Abstract
The frontal cortex mediates cognitive control and motivation to shape human behavior. It is generally observed that medial frontal areas are involved in motivational aspects of behavior, whereas lateral frontal regions are involved in cognitive control. Recent models of cognitive control suggest a rostro-caudal gradient in lateral frontal regions, such that progressively more rostral (anterior) regions process more complex aspects of cognitive control. How motivation influences such a control hierarchy is still under debate. Although some researchers argue that both systems work in parallel, others argue in favor of an interaction between motivation and cognitive control. In the latter case it is yet unclear how motivation would affect the different levels of the control hierarchy. This was investigated in the present functional MRI study applying different levels of cognitive control under different motivational states (low vs high reward anticipation). Three levels of cognitive control were tested by varying rule complexity: stimulus-response mapping (low-level), flexible task updating (mid-level), and sustained cue-task associations (high-level). We found an interaction between levels of cognitive control and motivation in medial and lateral frontal subregions. Specifically, flexible updating (mid-level of control) showed the strongest beneficial effect of reward and only this level exhibited functional coupling between dopamine-rich midbrain regions and the lateral frontal cortex. These findings suggest that motivation differentially affects the levels of a control hierarchy, influencing recruitment of frontal cortical control regions depending on specific task demands.
Keywords: cognitive control, control hierarchy, fMRI, lateral frontal cortex, motivation, reward
Introduction
Our decisions are driven by multiple factors, such as attention, motivation, emotion, and cognitive control. However, how these factors interact is not well understood. In particular, it is still under debate whether motivation and cognitive control operate independently to influence decision making (Kouneiher et al., 2009) or if these cognitive systems can also interact (Dreisbach and Goschke, 2004; Pessoa and Engelmann, 2010; Aarts et al., 2011).
The lateral frontal cortex is a critical node in brain networks involved in cognitive control (Miller and Cohen, 2001; Fuster, 2004; Petrides, 2005; Duncan, 2010). Recent models of goal-directed behavior propose a hierarchical organization of the lateral frontal cortex as a function of different levels of cognitive control (Koechlin et al., 2003; Fuster, 2004; Badre and D'Esposito, 2009; Bahlmann et al., 2014). These models suggest that lower levels of cognitive control, such as choosing a specific motor response, are integrated within higher levels of cognitive control that guide behavior over longer time lags and at more complex levels of action contingency. Importantly, different levels of cognitive control are proposed to be represented in lateral frontal cortex along a rostro-caudal gradient (Koechlin et al., 2003; Badre and D'Esposito, 2007), but see (Reynolds et al., 2012; Crittenden and Duncan, 2014).
It has been suggested that positive (i.e., appetitive) motivation enhances cognitive control (Krawczyk et al., 2007; Pessoa, 2009; Jimura et al., 2010). Kouhneiher et al. (2009) tested different levels of cognitive control during varying magnitudes of motivational incentives and demonstrated that motivational processes recruited medial frontal subregions, whereas lateral frontal subregions responded to different levels of cognitive control. No behavioral or neural interactions between motivation and cognitive control were found, leading to the conclusion that motivation and cognitive control are two independent systems operating in parallel during decision making. However, these findings are incongruent with recent behavioral and neuroimaging studies suggesting that motivation has differential effects depending on the type or level of cognitive control (Dreisbach and Goschke, 2004; Rowe et al., 2007; for reviews, see Pessoa, 2009; Aarts et al., 2011). Nevertheless, these studies did not use different levels of cognitive control to engage the rostro-caudal gradient in the lateral frontal cortex.
In this study, which assesses the effect of motivation on different level of cognitive control, we tested two alternative hypotheses. First, motivation and cognitive control are modular and operate as two independent systems represented in distinct medial (motivation) and lateral (levels of cognitive control) frontal regions (Kouneiher et al., 2009; Charron and Koechlin, 2010). Alternatively, these systems interact (Dreisbach and Goschke, 2004; Pessoa and Engelmann, 2010; Aarts et al., 2011) such that motivation modifies levels of cognitive control differentially to shape human behavior and these motivation–cognition interactions can take place in both medial and lateral frontal regions. Given evidence suggesting that dopamine is critical for motivation (Berridge and Robinson, 1998) and cognitive control (Cools and D'Esposito, 2011), we also tested the hypothesis that functional connectivity between dopamine-rich midbrain regions and frontal cortex would differ as a function of motivation and levels of cognitive control.
Materials and Methods
Participants.
Twenty right-handed individuals (9 female, mean age = 22 years, SD = 3.2 years) participated in this experiment. Data from 4 additional participants were collected but excluded due to excessive movement artifacts (n = 1), poor behavioral performance (n = 2), and problems with data collection (i.e., only 3 of 6 runs were collected, n = 1). None of the participants had a history of a neurological, psychiatric, or a significant medical disorder. Informed consent was obtained from subjects in accordance with procedures approved by the Committees for Protection of Human Subjects at the University of California, Berkeley.
Experimental design.
Two different tasks were used in the present experiment (Fig. 1A). The digits 2, 4, 7, or 9 were presented on a screen. Participants either judged the parity of digits (i.e., are the digits odd or even) or the magnitude of digits (i.e., are the digits smaller or larger than 5). We chose these two tasks because they are frequently used in task-switching paradigms, they are equally difficult, and they are known to engage the lateral frontal cortex.
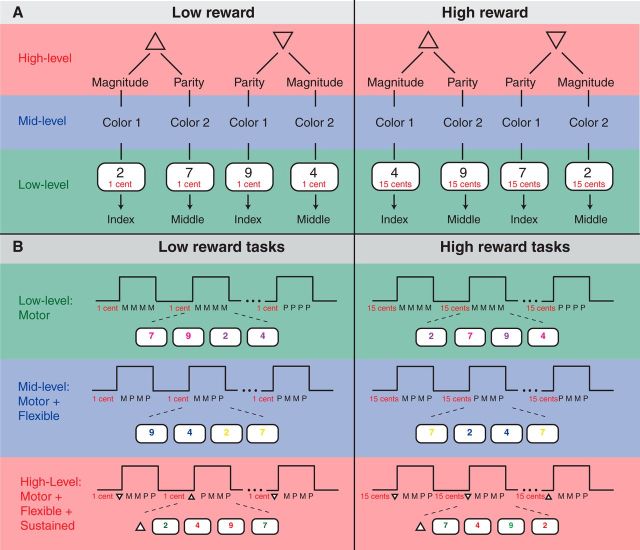
Figure 1.
Experimental design. A, Rule structure of the experiment. Digits were judged according to one of two tasks: Magnitude (>5?) and parity (odd or even?). Judgment was given with the index or middle finger. Low-level control: one task (e.g., Magnitude) was conducted in several blocks. Mid-level control: type of task was triggered by the color of the digit. Color 1 = magnitude and color 2 = parity. High-level control: An additional cue (triangle) determined the type of color-cue task association. Triangle with upward-tip: Color 1 = magnitude and color 2 = parity. Triangle with downward-tip: Color 1 = parity and color 2 = magnitude. Three levels of cognitive control were combined with low reward (left) and high reward (right). Low reward: participants earned 1 cent per correctly executed task. High reward: participants earned 15 cents. B, Examples for each of the six experimental conditions: In each condition, mini-blocks (MB) consisting of four consecutive trials were presented. Motivation cues (“1 cent” or “15 cents”) were always shown throughout the mini-block. Cognitive control level 1: Each MB comprised four magnitude (M) or four parity (P) tasks. Cognitive control level 2: M and P tasks were presented in random order in each MB. Digit-color (blue or yellow) determined the type of task. Cognitive control level 3: A triangle in front of the MB triggered the type of cue-task association. Upward-tip: green = P and red = M. Downward-tip: green = M and red = M.
Three different levels of cognitive control were tested: low, middle, and high levels of cognitive control varied as a function of rule complexity. Rule complexity refers to the manipulation of the relationship between task components (e.g., to associate a particular cue with a task) and drawing inferences about that relationship to make an appropriate response. In the present study, rule complexity was manipulated using three tasks that differed in the type of processing rules and that were nested in each other.
