was regulated by sevoflurane. Subsequently, the present study investigated whether sevoflurane exerts its function through the modulation of miR-27a. The results demonstrated that the overexpression of miR-27a via an injection with agomiR-27a produced similar protections as sevoflurane, while the inhibition of miR-27a suppressed the lung protective effects of sevoflurane in ALI mice. In addition, the present study identified that miR-27a inhibited Toll-like receptor 4 (TLR4) by binding to its 3′-untranslated region. Western blot analysis demonstrated that sevoflurane may ameliorate the inflammatory response by blocking the miR-27a/TLR4/MyD88/NF-κB signaling pathway. The present results indicate that sevoflurane may be a viable therapeutic option in the treatment of patients with ALI.
Keywords: sevoflurane, acute lung injury, microRNA-27a-3p, Toll-like receptor 4; MyD88; NF-κB signaling pathway
Introduction
Acute lung injury (ALI) is a critical complication of some clinical illnesses with high rates of morbidity and mortality, and it can lead to acute respiratory distress syndrome (1). ALI is characterized by an excessive inflammatory response, excessive neutrophil infiltration into the lung tissues, the release of pro-inflammatory cytokines, and lung endothelial and epithelial injuries, which cause edema and gas exchange deterioration (2-4). Therefore, the inhibition of the excessive inflammatory response may be an effective approach for the prevention and treatment of ALI.
Numerous studies have reported that volatile anesthetic can provide protective effects in various tissues and organs (5-7). Minguet et al (8) demonstrated that volatile anesthetics could protect against ischemia-reperfusion (IR) injury in the heart. Sevoflurane, a volatile anesthetic, is most commonly used as first line anesthesia (9). Despite being an inhaled agent, only a few studies have been reported using sevoflurane to attenuate lung injury (10-12). For example, Suter et al (13) revealed that sevoflurane can reduce the lung tissue edema and inflammatory cell infiltration induced by endotoxin. Ohsumi et al (14) demonstrated that sevoflurane exhibited significant protective effects against lung IR injury (IRI) via its anti-inflammatory activities in a rat lung transplantation model. In addition, sevoflurane was found to improve LPS-induced ALI by inhibiting lung inflammation in a series of in vivo and in vitro experiments (15,16). However, the mechanism of such effects on ALI is also still unclear.
MicroRNAs (miRNAs/miRs) are a class of endogenous small noncoding RNAs of ~21-23 nucleotides in length that repress the translation or induce the degradation of target mRNA by binding to the 3′-untranslated region (UTR) of target mRNAs (17,18). An increasing body of evidence has supported that miRNAs are implicated in certain inflammatory lung diseases. For example, Guo et al (19) reported that the upregulation of miR-125b significantly reduced lipopolysaccharide (LPS)-induced pulmonary inflammation in mice. Chen et al (20) demonstrated that miR-212-3p inhibited the LPS-induced inflammatory response by targeting high mobility group box-1 protein in murine macrophages. Recently, several studies have revealed that sevoflurane exerts its biological effects via modulation of miRNAs (21-23). Wu et al (22) revealed that sevoflurane protected against hepatic IRI by modulating miRNA-200c regulation in mice. Furthermore Wenlan et al (23) demonstrated that miR-34a-5p attenuated the protective effect of sevoflurane in hypoxia/reoxygenation-induced cardiomyocyte injury by targeting Syntaxin 1A. In another study, Lv et al (21) reported that the downregulation of miR-27a-3p expression ameliorated sevoflurane-induced neurotoxicity as well as learning and memory impairment in neonatal mice. However, whether miRNAs participate in the anti-inflammatory activity of sevoflurane in ALI remains unclear.
In the present study, the aim was to assess the possible protective effects of sevoflurane against LPS-induced lung injury and to elucidate the possible underlying mechanisms. The results from the in vivo experiments suggest that sevoflurane attenuates LPS-induced ALI through the inhibition of miR-27a/Toll-like receptor 4 (TLR4)/MyD88/NF-κB signaling pathway activation. Therefore, the present study suggests that sevoflurane may be a potential approach for ALI treatment.
Materials and methods
Animals
Male BALB/c mice (n=84), weighing 20-30 g, aged 8-12 weeks, were purchased from the Shanghai Experimental Animal Center. All surgical and care procedures were approved by the Animal Care and Use Committee of the First Affiliated Hospital of Xinxiang Medical University. All mice were maintained in a temperature-controlled room (22±2°C) with a 12-h light/dark cycle, a relative humidity of 40-60% and free access to food and water.
Experimental design and LPS induction
The mice were randomized into 7 experimental groups (n=6/group): Group 1, the Sham group who received saline (0.3 ml, intragastrically); group 2, the ALI group who only received LPS (5 mg/kg body weight, intranasally; Escherichia coli serotype 055:B5; Sigma-Aldrich; Merck KGaA) in 300 µl PBS for sensitization (24); group 3, the sevoflurane group (SEVO group), in which mice inhaled 3% sevoflurane (Sigma-Aldrich; Merck KGaA) for 4 h; group 4, ALI + SEVO group (3% sevoflurane + LPS), in which mice were treated the same as the ALI group but were administered 3% sevoflurane for 4 h, starting 2 h after LPS treatment (25); group 5, ALI + agomir-27a group (8 mg/kg agomir-27a + LPS), in which mice were injected with agomir-27a (8 mg/kg) by tail intravenous injection 24 h prior to LPS treatment, and then repeatedly injected every 24 h for 3 day; group 6, ALI + SEVO + antagomir-27a group (3% sevoflurane + 8 mg/kg antagomir-27a + LPS), in which mice were treated in the same way as group 5 (8 mg/kg antagomir-27a + LPS) but were also administered 3% sevoflurane for 4 h, starting 2 h after LPS treatment; group 7, ALI + SEVO + antagomir-negative control (NC) group (3% sevoflurane + 8 mg/kg antagomir-NC + LPS), in which mice were treated in the same way as group 5 (8 mg/kg antagomir-NC + LPS) but were also administered 3% sevoflurane for 4 h, starting at 2 h after LPS treatment. Animals were sacrificed 3 days after treatment with LPS or saline, and the lung tissues at 6, 12 and 24 h, and bronchoalveolar lavage fluid (BALF) at 12 h were collected for analysis. Antagomir-27a and agomir-27a were designed and synthesized by Guangzhou RiboBio Co., Ltd. The doses of miRNA were selected based on previous studies (26-29). The lung tissues were snap-frozen and stored at -80°C until subsequent use.
To evaluate the transfection efficiency of miR-27a in vivo, 12 mice were randomized into another 4 experimental groups (n=3/group). Agomir-27a/agomir-NC groups (Antagomir-27a/antagomir-NC groups), in which all mice were given agomir-27a/agomir-NC (Antagomir-27a/antagomir-NC groups; 8 mg/kg) via tail intravenous administration every 24 h for 3 days; after a further 3 days, animals were sacrificed and the lung tissues were extracted.
The survival experiment
The survival experiments were performed in all 7 groups of mice (the Sham, ALI, SEVO, ALI + SEVO, ALI + agomir-27a, ALI + SEVO + antagomir-27a and ALI + SEVO + antagomir-NC groups; n=10/each group). Mice were treated as aforementioned. Mice were monitored regularly, and survival was recorded over a period of 7 days.
Bronchoalveolar lavage collection
BALF was obtained by flushing the right lung lobes with 1.0 ml PBS. In total, ~0.8 ml of BALF was recovered without significant differences between groups. After centrifugation for 10 min at 600 g at 4°C, the pellet was re-suspended in PBS. Total cell numbers were counted using a hemocytometer. At least 200 cells were counted per sample. The supernatants were collected and immediately frozen on dry ice and stored at -80°C for cytokine measurements using mouse ELISA kits (R&D Systems, Inc.) for interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α.
