Abstract
Dendrite development of newborn granule cells (GCs) in the dentate gyrus of adult hippocampus is critical for their incorporation into existing hippocampal circuits, but the cellular mechanisms regulating their dendrite development remains largely unclear. In this study, we examined the function of brain-derived neurotrophic factor (BDNF), which is expressed in adult-born GCs, in regulating their dendrite morphogenesis. Using retrovirus-mediated gene transfection, we found that deletion and overexpression of BDNF in adult-born GCs resulted in the reduction and elevation of dendrite growth, respectively. This effect was mainly due to the autocrine rather than paracrine action of BDNF, because deletion of BDNF only in the newborn GCs resulted in dendrite abnormality of these neurons to a similar extent as that observed in conditional knockout (cKO) mice with BDNF deleted in the entire forebrain. Furthermore, selective expression of BDNF in adult-born GCs in BDNF cKO mice fully restored normal dendrite development. The BDNF autocrine action was also required for the development of normal density of spines and normal percentage of spines containing the postsynaptic marker PSD-95, suggesting autocrine BDNF regulation of synaptogenesis. Furthermore, increased dendrite growth of adult-born GCs caused by voluntary exercise was abolished by BDNF deletion specifically in these neurons and elevated dendrite growth due to BDNF overexpression in these neurons was prevented by reducing neuronal activity with coexpression of inward rectifier potassium channels, consistent with activity-dependent autocrine BDNF secretion. Therefore, BDNF expressed in adult-born GCs plays a critical role in dendrite development by acting as an autocrine factor.
Keywords: autocrine action, BDNF, dendrite development
Introduction
In the hippocampus of adult mammalian brain, newborn granule cells (GCs) are continuously being generated in the subgranular zone (SGZ) of the dentate gyrus and incorporated into the existing neural circuits (van Praag et al., 2002; Ming and Song, 2005; Zhao et al., 2008). These adult-born GCs contribute to cognitive functions (Clelland et al., 2009; Aimone et al., 2011; Sahay et al., 2011; Nakashiba et al., 2012) and emotional regulation (Santarelli et al., 2003; Snyder et al., 2011) of the animal. Once generated at SGZ, the soma of the newborn GC remains in the hilar side of the GC layer and extends highly branched dendrites toward the molecular layer, where axonal inputs from the entorhinal cortex are received (Zhao et al., 2006; Faulkner et al., 2008). A single axon is also initiated from the soma and projects through the hilus toward the CA3 area, providing the output to CA3 pyramidal cells (Hastings and Gould, 1999; Markakis and Gage, 1999; Toni et al., 2007). The physiological functions of adult newborn neurons depend critically on the development of dendrite/axon morphology that is required for their proper integration into existing neural circuits. Therefore, it is of great interest to study the developmental process and the regulatory mechanisms underlying the morphogenesis of adult-born neurons in the hippocampus. In this study, we focused on the role of brain-derived neurotrophic factor (BDNF) in regulating dendrite development.
As a member of the neurotrophin family, BDNF was initially identified as a secreted factor that promotes the survival and differentiation of selective populations of neurons (Barde et al., 1982; Huang and Reichardt, 2001). However, BDNF was later shown to act as an autocrine factor for promoting neuronal survival during target-independent stage of development (Acheson et al., 1995; Davies and Wright, 1995). A recent study also showed that BDNF could act as a self-amplifying autocrine factor in promoting axon growth of cultured embryonic hippocampal neurons (Cheng et al., 2011). Both BDNF and its receptor, TrkB, are expressed in the adult hippocampus (Li et al., 2008). Deletion of TrkB in adult neural progenitors reduced the dendrite and spine growth in adult-born GCs (Bergami et al., 2008), suggesting the importance of BDNF/TrkB signaling for dendrite morphogenesis of adult-born neurons. However, it is unclear whether BDNF's action is autocrine, paracrine, or both.
In this study, using a combination of retrovirus-mediated gene transfection and transgenic methods, we demonstrate that endogenous BDNF in adult-born hippocampal GCs acts primarily through an autocrine rather than paracrine action in promoting dendrite development. Furthermore, we found that elevated dendrite growth in these neurons caused by voluntary exercise also depends on autocrine BDNF action.
Materials and Methods
Transgenic animals.
Floxed BDNF mice were originally produced by Dr. Alexei Morozov's laboratory of Howard Hughes Medical Institute and provided by Dr. Bai Lu. Nestin-CreER mice and Rosa26-YFP mice were provided from the colony in Amelia J. Eisch's laboratory at the University of Texas Southwestern Medical Center. Rosa26-tdTomato transgenic mice and Nestin-Cre transgenic mice were purchased from The Jackson Laboratory. The running animals lived in standard cages with one running wheel with one mouse per cage. The runners were carefully observed each day before perfused. All animal care followed institutional guidelines and the animal protocols were approved by the Animal Care Facilities, Shanghai Institute for Biological Sciences, Chinese Academy of Sciences.
Retrovirus-mediated transfection of adult-born neurons and tamoxifen induction.
Retroviral vectors were modified from the murine Moloney leukemia virus-based retroviral vector CAG-GFP (Tashiro et al., 2006). For gene overexpression, cDNA of BDNF or Cre recombinase were coexpressed with GFP via E2A linker element under the control of the Ubiquitin C (Ubi) promoter; cDNA of inward rectifier potassium channel Kir2.1 (Kubo et al., 1993; Johns et al., 1999), or a nonconducting form of Kir2.1 (mutant Kir2.1; Burrone et al., 2002) was coexpressed with mCherry via E2A linker element under the control of Ubi promoter. The concentrated viral solution (107 particles/ml) was produced with 293gp as the packaging cell line by cotransfection of retroviral vectors together with pCMV-gp and pCMV-vsv-g plasmids, followed by ultraspeed centrifugation. The virus solution (1.5 μl/hemisphere) was delivered to the adult mouse dentate gyrus of each hemisphere via stereotaxic injection (injection site: posterior, 2 mm from bregma; lateral, 1.5 mm; ventral, 2.25 mm from the brain surface). The mice used were male C57BL/6 and were 8 weeks old at the time of surgery unless stated otherwise.
For inducible BDNF deletion, tamoxifen (TAM; Sigma) was dissolved in a 90% sunflower oil and 10% ethanol mixture at 30 mg/ml. TAM was injected intraperitoneally to 7-week-old mice at 180 mg/kg daily for 3 consecutive days. All injected mice were observed daily for neurological-related abnormality.
Immunohistochemistry and confocal imaging.
