Abstract
Background
Advances have been made in identifying genetic etiologies and maternal risk factors of congenital heart defects (CHDs), while few literatures are available regarding paternal risk factors for CHDs. Thus, we aim to conduct a meta‐analysis and systematic review about the non‐genetic paternal risk factors for CHDs.
Methods
We searched the PubMed, MEDLINE, and Cochrane Library online databases and identified 31 studies published between 1990 and 2018 according to the inclusion criteria. Paternal risk factors were divided into subgroups, and summarized odd ratios (OR) were calculated.
Results
Paternal age between 24 and 29 years decreased the risk of CHDs in the offspring (OR = 0.90 [0.82, 0.98]), while paternal age ≥ 35 years old increased the risk of CHDs (35‐39 years old: OR = 1.14 [1.09, 1.19], and ≥ 40 years: OR = 1.27 [1.14, 1.42]). Paternal cigarette smoking increased the risk of CHDs in a dose‐dependent way. Paternal wine drinking (OR = 1.47 [1.05, 2.07]) and exposure to chemical agents or drugs (OR = 2.15 [1.53, 3.02]) also increased the risk of CHDs. Some specific paternal occupations were also associated with increased risk for CHDs or CHD subtypes including factory workers, janitors, painters, and plywood mill workers.
Conclusions
This meta‐analysis and systematic review suggested that advanced paternal age, cigarette smoking, wine drinking, exposure to chemical agents or drugs and some specific occupations were associated with an increased risk of CHDs. More measures should be taken to reduce occupational and environment exposures. At the same time, fertility at certain age and establishment of healthy life habits are strongly recommended.
Keywords: congenital heart defects, meta‐analysis, paternal risk factors
1. INTRODUCTION
Congenital heart defects (CHDs) are groups of congenital cardiovascular disorders or diseases that affect about 1% of live births worldwide,1 which were also the leading cause of infant deaths.2 Over the past decades, there have been advances in the understanding of the risk factors for CHDs, that both genetic and non‐genetic risk factors are associated with the prevalence of CHDs. In the past, most investigations focused on maternal and genetic factors, while paternal factors attracted less attention. However, evidences suggested that paternal age, cigarette smoking, wine drinking, and occupational/environment exposures might have associations with various birth defects including CHDs.3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 Therefore, we aimed to provide a current review of paternal factors for CHDs.
2. MATERIALS AND METHODS
This report of systematic review and meta‐analysis followed the instructions of Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA).16
2.1. Search strategy
We searched the PubMed, MEDLINE, and Cochrane Library online databases. We used the selected search terms and the Medical Subject Headings (MeSH) that were related to “congenital heart defect,” “risk factor,” “exposure,” and “paternal”. In addition to these search terms, individual risk factors also were included in the search terms (eg, “age,” “smoking,” and “drinking”). Reference lists of articles were reviewed to get more potentially eligible articles.
2.2. Inclusion criteria and exclusion criteria
We selected articles that (a) were observational epidemiologic study (case‐control and cohort study), (b) examined the association between any paternal exposures (eg, paternal age, paternal smoking, paternal drinking, paternal occupation, and paternal exposure to chemical agents) and CHDs overall or any one of the CHD subtypes in infants, (c) were written either in English, Chinese, or French, and (d) reported ORs (ie, risk ratios [RR] or odds ratios [OR]) and associated 95% confidence intervals (CIs) or had raw data available.
The exclusion criteria were: articles that (a) did not examine the association between any paternal exposures and any CHD subtypes in infants, (b) did not reported ORs and associated 95% CIs or had no raw data available, and (c) we could get the full text.
In the case of multiple publications using the same database, we selected the study that contained the most comprehensive information (eg, longest study periods or most CHD subtypes analyzed).
2.3. Data extraction
The studies meeting the inclusion criteria were independently reviewed by two authors (JP, JW) to extract study characteristics (eg, authors, year of publication, geographic region, periods of data collection, study design, sample size, exposure data, exposure period around pregnancy) and measures of association (eg, OR, RR). Measures of association not available in the original article were calculated based on raw data. Discrepancies between the authors were resolved by discussion.
2.4. Statistical analysis
We tested for heterogeneity across studies using Cochran's Q‐test. If there was an evidence of heterogeneity (P < .1), we used a random‐effects model. Otherwise, we used a fixed‐effects analysis. The statistical analyses were performed with Review Manager Version 5.3 (Cochrane Collaboration, Baltimore, Maryland).
Subgroup analysis was performed based on the different paternal factor, and sensitivity analysis was conducted. Publication bias was evaluated visually by funnel plots.