Evaluation of lung permeability
Lung permeability was assessed using the Evans blue dye extravasation method, as described previously (30). Briefly, via the tail vein, 4 ml/kg of 2% Evans blue (Sigma-Aldrich; Merck KGaA) in normal saline was administered 10 h before the animals were euthanized. After adequate perfusion with normal saline, Evans blue dye was extracted from the lung using formamide for 18 h at 60°C and measured as the absorbance of the supernatant at 620 nm on a microplate reader (BioTek Instruments, Inc.) and was reported as the amount of Evans blue per wet tissue weight (µg/g).
Lung wet/dry (W/D) ratio
Mice were sacrificed, and the right lungs were excised and placed in an incubator at 80°C for 48 h to obtain the dry weight. The ratio of wet lung to dry lung was then calculated to assess tissue edema.
Histopathologic evaluation of lung tissues
Upper and lower lobe lung samples were excised 24 h after the LPS challenge, fixed with 10% formalin at room temperature for 24 h and microsectioned at 5 µm. Then, the tissues were embedded in paraffin and stained with hematoxylin and eosin at 37°C for 5 min. Finally, the lung injury score was assessed in a blinded fashion via semi-quantitative light microscopy evaluation as previously described (31). The images were captured with a Nikon E100 light microscope with ×200 magnification.
Measurement of pro-inflammatory cytokines
The levels of TNF-α (cat. no. MTA00B), IL-6 (cat. no. M6000B), and IL-1β (cat. no. MLB00C) in BALF were measured using mouse ELISA kits (R&D Systems, Inc.) according to the manufacturer's instructions protocol.
miRNA microarray
Total RNA was isolated from frozen lung tissue using the miRNeasy isolation kit (Qiagen, Inc.) following the manufacturer's protocol. The samples were analyzed via a miRCURY LNA™ Array (v.18.0; Agilent Technologies, Inc.). The procedure and images process method were conducted as described previously (32).
Reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was isolated from the left lung tissue samples using a miRNeasy mini kit (Qiagen, Inc.) following the manufacturer's protocol. RT was performed at 40°C for 45 min followed by incubation at 95°C for 5 min using a miRNA reverse transcription kit (Applied Biosystems; Thermo Fisher Scientific, Inc.). qPCR was performed using a standard protocol from the SYBR Green PCR kit (Toyobo Life Science) on an ABI PRISM 7500 Real-time PCR system (Thermo Fisher Scientific, Inc.). RT-qPCR was performed at 95°C for 10 min, followed by 40 cycles of amplification at 95°C for 2 sec and 60°C for 34 sec. The melting curve stage was performed at 95°C for 30 sec, 60°C for 30 min and 95°C for 30 sec. The primer sequences were as follows: miR-19a forward, 5′-TCA TCA CGC TGT GCA AAT CT-3′ and reverse, 5′-TAT GGT TGT TCT GCT CTC TGT CTC-3′; miR-149* forward, 5′-ACA CTC CAG CTG GGA GGG ACG GGG GC-3′ and reverse, 5′-CTC AAC TGG TGT CGT GGA-3′; miR-146a-5p forward, 5′-TGA GAA CTG AAT TCC ATG GGT-3′ and reverse, 5′-CGA GAA GCT TGC ATC ACC AGA GAA CG-3′; miR-122 forward 5′-GTG ACA ATG GTG GAA TGT GG-3′ and reverse, 5′-AAA GCA AAC GAT GCC AAG AC-3′; miR-27a forward 5′-TGC GGT TCA CAG TGG CTA AG-3′ and reverse, 5′-CTC AAC TGG TGT CGT GGA-3′; U6 forward, 5′-GCT TCG GCA GCA CAT ATA CTA AAA T-3′ and reverse, 5′CGC TTC ACG AAT TTG CGT GTC AT-3′. The results were quantified using the 2−ΔΔCq method (33).
Cells transfection
In order to up-or downregulate the expression of miR-27a, 50 nM miR-27a mimics, 50 nM miR-27a inhibitor and 50 nM their NC were synthesized by Shanghai GenePharma Co., Ltd. The sequences for these oligonucleotides were as follows: 5′-UUC ACA GUG GCU AAG UUC CGC-3′ for miR-27a mimics; 5′-GCG GAA CUU AGC CAC UGU GAA-3′ for miR-27a inhibitor; 5′-UUC UCC GAA CGU GUC ACG UTT-3′ for NC mimics; and 5′-CAG UAC UUUUGU GUA GUA CAA-3′ for the NC inhibitor. Cell transfection was conducted using Lipofectamine 2000™ reagent (Invitrogen; Thermo Fisher Scientific, Inc.) for 48 h following the manufacturer's protocol. At 24 h post-transfection, the cells were collected and used for the following analyses.
Bioinformatics
TargetScan (version 7.0; www.targetscan.org/), miRbase (version 21.0; www.mirbase.org/) and PicTar (version 2006; pictar.mdc-berlin.de) target gene prediction software were used to select MIF as a target gene of miR-27a.
Luciferase reporter assay
The 3′-UTR of TLR4 and the mutated sequence were inserted into the pGL3 control vector (Promega Corporation) to construct the wild-type (wt) TLR4-3′-UTR vector and mutant TLR4-3′-UTR vector, respectively. For luciferase reporter assay, 293T cells (American Type Culture Collection) in 24-well plates (5.0×105/well) and were transfected with either the wt or mut reporter vector, combined with miR-27a mimics or miR-27a inhibitor using Lipofectamine 2000™ (Invitrogen; Thermo Fisher Scientific, Inc.). At 48 h post-transfection, the dual-luciferase reporter assay system (Promega Corporation) was used to measure the luciferase activity. The pRL-TK plasmid (Promega Corporation) was used as a normalizing control. All experiments were performed in triplicate.
Western blot analysis
Lung tissue samples were lysed in RIPA buffer (Cell Signaling Technology, Inc.) supplemented with protease inhibitors (Roche Applied Science) for 30 min on ice. The protein concentrations were measured using a BCA protein assay kit (Beyotime Institute of Biotechnology). The protein samples (40 µg) were separated by 10% SDS-PAGE (Bio-Rad Laboratories, Inc.) and transferred onto a PVDF membrane, which was then blocked with 5% non-fat milk at room temperature for 1 h. Membranes were incubated with primary antibodies against Bcl-2 (cat. no. ab32124; 1:2,000), cleaved caspase-3 (cat. no. ab2302; 1:2,000), TLR4 (cat. no. ab22048; 1:2,000), nuclear-phospho (p)-p65 (Ser-536; cat. no. ab86299; 1:2,000), total p-65 (cat. no. ab140751; 1:2,000), p-IκBα (Ser-36; cat. no. ab133462; 1:5,000), total IκBα (cat. no. ab32518; 1:5,000), MyD88 (cat. no. ab199247; 1:2,000) and β-actin (cat. no. ab8226; 1:2,000; all Abcam) at 4°C overnight. After washing with PBS, the membrane was incubated with the appropriate horseradish peroxidase-conjugated secondary antibodies including goat anti-rabbit immunoglobulin (Ig)-G (cat. no. ab6721; 1:5,000; Abcam) and goat anti-mouse IgG (cat. no. ab6789; 1:5,000; Abcam) for 1 h at room temperature. A chemiluminescence detection system (EMD Millipore) was used for visualization of the results and quantification of the bands was performed using Quantity One software (version 4.4; Bio-Rad Laboratories, Inc.).
Statistical analysis
GraphPad Prism 5.0 (GraphPad Software, Inc.) was used to analyze the statistical analyses. Data are presented as the mean ± standard deviation. Statistical differences were analyzed using one-way analysis of variance with the Tukey's post hoc test. Survival studies were analyzed using the log rank test and the results are presented as the Kaplan-Meier curves. P<0.05 were considered to indicate a statistically significant difference.