Mice were anesthetized with 30% chloral hydrate and perfused transcardially with 0.9% saline followed by 4% paraformaldehyde. The brain samples were removed and postfixed with 4% paraformaldehyde, dehydrated in 30% sucrose, and sectioned into 30, 40, or 60 μm floating slices. One in six brain sections was used for immunostaining: brain sections were blocked with 1% BSA and 0.4% Triton-X in PBS for 2 h at room temperature and incubated with primary antibodies at 4°C overnight. After being washed 3 times with PBS, slices were incubated with secondary antibodies for 6 h at room temperature. Brain slices were heated at 92°C for 10 min in 0.01 m citrate buffer, pH 6.0, for antigen retrieval before immunostaining if necessary. Primary antibodies used were as follows: anti-BDNF (1:500, rabbit polyclonal IgG; Abcam), anti-PSD-95 (1:200, mouse monoclonal IgG; Millipore), anti-Doublecortin (1:100, goat polyclonal IgG; Santa Cruz Biotechnology), anti-Cre (1:500, mouse monoclonal IgG; Millipore), anti-DsRed (1:1000, rabbit polyclonal IgG; Clontech), anti-GFP (1:1000, rabbit polyclonal IgG; Invitrogen), and anti-GFP (1:1000, chicken polyclonal IgG; Abcam). Secondary antibodies used were as follows: Alexa Fluor donkey anti-rabbit IgG, Alexa Fluor-donkey anti-mouse IgG, Alexa Fluor-donkey anti-goat IgG, Alexa Fluor-donkey anti-chicken IgG (1:500; Invitrogen). For Golgi staining, we used FD Rapid Golgistain Kit (FD Neurotechnologies) and brain samples were sectioned into 150 μm slices for confocal microscopic imaging. For dendritic growth analysis, z-series at 0.5 μm intervals were acquired on a Nikon A1R laser scanning confocal microscope with a 40× oil-immersion lens. 2D maximal intensity projections of each z-series were created to measure the total dendritic length and total number of branching points. For qualitative assessment of axonal growth, the area of interest was taken by the same confocal microscope with a 20× lens at 1 μm intervals. For mossy fiber boutons analysis, images were taken with a 60× oil lens at 0.5 μm intervals. For spine growth analysis, images of dendritic processes at the outer molecular layer were acquired at 0.5 μm intervals with a 60× oil-immersion lens and a digital zoom of 3. For PSD-95 staining analysis, images of dendritic processes at the outer molecular layer were acquired at 0.3 μm intervals with a 60× oil-immersion lens and a digital zoom of 4. Images were processed with deconvolution method using Autoquant X software.
Western blotting.
Western blotting was performed using 15 μg of total protein from the supernatant of digested brain tissue. After 5% milk blocking, primary antibodies were against BDNF (1:500; Abcam) and actin (1:2000; Sigma) and visualized with HRP-conjugated secondary antibodies (1:10,000; Santa Cruz Biotechnology) followed by the ECL plus Western blotting detection system kit (GE Healthcare) as per manufacturer's instructions.
Data analysis.
For dendrite growth analysis, 2D images were traced manually using Neurolucida software. The branch structure analysis function of Neurolucida Explore software was used to measure the number of primary dendrites, the number of branching points, and the total dendrite length of the traced neuron. A total of 16–90 cells from three or four mice were analyzed for each experimental group. For quantitative measurements of axonal growth, the crossing point between pyramidale and a line connecting the ends of the two blades of the dentate gyrus was defined as the starting point; the growth of axonal fibers was determined as the average length of three longest axons (from the starting point along the border of stratum pyramidale) in the same section. A total of 12–18 sections from 3–4 mice were used for each data point. For mossy fiber bouton analysis, we analyzed boutons with diameter more than threefold greater than the diameter of the mossy fiber. Maximum projections of z series were created and then the area was traced with ImageJ. For spine density analysis (double blind), the length of each dendritic segment was measured by tracing the center of the dendritic shaft and the spine density was calculated by dividing the total counted number of spines by the length of measured dendritic segment. The results are presented as means ± SEM and statistical significance was determined using one-way ANOVA followed by Student's t test.
Results
Dendrite morphogenesis of adult-born GCs
In this study, we fluorescently labeled newborn granule neurons in the adult mouse hippocampus via stereotaxic injection of retrovirus-expressing GFP into the hilus of the dentate gyrus of 8-week-old male C57BL/6 mice (Tashiro et al., 2006). Hippocampal sections at different time points after viral injection were examined with confocal microscopy (Fig. 1A). Because only dividing cells could be infected by retrovirus and most of the GFP+ cells were born shortly after the time of viral injection (Zhao et al., 2006), we used the injection time to define the time of birth of these newborn neurons.
Figure 1.
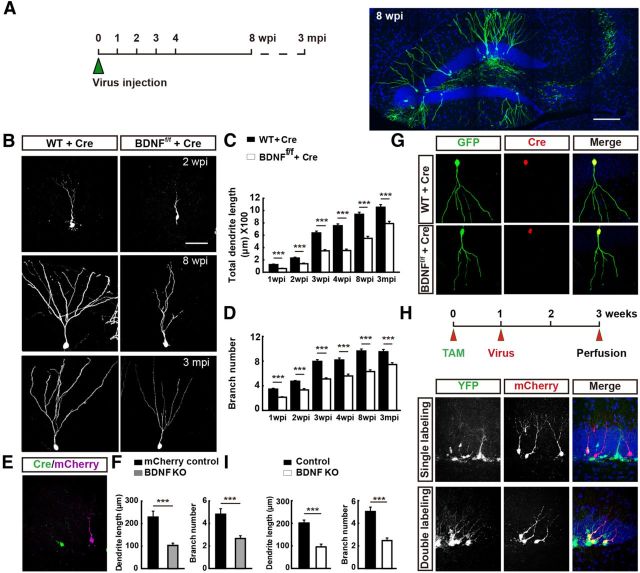
Effects of BDNF deletion in adult-born GCs on dendrite development. A, Schematic diagram showing the experimental procedure for labeling newborn GCs in adult mice through retrovirus-mediated gene transfection. Mice were perfused at different time points as indicated. mpi, Months postinfection. Sample confocal projection images (right) from newborn GCs (GFP +) in the hippocampus at 8 wpi. Blue: DAPI staining; green: GFP. Scale bar, 300 μm. B, Example images of newborn neurons expressing Cre recombinase and GFP at 2 wpi, 8 wpi and 3 mpi in WT (left) and BDNFf/f mice (right). Scale bar, 50 μm. C–D, Histograms showing total dendrite length and total branch number of WT (WT + Cre) and BDNFf/f (BDNFf/f + Cre) mice at each time point (mean ± SEM; n = 25–108 for each time point; 3–4 mice per point; ***p < 0.001, one-way ANOVA followed by Student's t test). E, Representative confocal image of adult-born GCs in the same slice from mice coinjected with retrovirus expressing Cre and GFP and retrovirus expressing only mCherry at 2 wpi. Magenta: normal newborn neuron expressing mCherry only; green: BDNF deleted neurons expressing both Cre and GFP; blue: DAPI staining. F, Graph depicts results of dendrite morphology analysis for all dendrites examined for neurons similar to those depicted in D (mean ± SEM; n = 16–30 neurons for each group; 3 mice each; ***p < 0.001, one-way ANOVA followed by Student's t test). G, Immunostaining of newborn GCs for Cre and GFP in adult DG from WT and BDNFf/f mice. Red: Cre; green: GFP; blue: DAPI. H, Schematic diagram of the experimental procedure (top). Representative confocal images of newborn GCs in adult BDNFf/f/Nestin-CreERT2/Rosa26-YFP mice through TAM induction and retrovirus-mediated gene transfection (bottom). I, Histograms showing total dendrite length and number of branch points for control and BDNF KO GCs similar to those depicted in G (mean ± SEM; n = 20–25 neurons for each group; 3 mice each; ***p < 0.001, one-way ANOVA followed by Student's t test).
