Acid-sensing ion channels (ASICs) are voltage-independent cation channels that are modulated by extracellular pH fluctuations. Lee et al. identify three neutral residues in the N terminus (T25, T39, and I40) that are critical for rapid resensitization of homomeric and heteromeric ASIC2a channels.
Abstract
Acid-sensing ion channels (ASICs), sensory molecules that continuously monitor the concentration of extracellular protons and initiate diverse intracellular responses through an influx of cations, are assembled from six subtypes that can differentially combine to form various trimeric channel complexes and elicit unique electrophysiological responses. For instance, homomeric ASIC1a channels have been shown to exhibit prolonged desensitization, and acid-evoked currents become smaller when the channels are repeatedly activated by extracellular protons, whereas homomeric or heteromeric ASIC2a channels continue to respond to repetitive acidic stimuli without exhibiting such desensitization. Although previous studies have provided evidence that both the desensitization of ASIC1a and rapid resensitization of ASIC2a commonly require domains that include the N terminus and the first transmembrane region of these channels, the biophysical basis of channel gating at the amino acid level has not been clearly determined. Here, we confirm that domain-swapping mutations replacing the N terminus of ASIC2a with that of ASIC2b result in de novo prolonged desensitization in homomeric channels following activation by extracellular protons. Such desensitization of chimeric ASIC2a mutants is due neither to internalization nor to degradation of the channel proteins. We use site-directed mutagenesis to narrow down the relevant portion of the N terminus of ASIC2a, identifying three amino acid residues within the N terminus (T25, T39, and I40) whose mutation is sufficient to phenocopy the desensitization exhibited by the chimeric mutants. A similar desensitization is observed in heteromeric ASICs containing the mutant subunit. These results suggest that T25, T39, and I40 of ASIC2a are key residues determining the rapid resensitization of homomeric and heteromeric ASIC2a channels upon proton activation.
Introduction
Acid-sensing ion channels (ASICs) are voltage-independent and proton-gated cation channels that respond to acidification of the extracellular environment under physiological and pathological conditions (Price et al., 1996; Waldmann et al., 1997; Yermolaieva et al., 2004; Xiong et al., 2008; Kweon and Suh, 2013; Wemmie et al., 2013; Zhou et al., 2015; Zhang et al., 2017; Qiang et al., 2018). Six subtypes of ASICs (ASIC1a, ASIC1b, ASIC2a, ASIC2b, ASIC3, and ASIC4) are generated by the transcription of four genes, followed by alternative splicing events. Three subunits of the same or different subtypes of ASIC gather to form a functional ion channel (Jasti et al., 2007; Gonzales et al., 2009). Depending on the composition of the subunits, homomeric and heteromeric channels display unique electrophysiological properties (Hesselager et al., 2004; Sherwood et al., 2011). For example, homomeric channels of ASIC1a exhibit a gradual decrease in peak currents in response to repetitive acidic stimuli (Gitterman et al., 2005; Neaga et al., 2005; Chen and Gründer, 2007; Li et al., 2012). This phenomenon has been suggested to be the result of a prolonged desensitization of homomeric ASIC1a channels following pore gating by extracellular protons (Li et al., 2012). Such desensitization has not been detected either in heteromeric channels involving ASIC1a or in homomeric channels consisting of other ASIC subtypes (Chen and Gründer, 2007).
