Abstract
It is well known that extensive osteoclast formation plays a key role in osteoporosis in post-menopausal women and the elderly. The suppression of extensive osteoclastogenesis and bone resorption may be an effective preventive strategy for osteoporosis. Zoledronic acid (ZOL) has been indicated to play an essential role in regulating bone mineral density and has already been used in large clinical trials. However, the effects of ZOL on osteoclastogenesis remain to be fully elucidated. Therefore, the present study aimed to determine the effects of ZOL on osteoclastogenesis, and to explore the corresponding signalling pathways. By using a cell viability assay, as well as in vitro osteoclastogenesis, immunofluorescence and resorption pit assays, we demonstrated that ZOL (0.1-5 µM) suppressed receptor activator of nuclear factor-κB ligand (RANKL)-induced osteoclast differentiation and bone resorptive activity. Furthermore, western blot analysis and reverse transcription-quantitative PCR indicated that ZOL inhibited the RANKL-induced activation of NF-κB and the phosphorylation of JNK in RAW264.7 cells, and subsequently decreased the expression of osteoclastogenesis-associated genes, including calcitonin receptor, tartrate-resistant acid phosphatase and dendritic cell-specific transmembrane protein. ZOL inhibited osteoclast formation and resorption in vitro by specifically suppressing NF-κB and JNK signalling. On the whole, the findings of this study indicate that ZOL may serve as a potential agent for the treatment of osteoclast-associated diseases, including osteoporosis.
Keywords: osteoclast, receptor activator of nuclear-κB ligand, zoledronic acid, osteoporosis, bone resorption
Introduction
Bone is a solid yet dynamic organ, which is in a constant state of remodelling. These remodelling activities occur throughout the entire lifespan of an individual and are tightly regulated by the cooperation of osteoblast-mediated disruption and the osteoclast-mediated facilitation of bone resorption. These two cell types are normally in balance to maintain homeostasis and warrant a constant amount of healthy bone (1). However, the imbalance between bone formation and resorption may lead to severe skeletal diseases, including osteoporosis and osteopetrosis (2,3).
Osteoclasts are members of the monocyte/macrophage hematopoietic lineage with specific morphological characteristics, including multiple nuclei and ruffled borders (4,5). Their number and resorptive function are usually increased in osteoporosis. Since osteoclasts are unique in their ability to resorb bone (6), the formation of mature multi-nucleated osteoclasts is a critical event in the development of osteoporosis (7,8). Furthermore, osteoclasts have become one of the key targets for the treatment of osteoporosis. The formation of osteoclasts includes two critical steps, namely a commitment of the mono-nuclear cell lineages to become pre-osteoclasts and cell-cell fusion to generate multinucleated giant osteoclasts (9). Each step may serve as a potential target for therapeutic intervention on osteoporosis. However, it has been verified that intervention on the first step may have severe adverse effects on the hematopoietic system (10). The strategy of interfering with the second step has been confirmed to attenuate the efficiency of bone resorption by decreasing the actin-rich structure of podosomes (10). Fusion failure may lead to an obvious reduction of bone-resorbing activity and an increase in bone mass, as observed in osteopetrosis (11). However, excessive bone resorption by osteoclasts is also involved in the pathogenesis of bone-associated disorders, including osteoporosis.
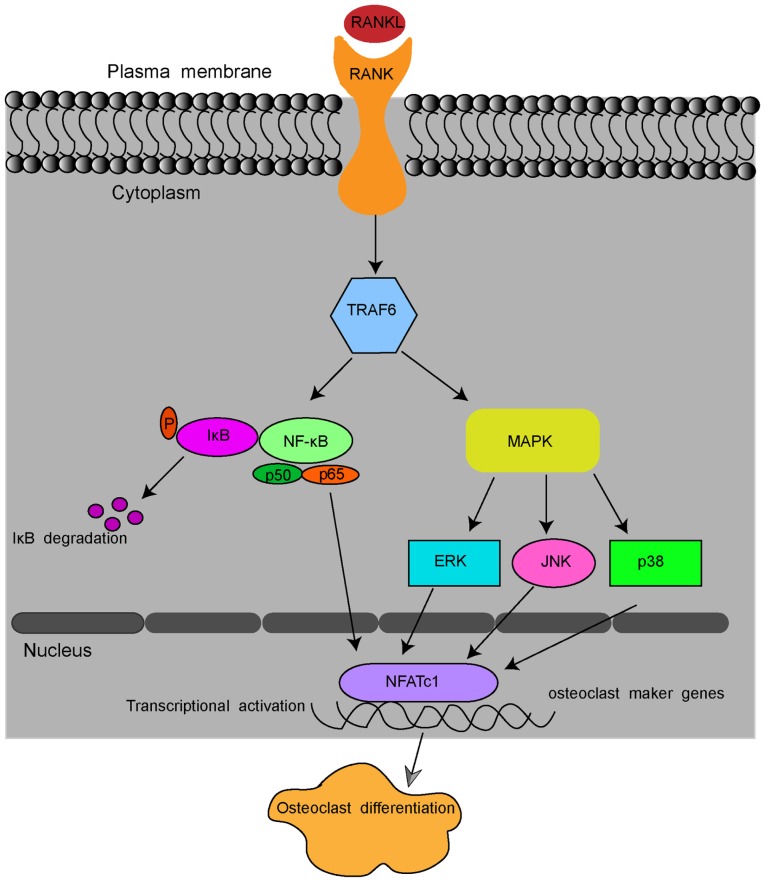
Pre-osteoclastic RAW264.7 cells may be induced into osteoclasts by receptor activator of nuclear factor-κB ligand (RANKL). RANKL is a member of the tumour necrosis factor (TNF) family, and it is the most significant for the process of osteoclast formation and activation (12,13). After binding to its receptor, RANK, RANKL stimulates the osteoclastic differentiation of monocyte macrophages and the maturation of osteoclasts (14,15). In detail, RANKL binding to RANK recruits TNF receptor-associated factor 6 (TRAF6) and sequentially activates the transcription factors, NF-κB, and several inflammation-associated mitogen-activated protein kinase (MAPK) pathways, including extracellular signal-regulated kinase (ERK)1/2, c-Jun N-terminal kinase (JNK) and p38 pathways (16-18). These pathways, in turn, stimulate the key transcription factors nuclear factor of activated T cells 1 (NFATc1) and c-Fos (19,20). Stimulated NFATc1 translocates into the nucleus and activates the expression of osteoclast marker genes, including RANK, calcitonin receptor (CTR), tartrate-resistant acid phosphatase (TRAP) and dendritic cell-specific transmembrane protein (DC-STAMP), which enable osteoclastogenesis and bone resorption by osteoclasts (Fig. 1) (6,21).
Figure 1.
Schematic presentation of RANKL-induced osteoclast formation. By binding to RANK, RANKL recruits TRAF6 and sequentially activates transcription factors NF-κB, ERK1/2, JNK and p38 pathways. The signal is then transmitted to NFATc1 and c-Fos. Stimulated NFATc1 translocates into the nucleus and activates the expression of osteoclast marker genes, including RANK, CTR, TRAP and DC-STAMP. RANKL, receptor activator of nuclear κB ligand; CTR, calcitonin receptor; TRAP, tartrate-resistant acid phosphatase; DC-STAMP, dendritic cell-specific transmembrane protein; NFATc1, nuclear factor of activated T cells 1; TRAF6, tumour necrosis factor-associated factor 6. NF-κB, nuclear factor-κB; ERK1/2, extracellular regulated protein kinases; JNK, c-Jun N-terminal kinase.
