Abstract
The abnormal activation of the downstream signaling pathways of epidermal growth factor receptor (EGFR) that are independent of EGFR, contribute to the acquisition of EGFR-tyrosine kinase inhibitor (TKI) resistance in non-small cell lung cancer (NSCLC). The serine/threonine protein kinase casein kinase II (CK2) phosphorylates and modulates several members of the EGFR downstream signaling pathways. Thus, the purpose of the current study was to investigate the effects of the addition of quinalizarin (a specific CK2 inhibitor) to icotinib (an EGFR-TKI) on the proliferation and apoptosis of four NSCLC cell lines and its underlying mechanisms. The human lung adenocarcinoma cell lines HCC827, A549, H1650 and H1975 were employed to represent the EGFR-TKI-sensitive EGFR (EGFR-sensitive) mutation, wild-type EGFR and the EGFR-TKI-resistant EGFR (EGFR-resistant) mutations. The cell viability was determined by the MTT assay. Cell apoptosis was detected by flow cytom-etry using the Annexin V-enhanced green fluorescent protein Apoptosis Detection kit. The level of proteins in the EGFR downstream pathway was observed using a western blot assay. The results showed that the cells with the EGFR-sensitive mutation (HCC827, EGFR E716-A750del) were more sensitive to icotinib compared with those possessing the EGFR wild-type (A549) and the EGFR-resistant mutations (H1650, EGFR E716-A750del and PTEN lost; H1975, EGFR L858R+T790M). Quinalizarin inhibited proliferation and promoted apoptosis in the cells with the EGFR wild-type and resistant mutations, and the addition of quinalizarin to icotinib partially restored their sensitivity to icotinib. Quinalizarin and/or icotinib increased the apoptotic rates in the EGFR-TKI resistant cells, and the combination of these reduced the level of protein downstream of EGFR, including phosphorylated (p-AKT) and p-(ERK). In conclusion, quinalizarin may partially sensitize cells to icotinib by inhibiting proliferation and promoting apoptosis mediated by AKT and ERK in EGFR-TKI resistant NSCLC cell lines.
Keywords: protein kinase casein kinase II, quinalizarin, icotinib, proliferation, apoptosis
Introduction
Non-small cell lung cancer (NSCLC) accounts for approximately 85% of lung cancer cases and 10-30% of patients with NSCLC bear activating mutations in the epidermal growth factor receptor gene (EGFR) (1). A prospective, multinational PIONEER study confirmed that there is an even higher EGFR mutation frequency (51.4% overall) in tumors from Asian patients with lung adenocarcinoma compared with their Caucasian counterparts (2). Almost 75% of patients with activated EGFR mutations have a longer median overall survival and better response rates when they are treated with an EGFR-tyrosine kinase inhibitor (EGFR-TKI) compared with only traditional platinum-based chemotherapy (3-6). Regretfully, most invariably develop or 'acquire' resistance to these agents during the treatment course (7).
Icotinib (also known as BPI-2009H and Conmana) is the first oral quinazoline compound that has an established survival benefit and fewer side effects in Chinese patients with NSCLC (8,9). A network meta-analysis demonstrated that icotinib shares equivalent efficacies with erlotinib, gefitinib and afatinib, but has a lower toxicity (10). The double-blind, head-to-head phase III ICOGEN study indicated that icotinib demonstrated an improved median progression-free survival compared with gefitinib and was also associated with fewer adverse events compared gefitinib when considering all grades of reactions together (11).
By acting on signaling pathways, including PI3K-AKT-mTOR, Ras-Raf-MEK-ERK and STAT, an EGFR-TKI regulates cell proliferation, apoptosis, invasion, migration and angiogenesis (12). A growing body of evidence has elucidated the mechanism of EGFR-TKI resistance (13). Although almost half of all TKI resistance is caused by a secondary T790M mutation (14), the abnormal activation, independent of EGFR, of EGFR's downstream signaling pathways, such as PI3K-AKT-mTOR (15), also contributes to the acquisition of resistance.
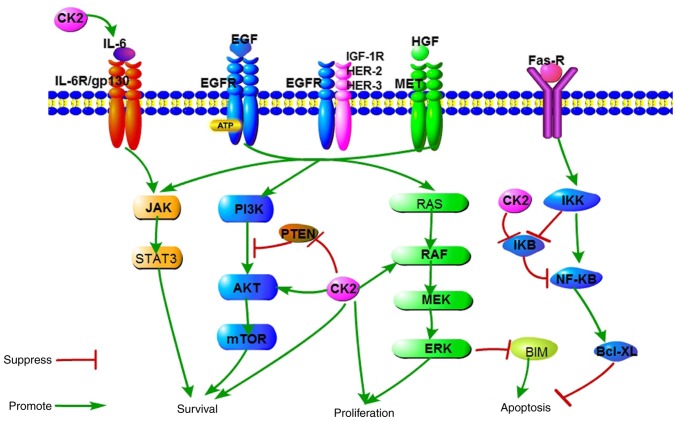
The protein kinase casein kinase II (CK2) is an evolutionary, highly conserved serine/threonine kinase that phosphorylates and interacts with more than 300 proteins (16). It is noteworthy that several members of the EGFR downstream singling pathways (Fig. 1), including PTEN, ribosomal protein S6 kinase β-1 (S6) and AKT within the PI3K-AKT-mTOR signaling pathway, have been previously reported to be phosphorylated or modulated by CK2 (17,18). Quinalizarin is known as a potent, selective and cell-permeable inhibitor of CK2 (19). A previous study revealed that quinalizarin reduced cell viability, suppressed migration and accelerated apoptosis in different human lung cancer cell lines with wild-type EGFR and EGFR-resistant mutations, as well as for those with an EGFR-sensitive mutation (20). Therefore, the authors of the current study hypothesized that a top-down inhibition of EGFR, combined with the lateral suppression of its multiple downstream pathways by targeting CK2 would create a pharmacologic synthetic lethal event and result in the resistance to EGFR-TKIs being overcome. The purpose of the current study was to investigate the effects of icotinib and quinalizarin on proliferation and apoptosis in four human lung adenocarcinoma cell lines (A549, HCC827, H1650 and H1975) with different EGFR genotypes, as well as to reveal quinalizarin's underlying mechanisms.
Figure 1.
A schematic representation of signaling pathways responsible for cell survival, proliferation and apoptosis, which are regulated by EGFR and CK2. CK2, casein kinase II; EGF, epidermal growth factor; EGFR, epidermal growth factor receptor; MEK, dual specificity mitogen-activated protein kinase kinase; IκB, NF-κ-B inhibitor; IKK, IκB kinase; BIM, Bcl-2-like protein 11; IL-6R, interleukin-6 receptor; IGF-1R, insulin-like growth factor 1 receptor; HER, receptor tyrosine-protein kinase erbB-4; HGF, hepatocyte growth factor; MET, hepatocyte growth factor receptor.