The low level of cognitive control was composed of a stimulus–response mapping task during which participants perceived a stimulus (digit presented on the screen) and gave a corresponding response (button press). This level of cognitive control consisted of only one rule (e.g., if stimulus A, then press button 1), therefore exhibiting the lowest rule. At this level, each trial in a block was composed of the same task. We presented the words “odd or even” or “small or large” in addition to the digits on the screen to indicate the parity block and a magnitude block. Pink or purple colors were randomly assigned to the digits because the color information was arbitrary in this condition.
At the middle level of cognitive control, participants switched between two tasks based on a color cue (e.g., blue or yellow color of a digit). This task-switching assignment comprised two rules, e.g., if color 1 and if stimulus A, then press button 1. The middle level of cognitive control exhibited increased rule complexity relative to the low level of cognitive control. At this level, the color blue or yellow of a digit indicated the magnitude or parity task.
At the high level of cognitive control, a second order cue determined which cue-task assignment was valid for a certain amount of time (i.e., over four trials). This rule maintenance task comprised of three rules, e.g., if cue-cue A, and if color 1, and if stimulus A, then press button 1. The high level of cognitive control exhibited the highest rule complexity in the present experiment. At this level, a cue-cue (a triangle pointing up or pointing down) at the beginning of a block indicated which color cue (green or red) determined the type of task. A triangle pointing up specified that the color green of a digit indicated the magnitude task and the color red of a digit indicated the parity task. In contrast, a triangle pointing down specified that the color red indicated the magnitude task and the digit color green indicated the parity task.
Rules of the three different levels of cognitive control are nested in each other such that magnitude judgment (low-level rule) is nested in the task-switching rule (mid-level rule), which is nested in the task-set maintenance rule (high-level rule).
Motivation was manipulated using a low and a high reward cue. In the high motivation condition, participants were told they could earn 15 cents for each correct trial. In the low motivation condition, participants were told they could earn 1 cent for each correct trial.
This experimental manipulation resulted in a 3 × 2 design with the factors Cognition (low, mid, and high levels) and Motivation (low and high motivation). Therefore, we generated six different conditions, namely low control and low motivation, middle control and low motivation, high control and low motivation, low control and high motivation, middle control and high motivation, and high control and high motivation conditions. We divided the experiment into several blocks (18 blocks per condition). One block was composed of four trials of one condition (e.g., low control and low motivation condition; Fig. 1B).
Procedure.
Each block started with a triangle shape (2 s). In the high level of cognitive control blocks, this shape represented the cue-cue and was either a triangle with a downward tip or an upward tip. In the middle and low level of cognitive control conditions, triangles randomly pointed either to the left or to the right (these shapes were arbitrary). Next, a digit (“2,” “4,” “7,” or “9”), a motivation cue (“1 cent” or “15 cents” in red), and a response-mapping screen (“odd/even” and “small/large”) were presented (3 s). Participants gave their response with a button press using the right-hand index (i.e., “odd/small”) or middle (i.e., “even/large”) finger. We used only the digits 2, 4, 7, and 9 because they are the only digits between 1 and 10 that can be unambiguously judged in either of the two tasks (e.g., “odd” and “small” were the same button, so only even numbers that were smaller than 5 were used). Subsequently, a feedback “correct” or “wrong” was presented (0.5 s). Finally, at the end of a block, feedback was given indicating the amount of money participants gained in a block (0.5 s). Note that the motivation cue (“1 cent” or “15 cents”) was permanently presented in a given block to ensure that participants did not have to memorize the motivation information in addition to the control task information. Each block lasted 18 s, followed by a resting block of the same duration. We divided the experiment into six sessions with 18 blocks each (three blocks per condition). Each session lasted ∼11 min. To exclude the possibility that participants applied other rules than intended in this experiment, we asked in an after-scan questionnaire: “Did you use any specific strategy to do the task?” None of the participants reported a divergent rule application.
We counterbalanced the number of task switches per block in the mid-level and high-level cognitive control conditions. Blocks were presented in randomized order. Before the testing session, training was given, starting with the low level, then the middle level, and finally the high level of cognitive control blocks. Next, blocks of the three levels of cognitive control were presented in random order. No reward cues were presented during training. Training was given to ensure that participants only entered the experiment when they reached a certain behavioral criterion (i.e., 90% correct answers in 10 consecutive trials). Training lasted ∼50 min. Preceding the test session, we informed participants about the possibility of gaining money during the experiment and gave an example of experimental blocks with motivation cues. Participants could earn maximally $28. We added the gained money to their paycheck in addition to the payment for the behavioral training ($10) and the testing session ($20) at the end of the experiment.
fMRI image acquisition.
Data were collected on a Siemens Magnetom Trio 3T MR Scanner at the Henry H. Wheeler, Jr, Brain Imaging Center at the University of California, Berkeley. A 32-channel head coil was used. Anatomical images consisted of 160 slices acquired using a T1-weighted Magnetization Prepared Rapid Acquisition Gradient Echo (MPRAGE) protocol (TR = 2300 ms; TE = 2.98 ms; FOV = 256 mm; matrix size = 256 × 256; voxel size = 1 mm3). Functional MRI scanning was performed using a T2*-weighted BOLD-sensitive gradient echo echoplanar imaging sequence (TR = 1.8 s, TE = 18 ms, α = 40°, FOV = 19.2 cm, 64 × 64 matrix, resulting in an in-plane resolution of 3 mm × 3 mm). We acquired 36 axial slices (thickness: 3 mm with 10% interslice gap) covering the whole brain (descending acquisition). Participants viewed projected stimuli via a mirror mounted on the head coil and manual responses were obtained using a fiber optic response pad. Six functional sessions with 348 volumes were collected. The fMRI experiment lasted ∼70 min.
Image processing.
MRI data were analyzed using SPM8 (available at http://www.fil.ion.ucl.ac.uk/spm). Preprocessing comprised realignment (reference scan = first slice, six parameters rigid body spatial transformation using second degree B-spline interpolation) and unwarp (fourth-degree B-spline interpolation). Next, slice-timing correction to the first slice and coregistration of the individual T1 image to the mean functional image was performed. Finally, segmentation of the individual T1 image, normalization to MNI space, and smoothing with 8 mm full-width at half-maximum Gaussian kernel was conducted. Normalizing an individual structural T1 image to the SPM8 T1 brain template was processed in two steps: segmentation of the structural T1 image into gray matter, white matter, and CSF and estimation of normalization parameters for the segmented images and writing the normalized images with these parameters. This procedure transformed the structural images and all EPI volumes into a common stereotactic space to allow for multisubject analyses. Voxel size was interpolated during preprocessing to isotropic 3 mm3.
fMRI statistical analysis.
BOLD signal change between conditions was analyzed using the general linear model approach implemented in SPM8. A block design matrix including all conditions of interest was specified using the canonical hemodynamic response function with time and dispersion derivatives. In addition, six motion parameters and sessions were modeled as covariates. Confounds of global signal changes were removed by applying a high-pass filter with a cutoff frequency of 128 s. In total, there were 18 blocks per condition, each lasting 18 s. The onset of an epoch was set to the first stimulus (i.e., triangle) in each condition. The resulting individual contrast images were submitted to the second level analysis. We conducted a 3 × 2 ANOVA (flexible factorial design implemented in SPM8) with the factor Cognition (low, middle, and high level of cognitive control) and the factor Motivation (low and high motivation). To protect against false-positive activations a correction for multiple comparisons was performed at voxel level using familywise error (FWE) correction. If not mentioned otherwise, a FWE-corrected threshold of p < 0.05 was applied. Given our a priori hypothesis of activation of medial frontal regions, we used a small volume (SV) correction for the interaction effect (Cognition × Motivation). Volumes for correction of the interaction effect were generated using the Marsbar toolbox (http://marsbar.sourceforge.net/) and anatomical ROIs based on the Automated Anatomical Labeling (AAL) atlas (Tzourio-Mazoyer et al., 2002). The second-level analysis of the interaction effect was restricted to the medial frontal region, including bilateral supplementary motor area, bilateral medial superior frontal gyrus, bilateral anterior cingulate cortex, and bilateral mid-cingulate cortex (containing 5039 voxels) based on the AAL atlas. All coordinates for peak activations were visualized with the software MRIcron (http://www.mccauslandcenter.sc.edu/mricro/mricron/).
