Abstract
Natural compounds isolated from medicinal herbs and plants have immense significance in maintaining bone health. Hydrolysable tannins have been shown to possess a variety of medicinal properties including antiviral, anticancer, and anti-osteoclastogenic activities. As a part of a study on the discovery of alternative agent against skeletal diseases, we isolated a hydrolysable tannin, 2-O-digalloyl-1,3,4,6-tetra-O-galloyl- β-D-glucose (DTOGG), from Galla Rhois and examined the effect on osteoclast formation and function. We found that DTOGG significantly inhibited receptor activator of nuclear factor-κB ligand (RANKL)-induced osteoclast differentiation by downregulating the expression of the key regulator in osteoclastogenesis as well as osteoclast-related genes. Analysis of RANKL/RANK signaling revealed that DTOGG impaired activation of IκBα and p65 in the nuclear factor kappa-light-chain- enhancer of activated B cells (NF-κB) signaling pathway. Furthermore, DTOGG reduced bone resorbing activity of osteoclasts, compared to the vehicle-treated control. These results suggest that DTOGG could be a useful natural compound to manage osteoclast-mediated skeletal diseases.
Keywords: Bone resorption, DTOGG, Hydrolysable tannins, NF-κB, Osteoclast differentiation
INTRODUCTION
The elaborate equipoise between osteoblastic bone formation and osteoclastic bone resorption is essential in the maintenance of bone homeostasis. Disruption of the balance caused by abnormal osteoclast activity leads to osteolytic bone diseases. The bone-resorbing osteoclasts are multinucleated giant cells formed through several processes, including the proliferation and differentiation of monocyte/macrophage lineage precursor cells into mononuclear preosteoclasts, as well as cellular fusion of preosteoclasts (1, 2). Osteoclast formation requires macrophage colony-stimulating factor (M-CSF) and the receptor activator of NF-κB ligand (RANKL) (3). M-CSF primarily supports the proliferation and survival of osteoclast precursors, and RANKL is responsible for the differentiation and resorbing function of osteoclasts (4). RANKL-RANK interaction triggers the activation of mitogen-activated protein kinases (MAPKs), such as p38, extracellular signal-regulated kinase (ERK), and c-Jun N-terminal kinase (JNK), and NF-κB, which is followed by the induction and activation of nuclear factor of activated T-cell c1 (NFATc1) (5). Eventually, NFATc1 upregulates the expression of osteoclast-related genes, such as tartrate-resistant acid phosphatase (TRAP/ACP5), cathepsin K (CTSK), dendritic cell-specific transmembrane protein (DCSTAMP), and matrix metallopeptidases (MMP) (6). Therefore, RANKL/RANK signaling pathways have been recognized as potential therapeutic targets in the development of novel drugs to treat osteolytic bone diseases.
There is a growing interest in natural products for drug discovery, owing to their specific biological and pharmaceutical properties. Galla Rhois is the excrescence caused by aphids, and several studies have reported that Galla Rhois and its constituents exhibit biological and pharmacological activities, including antimicrobial, antibacterial, antifungal, anticancer, and anti-inflammatory activities (7, 8). Owing to its numerous medicinal activities, Galla Rhois has been used to treat bleeding, diarrhea, and coughing in the folk medicine of Korea and other Asian countries (9). In a previous study on the discovery of naturally occurring compounds as potent anti-obesity agents, we isolated seven bioactive phenolic compounds from Galla Rhois, and reported that 2-O-digalloyl- 1,3,4,6-tetra-O-galloyl-β-D-glucose (DTOGG), which is composed of six galloyl groups in the glucose core, strongly inhibits pancreatic lipase activity and adipocyte differentiation in 3T3-L1 cells (10). Epidemiological and animal studies have revealed that obesity affects bone metabolism and is closely associated with diverse musculoskeletal disorders, such as osteoarthritis, osteoporosis, and rheumatoid arthritis (11). Furthermore, body fat mass and serum lipid are risk factors for osteoporosis and related fractures (12). Xanthohumol (XN), a natural compound isolated from the hops plant, exhibits not only anti-obesity effects partly via the AMPK signaling pathway but also anti-osteoclastogenic activity through RANK/TRAF6 signaling pathways (13, 14). In addition, barley, a natural food with health benefits, has been reported to show both anti-obesity and anti-bone-resorptive effects (15). However, to date, the role of DTOGG in osteoclast differentiation has not been investigated.
To discover a natural alternative drug for osteolytic bone diseases, we examined the effect of DTOGG on RANKL-mediated osteoclastogenesis in bone marrow-derived macrophages (BMMs) and the bone-resorbing function of mature osteoclasts. We further investigated the modes of action of DTOGG on osteoclast differentiation and function.