Materials and methods
Cell lines
Human lung adenocarcinoma A549 (wild-type EGFR), HCC827 (EGFR E716-A750del), NCI-H1975 (EGFR L858R+T790M), NCI-H1650 (EGFR E716-A750del and PTEN lost) cells were purchased from the American Type Culture Collection (Manassas, VA, USA) and were used within 3 months of resuscitation. The cells were cultured in RPMI 1640 supplemented with 10% fetal calf serum (FCS) and 1% penicillin/streptomycin (all Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), which will be termed culture medium henceforth, in a humidified atmosphere of 5% CO2 and 95% air at 37°C.
Reagents
Icotinib was from Betta Pharmaceuticals Co., Ltd. (Hangzhou, China). The specific CK2 inhibitor quinalizarin was purchased from Merck KGaA (Darmstadt, Germany).
Cell viability assays
For each cell line, the cells were harvested at the logarithmic phase and were then seeded into a 96-well plate at a density of 6,000 cells per well with 100 µl culture medium. The cells were cultured at 37°C with 5% CO2 overnight, and then various concentrations of icotinib and/or quinalizarin were added to each well (6.25, 12.5, 25, 50 and 100 µM), with a total of 200 µl culture medium and were further incubated for 48 h. Thereafter, 20 µl of the MTT solution (Wuhan Boster Biological Technology, Ltd., Wuhan, China) was added into each well. A total of 4 h later, 150 µl dimethyl sulfoxide (DMSO) was used to dissolve the crystals. The plates were placed on a low-speed shaker for 10 min to fully dissolve the crystals. The optical density (OD) of each well was read at a wavelength of 490 nm using a microplate reader. A cell viability curve was plotted using the concentration as the x-axis and the cell viability rate as the y-axis. The blank control group was incubated in culture medium without treatment. The values were calculated as follows: Cell survival rate=(OD value of experimental well-OD value of blank control well)/(OD value of no drug control well-OD value of blank control well) ×100%.
Each experiment was independently repeated at least three times for each cell line. The dose-effect relationship was fitted using curve regression models to obtain the 50% inhibitory concentration of 6.25, 12.5, 25, 50 and 100 µM icotinib, quinalizarin, and icotinib and quinalizarin combined by GraphPad Prism software (version 5.0; GraphPad Software, Inc., San Diego, CA, USA).
Apoptosis assays
Annexin V-enhanced green fluorescent protein (EGFP)-propidium iodide (PI) staining and flow cytometry were performed to detect cell apoptosis. Cells (1×104/cm2) in the logarithmic growth phase were plated into 12-well plates with fresh culture medium. After 24 h, for attachment, the cells were washed with PBS two to three times, and then 6.25, 12.5, 25, 50 and 100 µM icotinib and/or quinalizarin were added to EGFR-resistant cell lines. The cells were cultured with a trypsin enzyme digesting technique 48 h later and cell apoptosis was assessed using an Annexin V-EGFP Apoptosis Detection kit (cat. no. C1067M; Beyotime Institute of Biotechnology, Shanghai, China) following the product specifications.
Cell morphology
H1650, H1975 or A549 cells (1×104/cm2) were seeded into a 6-well plate for 24 h, washed with PBS two to three times, and then 6.25, 12.5, 25, 50 and 100 µM icotinib and/or quinalizarin were added. After 48 h, the cells were fixed with 4% paraformaldehyde at room temperature for 20 min, morphology was observed by a light microscope (IX71; Olympus Optical, Tokyo, Japan) at a magnification of ×100 and images were taken.
Western blot analysis
The cells (1×104/cm2) were seeded into a 6-well plate for 24 h. Then, different concentrations of drugs (50 µM icotinib or/and quinalizarin) were applied to the cells and they were further incubated 24 h. Thereafter, the cells were lysed in radioimmunoprecipitation assay buffer, phenylmethylsulfonyl fluoride and a phosphatase inhibitor cocktail, which were purchased from Wuhan Google Biological Technology Co., Ltd. (Wuhan, China). The protein concentration was measured on a microplate reader according to the bicinchoninic acid method. Protein (50 µg/lane) from the cell lysates were electrophoresed by SDS-PAGE on a 12% gel and were transferred to a polyvinylidene difluoride membrane. The membrane was blocked with 5% dry milk at room temperature for 1 h and was then incubated with anti-p-ERK (cat. no. 4370S), anti-EGFR (cat. no. ab289; Abcam, Cambridge, MA, USA), anti-CK2α (#212), anti-CK2β (#269), anti-AKT (cat. no. 1081-1; Epitomics; Abcam), anti-p-AKT (pS473; cat. no. 2118-1; Epitomics; Abcam) anti-ERK (cat. no. BS1112; Bioworld Technology, Inc., St. Louis Park, MN, USA), anti-p-EGFR (cat. no. AF3048, Affinity), anti-p-forkhead box protein O1 (FoxO1; cat. no. 9461), anti-p-S6 (cat. no. 2211; both Cell Signaling Technology, Inc.) or anti-β-actin (cat. no. sc-1616r; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) antibodies diluted at 1:1,000 in 5% bovine serum albumin (BSA; cat. no. AR1006; Wuhan Boster Biological Technology, Ltd.) at 4°C overnight. After washing with 1X PBS, the membrane was incubated with horseradish peroxidase-conjugated anti-mouse (cat. no. 5450-0011) or anti-rabbit (cat. no. 5220-0336) secondary antibodies (Kirkegaard & Perry Laboratories; SeraCare Life Sciences, Inc., Milford, MA, USA) diluted at 1:500 in 5% BSA at room temperature for 1 h. The proteins were observed with Western Blotting Luminol Reagent (sc-2048; Santa Cruz Biotechnology, Inc.). The intensity of the bands underwent densitometric analysis and they were calculated using AlphaEase FC software (version 5.0; Alpha Innotech Corporation, San Leandro, CA, USA). Anti-CK2α (#212) and anti-CK2β (#269) antibodies were generated as described previously (21) and were kind gifts from Professor Mathias Montenarh from Medical Biochemistry and Molecular Biology, Saarland University (Homburg, Germany).
Statistical analysis
Data are presented as mean ± standard deviation. All the statistical analyses were performed with two-way analysis of variance followed by Bonferroni test using GraphPad Prism software (version 5.0). Statistical diagrams were generated using GraphPad Prism software (version 5.0). P<0.05 indicated that the difference between groups was statistically significant.