3. RESULTS
3.1. Study selection
We identified 31 studies3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34 published between 1990 and 2018 according to the inclusion criteria. Study selection was summarized in Figure 1. Forty‐four studies were selected and retrieved for a full review. Five studies did not report OR and were excluded. Four studies were excluded since we had no access to full text. One study was only available in Lithuanian and was excluded. Finally, we included 31 studies for the meta‐analysis and systematic review.
Figure 1.
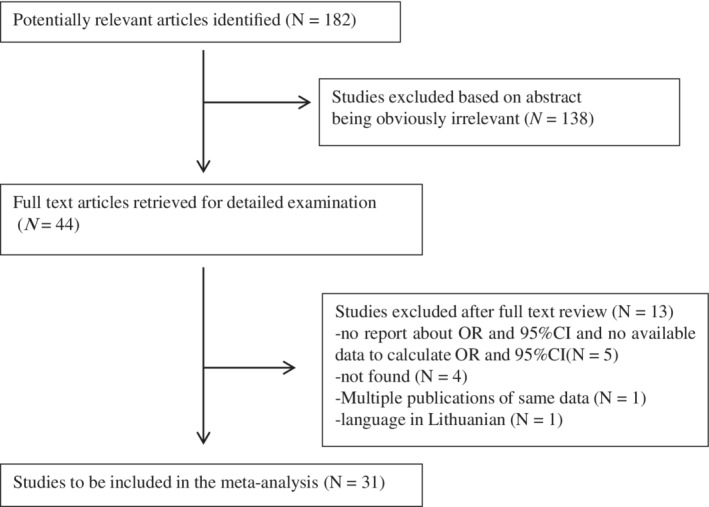
Flow chart of study selection process. CI, confidence intervals; OR, odd ratios
3.2. Study characteristics
The characteristics of the included studies were summarized in Table 1. There were two cohort studies and 29 case‐control studies published between 1990 and 2018. The included studies had been performed in the United States, the United Kingdom, France, Egypt, Norway, the Netherlands, Sweden, Italy, Greek, Poland, Lithuania, Canada, China, and India. Risk factors were divided into five broad categories: paternal age, paternal cigarette smoking, paternal wine‐drinking, paternal occupation, and paternal exposure to chemical agents.
Table 1.
The characteristics of the included studies
| Study | Study period | Study location | Study design | No. of cases | No. of controls | Exposure | Cardiac defects | Exposure period | NOS |
|---|---|---|---|---|---|---|---|---|---|
| 2018‐Liu | 2004‐2014 | China | Case‐control | 4726 | 4726 | Drinking | All CHDs | B3‐P3 | 7 |
| 2017‐Li | 2013‐2014 | China | Case‐control | 119 | 239 | Chronic disease | All CHDs | B6 | 6 |
| Exposure to occupational hazards | |||||||||
| 2016‐Silver | 1983‐2001 | USA | Cohort | NA | NA | Age | VSD | B3‐P1 | 8 |
| Metal | VSD | ||||||||
| Lead | VSD | ||||||||
| Chlorinated hydrocarbons | VSD | ||||||||
| 2016‐Ou | 2004‐2013 | China | Case‐control | 4034 | 4034 | Drinking | All CHDs | B3‐P3 | 7 |
| 1566 | 4034 | Drinking | VSD | 7 | |||||
| 1028 | 4034 | Drinking | ASD | ||||||
| 212 | 4034 | Drinking | PA | ||||||
| 143 | 4034 | Drinking | TGA | ||||||
| 2016‐Liu | 2014‐2015 | China | Case‐control | 80 | 160 | Drinking | All CHDs | B6 | 7 |
| Smoking | |||||||||
| Age | |||||||||
| 2016‐Abqari | 2014–2015 | India | Case‐control | 400 | 754 | Age | All CHDs | ‐ | 6 |
| 2015‐Wang | 2012‐2013 | China | Case‐control | 761 | 609 | Age | All CHDs | B3‐P3 | 8 |
| Smoking | |||||||||
| Drinking | |||||||||
| Pesticides | |||||||||
| Polychlorinated compounds | |||||||||
| Phthalates | |||||||||
| Alkylphenolic compounds | |||||||||
| Bisphenol A | |||||||||
| Heavy metals | |||||||||
| 2015‐Qu | 2004‐2012 | China | Case‐control | 3038 | 3038 | Occupation | All CHDs | B3 | 8 |
| 2015‐Chen | 2012–2013 | China | Case‐control | 435 | 574 | Age | All CHDs | ‐ | 6 |
| 2014‐Wijnands | 2013‐ | Netherlands | Case‐control | 114 | 484 | Phthalates | All CHDs | B1‐P2 | 6 |
| 2013‐Nie | 2004‐2011 | China | Case‐control | 2568 | 2568 | Antibiotics | All CHDs | B3 | 7 |
| Drinking | |||||||||
| Chemical agent contact | |||||||||