RESULTS
Effect of DTOGG on RANKL-induced osteoclastogenesis
To clarify whether DTOGG modulates the differentiation of BMMs into osteoclasts, BMMs were treated with DTOGG in the presence of M-CSF and RANKL. As expected, M-CSF and RANKL stimulated the formation of TRAP-positive multinucleated cells in the vehicle-treated control at day 4 (Fig. 1C). However, the addition of DTOGG significantly prevented the differentiation of BMMs into osteoclasts, and an approximately 96% reduction was observed in the group treated with 10 μM DTOGG (Fig. 1D). We next evaluated the effect of DTOGG on the viability of BMMs to confirm whether the anti-osteoclastogenic effect of DTOGG was due to its cytotoxic activity. DTOGG at concentrations of up to 10 μM was not cytotoxic to murine BMMs (Fig. 1B and Supplementary Fig. 1). Hence, DTOGG may specifically inhibit osteoclast differentiation in vitro.
Fig. 1.
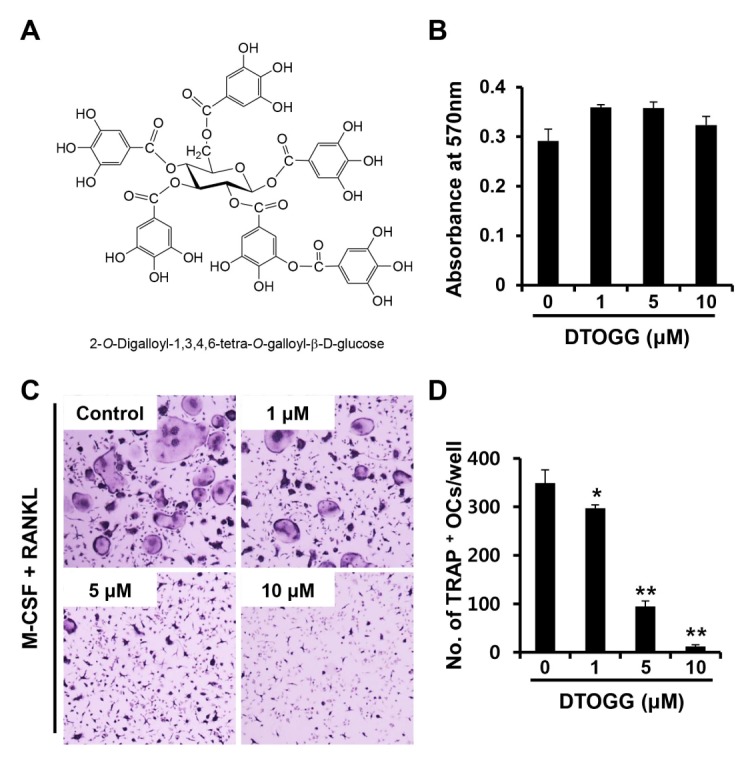
DTOGG suppressed RANKL-induced osteoclast formation. (A) Chemical structure of 2-O-digalloyl-1,3,4,6-tetra-O-galloyl-β-D-glucose (DTOGG). (B) BMMs were incubated with M-CSF (10 ng/ml) and different concentrations of DTOGG for 3 days. Cell viability was measured by the MTT assay. (C) Bone marrow-derived macrophages (BMMs) were cultured in osteoclastogenic medium containing M-CSF (10 ng/ml) and RANKL (20 ng/ml) in the presence of DTOGG (1, 5, or 10 μM) or vehicle. The cells were stained to examine TRAP activity. (D) The number of TRAP-positive osteoclasts was counted. The results are representative of three independent experiments. **P < 0.01 and *P < 0.05 versus vehicle-treated control (two-tailed Student’s t-test).
Effect of DTOGG on osteoclastic bone resorption
To investigate the effect of DTOGG on the bone-resorbing function of mature osteoclasts, we performed the resorption pit formation assay. BMMs were seeded on bone slices and differentiated into osteoclasts in osteoclast-inducing media for 3 days. Next, DTOGG at 10 μM was added to the osteoclast-inducing media for an additional day. We observed that resorbed area was significantly greater in the vehicle-treated control than in DTOGG-treated cells (Fig. 2A and 2B left graph). On the contrary, the number of osteoclasts was not changed (Fig. 2B right graph). These results suggested that DTOGG suppressed the bone-resorbing activity of osteoclasts.
Fig. 2.
