Abstract
Macrophages play an essential role not only in mediating the first line of defense but also in maintaining tissue homeostasis. In response to extrinsic factors derived from a given tissue, macrophages activate different functional programs to produce polarized macrophage populations responsible for inducing inflammation against microbes, removing cellular debris, and tissue repair. However, accumulating evidence has revealed that macrophage polarization is pivotal in the pathophysiology of metabolic syndromes and cancer, as well as in infectious and autoimmune diseases. Recent advances in transcriptomic and metabolomic studies have highlighted the link between metabolic rewiring of macrophages and their functional plasticity. These findings imply that metabolic adaption to their surrounding microenvironment instructs activation of macrophages with functionally distinct phenotypes, which in turn probably leads to the pathogenesis of a wide spectrum of diseases. In this review, we have introduced emerging concepts in immunometabolism with focus on the impact on functional activation of macrophages. Furthermore, we have discussed the implication of macrophage plasticity on the pathogenesis of metabolic syndromes and cancer, and how the disease microenvironment manipulates macrophage metabolism with regard to the pathophysiology.
Keywords: Atherosclerosis, Cancer, Macrophage, Macrophage polarization, Metabolism, Obesity
INTRODUCTION
Since the discovery of “phagocytes” in the larvae of starfish by a Russian zoologist, Metchnikoff, macrophages have been regarded as the key players in mediating the first line of defense. Study on macrophages as scavenger cells of the innate immune system was considerably progressed by a cell biologist, Zanvil Cohn who observed that macrophages engulf and digest invading microbes, as well as toxins and dead cells (1). Beyond phagocytosis, Cohn and his colleagues stated other vital function that macrophages are not just eaters but they also secrete a wide spectrum of bioactive substances into their surrounding environment, thereby affecting the activity of other cells (2). This provision was supported by the discovery of pattern recognition receptors such as Toll-like receptors (TLRs) by Beutler, Akira, and others: TLRs sense microbial and other defined ligands in response to infection and elicit a response pathway to alter gene expression and cytokine production (3–5). This link between microbial recognition and programming of activated macrophages culminates in shaping an immune microenvironment to collaborate with other innate and adaptive immune populations.
Apart from sentinel and proinflammatory functions, macrophages play a role in aseptic conditions. Tissue-resident macrophages are highly adapted to their tissue-specific purpose in order to maintain tissue homeostasis: microglia removes apoptotic neurons to support the neuronal network (6), and osteoclasts are essential for the continuous bone resorption process (7). Lung alveolar macrophages are responsible for the uptake of surfactant and removal of particles from the alveoli (8), and Kupffer cells are important for the uptake of dying red blood cells from the circulation and iron recycling (9). Macrophages also perform a trophic function in wound healing and muscle regeneration. After removal of damaged tissues, macrophages produce a number of soluble factors followed by extracellular matrix remodeling, angiogenesis, and tissue growth (10–13). This functional diversity and tissue specialization reflect “heterogeneity” in the macrophage compartment, and raises the question on the generation of functionally distinct macrophage populations. Recent advance in animal models and lineage tracing techniques have provided an explanation on the ontogeny of macrophage populations. For example, microglia in the adult brains is reported to arise predominantly from Yolk sac progenitors (14), and Kupffer cells and alveolar macrophages are populated from fetal liver progenitors (15–17). While tissue-resident macrophages are maintained independently of bone marrow (BM) hematopoiesis, adoptively transferred BM progenitors or monocytes could replenish tissue macrophage populations in response to injury such as irradiation (18, 19). Nonetheless, it remains unanswered what decides the fate of macrophages and how they are functionally adapted to the tissue community where they reside.
The demand for activation of immune cells in response to cytokine milieu in infected or damaged tissues is widely appreciated, but emerging evidence indicates that metabolic demand also contributes to the functional commitment of leukocytes. For example, limited glucose availability due to the competition with tumor cells attenuates Interferon-γ (IFN-γ) production in tumor-infiltrating T cells (20), while hypoxia and the resulting HIF-1α induction leads to increased IL-10 production by upregulation of Treg cells (21, 22). Importantly, advances in the field of immunometabolism have established the concept that metabolic regulation shapes T cell responses, and activation of naïve T cells is wired with metabolic reprogramming to generate functionally distinct effector T cell subsets or memory cells (23). This implies that immune cells are inherently capable of metabolically adapting to a given tissue microenvironment to become engaged in the immune response. With regards to T cells, immunometabolism of macrophages is receiving considerable attention, in view of its implication in activation and function of macrophages in a given tissue microenvironment. In this review, we have provided a general but comprehensive overview of metabolic regulation in shaping the identity of macrophages with regards to the functional activation. Also, we have discussed recent studies on the interplay between multiple aspects of macrophage polarization in relation to several disease conditions.
FUNCTIONAL DIVERSITY OF MACROPHAGES
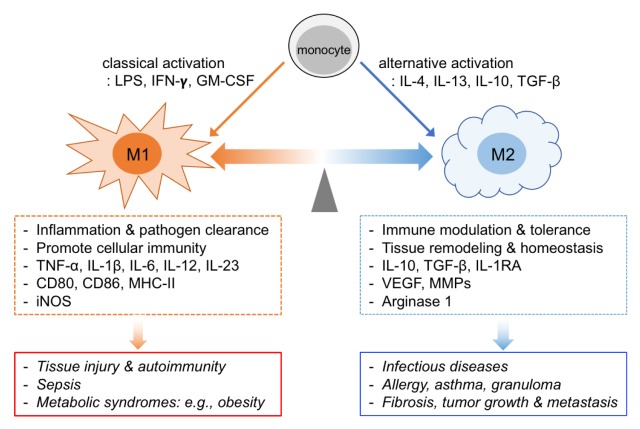
Macrophages are present in all the tissues and exhibit a wide range of functional diversity, and their heterogeneity is closely involved in health and diseases. “Activation” of macrophages was proposed by Mackaness who observed the enhanced antibacterial activity of phagocytes against Listeria in response to secondary infection (24). This classically activated (“M1”) macrophages can be induced in a culture dish by treating monocytes with lipopolysaccharide (LPS) or IFN-γ, and are characterized by secretion of proinflammatory cytokines including tumor necrosis factor-alpha (TNF-α), Interleukin-1β (IL-1β), IL-6, IL-12, and IL-18 (25) (Fig. 1). These cytokines facilitate type 1 T cell response of antigen-activated T cells, and as a result, support immune responses against intracellular pathogens and neoplastic growth. M1 macrophages also enhance antimicrobial activity by upregulation of superoxide burst (ROS), generation of reactive nitrogen intermediates (NO), and increased production of antimicrobial peptides (26).
Fig. 1.
Functional plasticity of macrophages. Monocytes can be activated in vitro by cytokines and microbial factors, and differentiate into either classically activated (M1) or alternatively activated macrophages (M2). Importantly, in vivo, macrophages exhibit phenotypic heterogeneity and plasticity during homeostasis and pathogenesis.
In the late 1990s, it was found that IL-4 upregulated the mannose receptor and reduced proinflammatory cytokines in human and mouse macrophages, which was apparently different from those activated with LPS or IFN-γ (27). This alternatively activated (“M2”) macrophages produce anti-inflammatory substances such as IL-10, TGF-β, IL-1RA, and glucocorticoid, and are linked to the process of resolving inflammation and maintaining the immune tolerance (Fig. 1) (28). M2 macrophages are frequently associated with the generation of vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), and platelet-derived growth and factor (PDGF), which contribute to the trophic function in the damaged tissues (29). Another important feature of M2 macrophages is a distinct arginine metabolism; induction of nitric oxide synthase 2 (NOS2) in M1 macrophages metabolizes arginine to produce NO and upregulation of arginase activity in M2 macrophages alternatively processes arginine into ornithine which is used to produce polyamines (30). Thus, the competition between NOS and arginase for the available arginine may provide a molecular rheostat in regulating macrophage polarization.
The concept of classically (M1) and alternatively (M2) activated macrophages was originally proposed by Mills (31), which states that macrophages activated in mouse strains with Th1 (e.g., C57BL/6) and Th2 (e.g., BALB/c) backgrounds differ qualitatively in their ability to influence inflammatory reactions. Although the M1 and M2 paradigm has been providing a useful guide for studying macrophage polarization and function, it is now considered as an oversimplification. The current knowledge of M1 and M2 concept is derived from extremely polarized macrophages generated in culture with a defined set of factors (32). This classification does not consider the source and tissue microenvironmental factors. Moreover, it does not fit into in vivo situation because macrophages in tissues exist as a “spectrum” of macrophage populations according to the magnitudes of its functional properties (33). Although further grouping and nomenclature of macrophage populations based on stimuli and gene expression profiles were recently suggested by Mantovani and others (34, 35), they still have a limitation.