| Smoking | |||||||||
| Virus infection | |||||||||
| Age | |||||||||
| 2013‐Fung | 2008‐2011 | Canada | Case‐control | 1339 | 199 | Smoking | All CHDs | B3‐P3 | 6 |
| 2013‐Deng | 2010‐2012 | China | Case‐control | 284 | 422 | Smoking | All CHDs | B3‐P3 | 6 |
| 75 | 147 | Smoking | Septal defects | ||||||
| 72 | 147 | Smoking | Conotruncal defects | ||||||
| 31 | 147 | Smoking | LVOTO | ||||||
| 28 | 147 | Smoking | RVOTO | ||||||
| 2012‐Snijder | 2003‐2010 | The Netherlands | Case‐control | 421 | 477 | Pesticides | All CHDs | B1‐P2 | 8 |
| Polychlorinated compounds | |||||||||
| Phthalates | |||||||||
| AlkylphenOlic compounds | |||||||||
| Heavy metals | |||||||||
| 2012‐Chehab | 2006‐2010 | France | Case‐control | 2466 | 793 | smoking | All CHDs | NA | 6 |
| 2011‐Karatza | 2006‐2009 | Greek | Case‐control | 157 | 208 | Smoking | All CHDs | B1‐P3 | 7 |
| 2011‐Cresci | 2008‐2010 | Italy | Case‐control | 330 | 330 | Diagnostic X‐ray exposure | All CHDs | B3 | 6 |
| Drinking | |||||||||
| Exposure to toxicants | |||||||||
| 2010‐Kuciene | 1995‐2005 | Lithuania | Case‐control | 261 | 1122 | Smoking | All CHDs | ‐ | 6 |
| 2010‐Green | 1997‐2004 | USA | Case‐control | 740 | 5839 | Age | PVS | ‐ | 6 |
| 1011 | 5839 | Age | RVOTO | ||||||
| 2009‐Materna | 1998‐2002 | Poland | Case‐control | 2451 | 6231 | Age | All CHDs | ‐ | 6 |
| 2007‐yang | 1999‐2000 | USA | Cohort | 6797 | 5 360 532 | Age | All CHDs | ‐ | 7 |
| 2006‐Kuehl | 1981‐1989 | USA | Case‐control | 142 | 3572 | Age | HLHS | B6 | 6 |
| 2004‐Kazaura | 1967‐1998 | Norway | Case‐control | 3628 | 13 668 | Age | All CHDs | ‐ | 6 |
| 2002‐Cedergren | 1982‐1996 | Sweden | Case‐control | 269 | 524 | Age | All CHDs | ‐ | 7 |
| 2001‐Loffredo | 1981–1989 | USA | Case‐control | 641 | 3549 | Painting | Isolated membranous VSD | B3‐P3 | 6 |
| 2000‐Bassili | 1995‐1997 | Egypt | Case‐control | 894 | 894 | Age | All CHDs | ‐ | 7 |
| 1997‐Ewing | 1981–1989 | USA | Case‐control | 643 | 3551 | Age | Isolated membranous VSD | B6 | 7 |
| Marijuana use | Isolated membranous VSD | ||||||||
| Cocaine use | Isolated membranous VSD | ||||||||
| Smoking | Isolated membranous VSD | ||||||||
| Drinking | Isolated membranous VSD | ||||||||
| 1996‐Aronson | 1979‐1986 | Canada | Nested Case–control cohort | 9340 | 9340 | Fire fighter | All CHDs | ‐ | 8 |
| 1994‐Olshan | 1952‐1973 | UK | Case‐control | 4110 | 8220 | Age | VSD | ‐ | 7 |
| Age | ASD | ||||||||
| 1991‐Zhan | 1986‐1987 | China | Case–control | 497 | 6222 | Age | All CHDs | ‐ | 7 |
| 1991‐Olshan | 1952–1973 | UK | Case‐control | 1081 | 2272 | Occupation | ASD | ‐ | 7 |
| 657 | 1213 | Occupation | VSD | 8 | |||||
| 1125 | 2309 | Occupation | PDA | ||||||
| 594 | 1256 | Occupation | Other CHDs |
Abbreviations: ASD, atrial septal defect; HLHS, hypoplastic left heart syndrome; LVOTO, left ventricular outflow tract obstructions; NOS, Newcastle‐Ottawa Scale; PA, pulmonary atresia; PDA, patent ductus arteriosus; PVS, pulmonary valve stenosis; RVOTO, right ventricular outflow tract obstructions; TGA, transposition of great artery; VSD, ventricular septal defect.