We found that GFP+ cells were mostly located at the hilar border of the GC layer (Fig. 1). Their apical dendrites extended toward the molecular layer and increased in complexity with time, with the overall morphology resembling that of the mature GCs by 8 weeks postinfection (wpi).
To quantify dendrite growth, we measured the total dendrite length and the total number of branch points at different times after birth for all GFP+ neurons. These measurements were likely to have underestimated the true values of dendrite growth and branching because the sections we used had a thickness of only 60 μm. Nevertheless, we found progressive increase in the dendrite length and the number of branch points over the first 8 wpi (Fig. 1C,D). The first-order dendrite length (soma to the first branch point) reached a plateau level by 2 wpi (data not shown).
BDNF deletion in newborn neurons impedes dendrite growth
First, we investigated whether endogenous BDNF expressed in the adult-born GCs plays a role in regulating its own dendrite development. Newborn neurons in adult BDNF-flox (BDNFf/f) mice (Zakharenko et al., 2003) were infected with a retrovirus vector containing Ubi promoter-driven Cre recombinase and GFP genes (linked by an E2A element). Cells labeled with GFP were the cells with BDNF gene deletion. We have examined the efficiency of Cre recombinase action by injecting the retroviral vector into the hippocampus of adult Rosa26-tdTomato reporter mice in which tdTomato expression depends on Cre-mediated recombination and found that GFP+ neurons also expressed Cre and tdTomato, indicating successful recombination. This result was reported previously (Huang et al., 2014).
When we injected retrovirus expressing Cre recombinase and GFP into the hippocampus of adult BDNFf/f mice (Fig. 1G), we found marked impairment of dendrite development of GFP+ neurons at all time points between 1 and 8 wpi and at 3 months after retrovirus infection (Fig. 1B), as shown by significantly reduced total dendrite length and total number of branch points compared with control GFP+ GCs infected with the same retroviral vector in wild-type (WT) mice (Fig. 1C,D). The presence of dendritic defects in 3-month-old adult-born neurons indicates that the BDNF deficiency had resulted in a long-lasting dendrite impairment, rather than simply being a delay in the tempo of dendrite maturation. In some experiments, we injected the Cre-GFP retrovirus together with a retrovirus expressing only mCherry into the hippocampal hilus of adult BDNFf/f mice and obtained both BDNF-deleted newborn neurons (GFP+) and normal BDNF-expressing newborn neurons (mCherry+) in the same hippocampal section (Fig. 1E). To examine potential differential morphological effects caused simply by expressing different fluorescent proteins, the same retrovirus mixture was also injected into WT mice as a control. After 2 weeks, we found that the total dendrite length and total number of branch points in BDNF-deleted (GFP+) neurons were significantly lower than those of normal BDNF-expressing (mCherry+) neurons within the same sections of BDNFf/f mice (Fig. 1F). No significant difference in dendrite development was observed between GFP+ and mCherry+ neurons in sections from WT mice infected with the same mixture of viral vectors (data not shown).
In addition to retrovirus-mediated BDNF knockout in the dentate gyrus, we also used an inducible gene knockout system, Nestin-CreERT2/BDNFf/f/Rosa26-YFP (BDNF-cKO), to specifically delete BDNF allele in the adult stage (Ables et al., 2010). TAM was injected into the abdomen of adult transgenic mice for 3 consecutive days and then retrovirus expressing mCherry was injected into the DG 1 week later as a marker for the age of newborn GCs (Fig. 1H). We found that the adult newborn GCs expressing both YFP and mCherry exhibited defective dendrite development in both the total dendrite length and total number of branch points (Fig. 1I), similar to that found in newborn GCs with retrovirus-mediated BDNF KO (Fig. 1C,D). These results support the notion that endogenous expression of BDNF in adult-born neurons is critical for their own dendrite development, presumably via autocrine action after BDNF secretion.
Autocrine versus paracrine action of BDNF in dendrite development
To further examine whether BDNF secreted by other neurons within the hippocampus also contributes to the dendrite development of adult-born GCs, we examined the effect of conditional deletion of BDNF in the forebrain by crossing BDNFf/f mice with Nestin-Cre transgenic mice, yielding mice with BDNF deletion in all neural progenitor cells (“BDNFf/f/Nestin-Cre+” referred to as “BDNF-cKO mice” hereafter; Tronche et al., 1999). Immunostaining for BDNF in adult hippocampal sections of control mice (obtained by crossing BDNFf/f with BDNFf/−/Nestin-Cre+ mice) showed a high level of BDNF expression in the entire adult DG (Fig. 2A) and coexpression of BDNF with doublecortin (Fig. 2B), which marked immature neurons. In contrast, BDNF expression was largely absent in BDNF-cKO mice (Fig. 2A,B). This was confirmed further by Western blotting of BDNF expression in the hippocampal tissue (Fig. 2C).
Figure 2.
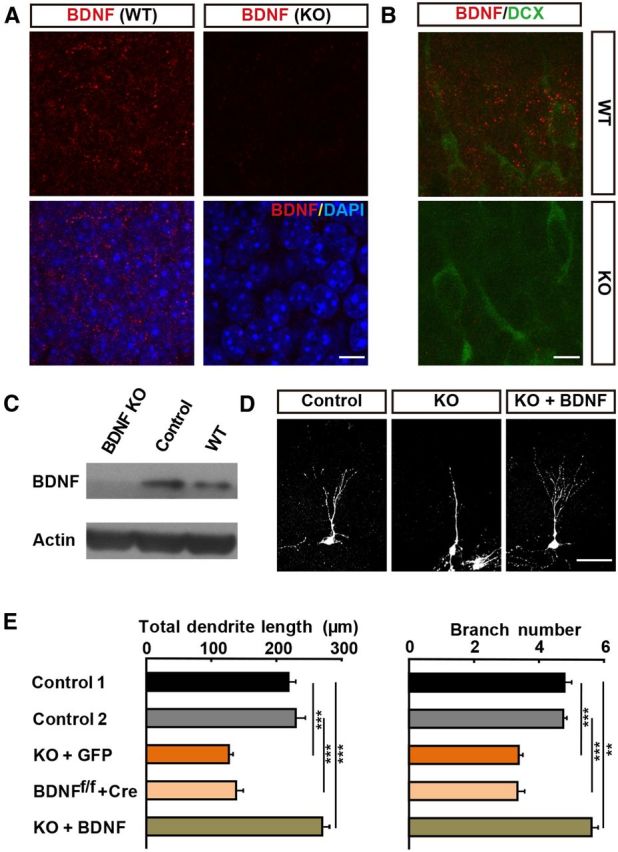
Impairment of dendrite development in BDNFf/f/nestin-Cre transgenic mice. A, BDNF immunostaining of adult DG sections from WT and BDNFf/f/Nestin-Cre mice. Red: BDNF; blue: DAPI. Scale bar, 10 μm. B, Coimmunostaining for BDNF (red) and doublecortin (Dcx, green) demonstrated BDNF expression in differentiating Dcx+ neurons. Scale bar, 10 μm. C, Elimination of BDNF protein expression in whole forebrain is demonstrated by Western analysis. BDNF KO, BDNFf/f/Nestin-Cre+ mice; control, BDNFf/f/Nestin-Cre− mice. D, Representative images of GFP-labeled newborn neurons in BDNFf/f/Nestin-Cre+ mice (KO) and its control littermates (Control), as well as newborn neurons in KO mice transfected with the retrovirus expressing BDNF-GFP (KO + BDNF), at 2 wpi. Scale bar, 50 μm. E, Summary of results on dendrite development in all experiments similar to those depicted in D as well as those described in A (mean ± SEM; n = 70–90 neurons for each group; 3–4 animals per group; **p < 0.01; ***p < 0.001, one-way ANOVA followed by Student's t test). Control 1: BDNFf/f/Nestin-Cre− + GFP, Control 2: WT + Cre.