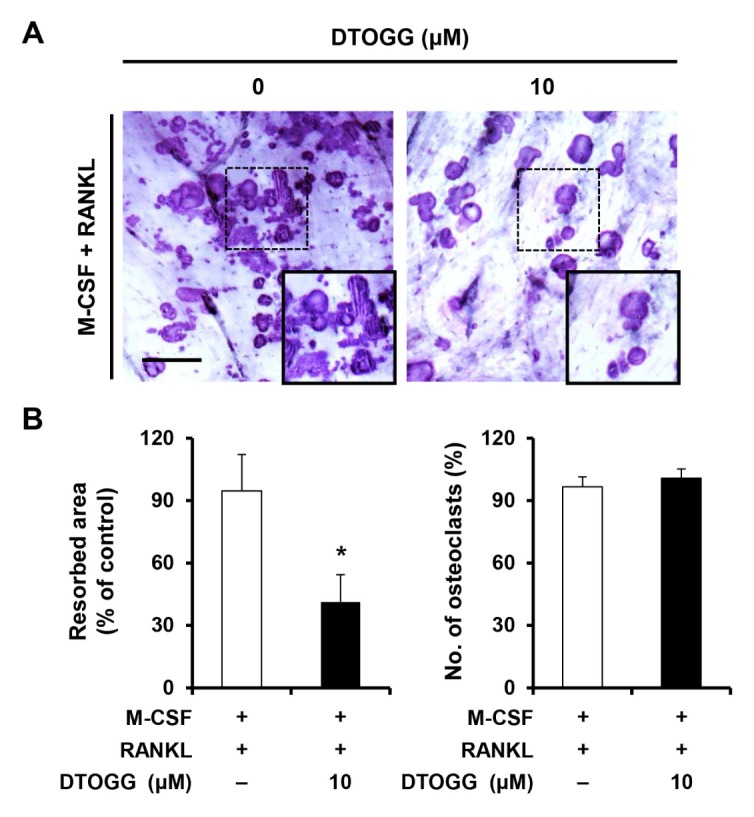
DTOGG attenuated the formation of resorption pits. (A) Bone marrow-derived macrophages were seeded onto bone slices and cultured in an osteoclastogenic medium to induce osteoclast differentiation. After 3 days, the cells were treated with DTOGG (10 μM) and incubated for 24 h. Next, the cells were washed, and resorbed areas were visualized by staining with Mayer’s hematoxylin. Scale bar, 100 μm. (B) Resorption areas (left) were quantified using the i-Solution program, and the number of multinucleated osteoclasts (right) was scored. The results are representative of two independent experiments. *P < 0.05 versus vehicle-treated control (two-tailed Student’s t-test).
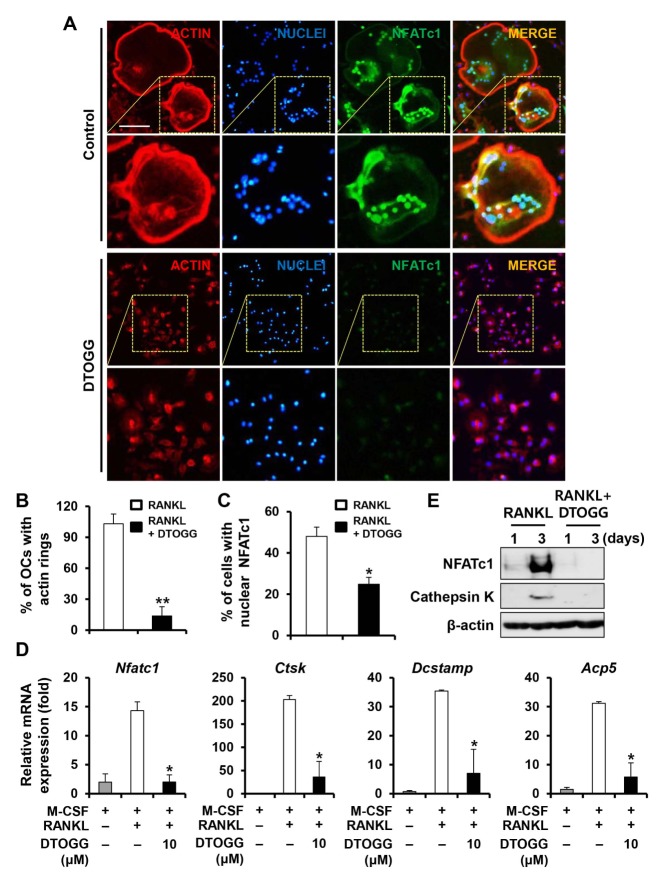
Effect of DTOGG on the formation of actin rings and expression of osteoclast marker genes
The actin ring is a morphological feature of osteoclasts and essential for osteoclastic bone resorption (16). We observed that vehicle-treated control cells formed circular actin rings around the cell periphery (Fig. 3A). On the contrary, cells treated with DTOGG hardly formed peripheral actin rings (Fig. 3A and 3B).
Fig. 3.