Results
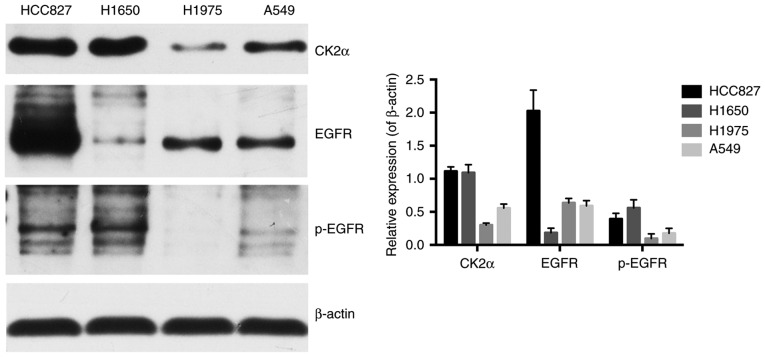
Cell lines with different EGFR genotypes have different basal expressions of CK2 and EGFR
To explore whether targeting CK2 eliminates icotinib-resistance, the basal protein expression of the catalytic CK2 subunit and EGFR were assessed in the four aforementioned lung adenocarcinoma cell lines with different EGFR genotypes, namely, A549 (wild-type EGFR), HCC827 (EGFR E716-A750del), NCI-H1975 (EGFR L858R+T790M) and NCI-H1650 (EGFR E716-A750del and PTEN lost). HCC827 has been demonstrated to be sensitive to EGFR-TKIs, while A549, NCI-H1975 and NCI-H1650 are known as EGFR-TKI resistant cell lines (22,23).
As presented in Fig. 2, the protein expression of the CK2 catalytic subunit α was strong and was clearly overexpressed in the HCC827 and NCI-H1650 cells compared with the A549 cells. It was notable that in the NCI-H1975 cells, there was markedly less CK2α protein expression compared with the other cells. Total EGFR and p-EGFR expression levels were also detected. The results revealed that EGFR expression within those four cell lines differed greatly, with the HCC827 cells containing the highest amount of total EGFR and the second highest amount of the phosphorylated form of EGFR. On the other hand, the lowest amount of total EGFR expression and the highest amount of the phosphorylated form were observed in NCI-H1650 cells. In addition, total EGFR expression in A549 and NCI-H1975 cells was relatively the same, which were markedly lower compared with in the HCC827 cells and markedly higher compared with the NCI-H1650 cells. The amount of phosphorylated form of EGFR in A549 and NCI-H1975 cells was clearly lower when compared with the HCC827 and NCI-H1650 cells, with the least amount in the NCI-H1975 cells.
Figure 2.
Cell lines with different EGFR genotypes have different basal expressions of CK2 and EGFR. Western blotting images, and quantification of CK2α, EGFR and p-EGFR protein expression. β-actin was used as a loading control. The mean ± standard deviation was calculated for three independent experiments. CK2α, casein kinase II subunit α; EGFR, epidermal growth factor receptor; p-, phosphorylated.
Sensitivity to icotinib is different in various lung adenocarcinoma cell lines and is closely associated with the EGFR genotype
To test the sensitivity of icotinib in different human lung adenocarcinoma cell lines with various EGFR genotypes, the cells were exposed to various concentrations of icotinib for 48 h. Then, the MTT assay was used to determine the cell viability. Fig. 3 demonstrates that the HCC827 cells were sensitive to icotinib, while the other three cell lines were resistant to icotinib. The sensitivity of the cells to icotinib depended on their EGFR genotypes, as described previously. Moreover, the levels of EGFR and p-EGFR were also closely related with the different EGFR genotypes. The H1975 cells, which are EGFR-mutant on both L858R and T790M, showed the lowest level of p-EGFR (Fig. 2) and the least sensitivity to the icotinib (Fig. 3).
Figure 3.
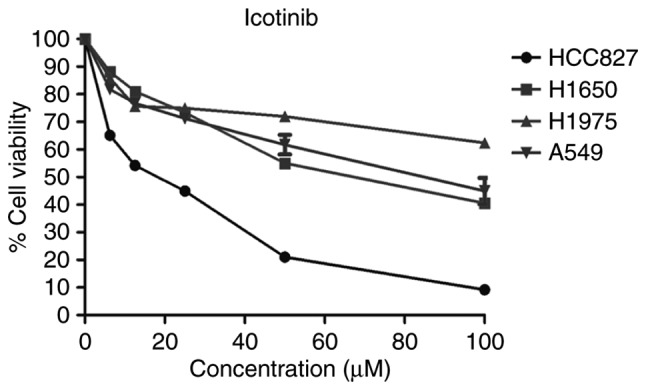
Sensitivity to icotinib is different in various lung adenocarcinoma cell lines and is closely associated with the EGFR genotype. The cells were treated with different concentrations of icotinib. Cell viability was assessed using an MTT assay after 48 h. Data are expressed as a percentage in relation to dimethyl sulfoxide. The mean ± standard deviation was calculated from three independent experiments. EGFR, epidermal growth factor receptor.
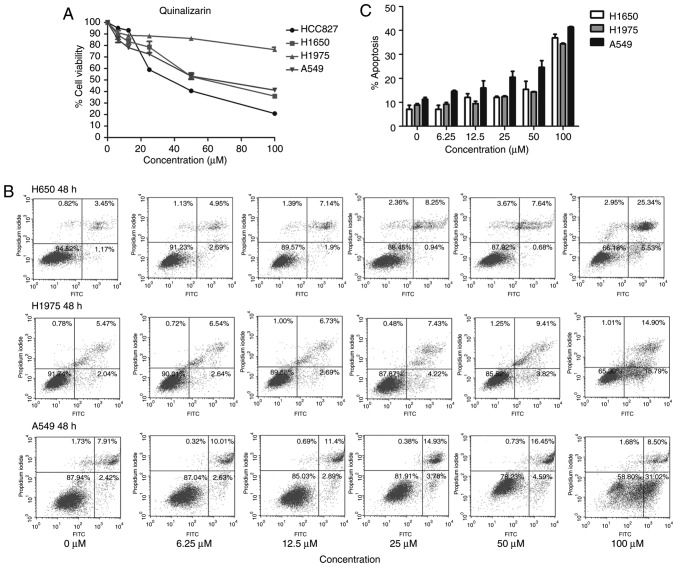
Quinalizarin inhibits the cell viability of all four cell lines and promotes apoptosis in cell lines with wild-type EGFR and EGFR-resistant mutations
The MTT assay was also used to explore the influence of quinalizarin on these four cell lines; the results showed that quinalizarin suppressed cell viability. Additionally, CK2α expression was negatively associated with cell viability (Figs. 2 and 4A). The highest inhibition rate was found in the HCC827 cells, which are known to bear an EGFR-sensitive mutation. Thereafter, the total apoptosis rates of the resistant cell lines were tested by flow cytometry after treatment with quinalizarin for 48 h (Fig. 4B). The result showed that quinalizarin promoted apoptosis in H1650, H1975 and A549 cells, with the strongest effect being shown in the A549 cells bearing the EGFR wild-type genotype (Fig. 4C).