Region of interest analysis.
The present study aimed to identify medial and lateral frontal regions that exhibit a (putative) interaction between motivation and cognitive control. To do so, we examined regions of interest (ROIs) in medial and lateral frontal subregions. To avoid the risk of circular analysis (Kriegeskorte et al., 2009), we defined ROIs in lateral frontal cortex based on a previous experiment investigating hierarchical cognitive control in lateral frontal cortex (Bahlmann et al., 2014). In that experiment, we found activity in anterior inferior frontal sulcus (aIFS, MNI coordinate: −48, 47, 13), in mid-inferior frontal sulcus (mIFS, MNI coordinates: −48, 32, 31), and in ventral premotor cortex (vPM, MNI coordinates: −45, 5, 34). These three lateral frontal regions were activated for the processing of the highest level of cognitive control (aIFS), middle level of cognitive control (mIFS), and low level of cognitive control (vPM). The ROI in medial frontal cortex (i.e., presupplementary (pre-SMA, MNI coordinates: −6, 11, 52) was generated based on the contrast of all conditions versus baseline. This contrast explores the variance explained by all experimental conditions and thus reduces the risk of circular analysis. In addition, two medial frontal ROIs derived from the study by Kouneiher et al. (2009) were generated [pre-SMA, MNI coordinates: −5, −5, 55; dorsal anterior cingulate cortex (dACC), MNI coordinates: 10, 20, 40). ROIs were generated with the Marsbar toolbox (http://marsbar.sourceforge.net/). BOLD signal change was extracted from 6 mm spherical volumes in aIFS, mIFS, vPM, and pre-SMA from each condition in each participant. Percentage of BOLD signal change was calculated using the Marsbar toolbox and applying the following steps. First, β values of the hemodynamic response function of each event were extracted. Second, a regressor was created representing the estimation of the height of the hemodynamic response function of each event using the duration of the event. Third, β values of the hemodynamic response function were multiplied with the new regressor to obtain the estimated event response for each single event. The maximum height of the reconstructed event represented the size of the response. Finally, the arbitrary value of the size of the response was scaled by the mean signal in each ROI (divide each size value by mean signal and multiply by 100). Mean signal change values were exported for analysis using SPSS. A 3 × 2 ANOVA on the percentage signal change was conducted with the factors Cognition (low, middle, and high level of cognitive control) and the factor Motivation (low and high motivation). The Greenhouse–Geisser correction (Greenhouse and Geisser, 1959) was always applied when evaluating effects with more than one degree of freedom in the numerator. In such cases, the uncorrected degrees of freedom (df), the corrected p values, and the correction factor ε are reported.
Psychophysiological interaction analysis.
To explore functional coupling between different brain regions, we conducted a psychophysiological interaction (PPI) analysis. Beyond classical GLM analysis, a PPI analysis provides insights into the correlation of time courses of distinct brain regions independent of the experimental manipulation (Friston et al., 1997). This method detects regions with activation that could be explained by the activation pattern of a seed region in interplay with a specific cognitive or sensory process. In the present study, we aimed to identify the cortical and subcortical networks that are involved in the processing of high motivation trials versus low motivation trials. Moreover, our aim was to test if three levels of cognitive control engage different networks when comparing high versus low motivation trials.
Midbrain dopaminergic neurons fire during the anticipation of reward (Schultz, 2002). Therefore, we chose the midbrain as a seed region in the PPI analysis to assess motivation-dependent connectivity. The seed region in the midbrain was determined by the contrast of all conditions versus baseline in the whole-brain random-effects analysis. For each participant, the center of the volume of interest (VOI) was set to the peak voxel (12, −25, −11) in the midbrain. Each VOI had a sphere of 6 mm radius. The design matrix of each participant comprised three PPI regressors and six movement parameters (three translation, three rotation). The physiological variable of the PPI was the first regressor. It was the time series of the first eigenvariate of the BOLD signal. The signal was high-pass filtered to account for global signal changes and mean corrected. The psychological variable was the second regressor. This was the experimental context vector convolved with the canonical hemodynamic response function. Trials of conditions with a high motivation were given the value 1, those of conditions with a low motivation were given the value −1, and those of all other conditions 0. The interaction between the physiological and psychological variable of the PPI was the third regressor. This regressor was created by deconvolving the seed VOI time series, making an element-wise product of it with the psychological variable, convolving with the canonical hemodynamic response function, and orthogonalizing with regard to the other two PPI regressors (Gitelman et al., 2003). The voxels that had a significant context-dependent increase in coupling with the seed region were identified by a t-contrast on the third PPI regressor. Individual contrast images entered the second-level random effect analysis, using one-sample t tests. Six different second-level random effect analyses were applied. We tested the effect of motivation on three levels of cognitive control, i.e., the effect of high motivation versus low motivation in low, middle, and high level of cognitive control (= three random effect analyses). The opposite effects (low motivation vs high motivation) were also tested (= another three random effect analyses). Given our hypothesis on a possible functional coupling between midbrain and lateral frontal areas, a small-volume correction was applied in the PPI analysis. The second-level analysis of the PPI analysis was restricted to left lateral frontal regions, including premotor cortex, inferior frontal gyrus, middle frontal gyrus, and middle orbito-frontal gyrus (containing 4044 voxels). Statistical inference was drawn similar to the whole-brain ANOVA (i.e., p < 0.05, FEW corrected).
Results
Behavioral data
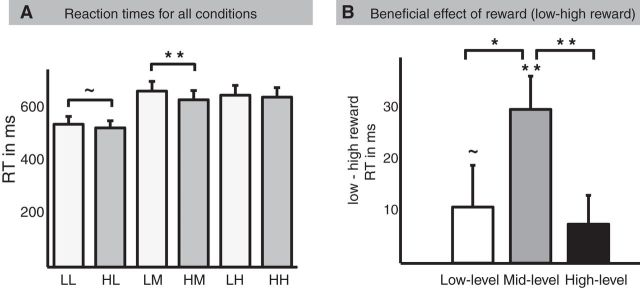
We analyzed reaction times (RT) and error rates (ER) on the factor Cognition (low, middle, high level of cognitive control) and the factor Motivation (low, high motivation). The ANOVA on RT revealed a significant Cognition by Motivation interaction (F(2,38) = 6.5, p = 0.006, ε = 0.855, Greenhouse-Geisser corrected), a main effect of Motivation (F(1,19) = 11.85, p = 0.003), and a main effect of Cognition (F(2,38) = 58.86, p < 0.001, ε = 0.919, Greenhouse-Geisser corrected). RTs of low motivation trials (mean = 620 ms, SD = 152 ms) differed from high motivation trials (mean = 602, SD = 144). RTs of low-level (mean = 534 ms, SD = 126 ms), mid-level (mean = 651 ms, SD = 160 ms), and high-level cognitive control (mean = 648 ms, SD = 163 ms) also differed. Based on the interaction effect, paired-sample t tests between low and high motivation on the three levels of cognitive control were performed (Fig. 2). We found a significant difference between low and high motivation trials in the middle level of cognitive control on RT (t(19) = 4.78, p < 0.001), demonstrating that participants were faster on high motivation trials compared with low motivation trials, suggesting a beneficial effect of motivation at this level of cognitive control. This effect was marginally significant in the low level of cognitive control on RT (t(19) = 1.87, p = 0.07) and was not significant in the high level of cognitive control. In addition, we compared the three levels of cognitive control directly on the difference of low and high motivation. These tests demonstrated significant differences of the beneficial effect of motivation between low-level and mid-level cognitive control on RT (t(19) = 2.23, p = 0.04). The beneficial effect of motivation also differed between the middle and high level of cognitive control on RT (t(19) = 4.29, p < 0.001). The ANOVA on ER revealed a significant main effect of Motivation (F(1,19) = 15.48, p = 0.001) and main effect of Cognition (F(2,38) = 14.63, p < 0.001, ε = 0.949, Greenhouse-Geisser corrected). Participants conducted fewer errors on high motivation trials (5.6% errors) than on low motivation trials (7.2% errors). They also conducted fewer errors on low-level cognitive control trials (4.1% errors) compared with mid-level cognitive control trials (7.4% errors) and high-level cognitive control trials (7.6% errors). The interaction effect between Cognition and Motivation did not reach significance (F(2,38) = 2.12, p = 0.15, ε = 0.709, Greenhouse-Geisser corrected; Table 1).