We then injected GFP-expressing retrovirus to label newborn GCs in adult BDNF-cKO mice. At 2 wpi, we found that GFP+ newborn neurons showed marked impairment of dendrite development, with lower total dendrite length and total number of branch points than those newborn neurons in Nestin-Cre-negative mice (Fig. 2E). The extents of reduction were similar to those found in the above studies when BDNF was deleted only in newborn neurons (Fig. 1C,D), suggesting that the contribution of BDNF from other neurons within the DG may not be significant. In other words, BDNF may act primarily in an autocrine rather than a paracrine manner.
To further determine the paracrine contribution of BDNF secreted by other cells on adult-born neurons, we expressed exogenous BDNF in adult-born neurons of BDNF-cKO mice in which paracrine contribution of BDNF should be absent. We found that the dendrite abnormalities observed in BDNF-cKO mice was completely prevented (Fig. 2D,E), with both total dendrite length and total number of branching points actually exceeded that of the adult-born neurons in Nestin-Cre-negative mice. Therefore, autocrine action of BDNF is sufficient to support the normal dendrite development of adult newborn neurons.
BDNF overexpression promotes dendrite growth
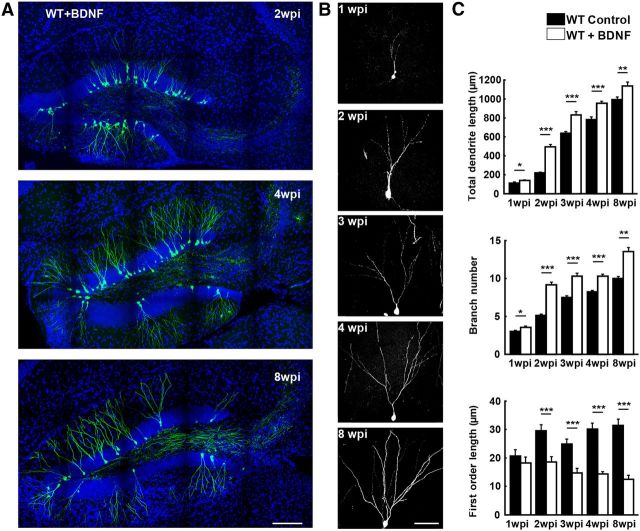
To further examine the BDNF autocrine action on the development of adult newborn neurons, we overexpressed BDNF in adult-born GCs by injecting retrovirus-expressing BDNF and GFP. Retrovirus expressing only GFP was also injected into the hippocampus of adult WT mice as a control. We found that both the total dendrite length and total number of branch points were significantly increased in single BDNF-overexpressing neurons at all stages examined (1–8 weeks after birth) compared with those found in control mice expressing only GFP in adult-born neurons (Fig. 3). In addition, the first-order dendrite length was shorter in BDNF-overexpressing neurons than that found in control neurons, indicating that BDNF accelerates branch formation (Fig. 3C).
Figure 3.
BDNF overexpression enhances dendrite growth of adult newborn neurons. A, Samples of confocal images showing axons from BDNF-overexpressing newborn dentate GCs (GFP +) in the adult hippocampus at 2, 4 and 8 wpi. Blue: DAPI staining; green: GFP. Scale bar, 300 μm. B, Examples of single GFP-labeled BDNF-overexpressing adult-born neurons at 1, 2, 3, 4, 8 wpi. Scale bar, 50 μm. C, Summary of results on dendrite development from all experiments similar to those described in B (mean ± SEM; n = 63–90 neurons for each group; 3–4 animals per group; *p < 0.05; **p < 0.01; ***p < 0.001, one-way ANOVA followed by Student's t test).
Effects of BDNF on axon growth
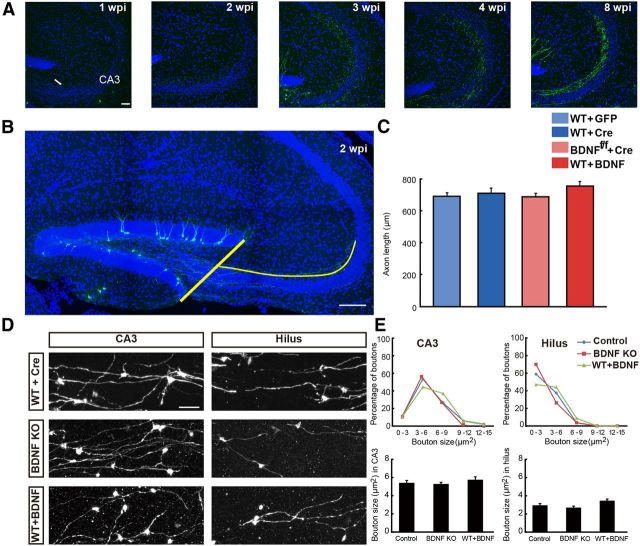
There was concurrent growth of axon during the dendrite development. We observed axons arriving CA3 area at 1 wpi, with the number of arriving axons increased with time afterward (Fig. 4A). For quantitative measurements of axonal growth, the growth of axonal fibers was determined by the average length of three longest axons projected to the CA3 area in the same section (Fig. 4B). By this assay, we did not observe any significant difference in axon growth of adult-born GCs between the WT control mice and either BDNF KO or BDNF overexpression mice (Fig. 4C).
Figure 4.
No apparent autocrine BDNF effect on axon growth. A, Samples of confocal images of adult-born GC axons in the CA3 area at 1, 2, 3, 4, and 8 wpi. Arrow indicates projected axons; blue: DAPI staining; green: GFP. Scale bar, 50 μm. B, Illustration of the measurement on GC axon length (blue line) from the edge of DG to the CA3 area at 2 wpi. Scale bar, 300 μm. C, Summary of results on axon length in mice with BDNF KO and BDNF overexpression in adult-born GCs (mean ± SEM; n = 12–18 sections for each group; 3–4 mice each; one-way ANOVA followed by Student's t test). D, Examples of mossy fiber boutons of control (WT + Cre) and BDNF KO (BDNF KO) and BDNF-overexpressing (WT + BDNF) adult-born GCs in the CA3 and hilus area. Scale bar, 50 μm. E, Distribution of the size of mossy fiber boutons for control and BDNF KO or overexpression adult-born GCs to those depicted in D. Mossy fiber boutons were grouped according to their size and the percentage of boutons in each size group was quantified. The average sizes of the mossy fiber boutons in each group were analyzed (mean ± SEM; n = 54–95 boutons from 3 animals per each group; one-way ANOVA followed by Student's t test).