B#, month before conception, B, unspecified time before conception, P#, month during pregnancy.
3.3. Paternal age
Eleven studies focused on paternal age as a risk factor for CHDs in offspring.4, 5, 10, 11, 12, 13, 15, 19, 25, 30, 32 Four studies evaluated the effect of advanced paternal age on the risk of CHDs, and the pooled OR is 1.02 (1.00, 1.04).
In addition, eight studies categorized paternal age into different age groups and we summarized the same age group, namely, <20, 20 to 24, 25 to 29, 30 to 34, 35 to 39, and ≥40 years of age. As shown in Table 2 and TABLE S1, paternal age older than 35 years was associated with higher risk of CHDs in offspring (OR for 35‐39 years: 1.14 [1.09, 1.19], OR for ≥40 years: 1.27 [1.14, 1.42]). On the contrary, paternal age of 25 to 29 years was associated with the lowest risk (OR = 0.90 [0.82, 0.98]).
Table 2.
The results of subgroup analysis of non‐genetic paternal factors on congenital heart defects
| Exposure | No. of cases | No. of controls | Summary odds ratio (95% CI) | Heterogeneity P‐value | Funnel plot | |
|---|---|---|---|---|---|---|
| Age (years) | 7137 | 860 802 | 1.02 (1.00, 1.04) | .04 | RE | Symmetric |
| <20 | 495 | 228 352 | 1.06 (0.72, 1.54) | .01 | RE | Symmetric |
| 20‐24 | 2978 | 1 120 362 | 0.90 (0.80, 1.02) | <.0001 | RE | Symmetric |
| 25‐29 | 5745 | 1 740 888 | 0.90 (0.82, 0.98) | <.0001 | RE | Symmetric |
| 30‐34 | 4816 | 1 635 132 | 0.99 (0.90, 1.08) | .0002 | RE | Symmetric |
| 35‐39 | 2816 | 987 206 | 1.14 (1.09, 1.19) | .45 | FE | Symmetric |
| ≥40 | 2032 | 523 839 | 1.27 (1.14, 1.42) | .0001 | RE | Symmetric |
| Smoking (cigarette/day) | 8709 | 14 456 | 1.42 (1.17, 1.74) | <.0001 | RE | Asymmetric |
| 1‐9 | 434 | 597 | 1.19 (0.82, 1.71) | .003 | RE | Asymmetric |
| 10‐19 | 467 | 495 | 1.41 (1.20, 1.67) | .15 | FE | Symmetric |
| 20‐ | 1131 | 730 | 1.75 (1.10, 2.80) | <.0001 | RE | Asymmetric |
| Drinking | 13 406 | 16 430 | 1.47 (1.05, 2.07) | <.0001 | RE | Symmetric |
| Toxicant | NA | NA | 2.15 (1.53, 3.02) | <.0001 | RE | Symmetric |
Abbreviations: CI, confidence interval; FE, fixed effects model; NA, not available; OR, odds ratio; RE, random effects model.
3.4. Paternal cigarette smoking
Maternal‐smoking is now a well‐proved risk factor for CHDs.35 Similarly, paternal smoking also attracted growing concerns. Ten studies7, 8, 9, 11, 14, 15, 20, 21, 23, 27 evaluated the role of paternal smoking in the origin of CHDs and the summarized OR was 1.42 (1.17, 1.74) (Table 2, TABLE S2). Furthermore, based on the amount of cigarette smoking per day, the paternal smokers were also divided into three groups as follows: light smoking (1‐9 cigarettes per day), medium smoking (10‐19 cigarettes per day), and heavy smoking (≥20 cigarettes per day), and the pooled OR was 1.19 (0.82, 1.71), 1.41 (1.20, 1.67), and 1.75 (1.10, 2.80), respectively. This suggested that paternal smoking was associated with increased risk of having offspring with CHDs and this association was dose‐dependent.