DTOGG inhibited actin ring formation and the mRNA expression of osteoclastic marker genes. (A) Bone marrow-derived macrophages were plated on glass coverslips and incubated with M-CSF (10 ng/ml) and RANKL (20 ng/ml) in the presence or absence of 10 μM DTOGG. Localization of NFATc1 (green) was investigated by immunostaining. Nuclei and F-actin were stained with DAPI and rhodamine-conjugated phalloidin, respectively. Yellow dashed box: magnified region. Scale bar, 50 μm. Quantitative analysis of percentage of (B) osteoclast containing actin rings and (C) cells with nuclear NFATc1. (D) The mRNA expression of NFATc1 (Nfatc1), Cathepsin K (Ctsk), DC-STAMP (Dcstamp), and TRAP (Acp5) was evaluated using real-time PCR. (E) Immunoblotting was performed to assess the protein expression levels of NFATc1 and cathepsin K. The results are representative of three independent experiments. **P < 0.01 and *P < 0.05 versus vehicle-treated control (two-tailed Student’s t-test).
Because NFATc1 acts as a major transcription factor in osteoclastogenesis and induces its target genes associated with osteoclast differentiation and function (17), we therefore investigated the effect of DTOGG on NFATc1 nuclear localization and the mRNA expression of its target genes. As shown in Fig. 3A and 3C, the number of nuclear NFATc1- positive cells was significantly reduced by DTOGG treatment. Consistent with the DTOGG-induced reduction of NFATc1 mRNA and protein levels, the mRNA expression of osteoclast-related genes, such as Acp5, Ctsk, and Dcstamp, was significantly downregulated in DTOGG-treated cells (Fig. 3D and 3E).
Effect of DTOGG on RANKL-stimulated intracellular signaling
Activation of MAPKs and NF-κB signaling pathways, two major intracellular signaling for osteoclast formation, are triggered by the binding of RANKL to its receptor, RANK (18). To examine the molecular mechanism of the anti-osteoclastogenic action of DTOGG, BMMs were pretreated with 10 μM DTOGG and stimulated with RANKL. Next, we examined the activation of MAPKs and NF-κB signaling pathways. Phosphorylation of p38, ERK, and JNK was observed in both the vehicle control and DTOGG-pretreated cells (Fig. 4A). However, RANKL-stimulated phosphorylation of IκBα and p65 was lower in cells pretreated with DTOGG than in vehicle-treated control cells (Fig. 4B). These data indicated that DTOGG exerted an anti-osteoclastogenic effect by regulating the NF-κB signaling pathway.
Fig. 4.
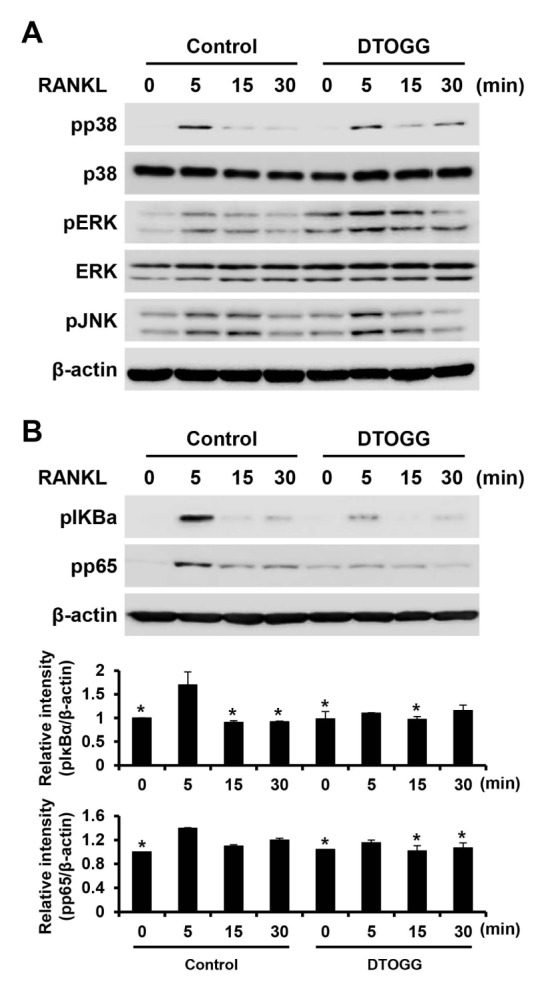
DTOGG suppressed RANKL-induced NF-κB signaling pathway. (A, B) Bone marrow-derived macrophages were cultured in a serum-free medium, pretreated with or without 10 μM DTOGG for 1 h, and stimulated with 50 ng/ml RANKL. The expression of (A) phosphorylated-p38, -ERK, -JNK, (B) -IκBα, and -p65 was analyzed by western blotting. β-actin was used as the loading control. The graphs show the quantification of band intensities for phosphorylated-IκBα and -p65 levels to β-actin. The results are representative of three independent experiments. *P < 0.05 versus vehicle-treated control (one-way ANOVA/Tukey).