Zoledronic acid (ZOL), a third-generation, nitrogen-containing, long-acting bisphosphonate, has been identified to significantly increase bone mineral density. It is used for the treatment of osteoporosis and decreases the incidence of osteoporotic fractures in patients with post-menopausal conditions when applied systemically by intravenous infusion once a year (22,23). Such agents are known to act by inhibiting osteoclast proliferation and inducing the apoptosis of osteoclasts (24,25). There are pioneer studies on the effect of nitrogen-containing bisphosphonates, e.g., minodronate and alendronate, on osteoclastogenesis (26,27). However, the mechanisms through which ZOL inhibits osteoclastogenesis remain to be fully elucidated (26,28). The present study aimed to further explore the effects of ZOL on RANKL-induced osteoclast differentiation and bone resorption activity in vitro, and to further explore the underlying mechanisms.
Materials and methods
Cells, reagents and antibodies
The RAW264.7 mouse macrophage (osteoclast precursor) cell line was obtained from the American Type Culture Collection. Foetal bovine serum (FBS) and the alpha modification of Eagle's medium (α-MEM) were purchased from Gibco-BRL (Thermo Fisher Scientific, Inc.). The cell counting kit (CCK-8) was purchased from Dojindo Laboratories. ZOL (dissolved in purified water) and a TRAP staining kit (387A, Sigma-Aldrich) were obtained from Sigma-Aldrich (Merck KGaA). DAPI and TRITC phalloidin were purchased from Beijing Solarbio. The recombinant human soluble RANK ligand (sRANKL) was purchased from R&D Systems. Polyvinylidene difluoride (PVDF) membranes were obtained from EMD Millipore. Specific antibodies against p38 (#8690), phospho-p38 (p-p38, #4511) (Thr180/Tyr182), IκBα (#4812), phospho-IκBα (p-IkBa, #2859) (Ser32), extracellular signal-regulated kinase 1/2 (ERK1/2, #9102), phospho-ERK (p-ERK, #4370) (Thr202/Tyr204), c-Jun N-terminal kinase (JNK, #9252), phospho-JNK (p-JNK, #4668) (Thr183/Tyr185), p65(#8242), phospho-p65 (p-p65, #3033) were obtained from Cell Signaling Technology. HRP-conjugated goat- anti-rabbit IgG secondary antibody (#014-090P) was purchased from Bioprimacy.
Cell culture
The RAW264.7 cells were cultured in α-MEM supplemented with antibiotics (100 units of penicillin and 100 µg/ml streptomycin, purchased from HyClone) and 10% heat-inactivated FBS at 37°C in a humidified atmosphere of 95% air and 5% CO2. The cells were used after 3-5 passages in α-MEM.
Cell viability assay
The effects of various concentrations of ZOL on the growth and viability of RAW264.7 cells in the presence or absence of RANKL were evaluated using a CCK-8 kit. In brief, the RAW264.7 cells were seeded into 3 96-well plates, at a density of 3×103 cells/well and cultured in α-MEM supplemented with 10% FBS and 1% penicillin and streptomycin for 24 h. The medium was discarded and serially diluted ZOL (0, 0.1, 1, 5, 15, 30 and 50 µM) with or without 100 ng/ml RANKL were added to the cells at the same time, followed by further incubation at 37°C for 24, 48 or 72 h, respectively. A total of 10 µl CCK-8 reagent was added to each well, and the cells were incubated for an additional 2 h at 37°C with 5% CO2. The optical density at 450 nm was read on an ELX800 microplate reader (BioTek Instruments), and the background reading (medium) was subtracted. Six replicates were used for each condition, and the experiments were repeated at least 3 times. The cell growth curves of the ZOL-treated cells were generated using GraphPad Prism 6.0 (GraphPad Software, Inc).
In vitro osteoclastogenesis assays
RAW264.7 cells differentiate into osteoclast-like cells in the presence of RANKL. The cells were seeded in 96-well tissue culture plates at a density of 1.5×103 cells/well with α-MEM (supplemented with 10% FBS and 1% penicillin-streptomycin) and incubated at 37°C overnight to allow the cells attach to the inner surface of a 6-well plate, and the cell culture was then supplemented with (RANKL group) or without (RANKL-free and ZOL-free, denoted vehicle group) 100 ng/ml RANKL and various concentrations of ZOL (0, 0.1, 1 or 5 µM) for 5 days at 37°C, and the cell culture medium were replaced with fresh complete medium every 2 days until a large number of mature osteoclasts formed in the group treated with RANKL only. In addition, RAW264.7 cells treated with or without 1 µM ZOL and 100 ng/ml RANKL were cultured for 3, 5 or 7 days at 37°C. To determine osteoclast differentiation at the end of each incubation, the cells were washed twice and fixed with 4% paraformaldehyde for 20 min. The TRAP staining kit was then used to stain for TRAP, an osteoclast marker, according to the manufacturer's instructions. TRAP-positive multinucleated cells with >3 nuclei identified under an inverted microscope (Olympus IX 51) were considered as osteoclast-like cells.
Immunofluorescence
RAW264.7 cells, cultured on glass coverslips, were treated with or without 100 ng/ml RANKL and 0, 0.1 or 5 µM ZOL until mature osteoclasts appeared in the control wells. To detect the formation of F-actin rings and nuclei, the cells were stained with TRITC phalloidin and DAPI, and analysed according to the manufacturer's instructions. In brief, the osteoclasts were fixed with 4.0% paraformaldehyde in PBS for 20 min. After washing with PBS 3 times, the cells were permeabilized using 0.25% (v/v) Triton X-100 for 5 min, followed by blocking in blocking buffer (3% bovine serum albumin in PBS, Thermo Fisher Scientific) for 1 h and then washed 3 times with PBS again. F-actin rings were stained with TRITC rhodamine-conjugated TRITC phalloidin and the nuclei with DAPI at room temperature for 30 min or 30 sec, respectively. Finally, the cells were washed with PBS and observed under a fluorescence microscope (BX51; Olympus) and the fluorescence images were obtained using Zeiss ZEN software (Zen 2.6, Zeiss AG).
Resorption pit assay
The resorptive function of the mature osteoclasts derived from the RANKL-differentiated RAW264.7 cells was analysed on sterile bovine bone slices (IDS Nordic), which were placed in 96-well plates with 3 replicates for each condition. The RAW264.7 cells were plated at a density of 1.5×103 cells/well onto the bovine bone slices. The cells were treated with 100 ng/ml RANKL and 0, 0.1 or 5 µM ZOL to induce osteoclast differentiation. After 10 days of culture (medium was changed every 48 h), all cells were removed from the bone slices, and the resorption pits were then visualised under a scanning electron microscope (Hitachi E-1010). The total number and area of resorption pits was quantified and compared using Image J software 6.0 (National Institutes of Health).
RNA extraction and reverse transcription-quantitative (RT-qPCR)
The RAW264.7 cells were seeded onto 6-well plates at a density of 1×105 cells per well and cultured in complete α-MEM in the presence or absence of 100 ng/ml RANKL. These cells were then incubated with 0, 0.1, 1 or 5 µM ZOL at 37°C for 3 days until mature osteoclasts formed. The cells were transferred into a tube containing TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and total RNA was isolated according to the manufacturer's instructions. Complementary DNA was synthesised from 1 mg total RNA using PrimeScript™ reverse transcriptase (2690A, Takara Bio Inc.) and stored at −70°C until further use. RT-qPCR was performed to verify the differential expression of the specific genes during osteoclast formation or of GAPDH using the SYBR® Premix Ex Taq™ kit (Takara Bio Inc.). For the analysis of mRNAs encoding osteoclastogenic proteins and osteoclast-specific markers, TRAP, CTR, RANK, NFATc1, c-Fos, DC-STAMP and GAPDH were amplified. The specific primer sequences are listed in Table I.