IMMUNOMETABOLISM OF MACROPHAGES
A growing body of evidence emphasizes the importance of metabolism in fate decision of immune populations. When placed under a stress condition, cells should adjust to catabolic and anabolic activities to meet the energy demand and support the production of biomolecules necessary for their growth and function. Metabolic shifts during T cell activation and differentiation have been well studied. Antigen-experienced T cells promote aerobic glycolysis, fatty acid synthesis, and amino acid metabolism to support their clonal expansion and cytokine production (36, 37). On the other hand, naïve and memory T cells predominantly utilize lipid oxidation and oxidative phosphorylation to survive in a quiescent state (38). However, it is still unclear how metabolic divergence supports the differentiation of T cells into their different functional effector subsets (e.g., Th1, Th2, and Th17 CD4 T cells), although several reports suggest that metabolic programming of helper T cells depends on the microenvironment in their anatomical location (39). Immunometabolism of macrophages is also a growing field in immunology, especially in the context of its impact on functional activation of macrophages.
Classically activated (M1) macrophages
Integrated transcriptomic and metabolomic analyses indicate that IFN-γ and LPS rapidly induce activation of the glycolytic pathway in macrophages (Fig. 2). The increased glycolytic activity is largely dependent on the stabilization of HIF-1α, a transcription factor responsible for the expression of hexokinase (HK) 1, HK2, glucose transporter (GLUT) 1, GLUT3, lactate dehydrogenase A, and pyruvate kinase M2 (40). For example, overexpression of GLUT-1 in RAW 264.7 cells confer an inflammatory phenotype as shown by increased expression of proinflammatory cytokines such as G-CSF and TNF-α, and production of ROS (41). Similarly, an abnormal increase in the uptake and utilization of glucose induces excessive and prolonged secretion of IL-6 and IL-1β, resulting in chronic inflammatory conditions such as atherosclerotic artery disease (42). Conversely, in the study conducted by Semba et al., inhibition of glucose metabolism by a chemical inhibitor of pyruvate dehydrogenase kinase suppressed the expression of inflammatory cytokine such as IL-1β in a sepsis model, and impaired migration of macrophages into the hypoxic tumor microenvironment in mice transplanted with a lung carcinoma cell line (43). These data suggest that metabolic adaptation to glucose availability in a given tissue is important for the polarization of macrophages into an inflammatory phenotype.
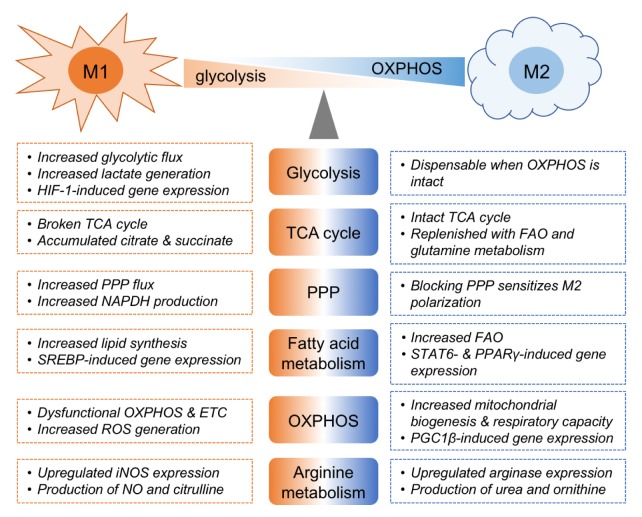
Fig. 2.
Metabolic regulation of macrophages. Metabolic features of M1 and M2 macrophages are indicated. OXPHOS: oxidative phosphorylation, TCA cycle: tricarboxylic acid cycle, PPP: pentose monophosphate pathway, FAO: fatty acid oxidation, ETC: electron transport chain.
As for the Warburg effect, truncated TCA cycle and mitochondrial dysfunction were generally considered as a metabolic feature of M1 macrophages (44). While the TCA cycle is a major metabolic pathway and highly efficient to generate ATP in most of the quiescent cells, it also provides intermediates necessary for biosynthetic reactions in actively proliferating tumor cells. Likewise, the TCA cycle in M1 macrophages is typically broken in the two reactions catalyzed by isocitrate dehydrogenase and succinate dehydrogenase, leading to the accumulation of citrate and succinate (Fig. 2) (45). The accumulated citrate can be utilized for the production of fatty acid, which is essential for membrane biogenesis and granule formation (46). Excessively produced citrate is also involved directly or indirectly in the generation of inflammatory effector molecules such as NO and prostaglandin (47). The resulting production of NO is reported to negatively modulate mitochondrial activity by disrupting electron transport chain in macrophages and dendritic cells (48, 49). Itaconic acid converted from citrate by the enzyme immunoresponsive gene 1 (IRG1), has an antimicrobial effect on Salmonella enterica and Mycobacterium tuberculosis through the inhibition of the glyoxylate shunt which is essential for the growth of some fungi and bacteria (50). Moreover, itaconate can inhibit succinate dehydrogenase, which is responsible for the increase in succinate level (51, 52). Succinate is another metabolite accumulated from the broken TCA cycle and associated with the proinflammatory function of M1 macrophages. Tannahill et al. have shown that increased level of succinate in LPS-treated macrophages enhanced IL-1β production by stabilizing HIF-1α (53).
The primary outcomes of the pentose phosphate pathway (PPP) include conversion of glycolytic intermediates to precursors of nucleotides and amino acids, and generation of reducing power in the form of NADPH, which is required for reductive anabolism (54). NADPH is actively utilized by activating macrophages for lipid biosynthesis to support the ER and cytokine secretion (55). It is also required for other inflammatory functions of macrophages. For example, inflammatory macrophages use NADPH as a reducing agent in the production of ROS and NO by NADPH oxidase and iNOS enzyme, respectively (56). The importance of PPP in M1 macrophages is highlighted in the work conducted by Haschemi et al. (57). The researchers stated that LPS-induced activation of macrophages is characterized by increased glycolytic and PPP flux, which is balanced by sedoheptulose kinase and carbohydrate kinase-like protein (CARKL). Overexpression of CARKL in RAW264.7 cells limited PPP flux and resulted in substantially impaired inflammatory responses, while CARKL loss was sufficient to amplify M1 polarization. A recent study by Baardman et al. further supports the obligation of PPP in M1 macrophages, by revealing that suppression of PPP in macrophages attenuated LPS-induction of oxidative stress responses and inflammatory cytokines in a hypercholesterolemic mouse model (58).
Mitochondrial oxidative phosphorylation (OXPHOS), coupled with TCA cycle, is slower but produces a greater amount of ATP through the electron transport chain (ETC). Even with the increased glycolytic rate, OXPHOS in M1 macrophages is largely impaired (Fig. 2), which is in sharp contrast to the induction of OXPHOS in the M2 macrophages (59). As described above, NO is not only an antimicrobial agent but also plays a role in the metabolic modulation of M1 macrophages. NO modifies ETC complex I with S-nitrosylation and inhibits mitochondrial respiration (60). Intriguingly, Van den Bossche et al. recently showed that inhibition of iNOS using a chemical inhibitor or genetic ablation markedly improved mitochondrial function in M1 macrophages, which promoted IL-4-induced repolarization of M1 into M2 (49). On the contrary, macrophages lacking PPARγ, an orphan nuclear receptor regulating mitochondrial function attenuated respiration rate and produced elevated levels of TNF-α even in the absence of inflammatory stimuli (61). These results imply that a metabolic shift towards glycolysis and OXPHOS instruct differentiation of macrophages into different functional subsets.
While mitochondrial fatty acid oxidation (FAO) and OXPHOS are essential for maintaining quiescent “resting” cells such as naïve T cells, lipid biosynthesis is a prerequisite for proinflammatory responses of macrophages. Lipogenesis is predominantly regulated by the sterol regulatory element binding proteins (SREBPs) which are key transcription factors responsible for the biosynthesis of fatty acids and cholesterol (62, 63). SREBP1 activity in macrophages can be induced by LPS and inflammatory cytokines, leading to the production of mature IL-1β and IL-18 by supporting NLRP3 inflammasome. Conversely, SREBP1c-deficient macrophages mice produce less IL-1β in response to LPS, which confers resistance to LPS-induced septic shock in mice (64). In addition, foam cells, a hallmark of atherosclerotic lesions, accumulate triglycerides and cholesterol esters by increasing de novo synthesis of fatty acids, resulting in the pathogenesis of chronic inflammatory diseases by secreting proinflammatory cytokines and chemokines (65). As commented above, glycolysis-derived glycerol, PPP-derived NADPH, and an excess amount of citrate due to the broken TCA cycle in M1 macrophages are utilized for lipid biosynthesis and lead to an intensification of the metabolic programming responsible for proinflammatory responses.