ASICs contain two transmembrane domains (TMs), a large extracellular loop between the TMs, and short cytoplasmic N and C termini. The functional roles of these domains and particular amino acid residues in ASICs have been revealed by using site-directed mutagenesis as well as domain-swapping between ASIC subtypes, particularly splicing variants. ASIC2a and ASIC2b, both encoded by accn1, share most amino acid sequences, except for the region extending from the N terminus to the initial one third of the extracellular loop. It has been shown that proton sensitivity of homomeric ASIC2a channels is determined by five amino acids located within the initial segment of the loop (H72, D77, E78, H109, and H180), and translocation of the channel proteins to the plasma membrane is mediated by TM1 and an additional 17 amino acids following TM1 (Baron et al., 2001; Smith et al., 2007; Kweon et al., 2016). On the other hand, ion selectivity and kinetics of desensitization and resensitization have been shown to be mediated by the N terminus of ASICs. For example, replacing the N terminus of ASIC2a with the comparable sequence in ASIC2b resulted in de novo prolonged desensitization of the channel in response to repetitive acidic stimuli, which was more severe than that observed with homomeric ASIC1a channels (Schuhmacher et al., 2015; Kweon et al., 2016). This finding suggests that the N terminus of ASIC2a is necessary for quick recovery of channel activity when the channel is desensitized by extracellular protons. Interestingly, a previous study has shown that mutations of the first 25 amino acids at the N terminus or specific residues in the initial segment at the TM1 of ASIC1a lead to nondesensitized currents in response to consecutive acidic stimuli, demonstrating the requirement for the N terminus and TM1 of ASIC1a for such desensitization to occur (Chen and Gründer, 2007; Li et al., 2012). Chen and Gründer (2007) further suggested that S23 and S25 residues in the N terminus play a key role in triggering the prolonged desensitization of ASIC1a, although experimental evidence was not provided. Even though these observations have provided evidence that the N termini of ASICs are involved in the regulation of both channel desensitization and recovery from desensitization, the specific amino acids within the N termini that mediate those processes in homomeric and heteromeric ASIC channels have yet to be determined.
Here, we have identified three amino acid residues within the N terminus of ASIC2a that are necessary for rapid recovery from desensitization in both homomeric and heteromeric ASIC2a during repetitive proton activation. Using an ASIC1a mutant in which the three amino acids in ASIC2a replace the respective residues in ASIC1a, we have further discovered that distinct amino acids in the N terminus are responsible for the process of desensitization and rapid recovery in ASIC1a and ASIC2a, respectively.
Materials and methods
Complementary DNAs (cDNAs) and molecular cloning
Mouse cDNAs for ASIC1a, ASIC2a, and ASIC2b have been described previously (Kweon et al., 2015, 2016). Chimeric ASIC mutants were generated using an overlap extension PCR strategy (Lee et al., 2010; Kweon et al., 2016). PCR products were ligated into pEGFP-C1 (Clontech) using T4 DNA ligase (New England Biolabs). In the concatemer of ASIC2a-Mut2-ASIC2a, individual subunits are linked by one asparagine followed by seven successive residues of glutamine (van Bemmelen et al., 2015). Point mutations and deletion of target sequences in ASIC2a mutants were generated by inverse PCR reactions with Pfu Turbo DNA polymerase (Agilent Technologies), followed by digestion with DpnI (Agilent Technologies). The digested products were phosphorylated at the 5′ end using T4 polynucleotide kinase (Enzynomics) and ligated into pEGFP-C1 (Clontech) using T4 DNA ligase (New England Biolabs). The primers used to generate the ASIC mutants and concatemer are listed in Tables S1, S2, and S3. Mutations were confirmed by DNA sequencing.
Cell culture and transfection
HEK293T cells were obtained from Bertil Hille (University of Washington School of Medicine, Seattle, WA). The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; HyClone, Thermo Fisher Scientific) with 10% FBS (HyClone, Thermo Fisher Scientific) and 0.2% penicillin/streptomycin (HyClone, Thermo Fisher Scientific) at 37°C under 5% CO2. HEK293T cells were transiently transfected with 200 ng of a cDNA using Lipofectamine 2000 (Invitrogen). To express heteromeric channels, the cells were transfected with a 1:1 mixture of plasmids containing different subtypes of ASICs. After transfection, the cells were transferred to coverslips coated with poly-l-lysine and used for voltage-clamp recordings performed 24–36 h after transfection.