Table I.
Sequences of primers used in RT-qPCR.
| Primer | Gene sequence |
|---|---|
| Mouse CTR forward | 5′-GTCCAGAGTGAAAAGGCGGA-3′ |
| Mouse CTR reverse | 5′-AGGGCAACTGATGAATCCGG-3′ |
| Mouse TRAP forward | 5′-AAGAGATCGCCAGAACCGTG-3′ |
| Mouse TRAP reverse | 5′-TTCCAGCCAGCACATACCAG-3′ |
| Mouse RANK forward | 5′-TTCGACTGGTTCACTGCTCC-3′ |
| Mouse RANK reverse | 5′-TCAGGTGCTTTTCAGGGGAC-3′ |
| Mouse DC-STAMP forward | 5′-CCCTTGGGCTGTTCTTCCTT-3′ |
| Mouse DC-STAMP reverse | 5′-AGGAATGCAGCTCGGTTCAA-3′ |
| Mouse NFATc1 forward | 5′-GACCGAAGATACCTGGCTCG-3′ |
| Mouse NFATc1 reverse | 5′-GTCAGAAGTGGGTGGAGTGG-3′ |
| Mouse c-Fos forward | 5′-CCGGTTCCTTCTATGCAGCA-3′ |
| Mouse c-Fos reverse | 5′-GCTTGGGAAGGAGTCAGCTT-3′ |
| Mouse GAPDH forward | 5′-GGTTGTCTCCTGCGACTTCA-3′ |
| Mouse GAPDH reverse | 5'-TGGTCCAGGGTTTCTTACTCC-3′ |
CTR, calcitonin receptor; TRAP, tartrate-resistant acid phosphatase; RANK, receptor activator of nuclear factor κB; DC-STAMP, dendritic cell-specific transmembrane protein; NFATc1, nuclear factor of activated T cells 1.
The thermocycling conditions for PCR were as follows: Initial denaturation for 1 min at 95°C, followed by 40 cycles of 95°C for 15 sec and extension at 60°C for 1 min. The 2-∆∆Cq method (29) was used to calculate relative mRNA expression as described previously, and each sample was run and analysed in triplicate. The expression levels of each gene in all experimental groups were normalised to the endogenous reference gene (GAPDH) and indicated as relative fold changes of the control.
Protein preparation and western blot analysis
The RAW264.7 cells were seeded in 6-well plates at a density of 1×106 cells/well, and following incubation at 37°C overnight, the cells were pre-treated with or without 5 µM ZOL for 4 h, and then cultured with 100 ng/ml RANKL for a further 0, 5, 10, 20, 30 or 60 min. Whole-cell lysates were prepared from harvested cells using radioimmunoprecipitation assay buffer consisting of 150 mM NaCl, 50 mM Tris-HCl, 5 mM EDTA, 1% Triton X-100, 1 mM sodium vanadate, 1 mM sodium fluoride, 1% deoxycholate and protease inhibitors. Cell debris was removed by centrifugation at 12,000 × g at 4°C for 10 min. The lysates were boiled in the loading buffer for 10 min and the protein concentrations in the whole-cell extracts were quantified using the bicinchoninic acid method (Beijing Solarbio). Total protein (30 µg per lane) was then separated by 10% SDS-PAGE and transferred onto PVDF membranes. After blocking in 5% non-fat milk in Tris-buffered saline containing Tween-20 at room temperature for 2 h, the membranes were incubated with a 1:1,000 dilution of the indicated primary antibodies at 4°C overnight, followed by horseradish-peroxidase-conjugated secondary antibodies diluted at 1:10,000 in the blocking buffer at room temperature for 1 h. After washing, the membranes were soaked in enhanced chemiluminescence solution (ECL, Millipore) for 1 min, and the bands were detected using the Gene Gnome Imaging System (Syngene). The band intensities were quantified using ImageJ software 1.48 q (NIH). Phosphorylated proteins (e.g., p-p65, p-IkBa, p-ERK1/2, p-p38 and p-JNK) were visualized by its specific primary antibody and corresponding secondary antibody. To detect total protein in the same membrane, antibodies, detecting each phosphorylated protein, were stripped from the membranes by using Stripping Buffer purchased from Solarbio® LIFE SCIENCE (SW3020, Beijing). Antibody-free membranes were then re-incubated with antibodies binding to total proteins (e.g., p65, IκBa, p38, JNK). The GAPDH protein was used as an internal reference.
Statistical analysis
All experiments were performed 3 times and values are expressed as the means ± standard deviation. The groups were compared using one-way or two-way ANOVA analysis of variance followed by Tukey's post-hoc test for multiple comparisons. All data analyses were performed with GraphPad Prism 6.0 (GraphPad Software, Inc) and differences between means were considered statistically significant at P<0.05.
Results
Cytotoxic effects of ZOL on RAW264.7 cells and osteoclasts
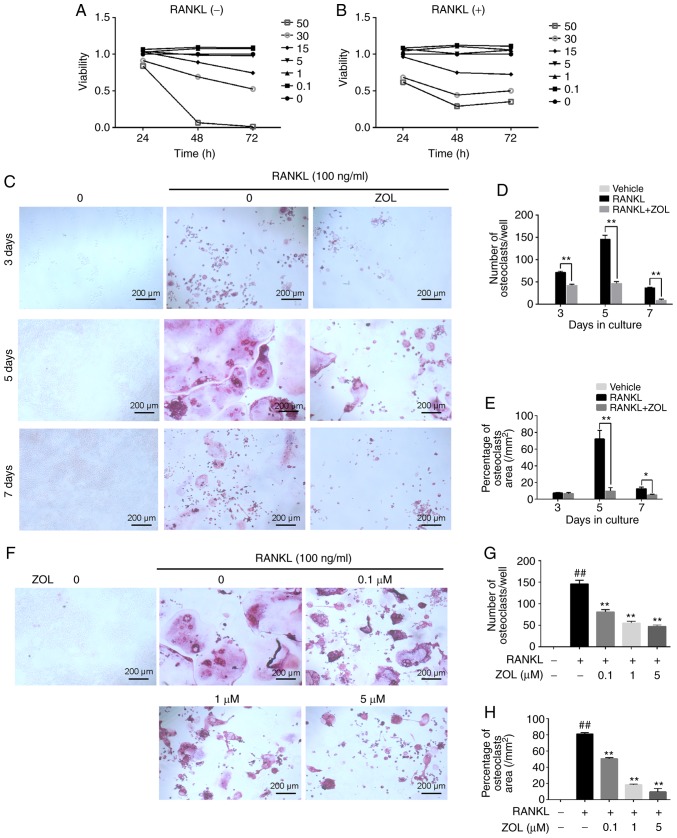
The effect of ZOL on the viability of RAW264.7 cells in the presence or absence of RANKL was assessed using a CCK-8 assay. First, the cytotoxicity of ZOL (0, 0.1, 1, 5, 15, 30 and 50 µM) on RAW264.7 cells without RANKL was analysed. The results indicated that the cell growth was suppressed by ZOL at concentrations of 15, 30 and 50 µM (Fig. 2A). Subsequently, the cytotoxicity of ZOL (at the same concentrations) in the presence of 100 ng/ml RANKL was assessed. As expected, the result was similar to that observed in the RANKL-free group (Fig. 2B).
Figure 2.