Alternatively activated (M2) macrophages
The key metabolic signature of alternatively activated macrophages is the consumption of fatty acid and increase in the mitochondrial respiratory capacity, while M1 macrophages preferentially derive ATP from glycolysis (Fig. 2). In 2006, Vats and colleagues found that IL-4 potently enhanced fatty acid uptake and induced the genetic program for oxidative metabolism in macrophages (66). The researchers suggested that IL-4 induction of genes involved in FAO and mitochondrial biogenesis are mediated by signal transducer and activator of transcription 6 (STAT6), and PPARγ-coactivator-1β (PGC-1β) in a feedforward fashion. Later, the importance of lipolysis in M2 macrophages was emphasized by revisiting the roles of the lysosomal acid lipase (LAL) and scavenger receptor CD36 (67). IL-4 treatment induced lipolysis of triacylglycerols in macrophages as shown by increased extracellular glycerol and ablation of genes encoding LAL led to substantial attenuation of the expression of M2 markers CD206 (mannose receptor), CD301 (C-type lectin) and programmed cell death 1 ligand 2 (PD-L2). Furthermore, they revealed that CD36 serves a role in the increased fatty acid oxidation in M2 macrophages by facilitating the uptake of triacylglycerols. The lipid metabolism mediated by LAL and CD36 was closely associated with functional responses of M2 macrophages to helminthic infection. Etomoxir, a chemical inhibitor of the mitochondrial enzyme carnitine palmitoyl-transferase 1 (CTP-1), has been broadly used to block fatty acid oxidation and was reported to be effective in suppression of IL-4-induced activation of M2 macrophages. In line with this result, enforced expression of constitutively-active CTP-1 in RAW264.7 cells blunted induction of proinflammatory cytokines and ROS damage in RAW264.7 cells incubated with palmitate (68).
On the contrary, recent studies argue the requirement for fatty acid oxidation in M2 macrophages. Macrophage-specific deletion of CTP-2 did not affect the expression of CD206, CD301, and arginase 1 in IL-4-activated macrophages, although the macrophages lacked the capacity for FAO (69). Further works have provided evidence that human macrophages may not depend on fatty acid oxidation as etomoxir did not inhibit M2 polarization of human monocyte-derived macrophages in contrast to mouse bone marrow-derived macrophages (70). Indeed, IL-4 treatment induced only moderate changes in mitochondrial metabolism and FAO, without marked shifting of glycolysis towards OXPHOS in human macrophages. Therefore, the impact of fatty acid utilization in the polarization and function of M2 macrophages remains a debatable topic.
Initially, it was believed that alternatively activated macrophages rely solely on fatty acid oxidation and are independent of glucose metabolism processed by glycolysis (Fig. 2). However, recent studies have claimed that glycolysis is also critical for the polarization of M2 macrophages. Pretreatment of mouse BMDM with 2-deoxyglucose diminished IL-4-induced expression of M2 markers including PD-L2 and RELMα, even in the presence of TGs and fatty acids (71). Further, metabolomic analysis revealed that IL-4 upregulated glycolysis, glycolytic shunts in macrophages, and the altered metabolic flux are mediated by Akt-mTOR signaling (72). Thus, inhibition of glucose uptake using 2-deoxyglucose and Akt inhibitor could suppress the IL-4-induced expression of M2 marker genes without affecting β-oxidation rate. However, Wang and colleagues showed that depletion of glucose or substitution of glucose with galactose had no effect on IL-4-induced polarization of M2 macrophages (73). In contrast to treatment with 2-deoxyglucose, glucose depletion or substitution with galactose did not impair OXPHOS while suppression of glycolysis was observed, as OXPHOS was connected normally with the TCA cycle being powered by glutamine in the experimental setting. Hence, it is likely that M2 macrophages seem to flexibly adapt to their bioenergetics programs, which depends on nutrient availability.
Glutamine is a versatile amino acid and glutamine metabolism is critical for many cellular functions. For example, glutamate that is generated by glutaminase is preferentially used as an anaplerotic precursor to maintain the TCA cycling in the highly proliferative cancer cells (74). Glutamine can also be utilized for the biosynthesis of amino acids and nucleotides by participating in nitrogen-donating reactions, and acetyl-CoA production via reductive carboxylation of 2-ketoglutarate. Glutamine depletion in human and mouse macrophage culture affects the production of inflammatory cytokines such as TNF-α and IL-8, in parallel with attenuated oxygen consumption rate (75). Glutamine metabolism is also associated with NO production, as arginine is catalyzed by iNOS to generate NO and intracellular arginine is supplied mainly by conversion of glutamine (76). Interestingly, Palmieri et al. provided evidence that glutamine synthetase (GS) is upregulated in the alternatively activated macrophages, and that it modulates macrophage polarization towards M2 phenotype (77). Indeed, chemical inhibition of GS and genetic depletion of GS blocked IL-10-induced expression of M2 markers such as CD206, while promoted induction of proinflammatory mediators including TNF-α and iNOS through the stabilization of HIF-1α. This data indicates that conversion of glutamate to glutamine is relevant for the immunomodulatory function of M2 macrophages, reinforcing the impact of glutamine metabolism on the polarization of macrophages.
Arginine metabolism is a key modulator in the regulation of innate and adaptive immune responses. In macrophages, iNOS is transcriptionally upregulated upon LPS or IFN-γ stimulation, and metabolizes arginine to generate NO, which is associated with M1 phenotype (78). On the other hand, arginine can be alternatively metabolized by arginase to generate ornithine and urea. Ornithine is utilized for the production of polyamine and proline which are essential for cell growth and collagen synthesis (30). Thus, arginine catabolism mediated by arginase 1 contributes towards wound healing process and trophic function of macrophages. iNOS and arginase compete for the available intracellular arginine, thus proving that relative expression of iNOS and arginase 1 is pivotal for distinct functional activation of macrophages via the production of different metabolites.
METABOLIC DISEASES AND MACROPHAGES
Functional activation of macrophages is important for the maintenance of tissue homeostasis and dealing with pathologic conditions such as infection and neoplastic growth. In the early phase of infection caused by pathogenic bacteria and fungus, macrophages with M1-like phenotype are predominantly activated, and then M2-like macrophages repopulate in the infected area (79), supporting the protective mechanism to counteract overwhelming inflammation. During the progression of many cancers, chronic non-resolving inflammation in tumor microenvironment coaxes macrophages to repopulate with M2-like phenotypes, which exert a role in tumorigenesis (80). These imply that the surrounding microenvironment instructs activation of macrophages with functionally distinct phenotypes, which in turn probably leads to pathology. Further, we will discuss the implication of macrophage plasticity on the pathogenesis of metabolic syndromes such as obesity and atherosclerosis, and cancer with regard to metabolic regulation of macrophages.
Obesity
It has been becoming evident that inflammation within adipose tissue is strongly associated with the pathogenesis of metabolic syndromes. For example, TNF-α is overexpressed in adipose tissue of obese humans and mice, and inhibition of TNF-α results was reported to improve insulin sensitivity and glucose tolerance in a mouse model (81, 82). Anti-TNF-α treatment of rheumatoid arthritis was reported to have a secondary effect on insulin sensitivity although it was uncertain whether TNF-α neutralization alone is beneficial for obesity (83). Adipose tissues contain innate immune cells including macrophages that contribute towards the maintenance of tissue homeostasis via their immunomodulatory functions in lean individuals (84). On the other hand, in obese tissues, increased numbers and size of adipocytes, and higher levels of free fatty acid and hypoxia within the adipose tissues lead to recruitment and activation of some immune cell subsets, eventually causing chronic inflammation and metabolic disorders such as insulin resistance and type 2 diabetes (85). Thus, changes in adipose tissue microenvironment during the progression of obesity seems to exploit the quantity and quality of inflammatory responses.
Macrophages in adipose tissue consists of two major populations, tissue-resistant and bone marrow-derived “recruited” macrophages. Comparison of the number of macrophages within the visceral adipose tissues of lean and obese mice revealed a dramatic increase in the number of macrophages (40–50% of adipose tissue) in severe obesity mainly due to the recruitment of monocytes and expansion of the recruited macrophages (86). Moreover, a translational study showed a correlation between drastic weight loss in obese individuals and a decrease in the number of macrophages in white adipose tissues, remaining macrophage subsets that produce IL-10 (87). Adipose tissue macrophages in obese individuals are predominantly with M1 phenotype expressing TNF-α, IL-1β, and IL-6, which are involved in metabolic disorders. In obese tissues, increased levels of free fatty acid promote proinflammatory responses of macrophages, mediated by NLRP3 inflammasome pathway and via the proteolytic maturation of IL-1β and IL-18 (88). Elevated plasma levels of IL-1β and IL-1 receptor antagonist (IL-1RA) are strongly associated with human obesity (89, 90), and blockade of IL-1β by the administration of IL-1RA in obese mice led to an improvement in glucose tolerance and hyperglycemia (91). Conversely, tissue-resident macrophages in lean tissues are largely regarded to produce anti-inflammatory cytokines such as IL-10, which blunt inflammation responses (92). Roberto et al. reported that administration of M2-polarizing cytokine IL-4 to obese mice attenuated adipose tissue inflammation, reduced weight gain, and improved insulin sensitivity (93). They showed that IL-4-induced activation of STAT6 represses catabolic metabolism driven by PPARα, suggesting a link between metabolic respiration and inflammatory phenotype of macrophages. Similarly, CD14+ CD16− CD163+ macrophages in adipose tissues of lean individuals were found to be of M2 phenotypes and negatively correlated with body mass index (BMI); however, it remains unclear if the population is tissue-resident or derived from monocytes (94).