3.5. Paternal wine drinking
Seven studies6, 11, 14, 15, 17, 20, 21 evaluated the effect of paternal alcohol consumption on CHDs. The summarized OR was 1.47 (1.05, 2.07) (Table 2, TABLE S3), indicating that paternal alcohol intake was a risk factor for CHDs in the offspring. However, the definition of paternal wine drinking was various from studies. The most common definition was defined by drinking capacity, that is, wine drinking mean a reported alcohol intake of on average at least 50 mL per day or per time without specifying wine.6, 11, 15, 20, 21 Others defined wine drinking by the amount of wine categories.11, 14 Only one study did not specify the definition of wine drinking.17
3.6. Paternal exposure to chemical agents or drugs
Seven studies3, 11, 14, 15, 20, 22, 23 evaluated the effect of paternal exposure to chemical agents or drugs on CHDs. These toxic chemical agents including pesticides, polychlorinated compounds, phthalates, alkyl phenolic compounds, bisphenol A, heavy metals,15 hydrocarbons,3 marijuana, and cocaine.11 After meta‐analysis, we found that paternal exposure to chemical agents or drugs had a strong association with increased risk of CHDs (OR = 2.15 [1.53, 3.02]) (Table 2, TABLE S4).
3.7. Paternal occupation
Some occupations like factory workers (left‐to‐right shunt CHDs [OR = 1.46, 95% CI: 1.23‐1.73] and left ventricular outflow tract obstruction CHDs [OR = 6.01, 95% CI: 1.05‐34.59], janitors ventricular septal defects [OR = 2.45], other heart defects [OR = 2.35], atrial septal defects [OR = 2.03]), painters (patent ductus arteriosus [OR = 2.34]) and plywood mill workers (patent ductus arteriosus [OR = 2.52]) might increase the risk of CHDs.20, 34 However, inconsistent results were shown in the investigations about the association between fire fighters and the risk of CHDs in offspring. An exploratory case‐control study from British Columbia reported statistically significant increased risk for ventricular and atrial septal defects among offspring of male fire fighters, compared to all other paternal occupations and to policemen.36 However, another investigation conducted in Metropolitan Toronto did not support the hypothesis of elevated risk of CHDs among the offspring of fire fighters.33
3.8. Other paternal risk factors
Apart from the above paternal risk factors, there are also several studies concerned about other paternal risk factors, such as chronic disease, viral infection, etc. Paternal chronic disease was another risk factor for CHDs (OR = 4.87, 95% CI: 1.23‐19.24), according to the findings of Li's investigation.18 And paternal virus infection (OR = 2.46, 95% CI: 1.13‐5.35), antibiotics usage (OR = l0.04, 95% CI: 1.28‐78.45) may also increase the risk of CHDs.23 On the other hand, evidences suggested that some paternal factors might not be the risk factors for CHDs. Paternal diagnostic X‐ray exposure may not increase the risk of CHDs (OR = 1.3, 95% CI: 0.8‐2.1).14
4. DISCUSSION
More and more evidence showed that not only maternal factors but also some paternal factors were associated with increased risk of CHDs. Nevertheless, there was little review or meta‐analysis focused on the non‐genetic paternal factors for CHDs, and our study made up this blank. We analyzed almost all the current literature and made a relatively comprehensive summary about the non‐genetic paternal factors for CHDs. After subgroup analysis, we found that advanced paternal age, cigarette smoking, wine drinking, some occupations, and exposure to chemical agents and drugs were still associated with the increased risk of CHDs.
Advanced paternal age was previously found to be associated with increased DNA mutations and chromosomal aberrations in sperm.37 Genetic changes in sperm associated with advanced paternal age could lead to an increased risk for birth defects in offspring.10 Consistent with these findings, we found that advanced paternal age (≥35 years) was associated with increased risk of CHDs. On the contrary, paternal age between 25 to 29 years decreased the risk of CHDs. This suggested that a certain reproductive age might be helpful to reduce the prevalence of CHDs, which could help to provide evidence for governmental health policy. In addition, these conclusions still need further cohort studies with larger sample to confirm.
Cigarette smoking is a well‐known teratogenic risk factor for birth defects and it can affect a number of developing structures.35 Nicotine, the main toxic agent during smoking, could affect sperm activity greatly and lead to chromosome aberration, which might affect the fetal development, and result in the occurrence of cardiac malformations.38 Besides, paternal smoking could induce maternal passive smoking, which could also increase the risk of CHDs.39 Consistently, Deng et al found that the avoidance behavior of paternal smokers might decrease the risk of selected CHDs.7
Apart from smoking, paternal wine drinking was also associated with increased risk of CHDs. However, drinking might be a temporary risk factor because Liu et al showed no evidence that wine‐drinking history would increase the risk of CHDs (OR = 1.087, 0.618‐1.913).21 The association between paternal wine drinking and CHDs in the offspring might need further validation in large cohort studies.