ZOL inhibits RANKL-induced osteoclast differentiation without cytotoxicity. RAW264.7 cells were cultured with ZOL (0, 0.1, 1, 5, 15, 30 or 50 µM). (A and B) A Cell Counting Kit-8 assay was performed to determine cell viability at various time-points (24, 48 and 72 h). The results (A) without or (B) with 100 ng/ml RANKL were plotted as cellular growth curves. (C) RAW264.7 cells were incubated with different concentrations of ZOL (0, 0.1, 1 and 5 µM) in the presence or absence of 100 ng/ml RANKL for 5 days, and then stained using a TRAP staining kit. (D and E) The numbers and percentages of osteoclasts were determined. (F) RAW264.7 cells were cultured with or without 1 µM ZOL, and then stained for TRAP at 3, 5 and 7 days, respectively. (G and H) Osteoclast numbers and area percentage were counted at 3, 5 and 7 days, respectively. ##P<0.01 vs. the vehicle group; *P<0.05, **P<0.01 vs. the RANKL-only group. ZOL, zoledronic acid; RANKL, receptor activator of nuclear-κB ligand; TRAP, tartrate-resistant acid phosphatase.
ZOL suppresses the RANKL-induced osteoclastic differentiation of RAW264.7 cells
After excluding the possibility that the inhibitory effects of ZOL on TRAP activity were due to cytotoxicity at concentrations of up to 5 µM, the effects of ZOL on RANKL-induced osteoclastogenesis were assessed. In the RANKL group, the RAW264.7 cells exhibited characteristic morphological changes toward osteoclasts at day 3 of RANKL-induced differentiation, with increasing cell-cell fusion into large and multinucleate TRAP-positive osteoclast cells, reaching completion at day 5 (Fig. 2C). However, the number of osteoclasts (Fig. 2D) and percentage of the osteoclast area (Fig. 2E) was significantly suppressed by incubation with 1 µM ZOL for different periods of time (3, 5 and 7 days). Furthermore, ZOL suppressed osteoclastogenesis in a dose-dependent manner (Fig. 2F-H). Taken together, these results suggest that ZOL inhibits osteoclast formation.
ZOL inhibits the formation of F-actin rings and multiple nuclei
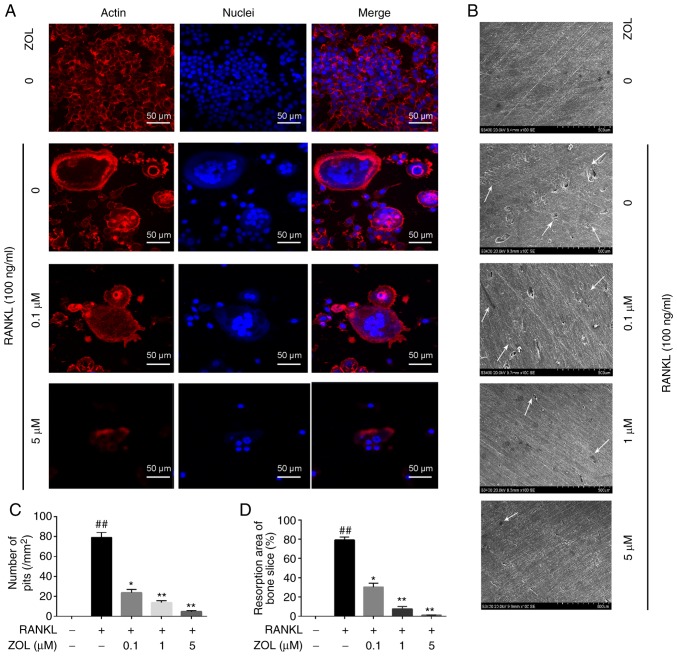
Mature osteoclasts contain actin ring structures that create sealing zones between the cells and bone matrix, and it is a prerequisite for osteoclast bone resorption (30,31). Thus, immunofluorescence analysis was performed to examine the effects of ZOL on F-actin rings and cell nuclei. Well-structured F-actin rings were observed by confocal fluorescence microscopy in the sealing zones of RANKL induced osteoclasts (Fig. 3A). However, the formation of the F-actin ring and the gathering of nuclei was markedly inhibited by ZOL in a concentration-dependent manner. Therefore, osteoclast morphology appeared abnormal or immature (Fig. 3A).
Figure 3.
ZOL inhibits RANKL-induced gathering of nuclei, F-actin ring formation and bone resorptive activity. (A) RAW264.7 cells were treated with various concentrations of ZOL in the presence or absence of 100 ng/ml RANKL until mature osteoclasts were observed. Cell nuclei and F-actin rings were stained with DAPI and TRITC phalloidin, respectively. Fluorescence was detected by using a confocal microscope. (B) RAW264.7 cells were treated with various concentrations of ZOL in the presence or absence of 100 ng/ml RANKL until mature osteoclasts formed. Bone resorption pits (indicated by white arrows) were visualized under a scanning electron microscope. (C) The number of pits and (D) the resorption area was determined. ##P<0.01 vs. the vehicle group; *P<0.05, **P<0.01 vs. the RANKL-only group. ZOL, zoledronic acid; RANKL, receptor activator of nuclear-κB ligand.
Effects of ZOL on bone resorption in RANKL-induced RAW264.7 cells
We then investigated whether ZOL modulates mature osteoclast activity by performing a resorption pit assay. RAW264.7 cells were plated on bone slices, which were treated with various concentrations of ZOL in the presence or absence of 100 ng/ml RANKL. The results indicated that the area of osteoclast bone resorption pits was markedly decreased by ZOL in a dose-dependent manner compared with the ZOL-free group. Furthermore, almost no resorption pits were observed in the groups treated with 5 µM ZOL (Fig. 3B-D). These results suggested that treatment with ZOL markedly attenuates the bone-resorption activity of osteoclasts. This may, at least partially, be explained by the effect of ZOL to impair osteoclastogenesis.
Effects of ZOL on the mRNA expression of osteoclast differentiation-specific genes in RAW264.7 cells
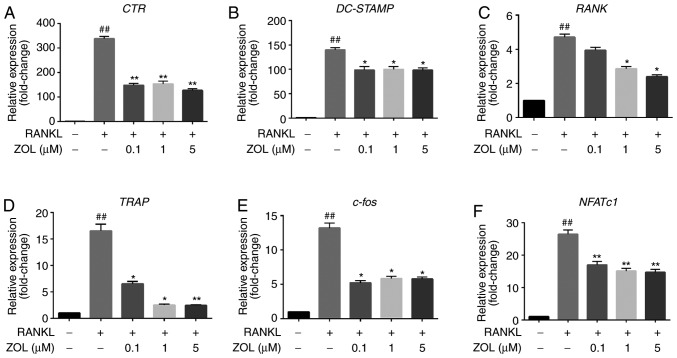
To further elucidate the effects of ZOL on osteoclast formation and resorptive function, the expression of specific osteoclast differentiation-associated genes in ZOL-treated cells was assessed by RT-qPCR. It was indicated that treatment with RANKL markedly increased the expression levels of CTR, RANK, TRAP, DC-STAMP, NFATc1 and c-Fos. However, this upregulation was significantly suppressed by ZOL in a dose-dependent manner during osteoclastogenesis compared with that in the ZOL-free group (Fig. 4). These results suggest that ZOL inhibits the expression of RANKL-induced genes involved in osteoclast differentiation and function.
Figure 4.
ZOL inhibits RANKL-induced osteoclast-specific gene expression. RAW264.7 cells were cultured with or without 100 ng/ml RANKL and treated with 0, 0.1, 1 or 5 µM ZOL. (A-F) The expression of osteoclast-specific genes (CTR, DC-STAMP, RANK, TRAP, c-Fos and NFATc1) was detected by RT-qPCR. Results were normalized to the expression of the GAPDH gene. ##P<0.01 vs. the vehicle group; *P<0.05, **P<0.01 vs. the RANKL-only group. CTR, calcitonin receptor; TRAP, tartrate-resistant acid phosphatase; DC-STAMP, dendritic cell-specific transmembrane protein; ZOL, zoledronic acid; RANKL, receptor activator of nuclear-κB ligand; NFATc1, nuclear factor of activated T cells 1.