A recent transcriptome study combined with extracellular flux analysis provided insight on the metabolic signature of macrophages in obesity (95). Adipose tissue macrophages in obese mice are characterized by increased activation of both glycolysis and OXPHOS, which is distinct from LPS-induced M1 macrophages but resembles human visceral adipose macrophages of obese individuals with type 2 diabetes. Based on the use of chemical inhibitors, interfering with the metabolic routes, it was demonstrated that glycolysis is predominantly involved in the secretion of proinflammatory cytokines such as IL-6 in obese tissue, thus raising a possibility that activation of OXPHOS in obesity might not be responsible for the inflammatory phenotype. Despite the importance of prevention of lipotoxicity, the removal of extracellular lipid and uptake of free fatty acids contribute to the inflammatory responses of macrophages (96). Lipid-laden macrophages accumulate as adiposity increases and promote M1-like activation (97), whereas omega-3 polyunsaturated fatty acid exerts an anti-inflammatory effect by antagonizing TLR4 signaling (98). Intriguingly, Xu et al. proposed that lipid accumulation in adipose tissue macrophages induces lysosome biogenesis and enhances lipid catabolism, independently from activation of inflammatory responses (99). Apparently, adipose tissue macrophages undergo lipid catabolism during the progression of obesity, and a failure to adapt to the sustained metabolic stress likely leads to inflammation and adipocyte dysfunction.
Adipocyte-derived hormones such as leptin and adiponectin are implicated in inflammation and macrophage plasticity. Leptin has been extensively studied because of its essential role in energy metabolism. In addition to the neuroendocrine effect, leptin was shown to modulate immunity as both leptin- and leptin receptor-deficient mice are not only obese but also have altered immune phenotypes including thymic atrophy and impaired T cell responses (100). Moreover, administration of leptin reversed the immunosuppressive effect induced by starvation, suggesting leptin as a proinflammatory factor. Monocytes and macrophages also express leptin receptor (OB-R) and in response to leptin, proliferate and produce proinflammatory molecules such as TNF-α, IL-6, and CXCL10 (101). Thus, beyond a fasting signal, leptin mediates local and systemic inflammation which contributes to metabolic syndrome-associated pathology. Adiponectin regulates a number of metabolic processes including glucose uptake and fatty acid oxidation, and decreased adiponectin secretion is associated with hypertension and type 2 diabetes (102). Unlike leptin, adiponectin was reported to suppress NF-κB-dependent expression of proinflammatory cytokines (103) and promote macrophage polarization towards M2-like phenotype possibly through the regulation of AMPK and PPARα (104). However, other studies argue that adiponectin augments expression of proinflammatory cytokines including TNF-α, IL-6, and IL-12 without affecting M2 polarization of macrophages (105). Whether adiponectin is proinflammatory or anti-inflammatory in macrophages remains to be determined.
Atherosclerosis
Atherosclerosis can be initiated by cholesterol deposition but the progression requires activation of myeloid cells and chronic inflammation. Atherosclerotic lesion recruits circulating monocytes into an atherosclerotic lesion of the artery wall, where they differentiate into macrophages, remove lipoproteins and cell debris and finally transform into cholesterol-rich foam cells (106). The atherosclerotic milieu is rich in cholesterol crystals, oxidized lipids and DAMP ligands released by dead cells, which shapes distinct microenvironment for macrophages in the plaque. Increased glucose metabolism is apparent in human and mouse atherosclerosis (107). In particular, elevated glucose uptake increased expression of GLUT-1 and PKM2, and accumulated lactate production contribute to the inflammatory phenotype of macrophages (108). Bone marrow transplantation experiments using ApoE-deficient mice demonstrated that Glut1 deficiency in monocyte/macrophages reduced glycolytic flux and delayed the progression of atherosclerosis (109). Effect of the metabolic alterations in atherosclerotic plaque on human macrophages was well defined by Shirai and colleagues (42). In response to LPS and IFN-γ, monocytes isolated from atherosclerotic patients produced more IL-6, IL-1β, and ROS compared to healthy individuals, and this hyperinflammatory phenotype was dependent on glycolytic flux, proposing the concept of “monocyte priming” in relation to cellular metabolism.
Marked upregulation of glucose metabolism in atherosclerosis raised a question that to what extent glucose-rich condition such as diabetes affects the inflammatory phenotype of macrophages. Both type 1 and type 2 diabetes mellitus are independent risk factors for atherosclerosis, but hyperglycemia associated with diabetes strongly predisposes to atherosclerosis (110). Hyperglycemia and increased glucose availability contribute to enhanced glucose metabolism and inflammatory activation of macrophages. Type 1 diabetes-associated hyperglycemia promotes myelopoiesis and impairs the resolution of atherosclerotic plaques (111). Conversely, treatment of hyperglycemia was shown to reduce monocyte recruitment into the plaques and ameliorate atherosclerosis in a mouse model (112). Intriguingly, Nishizawa et al. reported that overexpression of GLUT1 in macrophages upregulated glycolysis and PPP, but did not aggravate atherosclerosis in LDL receptor (Ldlr)-deficient mice (113). Along with the effect of GLUT1 depletion (109), these results imply that increased glycolysis is necessary but not sufficient for inflammatory activation of macrophages in atherosclerosis.
Oxidative stress and mitochondrial dysfunction are involved in inflammatory responses, thereby promoting atherosclerosis. By clearing the accumulated oxidized LDL and phospholipid in atherosclerotic plaques, macrophages generate an excessive amount of ROS through the mitochondrial oxidative metabolism and cause mitochondrial damage (114). Subsequently, mitochondrial dysfunction blocks OXPHOS, resulting in attenuated activation of M2-like macrophages. Suppression of oxidative stress by ectopic expression of catalase was shown to reduce aortic lesion in Ldlr KO mice, in parallel with decreased inflammatory macrophages (115). In support of this, other study reported that upregulating mitochondrial oxidative metabolism in the plaque macrophages by IL-13 administration mitigated atherosclerosis in Ldlr KO mice (116). The limited supply of oxygen in atherosclerotic lesion also contributes to inflammatory responses of macrophages. Under the hypoxic condition, macrophages suppress fatty acid oxidation and increase biosynthesis of lipids and cholesterol, which are subsequently accumulated in the form of cytosolic lipid droplets in macrophages (117). In advanced human atherosclerosis, hypoxia-induced expression of HIF-1α and VEGF was observed especially within the macrophage-rich region of the lesions (118), whereas HIF-1α depletion in myeloid cells mitigated atherosclerosis in Ldlr KO mice (119). Therefore, bioenergetic of macrophage might be an efficient therapeutic target to treat atherosclerosis.
Cancer
The macrophage is one of the most prominent populations in the tumor stroma, and abundance of tumor-associated macrophages (TAMs) was correlated with clinical outcomes in many cancers (120). During tumorigenesis, circulating monocytes are recruited at tumor sites by tumor-derived chemoattractants including CCL2 (MCP-1), CCL3 (MIP-1), CXCL12 (SDF-1), and CSF-1, and differentiate into TAMs (121). In addition to monocyte-derived macrophages, tissue-resident macrophages consist of TAMs, but it is still unclear whether these two populations have different roles in tumor growth. TAMs in mouse and human cancers largely expresses M2-like phenotypes that include arginase, IL-10, and PDGF-BB, which induces immunosuppression and fibrosis within the tumor microenvironment (122). Macrophage depletion studies have emphasized the pro-tumoral potential of TAMs. For example, genetic deletion of CSF-1 in a mammary carcinoma model delayed development of invasive metastatic carcinoma, while transgenic expression of CSF-1 accelerated pulmonary metastasis (123). Nonetheless, TAMs are heterogeneous with respect to their plasticity. In fact, the prevalence of macrophages with M1-like phenotypes expressing IL-12, TNF-α, and iNOS has been reported to correlate with favorable clinical outcomes in many human cancers (124).