Some occupations like factory workers, painters, and plywood mill workers, probably suffered occupational exposures and Cresci's investigation suggested that occupational/environmental exposures increased the risk of CHDs.14 Several studies have shown that toxicant compounds could induce oxidative DNA damage, mutations, and chromosomal aberrations, such as DNA strand breaks and aneuploidy in human seminal fluid. And they have detected teratogenic, carcinogenic, and endocrine disrupting agents, such as pesticide residues, heavy metal organic solvents (benzene, toluene, and xylene), nicotine, aromatic hydrocarbons, and precursors of mutagenic nitrosamines in human seminal fluid.40 However, with the changing of natural and work environment, the situation may be different when it comes to how the current paternal occupation and exposure to chemical agents affect the prevalence of CHDs. And this needs further researches to explore.
The expose period defined by most identified studies is 3 months before conception.3, 6, 7, 14, 15, 17, 20, 23, 24, 31 The duration of spermatogenesis in human is 72 to 74 days, involving differentiation of the germ cells through several stages of meiosis and mitosis, some of which may be more vulnerable to cytotoxic damage or alterations in the DNA sequence.28 Thus, 3 months before conception could be a critical period of paternal risk factors for CHDs. However, the situation is different when it comes to smoking. In Liu's study, both paternal smoking history (OR = 2.687 [1.538‐4.692]) and paternal smoking half a year before pregnancy (OR = 2.889 [1.589‐5.254]) increased the risk of CHDs.21 Therefore, some paternal factors may have long‐term effects on CHDs.
This study identified articles mostly from the main continents, which were representative. However, there was still evidence of heterogeneity across studies even though subgroup analyses were performed. The probable reason might be the distinct subtypes of CHDs, which could obscure findings when subtypes were “lumped” into a common phenotype to increase study power. For publication bias, the funnel plot of smoking subgroup showed asymmetry, which indicated publication bias. However, for the rest of subgroups, the funnel plots were basically symmetric.
5. CONCLUSIONS
In conclusion, we summarized all the articles about non‐genetic paternal risk factors for CHDs and found that advanced paternal age, cigarette smoking, wine drinking, some occupations, and exposure to chemical agents and drugs would increase the risk of CHDs. It is important and urgent to encourage fertility at certain age, building a healthy life habit, beginning with quitting smoking and drinking, and trying to avoid occupational and environment exposures.
CONFLICT OF INTEREST
The authors declare no potential conflict of interests.
Supporting information
TABLE S1 Forest plot: the association between paternal age and the prevalence of CHDs in offspring. CI: confidence intervals
TABLE S2 Forest plot: the association between paternal cigarette smoking and the prevalence of CHDs in offspring. CI: confidence intervals
TABLE S3 Forest plot: the association between paternal wine drinking and the prevalence of CHDs in offspring. CI: confidence intervals
TABLE S4 Forest plot: the association between paternal exposure to chemical agents or drugs and the prevalence of CHDs in offspring. CI: confidence intervals
ACKNOWLEDGMENT
We would like to thank the support of Department of Pediatric Cardiology, Xinhua Hospital, affiliated to Shanghai Jiao Tong University School of Medicine. Moreover, we are grateful to grants from Shanghai Municipal Commission of Health and Family Planning (20184Y0062) and Shanghai Jiao Tong University School of Medicine (DLY201609) for financial support of this Research Project.
Peng J, Meng Z, Zhou S, et al. The non‐genetic paternal factors for congenital heart defects: A systematic review and meta‐analysis. Clin Cardiol. 2019;42:684–691. 10.1002/clc.23194
Funding information Shanghai Municipal Commission of Health and Family Planning, Grant/Award Number: 20184Y0062, DLY201609; School of Medicine; Shanghai Jiao Tong University
Contributor Information
Jian Wang, Email: wangjian@xinhuamed.com.cn.
Kun Sun, Email: sunkun@xinhuamed.com.cn.