Electrophysiology
Whole-cell patch-clamp recordings were performed using a HEKA EPC-10 amplifier with pulse software (HEKA Elektronik) at room temperature (22–25°C). In all recordings, transfected cells were clamped at −70 mV. Borosilicate glass pipettes (Sutter Instrument) were pulled using a Flaming/Brown micropipette puller (P-97; Sutter Instrument Co.). Glass micropipettes with a resistance of 2–3 MΩ were used for recording. The pipette solution contained (in mM) 140 KCl, 5 MgCl2, 10 HEPES, and 0.1 1,2-bis(2-aminophenoxy) ethane-N,N,N’,N’-tetraacetic acid, 3 Na2ATP, and 0.1 Na3GTP and was adjusted to pH 7.4 with KOH. The external solution contained (in mM) 160 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, and 10 HEPES and was adjusted to pH 7.4 with tetramethylammonium hydroxide. 2-(N-morpholino)ethanesulfonic acid (MES) replaced HEPES in the external solution when the pH of a solution was <5.0. During recording, the external solutions were delivered to a Quick Change Chamber Narrow Slotted Bath (RC-46SNLP; Warner Instruments) using a Six-channel Pinch Valve Controller System (VC-6; Warner Instruments). Chemicals were purchased from Sigma-Aldrich and Merck, except for HEPES (Calbiochem) and MES (Alfa Aesar).
Primaquine test
Transfected HEK293 cells were pretreated for 1 h with primaquine (50 µM; LKT Laboratories), and the bath solution was continuously perfused with primaquine over the course of the recording. Control cells were not treated with primaquine. Acidic stimuli were delivered by switching the bath solution from pH 7.4 to pH 4.0 for 20 s.
Plasma membrane isolation and Western blotting
Transfected HEK293T cells were treated twice for 20 s with either pH-4.0 or pH-7.4 bath solution. The subcellular fraction containing the plasma membrane was isolated using a Plasma Membrane Protein Extraction kit (ab65400; Abcam) according to the manufacturer’s instructions. For Western blotting, protein samples were resolved on a 10% SDS-polyacrylamide gel, then transferred to a polyvinylidene fluoride membrane (Bio-Rad) for 35 min at 20 V in a buffer containing 25 mM Tris base (Bio-Rad), 192 mM glycine (Bio-Rad), and 20% (vol/vol) methanol. The membrane was blocked with 5% skim milk for an hour and incubated with a primary antibody at room temperature for 1 h or at 4°C overnight. After being washed three times, the membrane was incubated with a secondary antibody conjugated with horseradish peroxidase and then visualized using the ECL detection system (Bio-Rad). The antibodies used were anti-GFP (4B10B2; Thermo Fisher Scientific, 1:2,000), anti-E-cadherin (NBP1-42793; Novus Biologicals, 1:1,000), anti-GAPDH (2118; Cell Signaling, 1:5,000), anti-mouse IgG (Cell Signaling Technology, 1:3,000), and anti-rabbit IgG (Thermo Fisher Scientific, 1:5,000). ImageJ software (National Institutes of Health) was used for quantification.
Statistical analysis
Data are presented as means ± SEM. The Mann–Whitney U test as well as one- and two-way ANOVA with post hoc multiple comparisons corrections were performed and statistically analyzed using software including FitMaster (HEKA Elektronik), IGOR Pro (WaveMetrics), and GraphPad Prism (GraphPad Software). Differences were considered significant at *, P < 0.05; **, P < 0.01; ***, P < 0.001; and ****, P < 0.0001. n.s. and n indicate statistically not significant and the number of cells or samples, respectively.
Online supplemental material
Fig. S1 shows the acid-evoked currents exhibited by additional ASIC2a mutants that are used to identify the amino acid residues responsible for desensitization and resensitization of the channel. Fig. S2 shows the sequences of ASIC mutants used in the study in comparison with those of wild-type ASIC2a and ASIC2b. Tables S1, S2, and S3 list the information of primers and templates used to generate ASIC mutants.