Results
Sevoflurane protects against LPS-induced ALI in mice
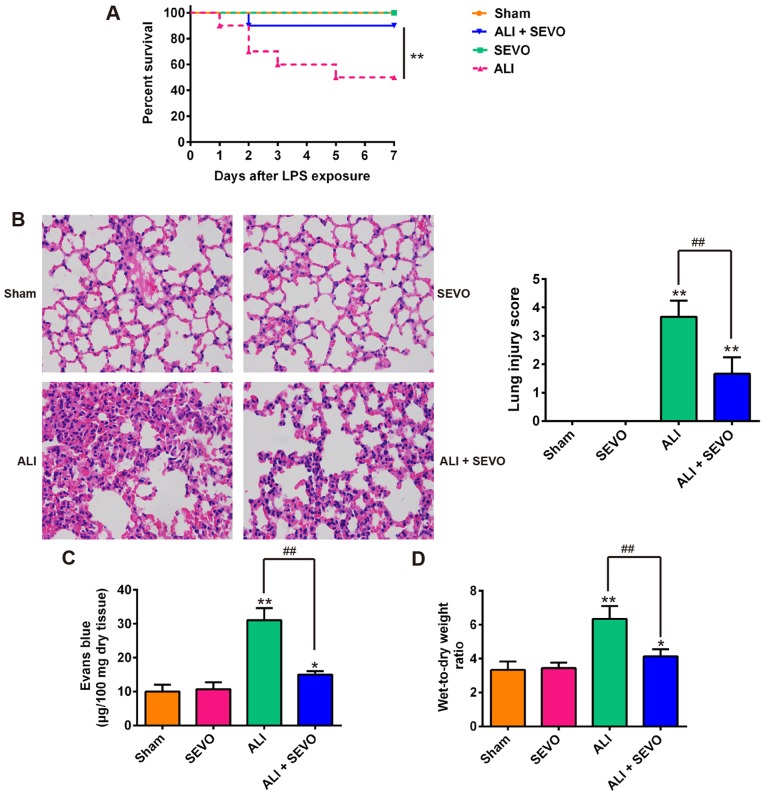
Previous studies on rat and mice have revealed that sevoflurane is able to improve different types of injuries, including lung, liver and renal injuries (10,34-36). In the present study, a mouse model of LPS-induced ALI was used to evaluate the therapeutic effects of sevoflurane. As shown in Fig. 1A, 50% of the LPS-induced ALI mice succumbed within 7 days whilst the survival rate of the ALI + sevoflurane mice was significantly higher than that of the ALI group. Then, the present study analyzed the histopathological changes in the lungs of ALI mice. Lung inflammatory responses including interstitial infiltration of inflammatory cells and thickening of the alveolar walls observed in the lung tissues of LPS-induced ALI mice were markedly reduced following sevoflurane treatment (Fig. 1B-D). Subsequently, the histopathological changes were scored and the result revealed that sevoflurane clearly ameliorated the histological damage (Fig. 1B). In addition, Evans blue dye was employed to evaluate the permeability of lung vasculature and it was observed that the LPS-induced increase in capillary permeability was significantly abolished by sevoflurane (Fig. 1C). Furthermore, the lung W/D ratio (an indicator of the extent of lung edema) of mice in the ALI group was higher than that of Sham mice, whilst sevoflurane significantly reduced the W/D ratio of LPS-stimulated ALI mice compared with the ALI group (Fig. 1D). These results indicated that the mouse model of ALI was successfully established and that sevoflurane treatment can protect LPS induced ALI in mice.
Figure 1.
Sevoflurane protects against LPS-induced lung injury. Mice were divided into 4 groups: The Sham, SEVO, ALI and ALI + SEVO groups. (A) Animal survival was recorded at the indicated time points (n=10/group). **P<0.01, as indicated. (B) Lung slides were stained with hematoxylin and eosin (magnification, ×200) to assess lung damage and the histopathological changes were scored (n=6 mice/group). (C) The capillary permeability was evaluated by Evans Blue assay (n=6 mice/group). (D) The ratio of wet lung to dry lung was calculated to evaluate lung tissue edema (n=6 mice/group). Data are presented as the mean ± standard deviation of three independent experiments. *P<0.05 and **P<0.01 vs. Sham group; ##P<0.01, as indicated. LPS, lipopolysaccharide; SEVO, the sevoflurane treated group; ALI, acute lung injury.
Sevoflurane inhibits LPS induced inflammation and apoptosis in mice
An excessive inflammatory response and increased endothelial cell permeability contribute to vascular leakage in ALI, which is considered to be central to the pathogenesis of ALI (37). Thus, the present study then examined the protective effects of sevoflurane on vascular leakage in ALI mice by measuring the total cell counts in BALF using a hemocytometer. As shown in Fig. 2A, LPS caused a significant increase in the total cell counts in BALF compared with the Sham group, whereas sevoflurane lead to a reduction in the LPS-induced total cell counts in the LPS and sevoflurane treated group. To further investigate the anti-inflammatory effects of sevoflurane, the present study measured the expression levels of inflammatory factors (IL-1β, IL-6 and TNF-α) in BALF via ELISA. The results demonstrated that the concentrations of these pro-inflammatory factors in the ALI group were significantly increased compared with the Sham group, whereas sevoflurane pretreatment significantly ameliorated the levels of these inflammatory cytokines (Fig. 2B-D). Additionally, the levels of the pro-apoptotic marker cleaved caspase-3 and anti-apoptotic marker Bcl-2 were also analyzed by western blotting in BALF. As presented in Fig. 2E, LPS administration increased the levels of the pro-apoptotic protein, cleaved caspase 3 and decreased the level of the anti-apoptotic protein Bcl2 compared with the Sham group. On the other hand, sevoflurane pretreatment induced a significant decrease in the levels of cleaved caspase-3 as well as a marked increase in the level of Bcl2 when compared with the ALI group. These results suggest that sevoflurane attenuates LPS-induced lung injury by suppressing the inflammatory response and reducing apoptosis.
Figure 2.
Sevoflurane inhibits inflammation and apoptosis in ALI mice. Mice were divided into 4 groups: The Sham, SEVO, ALI and ALI + SEVO groups (n=6 mice/group). (A) The total counts of cells from BALF were counted using a hemocytometer. (B-D) The protein levels of (B) IL-1β, (C) IL-6 and (D) TNF-α were assessed by ELISA. (E) The expression of cleaved caspase 3 and Bcl-2 was measured by western blotting. Data are presented as the mean ± standard deviation of three independent experiments. *P<0.05 and **P<0.01 vs. Sham group; ##P<0.01, as indicated. SEVO, the sevoflurane treated group; ALI, acute lung injury; IL, interleukin; TNF, tumor necrosis factor; BALF, bronchoalveolar lavage fluid.
Sevoflurane upregulates the expression of miR-27a
Previous studies have reported that various aberrantly expressed miRNAs are associated with ALI and inflammatory responses (38,39). To determine the potential involvement of miRNAs in ALI in mice, the present study used microarray analysis to determine the miRNA levels in lung tissues between ALI and Sham groups. The miRNA microarray identified 25 miRNAs that were upregulated and 25 miRNAs that were downregulated in the ALI group, compared with the Sham group (Fig. 3A). To determine whether sevoflurane could alter the expressions of some miRNAs obtained from the miRNA microarray data, a total of 6 miRNAs were selected including miR-1246 (40), miR-149* (41), miR-122 (42), miR-19a (43), miR-146a-5p (44) and miR-27a (45) as the candidate miRNAs as they have been shown to play a critical role in inflammation regulation. As shown in Fig. 3B sevoflurane only upregulated the expression of miR-27a, while the other miRNAs exhibited no evident changes in expression after sevoflurane treatment. To further investigate the effect of sevoflurane on the expression of miR-27a in ALI mice, RT-qPCR was performed in a larger scale sample. As shown in Fig. 3C, miR-27a expression was significantly decreased after LPS treatment (ALI group); however, in the sevoflurane pretreatment group (ALI + SEVO) the level of miR-27a increased significantly when compared with the ALI group. As miR-27a has previously been reported to be upregulated by sevoflurane in a sevoflurane-induced anesthetic mouse model (21), it seems plausible that the observed protective effects of sevoflurane may be exerted through upregulating the expression of miR-27a in ALI.