In addition to axon growth, we also quantified the morphology of axon boutons in the CA3 and the hilus area to determine whether BDNF deletion or overexpression leads to a difference in synaptic output of adult-born GCs. Based on the finding that the size of the mossy fiber boutons of adult-born GCs reaches a plateau at 4 weeks after infection (Faulkner et al., 2008; Toni et al., 2007), we chose this time point to examine the size of boutons in BDNF KO and BDNF-overexpressing adult-born GCs. We found that the average size of the mossy fiber boutons in CA3 was significantly larger than that in the hilus in WT mice (Fig. 4D,E), consistent with previous findings. However, we observed no difference in the size of axon boutons between adult-born GCs in WT control mice and BDNF KO or BDNF-overexpressing adult-born GCs in both CA3 and hilus area (Fig. 4E). Together, these studies did not detect any evidence for BDNF autocrine action on either axon growth or presynaptic bouton development of adult-born GCs in the present experimental system.
Effects of BDNF on spine development
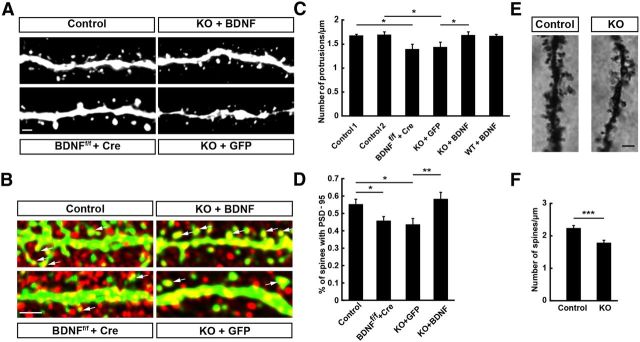
Because the overall morphology of newborn neurons resembled that of typical mature granule neurons by 4 wpi, we have also chosen this time point to examine the development of dendritic spines under various manipulations of BDNF expression. We found that most spines appeared to be thin protrusions with small heads and there were only a few spines with mushroom morphology (Fig. 5A), consistent with previous findings (Zhao et al., 2006). Compared with that found in control mice, we found that spine density of adult-born GCs was significantly reduced to a similarly low level when BDNF was deleted in either adult-born GCs themselves or in all DG neurons (Fig. 5C). Furthermore, expression of exogenous BDNF in adult-born GCs in BDNF-cKO mice resulted in normal spine development compared with controls, although the same overexpression of BDNF in WT mice had no detectable effect on spine development (Fig. 5C), suggesting that the endogenous BDNF may have already exerted a maximal effect on spine development. Together, these findings support the notion that the autocrine rather than paracrine action of BDNF is a dominant factor in spine development of adult-born GCs.
Figure 5.
Impairment of spine growth in newborn GCs of BDNF KO mice. A, Left, Representative images of GFP-labeled dendritic segments of newborn neurons expressing Cre recombinase in BDNFf/f mice (BDNFf/f + Cre) and its control group (control). Right, Representative images of GFP-labeled dendritic segments in BDNFf/f /Nestin-Cre+ mice (KO) and newborn neurons in KO mice transfected with the retrovirus expressing BDNF-GFP (KO+BDNF) at 4 wpi. Scale bar, 1 μm. B, PSD-95 immunostaining of newborn GCs in adult DG sections from experimental groups depicted in A. Arrows indicate PSD-95 clusters in spines. Red: PSD-95; green: GFP. Scale bar, 5 μm. C, Graph summarizing results on spine density for dendritic segments examined for neurons similar to those depicted in A (mean ± SEM; n = 19–25 segments from 3 mice for each group; *p < 0.05, one-way ANOVA followed by Student's t test). Control 1: WT + Cre; Control 2: BDNFf/f/Nestin-cre− + GFP. D, Graph summarizing the percentage of spines with PSD-95 clusters in dendritic segments depicted in B (mean ± SEM; n = 25–41 segments from 3 mice for each group; *p < 0.05, one-way ANOVA followed by Student's t test). E, Representative Golgi-staining images of dendrite segments of mature GCs in BDNFf/f /Nestin-Cre+ mice (KO) and its control littermates. Scale bar, 2 μm. F, Graph summarizing results on spine density for dendritic segments examined for neurons similar to those depicted in E (mean ± SEM; n = 21–34 segments from 3 mice for each group; ***p < 0.001, one-way ANOVA followed by Student's t test).
We have also used Golgi staining to examine the morphology of mature GCs in BDNF cKO mice. Given the very small percentage of new neurons among all existing mature GCs in the adult hippocampus and by selecting labeled neurons away from the DG inner layer (where most newborn neurons reside), the Golgi-stained neurons we examined are likely to be mature GCs in the hippocampus. We found that the morphology of these GCs in BDNF KO mice was apparently normal and that the spine density of GCs was significantly reduced in BDNF cKO mice compared with those in control WT mice (Fig. 5E,F). These results support the notion that BDNF plays an important role in spine formation during embryonic development and in the adult.
Dendritic spines are the postsynaptic sites of excitatory synapses. We thus further examined the expression of the postsynaptic scaffolding protein PSD-95 of excitatory synapses (Fig. 5B) and found that the percentage of spines with PSD-95 clusters in adult-born GCs in BDNF cKO mice was reduced to a level similar to that found in mice with BDNF deletion only in adult-born GCs. Furthermore, expression of exogenous BDNF in adult-born GCs in BDNF cKO mice resulted in normal PSD-95 postsynaptic localization compared with WT control GCs (Fig. 5D). These results indicate that BDNF contributes to the development of excitatory synapses.
Activity-dependent BDNF regulation of dendrite development
Previous studies have shown that running increases BDNF expression in the adult brain (Vivar et al., 2012) and promotes the maturation of adult-born GCs (Piatti et al., 2011). To investigate whether exercise-associated activity depends on the BDNF regulation of dendrite development, we examined the morphological effects of deleting BDNF only in adult-born GCs in mice that have access to a running wheel. We found that voluntary exercise increased the total dendrite length and total number of branch points in WT mice after 2 weeks of exposure to the running wheel (see Materials and Methods, Fig. 6A). In contrast, we found no such dendrite-promoting effects of voluntary exercise in adult-born GCs with BDNF deletion (Fig. 6B). Instead, the dendrite development was impeded to the same level as that found in mice without running (Fig. 1C,D). These results suggest that running-induced elevation of dendrite growth depends on the autocrine action of BDNF.
Figure 6.