Results
Substitution of the N terminus of ASIC2a with that of ASIC2b results in the prolonged desensitization of the channel following an initial response to extracellular protons
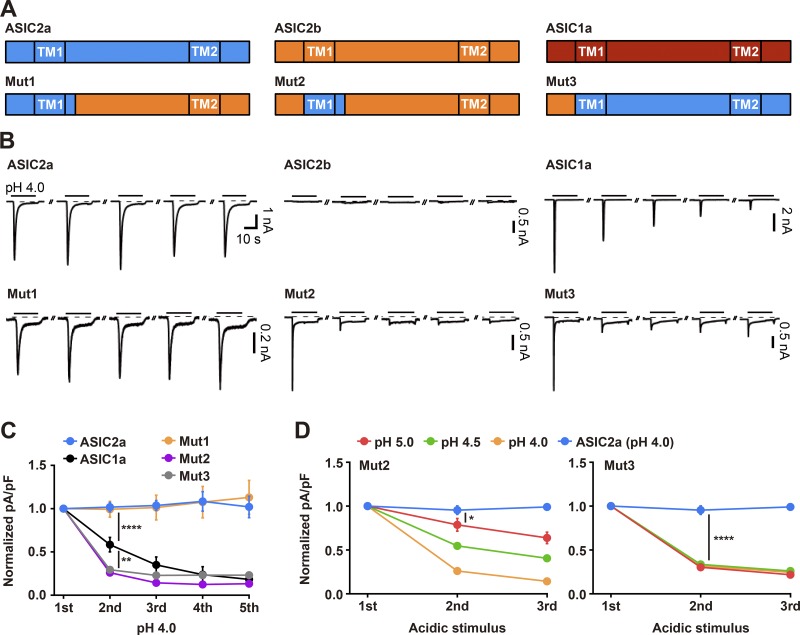
To obtain a biophysical understanding of the mechanisms underlying desensitization and resensitization of ASIC2a, we focused on the N terminus and TM1 of ASIC2a because previous studies performed by others and us have provided evidence that this region is necessary for generating nondesensitized peak currents in response to repetitive acidic stimuli. Voltage-clamp recordings were performed on HEK293T cells transiently transfected with either a wild-type ASIC or a chimeric mutant containing mixed sequences of ASIC2a and ASIC2b, while acidic stimuli were delivered by switching the bath solution from pH 7.4 to pH 4.0 for 20 s every 2 min (Fig. 1, A and B). Consistent with previous results, acid-evoked currents were not desensitized over the course of five consecutive treatments with acidic stimuli in cells transfected with wild-type ASIC2a, whereas a gradual and significant reduction in peak currents was observed in cells transfected with wild-type ASIC1a (Fig. 1, B and C). No currents were elicited by an exposure to protons in cells transfected with wild-type ASIC2b, mainly because of the impaired translocation of the homomeric ion channel to the plasma membrane, as we have previously demonstrated (Fig. 1 B; Kweon et al., 2016). As in the case of wild-type ASIC2a, cells transfected with Mut1, in which the N terminus, the TM1, and the proximal post-TM1 region (17 amino acids) of ASIC2b were replaced with the comparable sequences of ASIC2a, did not exhibit the desensitized currents (Fig. 1, B and C). In contrast, cells transfected with Mut2, in which the N terminus of Mut1 was further replaced by the comparable sequence in ASIC2b, exhibited severe prolonged desensitization and became almost unresponsive to protons after the initial acidic stimulus (Fig. 1, B and C). Similarly, cells transfected with Mut3, which is equivalent to ASIC2a except for its N terminus, which comes from ASIC2b, displayed severe desensitized currents similar to those observed with Mut2 (Fig. 1, B and C). Taken together, these data suggest that the N terminus of ASIC2 subtype plays an important role in channel desensitization. The desensitization of Mut2 and Mut3 was also induced by weaker acidic stimuli, such as pH 4.5 and 5.0 (Fig. 1 D). Interestingly, cells transfected with Mut3 exhibited maximal desensitization in response to pH 5.0, whereas cells transfected with Mut2 showed a gradual decrease in peak currents in a pH-dependent manner.