Figure 2.
Reaction times analysis. Analysis of reaction times revealed a Cognition × Motivation interaction. A, Reaction times separately for the six conditions. LL = low reward low level, HL = high reward low level, LM = low reward middle level, HM = high reward middle level, LH = low reward high level, HH = high reward high level. B, Shown here is the beneficial effect of motivation separately for the three levels of cognitive control. High reaction times differences (low minus high motivation trials) indicate faster responses for high motivation trials and thus a benefit from reward.
Table 1.
Behavioral results
| LL | HL | LM | HM | LH | HH | |
|---|---|---|---|---|---|---|
| ER in % (SD) | 4.1 (4.3) | 4.1 (5.1) | 8.8 (6.7) | 6.0 (4.5) | 8.4 (5.1) | 6.6 (5.6) |
| RT in ms (SD) | 541 (133) | 527 (121) | 667 (165) | 634 (156) | 652 (166) | 644 (161) |
LL, Low reward low level; HL, high reward low level; LM, low reward middle level; HM, high reward middle level; LH, low reward high level; HH, high reward high level.
Functional MRI data
Whole-brain analysis
We conducted a whole-brain ANOVA with the factor Cognition (low, middle, and high level of cognitive control) and Motivation (low and high motivation) as implemented in SPM8. The main effect of Cognition revealed increased activity in left lateral frontal areas, including premotor cortex, mid-inferior frontal sulcus, and anterior-inferior frontal sulcus (whole-brain corrected FWE < 0.05; see Fig. 3A, Table 2 for additional activations). The main effect of Motivation revealed increased activity in the anterior cingulate cortex, bilateral thalamus, and midbrain (whole-brain corrected FWE < 0.05; see Fig. 3A, Table 2 for additional activations). The interaction effect of Cognition and Motivation revealed increased activity in right presupplementary motor area (∼BA 6/8, MNI coordinates: 12, 20, 49, F(2,95) = 10.83, Zmax = 3.85) and left medial superior frontal gyrus, expanding into anterior cingulate cortex (∼BA 8/32, MNI coordinates: −9, 23, 43, F(2,95) = 10.2, Zmax = 3.73), values were SV-FEW corrected.
Figure 3.
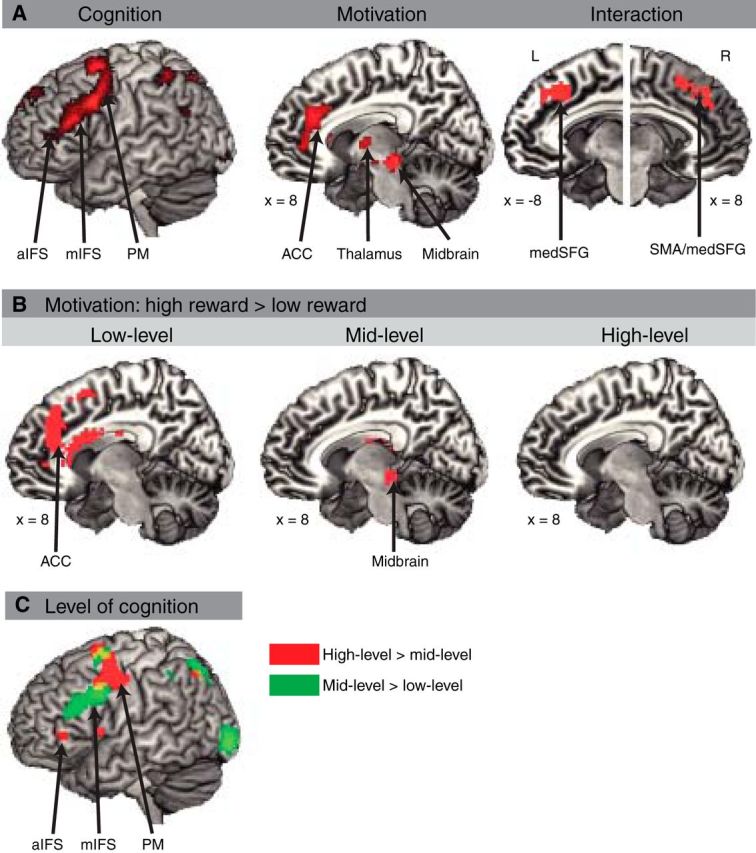
Activation pattern of whole brain analyses. A, Left, Main effect of Cognition. Each cognitive control condition collapsed across low and high motivation conditions. Middle, Main effect of Motivation. High versus low motivation collapsed across cognitive control conditions. Right, Interaction of Cognition and Motivation. Shown here and in subsequent figures are active clusters that surpassed a threshold of p < 0.001 (uncorrected) and 10 consecutive voxels per cluster. FWE-corrected values and coordinates are shown in the tables below. B, Motivation comparisons (i.e., high reward versus low reward) separately for the three levels of cognitive control. Left, Motivation effect in low-level cognitive control. Middle, Motivation effect in mid-level cognitive control. Right, Motivation effect in high-level cognitive control (no significant activation). C, Cognition comparisons collapsed across low and high motivation conditions. High-level versus mid-level (red) and mid-level versus low-level (blue) conditions are shown.
Table 2.
Whole-brain main effects
| Brain region | BA | Coordinate | F(1,95) | Zmax |
|---|---|---|---|---|
| Main effect of cognition | ||||
| L superior parietal gyrus | 7 | −24 −70 52 | 52.06 | 6.32 |
| L premotor cortex | 6 | −42 8 34 | 52.56 | 6.35 |
| L middle frontal gyrus | 8 | −30 5 61 | 50.95 | 6.26 |
| L inferior frontal sulcus | 44/45 | −45 26 22 | 38.85 | 5.57 |
| L supplementary motor area | 6/8 | −3 11 55 | 49.87 | 6.21 |
| R medial superior frontal gyrus | 10 | 3 62 10 | 42.18 | 5.78 |
| L anterior cingulate cortex | 32/10 | −3 50 4 | 28.91 | 4.88 |
| R calcarine sulcus | 18 | 21 −94 1 | 39.72 | 5.63 |
| L calcarine sulcus | 18 | −3 −88 4 | 29.75 | 4.94 |
| R cuneus | 17 | 6 −85 16 | 26.53 | 4.68 |
| L middle occipital gyrus | 18 | −27 −91 −2 | 33.93 | 5.25 |
| L inferior parietal gyrus | 40 | −42 −46 55 | 30.71 | 5.01 |
| L mid-cingulate cortex | 23 | 0 −22 40 | 30.4 | 4.99 |
| Main effect of motivation | ||||
| R midbrain | 12 −25 −11 | 33.8 | 5.24 | |
| R hippocampus | 27 | 21 −28 −2 | 19.07 | 4 |
| L hippocampus | 27 | −21 −28 −5 | 26.29 | 4.66 |
| R occipital lobe | 18 | 6 −94 10 | 25.8 | 4.62 |
| R thalamus | 0 −10 7 | 25.62 | 4.61 | |
| L thalamus | −18 −22 16 | 24.97 | 4.55 | |
| R anterior insula | 47 | 36 26 −5 | 24.37 | 4.5 |
| R anterior cingulate cortex | 32 | 9 35 22 | 23.71 | 4.44 |
| R middle frontal gyrus | 9/46 | 36 44 37 | 23.34 | 4.41 |
L, Left hemisphere; R, right hemisphere; p < 0.05, FWE corrected. Anatomical areas, approximate Brodmann's area (BA), mean x, y, and z Montreal Neurological Institute (MNI) coordinates, F values of whole-brain ANOVA, and maximal Z values of the significant activations are presented.