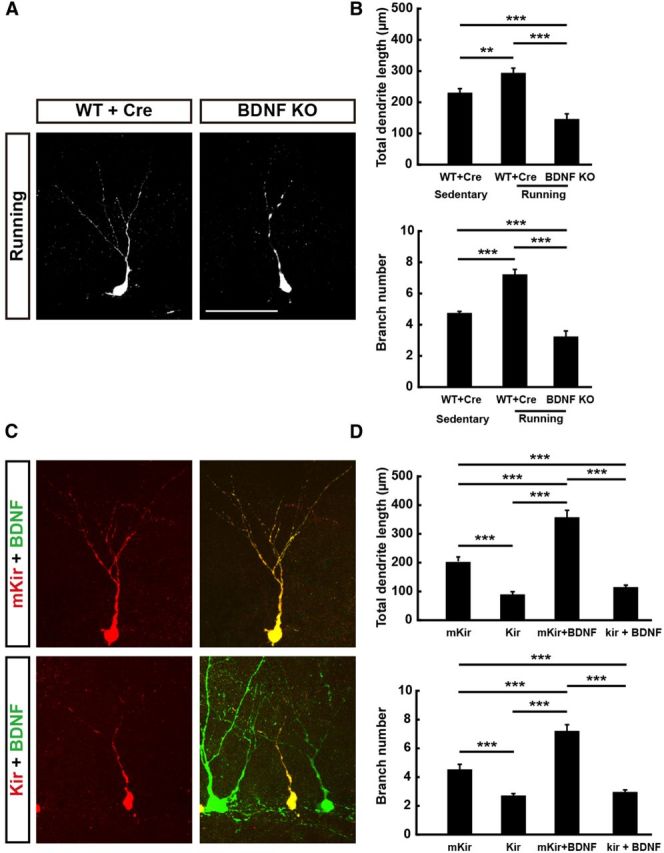
Activity-dependent BDNF regulation on dendrite development. A, Representative images of GFP-labeled newborn neurons in WT mice (WT + Cre) and BDNF KO neurons in BDNF-flox mice (BDNF KO) that have access to a running wheel (running) or not (sedentary) at 2 wpi. Scale bar, 50 μm. B, Histograms showing total dendrite length and total branch number of examined groups depicted in A (mean ± SEM; n = 29–60 neurons from 3–4 mice for each group; ***p < 0.001, one-way ANOVA followed by Student's t test). C, Representative confocal image of adult-born GCs from WT mice coinjected with retrovirus expressing mKir/Kir2.1 and mCherry and retrovirus expressing BDNF and GFP at 2 wpi. Red: newborn neuron expressing both mKir/Kir2.1 and mCherry; green: BDNF overexpressing neurons expressing both BDNF and GFP. D, Graph summarizing results on dendrite growth for neurons similar to those depicted in C (mean ± SEM; n = 21–76 neurons from 3–4 mice for each group; ***p < 0.001, one-way ANOVA followed by Student's t test).
To further address the role of neuronal activity in regulating dendrite growth, we overexpressed inward rectifier K+ channel Kir2.1 to prevent neuronal depolarization in adult-born GCs and found that the dendrite growth was significantly reduced but a mutant form of nonconductive Kir2.1 (mKir) was overexpressed (see Materials and Methods) and had no effect on dendrite development (Fig. 6D). Furthermore, coexpressing Kir2.1 and BDNF in adult-born GCs prevented the dendrite-promoting effect found when BDNF was expressed alone in these GCs (Fig. 6C,D). In contrast, coexpression of mKir with BDNF was ineffective in preventing the BDNF promotion effect (Fig. 6D). Together, these results suggest that voluntary exercise elevates dendrite growth of adult-born GCs via autocrine action of BDNF, which in turn depends on the electrical activity of these neurons.
Discussion
In this study, we found that endogenous BDNF acts as an autocrine factor during in vivo dendrite development of adult-born neurons of mouse hippocampus. The use of retrovirus-mediated gene transduction in combination with transgenic mice allowed us to analyze the function of BDNF autocrine versus paracrine action during the early stages of dendrite morphogenesis. Previous studies have already implicated the role of BDNF/TrkB signaling in neurogenesis and dendrite growth of adult-born GCs in adult mouse hippocampus (Lee et al., 2002; Rossi et al., 2006; Bergami et al., 2008; Li et al., 2008; Waterhouse et al., 2012). Our results now demonstrate that TrkB signaling is activated mainly via autocrine rather than paracrine action of BDNF in these neurons.
There is growing evidence that BDNF plays an important role in neural circuit development and plasticity in both developing and mature brain (Park and Poo, 2013). For examples, BDNF promotes the growth and complexity of dendritic arbors of cortical neurons in visual cortex slices (Horch et al., 1999; Horch, 2004). In vivo dendrite development of embyronic hippocampal GCs also depends on BDNF because long-term elevated BDNF expression in transgenic mice led to increased dendrite growth during the postnatal period (Tolwani et al., 2002). However, none of these previous studies has addressed the issue of whether BDNF acts as an autocrine factor, a paracrine factor, or both. A critical property for BDNF for its action as an autocrine factor is that it is highly positively charged (pI ≈ 9), allowing immediate binding of secreted BDNF to the surface of the secreting cell itself (Blöchl and Thoenen, 1995) or the extracellular matrix near the secretion sites. In the present study, we have provided several lines of evidence supporting the notion that autocrine action of BDNF is predominant in dendrite morphogenesis of adult-born GCs. First, endogenous expression of BDNF in adult-born GCs is critical for its own dendrite development. Second, the extent of the reduction in dendrite growth when BDNF was deleted in the entire DG (of BDNF-cKO mice) was similar to that found when BDNF was deleted only in adult-born neurons. Third, expression of BDNF only in adult-born GCs restored normal dendrite development in BDNF-cKO mice, indicating that BDNF autocrine action by itself is sufficient to support the normal dendrite development. Fourth, overexpression of BDNF only in adult-born GCs also promoted their dendrite growth and accelerated the formation of the first dendritic branch. Finally, the elevated dendrite growth in adult-born GCs induced by voluntary exercise also depends on the autocrine action of BDNF. Our finding that coexpressing Kir2.1 with BDNF in adult-born GCs prevented the autocrine BDNF effect in promoting dendrite growth is consistent with the activity-dependent secretion of BDNF found in a variety of neurons (Park and Poo, 2013) and further supports the notion that autocrine BDNF action is regulated by neuronal activity.
Previous in vitro studies have shown that the survival (Davies and Wright, 1995; Davies, 1996) and axon growth (Cheng et al., 2011) of embryonic neurons depended on autocrine action of BDNF. Our in vivo results here further indicate that the autocrine action of BDNF is also in operation during morphogenesis of adult-born GCs in the hippocampus.