DISCUSSION
Because prolonged treatment with synthetic antiresorptive drugs, including bisphosphonate, can cause serious side effects, there is a growing interest in natural products that may be more appropriate as long-term treatments for osteoclast-mediated bone disorders. A number of bioactive compounds, including flavonoids, terpenoids, polyphenols, and glucosides, which are derived from natural plant or herbs, have been identified to possess therapeutic and protective effects against bone diseases (19). These compounds downregulate RANKL-mediated osteoclast differentiation and/or bone resorption by modulating various signaling pathways and regulating the expression of critical transcription factors and osteoclast specific markers. Among them, tannins, polyphenolic compounds, have been reported to exhibit beneficial effects, such as antiviral, anticancer, and antioxidant effects (20–22). As a part of a study on the discovery of novel anti-osteoclastic drugs from natural products, we isolated DTOGG, a hydrolysable tannin, and examined its role in osteoclast formation and function.
Osteoclasts are formed from monocytes/macrophages through cell commitment and cell fusion processes (23). DTOGG significantly impaired the formation of multinucleated osteoclasts in a dose-dependent manner (Fig. 1C). Osteoclasts express specific genes required for proper osteoclast differentiation and bone-resorbing function, such as Dcstamp and Ctsk. Osteoclasts isolated from mice lacking DC-STAMP fail to fuse and form multinucleated giant cells, which leads to impaired bone-resorbing activity (24). Cathepsin K is the primary bone-degrading enzyme, and cathepsin K deficiency causes dysfunctional bone resorption, resulting in skeletal abnormalities (25). DTOGG treatment both suppressed the induction of osteoclastogenesis-associated genes in the presence of RANKL (Fig. 3D) and attenuated the formation of multinucleated osteoclasts (Fig. 1D), indicating that this compound affected osteoclast formation.
It is well established that NFATc1 is the key transcription factor of RANKL-mediated osteoclastogenesis, which encourages the expression of osteoclast-specific genes (5). MAPKs and NF-κB, downstream effector molecules of the RANKL/RANK signaling, are responsible for the induction of NFATc1 (26). Especially, deficiencies in both the p50 and p52 subunits of NF-κB leads to the development of osteopetrosis due to failure of osteoclast formation (27). A further study showed that osteoclastogenic cytokines, such as RANKL, are unable to differentiate osteoclast precursors derived from NF-κB double-knockout mice into osteoclasts (28). These reports established that NF-κB signaling is essential for the differentiation and bone-resorbing function of osteoclasts. In this study, DTOGG did not prevent the phosphorylation of MAPKs (Fig. 4A). However, the NF-κB signaling pathway was dramatically suppressed by pretreatment with DTOGG (Fig. 4B). As expected, the mRNA expression and nuclear localization of NFATc1 were significantly decreased by DTOGG (Fig. 3), suggesting that the anti-osteoclastogenic effect of DTOGG was mediated through the inhibition of NF-κB pathway.
The principal function of osteoclasts is to break down calcified bone tissues and maintain bone and mineral homeostasis, a process called bone resorption. For proper bone resorption, the formation of actin rings is required to seal bone-resorbing area (16). Through phalloidin staining, we revealed that DTOGG prevented RANKL-induced formation of actin rings (Fig. 3A). Addition of DTOGG significantly affected the bone-resorbing capacity of osteoclasts (Fig. 2), indicating that DTOGG possessed both anti-osteoclastogenic and anti-resorptive properties.
In a previous study, we reported that furosin, a hydrolysable tannin, possesses an anti-osteoclastogenic activity; at a concentration of 10 μg/ml, it significantly suppresses the differentiation of BMMs or Raw264.7 cells into osteoclasts by modulating RANKL-induced activation of p38 and JNK signaling pathways (29). However, DTOGG exerted a similar inhibitory effect on osteoclast differentiation and bone resorption at a lower concentration (10 μM) than that of furosin, indicating that DTOGG was a more effective anti-osteoclastogenic and anti-resorptive agent than furosin.
In conclusion, DTOGG suppressed RANKL-induced osteoclastogenesis and the bone-resorbing activity of osteoclasts. The key inhibitory mechanisms of DTOGG include the inhibition of RANKL-stimulated NF-κB signaling pathway and downregulation of NFATc1.