Figure 4.
Quinalizarin inhibits the cell viability of all four cell lines and promotes apoptosis in cell lines with wild-type EGFR and EGFR-resistant mutations. The cells were treated with different concentrations of quinalizarin. (A) Cell viability was assessed using an MTT assay after 48 h. Data are expressed as a percentage in relation to dimethyl sulfoxide. (B) Cell apoptosis was measured by a flow cytometry. The lower left square represents living cells, the lower right square represents early apoptotic cells, the upper right square represents late apoptotic cells and the upper left square represents necrotic cells. The control group was treated with the equal concentration of dimethyl sulfoxide. (C) Quantification of total apoptosis rates. The mean ± standard deviation was calculated from three independent experiments. EGFR, epidermal growth factor receptor.
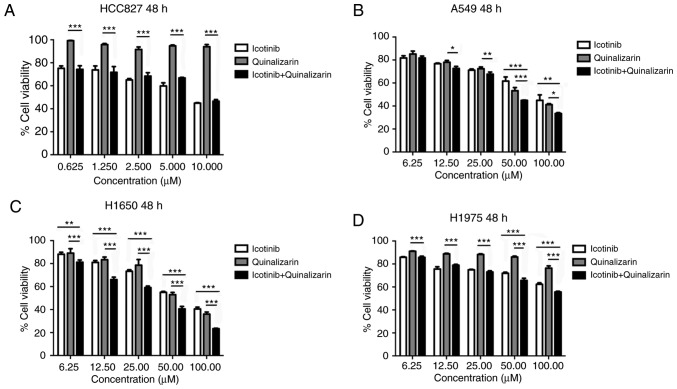
Quinalizarin enhances the suppression of cell viability mediated by icotinib in both primary and secondary drug resistant cell lines
From the above experiments, the authors of the current study found that quinalizarin and icotinib, individually, suppressed the viability of four different lung adenocarcinoma cells. The cell viability after the cells were treated with a combination of quinalizarin and icotinib was then evaluated (Fig. 5). Fig. 5A shows that quinalizarin did not enhance the reduction of cell viability mediated by icotinib in the HCC827 cells. However, quinalizarin enhanced the suppression of cell viability mediated by icotinib in A549, H1650 and H1975 cell lines, which were shown to be primary or secondary EGFR-TKI resistant cell lines. When treated with 100 µM quinalizarin and icotinib, the viability of A549 cells were significantly lower than that of cells treated with quinalizarin (P<0.05) or icotinib (P<0.01) alone (Fig. 5B). When treated with 100 µM quinalizarin and icotinib, the viability of H1650 and H1975 cells were significantly lower than that of cells treated with quinalizarin or icotinib alone (all P<0.001; Fig. 5C and D). Moreover, the suppression effect was more prominent in the H1650 cells.
Figure 5.
Quinalizarin enhances the suppression of cell viability mediated by icotinib in both primary and secondary drug resistant cell lines. The cells were treated with different concentrations of quinalizarin. The control group was treated with the equal concentration of dimethyl sulfoxide. The viability of (A) HCC827, (B) A549, (C) H1650 and (D) H1975 cells was assessed using an MTT assay after 48 h. The mean ± standard deviation was calculated from three independent experiments. (*P<0.05, **P<0.01, ***P<0.001).
Quinalizarin increases the apoptosis rate of EGFR-resistant cells when treated together with icotinib
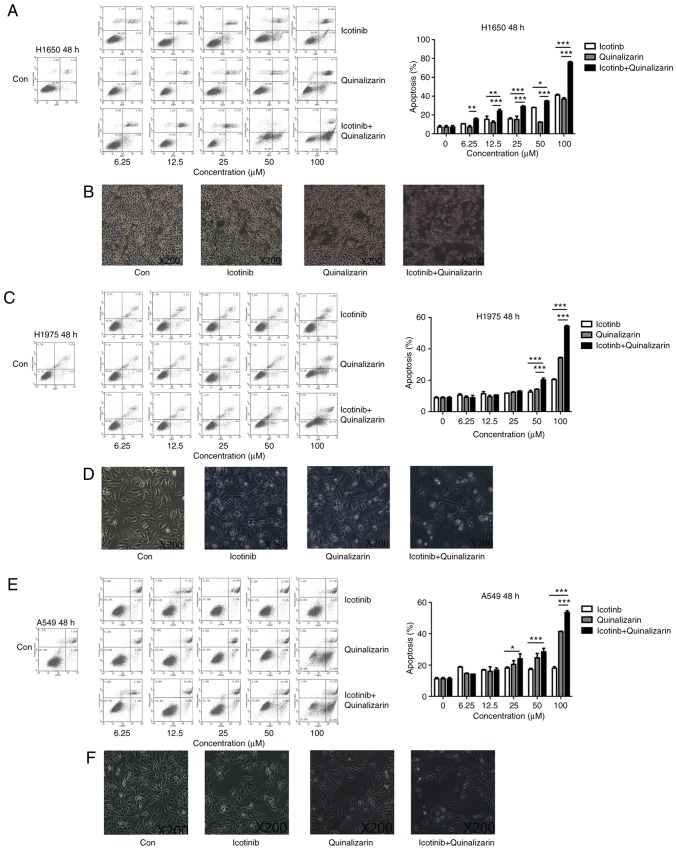
As described previously, quinalizarin enhanced the suppression of cell proliferation mediated by icotinib in both primary and secondary drug resistant cell lines. To determine how these effects occur, flow cytometry was performed to assess the apoptosis rates after the cells were treated with quinalizarin and icotinib. Fig. 6A shows that the combination of 100 µM quinalizarin and icotinib significantly increased the total apoptosis rate of the H1650 cells compared with quinalizarin or icotinib alone (both P<0.001). In addition, Fig. 6B shows that H1650 cell morphology changed after the cells were treated with both quinalizarin and icotinib. The cells treated with the combination of the drugs were small and round, and some of them showed obvious vacuolization. These changes are considered signs of cell apoptosis. Thus, the authors of the current study deduced that quinalizarin, together with icotinib, mediated cell apoptosis and increased the apoptosis rate in the H1650 cells. Similar results were also observed in H1975 (Fig. 6C and D) and A549 (Fig. 6E and F).
Figure 6.