Tumor cells evolutionally adapt metabolism to respond to high energy demand required for their growth and proliferation. The altered energy metabolism of cancer cells is called the “Warburg effect” and is characterized by preferential upregulation of glycolysis even in the aerobic condition (125). Since tumor cells and TAMs share nutrients and metabolites present in their microenvironment, their metabolisms inevitably affect each other. Extracellular accumulation of lactate from tumor cells is sufficient to upregulate the expression of arginase and VEGF in macrophages, which in turn suppresses antitumoral immune responses and induces angiogenesis (126). An in vitro study showed that human monocytes gained abilities to induce angiogenesis and epithelial-mesenchymal transition upon culturing with conditioned media from a pancreatic ductal adenocarcinoma cell line (127). Interestingly, the tumor-conditioned macrophages exhibited elevated glycolytic features and inhibition of glycolytic activity using 2-deoxyglucose and hexokinase II inhibitor blunted the pro-metastatic phenotype. This study claimed that glycolytic metabolism is important for pro-tumoral M2-like phenotype of TAMs, which is in sharp contrast to the metabolic shift of M2 macrophages toward oxidative mitochondrial metabolism. On the other hand, Wenes et al. revealed a link between TAM metabolism and tumor vasculature in the hypoxic tumor microenvironment (128). The researchers observed increased expression of REDD1, a negative regulator of mTOR in TAMs within the hypoxic regions of several tumors and that genetic ablation of REDD1 in TAMs prevented abnormal angiogenesis and metastasis. Mechanistically, REDD1-deficient TAMs induced mTOR-dependent upregulation of glucose uptake and glycolysis, leading to outcompete endothelial cells for glucose. Further study is needed to determine whether upregulation of glucose metabolism via mTOR activation is sufficient to repolarize TAMs toward anti-tumoral M1-like phenotype.
In addition to M2-like macrophages, TAMs are comprised of macrophages with M1-like phenotype. However, M1-like macrophages are reported to be limited to the normoxic region while M2-like TAMs are accumulated in the hypoxic region of the tumor (129). A recent study has revealed that exosomes released from hypoxic tumor region but not from normoxic region promote M2-like macrophage polarization (130). The finding that M1-like TAMs possessing anti-tumoral activity stay within the tumor has suggested a novel therapeutic approach to hijack TAMs against cancer. Pyonteck and colleagues demonstrated that inhibition of the CSF-1 receptor to target TAMs in a glioblastoma model suppressed the expression of M2 markers without depleting TAMs, and regressed established tumors (131). Similarly, Casazza et al. showed that depletion of neuropilin-1, a chemotactic receptor impeded Semaphorin 3A-mediated migration of TAMs into a hypoxic niche within the tumor (132). Instead, it led to the accumulation of TAMs in the normoxic region and reprogramming of M1-like macrophages, eventually causing inhibition of tumor growth and metastasis.
CONCLUDING REMARKS
Development and progression of various diseases including cancers and autoimmune disorders involve inappropriate activation of macrophages. The growing body of knowledge on immunometabolism, particularly of macrophages, has presented metabolic reprogramming as an attractive therapeutic target for disease conditions (Table 1). For example, targeting microRNA (miR-33) regulating FAO has demonstrated a protective effect on atherosclerosis by promoting M2-like macrophages (133). CpG oligonucleotide, agonist TLR-9 ligand has the potential to orchestrate immunity towards type 1 immune responses. Treatment with CpG oligonucleotides enhanced the anti-tumoral capacity of TAMs through the upregulation of glycolytic flux and OXPHOS, hence causing suppression of tumor growth in pancreatic cancer models (134). These findings shed light on the importance of metabolic modulation of macrophages for broadening the therapeutic options to treat metabolic syndromes, cancers, and autoimmune disorders. Furthermore, considering low response rates and high cost of current cancer immunotherapeutics, targeting macrophage metabolism could emerge as a promising option to synergize with immune checkpoint inhibitors. The major hurdle of this approach would be specific targeting of metabolic inhibitors to macrophages while sparing other cells. In addition, it should be considered that macrophages are very plastic within the tissue microenvironment unlike the in vitro derived macrophages. Though many questions remain regarding the metabolic feature of tissue-specific macrophages, an exciting venue has opened for the development of novel therapeutic strategies for metabolic reprogramming of macrophages.
Table 1.
Therapeutic targeting of macrophage metabolism for obesity and atherosclerosis
| Treatment | Metabolic change | Preclinical outcomes | Ref | |
|---|---|---|---|---|
| Obesity | IL-4 | Upregulates FAO and OXPHOS | Improved insulin sensitivity | (93) |
| IL-33 | Increases mitochondrial biogenesis | Prevention of HFD-induced insulin resistance | (135) | |
| ω-3 fatty acid | Fatty acid re-esterification and enhanced FAO | Improved diabetes and hepatic steatosis | (136) | |
| NADPH oxidase inhibitor | Reduces ROS and prevents OXPHOS dysfunction | Improved glucose and insulin tolerance | (137) | |
| Notch inhibitor (DAPT) | Impairs glucose oxidation and ROS generation | Notch1 deficiency mitigates steatohepatitis | (138) | |
| mTOR inhibitor | Torin but not rapamycin suppresses glucose uptake | Resveratrol prevents glucose tolerance | (71, 139) | |
| Atherosclerosis | IL-13 | Increases mitochondrial biogenesis and activity | Reduced macrophagesin atherosclerotic plaques | (116) |
| LXR agonist (desmosterol) | Alters fatty acid and cholesterol metabolism | Deactivated foam cells with reduced inflammation | (140) | |
| PKM2 inhibitor | PKM2 deficiency reduces glycolysis | Mitigated atherosclerotic lesion formation | (141) | |
| AMPK activator (anti-miR-33) | Increases OXPHOS and reduces glycolysis | Regressed atherosclerotic plaques | (133) | |
| Autophagy | Suppresses glucose consumption | ATG5 deficiency enhanced atherosclerotic plaque formation | (142) |
ACKNOWLEDGEMENTS
This research was supported by Basic Science Research Program (NRF-2018R1D1A1B07048260) funded by the Ministry of Education through the National Research Foundation; Korean Mouse Phenotyping Project (NRF-2014M3A9D5A0 1073841) funded by the Ministry of Science through the National Research Foundation.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Cohn ZA, Benson B. The Differentiation of Mononuclear Phagocytes. Morphology, Cytochemistry, and Biochemistry. J Exp Med. 1965;121:153–170. doi: 10.1084/jem.121.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rouzer CA, Scott WA, Hamill AL, Liu FT, Katz DH, Cohn ZA. Secretion of leukotriene C and other arachidonic acid metabolites by macrophages challenged with immunoglobulin E immune complexes. J Exp Med. 1982;156:1077–1086. doi: 10.1084/jem.156.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 4.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 5.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 6.Witting A, Muller P, Herrmann A, Kettenmann H, Nolte C. Phagocytic clearance of apoptotic neurons by Microglia/Brain macrophages in vitro: involvement of lectin-, integrin-, and phosphatidylserine-mediated recognition. J Neurochem. 2000;75:1060–1070. doi: 10.1046/j.1471-4159.2000.0751060.x. [DOI] [PubMed] [Google Scholar]
- 7.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 8.van Iwaarden JF, Claassen E, Jeurissen SH, Haagsman HP, Kraal G. Alveolar macrophages, surfactant lipids, and surfactant protein B regulate the induction of immune responses via the airways. Am J Respir Cell Mol Biol. 2001;24:452–458. doi: 10.1165/ajrcmb.24.4.4239. [DOI] [PubMed] [Google Scholar]
- 9.Willekens FL, Werre JM, Kruijt JK, et al. Liver Kupffer cells rapidly remove red blood cell-derived vesicles from the circulation by scavenger receptors. Blood. 2005;105:2141–2145. doi: 10.1182/blood-2004-04-1578. [DOI] [PubMed] [Google Scholar]
- 10.Leibovich SJ, Ross R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am J Pathol. 1975;78:71–100. [PMC free article] [PubMed] [Google Scholar]
- 11.Polverini PJ, Cotran PS, Gimbrone MA, Jr, Unanue ER. Activated macrophages induce vascular proliferation. Nature. 1977;269:804–806. doi: 10.1038/269804a0. [DOI] [PubMed] [Google Scholar]
- 12.Hunt TK, Knighton DR, Thakral KK, Goodson WH, 3rd, Andrews WS. Studies on inflammation and wound healing: angiogenesis and collagen synthesis stimulated in vivo by resident and activated wound macrophages. Surgery. 1984;96:48–54. [PubMed] [Google Scholar]
- 13.Koh TJ, DiPietro LA. Inflammation and wound healing: the role of the macrophage. Expert Rev Mol Med. 2011;13:e23. doi: 10.1017/S1462399411001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alliot F, Godin I, Pessac B. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Brain Res Dev Brain Res. 1999;117:145–152. doi: 10.1016/S0165-3806(99)00113-3. [DOI] [PubMed] [Google Scholar]