To further investigate the nature of the observed interaction effect, we conducted contrasts between high and low motivation conditions on each level of cognitive control (whole-brain corrected (FWE < 0.05). The contrast between high versus low motivation in low-level cognitive control revealed increased activity the anterior cingulate cortex and medial superior frontal gyrus. The contrast between high versus low motivation in mid-level cognitive control revealed increased activity in the right midbrain and right caudate nucleus (see Fig. 3B, Table 3 for additional activations). The contrast between high versus low motivation in high-level cognitive control revealed no differences in activity in any brain region.
Table 3.
Motivation effects
| Brain region | BA | Coordinate | t-value | Zmax |
|---|---|---|---|---|
| Low-level: high versus low motivation | ||||
| R anterior cingulate cortex | 32 | 12 38 22 | 5.34 | 4.98 |
| L middle occipital gyrus | 18/19 | −21 −91 −2 | 5.34 | 4.98 |
| L medial superior frontal gyrus/white matter | 6/48 | −24 20 28 | 5.33 | 4.98 |
| L mid-cingulate cortex | 32 | −12 8 46 | 5.08 | 4.76 |
| L premotor cortex | 6 | −30 5 46 | 5.08 | 4.76 |
| R premotor cortex | 8 | 30 5 46 | 5.06 | 4.74 |
| L anterior insula/white matter | 47 | −27 32 4 | 5.05 | 4.74 |
| Mid-level: high versus low motivation | ||||
| R midbrain | 12 −22 −11 | 4.86 | 4.58 | |
| R caudate (tail)/thalamus | 15 −13 19 | 4.49 | 4.26 | |
L, Left hemisphere; R, right hemisphere; p < 0.05, FWE corrected. Anatomical areas, approximate Brodmann's area (BA), mean x, y, and z Montreal Neurological Institute (MNI) coordinates, F values of whole-brain ANOVA, and maximal Z values of the significant activations are presented.
After the whole-brain corrected (FWE < 0.05) main effects of Cognition reported above, we tested the contrasts between two levels of control in exploratory analyses (p < 0.001 uncorrected). The contrast between high and middle levels of control revealed increased activity the left anterior inferior frontal sulcus (i.e., rostral lateral frontal cortex) and left premotor cortex. The contrast between middle and low levels revealed increased activity in mid-inferior frontal sulcus (i.e., mid-lateral frontal cortex) and premotor cortex (i.e., caudal lateral frontal cortex; see Fig. 3C, Table 4 for additional activations).
Table 4.
Cognition effects
| Brain region | BA | Coordinate | t-value | Zmax |
|---|---|---|---|---|
| High-level versus mid-level of cognitive control | ||||
| L premotor cortex | 6 | −45 2 46 | 5.25 | 4.91 |
| L presupplementary motor area | 6/8 | −3 11 58 | 5.2 | 4.86 |
| L caudate | −12 20 4 | 4.17 | 3.98 | |
| L precuneus | 7 | −9 −64 49 | 4.41 | 4.2 |
| L middle temporal gyrus | 22 | −54 −43 4 | 3.86 | 3.71 |
| L thalamus | −3 −22 16 | 3.76 | 3.62 | |
| L inferior parietal gyrus | 7 | −30 −67 46 | 3.69 | 3.55 |
| L anterior inferior frontal sulcus | 47 | −42 41 −2 | 3.37 | 3.27 |
| Mid-level versus low-level of cognitive control | ||||
| R calcarine sulcus | 18 | 21 −94 1 | 6.84 | 6.15 |
| L middle occipital gyrus | 17/18 | −27 −91 −2 | 6.78 | 6.11 |
| R inferior occipital gyrus | 17/18 | 27 −91 −8 | 6.44 | 5.85 |
| L premotor cortex | 6 | −39 8 31 | 6.44 | 5.85 |
| L mid-inferior frontal sulcus | 45 | −45 32 19 | 4.87 | 4.59 |
| L superior parietal gyrus | 7 | −24 −70 52 | 5.91 | 5.44 |
| L precuneus | 7 | −12 −73 49 | 5.62 | 5.21 |
| L premotor cortex/middle frontal gyrus | 6/8 | −30 5 64 | 5.23 | 4.89 |
L, Left hemisphere; R, right hemisphere; p < 0.05, FWE corrected. Anatomical areas, approximate Brodmann's area (BA), mean x, y, and z Montreal Neurological Institute (MNI) coordinates, F values of whole-brain ANOVA, and maximal Z values of the significant activations are presented.
Next, we investigated the nature of the Cognition and Motivation interaction by applying 2 × 2 interactions. The 2 × 2 interaction between Cognition (low, middle level of cognitive control) and Motivation (low, high motivation) revealed activation in medial frontal cortex at an uncorrected threshold (p < 0.001, uncorrected; Fig. 3D).
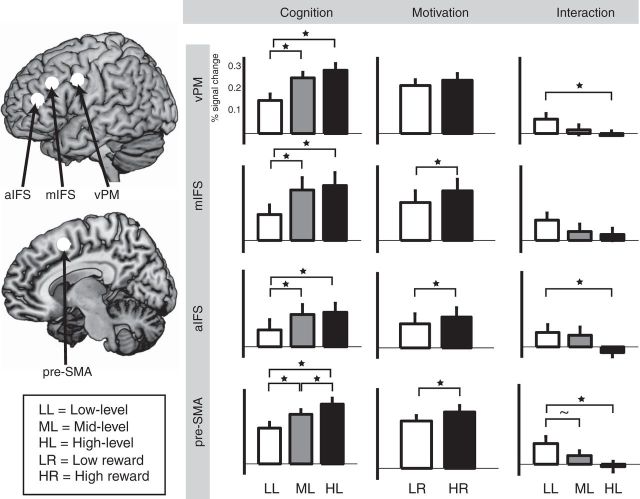
ROI analysis
ROI analysis (Fig. 4) was performed in three independently defined lateral frontal subregions, the aIFS, mIFS, and vPM, derived from a previous study investigating into a rostro-caudal gradient of cognitive control (Bahlmann et al., 2014). A medial frontal ROI in pre-SMA was generated based on the contrast of all conditions versus baseline. The ANOVA on factor Cognition (low, middle, and high level of cognitive control) and the factor Motivation (low and high motivation) revealed an interaction effect in pre-SMA (F(2,38) = 4.46, p = 0.02, ε = 0.897) and a marginally significant interaction in vPM (F(2,38) = 2.74, p = 0.08, ε = 0.946) and in aIFS (F(2,38) = 2.63, p = 0.09, ε = 0.840). Based on these interactions, paired-sample t tests were applied comparing the effect of Motivation (high vs low motivation) between the three levels of cognitive control (low, middle, and high level). We found a significant difference of the motivation effect between low-level and high-level cognitive control in pre-SMA (t(19) = 2.59, p = 0.02), in aIFS (t(19) = 2.81, p = 0.01), and in vPM (t(19) = 2.12, p = 0.04). This effect was not significant in mIFS (t(19) = 1.65, p = 0.1). In addition, in pre-SMA, the motivation effect was marginally significant between the low and middle levels of cognitive control (t(19) = 1.81, p = 0.08).
Figure 4.
ROI analyses. Percentage of BOLD signal change of ROIs in lateral frontal cortex: vPM, mIFS, aIFS, and SMA. Left, Cognition effects. Analysis of BOLD signal change for the three levels of cognitive control collapsed across low and high motivation conditions. Middle column, Motivation effects. Analysis of BOLD signal change for low and high motivation collapsed across three levels of cognitive control. Right column, Interaction of Cognition and Motivation. Difference between high and low motivation BOLD signal change separately for the three levels of cognitive control.