Quinalizarin increases the apoptosis rate of EGFR-resistant cells when treated together with icotinib. Wild-type EGFR and EGFR-resistant cells were treated with different concentration of icotinib and/or quinalizairn. The control group was treated with the equal concentration of dimethyl sulf-oxide. (A) H1650 cell apoptosis was measured by a flow cytometry after 48 h; total apoptosis rates were then quantified. (B) Images of H1650 cell morphology. (C) H1975 cell apoptosis was measured by a flow cytometry after 48 h; total apoptosis rates were then quantified. (D) Images of H1975 cell morphology. (E) A549 cell apoptosis was measured by a flow cytometry after 48 h; total apoptosis rates were then quantified. (F) Images of A549 cell morphology. In the flow cytometry images, the lower left square represents living cells, the lower right square represents early apoptotic cells, the upper right square represents late apoptotic cells and the upper left square represents necrotic cells. Magnification, ×200. The mean ± standard deviation was calculated from three independent experiments. (*P<0.05, **P<0.01, ***P<0.001). EGFR, epidermal growth factor receptor.
The total apoptosis rate and cell morphology in H1650, H1975 and A549 were shown in Fig. 6. Data showed that the combination treatment enhanced the total apoptosis rate compared with quinalizarin or icotinib alone in all cell lines (P<0.0001 at 100 µM). The results also suggested that the largest increase in the total apoptosis rate was in the H1650 cells after they were treated with both drugs.
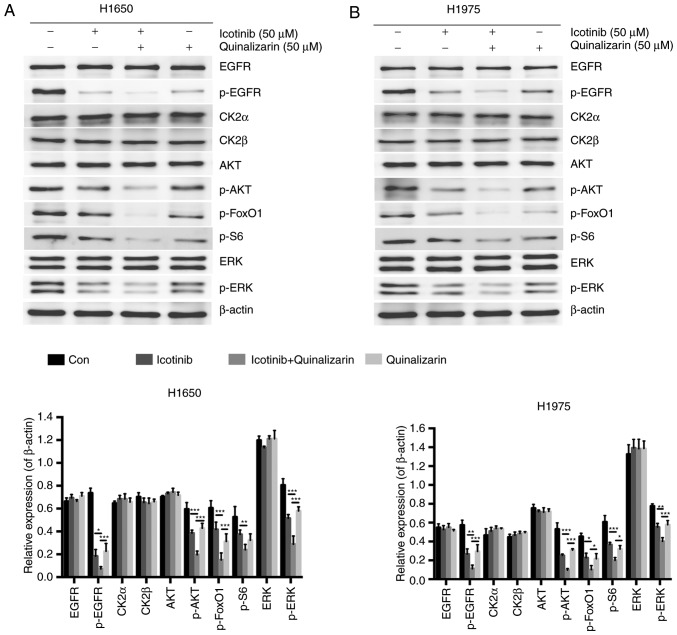
Quinalizarin and icotinib together decrease AKT and ERK protein expression in EGFR-resistant cell lines
It was shown earlier that quinalizarin may sensitize lung adenocar-cinoma cancer cells to icotinib by inhibiting cell viability and promoting apoptosis. To further explore the underlying mechanisms, a western blot analysis was performed to assess whether the combination of these two drugs resulted in a reduction of the EGFR signaling pathways. A previous study showed that the efficacy of EGFR-TKIs was partly due to the modulation of the PI3K-AKT-mTOR and the Ras-Raf-MEK-ERK signaling pathways (12). Several specific CK2 inhibitors have also already been shown to work with these two pathways (24,25). Since AKT and ERK are regarded as key members of these pathways, the authors of the current study mainly evaluated the levels of EGFR, CK2α, CK2β, AKT and ERK, and the corresponding phosphorylated forms of EGFR, AKT, ERK, FoxO1 and S6 (Fig. 7); FoxO1 and S6 are downstream of AKT (26).
Figure 7.
Quinalizarin and icotinib together decrease AKT and ERK protein expression in EGFR-resistant cell lines. EGFR-resistant cells were treated with 50 µM icotinib and/or 50 µM quinalizairn. The control group was treated with the equal concentration of dimethyl sulfoxide. Western blotting images and quantification of protein expression from (A) H1650 and (B) H1975 cells. β-actin was used as a loading control. The mean ± standard deviation was calculated for three independent experiments. (*P<0.05, **P<0.01, ***P<0.001). EGFR, epidermal growth factor receptor; CK2α/β, casein kinase II subunit α/β; p-, phosphorylated; FoxO1, forkhead box protein O1; S6, ribosomal protein S6 kinase β-1.
The results showed that p-EGFR was significantly down-regulated by combining 50 µM quinalizarin and 50 µM icotinib compared with quinalizarin alone (P<0.05 in H1650 and P<0.01 in H1975) and icotinib alone (both P<0.001). The combination treatment also significantly decreased the expression of p-AKT in both cells lines (all P<0.001), p-ERK in H1650 cells (both P<0.001) and in H1975 cells (P<0.01 for icotinib alone and P<0.001 for quinalizarin alone), p-FoxO1 in H1650 cells (both P<0.001) and in H1975 cells (both P<0.05), and p-S6 in H1650 (P<0.01 for icotinib alone) and in H1975 cells (P<0.05 for quinalizarin alone and P<0.001 for icotinib alone).
Discussion
EGFR-TKIs provide a way to improve the treatment outcomes for lethal NSCLC (4-6). However, the critical problems related to EGFR-TKIs is unavoidable drug resistance, which prevents patients from further benefits (22). The authors of the current study illustrated several ways in which EGFR-TKIs work and discussed the possible mechanisms devoted to the drug resistance.
In accordance with a great body of literature, the current study found that the protein kinase CK2 was almost connected, either indirect or directly, to all analysed pathways, including PTEN, S6 and AKT within the PI3K-AKT-mTOR signaling pathway (17,18) as well as RAF within the Ras-Raf MEK-ERK downstream pathway (27). CK2 has recently emerged as a promising target for lung cancer therapy (28). It is a ubiquitous serine/threonine protein kinase that has been demonstrated to be involved in cell growth and proliferation, as well as in inhibiting apoptosis (16,25). Other studies have revealed that the activity of CK2 is up to 2-3-fold higher in lung cancer cells compared with normal lung tissues (20,29). Hung et al (30) revealed that the CK2 inhibitor suppressed lung cancer growth in a murine xenograft model. Li et al (31) found that the CK2 inhibitor quinalizarin reduced cell proliferation and induced apoptosis in NSCLC cells. Kim and Kim (32) reported that the CK2 inhibitor CX-4945 suppressed the proliferative activity of human cancer cells. Ku et al (33) showed that CX-4945 induced cell migration and suppressed metastasis in A549 human lung cancer cells. Di Maira et al (34) demonstrated that the inhibition of CK2 reversed the multidrug resistance in a CEM cell line with a high CK2 level. Thus, the authors of the current study deduced that CK2 inhibitors might not only have antineoplastic effects but also partly reverse the EGFR-TKI-resistance of human lung adenocarcinoma cell lines.