ZOL inhibits NF-κB and JNK signalling
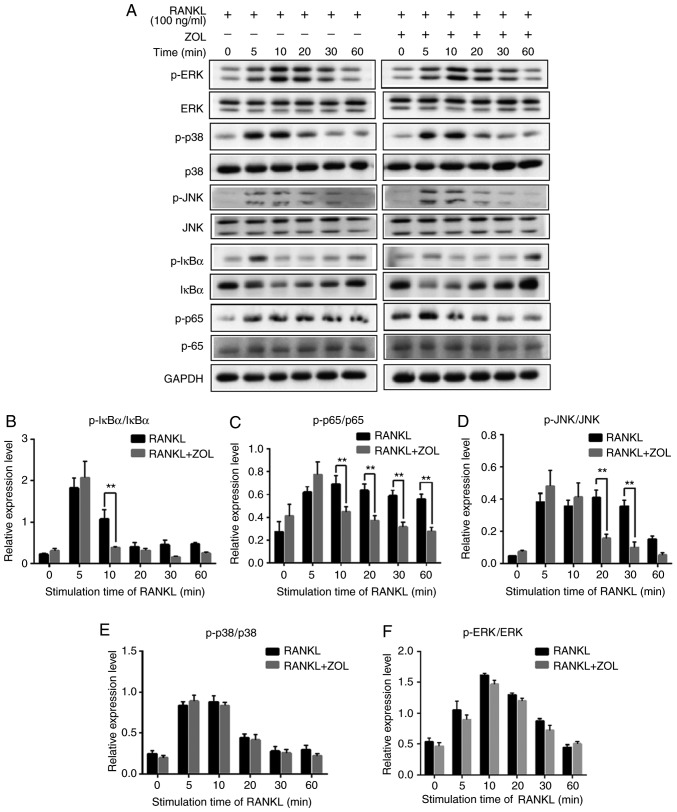
Previous studies have revealed that NF-κB, p38, ERK1/2 and JNK play critical roles in osteoclast differentiation (Fig. 1) (32-34). To explore the pathways through which ZOL regulates osteoclastogenesis, the protein levels of RANKL-induced signalling pathways were investigated by western blot analysis. As presented in Fig. 5A, the rapid activation of NF-κB was detected by the phosphorylation of IκBα, the inhibitor of NF-κB, at 5 min following RANKL exposure. As expected, RANKL treatment induced a significant increase in the RANKL-induced phosphorylation of p65. In addition, induction with RANKL markedly increased the phosphorylation levels of p38, ERK and JNK, which exhibited a maximum increase at 10 or 20 min (Fig. 5A).
Figure 5.
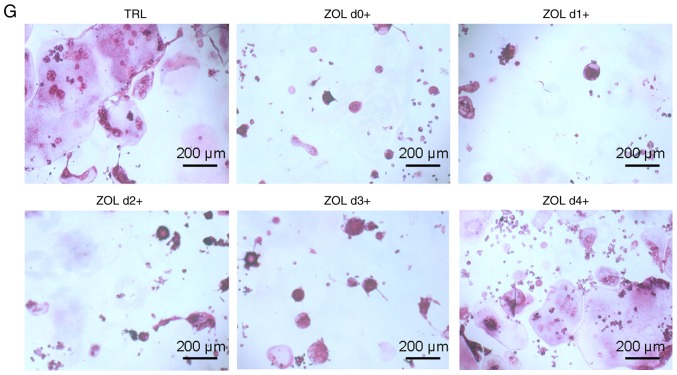
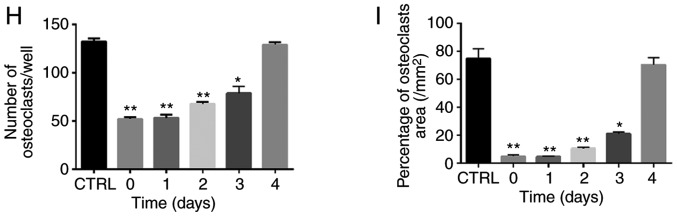
ZOL specifically attenuates RANKL-induced NF-κB and JNK signalling. (A) RAW264.7 cells were induced with RANKL for the indicated times following pre-treatment with ZOL (5 µM) for 4 h. Western blot analysis was performed using specific antibodies. The band intensities were quantified using Image J software. The ratios of band intensity of (B) p-IκBα/IκBα, (C) p-p65/p65, (D) p-JNK/JNK, (E) p-p38/p38 and (F) p-ERK/ERK are shown. (G) ZOL inhibited osteoclast differentiation at an early stage. RAW264.7 cells were treated with or without 5 µM ZOL at days 0, 1, 3 and 4. They were subsequently treated with 100 ng/ml RANKL for 5 days, and then subjected to TRAP staining. (H) The number and (I) area of osteoclasts were quantified. *P<0.05, **P<0.01 vs. the RANKL-only group. TRAP, tartrate-resistant acid phosphatase; ZOL, zoledronic acid; RANKL, receptor activator of nuclear-κB ligand.
To further examine the influence of ZOL on NF-κB and MAPK mediated osteoclast differentiation, the intensity of each phosphorylated protein was divided by the intensity of corresponding total protein in both RANKL and RANKL+ZOL treated groups. The results revealed that ZOL significant inhibited RANKL-dependent phosphorylation of IκBα (Fig. 5B) and p65 (Fig. 5C). Among the MAPK family proteins, the phosphorylation of JNK (Fig. 5D) was significantly inhibited by ZOL. However, the phosphorylation of p38 and ERK proteins were not significantly affected by ZOL (Fig. 5E and F). Thus, these results indicate that ZOL may inhibit NF-κB and JNK signalling by reducing the levels of p-IκBα, p-p65 and p-JNK. In other words, ZOL may reduce the formation of osteoclasts by suppressing the RANKL-induced activation of the NF-κB and JNK signalling pathways.
As reported previously, JNK and NF-κB signalling play a vital role at the early stage of osteoclast differentiation (35-37). Thus, it was further explored whether ZOL also suppresses early-stage osteoclast formation. Following the addition of RANKL, the RAW264.7 cells were treated with ZOL on days 0, 1, 2, 3 and 4. It was observed that treatment with ZOL at the early stage resulted in a prominent decrease in osteoclastogenesis in the RAW264.7 cells (Fig. 5G). However, the extent of osteoclastogenesis was comparable to that in the control group if ZOL was added at a later stage (Fig. 5H and I). These results thus indicate that ZOL suppresses the early stage of osteoclast formation, which is consistent with the findings of western blot analysis, according to which ZOL suppressed NF-κB and JNK signalling at the early stage of osteoclast differentiation.