Figure 1.
Absence of the N terminus of ASIC2a causes de novo prolonged desensitization of the ion channel in response to repetitive exposure to extracellular protons. (A) Schematic showing ASICs and their chimeric mutants used in this study. (B) Currents elicited by pH 4.0 for 20 s (as indicated with black lines above traces) in HEK293T cells expressing either a wild-type ASIC or a chimeric mutant. Acidic stimuli were delivered by switching the bath solution from pH 7.4 to pH 4.0 at 2-min intervals. The dashed line indicates the zero-current level. (C) Peak current densities elicited by five successive applications of pH 4.0, normalized to those evoked by the initial stimulus. Note that ASIC2a chimeras missing the N terminus of ASIC2a (Mut2 and Mut3) exhibit a significant reduction in peak currents upon response to repeated acidic stimuli. n = 6–11 for each group. (D) Peak current densities elicited by three successive treatments with protons (pH 4.0, 4.5, or 5.0), normalized to those evoked by the initial stimulus. n = 6–8 for each group. *, P < 0.05; **, P < 0.01; ****, P < 0.0001, two-way ANOVA followed by Tukey’s multiple comparisons corrections.
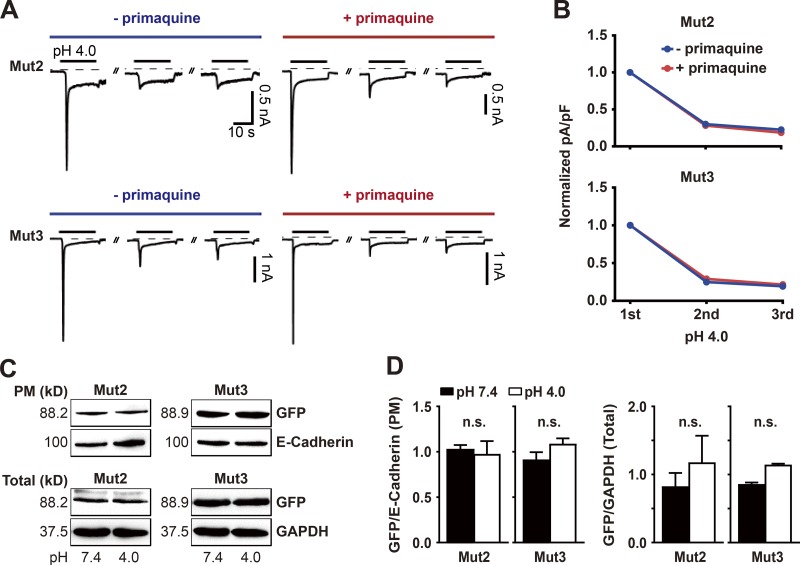
The prolonged desensitization of the ASIC2a mutants is not caused by either internalization or degradation of the ion channels following exposure to extracellular protons
To determine whether the prolonged desensitization exhibited by the chimeric mutants Mut2 and Mut3 is the result of a rapid internalization of these ion channels into the cytoplasmic compartment following the initial acidic stimulus, we applied a potent inhibitor of endocytosis, 50 µM primaquine, to the bath solution at pH 7.4 for 1 h before and during three consecutive applications of pH 4.0 for 20 s, with a 2-min interval at pH 7.4 between stimuli (Hiebsch et al., 1991; Volk et al., 2004). Voltage-clamp recordings performed on cells transfected with either Mut2 or Mut3 revealed that the prolonged desensitization continued to occur in primaquine-treated cells, with the amount of acid-evoked current being almost identical to that displayed by untreated controls (Fig. 2, A and B). This result suggests that the desensitization exhibited by these chimeric mutants is not the result of an internalization of the ion channels from the plasma membrane. Western blotting demonstrated that the amounts of Mut2 and Mut3 detected by a GFP tag from the purified subcellular fraction of plasma membrane containing E-cadherin did not change significantly in cells treated with pH 4.0 for 20 s, compared with untreated controls (Fig. 2, C and D). Furthermore, the total amounts of Mut2 and Mut3 in transfected cells, normalized to the amount of GAPDH, were unaffected by acidic stimulation. These results suggest that the prolonged desensitization displayed by homomeric channels of Mut2 and Mut3 is due neither to an internalization of these chimeric mutants from the plasma membrane nor to their degradation in transfected cells as a result of the acidic stimuli.