The current study showed that the sensitivity of lung adenocarcinoma cells to icotinib was different between the cell lines and was associated with various EGFR mutation types. The cells with an EGFR-sensitive mutation (HCC827) were more sensitive to icotinib than those that possessed wild-type EGFR (A549) and those with an EGFR-resistant mutation (H1650 and H1975). The reason that icotinib prevented the tumor from growing is because it competed for EGFR binding and inhibited the downstream signaling pathways, such as PI3K-AKT-mTOR, Ras-Raf-Mek-ERK and STAT. The current study demonstrated that the downstream signaling pathways were normally overexpressed or activated in the EGFR-sensitive mutant cell line (HCC827) and these pathways play a prominent role in cell growth (35). The inhibition of these pathways showed a more remarkable anti-tumor effect and HCC827 cells were more sensitive to icotinib. The A549, which possesses wild-type EGFR, has fewer activated basal pathways and is less sensitive to EGFR-TKIs (36,37). The H1975 cell line possesses a T790M mutation that changes the binding site on EGFR and weakens the competition of EGFR-TKIs (23). The H1650 cell line does not have PTEN expression, which negatively regulates the PI3K-AKT-mTOR signaling pathway (38). In the current study, it was not surprising to find that H1975 and H1650 cells were resistant to icotinib, an EGFR-TKI.
In the current study, quinalizarin inhibited cell viabilty in the cell lines that possessed wild-type EGFR and were EGFR-resistant, as well as in the EGFR-sensitive cells. Quinalizarin also promoted apoptosis in the cell lines that possessed wild-type EGFR and were EGFR-resistant. CK2 plays an important role in the activation of the Ras-Raf-MEK-ERK signaling pathway (39,40). CK2 inhibitors suppress the PI3K-AKT-mTOR signaling pathway and promote apoptosis (24,41). The Ras-Raf-MEK-ERK and PI3K-AKT-mTOR signaling pathways are important for proliferation and apoptosis (42-44), which is why quinalizarin inhibited cell proliferation and promote apoptosis in these cells.
Additionally, in the current study, quinalizarin synergized with anti-tumor effects mediated by icotinib. The combination of quinalizarin with icotinib had a greater inhibitory effect on cell viability and further promoted apoptosis in the lung adenocarcinoma cells that were EGFR-resistant. The results of the current study were in line with the previous literature, which indicated that CK2 inhibition suppresses the PI3K-AKT-mTOR signaling pathway and promotes apoptosis by itself (41). Bliesath et al (45) insisted that the combination of EGFR-TKIs and CX-4945 shows better antitumor effects by suppressing the PI3K-AKT-mTOR signaling pathway compared with EGFR-TKI alone. In line with the study, the current results showed again that combining a CK2 inhibitor and icotinib together had better antitumor activity than icotinib alone, which might be explained by the fact that the combination treatment inhibited the activity of the PI3K-AKT-mTOR signaling pathway to a greater degree. So et al (46) reported that the CK2 inhibitor CX-4945 synergized with EGFR-TKIs on EGFR-mutant lung cancer cells (exon 19del and T790M), which were established as secondary resistant cell lines to EGFR-TKIs. In accordance with the aforementioned study, the current study demonstrated the potential ways in which quinalizarin was able to make the H1975 cells sensitive to icotinib. PTEN loss (H1650) has been shown to contribute to EGFR-TKI resistance (38) and CK2 further induces the stability of PTEN (18), which may be another way that quinalizarin acts to sensitize H1650 cells to icotinib.
Further experiments on the exact mechanism of the combination treatment were also conducted. The result showed that p-AKT and p-ERK were obviously downregulated when the cells were treated with both quinalizarin and icotinib. The Ras-Raf-MEK-ERK and PI3K-AKT-mTOR signaling pathways are main pathways that regulate cell survival and proliferation, and AKT and ERK are the key members of these two pathways. A number of studies insist that EGFR-TKIs play a pivotal role in anti-cancer therapy mainly by acting on these two signaling pathways (47,48). Similarly, in the current study, CK2 inhibition by quinalizarin also had an impact on these two signaling pathways. Thus, it is reasonable to conclude that quinalizarin may sensitize cells to icotinib by inhibiting the proliferation and promoting the apoptosis mediated by AKT and ERK in EGFR-TKI-resistant human adenocarcinoma cell lines.
Acknowledgments
The authors sincerely thank Professor Mathias Montenarh from Medical Biochemistry and Molecular Biology, Saarland University (Homburg, Germany) for his time, support and valuable suggestions throughout the development of this paper.