Discussion
Osteoporosis is a silent disease and remains a major health concern; it is characterized by low bone mineral density and quality, as well as an abnormal microarchitecture of bone tissue (38). A previous study indicated that nitrogen-containing bisphosphonates, e.g., minodronate and alendronate, inhibit osteoclastogenesis (26,27). ZOL belongs to the class of nitrogen-containing bisphosphonates and is widely used to prevent bone loss. Kimachi et al (26) indicated that ZOL inhibited RANK expression and the migration of osteoclast precursors during osteoclastogenesis, and that the inhibitory effects on RANK expression were likely to be associated with the suppression of the NF-κB pathway. However, the mechanisms of the inhibitory effects of ZOL on osteoclastogenesis remain to be fully elucidated (26,28). In the present study, the effects of ZOL on osteoclastogenesis were explored by using RANKL-induced RAW264.7 cells as a model. The results indicated that ZOL inhibited osteoclast formation in dose-dependent manner at the early stage. It was also demonstrated to impair the formation of the actin cytoskeleton and the bone resorption ability of RANKL-induced Raw264.7 cells. Furthermore, it was revealed that ZOL inhibited osteoclastogenesis through the NF-κB and JNK pathways, as indicated by the inhibition of the RANKL-induced expression CTR, RANK, TRAP, DC-STAMP, NFATc1 and c-Fos genes by ZOL.
The NF-κB signalling pathway may be activated by the binding of RANKL to RANK (39,40). RANKL/RANK/TRAF6 signalling may activate IKK and subsequently, IκB-α becomes phosphorylated and is degraded (20). As a result, NF-κB is released and translocated to the nucleus to increase the expression of NFATc1 and c-Fos, which have been identified as two important transcription factors that regulate osteoclast formation via initiating the transcription of certain downstream targets that are osteoclastogenesis-associated genes (41-43). Previous studies using gene knock-out experiments have indicated that mice lacking NF-κB dimmers may not form osteoclasts normally and present with serious osteopetrosis (44,45). The present study suggested that ZOL exerts an inhibitory effect on the RANKL-induced degradation of IκB-α and phosphorylation. These results suggest that ZOL may attenuate RANKL-induced osteoclastogenesis by blocking the NF-κB signalling pathway.
Another group of essential signalling pathways, namely MAPKs, are downstream pathways of RANKL/RANK/TRAF6 signalling. The RANKL-RANK interaction results in the phosphorylation of MAPKs, including JNK, p38 and ERK, promoting the activation of c-Fos and facilitating the translocation of activator protein-1, an essential translation factor for osteoclast formation. Previous studies have confirmed that inhibitors of p38, JNK or ERK inhibit osteoclast formation (46,47). In the present study, ZOL diminished the phosphorylation of JNK induced by RANKL. It may be speculated that the blockade or downregulation of NF-κB and JNK signalling pathways by ZOL may result in decreased expression of downstream molecules required for osteoclast differentiation. Thus, it may be suggested that ZOL exerts a marked inhibitory activity on osteoclast differentiation through the inhibition of NF-κB and JNK signalling. The present results not only testified the conclusion drawn in the study by Kimachi et al (26), but also further indicated that the JNK pathway was inhibited by ZOL. However, further studies are required to determine the biological efficacy of ZOL in in vitro or in vivo models and selective inhibitors of NF-κB or JNK should also be administrated to investigate the expression of associated proteins, so as to further verify the present results.
In recent years, bisphosphonate therapy has been prescribed for an increasing number of patients with osteoporosis and bone cancer metastasis. Despite these significant advances, the evidence for bisphosphonate-related osteonecrosis of the jaw (BRONJ), first noted in 2003 and now widely recognised as a complication of bisphosphonate therapy, has been increasingly regarded as a limitation (26). The majority of reported cases of bisphosphonate osteonecrosis were caused by dental extractions, intraoral surgical intervention or mucosal trauma (12). Previous studies have also reported on the development of BRONJ along with bacterial infection (48-50). However, the pathogenic mechanisms of BRONJ remain elusive and successful treatments are currently unavailable. In the present study, the cytotoxic effects of ZOL on RAW264.7 cells and osteoclasts were first explored. It was revealed that low concentrations of ZOL (0.1-5 µM) were non-toxic, but suppressed osteoclast formation in a dose- and time-dependent manner during osteoclast precursor differentiation. Based on this finding, it can by hypothesized that exposure to ZOL at appropriate dosages and for suitable durations may provide a benefit in the treatment of osteoporosis. However, beyond this, it may be expected to have severe side-effects, e.g., BRONJ. Thus, it is suggested that avoiding overexposure to ZOL may be an effective way to avoid BRONJ. In addition, clinical investigations are still required to develop novel therapeutic agents that do not cause these side-effects.
In dental implantation, osteoporosis may result in poor primary stability and subsequent prosthetic loosening, as well as severe inflammatory bone loss, e.g., peri-implantitis. Therefore, it is widely discussed whether patients who have osteoporosis are suitable for tooth implantation. Numerous attempts have been made to identify a reliable therapeutic strategy to prevent osteoporosis (51-53). Since the RANKL/RANK interaction is mechanistically involved in the pathological processes of bone loss, it has received a large amount of attention. RANKL targeted therapy has been a valid target for the modulation of bone formation and resorption as an approach for the development of anti-osteoporotic and anti-resorptive drugs. For instance, the Food and Drug Administration of the USA has approved the anti-RANKL monoclonal antibody denosumab, which acts by decreasing bone resorption, for the treatment of post-menopausal women with osteoporosis (43,54,55). Furthermore, a large international clinical trial demonstrated that osteoporotic patients treated with ZOL exhibited significant improvements in bone mineral density and bone metabolism markers. Treatment with ZOL reduces the risk of vertebral fracture by 70% and hip fracture by 41% over 3 years relative to placebo (56,57). Therefore, discovering the underlying mechanisms of the effects of ZOL to prevent bone loss may further promote the development of drugs for the treatment of osteoporosis.
In conclusion, the present results may shed light on the mechanisms of action of ZOL and the pathology of BRONJ. The optimal dosage and timing of ZOL administration should be further determined to enhance the prospects of this drug as a candidate for the treatment of osteoporosis.
Acknowledgments
Not applicable.
Funding
The present study was supported by the Natural Science Foundation of China (grant nos. 81660179, 31560318 and U1812403) and the Science and Technology Foundation of Guizhou Province [grant no. (2016)1124].
Availability of data and materials
All data generated or analysed during this study are included in this published article or are available from the corresponding author on reasonable request.