We also found a main effect of Motivation in pre-SMA (F(1,19) = 6.02, p = 0.02), in aIFS (F(1,19) = 4.81, p = 0.04), and in mIFS (F(1,19) = 5.71, p = 0.03). This effect did not reach significance in vPM (F(1,19) = 2.85, p = 0.1). Finally, the main effect of Cognition reached significance in all of the four ROIs: aIFS (F(2,38) = 3.19, p = 0.05, ε = 0.831), mIFS (F(2,38) = 8.46, p = 0.003, ε = 0.708), vPM (F(2,38) = 19.45, p < 0.001, ε = 0.743), and pre-SMA (F(2,38) = 20.99, p < 0.001, ε = 0.778). A comparison of the three factor steps was conducted based on this main effect. A significant difference between high and middle level of cognitive control (collapsed across motivation) was found in pre-SMA (t(19) = 3.97, p < 0.001). In addition, a significant difference between the high and low level of cognitive control was found in pre-SMA (t(19) = 6.03, p < 0.001), in mIFS (t(19) = 3.46, p = 0.003), and in vPM (t(19) = 4.88, p < 0.001). This comparison was marginally significant in aIFS (t(19) = 1.94, p = 0.07). Finally, mid-level and low-level cognitive control significantly differed in all four ROIs: pre-SMA (t(19) = 4.61, p < 0.001), aIFS (t(19) = 2.13, p = 0.04), mIFS (t(19) = 4.63, p < 0.001), and vPM (t(19) = 0.4.71, p < 0.001).
The ANOVA in medial frontal ROIs derived from the study by Kouneiher et al. (2009) revealed a main effect of Motivation in dACC (F(2,38) = 20.26, p < 0.001) and no effect in SMA (F < 2.4).
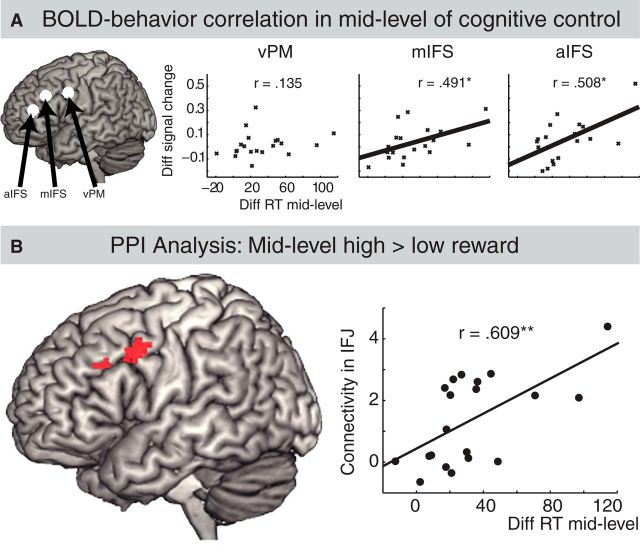
Relationship between BOLD activity and behavior
Based on the interaction between levels of cognitive control and motivation in the ROI analysis, we further investigated activity in the three lateral frontal subregions as a function of individual behavioral differences. We analyzed the relationship between the beneficial effects of motivation at mid-level control condition (reaction times differences between high and low motivation trials) and differences in BOLD signal (percentage signal change of the difference between high and low motivation trials) using a correlation analysis of individual RT values and individual BOLD changes in aIFS, mIFS, and vPM. We applied parametric (Pearson's r), as well as nonparametric (Spearman's rho and Kandell's tau) correlation coefficients. These correlations were significant in mIFS (r = 0.491, p = 0.03; rho = 0.505, p = 0.02; tau = 0.368, p = 0.03) and aIFS (r = 0.508, p = 0.02; rho = 0.558, p = 0.01; tau = 0.357, p = 0.3) but not in vPM (r = 0.135, rho = 0.09, tau = 0.08; Fig. 5A).
Figure 5.
Correlation and PPI analysis. A, Correlation between individual behavioral and BOLD signal change in mid-level of cognitive control. The x-axis shows individual (i.e., for each participant) behavioral difference between high and low motivation conditions separately for the mid-level control condition. High values relate to strong behavioral effects of motivation. The y-zxis shows individual BOLD signal difference between high and low motivation conditions separately for the mid-level control condition. High values relate to higher activity for motivation. B, PPI analysis. Left, Results of the PPI analysis on mid-level cognitive control condition (high vs low motivation) with midbrain as seed region. Note that we found no significant effect on the analysis in low-level and high-level cognitive control conditions. Right, Correlation between individual connectivity values of IFJ and individual behavioral values (i.e., reaction time differences between high and low motivation conditions in mid-level cognitive control condition).
PPI analysis
The PPI analysis aimed to identify brain regions that are functionally coupled with the midbrain, which contains dopamine neurons involved in motivational processing (Schultz, 2002). The midbrain seed was generated based on the contrast of all conditions versus baseline. We aimed to detect a possible coupling between the midbrain seed region and the lateral frontal cortex as a function of the interaction between motivation and cognitive control. To do so, six different analyses were done, namely a PPI analysis on the difference of high minus low motivation in low, middle, and high level of cognitive control and the opposite contrasts (i.e., low vs high motivation in low, middle, and high level of cognitive control). Only the contrast of high versus low motivation in mid-level cognitive control condition revealed a significant effect located in left inferior frontal junction (∼BA 6/44, MNI coordinate: −54, 8, 40, SV-FEW corrected). The inferior frontal junction is approximately located between the vPM and mIFS ROI of the lateral frontal cortex (Fig. 5B, left). Next, comparisons of individual connectivity values and individual RT values were conducted. Connectivity values were extracted from left inferior frontal junction, representing the functional coupling between this region and the midbrain seed regions. Behavioral values represent the individual beneficial effects of motivation in middle level of cognitive control; that is, reaction times differences between low and high motivation trials in middle level of cognitive control were used. Pearson product-moment coefficient analysis revealed a significant correlation between connection values and the beneficial effect of motivation in middle level of cognitive control (r = 0.609, p = 0.004; Fig. 5B, right). This effect was not significant in the low (r = 0.174) or high (r = 0.283) level of cognitive control.
Discussion
This study identified an interaction between motivation and different levels of cognitive control. The interaction was observed in both the behavioral and neural data. Behaviorally, different levels of cognitive control showed different beneficial effects of reward. We found a robust decrease of reaction times for high versus low reward anticipation at the middle level cognitive control trials, which was significantly smaller in the low- and high-level cognitive control trials. Neural correlates of the interaction between motivation and cognitive control were found in medial and lateral frontal cortex. More specifically, these regions exhibited increased activity for high versus low reward anticipation, which was stronger at the low level of cognitive control trials compared with the other two control levels. Finally, dopamine-rich midbrain regions and lateral frontal cortex were functionally coupled as a function of the beneficial effect of reward only for the mid-level cognitive control.