- 15.Naito M, Hasegawa G, Takahashi K. Development, differentiation, and maturation of Kupffer cells. Microsc Res Tech. 1997;39:350–364. doi: 10.1002/(SICI)1097-0029(19971115)39:4<350::AID-JEMT5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 16.Guilliams M, De Kleer I, Henri S, et al. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J Exp Med. 2013;210:1977–1992. doi: 10.1084/jem.20131199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ginhoux F, Guilliams M. Tissue-Resident Macrophage Ontogeny and Homeostasis. Immunity. 2016;44:439–449. doi: 10.1016/j.immuni.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 18.Matute-Bello G, Lee JS, Frevert CW, et al. Optimal timing to repopulation of resident alveolar macrophages with donor cells following total body irradiation and bone marrow transplantation in mice. J Immunol Methods. 2004;292:25–34. doi: 10.1016/j.jim.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 19.Scott CL, Zheng F, De Baetselier P, et al. Bone marrow-derived monocytes give rise to self-renewing and fully differentiated Kupffer cells. Nat Commun. 2016;7:10321. doi: 10.1038/ncomms10321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang CH, Qiu J, O’Sullivan D, et al. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell. 2015;162:1229–1241. doi: 10.1016/j.cell.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Westendorf AM, Skibbe K, Adamczyk A, et al. Hypoxia Enhances Immunosuppression by Inhibiting CD4+ Effector T Cell Function and Promoting Treg Activity. Cell Physiol Biochem. 2017;41:1271–1284. doi: 10.1159/000464429. [DOI] [PubMed] [Google Scholar]
- 22.Clambey ET, McNamee EN, Westrich JA, et al. Hypoxia-inducible factor-1 alpha-dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa. Proc Natl Acad Sci U S A. 2012;109:E2784–2793. doi: 10.1073/pnas.1202366109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bantug GR, Galluzzi L, Kroemer G, Hess C. The spectrum of T cell metabolism in health and disease. Nat Rev Immunol. 2018;18:19–34. doi: 10.1038/nri.2017.99. [DOI] [PubMed] [Google Scholar]
- 24.Mackaness GB. Cellular resistance to infection. J Exp Med. 1962;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992;176:287–292. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Wynn TA, Vannella KM. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity. 2016;44:450–462. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rath M, Muller I, Kropf P, Closs EI, Munder M. Metabolism via Arginase or Nitric Oxide Synthase: Two Competing Arginine Pathways in Macrophages. Front Immunol. 2014;5:532. doi: 10.3389/fimmu.2014.00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166–6173. doi: 10.4049/jimmunol.164.12.6166. [DOI] [PubMed] [Google Scholar]
- 32.Murray PJ, Allen JE, Biswas SK, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 35.Roszer T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediators Inflamm. 20152015 doi: 10.1155/2015/816460. 816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pearce EL. Metabolism in T cell activation and differentiation. Curr Opin Immunol. 2010;22:314–320. doi: 10.1016/j.coi.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buck MD, O’Sullivan D, Pearce EL. T cell metabolism drives immunity. J Exp Med. 2015;212:1345–1360. doi: 10.1084/jem.20151159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Sullivan D, van der Windt GJ, Huang SC, et al. Memory CD8(+) T cells use cell-intrinsic lipolysis to support the metabolic programming necessary for development. Immunity. 2014;41:75–88. doi: 10.1016/j.immuni.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slack M, Wang T, Wang R. T cell metabolic reprogramming and plasticity. Mol Immunol. 2015;68:507–512. doi: 10.1016/j.molimm.2015.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang T, Liu H, Lian G, Zhang SY, Wang X, Jiang C. HIF1alpha-Induced Glycolysis Metabolism Is Essential to the Activation of Inflammatory Macrophages. Mediators Inflamm. 20172017 doi: 10.1155/2017/9029327. 9029327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freemerman AJ, Johnson AR, Sacks GN, et al. Metabolic reprogramming of macrophages: glucose transporter 1 (GLUT1)-mediated glucose metabolism drives a proinflammatory phenotype. J Biol Chem. 2014;289:7884–7896. doi: 10.1074/jbc.M113.522037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shirai T, Nazarewicz RR, Wallis BB, et al. The glycolytic enzyme PKM2 bridges metabolic and inflammatory dysfunction in coronary artery disease. J Exp Med. 2016;213:337–354. doi: 10.1084/jem.20150900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Semba H, Takeda N, Isagawa T, et al. HIF-1alpha-PDK1 axis-induced active glycolysis plays an essential role in macrophage migratory capacity. Nat Commun. 2016;7:11635. doi: 10.1038/ncomms11635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Geeraerts X, Bolli E, Fendt SM, Van Ginderachter JA. Macrophage Metabolism As Therapeutic Target for Cancer, Atherosclerosis, and Obesity. Front Immunol. 2017;8:289. doi: 10.3389/fimmu.2017.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ryan DG, O’Neill LAJ. Krebs cycle rewired for macrophage and dendritic cell effector functions. FEBS Lett. 2017;591:2992–3006. doi: 10.1002/1873-3468.12744. [DOI] [PubMed] [Google Scholar]
- 46.Williams NC, O’Neill LAJ. A Role for the Krebs Cycle Intermediate Citrate in Metabolic Reprogramming in Innate Immunity and Inflammation. Front Immunol. 2018;9:141. doi: 10.3389/fimmu.2018.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Infantino V, Convertini P, Cucci L, et al. The mitochondrial citrate carrier: a new player in inflammation. Biochem J. 2011;438:433–436. doi: 10.1042/BJ20111275. [DOI] [PubMed] [Google Scholar]
- 48.Everts B, Amiel E, van der Windt GJ, et al. Commitment to glycolysis sustains survival of NO-producing inflammatory dendritic cells. Blood. 2012;120:1422–1431. doi: 10.1182/blood-2012-03-419747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van den Bossche J, Baardman J, Otto NA, et al. Mitochondrial Dysfunction Prevents Repolarization of Inflammatory Macrophages. Cell Rep. 2016;17:684–696. doi: 10.1016/j.celrep.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 50.Michelucci A, Cordes T, Ghelfi J, et al. Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production. Proc Natl Acad Sci U S A. 2013;110:7820–7825. doi: 10.1073/pnas.1218599110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cordes T, Wallace M, Michelucci A, et al. Immunoresponsive Gene 1 and Itaconate Inhibit Succinate Dehydrogenase to Modulate Intracellular Succinate Levels. J Biol Chem. 2016;291:14274–14284. doi: 10.1074/jbc.M115.685792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lampropoulou V, Sergushichev A, Bambouskova M, et al. Itaconate Links Inhibition of Succinate Dehydrogenase with Macrophage Metabolic Remodeling and Regulation of Inflammation. Cell Metab. 2016;24:158–166. doi: 10.1016/j.cmet.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tannahill GM, Curtis AM, Adamik J, et al. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature. 2013;496:238–242. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eggleston LV, Krebs HA. Regulation of the pentose phosphate cycle. Biochem J. 1974;138:425–435. doi: 10.1042/bj1380425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Everts B, Amiel E, Huang SC, et al. TLR-driven early glycolytic reprogramming via the kinases TBK1-IKKvarepsilon supports the anabolic demands of dendritic cell activation. Nat Immunol. 2014;15:323–332. doi: 10.1038/ni.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koo SJ, Szczesny B, Wan X, Putluri N, Garg NJ. Pentose Phosphate Shunt Modulates Reactive Oxygen Species and Nitric Oxide Production Controlling Trypanosoma cruzi in Macrophages. Front Immunol. 2018;9:202. doi: 10.3389/fimmu.2018.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haschemi A, Kosma P, Gille L, et al. The sedoheptulose kinase CARKL directs macrophage polarization through control of glucose metabolism. Cell Metab. 2012;15:813–826. doi: 10.1016/j.cmet.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baardman J, Verberk SGS, Prange KHM, et al. A Defective Pentose Phosphate Pathway Reduces Inflammatory Macrophage Responses during Hypercholesterolemia. Cell Rep. 2018;25:2044–2052 e2045. doi: 10.1016/j.celrep.2018.10.092. [DOI] [PubMed] [Google Scholar]