Authors' contributions
JL, WH and ZZG conceived and designed the research. XLH, LYH, YTC, FL, QZ, CW and QHS performed the experiments. WH, XLH and LJ wrote the manuscript. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Kim HS, Suh KS, Sul D, Kim BJ, Lee SK, Jung WW. The inhibitory effect and the molecular mechanism of glabridin on RANKL-induced osteoclastogenesis in RAW264.7 cells. Int J Mol Med. 2012;29:169–177. doi: 10.3892/ijmm.2011.822. [DOI] [PubMed] [Google Scholar]
- 2.Villa A, Guerrini MM, Cassani B, Pangrazio A, Sobacchi C. Infantile malignant, autosomal recessive osteopetrosis: The rich and the poor. Calcif Tissue Int. 2009;84:1–12. doi: 10.1007/s00223-008-9196-4. [DOI] [PubMed] [Google Scholar]
- 3.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 4.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 5.Nijweide PJ, Burger EH, Feyen JH. Cells of bone: Proliferation, differentiation, and hormonal regulation. Physiol Rev. 1986;66:855–886. doi: 10.1152/physrev.1986.66.4.855. [DOI] [PubMed] [Google Scholar]
- 6.Soysa NS, Alles N, Aoki K, Ohya K. Osteoclast formation and differentiation: An overview. J Med Dent Sci. 2012;59:65–74. [PubMed] [Google Scholar]
- 7.Zeng Z, Zhang C, Chen J. Lentivirus-mediated RNA interference of DC-STAMP expression inhibits the fusion and resorptive activity of human osteoclasts. J Bone Miner Metab. 2013;31:409–416. doi: 10.1007/s00774-013-0434-0. [DOI] [PubMed] [Google Scholar]
- 8.Zeng XZ, He LG, Wang S, Wang K, Zhang YY, Tao L, Li XJ, Liu SW. Aconine inhibits RANKL-induced osteoclast differentiation in RAW264.7 cells by suppressing NF-κB and NFATc1 activation and DC-STAMP expression. Acta Pharmacol Sin. 2016;37:255–263. doi: 10.1038/aps.2015.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oikawa T, Kuroda Y, Matsuo K. Regulation of osteoclasts by membrane-derived lipid mediators. Cell Mol Life Sci. 2013;70:3341–3353. doi: 10.1007/s00018-012-1238-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liou YM, Chan CL, Huang R, Wang CA. Effect of l-caldesmon on osteoclastogenesis in RANKL-induced RAW264.7 cells. J Cell Physiol. 2018;233:6888–6901. doi: 10.1002/jcp.26452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Islam R, Bae HS, Yoon WJ, Woo KM, Baek JH, Kim HH, Uchida T, Ryoo HM. Pin1 regulates osteoclast fusion through suppression of the master regulator of cell fusion DC-STAMP. J Cell Physiol. 2014;229:2166–2174. doi: 10.1002/jcp.24679. [DOI] [PubMed] [Google Scholar]
- 12.Abe K, Yoshimura Y, Deyama Y, Kikuiri T, Hasegawa T, Tei K, Shinoda H, Suzuki K, Kitagawa Y. Effects of bisphosphonates on osteoclastogenesis in RAW264.7 cells. Int J Mol Med. 2012;29:1007–1015. doi: 10.3892/ijmm.2012.952. [DOI] [PubMed] [Google Scholar]
- 13.Mediero A, Perez-Aso M, Cronstein BN. Activation of adenosine A(2A) receptor reduces osteoclast formation via PKA- and ERK1/2-mediated suppression of NFκB nuclear trans-location. Br J Pharmacol. 2013;169:1372–1388. doi: 10.1111/bph.12227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/S0092-8674(00)81569-X. [DOI] [PubMed] [Google Scholar]
- 15.Yuan M, Chen J, Zeng Z. Knockdown of macrophage inhibitory cytokine-1 in RPMI-8226 human multiple myeloma cells inhibits osteoclastic differentiation through inhibiting the RANKL-Erk1/2 signaling pathway. Mol Med Rep. 2016;14:5199–5204. doi: 10.3892/mmr.2016.5879. [DOI] [PubMed] [Google Scholar]
- 16.Wada T, Nakashima T, Hiroshi N, Penninger JM. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol Med. 2006;12:17–25. doi: 10.1016/j.molmed.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Huang P, Han J, Hui L. MAPK signaling in inflammation- associated cancer development. Protein Cell. 2010;1:218–226. doi: 10.1007/s13238-010-0019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ihn HJ, Lee D, Lee T, Shin HI, Bae YC, Kim SH, Park EK. The 1,2,3-triazole derivative KP-A021 suppresses osteoclast differentiation and function by inhibiting RANKL-mediated MEK-ERK signaling pathway. Exp Biol Med (Maywood) 2015;240:1690–1697. doi: 10.1177/1535370215576310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Negishi-Koga T, Takayanagi H. Ca2+-NFATc1 signaling is an essential axis of osteoclast differentiation. Immunol Rev. 2009;231:241–256. doi: 10.1111/j.1600-065X.2009.00821.x. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Wang Z, Xie X, Wang J, Wang Y, Peng QS, Zhang M, Wu D, Liu N, Wang HB, Sun WC. Tatarinan N inhibits osteoclast differentiation through attenuating NF-κB, MAPKs and Ca2+-dependent signaling. Int Immunopharmacol. 2018;65:199–211. doi: 10.1016/j.intimp.2018.09.030. [DOI] [PubMed] [Google Scholar]
- 21.Nakashima T, Takayanagi H. Osteoimmunology: Crosstalk between the immune and bone systems. J Clin Immunol. 2009;29:555–567. doi: 10.1007/s10875-009-9316-6. [DOI] [PubMed] [Google Scholar]
- 22.Lambrinoudaki I, Vlachou S, Galapi F, Papadimitriou D, Papadias K. Once-yearly zoledronic acid in the prevention of osteoporotic bone fractures in postmenopausal women. Clin Interv Aging. 2008;3:445–451. doi: 10.2147/CIA.S2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dalle Carbonare L, Zanatta M, Gasparetto A, Valenti MT. Safety and tolerability of zoledronic acid and other bisphosphonates in osteoporosis management. Drug Healthc Patient Saf. 2010;2:121–137. doi: 10.2147/DHPS.S6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benford HL, McGowan NW, Helfrich MH, Nuttall ME, Rogers MJ. Visualization of bisphosphonate-induced caspase-3 activity in apoptotic osteoclasts in vitro. Bone. 2001;28:465–473. doi: 10.1016/S8756-3282(01)00412-4. [DOI] [PubMed] [Google Scholar]
- 25.Yasen M, Li X, Jiang L, Yuan W, Che W, Dong J. Effect of zoledronic acid on spinal fusion outcomes in an ovariectomized rat model of osteoporosis. J Orthop Res. 2015;33:1297–1304. doi: 10.1002/jor.22763. [DOI] [PubMed] [Google Scholar]
- 26.Kimachi K, Kajiya H, Nakayama S, Ikebe T, Okabe K. Zoledronic acid inhibits RANK expression and migration of osteoclast precursors during osteoclastogenesis. Naunyn Schmiedebergs Arch Pharmacol. 2011;383:297–308. doi: 10.1007/s00210-010-0596-4. [DOI] [PubMed] [Google Scholar]
- 27.Tsubaki M, Komai M, Itoh T, Imano M, Sakamoto K, Shimaoka H, Takeda T, Ogawa N, Mashimo K, Fujiwara D, et al. Nitrogen-containing bisphosphonates inhibit RANKL- and M-CSF-induced osteoclast formation through the inhibition of ERK1/2 and Akt activation. J Biomed Sci. 2014;21:10. doi: 10.1186/1423-0127-21-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tai TW, Su FC, Chen CY, Jou IM, Lin CF. Activation of p38 MAPK-regulated Bcl-xL signaling increases survival against zoledronic acid-induced apoptosis in osteoclast precursors. Bone. 2014;67:166–174. doi: 10.1016/j.bone.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.Teitelbaum SL. Osteoclasts: What do they do and how do they do it? Am J Pathol. 2007;170:427–435. doi: 10.2353/ajpath.2007.060834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jurdic P, Saltel F, Chabadel A, Destaing O. Podosome and sealing zone: Specificity of the osteoclast model. Eur J Cell Biol. 2006;85:195–202. doi: 10.1016/j.ejcb.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 32.Stevenson DA, Schwarz EL, Carey JC, Viskochil DH, Hanson H, Bauer S, Weng HY, Greene T, Reinker K, Swensen J, et al. Bone resorption in syndromes of the Ras/MAPK pathway. Clin Genet. 2011;80:566–573. doi: 10.1111/j.1399-0004.2010.01619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suda T, Kobayashi K, Jimi E, Udagawa N, Takahashi N. The molecular basis of osteoclast differentiation and activation. Novartis Found Symp. 2001;232:235–247. doi: 10.1002/0470846658.ch16. discussion 247-250. [DOI] [PubMed] [Google Scholar]