Previous studies have found that motivation has an enhancing effect on cognitive control. This effect is demonstrated by faster reaction times for high relative to low reward expectation (Della Libera and Chelazzi, 2006; Dreisbach, 2006), increased spiking of frontal neurons in monkey (Kobayashi et al., 2002; Roesch and Olson, 2003) and increased BOLD response in caudal lateral frontal areas in humans (Pochon et al., 2002; Taylor et al., 2004; Krawczyk et al., 2007; Engelmann et al., 2009; Jimura et al., 2010). Moreover, a recent neuroimaging study revealed a rostro-caudal gradient in medial frontal cortex as a function of different value-based control decisions (Venkatraman et al., 2009). The present study investigated the enhancing effect of motivation on cognitive control by not only manipulating differences in reward anticipation, but also by using multiple levels of cognitive control. Our whole-brain ANOVA on Cognition (low, middle, high level of cognitive control) and Motivation (low, high reward) revealed three different activation patterns. First, different levels of cognitive control activated different subregions in lateral frontal cortex. Exploratory whole-brain analyses revealed that high-level versus mid-level cognitive control aIFS and mid-level versus low-level cognitive control activated mIFS (Fig. 3C), corroborating previous studies suggesting a rostro-caudal gradient in (lateral) frontal cortex for different levels of cognitive control despite using very different tasks (Koechlin et al., 2003; Badre and D'Esposito, 2007; Venkatraman et al., 2009; Bahlmann et al., 2014). However, the statistically independent ROI analysis in aIFS did not reveal differences in hemodynamic responses between the high and middle levels (Fig. 4). Further research is needed to investigate these conflicting results. Second, a main effect of motivation was found in medial frontal cortex, subcortical regions, and the midbrain, consistent with previous studies on the anticipation of monetary reward (Knutson et al., 2003; Kirsch et al., 2003; Zink et al., 2004; Rushworth et al., 2011). Third, in our whole-brain analysis, an interaction effect between Cognition and Motivation was found in medial frontal cortex (medial superior frontal gyrus; Fig. 3A). In our ROI analyses, interaction effects were also observed in lateral frontal subregions (Fig. 4). Moreover, behaviorally, the effects of motivation were enhanced for the middle level of control especially relative to the high level of control (Fig. 2), although the difference of control complexity between these two levels is smaller compared with the difference between low and middle levels of control. Therefore, our findings are consistent with the notion that motivation shapes cognitive control dependent on the nature of the control process (Pessoa and Engelmann, 2010; Aarts et al., 2011). Accordingly, levels of cognitive control and motivation might not represent two independent and parallel processes, as was suggested previously (Kouneiher et al., 2009).
Our results demonstrated that the enhancing effect of motivation differs as a function of levels of control. Regarding specific task properties, the low level of cognitive control condition consisted of a simple S-R mapping rule. The mid-level control condition was more complex, exhibiting flexible updating of task information by using a cue-task association rule. The high-level control condition was most complex, because a second order cue determined which cue-task assignment was valid for a certain amount of time. Behaviorally, the beneficial effect of reward was significantly stronger in the middle level of control relative to the other levels (Fig. 2). This interaction between motivation and cognition might result from differences in cognitive load of the different tasks at hand. High-level control tasks may be too demanding to generate an additional enhancing effect of motivation. These higher demands on cognitive control might result from increased, ineffective performance monitoring during high-level control. Consistent with this interpretation, increased performance monitoring was shown to diminish task performance for high relative to low monetary incentives (i.e., choking under pressure was observed, Lee and Grafton, 2015). In contrast, performance of low-level control tasks might already be very high such that variance differences of RT between high and low motivation trials are no longer detectable. In terms of BOLD responses, we did observe greatest effects of motivation in the low-level control condition in our ROIs (Fig. 4), suggesting that our behavioral effects in this level indeed reflected ceiling effects of performance. Our middle level of cognitive control might have been optimal in terms of performance monitoring and room for improvement, suggesting that both too little (low-level tasks) and too high (high-level tasks) cognitive control complexity diminish the motivation effect in speed of responding (i.e., an inverted-U shape function). On the contrary, the BOLD responses showed a linear effect of motivation, suggesting that increased control (and performance monitoring) demands result in decreased effects of motivation. In addition to these hierarchical effects, the middle level of cognitive control is characterized by flexible task-set updating, which might represent an optimal cognitive task demand to generate beneficial effects of positive motivation or emotion, as was observed in earlier studies (Dreisbach and Goschke, 2004; Dreisbach, 2006; Aarts et al., 2010, 2012; van Holstein et al., 2011; van Wouwe et al., 2011). Therefore, we found a distinctive influence of motivation on the control hierarchy such that different levels of this hierarchy gain differently from motivation.
An unexpected finding is that the beneficial effect of reward in rostral frontal areas (i.e., aIFS) was highest in the low-level cognitive control condition (Fig. 4). These results suggest that low-level cognitive control tasks also engage rostral (i.e., aIFS) regions when relatively high rewards are at stake. However, the aIFS activity was still significantly lower for the low-level compared with the high-level cognitive control tasks, which is consistent with a rostro-caudal gradient in lateral frontal cortex. A speculative post hoc interpretation could be that motivation might shape the rostro-caudal organization in lateral frontal cortex such that rostral regions become engaged in lower cognitive control processes when the prospect of earning a reward is high. Therefore, rostral regions do not only represent higher control complexity, but also lower ones when motivation is high.
In the present study, individual behavioral differences of the beneficial effect of motivation predicted brain activity changes during reward anticipation only at the middle level of cognitive control in two left lateral subregions (i.e., mIFS and aIFS; Fig. 5A). Moreover, PPI analyses at each level of cognitive control as a function of motivation revealed functional coupling between a midbrain seed region and left lateral cortex (i.e., IFJ) only on the middle level of cognitive control (Fig. 5B). The midbrain seed region was chosen because this region synthesizes dopamine and is crucial for motivation (Berridge and Robinson, 1998). Dopamine is also well known to play an important role in cognitive control (Cohen et al., 2002; Bilder et al., 2004; D'Esposito, 2007), for example, during control of flexible task updating and its modulation by motivation (Aarts et al., 2011). Please note that we cannot identify a specific midbrain nucleus with the imaging technique we implemented (Eapen et al., 2011). The IFJ plays an important role in the processing of cognitive control tasks requiring flexible updating such as task-switching or Stroop tasks (for review, see Brass et al., 2005). Moreover, a recent study on the influence of genetic differences on cognitive control processing found that the density of dopamine D2 receptors is correlated with increased activity in IFJ during flexible task updating (Stelzel et al., 2010). Our results revealed functional connectivity between midbrain and IFJ as a function of motivation during flexible updating (i.e., mid-level control). This finding suggests that the dopamine-producing midbrain region that is critical for motivation is functionally coupled to the IFJ, which has been shown to be sensitive to dopamine D2 density differences during flexible updating. In addition, motivational processes during flexible updating were significantly correlated with connectivity strength between midbrain and IFJ such that stronger beneficial effects of motivation were associated with higher connectivity between midbrain and IFS (Fig. 5B). Midbrain and IFJ might be part of a network integrating motivational and cognitive processes to shape human goal-directed behavior such that motivation enhances midbrain projections within the dopaminergic brain circuit to the IFJ if flexible updating of task information is needed.
Footnotes
This work was supported by the German Research Foundation DFG (Grant BA 4871/1-1) and by the German National Academy of Sciences Leopoldina (Grant PPDS 2009-20). E.A. was supported by the Niels Stensen foundation.
The authors declare no competing financial interests.