Funding
This study was supported by The National Natural Science Foundation of China (grant no. 81101690 to RM and grant no. 81672979 to GW), The Foundation for Fostering Key Talents from Middle-aged and Young Medical Personnel in Wuhan (2016), Natural Science Foundation of Hubei Province of China (grant no. 2016cfb426).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
GW and RM conceived and designed the experiment. KL and FZ performed the experiments. KL and YZ analyzed the data. QL and ZL interpreted the results and wrote the draft of the manuscript. SZ performed the MTT assay and LL performed part of the western blot analysis. SZ and LL wrote the final vision of the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bareschino MA, Schettino C, Rossi A, Maione P, Sacco PC, Zeppa R, Gridelli C. Treatment of advanced non small cell lung cancer. J Thorac Dis. 2011;3:122–133. doi: 10.3978/j.issn.2072-1439.2010.12.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi Y, Au JS, Thongprasert S, Srinivasan S, Tsai CM, Khoa MT, Heeroma K, Itoh Y, Cornelio G, Yang PC. A prospective, molecular epidemiology study of EGFR mutations in asian patients with advanced non-small-cell lung cancer of adeno-carcinoma histology (PIONEER) J Thorac Oncol. 2014;9:154–162. doi: 10.1097/JTO.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu YL, Chu DT, Han B, Liu X, Zhang L, Zhou C, Liao M, Mok T, Jiang H, Duffield E, Fukuoka M. Phase III, randomized, open-label, first-line study in asia of gefitinib versus carbo-platin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer: Evaluation of patients recruited from mainland China. Asia-Pac J Clin Oncol. 2012;8:232–243. doi: 10.1111/j.1743-7563.2012.01518.x. [DOI] [PubMed] [Google Scholar]
- 4.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 5.Thongprasert S, Duffield E, Saijo N, Wu YL, Yang JC, Chu DT, Liao M, Chen YM, Kuo HP, Negoro S, et al. Health-related quality-of-life in a randomized phase III first-line study of gefi-tinib versus carboplatin/paclitaxel in clinically selected patients from Asia with advanced NSCLC (IPASS) J Thorac Oncol. 2011;6:1872–1880. doi: 10.1097/JTO.0b013e31822adaf7. [DOI] [PubMed] [Google Scholar]
- 6.Ellis PM, Coakley N, Feld R, Kuruvilla S, Ung YC. Use of the epidermal growth factor receptor inhibitors gefitinib, erlotinib, afatinib, dacomitinib, and icotinib in the treatment of non-small-cell lung cancer: A systematic review. Curr Oncol. 2015;22:e183–e215. doi: 10.3747/co.22.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackman D, Pao W, Riely GJ, Engelman JA, Kris MG, Janne PA, Lynch T, Johnson BE, Miller VA. Clinical definition of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. J Clin Oncol. 2010;28:357–360. doi: 10.1200/JCO.2009.24.7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao Z, Chen W, Zhang X, Cai P, Fang X, Xu Q, Sun Y, Gu Y. Icotinib, a potent and specific EGFR tyrosine kinase inhibitor, inhibits growth of squamous cell carcinoma cell line A431 through negatively regulating AKT signaling. Biomed Pharmacother. 2013;67:351–356. doi: 10.1016/j.biopha.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 9.Chen X, Zhu Q, Liu Y, Liu P, Yin Y, Guo R, Lu K, Gu Y, Liu L, Wang J, et al. Icotinib is an active treatment of non-small-cell lung cancer: A retrospective study. PLoS One. 2014;9:e95897. doi: 10.1371/journal.pone.0095897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang W, Wu X, Fang W, Zhao Y, Yang Y, Hu Z, Xue C, Zhang J, Zhang J, Ma Y, et al. Network meta-analysis of erlotinib, gefitinib, afatinib and icotinib in patients with advanced non-small-cell lung cancer harboring EGFR mutations. PLoS One. 2014;9:e85245. doi: 10.1371/journal.pone.0085245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi Y, Zhang L, Liu X, Zhou C, Zhang L, Zhang S, Wang D, Li Q, Qin S, Hu C, et al. Icotinib versus gefitinib in previously treated advanced non-small-cell lung cancer (ICOGEN): A randomised, double-blind phase 3 non-inferiority trial. Lancet Oncol. 2013;14:953–961. doi: 10.1016/S1470-2045(13)70355-3. [DOI] [PubMed] [Google Scholar]
- 12.Antonicelli A, Cafarotti S, Indini A, Galli A, Russo A, Cesario A, Lococo FM, Russo P, Mainini AF, Bonifati LG, et al. EGFR-targeted therapy for non-small cell lung cancer: Focus on EGFR oncogenic mutation. Int J Med Sci. 2013;10:320–330. doi: 10.7150/ijms.4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou C, Yao LD. Strategies to improve outcomes of patients with EGRF-mutant non-small cell lung cancer: Review of the literature. J Thorac Oncol. 2016;11:174–186. doi: 10.1016/j.jtho.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Yun CH, Mengwasser KE, Toms AV, Woo MS, Greulich H, Wong KK, Meyerson M, Eck MJ. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci USA. 2008;105:2070–2075. doi: 10.1073/pnas.0709662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, Bergethon K, Shaw AT, Gettinger S, Cosper AK, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3:75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meggio F, Pinna LA. One-thousand-and-one substrates of protein kinase CK2? FASEB J. 2003;17:349–368. doi: 10.1096/fj.02-0473rev. [DOI] [PubMed] [Google Scholar]
- 17.Ortega CE, Seidner Y, Dominguez I. Mining CK2 in cancer. PLoS One. 2014;9:e115609. doi: 10.1371/journal.pone.0115609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torres J, Pulido R. The tumor suppressor PTEN is phosphorylated by the protein kinase CK2 at its C terminus. implications for PTEN stability to proteasome-mediated degradation. J Biol Chem. 2001;276:993–998. doi: 10.1074/jbc.M009134200. [DOI] [PubMed] [Google Scholar]
- 19.Cozza G, Mazzorana M, Papinutto E, Bain J, Elliott M, di Maira G, Gianoncelli A, Pagano MA, Sarno S, Ruzzene M, et al. Quinalizarin as a potent, selective and cell-permeable inhibitor of protein kinase CK2. Biochem J. 2009;421:387–395. doi: 10.1042/BJ20090069. [DOI] [PubMed] [Google Scholar]
- 20.Daya-Makin M, Sanghera JS, Mogentale TL, Lipp M, Parchomchuk J, Hogg JC, Pelech SL. Activation of a tumor-associated protein kinase (p40TAK) and casein kinase 2 in human squamous cell carcinomas and adenocarcinomas of the lung. Cancer Res. 1994;54:2262–2268. [PubMed] [Google Scholar]
- 21.Faust M, Schuster N, Montenarh M. Specific binding of protein kinase CK2 catalytic subunits to tubulin. FEBS Lett. 1999;462:51–56. doi: 10.1016/S0014-5793(99)01492-1. [DOI] [PubMed] [Google Scholar]