Figure 2.
The prolonged desensitization of the ASIC2a mutants is not due to an internalization of the ion channels in response to extracellular protons. (A) Currents elicited by three consecutive applications of pH 4.0 for 20 s in HEK293T cells expressing either Mut2 (upper) or Mut3 (lower) in the absence (blue) or presence (red) of 50 µM primaquine, begun 1 h before the recordings. (B) Peak current densities in cells expressing either Mut2 (upper) or Mut3 (lower) were normalized to those evoked by the initial acidic stimulus in the absence (blue) or presence (red) of 50 µM primaquine. n = 5 for each group. Acidic stimuli were delivered by switching the bath solution from pH 7.4 to pH 4.0 at 2-min intervals. (C and D) Western blotting showing the amounts of GFP-tagged Mut2 and Mut3 in the plasma membrane (PM) and total cell lysate (Total) following application of pH 7.4 versus pH 4.0. E-cadherin and GAPDH are markers used to normalize the amounts of Mut2 and Mut3 in the plasma membrane and total cell lysate, respectively. n = 3 for each group. n.s. not significant; Mann–Whitney U test.
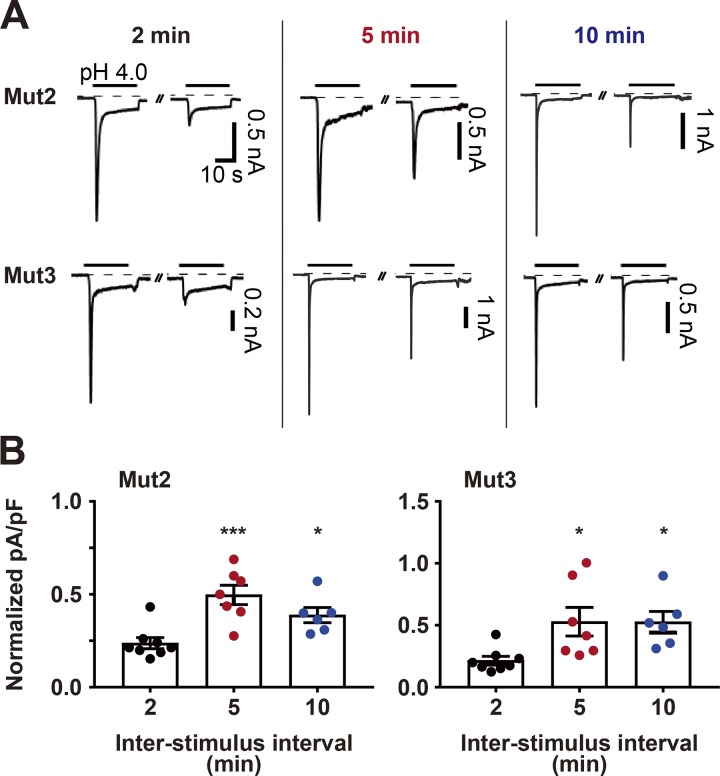
The prolonged desensitization exhibited by the ASIC2a mutants can be partially relieved in a resting time-dependent manner
It has been reported that the currents of homomeric ASIC1a channels can be partially recovered from the prolonged desensitization, depending on the duration of the poststimulus resting period (Li et al., 2012). To examine whether the desensitization exhibited by Mut2 and Mut3 can also be relieved after a long rest at pH 7.4, we delivered pH 4.0 twice to cells transfected with either Mut2 or Mut3, with a 2-, 5-, or 10-min interstimulus interval (Fig. 3 A). Comparison of the peak currents elicited during the second pH 4.0 treatment, normalized to the initial response, showed that a 5-min rest period led to significantly larger acid-evoked currents than did a 2-min rest (Fig. 3 B). A 10-min rest resulted in no further increase in acid-evoked currents compared with a 5-min rest (Fig. 3 B). These results suggest that partial recovery from desensitization in these chimeric mutants can occur in a resting time-dependent manner.