References
- Aarts E, Roelofs A, Franke B, Rijpkema M, Fernández G, Helmich RC, Cools R. Striatal dopamine mediates the interface between motivational and cognitive control in humans: evidence from genetic imaging. Neurosychopharmacology. 2010;35:1943–1951. doi: 10.1038/npp.2010.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarts E, van Holstein M, Cools R. Striatal dopamine and the interface between motivation and cognition. Front Psychol. 2011;2:163. doi: 10.3389/fpsyg.2011.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarts E, Helmich RC, Janssen MJ, Oyen WJ, Bloem BR, Cools R. Aberrant reward processing in Parkinson's disease is associated with dopamine cell loss. Neuroimage. 2012;59:3339–3346. doi: 10.1016/j.neuroimage.2011.11.073. [DOI] [PubMed] [Google Scholar]
- Badre D, D'Esposito M. Functional magnetic resonance imaging evidence for a hierarchical organization of the prefrontal cortex. J Cogn Neurosci. 2007;19:2082–2099. doi: 10.1162/jocn.2007.19.12.2082. [DOI] [PubMed] [Google Scholar]
- Badre D, D'Esposito M. Is the rostro-caudal axis of the frontal lobe hierarchical? Nat Rev Neurosci. 2009;10:659–669. doi: 10.1038/nrn2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahlmann J, Blumenfeld RS, D'Esposito M. The rostro-caudal axis of frontal cortex is sensitive to the domain of stimulus information. Cereb Cortex. 2014 doi: 10.1093/cercor/bht419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–369. doi: 10.1016/S0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Volavka J, Lachman HM, Grace AA. The catechol-O-methyltransferase polymorphism: relations to the tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes. Neurosychopharmacology. 2004;29:1943–1961. doi: 10.1038/sj.npp.1300542. [DOI] [PubMed] [Google Scholar]
- Brass M, Derrfuss J, Forstmann B, von Cramon DY. The role of the inferior frontal junction area in cognitive control. Trends Cogn Sci. 2005;9:314–316. doi: 10.1016/j.tics.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Charron S, Koechlin E. Divided representation of concurrent goals in the human frontal lobes. Science. 2010;328:360–363. doi: 10.1126/science.1183614. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Braver TS, Brown JW. Computational perspectives on dopamine function in prefrontal cortex. Curr Opin Neurobiol. 2002;12:223–229. doi: 10.1016/S0959-4388(02)00314-8. [DOI] [PubMed] [Google Scholar]
- Cools R, D'Esposito M. Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry. 2011;69:e113–e125. doi: 10.1016/j.biopsych.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crittenden BM, Duncan J. Task difficulty manipulation reveals multiple demand activity but no frontal lobe hierarchy. Cereb Cortex. 2014;24:532–540. doi: 10.1093/cercor/bhs333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Libera C, Chelazzi L. Visual selective attention and the effects of monetary rewards. Psychol Sci. 2006;17:222–227. doi: 10.1111/j.1467-9280.2006.01689.x. [DOI] [PubMed] [Google Scholar]
- D'Esposito M. From cognitive to neural models of working memory. Philos Trans R Soc Lond B Biol Sci. 2007;362:761–772. doi: 10.1098/rstb.2007.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreisbach G. How positive affect modulates cognitive control: the costs and benefits of reduced maintenance capability. Brain Cogn. 2006;60:11–19. doi: 10.1016/j.bandc.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Dreisbach G, Goschke T. How positive affect modulates cognitive control: reduced perseveration at the cost of increased distractibility. J Exp Psychol Learn Mem Cogn. 2004;30:343–353. doi: 10.1037/0278-7393.30.2.343. [DOI] [PubMed] [Google Scholar]
- Duncan J. The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cogn Sci. 2010;14:172–179. doi: 10.1016/j.tics.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Eapen M, Zald DH, Gatenby JC, Ding Z, Gore JC. Using high-resolution MR imaging at 7T to evaluate the anatomy of the midbrain dopaminergic system. Am J Neuroradiol. 2011;32:688–694. doi: 10.3174/ajnr.A2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann JB, Damaraju E, Padmala S, Pessoa L. Combined effects of attention and motivation on visual task performance: transient and sustained motivational effects. Front Hum Neurosci. 2009;3:4. doi: 10.3389/neuro.09.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Upper processing stages of the perception-action cycle. Trends Cogn Sci. 2004;8:143–145. doi: 10.1016/j.tics.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Gitelman DR, Penny WD, Ashburner J, Friston KJ. Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution. Neuroimage. 2003;19:200–207. doi: 10.1016/S1053-8119(03)00058-2. [DOI] [PubMed] [Google Scholar]
- Greenhouse SW, Geisser S. On methods in the analysis of profile data. Psychometrika. 1959;24:95–112. doi: 10.1007/BF02289823. [DOI] [Google Scholar]
- Jimura K, Locke HS, Braver TS. Prefrontal cortex mediation of cognitive enhancement in rewarding motivational contexts. Proc Natl Acad Sci U S A. 2010;107:8871–8876. doi: 10.1073/pnas.1002007107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch P, Schienle A, Stark R, Sammer G, Blecker C, Walter B, Ott U, Burkart J, Vaitl D. Anticipation of reward in a nonaversive differential conditioning paradigm and the brain reward system: an event-related fMRI study. Neuroimage. 2003;20:1086–1095. doi: 10.1016/S1053-8119(03)00381-1. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. Neuroimage. 2003;18:263–272. doi: 10.1016/S1053-8119(02)00057-5. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Lauwereyns J, Koizumi M, Sakagami M, Hikosaka O. Influence of reward expectation on visuospatial processing in macaque lateral prefrontal cortex. J Neurophysiol. 2002;87:1488–1498. doi: 10.1152/jn.00472.2001. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science. 2003;302:1181–1185. doi: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- Kouneiher F, Charron S, Koechlin E. Motivation and cognitive control in the human prefrontal cortex. Nat Neurosci. 2009;12:939–945. doi: 10.1038/nn.2321. [DOI] [PubMed] [Google Scholar]
- Krawczyk DC, Gazzaley A, D'Esposito M. Reward modulation of prefrontal and visual association cortex during an incentive working memory task. Brain Res. 2007;1141:168–177. doi: 10.1016/j.brainres.2007.01.052. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci. 2009;12:535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TG, Grafton ST. Out of control: Diminished prefrontal activity coincides with impaired motor performance due to choking under pressure. Neuroimage. 2015;105:145–155. doi: 10.1016/j.neuroimage.2014.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Pessoa L. How do emotion and motivation direct executive control? Trends Cogn Sci. 2009;13:160–166. doi: 10.1016/j.tics.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Engelmann JB. Embedding reward signals into perception and cognition. Front Neurosci. 2010 doi: 10.3389/fnins.2010.00017. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M. Lateral prefrontal cortex: architectonic and functional organization. Philos Trans R Soc Lond B Biol Sci. 2005;360:781–795. doi: 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochon JB, Levy R, Fossati P, Lehericy S, Poline JB, Pillon B, Le Bihan D, Dubois B. The neural system that bridges reward and cognition in humans: an fMRI study. Proc Natl Acad Sci U S A. 2002;99:5669–5674. doi: 10.1073/pnas.082111099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JR, O'Reilly RC, Cohen JD, Braver TS. The function and organization of lateral prefrontal cortex: a test of competing hypotheses. PLoS One. 2012;7:e30284. doi: 10.1371/journal.pone.0030284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch MR, Olson CR. Impact of expected reward on neuronal activity in prefrontal cortex, frontal and supplementary eye fields and premotor cortex. J Neurophysiol. 2003;90:1766–1789. doi: 10.1152/jn.00019.2003. [DOI] [PubMed] [Google Scholar]
- Rowe G, Hirsh JB, Anderson AK. Positive affect increases the breadth of attentional selection. Proc Natl Acad Sci U S A. 2007;104:383–388. doi: 10.1073/pnas.0605198104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MF, Noonan MP, Boorman ED, Walton ME, Behrens TE. Frontal cortex and reward-guided learning and decision-making. Neuron. 2011;70:1054–1069. doi: 10.1016/j.neuron.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/S0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Stelzel C, Basten U, Montag C, Reuter M, Fiebach CJ. Frontostriatal involvement in task switching depends on genetic differences in d2 receptor density. J Neurosci. 2010;30:14205–14212. doi: 10.1523/JNEUROSCI.1062-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SF, Welsh RC, Wager TD, Phan KL, Fitzgerald KD, Gehring WJ. A functional neuroimaging study of motivation and executive function. Neuroimage. 2004;21:1045–1054. doi: 10.1016/j.neuroimage.2003.10.032. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Van Holstein M, Aarts E, van der Schaaf ME, Geurts DEM, Verkes RJ, Franke B, van Schouwenburg MR, Cools R. Human cognitive flexibility depends on dopamine D2 receptor signaling. Psychopharmacology (Berl) 2011;218:567–578. doi: 10.1007/s00213-011-2340-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wouwe NC, Band GP, Ridderinkhof KR. Positive affect modulates flexibility and evaluative control. J Cogn Neurosci. 2011;23:524–539. doi: 10.1162/jocn.2009.21380. [DOI] [PubMed] [Google Scholar]
- Venkatraman V, Rosati AG, Taren AA, Huettel SA. Resolving response, decision, and strategic control: evidence for a functional topography in dorsomedial prefrontal cortex. J Neurosci. 2009;29:13158–13164. doi: 10.1523/JNEUROSCI.2708-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Martin-Skurski ME, Chappelow JC, Berns GS. Human striatal responses to monetary reward depend on saliency. Neuron. 2004;42:509–517. doi: 10.1016/S0896-6273(04)00183-7. [DOI] [PubMed] [Google Scholar]