Figure 3.
miR-27a is upregulated in lung injury after sevoflurane treatment. (A) Heat map analysis of the miRNA expression in ALI group (n=2 mice) and Sham group (n=1 mice). Rows: Groups; Columns: miRNAs; Color key indicates the miRNA expression value, red indicates the highest expression and blue indicates the lowest expression. (B) Validation of the 6 differentially expressed miRNAs in the ALI (n=6 mice), ALI + SEVO (n=6 mice) and Sham groups (n=6 mice) based on the RT-qPCR assay. (C) The expression of miR-27a was further detected in the Sham (n=10 mice), ALI (n=20 mice) and ALI + SEVO groups (n=20 mice) by RT-qPCR analysis. The decreased expression of miR-27a-3p caused by LPS treatment was significantly reversed when pretreated with sevoflurane. Data are presented as the mean ± standard deviation of three independent experiments. *P<0.05 and **P<0.01 vs. Sham group; ##P<0.01, as indicated. SEVO, the sevoflurane treated group; ALI, acute lung injury; miRNA/miR, microRNA; RT-qPCR, reverse transcription-quantitative PCR.
miR-27a is involved in the therapeutic effects of sevoflurane in ALI mice
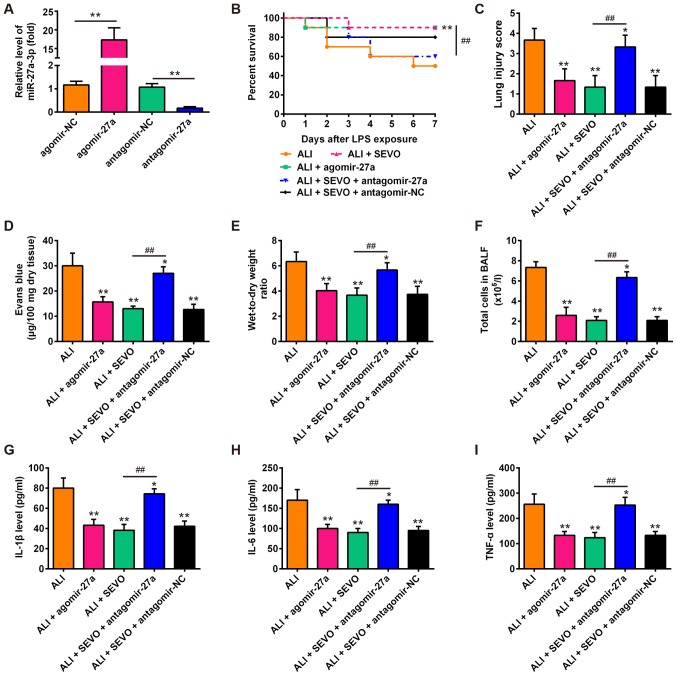
Previous studies have shown that miR-27a play important roles in several types of injuries, including traumatic brain injury (46), oxygen-glucose deprivation-induced injury (46) and LPS-induced sepsis injury (47). These studies warranted the investigation into whether the ectopic expression of miR-27a contributes to the therapeutic effects of sevoflurane in ALI mice. Since the stability of antagomir/agomir is stronger than inhibitor/mimics, the agomir/antagomir were used in the in vivo experiments. The present study overex-pressed and knocked down the expression of miR-27a via an injection of agomir-27a/antagomir-27a prior to LPS and sevoflurane treatment, and then observed the alterations in the therapeutic effects of sevoflurane in ALI mice. Firstly, it was observed that the expression of miR-27a was significantly increased or decreased following the injection of agomir-27a or antagomir-27a, respectively (Fig. 4A). As shown in Fig. 4B, mouse survival in the ALI + agomir-27a group was markedly improved compared with ALI group, which was similar to the effects of sevoflurane pretreatment observed. However, the survival rate of ALI + SEVO + antagomir-27a mice was significantly lower than that of the ALI + SEVO group. Furthermore, the agomir-27a injection improved LPS-induced lung injury, as evidenced by the reduced histological scores, capillary permeability and W/D ratio of lung in the lung tissues (Fig. 4C-E), which is also similar to the observed effects of sevoflurane. However, the improvement induced by sevoflurane in lung injury was abrogated when miR-27a was knocked down. In addition, the agomir-27a injection attenuated the LPS-induced inflammatory response including decreasing the total cell counts and reducing the levels of IL-1β, IL-6 and TNF-α in BALF (Fig. 4F-I); treatment with sevoflurane produced similar results. However, the inhibitory effects of sevoflurane on inflammatory response were abolished after the antagomir-27a injection. These results indicate that sevoflurane ameliorates LPS-induced ALI in mice by upregulating miR-27a.
Figure 4.
miR-27a is involved in the therapeutic effects of sevoflurane in ALI mice. (A) The expression of miR-27a was measured by reverse transcription-quantitative PCR after agomir-27a and antagomir-27a (8 mg/kg) treatment via tail intravenous injection in mice (n=3 mice/group). **P<0.01, as indicated. Then, mice were divided into 5 groups: The Sham, ALI group, ALI + SEVO, ALI + agomir-27a and ALI + SEVO + antagomir-27a groups (n=6 mice/group). (B) Animal survival was recorded at the indicated time points. (C) Lung slides were stained with hematoxylin and eosin to review lung damage and the histopathological changes were scored. (D) The capillary permeability was evaluated by Evans Blue assay. (E) The ratio of wet lung to dry lung was calculated to evaluate lung tissue edema. (F) The total counts of cells from BALF were counted using a hemocytometer. (G-I) The protein levels of (G) IL-1β, (H) IL-6 and (I) TNF-α were assessed by ELISA. All data are presented as the mean ± standard deviation of three independent experiments. *P<0.05 and **P<0.01 vs. Sham group; ##P<0.01, as indicated. SEVO, the sevoflurane treated group; ALI, acute lung injury; miR, microRNA; IL, interleukin; TNF, tumor necrosis factor; BALF, bronchoalveolar lavage fluid; NC, negative control.