MATERIALS AND METHODS
Mice and reagents
Six-week-old C57/B6L mice were obtained from Dae Han Bio Link (Chungbuk, Korea). All animal experiments were approved by the committees on the care and use of animals in research at Kyungpook National University, and were conducted in accordance with the guidelines for the care and use of laboratory animals. Primary antibodies against phospho-p38, p38, phospho-JNK, phospho-ERK, ERK, phospho-p65, and phospho -IκBα were purchased from Cell Signaling Technology (Danvers, MA). Anti-NFATc1 antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Recombinant M-CSF and RANKL were obtained from R&D Systems (Minneapolis, MN). Fetal bovine serum (FBS) and α-minimum essential medium (α-MEM) were purchased from Gibco BRL (Grand Island, NY). 2-O-digalloyl-1,3,4,6-tetra-O-galloyl-β-D-glucose (DTOGG, Fig. 1A) was isolated from Galla Rhois collected in Asan-si, South Korea, and its extraction and isolation methods were described previously (10).
Osteoclast generation
Mouse bone marrow cells harvested from the hind leg bones were cultured in α-MEM containing 10% FBS, and differentiated into bone marrow-derived macrophages (BMMs) in α-MEM supplemented with 10% FBS and 30 ng/ml M-CSF for 3 days, as previously described (30, 31). To generate osteoclasts, BMMs were incubated in an osteoclast-inducing media containing 20 ng/ml RANKL and 10 ng/ml M-CSF in the presence or absence of various doses of DTOGG. The media were changed every 2 days until multinucleated osteoclasts formed.
Cultured cells were fixed in 4% paraformaldehyde, and osteoclast formation was determined using an Acid Phosphatase, Leukocyte (TRAP) staining kit (Sigma-Aldrich, St. Louis, MO). TRAP-positive multinucleated cells with more than 3 nuclei were counted as osteoclast-like cells.
Cell viability assay
BMMs were incubated in α-MEM containing 10% FBS and M-CSF (10 ng/ml) with or without various concentrations of DTOGG for 3 days. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was added to the cells and formazan crystals were dissolved with dimethyl sulfoxide (DMSO) as described previously (32, 33). Cell viability was determined by measuring the absorbance at 570 nm using a 96-well microplate reader (BioRad, Hercules, CA).
Real-time PCR
BMMs were cultured in 6-well plates with or without 10 μM DTOGG in an osteoclast-inducing media. Total RNA was collected using TRI-solution (Bioscience, Seoul, Korea) according to the manufacturer’s instructions, and 1 μg of RNA was converted into cDNA using SuperScript II Reverse Transcriptase (Invitrogen, Carlsbad, CA). Real-time PCR was performed using a LightCycler 1.5 real-time PCR system (Roche Diagnostics, Basel, Switzerland) and the SYBR Premix Ex Taq (Takara Bio Inc., Shiga, Japan). The following primer sets were used: Acp5, 5’-TCCCCAATGCCCCATTC-3’ and 5’-CGGTTCTGGCGATCTCTTTG-3’; Ctsk, 5’-GGCTGTGGAGGC GGCTAT-3’ and 5’-AGAGTCAATGCCTCCGTTCTG-3’; Dcstamp, 5’-CTTCCGTGGGCCAGAAGTT-3’ and 5’-AGGCCAGTGCTG ACTAGGATGA-3’; Nfatc1, 5’-ACCACCTTTCCGCAACCA-3’ and 5’-TTCCGTTTCCCGTTGCA-3’.
Western blotting analysis
Cell lysates were prepared with RIPA buffer containing protease and phosphatase inhibitors. Protein concentration was determined using the Pierce BCA protein assay kit. Proteins (25 μg) were separated by 10% SDS-PAGE. The separated proteins were transferred to a nitrocellulose membrane (Whatman, Florham Park, NJ) (34). The membrane was immersed in TBS blocking buffer [3% non-fat dry milk in TBS-T (25 mM Tris-HCl, pH 7.4; 150 mM NaCl; and 0.1% Tween 20)] for 1 h and then incubated with specific primary antibodies at 4°C overnight. The membrane was incubated with HRP-conjugated secondary antibodies for 1 h. Protein signals were detected using a WesternBright ECL kit (Advansta, Menlo Park, CA) and a chemiluminescence imager (Azure Biosystems, Inc., Dublin, CA).
Immunofluorescence staining
BMMs were plated on glass coverslips and incubated in an osteoclastogenic media containing 10 ng/ml M-CSF and 20 ng/ml RANKL with 10 μM DTOGG or vehicle. The cells were fixed with 4% paraformaldehyde and permeabilized with Triton X-100. The cells were blocked with 3% bovine serum albumin (BSA) in PBS and incubated with NFATc1 primary antibody at 4°C overnight. At the next day, the cells were incubated with Alexa Fluor-488 conjugated secondary antibodies and F-actin and nuclei were stained, respectively, with rhodamine-conjugated phalloidin (Cytoskeleton, Denver, CO) and 4’,6-diamidino-2-phenylindole dihydrochloride (DAPI; Santa Cruz Biotechnology). The cells were observed with a Leica DM 2500 fluorescence microscope (Leica Microsystems, Wetzlar, Germany).