In this study, we did not observe any abnormality of axon development in adult-born neurons with BDNF deletion (Fig. 4). Due to technical limitation in controlling the number of transfected neurons in different mice, we have not been able to perform quantitative analysis on the autocrine action of BDNF in axon development, although the latter has been reported for isolated embryonic hippocampal neurons in cell cultures (Cheng et al., 2011). Autocrine secretion of BDNF from adult-born neurons could depend on neuronal activity due to early excitatory action of GABA released by DG interneurons (Ge et al., 2006; Ge et al., 2007a; Duan et al., 2008; Ge et al., 2008) or to excitatory glutamatergic synaptic inputs (Espósito et al., 2005; Ge et al., 2007b) onto developing dendrites of these newborn neurons. There is evidence in cultured hippocampal neurons that dendritic BDNF secretion is much more sensitive to neuronal and synaptic activities than axonal BDNF secretion (Matsuda et al., 2009). Therefore, autocrine BDNF secretion may be more prominent in regulating dendrite growth than axon growth in these developing neurons. However, due to the latency of retrovirus effects (2 d after injection), our study may have missed the time window for revealing the autocrine effect of BDNF on axon development. More quantitative analysis of axon growth is required to further clarify the role of autocrine BDNF action in axon development of these adult-born neurons.
Accumulating evidence shows that exercise could improve learning and memory (Vivar et al., 2012). Exercise-induced improvements in learning and memory depend on enhanced adult-hippocampal neurogenesis and increased activity-dependent synaptic plasticity while concomitantly increasing BDNF levels (Kobilo et al., 2011; Park and Poo, 2013). Previous studies have shown that running increases BDNF expression in the adult brain and promotes dendrite development in the adult DG (Lafenêtre et al., 2010). The effects of exercise on BDNF levels have been suggested to be regulated by activation of NMDA receptors that contain the ε1 subunit (Kitamura et al., 2003). Previous studies have also shown that Gadd45b, a neural-activity-induced immediate early gene, promotes epigenetic DNA demethylation and dendritic development of adult newborn neurons by expressing corresponding genes critical for adult neurogenesis, including BDNF (Ma et al., 2009). Using retrovirus-mediated labeling of newborn neurons, we found that running leads to a phenotype similar to that observed in adult-born GCs overexpressing BDNF. Interestingly, the increased dendrite growth observed in mice with access to a running wheel was abolished when BDNF was deleted in adult-born GCs, suggesting that running-induced elevated dendrite growth mainly depends on the autocrine effect of BDNF in adult-born GCs. Previous study has shown that adult-born neurons in mice with voluntary exercise (running) exhibited a delayed maturation when their neuronal activity was reduced by the cell-autonomous overexpression of Kir2.1 (Piatti et al., 2011), suggesting that the neuronal activity in these neurons induces the effects caused by running. In this study, we found that autonomous neuronal activity is also required for the autocrine effect of BDNF on dendrite development in adult-born GCs because the elevated dendrite growth caused by BDNF overexpression was abolished when neuronal activity was suppressed by coexpressing Kir2.1 in adult-born GCs. More importantly, reducing neuronal activity leads to a morphologic phenotype similar to that seen in BDNF KO adult-born GCs. Together, these results support the notion that the increased dendrite growth of adult-born GCs caused by voluntary exercise is due to enhanced autocrine action of BDNF, which is mediated by elevated neuronal activity in these cells. Antidepressant drugs have also been shown to increase hippocampal expression of BDNF, which is implicated in the mechanism of action of antidepressants (Chen et al., 2001; Sairanen et al., 2005). Our results suggest that autocrine action of BDNF on dendrite morphogenesis may also contribute to the effects of antidepressant drugs.
Footnotes
This work was supported by the Ministry of Science and Technology (973 Program Grant 2011CBA00400) and the Chinese Academy of Sciences (Strategic Priority Research Program Grant XDB02020001). We thank F.H. Gage (Salk Institute) for providing the constructs of plasmids for viral packaging and A.J. Eisch (University of Texas Southwestern Medical Center) for providing Nestin-CreERT2 transgenic mice.
The authors declare no competing financial interests.
References
- Ables JL, Decarolis NA, Johnson MA, Rivera PD, Gao Z, Cooper DC, Radtke F, Hsieh J, Eisch AJ. Notch1 is required for maintenance of the reservoir of adult hippocampal stem cells. J Neurosci. 2010;30:10484–10492. doi: 10.1523/JNEUROSCI.4721-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acheson A, Conover JC, Fandl JP, DeChiara TM, Russell M, Thadani A, Squinto SP, Yancopoulos GD, Lindsay RM. A BDNF autocrine loop in adult sensory neurons prevents cell death. Nature. 1995;374:450–453. doi: 10.1038/374450a0. [DOI] [PubMed] [Google Scholar]
- Aimone JB, Deng W, Gage FH. Resolving new memories: a critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron. 2011;70:589–596. doi: 10.1016/j.neuron.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barde YA, Edgar D, Thoenen H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1982;1:549–553. doi: 10.1002/j.1460-2075.1982.tb01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergami M, Rimondini R, Santi S, Blum R, Götz M, Canossa M. Deletion of TrkB in adult progenitors alters newborn neuron integration into hippocampal circuits and increases anxiety-like behavior. Proc Natl Acad Sci U S A. 2008;105:15570–15575. doi: 10.1073/pnas.0803702105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blöchl A, Thoenen H. Characterization of nerve growth factor (NGF) release from hippocampal neurons: evidence for a constitutive and an unconventional sodium-dependent regulated pathway. Eur J Neurosci. 1995;7:1220–1228. doi: 10.1111/j.1460-9568.1995.tb01112.x. [DOI] [PubMed] [Google Scholar]
- Burrone J, O'Byrne M, Murthy VN. Multiple forms of synaptic plasticity triggered by selective suppression of activity in individual neurons. Nature. 2002;420:414–418. doi: 10.1038/nature01242. [DOI] [PubMed] [Google Scholar]
- Chen B, Dowlatshahi D, MacQueen GM, Wang JF, Young LT. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol Psychiatry. 2001;50:260–265. doi: 10.1016/S0006-3223(01)01083-6. [DOI] [PubMed] [Google Scholar]
- Cheng PL, Song AH, Wong YH, Wang S, Zhang X, Poo MM. Self-amplifying autocrine actions of BDNF in axon development. Proc Natl Acad Sci U S A. 2011;108:18430–18435. doi: 10.1073/pnas.1115907108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies AM. Paracrine and autocrine actions of neurotrophic factors. Neurochem Res. 1996;21:749–753. doi: 10.1007/BF02532296. [DOI] [PubMed] [Google Scholar]
- Davies AM, Wright EM. Neurotrophic factors: neurotrophin autocrine loops. Curr Biol. 1995;5:723–726. doi: 10.1016/S0960-9822(95)00144-8. [DOI] [PubMed] [Google Scholar]