- 59.Galvan-Pena S, O’Neill LA. Metabolic reprograming in macrophage polarization. Front Immunol. 2014;5:420. doi: 10.3389/fimmu.2014.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clementi E, Brown GC, Feelisch M, Moncada S. Persistent inhibition of cell respiration by nitric oxide: crucial role of S-nitrosylation of mitochondrial complex I and protective action of glutathione. Proc Natl Acad Sci U S A. 1998;95:7631–7636. doi: 10.1073/pnas.95.13.7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Heming M, Gran S, Jauch SL, et al. Peroxisome Proliferator-Activated Receptor-gamma Modulates the Response of Macrophages to Lipopolysaccharide and Glucocorticoids. Front Immunol. 2018;9:893. doi: 10.3389/fimmu.2018.00893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee JH, Phelan P, Shin M, et al. SREBP-1a-stimulated lipid synthesis is required for macrophage phagocytosis downstream of TLR4-directed mTORC1. Proc Natl Acad Sci U S A. 2018;115:E12228–E12234. doi: 10.1073/pnas.1813458115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125–1131. doi: 10.1172/JCI0215593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Im SS, Yousef L, Blaschitz C, et al. Linking lipid metabolism to the innate immune response in macrophages through sterol regulatory element binding protein-1a. Cell Metab. 2011;13:540–549. doi: 10.1016/j.cmet.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schneider JG, Yang Z, Chakravarthy MV, et al. Macrophage fatty-acid synthase deficiency decreases diet-induced atherosclerosis. J Biol Chem. 2010;285:23398–23409. doi: 10.1074/jbc.M110.100321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vats D, Mukundan L, Odegaard JI, et al. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab. 2006;4:13–24. doi: 10.1016/j.cmet.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang SC, Everts B, Ivanova Y, et al. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat Immunol. 2014;15:846–855. doi: 10.1038/ni.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Malandrino MI, Fucho R, Weber M, et al. Enhanced fatty acid oxidation in adipocytes and macrophages reduces lipid-induced triglyceride accumulation and inflammation. Am J Physiol Endocrinol Metab. 2015;308:E756–769. doi: 10.1152/ajpendo.00362.2014. [DOI] [PubMed] [Google Scholar]
- 69.Nomura M, Liu J, Rovira II, et al. Fatty acid oxidation in macrophage polarization. Nat Immunol. 2016;17:216–217. doi: 10.1038/ni.3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Namgaladze D, Brune B. Fatty acid oxidation is dispensable for human macrophage IL-4-induced polarization. Biochim Biophys Acta. 20141841:1329–1335. doi: 10.1016/j.bbalip.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 71.Huang SC, Smith AM, Everts B, et al. Metabolic Reprogramming Mediated by the mTORC2-IRF4 Signaling Axis Is Essential for Macrophage Alternative Activation. Immunity. 2016;45:817–830. doi: 10.1016/j.immuni.2016.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Covarrubias AJ, Aksoylar HI, Yu J, et al. Akt-mTORC1 signaling regulates Acly to integrate metabolic input to control of macrophage activation. Elife. 2016;5:e11612. doi: 10.7554/eLife.11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang F, Zhang S, Vuckovic I, et al. Glycolytic Stimulation Is Not a Requirement for M2 Macrophage Differentiation. Cell Metab. 2018;28:463–475 e464. doi: 10.1016/j.cmet.2018.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang C, Ko B, Hensley CT, et al. Glutamine oxidation maintains the TCA cycle and cell survival during impaired mitochondrial pyruvate transport. Mol Cell. 2014;56:414–424. doi: 10.1016/j.molcel.2014.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wise DR, Thompson CB. Glutamine addiction: a new therapeutic target in cancer. Trends Biochem Sci. 2010;35:427–433. doi: 10.1016/j.tibs.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ligthart-Melis GC, van de Poll MC, Boelens PG, Dejong CH, Deutz NE, van Leeuwen PA. Glutamine is an important precursor for de novo synthesis of arginine in humans. Am J Clin Nutr. 2008;87:1282–1289. doi: 10.1093/ajcn/87.5.1282. [DOI] [PubMed] [Google Scholar]
- 77.Palmieri EM, Menga A, Martin-Perez R, et al. Pharmacologic or Genetic Targeting of Glutamine Synthetase Skews Macrophages toward an M1-like Phenotype and Inhibits Tumor Metastasis. Cell Rep. 2017;20:1654–1666. doi: 10.1016/j.celrep.2017.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mori M, Gotoh T. Arginine metabolic enzymes, nitric oxide and infection. J Nutr. 2004;134:2820S–2825S. doi: 10.1093/jn/134.10.2820S. discussion 2853S. [DOI] [PubMed] [Google Scholar]
- 79.Benoit M, Desnues B, Mege JL. Macrophage polarization in bacterial infections. J Immunol. 2008;181:3733–3739. doi: 10.4049/jimmunol.181.6.3733. [DOI] [PubMed] [Google Scholar]
- 80.Nardin A, Abastado JP. Macrophages and cancer. Front Biosci. 2008;13:3494–3505. doi: 10.2741/2944. [DOI] [PubMed] [Google Scholar]
- 81.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schreyer SA, Chua SC, Jr, LeBoeuf RC. Obesity and diabetes in TNF-alpha receptor- deficient mice. J Clin Invest. 1998;102:402–411. doi: 10.1172/JCI2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gonzalez-Gay MA, Gonzalez-Juanatey C, Vazquez-Rodriguez TR, Miranda-Filloy JA, Llorca J. Insulin resistance in rheumatoid arthritis: the impact of the anti-TNF-alpha therapy. Ann N Y Acad Sci. 2010;1193:153–159. doi: 10.1111/j.1749-6632.2009.05287.x. [DOI] [PubMed] [Google Scholar]
- 84.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pirzgalska RM, Domingos AI. Macrophages in obesity. Cell Immunol. 2018;330:183–187. doi: 10.1016/j.cellimm.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 86.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI200319246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Frikke-Schmidt H, Zamarron BF, O’Rourke RW, Sandoval DA, Lumeng CN, Seeley RJ. Weight loss independent changes in adipose tissue macrophage and T cell populations after sleeve gastrectomy in mice. Mol Metab. 2017;6:317–326. doi: 10.1016/j.molmet.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vandanmagsar B, Youm YH, Ravussin A, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Meier CA, Bobbioni E, Gabay C, Assimacopoulos-Jeannet F, Golay A, Dayer JM. IL-1 receptor antagonist serum levels are increased in human obesity: a possible link to the resistance to leptin? J Clin Endocrinol Metab. 2002;87:1184–1188. doi: 10.1210/jcem.87.3.8351. [DOI] [PubMed] [Google Scholar]
- 90.Spranger J, Kroke A, Mohlig M, et al. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes. 2003;52:812–817. doi: 10.2337/diabetes.52.3.812. [DOI] [PubMed] [Google Scholar]
- 91.Sauter NS, Schulthess FT, Galasso R, Castellani LW, Maedler K. The antiinflammatory cytokine interleukin-1 receptor antagonist protects from high-fat diet-induced hyperglycemia. Endocrinology. 2008;149:2208–2218. doi: 10.1210/en.2007-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Thomas D, Apovian C. Macrophage functions in lean and obese adipose tissue. Metabolism. 2017;72:120–143. doi: 10.1016/j.metabol.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ricardo-Gonzalez RR, Red Eagle A, Odegaard JI, et al. IL-4/STAT6 immune axis regulates peripheral nutrient metabolism and insulin sensitivity. Proc Natl Acad Sci U S A. 2010;107:22617–22622. doi: 10.1073/pnas.1009152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lesna IK, Cejkova S, Kralova A, et al. Human adipose tissue accumulation is associated with pro-inflammatory changes in subcutaneous rather than visceral adipose tissue. Nutr Diabetes. 2017;7:e264. doi: 10.1038/nutd.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Boutens L, Hooiveld GJ, Dhingra S, Cramer RA, Netea MG, Stienstra R. Unique metabolic activation of adipose tissue macrophages in obesity promotes inflammatory responses. Diabetologia. 2018;61:942–953. doi: 10.1007/s00125-017-4526-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Johnson AR, Qin Y, Cozzo AJ, et al. Metabolic reprogramming through fatty acid transport protein 1 (FATP1) regulates macrophage inflammatory potential and adipose inflammation. Mol Metab. 2016;5:506–526. doi: 10.1016/j.molmet.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shapiro H, Pecht T, Shaco-Levy R, et al. Adipose tissue foam cells are present in human obesity. J Clin Endocrinol Metab. 2013;98:1173–1181. doi: 10.1210/jc.2012-2745. [DOI] [PubMed] [Google Scholar]
- 98.Rogero MM, Calder PC. Obesity, Inflammation, Toll-Like Receptor 4 and Fatty Acids. Nutrients. 2018;10:432. doi: 10.3390/nu10040432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xu X, Grijalva A, Skowronski A, van Eijk M, Serlie MJ, Ferrante AW., Jr Obesity activates a program of lysosomal-dependent lipid metabolism in adipose tissue macrophages independently of classic activation. Cell Metab. 2013;18:816–830. doi: 10.1016/j.cmet.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hick RW, Gruver AL, Ventevogel MS, Haynes BF, Sempowski GD. Leptin selectively augments thymopoiesis in leptin deficiency and lipopolysaccharide-induced thymic atrophy. J Immunol. 2006;177:169–176. doi: 10.4049/jimmunol.177.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fernandez-Riejos P, Najib S, Santos-Alvarez J, et al. Role of leptin in the activation of immune cells. Mediators Inflamm. 20102010 doi: 10.1155/2010/568343. 568343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sheng T, Yang K. Adiponectin and its association with insulin resistance and type 2 diabetes. J Genet Genomics. 2008;35:321–326. doi: 10.1016/S1673-8527(08)60047-8. [DOI] [PubMed] [Google Scholar]
- 103.Ajuwon KM, Spurlock ME. Adiponectin inhibits LPS-induced NF-kappaB activation and IL-6 production and increases PPARgamma2 expression in adipocytes. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1220–1225. doi: 10.1152/ajpregu.00397.2004. [DOI] [PubMed] [Google Scholar]