- 34.Li DZ, Zhang QX, Dong XX, Li HD, Ma X. Treatment with hydrogen molecules prevents RANKL-induced osteoclast differentiation associated with inhibition of ROS formation and inactivation of MAPK, AKT and NF-kappa B pathways in murine RAW264.7 cells. J Bone Miner Metab. 2014;32:494–504. doi: 10.1007/s00774-013-0530-1. [DOI] [PubMed] [Google Scholar]
- 35.Ikeda F, Nishimura R, Matsubara T, Tanaka S, Inoue J, Reddy SV, Hata K, Yamashita K, Hiraga T, Watanabe T, et al. Critical roles of c-Jun signaling in regulation of NFAT family and RANKL-regulated osteoclast differentiation. J Clin Invest. 2004;114:475–484. doi: 10.1172/JCI200419657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ikeda F, Matsubara T, Tsurukai T, Hata K, Nishimura R, Yoneda T. JNK/c-Jun signaling mediates an anti-apoptotic effect of RANKL in osteoclasts. J Bone Miner Res. 2008;23:907–914. doi: 10.1359/jbmr.080211. [DOI] [PubMed] [Google Scholar]
- 37.Siddiqi MH, Siddiqi MZ, Kang S, Noh HY, Ahn S, Simu SY, Aziz MA, Sathishkumar N, Jiménez Pérez ZE, Yang DC. Inhibition of osteoclast differentiation by ginsenoside rg3 in RAW264.7 cells via RANKL, JNK and p38 MAPK pathways through a modulation of cathepsin k: An in silico and in vitro study. Phytother Res. 2015;29:1286–1294. doi: 10.1002/ptr.5374. [DOI] [PubMed] [Google Scholar]
- 38.Feng X, McDonald JM. Disorders of bone remodeling. Annu Rev Pathol. 2011;6:121–145. doi: 10.1146/annurev-pathol-011110-130203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu K, Lin TH, Liou HC, Lu DH, Chen YR, Fu WM, Yang RS. Dextromethorphan inhibits osteoclast differentiation by suppressing RANKL-induced nuclear factor-κB activation. Osteoporos Int. 2013;24:2201–2214. doi: 10.1007/s00198-013-2279-8. [DOI] [PubMed] [Google Scholar]
- 40.Kang MR, Jo SA, Yoon YD, Park KH, Oh SJ, Yun J, Lee CW, Nam KH, Kim Y, Han SB, et al. Agelasine D suppresses RANKL-induced osteoclastogenesis via down-regulation of c-Fos, NFATc1 and NF-κB. Mar Drugs. 2014;12:5643–5656. doi: 10.3390/md12115643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee CC, Liu FL, Chen CL, Chen TC, Chang DM, Huang HS. Discovery of 5-(2′,4′-difluorophenyl)-salicylanilides as new inhibitors of receptor activator of NF-κB ligand (RANKL)-induced osteoclastogenesis. Eur J Med Chem. 2015;98:115–126. doi: 10.1016/j.ejmech.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 42.Liu W, Zhang X. Receptor activator of nuclear factor-κB ligand (RANKL)/RANK/osteoprotegerin system in bone and other tissues (review) Mol Med Rep. 2015;11:3212–3218. doi: 10.3892/mmr.2015.3152. [DOI] [PubMed] [Google Scholar]
- 43.Zhao XL, Chen JJ, Si SY, Chen LF, Wang Z. T63 inhibits osteoclast differentiation through regulating MAPKs and Akt signaling pathways. Eur J Pharmacol. 2018;834:30–35. doi: 10.1016/j.ejphar.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 44.Kim HJ, Yoon KA, Lee MK, Kim SH, Lee IK, Kim SY. A novel small molecule, NecroX-7, inhibits osteoclast differentiation by suppressing NF-κB activity and c-Fos expression. Life Sci. 2012;91:928–934. doi: 10.1016/j.lfs.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 45.Leotoing L, Wauquier F, Guicheux J, Miot-Noirault E, Wittrant Y, Coxam V. The polyphenol fisetin protects bone by repressing NF-κB and MKP-1-dependent signaling pathways in osteoclasts. PLoS One. 2013;8:e68388. doi: 10.1371/journal.pone.0068388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kong X, Wu W, Yang Y, Wan H, Li X, Zhong M, Zhao H, Su X, Jia S, Ju D, Lin N. Total saponin from Anemone flaccida Fr. Schmidt abrogates osteoclast differentiation and bone resorption via the inhibition of RANKL-induced NF-κB, JNK and p38 MAPKs activation. J Transl Med. 2015;13:91. doi: 10.1186/s12967-015-0440-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu X, Liu N, Wang Y, Pan LC, Wu D, Peng Q, Zhang M, Wang HB, Sun WC. Tatarinan O, a lignin-like compound from the roots of Acorus tatarinowii Schott inhibits osteoclast differentiation through suppressing the expression of c-Fos and NFATc1. Int Immunopharmacol. 2016;34:212–219. doi: 10.1016/j.intimp.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 48.Fliefel R, Troltzsch M, Kuhnisch J, Ehrenfeld M, Otto S. Treatment strategies and outcomes of bisphosphonate-related osteonecrosis of the jaw (BRONJ) with characterization of patients: A systematic review. Int J Oral Maxillofac Surg. 2015;44:568–585. doi: 10.1016/j.ijom.2015.01.026. [DOI] [PubMed] [Google Scholar]
- 49.Sakaguchi O, Kokuryo S, Tsurushima H, Tanaka J, Habu M, Uehara M, Nishihara T, Tominaga K. Lipopolysaccharide aggravates bisphosphonate-induced osteonecrosis in rats. Int J Oral Maxillofac Surg. 2015;44:528–534. doi: 10.1016/j.ijom.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 50.Wachi T, Shuto T, Shinohara Y, Matono Y, Makihira S. Release of titanium ions from an implant surface and their effect on cytokine production related to alveolar bone resorption. Toxicology. 2015;327:1–9. doi: 10.1016/j.tox.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 51.Baek JM, Kim JY, Lee CH, Yoon KH, Lee MS. Methyl gallate inhibits osteoclast formation and function by suppressing Akt and Btk-PLCgamma2-Ca(2+) signaling and prevents lipo-polysaccharide-induced bone loss. Int J Mol Sci. 2017;18:E581. doi: 10.3390/ijms18030581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun X, Wei J, Lyu J, Bian T, Liu Z, Huang J, Pi F, Li C, Zhong Z. Bone-targeting drug delivery system of biomineral-binding liposomes loaded with icariin enhances the treatment for osteoporosis. J Nanobiotechnology. 2019;17:10. doi: 10.1186/s12951-019-0447-5. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 53.Yasuda H. The mechanism of anti-RANKL antibody in the treatment of metabolic bone diseases including osteoporosis-possible applications of anti-RANKL antibody to the treatment of cancer patients. Nihon Yakurigaku Zasshi. 2019;153:11–15. doi: 10.1254/fpj.153.11. In Japanese. [DOI] [PubMed] [Google Scholar]
- 54.Moen MD, Keam SJ. Denosumab: A review of its use in the treatment of postmenopausal osteoporosis. Drugs Aging. 2011;28:63–82. doi: 10.2165/11203300-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 55.Sidlauskas KM, Sutton EE, Biddle MA. Osteoporosis in men: Epidemiology and treatment with denosumab. Clin Interv Aging. 2014;9:593–601. doi: 10.2147/CIA.S51940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tai TW, Chen CY, Su FC, Tu YK, Tsai TT, Lin CF, Jou IM. Reactive oxygen species are required for zoledronic acid-induced apoptosis in osteoclast precursors and mature osteoclast-like cells. Sci Re. 2017;7:44245. doi: 10.1038/srep44245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lyles KW, Colon-Emeric CS, Magaziner JS, Adachi JD, Pieper CF, Mautalen C, Hyldstrup L, Recknor C, Nordsletten L, Moore KA, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357:1799–1809. doi: 10.1056/NEJMoa074941. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article or are available from the corresponding author on reasonable request.