REFERENCES
- 1. Sun J, Chen X, Chen H, Ma Z, Zhou J. Maternal alcohol consumption before and during pregnancy and the risks of congenital heart defects in offspring: a systematic review and meta‐analysis. Congenit Heart Dis. 2015;10:E216‐E224. [DOI] [PubMed] [Google Scholar]
- 2. Gilboa SM, Salemi JL, Nembhard WN, Fixler DE, Correa A. Mortality resulting from congenital heart disease among children and adults in the United States, 1999 to 2006. Circulation. 2010;122:2254‐2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Silver SR, Pinkerton LE, Rocheleau CM, Deddens JA, Michalski AM, Van Zutphen AR. Birth defects in infants born to employees of a microelectronics and business machine manufacturing facility. Birth Defects Res A Clin Mol Teratol. 2016;106:696‐707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yang Q, Wen SW, Leader A, Chen XK, Lipson J, Walker M. Paternal age and birth defects: how strong is the association? Hum Reprod. 2007;22:696‐701. [DOI] [PubMed] [Google Scholar]
- 5. Kazaura M, Lie RT, Skjaerven R. Paternal age and the risk of birth defects in Norway. Ann Epidemiol. 2004;14:566‐570. [DOI] [PubMed] [Google Scholar]
- 6. Ou Y, Mai J, Zhuang J, et al. Risk factors of different congenital heart defects in Guangdong, China. Pediatr Res. 2016;79:549‐558. [DOI] [PubMed] [Google Scholar]
- 7. Deng K, Liu Z, Lin Y, et al. Periconceptional paternal smoking and the risk of congenital heart defects: a case‐control study. Birth Defects Res A Clin Mol Teratol. 2013;97:210‐216. [DOI] [PubMed] [Google Scholar]
- 8. Chehab G, El‐Rassi I, Adhami A, et al. Parental smoking during early pregnancy and congenital heart defects. J Med Liban. 2012;60:14‐18. [PubMed] [Google Scholar]
- 9. Kuciene R, Dulskiene V. Parental cigarette smoking and the risk of congenital heart septal defects. Medicina (Kaunas). 2010;46:635‐641. [PubMed] [Google Scholar]
- 10. Green RF, Devine O, Crider KS, et al. Association of paternal age and risk for major congenital anomalies from the National Birth Defects Prevention Study, 1997 to 2004. Ann Epidemiol. 2010;20:241‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ewing CK, Loffredo CA, Beaty TH. Paternal risk factors for isolated membranous ventricular septal defects. Am J Med Genet. 1997;71:42‐46. [PubMed] [Google Scholar]
- 12. Olshan AF, Schnitzer PG, Baird PA. Paternal age and the risk of congenital heart defects. Teratology. 1994;50:80‐84. [DOI] [PubMed] [Google Scholar]
- 13. Zhan SY, Lian ZH, Zheng DZ, Gao L. Effect of fathers' age and birth order on occurrence of congenital heart disease. J Epidemiol Community Health. 1991;45:299‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cresci M, Foffa I, Ait‐Ali L, et al. Maternal and paternal environmental risk factors, metabolizing GSTM1 and GSTT1 polymorphisms, and congenital heart disease. Am J Cardiol. 2011;108:1625‐1631. [DOI] [PubMed] [Google Scholar]
- 15. Wang C, Zhan Y, Wang F, et al. Parental occupational exposures to endocrine disruptors and the risk of simple isolated congenital heart defects. Pediatr Cardiol. 2015;36:1024‐1037. [DOI] [PubMed] [Google Scholar]
- 16. Knobloch K, Yoon U, Vogt PM. Preferred reporting items for systematic reviews and meta‐analyses (PRISMA) statement and publication bias. J Craniomaxillofac Surg. 2011;39:91‐92. [DOI] [PubMed] [Google Scholar]
- 17. Liu X, Nie Z, Chen J, et al. Does maternal environmental tobacco smoke interact with social‐demographics and environmental factors on congenital heart defects? Environmental Pollution (Barking, Essex : 1987). 2018;234:214‐222. [DOI] [PubMed] [Google Scholar]
- 18. Li H, Luo M, Zheng J, et al. An artificial neural network prediction model of congenital heart disease based on risk factors: a hospital‐based case‐control study. Medicine. 2017;96:e6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Abqari S, Gupta A, Shahab T, Rabbani MU, Ali SM, Firdaus U. Profile and risk factors for congenital heart defects: a study in a tertiary care hospital. Ann Pediatr Cardiol. 2016;9:216‐221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Qu Y, Liu X, Mai J, et al. Analysis of environmental risk factors in congenital heart defects. Beijing da xue xue bao Yi xue ban. Journal of Peking University Health Sciences. 2015;47:420‐430. [PubMed] [Google Scholar]
- 21. Liu Z, Yan S. Relative factors for congenital heart disease: a case‐control study. Int J Clin Exp Med. 2016;9:16568‐16577. [Google Scholar]