TLR4 is a direct target of miR-27a
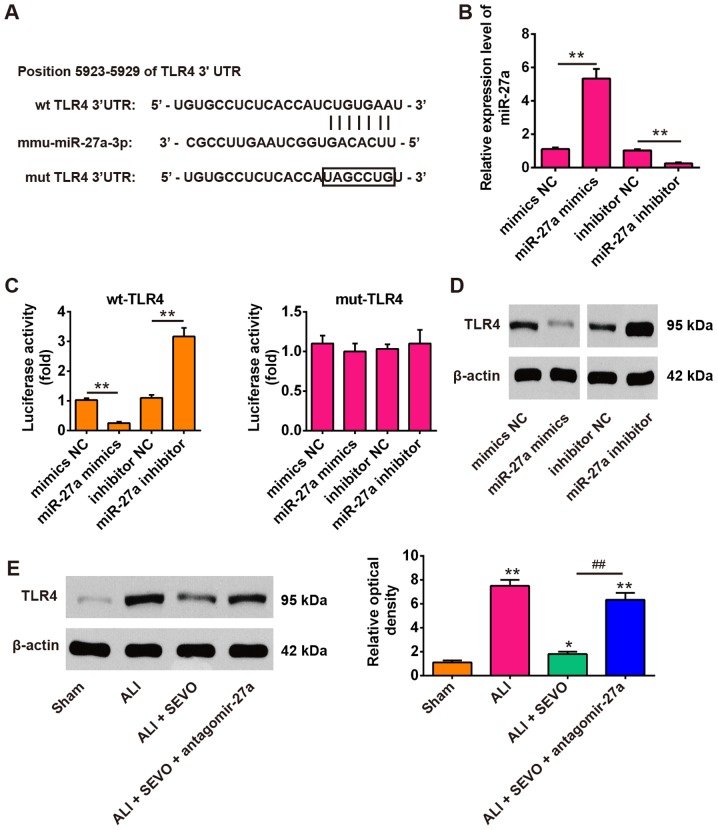
To explore the molecular mechanism by which miR-27a functions in the therapeutic effects of sevoflurane in ALI mice, the potential targets of miR-27a were screened using the TargetScan, miRBase and PicTar databases. According to the bioinformatics analysis, the present study focused on TLR4, an upstream positive regulator of the NF-κB signaling pathway. As shown in Fig. 5A, the RNA sequence alignment revealed that the 3′-UTR of TLR4 mRNA contained a complementary site for the seed region of miR-27a. To confirm the target reaction of miR-27a on the 3′-UTR of TLR4 mRNA, a luciferase activity assay was performed. The reporters were co-transfected with either miR-27a mimics/inhibitor or with NC mimics/inhibitor into 239T cells, and luciferase activity was then measured. It was observed that the miR-27a expression levels were significantly reduced in cells transfected with the miR-27a inhibitor, and were significantly increased in cells transfected with the miR-27a mimics (Fig. 5B). Then, it was revealed that the overexpression of miR-27a decreased the relative luciferase activity in the presence of the wt 3′-UTR, whereas knockdown of miR-27a increased the relative luciferase activity (Fig. 5C). Similarly, the luciferase activity did not change significantly when the targeted sequence of TLR4 was mutated in the miR-27a-binding site. To further confirm that TLR4 was negatively regulated by miR-27a, the TLR4 protein expression levels were analyzed by western blot analysis. The results demonstrated that the TLR4 levels were markedly downregulated after miR-27a mimics transfection, while transfection with the miR-27a inhibitor enhanced TLR4 expression (Fig. 5D). In addition, the present study also measured the expression of TLR4 in ALI mice treated with sevoflurane and/or antagomir-27a. When sevoflurane was applied to LPS treated mice, it was observed that the expression of TLR4 protein decreased significantly compared with the ALI group. However, antagomir-27a injection reversed the inhibitory effect of sevoflurane on the protein levels of TLR4 (Fig. 5E). Taken together, these results indicate that TLR4 is a functional target of miR-27a, suggesting that the miR-27a/TLR4 signaling axis may be involved in the therapeutic effects of sevoflurane in ALI mice.
Figure 5.
TLR4 was a direct target of miR-27a. (A) miR-27a-binding sequences in the 3′-UTR of TLR4 and mutated sites in the 3′-UTR of TLR4. (B) The transfection efficiency of miR-27a mimics/inhibitor was determined by reverse transcription-quantitative PCR. (C) miR-27a mimics suppressed the lucif-erase activities of constructs containing the 3′-UTR segment of TLR4, while miR-27a inhibitor significantly increased the luciferase activities of constructs containing the 3′-UTR segment of TLR4. All data are expressed as the mean ± standard deviation (n=3). **P<0.01, as indicated. (D) The expression of TLR4 was detected by western blotting after treatment with miR-27a mimics or miR-27a inhibitor. (E) The expression of TLR4 was detected by western blotting in 4 groups of mice: The Sham, ALI, ALI + SEVO and ALI + SEVO + antagomir-27a groups (n=6 mice/group). All data are presented as the mean ± standard deviation (n=6). *P<0.05 and **P<0.01 vs. Sham group; ##P<0.01, as indicated. UTR, untranslated region; TLF4, Toll-like receptor 4; SEVO, the sevoflurane treated group; ALI, acute lung injury; miR, microRNA; NC, negative control; wt, wild-type; mut, mutant.
The role of the NF-κB signaling pathway in the protective effects of sevoflurane
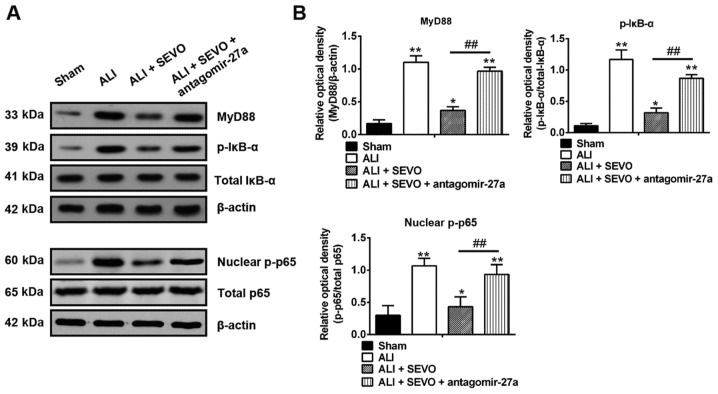
Previous studies have indicated that the activation of the NF-κB signaling pathway plays a pivotal role in the inflammatory response to ALI, whereby downregulation of NF-κB expression reduces the release of pro-inflammatory cytokines and attenuates ALI (48,49). Given that miR-27a directly targets the TLR4, a well-known upstream positive regulator of the NF-κB signaling pathway (50), the present study determined whether the miR-27a/TLR4/NF-κB axis was involved in the therapeutic effects of sevoflurane in ALI mice. The protein expressions of the NF-κB signaling pathway transcription factors including MyD88, nuclear p-p65, total p-65, p-IκB-α and total IκB-α in the lung tissues obtained from the different groups were determined by western blotting. The results revealed that the levels of MyD88, nuclear p-p65 and p-IκB-α were increased in ALI mice when compared with the Sham group, but these promotional effects were inhibited by sevoflurane (Fig. 6). However, inhibition of miR-27a by the antagomir-27a reversed the inhibitory effects of sevoflurane on the expressions of MyD88, nuclear p-p65 and p-IκB-α (Fig. 6A and B). These results suggest that sevoflurane blocked the activation of the NF-κB signaling pathway by upregulating the miR-27a/TLR4 axis.
Figure 6.
Sevoflurane blocks the activation of the TLR4/MyD88/NF-κB signaling pathway. (A) The protein expressions of the NF-κB signaling pathway transcription factors including MyD88, nuclear p-p65, total p-65, total IκB-α and p-IκB-α were detected by western blotting in four groups of mice: The Sham, ALI, ALI + SEVO and ALI + SEVO + antagomir-27a-3p groups (n=6 mice/group). (B) The bands were semi-quantitatively analyzed using Quantity One software, and normalized to β-actin, total p65 and total IκB-α density, respectively. Data are presented as the mean ± standard deviation of three independent experiments. *P<0.05 and **P<0.01 vs. Sham group; ##P<0.01, as indicated. TLF4, Toll-like receptor 4; SEVO, the sevoflurane treated group; ALI, acute lung injury; p-, phosphorylated.
Discussion
In the present study, it was demonstrated that sevoflurane effectively protected against LPS-induced ALI by inhibiting the inflammatory response in mice. Furthermore, sevoflurane increased the expression of miR-27a in the lung tissues of the LPS-induced ALI mice model. In addition, the present results demonstrated that the overexpression of miR-27a ameliorated LPS-induced ALI and inhibited the expression of the pro-inflammatory cytokines, TNF-α, IL-1β and IL-6. In addition, it was identified that miR-27a suppressed TLR4 by binding to its 3′-UTR, and it was also indicated that sevoflurane exerts an anti-inflammatory effect on LPS-induced ALI by suppressing the activation of the TLR4/MyD88/NF-κB signaling pathways.
To date, several in vivo studies have explored the effects of sevoflurane on lung tissue. For example, Steurer et al (51) showed that sevoflurane could attenuate LPS-induced injury by suppressing the inflammatory response in alveolar macrophages. In addition, Voigtsberger et al (52) reported that sevoflurane alleviated lung damage in an in vivo model of LPS-induced lung injury. Tang et al (53) demonstrated that sevoflurane could improve lung function post-acute lung injury by regulating immune homeostasis in mice. However, the relative mechanisms by which sevoflurane improve ALI remain unknown. In the present study, sevoflurane effectively reduced LPS-induced pulmonary inflammation and resulted in the significant reduction of LPS-induced increases in lung permeability and the W/D ratio of lung tissue, accompanied by a significant reduction in the histopathology changes of the lung. The present results demonstrated that sevoflurane could protect the lung from LPS-induced ALI by regulating the inflammatory responses. In addition, it was also revealed that the expression of the apoptosis-related protein cleaved caspase 3 was significantly decreased, while Bcl2 was increased in the sevoflurane treated ALI mice. However, the possible molecular mechanism requires further investigation to be fully understood.