- 104.Ohashi K, Parker JL, Ouchi N, et al. Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J Biol Chem. 2010;285:6153–6160. doi: 10.1074/jbc.M109.088708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tsatsanis C, Zacharioudaki V, Androulidaki A, et al. Adiponectin induces TNF-alpha and IL-6 in macrophages and promotes tolerance to itself and other pro-inflammatory stimuli. Biochem Biophys Res Commun. 2005;335:1254–1263. doi: 10.1016/j.bbrc.2005.07.197. [DOI] [PubMed] [Google Scholar]
- 106.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013;13:709–721. doi: 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen W, Bural GG, Torigian DA, Rader DJ, Alavi A. Emerging role of FDG-PET/CT in assessing atherosclerosis in large arteries. Eur J Nucl Med Mol Imaging. 2009;36:144–151. doi: 10.1007/s00259-008-0947-2. [DOI] [PubMed] [Google Scholar]
- 108.Folco EJ, Sheikine Y, Rocha VZ, et al. Hypoxia but not inflammation augments glucose uptake in human macrophages: Implications for imaging atherosclerosis with 18fluorine-labeled 2-deoxy-D-glucose positron emission tomography. J Am Coll Cardiol. 2011;58:603–614. doi: 10.1016/j.jacc.2011.03.044. [DOI] [PubMed] [Google Scholar]
- 109.Sarrazy V, Viaud M, Westerterp M, et al. Disruption of Glut1 in Hematopoietic Stem Cells Prevents Myelopoiesis and Enhanced Glucose Flux in Atheromatous Plaques of ApoE(−/−) Mice. Circ Res. 2016;118:1062–1077. doi: 10.1161/CIRCRESAHA.115.307599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chait A, Bornfeldt KE. Diabetes and atherosclerosis: is there a role for hyperglycemia? J Lipid Res. 2009;50(Suppl):S335–339. doi: 10.1194/jlr.R800059-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nagareddy PR, Murphy AJ, Stirzaker RA, et al. Hyperglycemia promotes myelopoiesis and impairs the resolution of atherosclerosis. Cell Metab. 2013;17:695–708. doi: 10.1016/j.cmet.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Terasaki M, Hiromura M, Mori Y, et al. Amelioration of Hyperglycemia with a Sodium-Glucose Cotransporter 2 Inhibitor Prevents Macrophage-Driven Atherosclerosis through Macrophage Foam Cell Formation Suppression in Type 1 and Type 2 Diabetic Mice. PLoS One. 2015;10:e0143396. doi: 10.1371/journal.pone.0143396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nishizawa T, Kanter JE, Kramer F, et al. Testing the role of myeloid cell glucose flux in inflammation and atherosclerosis. Cell Rep. 2014;7:356–365. doi: 10.1016/j.celrep.2014.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yang X, Li Y, Ren X, et al. Oxidative Stress-Mediated Atherosclerosis: Mechanisms and Therapies. Front Physiol. 2017;8:600. doi: 10.3389/fphys.2017.00600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang Y, Wang GZ, Rabinovitch PS, Tabas I. Macrophage mitochondrial oxidative stress promotes atherosclerosis and nuclear factor-kappaB-mediated inflammation in macrophages. Circ Res. 2014;114:421–433. doi: 10.1161/CIRCRESAHA.114.302153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cardilo-Reis L, Gruber S, Schreier SM, et al. Interleukin-13 protects from atherosclerosis and modulates plaque composition by skewing the macrophage phenotype. EMBO Mol Med. 2012;4:1072–1086. doi: 10.1002/emmm.201201374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang Y, Wang W, Wang N, Tall AR, Tabas I. Mitochondrial Oxidative Stress Promotes Atherosclerosis and Neutrophil Extracellular Traps in Aged Mice. Arterioscler Thromb Vasc Biol. 2017;37:e99–e107. doi: 10.1161/ATVBAHA.117.309580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vink A, Schoneveld AH, Lamers D, et al. HIF-1 alpha expression is associated with an atheromatous inflammatory plaque phenotype and upregulated in activated macrophages. Atherosclerosis. 2007;195:e69–75. doi: 10.1016/j.atherosclerosis.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 119.Aarup A, Pedersen TX, Junker N, et al. Hypoxia-Inducible Factor-1alpha Expression in Macrophages Promotes Development of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2016;36:1782–1790. doi: 10.1161/ATVBAHA.116.307830. [DOI] [PubMed] [Google Scholar]
- 120.Zhang QW, Liu L, Gong CY, et al. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS One. 2012;7:e50946. doi: 10.1371/journal.pone.0050946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Argyle D, Kitamura T. Targeting Macrophage-Recruiting Chemokines as a Novel Therapeutic Strategy to Prevent the Progression of Solid Tumors. Front Immunol. 2018;9:2629. doi: 10.3389/fimmu.2018.02629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (Basel) 2014;6:1670–1690. doi: 10.3390/cancers6031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193:727–740. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Quatromoni JG, Eruslanov E. Tumor-associated macrophages: function, phenotype, and link to prognosis in human lung cancer. Am J Transl Res. 2012;4:376–389. [PMC free article] [PubMed] [Google Scholar]
- 125.Liberti MV, Locasale JW. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem Sci. 2016;41:211–218. doi: 10.1016/j.tibs.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Romero-Garcia S, Moreno-Altamirano MM, Prado-Garcia H, Sanchez-Garcia FJ. Lactate Contribution to the Tumor Microenvironment: Mechanisms, Effects on Immune Cells and Therapeutic Relevance. Front Immunol. 2016;7:52. doi: 10.3389/fimmu.2016.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Penny HL, Sieow JL, Adriani G, et al. Warburg metabolism in tumor-conditioned macrophages promotes metastasis in human pancreatic ductal adenocarcinoma. Oncoimmunology. 2016;5:e1191731. doi: 10.1080/2162402X.2016.1191731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wenes M, Shang M, Di Matteo M, et al. Macrophage Metabolism Controls Tumor Blood Vessel Morphogenesis and Metastasis. Cell Metab. 2016;24:701–715. doi: 10.1016/j.cmet.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 129.Movahedi K, Laoui D, Gysemans C, et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70:5728–5739. doi: 10.1158/0008-5472.CAN-09-4672. [DOI] [PubMed] [Google Scholar]
- 130.Park JE, Dutta B, Tse SW, et al. Hypoxia-induced tumor exosomes promote M2-like macrophage polarization of infiltrating myeloid cells and microRNA-mediated metabolic shift. Oncogene. 2019 doi: 10.1038/s41388-019-0782-x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 131.Pyonteck SM, Akkari L, Schuhmacher AJ, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19:1264–1272. doi: 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Casazza A, Laoui D, Wenes M, et al. Impeding macrophage entry into hypoxic tumor areas by Sema3A/Nrp1 signaling blockade inhibits angiogenesis and restores antitumor immunity. Cancer Cell. 2013;24:695–709. doi: 10.1016/j.ccr.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 133.Ouimet M, Ediriweera HN, Gundra UM, et al. MicroRNA-33-dependent regulation of macrophage metabolism directs immune cell polarization in atherosclerosis. J Clin Invest. 2015;125:4334–4348. doi: 10.1172/JCI81676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liu M, O’Connor RS, Trefely S, Graham K, Snyder NW, Beatty GL. Metabolic rewiring of macrophages by CpG potentiates clearance of cancer cells and overcomes tumor-expressed CD47-mediated ‘don’t-eat-me’ signal. Nat Immunol. 2019;20:265–275. doi: 10.1038/s41590-018-0292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Miller AM, Asquith DL, Hueber AJ, et al. Interleukin-33 induces protective effects in adipose tissue inflammation during obesity in mice. Circ Res. 2010;107:650–658. doi: 10.1161/CIRCRESAHA.110.218867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rombaldova M, Janovska P, Kopecky J, Kuda O. Omega-3 fatty acids promote fatty acid utilization and production of pro-resolving lipid mediators in alternatively activated adipose tissue macrophages. Biochem Biophys Res Commun. 2017;490:1080–1085. doi: 10.1016/j.bbrc.2017.06.170. [DOI] [PubMed] [Google Scholar]
- 137.Furukawa S, Fujita T, Shimabukuro M, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Xu J, Chi F, Guo T, et al. NOTCH reprograms mitochondrial metabolism for proinflammatory macrophage activation. J Clin Invest. 2015;125:1579–1590. doi: 10.1172/JCI76468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jung MJ, Lee J, Shin NR, et al. Chronic Repression of mTOR Complex 2 Induces Changes in the Gut Microbiota of Diet-induced. Obese Mice Sci Rep. 2016;6:30887. doi: 10.1038/srep30887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Spann NJ, Garmire LX, McDonald JG, et al. Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell. 2012;151:138–152. doi: 10.1016/j.cell.2012.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Deng J, Lu S, Liu H, et al. Homocysteine Activates B Cells via Regulating PKM2-Dependent Metabolic Reprogramming. J Immunol. 2017;198:170–183. doi: 10.4049/jimmunol.1600613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Liao X, Sluimer JC, Wang Y, et al. Macrophage autophagy plays a protective role in advanced atherosclerosis. Cell Metab. 2012;15:545–553. doi: 10.1016/j.cmet.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]