- 22.Lee CK, Kim S, Lee JS, Lee JE, Kim SM, Yang IS, Kim HR, Lee JH, Kim S, Cho B. Next-generation sequencing reveals novel resistance mechanisms and molecular heterogeneity in EGFR-mutant non-small cell lung cancer with acquired resistance to EGFR-TKIs. Lung Cancer. 2017;113:106–114. doi: 10.1016/j.lungcan.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Cross DA, Ashton SE, Ghiorghiu S, Eberlein C, Nebhan CA, Spitzler PJ, Orme JP, Finlay MR, Ward RA, Mellor MJ, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014;4:1046–1061. doi: 10.1158/2159-8290.CD-14-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Maira G, Salvi M, Arrigoni G, Marin O, Sarno S, Brustolon F, Pinna LA, Ruzzene M. Protein kinase CK2 phosphorylates and upregulates Akt/PKB. Cell Death Differ. 2005;12:668–677. doi: 10.1038/sj.cdd.4401604. [DOI] [PubMed] [Google Scholar]
- 25.Duncan JS, Litchfield DW. Too much of a good thing: The role of protein kinase CK2 in tumorigenesis and prospects for therapeutic inhibition of CK2. Biochim Biophys Acta. 2008;1784:33–47. doi: 10.1016/j.bbapap.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 26.Hay N. Interplay between FOXO, TOR, and Akt. Biochim Biophys Acta. 2011;1813:1965–1970. doi: 10.1016/j.bbamcr.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu SG, Shih JY. Management of acquired resistance to EGFR TKI-targeted therapy in advanced non-small cell lung cancer. Mol Cancer. 2018;17:38. doi: 10.1186/s12943-018-0777-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chua MM, Ortega CE, Sheikh A, Lee M, Abdul-Rassoul H, Hartshorn KL, Dominguez I. CK2 in cancer: Cellular and biochemical mechanisms and potential therapeutic target. Pharmaceuticals (Basel) 2017;10:E18. doi: 10.3390/ph10010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yaylim I, Isbir T. Enhanced casein kinase II (CK II) activity in human lung tumours. Anticancer Res. 2002;22:215–218. [PubMed] [Google Scholar]
- 30.Hung MS, Xu Z, Chen Y, Smith E, Mao JH, Hsieh D, Lin YC, Yang CT, Jablons DM, You L. Hematein, a casein kinase II inhibitor, inhibits lung cancer tumor growth in a murine xenograft model. Int j Oncol. 2013;43:1517–1522. doi: 10.3892/ijo.2013.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Q, Li K, Yang T, Zhang S, Zhou Y, Li Z, Xiong J, Zhou F, Zhou X, Liu L, et al. Association of protein kinase CK2 inhibition with cellular radiosensitivity of non-small cell lung cancer. Sci Rep. 2017;7:16134. doi: 10.1038/s41598-017-16012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim J, Kim SH. Druggability of the CK2 inhibitor CX-4945 as an anticancer drug and beyond. Arch Pharm Res. 2012;35:1293–1296. doi: 10.1007/s12272-012-0800-9. [DOI] [PubMed] [Google Scholar]
- 33.Ku MJ, Park JW, Ryu BJ, Son YJ, Kim SH, Lee SY. CK2 inhibitor CX4945 induces sequential inactivation of proteins in the signaling pathways related with cell migration and suppresses metastasis of A549 human lung cancer cells. Bioorg Med Chem Lett. 2013;23:5609–5613. doi: 10.1016/j.bmcl.2013.08.043. [DOI] [PubMed] [Google Scholar]
- 34.Di Maira G, Brustolon F, Bertacchini J, Tosoni K, Marmiroli S, Pinna LA, Ruzzene M. Pharmacological inhibition of protein kinase CK2 reverts the multidrug resistance phenotype of a CEM cell line characterized by high CK2 level. Oncogene. 2007;26:6915–6926. doi: 10.1038/sj.onc.1210495. [DOI] [PubMed] [Google Scholar]
- 35.Asati V, Mahapatra DK, Bharti SK. PI3K/Akt/mTOR and Ras/Raf/MEK/ERK signaling pathways inhibitors as anticancer agents: Structural and pharmacological perspectives. Eur J Med Chem. 2016;109:314–341. doi: 10.1016/j.ejmech.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 36.Lieber M, Smith B, Szakal A, Nelson-Rees W, Todaro G. A continuous tumor-cell line from a human lung carcinoma with properties of type II alveolar epithelial cells. Int J Cancer. 1976;17:62–70. doi: 10.1002/ijc.2910170110. [DOI] [PubMed] [Google Scholar]
- 37.Greve G, Schiffmann I, Pfeifer D, Pantic M, Schuler J, Lubbert M. The pan-HDAC inhibitor panobinostat acts as a sensitizer for erlotinib activity in EGFR-mutated and -wildtype non-small cell lung cancer cells. BMC Cancer. 2015;15:947. doi: 10.1186/s12885-015-1967-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sos ML, Koker M, Weir BA, Heynck S, Rabinovsky R, Zander T, Seeger JM, Weiss J, Fischer F, Frommolt P, et al. PTEN loss contributes to erlotinib resistance in EGFR-mutant lung cancer by activation of Akt and EGFR. Cancer Res. 2009;69:3256–3261. doi: 10.1158/0008-5472.CAN-08-4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ritt DA, Zhou M, Conrads TP, Veenstra TD, Copeland TD, Morrison DK. CK2 is a component of the KSR1 scaffold complex that contributes to raf kinase activation. Curr Biol. 2007;17:179–184. doi: 10.1016/j.cub.2006.11.061. [DOI] [PubMed] [Google Scholar]
- 40.Parker R, Clifton-Bligh R, Molloy MP. Phosphoproteomics of MAPK inhibition in BRAF-mutated cells and a role for the lethal synergism of dual BRAF and CK2 inhibition. Mol Cancer Ther. 2014;13:1894–1906. doi: 10.1158/1535-7163.MCT-13-0938. [DOI] [PubMed] [Google Scholar]
- 41.So KS, Rho JK, Choi YJ, Kim SY, Choi CM, Chun YJ, Lee JC. AKT/mTOR down-regulation by CX-4945, a CK2 inhibitor, promotes apoptosis in chemorefractory non-small cell lung cancer cells. Anticancer Res. 2015;35:1537–1542. [PubMed] [Google Scholar]
- 42.Yip PY. Phosphatidylinositol 3-kinase-AKT-mammalian target of rapamycin (PI3K-Akt-mTOR) signaling pathway in non-small cell lung cancer. Transl Lung Cancer Res. 2015;4:165–176. doi: 10.3978/j.issn.2218-6751.2015.01.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reungwetwattana T, Dy GK. Targeted therapies in development for non-small cell lung cancer. J Carcinog. 2013;12:22. doi: 10.4103/1477-3163.123972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zer A, Leighl N. Promising targets and current clinical trials in metastatic non-squamous NSCLC. Front Oncol. 2014;4:329. doi: 10.3389/fonc.2014.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bliesath J, Huser N, Omori M, Bunag D, Proffitt C, Streiner N, Ho C, Siddiqui-Jain A, O'Brien SE, Lim JK, et al. Combined inhibition of EGFR and CK2 augments the attenuation of PI3K-Akt-mTOR signaling and the killing of cancer cells. Cancer Lett. 2012;322:113–118. doi: 10.1016/j.canlet.2012.02.032. [DOI] [PubMed] [Google Scholar]
- 46.So KS, Kim CH, Rho JK, Kim SY, Choi YJ, Song JS, Kim WS, Choi CM, Chun YJ, Lee JC. Autophagosome-mediated EGFR down-regulation induced by the CK2 inhibitor enhances the efficacy of EGFR-TKI on EGFR-mutant lung cancer cells with resistance by T790M. PLoS One. 2014;9:e114000. doi: 10.1371/journal.pone.0114000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ke EE, Wu YL. EGFR as a pharmacological target in EGFR-mutant non-small-cell lung cancer: Where do we stand now? Trends Pharmacol Sci. 2016;37:887–903. doi: 10.1016/j.tips.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 48.Morgillo F, Della Corte CM, Fasano M, Ciardiello F. Mechanisms of resistance to EGFR-targeted drugs: Lung cancer. ESMO Open. 2016;1:e000060. doi: 10.1136/esmoopen-2016-000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.