Previous studies had demonstrated that sevoflurane could attenuate LPS-induced ALI via its anti-inflammatory properties. Voigtsberger et al (52) reported that sevoflurane ameliorated the gas exchange and attenuated lung damage; this property appeared to be mediated via the inhibition of lung inflammation as indicated by the lower levels of cytokines and less recruitment of effector cells into the lung tissue. Schläpfer et al (54) revealed that sevoflurane exposure can positively influence the course of LPS-induced lung injury with regard to oxygenation and this effect was mediated by the anti-inflammatory properties of sevoflurane leading to less edema formation. Hofstetter et al (55) examined the anti-inflammatory effects of sevoflurane in an in vivo model of LPS-induced endotoxemia in rats. In this study, the administration of sevoflurane 15 min after stimulation with LPS resulted in a decrease in TNF-α and IL-1β plasma levels (55). However, the molecular mechanisms remain unknown.
Recently, an association has also been reported between the aberrant expression of miRNAs and exposure to anesthetics such as sevoflurane (56). For example, Tang et al (57) reported that miR-29a regulated LPS-induced inflammatory responses in murine macrophages through the Akt1/NF-κB signaling pathway. Therefore, given the important role of sevoflurane in the regulation of miRNAs, it is possible that miRNAs are also involved in the lung protective mechanisms of sevoflurane. In the present study, microarray screening revealed miR-27a to be one of the major miRNAs that were upregulated in ALI mice by sevoflurane treatment, and that this upregulation was time-dependent. Notably, previous studies have reported that downregulation of miR-27a expression attenuated sevoflurane-induced neuronal apoptosis, and learning and memory impairment via the upregulation of peroxisome proliferator-activated receptor-γ, which play important roles in sevoflurane mediated neurotoxicity and cognitive impairment effects (21,55). Thus, the present study focused on miR-27a for further study. Notably, it was confirmed that the agomir-27a injection produced similar protective effects when compared with sevoflurane, while the antagomir-27a injection suppressed the therapeutic effects of sevoflurane in ALI mice. Taken together, these results indicate that upregulation of miR-27a may contribute to sevoflurane-induced protective effects against LPS-induced ALI.
TLR4, a component of the primary innate immune receptor-mediated inflammatory signaling pathway, has been associated with various inflammation-related diseases including ALI (58-60). A previous study has shown that LPS could induce TLR4 activation and subsequently transmit a signal via an adaptor molecule, MyD88, leading to the activation of NF-κB, which in turn causes the upregulation of pro-inflammatory cytokines such as IL-1β, IL-6 and TNF-α (61). The present results demonstrated that TLR4 was a direct target of miR-27a. In addition, it was revealed that LPS induced the upregulation of TLR4, but the expression of TLR4 was markedly decreased after sevoflurane treatment. Notably, increased MyD88, nuclear p-p65 and p-IκB-α, and decreased IκB-α were detected in ALI mice, which was indicative of the activation of the NF-κB signaling pathway in ALI mice. However, pretreatment with sevoflurane decreased the expression of MyD88, nuclear p-p65 and p-IκB-α, and increased the expression of IκB-α in all ALI mice. Interestingly, the inactivation of NF-κB signaling in sevoflurane treated ALI mice was re-activated by an injection of the antagomir-27a. These results indicate that sevoflurane blocked the activation of the NF-κB signaling pathway through the miR-27a/TLR4 axis.
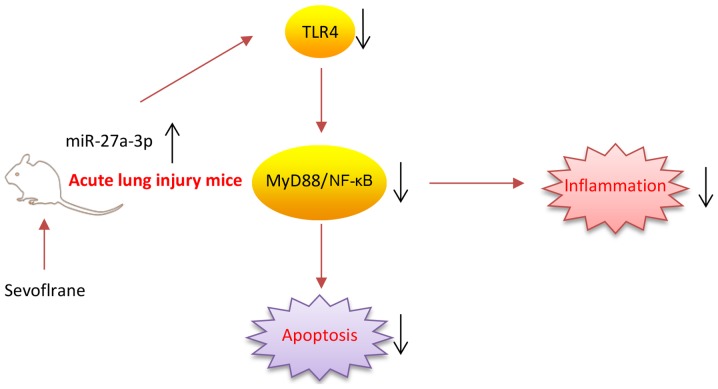
In conclusion, as illustrated in Fig. 7, the present results firstly demonstrated that sevoflurane effectively protected ALI against inflammation damage, which was largely dependent on the upregulation of miR-27a via suppressing the LPS-activated TLR4/MyD88/NF-κB signaling pathway. The present study provides beneficial evidence for the application of sevoflurane in the prevention of ALI. However, the application and efficacy of sevoflurane in clinical practice remains to be studied.
Figure 7.
Scheme summarizing the protective effects of sevoflurane on lipopolysaccharide-induced inflammation and apoptosis by suppressing the activation of the miR-27a/TLR4/MyD88/NF-κB signaling pathway. miR, microRNA; TLR4, Toll-like receptor 4.
Acknowledgments
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
XZ, JT, GL, XL and DS performed the experiments, analyzed the data and wrote the paper. YW conceived and designed the study, and contributed to data analysis and experimental materials. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by The First Affiliated Hospital of Xinxiang Medical University Ethics Committees (Henan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 2.Brigham KL. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Curr Infect Dis Rep. 2005;7:327–328. doi: 10.1007/s11908-005-0004-2. [DOI] [PubMed] [Google Scholar]
- 3.Bhatia M, Moochhala S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J Pathol. 2004;202:145–156. doi: 10.1002/path.1491. [DOI] [PubMed] [Google Scholar]
- 4.Grommes J, Soehnlein O. Contribution of neutrophils to acute lung injury. Mol Med. 2011;17:293–307. doi: 10.2119/molmed.2010.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kersten JR, Schmeling TJ, Hettrick DA, Pagel PS, Gross GJ, Warltier DC. Mechanism of myocardial protection by isoflurane. Role of adenosine triphosphate-regulated potassium (KATP) channels. Anesthesiology. 1996;85:794–807. doi: 10.1097/00000542-199610000-00015. discussion 27A. [DOI] [PubMed] [Google Scholar]
- 6.Kersten JR, Schmeling TJ, Pagel PS, Gross GJ, Warltier DC. Isoflurane mimics ischemic preconditioning via activation of K(ATP) channels: Reduction of myocardial infarct size with an acute memory phase. Anesthesiology. 1997;87:361–370. doi: 10.1097/00000542-199708000-00024. [DOI] [PubMed] [Google Scholar]
- 7.Schlack W, Preckel B, Barthel H, Obal D, Thamer V. Halothane reduces reperfusion injury after regional ischaemia in the rabbit heart in vivo. Br J Anaesth. 1997;79:88–96. doi: 10.1093/bja/79.1.88. [DOI] [PubMed] [Google Scholar]
- 8.Minguet G, Joris J, Lamy M. Preconditioning and protection against ischaemia-reperfusion in non-cardiac organs: A place for volatile anaesthetics? Eur J Anaesthesiol. 2007;24:733–745. doi: 10.1017/S0265021507000531. [DOI] [PubMed] [Google Scholar]
- 9.Kalimeris K, Zerva A, Matsota P, Nomikos T, Fragopoulou E, Politi AN, Karamitopoulou E, Kostopanagiotou G. Pretreatment with sevoflurane attenuates direct lung injury. Minerva Anestesiol. 2014;80:635–644. [PubMed] [Google Scholar]