Resorption pit assay
BMMs were plated on bone slices (IDS Nordic Bioscience, Herlev, Denmark) and cultured in the presence of M-CSF (10 ng/ml) and RANKL (20 ng/ml) for 3 days (35). Next, the cells were treated with 10 μM DTOGG or vehicle. After incubation for 24 h, the cells were removed, and resorbed pit area was visualized by hematoxylin staining and quantified using the i-Solution image analysis program (IMT i-Solution; Daejeon, Korea).
Statistical analysis
Experiments were conducted three times, and all data are expressed as mean ± standard deviation (SD) and compared by the two-tailed Student’s t-test or one-way analysis of variance (ANOVA) with Tukey’s multiple comparison post-hoc test. *P < 0.05 or **P < 0.01 was considered to be statistically significant.
SUPPLEMENTARY INFORMATION
ACKNOWLEDGEMENTS
This study was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean Government (MSIT) (2017R1A5A2015391), and the Bio & Medical Technology Development Program of the NRF, which was funded by the Korean Government (MSIT) (2017M3A9E4047244).
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Chambers TJ. Regulation of the differentiation and function of osteoclasts. J Pathol. 2000;192:4–13. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH645>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 2.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 3.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 4.Feng X, Teitelbaum SL. Osteoclasts: New Insights. Bone Res. 2013;1:11–26. doi: 10.4248/BR201301003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takayanagi H, Kim S, Koga T, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002;3:889–901. doi: 10.1016/S1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- 6.Nakashima T, Takayanagi H. New regulation mechanisms of osteoclast differentiation. Ann N Y Acad Sci. 2011;1240:E13–18. doi: 10.1111/j.1749-6632.2011.06373.x. [DOI] [PubMed] [Google Scholar]
- 7.Choi JG, Mun SH, Chahar HS, et al. Methyl gallate from Galla rhois successfully controls clinical isolates of Salmonella infection in both in vitro and in vivo systems. PLoS One. 2014;9:e102697. doi: 10.1371/journal.pone.0102697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Djakpo O, Yao W. Rhus chinensis and Galla Chinensis--folklore to modern evidence: review. Phytother Res. 2010;24:1739–1747. doi: 10.1002/ptr.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yim NH, Gu MJ, Hwang YH, Cho WK, Ma JY. Water extract of Galla Rhois with steaming process enhances apoptotic cell death in human colon cancer cells. Integr Med Res. 2016;5:284–292. doi: 10.1016/j.imr.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwon OJ, Bae JS, Lee HY, et al. Pancreatic lipase inhibitory gallotannins from Galla Rhois with inhibitory effects on adipocyte differentiation in 3T3-L1 cells. Molecules. 2013;18:10629–10638. doi: 10.3390/molecules180910629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anandacoomarasamy A, Caterson I, Sambrook P, Fransen M, March L. The impact of obesity on the musculoskeletal system. Int J Obes (Lond) 2008;32:211–222. doi: 10.1038/sj.ijo.0803715. [DOI] [PubMed] [Google Scholar]
- 12.Hsu YH, Venners SA, Terwedow HA, et al. Relation of body composition, fat mass, and serum lipids to osteoporotic fractures and bone mineral density in Chinese men and women. Am J Clin Nutr. 2006;83:146–154. doi: 10.1093/ajcn/83.1.146. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Zeng L, Xie J, et al. Inhibition of Osteoclastogenesis and Bone Resorption in vitro and in vivo by a prenylflavonoid xanthohumol from hops. Sci Rep. 2015;5:17605. doi: 10.1038/srep17605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samuels JS, Shashidharamurthy R, Rayalam S. Novel anti-obesity effects of beer hops compound xanthohumol: role of AMPK signaling pathway. Nutr Metab (Lond) 2018;15:42. doi: 10.1186/s12986-018-0277-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi SW, Kim SH, Lee KS, et al. Barley Seedling Extracts Inhibit RANKL-Induced Differentiation, Fusion, and Maturation of Osteoclasts in the Early-to-Late Stages of Osteoclastogenesis. Evid Based Complement Alternat Med. 20172017 doi: 10.1155/2017/6072573. 6072573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsubara T, Kokabu S, Nakatomi C, et al. The Actin-Binding Protein PPP1r18 Regulates Maturation, Actin Organization, and Bone Resorption Activity of Osteoclasts. Mol Cell Biol. 2018;38 doi: 10.1128/MCB.00425-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asagiri M, Sato K, Usami T, et al. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J Exp Med. 2005;202:1261–1269. doi: 10.1084/jem.20051150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wada T, Nakashima T, Hiroshi N, Penninger JM. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol Med. 2006;12:17–25. doi: 10.1016/j.molmed.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 19.An J, Hao D, Zhang Q, et al. Natural products for treatment of bone erosive diseases: The effects and mechanisms on inhibiting osteoclastogenesis and bone resorption. Int Immunopharmacol. 2016;36:118–131. doi: 10.1016/j.intimp.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 20.Cai Y, Zhang J, Chen NG, et al. Recent Advances in Anticancer Activities and Drug Delivery Systems of Tannins. Med Res Rev. 2017;37:665–701. doi: 10.1002/med.21422. [DOI] [PubMed] [Google Scholar]
- 21.Formagio AS, Volobuff CR, Santiago M, Cardoso CA, do Vieira MC, Valdevina Pereira Z. Evaluation of Antioxidant Activity, Total Flavonoids, Tannins and Phenolic Compounds in Psychotria Leaf Extracts. Antioxidants (Basel) 2014;3:745–757. doi: 10.3390/antiox3040745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ueda K, Kawabata R, Irie T, Nakai Y, Tohya Y, Sakaguchi T. Inactivation of pathogenic viruses by plant-derived tannins: strong effects of extracts from persimmon (Diospyros kaki) on a broad range of viruses. PLoS One. 2013;8:e55343. doi: 10.1371/journal.pone.0055343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yavropoulou MP, Yovos JG. Osteoclastogenesis-- current knowledge and future perspectives. J Musculoskelet Neuronal Interact. 2008;8:204–216. [PubMed] [Google Scholar]
- 24.Yagi M, Miyamoto T, Sawatani Y, et al. DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J Exp Med. 2005;202:345–351. doi: 10.1084/jem.20050645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gowen M, Lazner F, Dodds R, et al. Cathepsin K knockout mice develop osteopetrosis due to a deficit in matrix degradation but not demineralization. J Bone Miner Res. 1999;14:1654–1663. doi: 10.1359/jbmr.1999.14.10.1654. [DOI] [PubMed] [Google Scholar]
- 26.Asagiri M, Takayanagi H. The molecular understanding of osteoclast differentiation. Bone. 2007;40:251–264. doi: 10.1016/j.bone.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 27.Iotsova V, Caamano J, Loy J, Yang Y, Lewin A, Bravo R. Osteopetrosis in mice lacking NF-kappaB1 and NF-kappaB2. Nat Med. 1997;3:1285–1289. doi: 10.1038/nm1197-1285. [DOI] [PubMed] [Google Scholar]
- 28.Boyce BF, Xing L, Franzoso G, Siebenlist U. Required and nonessential functions of nuclear factor-kappa B in bone cells. Bone. 1999;25:137–139. doi: 10.1016/S8756-3282(99)00105-2. [DOI] [PubMed] [Google Scholar]
- 29.Park EK, Kim MS, Lee SH, et al. Furosin, an ellagitannin, suppresses RANKL-induced osteoclast differentiation and function through inhibition of MAP kinase activation and actin ring formation. Biochem Biophys Res Commun. 2004;325:1472–1480. doi: 10.1016/j.bbrc.2004.10.197. [DOI] [PubMed] [Google Scholar]
- 30.Ihn HJ, Lee T, Kim JA, et al. OCLI-023, a Novel Pyrimidine Compound, Suppresses Osteoclastogenesis In Vitro and Alveolar Bone Resorption In Vivo. PLoS One. 2017;12:e0170159. doi: 10.1371/journal.pone.0170159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ihn HJ, Lee T, Lee D, et al. A novel benzamide derivative protects ligature-induced alveolar bone erosion by inhibiting NFATc1-mediated osteoclastogenesis. Toxicol Appl Pharmacol. 2018;355:9–17. doi: 10.1016/j.taap.2018.06.017. [DOI] [PubMed] [Google Scholar]
- 32.Kim JA, Choi YA, Yun HS, Bae YC, Shin HI, Park EK. Extracellular calcium-binding peptide-modified ceramics stimulate regeneration of calvarial bone defects. Tissue Eng Regen Med. 2016;13:57–65. doi: 10.1007/s13770-015-9066-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Imran KM, Yoon D, Lee TJ, Kim YS. Medicarpin induces lipolysis via activation of Protein Kinase A in brown adipocytes. BMB Rep. 2018;51:249–254. doi: 10.5483/BMBRep.2018.51.5.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin SH, Kim H, Gu DR, et al. Actin-binding LIM protein 1 regulates receptor activator of NF-κB ligand-mediated osteoclast differentiation and motility. BMB Rep. 2018;51:356–361. doi: 10.5483/BMBRep.2018.51.7.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ihn HJ, Kim JA, Bae YC, Shin HI, Baek MC, Park EK. Afatinib ameliorates osteoclast differentiation and function through downregulation of RANK signaling pathways. BMB Rep. 2017;50:150–155. doi: 10.5483/BMBRep.2017.50.3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.