- Duan X, Kang E, Liu CY, Ming GL, Song H. Development of neural stem cell in the adult brain. Curr Opin Neurobiol. 2008;18:108–115. doi: 10.1016/j.conb.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espósito MS, Piatti VC, Laplagne DA, Morgenstern NA, Ferrari CC, Pitossi FJ, Schinder AF. Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J Neurosci. 2005;25:10074–10086. doi: 10.1523/JNEUROSCI.3114-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner RL, Jang MH, Liu XB, Duan X, Sailor KA, Kim JY, Ge S, Jones EG, Ming GL, Song H, Cheng HJ. Development of hippocampal mossy fiber synaptic outputs by new neurons in the adult brain. Proc Natl Acad Sci U S A. 2008;105:14157–14162. doi: 10.1073/pnas.0806658105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Pradhan DA, Ming GL, Song H. GABA sets the tempo for activity-dependent adult neurogenesis. Trends Neurosci. 2007a;30:1–8. doi: 10.1016/j.tins.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Ge S, Yang CH, Hsu KS, Ming GL, Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007b;54:559–566. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Sailor KA, Ming GL, Song H. Synaptic integration and plasticity of new neurons in the adult hippocampus. J Physiol. 2008;586:3759–3765. doi: 10.1113/jphysiol.2008.155655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings NB, Gould E. Rapid extension of axons into the CA3 region by adult-generated granule cells. J Comp Neurol. 1999;413:146–154. doi: 10.1002/(SICI)1096-9861(19991011)413:1<146::AID-CNE10>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Horch HW. Local effects of BDNF on dendritic growth. Rev Neurosci. 2004;15:117–129. doi: 10.1515/revneuro.2004.15.2.117. [DOI] [PubMed] [Google Scholar]
- Horch HW, Krüttgen A, Portbury SD, Katz LC. Destabilization of cortical dendrites and spines by BDNF. Neuron. 1999;23:353–364. doi: 10.1016/S0896-6273(00)80785-0. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, She L, Chang XY, Yang RR, Wang L, Ji HB, Jiao JW, Poo MM. Protein kinase LKB1 regulates polarized dendrite formation of adult hippocampal newborn neurons. Proc Natl Acad Sci U S A. 2014;111:469–474. doi: 10.1073/pnas.1321454111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns DC, Marx R, Mains RE, O'Rourke B, Marbán E. Inducible genetic suppression of neuronal excitability. J Neurosci. 1999;19:1691–1697. doi: 10.1523/JNEUROSCI.19-05-01691.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Mishina M, Sugiyama H. Enhancement of neurogenesis by running wheel exercises is suppressed in mice lacking NMDA receptor epsilon 1 subunit. Neurosci Res. 2003;47:55–63. doi: 10.1016/S0168-0102(03)00171-8. [DOI] [PubMed] [Google Scholar]
- Kobilo T, Liu QR, Gandhi K, Mughal M, Shaham Y, van Praag H. Running is the neurogenic and neurotrophic stimulus in environmental enrichment. Learn Mem. 2011;18:605–609. doi: 10.1101/lm.2283011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo Y, Baldwin TJ, Jan YN, Jan LY. Primary structure and functional expression of a mouse inward rectifier potassium channel. Nature. 1993;362:127–133. doi: 10.1038/362127a0. [DOI] [PubMed] [Google Scholar]
- Lafenêtre P, Leske O, Ma-Högemeie Z, Haghikia A, Bichler Z, Wahle P, Heumann R. Exercise can rescue recognition memory impairment in a model with reduced adult hippocampal neurogenesis. Front Behav Neurosci. 2010;3:34. doi: 10.3389/neuro.08.034.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem. 2002;82:1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- Li Y, Luikart BW, Birnbaum S, Chen J, Kwon CH, Kernie SG, Bassel-Duby R, Parada LF. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron. 2008;59:399–412. doi: 10.1016/j.neuron.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma DK, Jang MH, Guo JU, Kitabatake Y, Chang ML, Pow-Anpongkul N, Flavell RA, Lu B, Ming GL, Song H. Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science. 2009;323:1074–1077. doi: 10.1126/science.1166859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markakis EA, Gage FH. Adult-generated neurons in the dentate gyrus send axonal projections to field CA3 and are surrounded by synaptic vesicles. J Comp Neurol. 1999;406:449–460. [PubMed] [Google Scholar]
- Matsuda N, Lu H, Fukata Y, Noritake J, Gao H, Mukherjee S, Nemoto T, Fukata M, Poo MM. Differential activity-dependent secretion of brain-derived neurotrophic factor from axon and dendrite. J Neurosci. 2009;29:14185–14198. doi: 10.1523/JNEUROSCI.1863-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Nakashiba T, Cushman JD, Pelkey KA, Renaudineau S, Buhl DL, McHugh TJ, Rodriguez Barrera V, Chittajallu R, Iwamoto KS, McBain CJ, Fanselow MS, Tonegawa S. Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell. 2012;149:188–201. doi: 10.1016/j.cell.2012.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Poo MM. Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci. 2013;14:7–23. doi: 10.1038/nrc3653. [DOI] [PubMed] [Google Scholar]
- Piatti VC, Davies-Sala MG, Espósito MS, Mongiat LA, Trinchero MF, Schinder AF. The timing for neuronal maturation in the adult hippocampus is modulated by local network activity. J Neurosci. 2011;31:7715–7728. doi: 10.1523/JNEUROSCI.1380-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi C, Angelucci A, Costantin L, Braschi C, Mazzantini M, Babbini F, Fabbri ME, Tessarollo L, Maffei L, Berardi N, Caleo M. Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. Eur J Neurosci. 2006;24:1850–1856. doi: 10.1111/j.1460-9568.2006.05059.x. [DOI] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O'Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472:466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sairanen M, Lucas G, Ernfors P, Castrén M, Castrén E. Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J Neurosci. 2005;25:1089–1094. doi: 10.1523/JNEUROSCI.3741-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476:458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro A, Zhao C, Gage FH. Retrovirus-mediated single-cell gene knockout technique in adult newborn neurons in vivo. Nat Protoc. 2006;1:3049–3055. doi: 10.1038/nprot.2006.473. [DOI] [PubMed] [Google Scholar]
- Tolwani RJ, Buckmaster PS, Varma S, Cosgaya JM, Wu Y, Suri C, Shooter EM. BDNF overexpression increases dendrite complexity in hippocampal dentate gyrus. Neuroscience. 2002;114:795–805. doi: 10.1016/S0306-4522(02)00301-9. [DOI] [PubMed] [Google Scholar]
- Toni N, Teng EM, Bushong EA, Aimone JB, Zhao C, Consiglio A, van Praag H, Martone ME, Ellisman MH, Gage FH. Synapse formation on neurons born in the adult hippocampus. Nat Neurosci. 2007;10:727–734. doi: 10.1038/nn1908. [DOI] [PubMed] [Google Scholar]
- Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schütz G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivar C, Potter MC, van Praag H. All about running: synaptic plasticity, growth factors and adult hippocampal neurogenesis. Curr Top Behav Neurosci. 2012 doi: 10.1007/7854_2012_220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse EG, An JJ, Orefice LL, Baydyuk M, Liao GY, Zheng K, Lu B, Xu B. BDNF promotes differentiation and maturation of adult-born neurons through GABAergic transmission. J Neurosci. 2012;32:14318–14330. doi: 10.1523/JNEUROSCI.0709-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharenko SS, Patterson SL, Dragatsis I, Zeitlin SO, Siegelbaum SA, Kandel ER, Morozov A. Presynaptic BDNF required for a presynaptic but not postsynaptic component of LTP at hippocampal CA1-CA3 synapses. Neuron. 2003;39:975–990. doi: 10.1016/S0896-6273(03)00543-9. [DOI] [PubMed] [Google Scholar]
- Zhao C, Teng EM, Summers RG, Jr, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]