- 10.Liu R, Ishibe Y, Ueda M. Isoflurane-sevoflurane adminstration before ischemia attenuates ischemia-reperfusion-induced injury in isolated rat lungs. Anesthesiology. 2000;92:833–840. doi: 10.1097/00000542-200003000-00027. [DOI] [PubMed] [Google Scholar]
- 11.Kalb R, Schober P, Schwarte LA, Weimann J, Loer SA. Preconditioning, but not postconditioning, with Sevoflurane reduces pulmonary neutrophil accumulation after lower body ischaemia/reperfusion injury in rats. Eur J Anaesthesiol. 2008;25:454–459. doi: 10.1017/S0265021508003682. [DOI] [PubMed] [Google Scholar]
- 12.Casanova J, Garutti I, Simon C, Giraldez A, Martin B, Gonzalez G, Azcarate L, Garcia C, Vara E. The effects of anesthetic preconditioning with sevoflurane in an experimental lung autotransplant model in pigs. Anesth Analg. 2011;113:742–748. doi: 10.1213/ANE.0b013e3182288e01. [DOI] [PubMed] [Google Scholar]
- 13.Suter D, Spahn DR, Blumenthal S, Reyes L, Booy C, Z'graggen BR, Beck-Schimmer B. The immunomodulatory effect of sevoflurane in endotoxin-injured alveolar epithelial cells. Anesth Analg. 2007;104:638–645. doi: 10.1213/01.ane.0000255046.06058.58. [DOI] [PubMed] [Google Scholar]
- 14.Ohsumi A, Marseu K, Slinger P, McRae K, Kim H, Guan Z, Hwang DM, Liu M, Keshavjee S, Cypel M. Sevoflurane attenuates ischemia-reperfusion injury in a rat lung transplantation model. Ann Thorac Surg. 2017;103:1578–1586. doi: 10.1016/j.athoracsur.2016.10.062. [DOI] [PubMed] [Google Scholar]
- 15.Sun XJ, Li XQ, Wang XL, Tan WF, Wang JK. Sevoflurane inhibits nuclear factor-κB activation in lipopolysaccha-ride-induced acute inflammatory lung injury via toll-like receptor 4 signaling. PLoS One. 2015;10:e0122752. doi: 10.1371/journal.pone.0122752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song SY, Zhou B, Yang SM, Liu GZ, Tian JM, Yue XQ. Preventive effects of sevoflurane treatment on lung inflammation in rats. Asian Pac J Trop Med. 2013;6:53–56. doi: 10.1016/S1995-7645(12)60200-4. [DOI] [PubMed] [Google Scholar]
- 17.Bartel DP. MicroRNAs: Target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stefani G, Slack FJ. Small non-coding RNAs in animal development. Nat Rev Mol Cell Biol. 2008;9:219–230. doi: 10.1038/nrm2347. [DOI] [PubMed] [Google Scholar]
- 19.Guo Z, Gu Y, Wang C, Zhang J, Shan S, Gu X, Wang K, Han Y, Ren T. Enforced expression of miR-125b attenuates LPS-induced acute lung injury. Immunol Lett. 2014;162:18–26. doi: 10.1016/j.imlet.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 20.Chen W, Ma X, Zhang P, Li Q, Liang X, Liu J. MiR-212 3p inhibits LPS-induced inflammatory response through targeting HMGB1 in murine macrophages. Exp Cell Res. 2017;350:318–326. doi: 10.1016/j.yexcr.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 21.Lv X, Yan J, Jiang J, Zhou X, Lu Y, Jiang H. MicroRNA-27a 3p suppression of peroxisome proliferator-activated receptor-γ contributes to cognitive impairments resulting from sevoflurane treatment. J Neurochem. 2017;143:306–319. doi: 10.1111/jnc.14208. [DOI] [PubMed] [Google Scholar]
- 22.Wu Y, Gu C, Huang X. Sevoflurane protects against hepatic ischemia/reperfusion injury by modulating microRNA-200c regulation in mice. Biomed Pharmacother. 2016;84:1126–1136. doi: 10.1016/j.biopha.2016.10.024. [DOI] [PubMed] [Google Scholar]
- 23.Wenlan L, Zhongyuan X, Shaoqing L, Liying Z, Bo Z, Min L. MiR-34a-5p mediates sevoflurane preconditioning induced inhibition of hypoxia/reoxygenation injury through STX1A in cardiomyocytes. Biomed Pharmacother. 2018;102:153–159. doi: 10.1016/j.biopha.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Beck-Schimmer B, Madjdpour C, Kneller S, Ziegler U, Pasch T, Wüthrich RP, Ward PA, Schimmer RC. Role of alveolar epithelial ICAM-1 in lipopolysaccharide-induced lung inflammation. Eur Respir J. 2002;19:1142–1150. doi: 10.1183/09031936.02.00236602. [DOI] [PubMed] [Google Scholar]
- 25.Wang L, Ye Y, Su HB, Yang JP. The anesthetic agent sevoflurane attenuates pulmonary acute lung injury by modulating apoptotic pathways. Braz J Med Biol Res. 2017;50:e5747. doi: 10.1590/1414-431x20165747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao YZ, Tu YY, Chen X, Wang BL, Zhong YX, Liu MH. Protective effect of Ulinastatin against murine models of sepsis: Inhibition of TNF-α and IL-6 and augmentation of IL-10 and IL-13. Exp Toxicol Pathol. 2012;64:543–547. doi: 10.1016/j.etp.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 27.Gao C, Li R, Wang S. Ulinastatin protects pulmonary tissues from lipopolysaccharide-induced injury as an immunomodulator. J Trauma Acute Care Surg. 2012;72:169–176. doi: 10.1097/TA.0b013e318223c20f. [DOI] [PubMed] [Google Scholar]
- 28.Luo Y, Che W, Zhao M. Ulinastatin post-treatment attenuates lipopolysaccharide-induced acute lung injury in rats and human alveolar epithelial cells. Int J Mol Med. 2017;39:297–306. doi: 10.3892/ijmm.2016.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu Z, Zhang C, Cheng L, Hu M, Tao H, Song L. The microRNA miR-17 regulates lung FoxA1 expression during lipopolysaccharide-induced acute lung injury. Biochem Biophys Res Commun. 2014;445:48–53. doi: 10.1016/j.bbrc.2014.01.108. [DOI] [PubMed] [Google Scholar]
- 30.Duan Y, Learoyd J, Meliton AY, Leff AR, Zhu X. Inhibition of Pyk2 blocks lung inflammation and injury in a mouse model of acute lung injury. Respir Res. 2012;13:4. doi: 10.1186/1465-9921-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barreto TR, Costola-de-Souza C, Margatho RO, Queiroz-Hazarbassanov N, Rodrigues SC, Felício LF, Palermo-Neto J, Zager A. Repeated Domperidone treatment modulates pulmonary cytokines in LPS-induced acute lung injury in mice. Int Immunopharmacol. 2018;56:43–50. doi: 10.1016/j.intimp.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 32.Yang Q, Zhang D, Li Y, Li Y, Li Y. Paclitaxel alleviated liver injury of septic mice by alleviating inflammatory response via microRNA-27a/TAB3/NF-κB signaling pathway. Biomed Pharmacother. 2018;97:1424–1433. doi: 10.1016/j.biopha.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 34.Luo C, Yuan D, Zhao W, Chen H, Luo G, Su G, Hei Z. Sevoflurane ameliorates intestinal ischemia-reperfusion-induced lung injury by inhibiting the synergistic action between mast cell activation and oxidative stress. Mol Med Rep. 2015;12:1082–1090. doi: 10.3892/mmr.2015.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu C, Shen Z, Liu Y, Peng J, Miao L, Zeng W, Li Y. Sevoflurane protects against intestinal ischemia-reperfusion injury partly by phosphatidylinositol 3 kinases/Akt pathway in rats. Surgery. 2015;157:924–933. doi: 10.1016/j.surg.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 36.Zhao W, Gan X, Su G, Wanling G, Li S, Hei Z, Yang C, Wang H. The interaction between oxidative stress and mast cell activation plays a role in acute lung injuries induced by intestinal ischemia-reperfusion. J Surg Res. 2014;187:542–552. doi: 10.1016/j.jss.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 37.Zhou T, Garcia JG, Zhang W. Integrating microRNAs into a system biology approach to acute lung injury. Transl Res. 2011;157:180–190. doi: 10.1016/j.trsl.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin GS. Sepsis, severe sepsis and septic shock: Changes in incidence, pathogens and outcomes. Expert Rev Anti Infect Ther. 2012;10:701–706. doi: 10.1586/eri.12.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang RS, Hu GQ, Lin B, Lin ZY, Sun CC. MicroRNA-155 silencing enhances inflammatory response and lipid uptake in oxidized low-density lipoprotein-stimulated human THP-1 macrophages. J Investig Med. 2010;58:961–967. doi: 10.2310/JIM.0b013e3181ff46d7. [DOI] [PubMed] [Google Scholar]