- 22. Wijnands KPJ, Zeilmaker GA, Meijer WM, Helbing WA, Steegers‐Theunissen RPM. Periconceptional parental conditions and perimembranous ventricular septal defects in the offspring. Birth Defects Res A Clin Mol Teratol. 2014;100:944‐950. [DOI] [PubMed] [Google Scholar]
- 23. Nie ZQ, Ou YQ, Chen JM, et al. Risk factors of congenital heart defects in fetal and infants born from 2004 to 2011 in Guangdong. Zhonghua Xin Xue Guan Bing Za Zhi. 2013;41:704‐708. [PubMed] [Google Scholar]
- 24. Fung A, Manlhiot C, Naik S, et al. Impact of prenatal risk factors on congenital heart disease in the current era. J Am Heart Assoc. 2013;2:e000064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen X, Hu J, Wang J, et al. Clinical epidemiological study of peri‐conceptional multiple risk factors and congenital heart diseases. Zhonghua Yi Xue Za Zhi. 2015;95:701‐704. [PubMed] [Google Scholar]
- 26. Snijder CA, Vlot IJ, Burdorf A, et al. Congenital heart defects and parental occupational exposure to chemicals. Hum Reprod. 2012;27:1510‐1517. [DOI] [PubMed] [Google Scholar]
- 27. Karatza AA, Giannakopoulos I, Dassios TG, Belavgenis G, Mantagos SP, Varvarigou AA. Periconceptional tobacco smoking and isolated congenital heart defects in the neonatal period. Int J Cardiol. 2011;148:295‐299. [DOI] [PubMed] [Google Scholar]
- 28. Materna‐Kiryluk A, Wisniewska K, Badura‐Stronka M, et al. Parental age as a risk factor for isolated congenital malformations in a Polish population. Paediatr Perinat Epidemiol. 2009;23:29‐40. [DOI] [PubMed] [Google Scholar]
- 29. Kuehl KS, Loffredo CA. A cluster of hypoplastic left heart malformation in Baltimore, Maryland. Pediatr Cardiol. 2006;27:25‐31. [DOI] [PubMed] [Google Scholar]
- 30. Cedergren MI, Selbing AJ, Källén BAJ. Risk factors for cardiovascular malformation—a study based on prospectively collected data. Scand J Work Environ Health. 2002;28:12‐17. [DOI] [PubMed] [Google Scholar]
- 31. Loffredo CA, Hirata J, Wilson PD, Ferencz C, Lurie IW. Atrioventricular septal defects: possible etiologic differences between complete and partial defects. Teratology. 2001;63:87‐93. [DOI] [PubMed] [Google Scholar]
- 32. Bassili A, Mokhtar SA, Dabous NI, Zaher SR, Mokhtar MM, Zaki A. Risk factors for congenital heart diseases in Alexandria, Egypt. Eur J Epidemiol. 2000;16:805‐814. [DOI] [PubMed] [Google Scholar]
- 33. Aronson KJ, Dodds LA, Marrett L, Wall C. Congenital anomalies among the offspring of fire fighters. Am J Ind Med. 1996;30:83‐86. [DOI] [PubMed] [Google Scholar]
- 34. Olshan AF, Teschke K, Baird PA. Paternal occupation and congenital anomalies in offspring. Am J Ind Med. 1991;20:447‐475. [DOI] [PubMed] [Google Scholar]
- 35. Lee L, Lupo P. Maternal smoking during pregnancy and risk of congenital heart defects: a systematic review and a meta‐analysis. Circulation. 2012;125: 645‐657. [Google Scholar]
- 36. Olshan AF, Teschke K, Baird PA. Birth defects among offspring of firemen. Am J Epidemiol. 1990;131:312‐321. [DOI] [PubMed] [Google Scholar]
- 37. Puscheck EE, Jeyendran RS. The impact of male factor on recurrent pregnancy loss. Curr Opin Obstet Gynecol. 2007;19:222‐228. [DOI] [PubMed] [Google Scholar]
- 38. Correa A, Levis DM, Tinker SC, Cragan JD. Maternal cigarette smoking and congenital heart defects. J Pediatr. 2015;166:801‐804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu Y, Zhu B, Zhuo L, et al. Risk factors for congenital heart disease in chinese neonates: A meta analysis. Zhongguo Dang Dai Er Ke Za Zhi. 2017;19:754‐758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gianicolo EAL, Cresci M, Ait‐Ali L, Foffa I, Andreassi MG. Smoking and congenital heart disease: the epidemiological and biological link. Curr Pharm des. 2010;16:2572‐2577. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1 Forest plot: the association between paternal age and the prevalence of CHDs in offspring. CI: confidence intervals
TABLE S2 Forest plot: the association between paternal cigarette smoking and the prevalence of CHDs in offspring. CI: confidence intervals
TABLE S3 Forest plot: the association between paternal wine drinking and the prevalence of CHDs in offspring. CI: confidence intervals
TABLE S4 Forest plot: the association between paternal exposure to chemical agents or drugs and the prevalence of CHDs in offspring. CI: confidence intervals