- 40.Wu DP, Zhang JL, Wang JY, Cui MX, Jia JL, Liu XH, Liang QD. MiR-1246 promotes LPS-induced inflammatory injury in chondrogenic cells ATDC5 by targeting HNF4γ. Cell Physiol Biochem. 2017;43:2010–2021. doi: 10.1159/000484162. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Q, Su J, Wang Z, Qi H, Ge Z, Li Z, Chen WD, Wang YD. MicroRNA-149* suppresses hepatic inflammatory response through antagonizing STAT3 signaling pathway. Oncotarget. 2017;8:65397–65406. doi: 10.18632/oncotarget.18541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Satishchandran A, Ambade A, Rao S, Hsueh YC, Iracheta-Vellve A, Tornai D, Lowe P, Gyongyosi B, Li J, Catalano D, et al. MicroRNA 122, regulated by GRLH2, protects livers of mice and patients from ethanol-induced liver disease. Gastroenterology. 2018;154:238–252.e7. doi: 10.1053/j.gastro.2017.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen H, Li X, Liu S, Gu L, Zhou X. MircroRNA-19a promotes vascular inflammation and foam cell formation by targeting HBP-1 in atherogenesis. Sci Rep. 2017;7:12089. doi: 10.1038/s41598-017-12167-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lo WY, Peng CT, Wang HJ. MicroRNA-146a-5p mediates high glucose-induced endothelial inflammation via targeting interleukin-1 receptor-associated kinase 1 expression. Front Physiol. 2017;8:551. doi: 10.3389/fphys.2017.00551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lv YN, Ou-Yang AJ, Fu LS. MicroRNA-27a negatively modulates the inflammatory response in lipopolysaccharide-stimulated microglia by targeting TLR4 and IRAK4. Cell Mol Neurobiol. 2017;37:195–210. doi: 10.1007/s10571-016-0361-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cai Q, Wang T, Yang WJ, Fen X. Protective mechanisms of microRNA-27a against oxygen-glucose deprivation-induced injuries in hippocampal neurons. Neural Regen Res. 2016;11:1285–1292. doi: 10.4103/1673-5374.189194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xue WL, Bai X, Zhang L. rhTNFR:Fc increases Nrf2 expression via miR-27a mediation to protect myocardium against sepsis injury. Biochem Biophys Res Commun. 2015;464:855–861. doi: 10.1016/j.bbrc.2015.07.051. [DOI] [PubMed] [Google Scholar]
- 48.Wu C, Zhao J, Zhu G, Huang Y, Jin L. SiRNA directed against NF-κB inhibits mononuclear macrophage cells releasing proinflammatory cytokines in vitro. Mol Med Rep. 2017;16:9060–9066. doi: 10.3892/mmr.2017.7715. [DOI] [PubMed] [Google Scholar]
- 49.Zhu G, Xin X, Liu Y, Huang Y, Li K, Wu C. Geraniin attenuates LPS-induced acute lung injury via inhibiting NF-κB and activating Nrf2 signaling pathways. Oncotarget. 2017;8:22835–22841. doi: 10.18632/oncotarget.15227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kawasaki T, Kawai T. Toll-like receptor signaling pathways. Front Immunol. 2014;5:461. doi: 10.3389/fimmu.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steurer M, Schläpfer M, Steurer M, Z'graggen BR, Booy C, Reyes L, Spahn DR, Beck-Schimmer B. The volatile anaesthetic sevoflurane attenuates lipopolysaccharide-induced injury in alveolar macrophages. Clin Exp Immunol. 2009;155:224–230. doi: 10.1111/j.1365-2249.2008.03807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Voigtsberger S, Lachmann RA, Leutert AC, Schläpfer M, Booy C, Reyes L, Urner M, Schild J, Schimmer RC, Beck-Schimmer B. Sevoflurane ameliorates gas exchange and attenuates lung damage in experimental lipopolysaccharide-induced lung injury. Anesthesiology. 2009;111:1238–1248. doi: 10.1097/ALN.0b013e3181bdf857. [DOI] [PubMed] [Google Scholar]
- 53.Tang QF, Fang ZY, Shi CH. The protective effect and mechanism of sevoflurane on LPS-induced acute lung injury in mice. Am J Transl Res. 2017;9:1732–1742. [PMC free article] [PubMed] [Google Scholar]
- 54.Schläpfer M, Leutert AC, Voigtsberger S, Lachmann RA, Booy C, Beck-Schimmer B. Sevoflurane reduces severity of acute lung injury possibly by impairing formation of alveolar oedema. Clin Exp Immunol. 2012;168:125–134. doi: 10.1111/j.1365-2249.2012.04562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hofstetter C, Boost KA, Flondor M, Basagan-Mogol E, Betz C, Homann M, Muhl H, Pfeilschifter J, Zwissler B. Anti-inflammatory effects of sevoflurane and mild hypothermia in endotoxemic rats. Acta Anaesthesiol Scand. 2007;51:893–899. doi: 10.1111/j.1399-6576.2007.01353.x. [DOI] [PubMed] [Google Scholar]
- 56.Tanaka S, Ishikawa M, Arai M, Genda Y, Sakamoto A. Changes in microRNA expression in rat lungs caused by sevoflurane anesthesia: A TaqMan® low-density array study. Biomed Res. 2012;33:255–263. doi: 10.2220/biomedres.33.255. [DOI] [PubMed] [Google Scholar]
- 57.Tang B, Li X, Ren Y, Wang J, Xu D, Hang Y, Zhou T, Li F, Wang L. MicroRNA-29a regulates lipopolysaccharide (LPS)-induced inflammatory responses in murine macrophages through the Akt1/NF-κB pathway. Exp Cell Res. 2017;360:74–80. doi: 10.1016/j.yexcr.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 58.Li J, Bao L, Zha D, Zhang L, Gao P, Zhang J, Wu X. Oridonin protects against the inflammatory response in diabetic nephrop-athy by inhibiting the TLR4/p38-MAPK and TLR4/NF-κB signaling pathways. Int Immunopharmacol. 2018;55:9–19. doi: 10.1016/j.intimp.2017.11.040. [DOI] [PubMed] [Google Scholar]
- 59.Yan J, Li J, Zhang L, Sun Y, Jiang J, Huang Y, Xu H, Jiang H, Hu R. Nrf2 protects against acute lung injury and inflammation by modulating TLR4 and Akt signaling. Free Radic Biol Med. 2018;121:78–85. doi: 10.1016/j.freeradbiomed.2018.04.557. [DOI] [PubMed] [Google Scholar]
- 60.Deng G, He H, Chen Z, OuYang L, Xiao X, Ge J, Xiang B, Jiang S, Cheng S. Lianqinjiedu decoction attenuates LPS-induced inflammation and acute lung injury in rats via TLR4/NF-κB pathway. Biomed Pharmacother. 2017;96:148–152. doi: 10.1016/j.biopha.2017.09.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suzuki Y, Ruiz-Ortega M, Lorenzo O, Ruperez M, Esteban V, Egido J. Inflammation and angiotensin II. Int J Biochem Cell Biol. 2003;35:881–900. doi: 10.1016/S1357-2725(02)00271-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.