Figure 3.
The prolonged desensitization exhibited by the ASIC2a mutants can be partially reversed after a long resting period. (A) Currents elicited in HEK293T cells expressing Mut2 (upper) and Mut3 (lower) after two successive applications of pH 4.0 with variable interstimulus intervals: 2 min (black), 5 min (red), or 10 min (blue). Acidic stimuli were delivered by switching the bath solution from pH 7.4 to pH 4.0. (B) Peak current densities elicited by the second pH-4.0 treatment normalized to those elicited by the first pH-4.0 treatment. n = 6–8 for each group. *, P < 0.05; ***, P < 0.001, one-way ANOVA followed by Dunnett’s multiple comparisons corrections with 2-min interval.
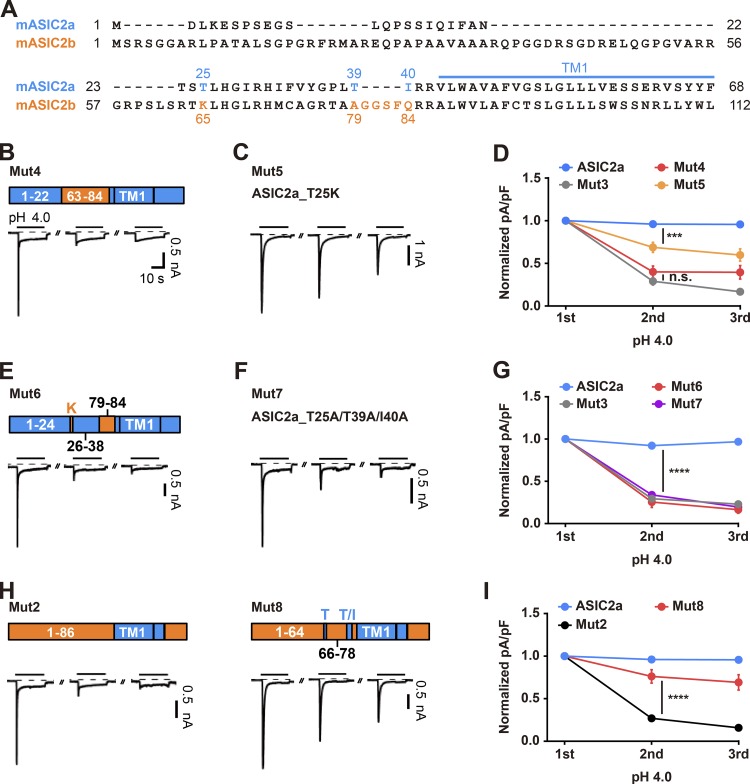
Mutation of three amino acid residues within the N terminus of ASIC2a (T25, T39, and I40) is sufficient for the prolonged desensitization of a homomeric ASIC2a channel
Voltage-clamp recordings of the chimeric mutants containing mixed sequences of ASIC2a and ASIC2b provided evidence that the N termini of the ASIC2a mutants play a critical role in the recovery from the desensitization of the channel. To further dissect the N terminus, we generated two additional chimeric mutants, Mut S1 and Mut S2, in which amino acids 1–22 of ASIC2a were switched to amino acids 1–35 and 1–62 of ASIC2b, respectively (Fig. S1, A and B). In response to three consecutive pH 4.0 treatments, both chimeras exhibited nondesensitized peak currents over the course of the recordings (Fig. S1, B and C). In contrast, another chimeric mutant, Mut4, in which amino acids 23–40 of ASIC2a were changed to amino acids 63–84 of ASIC2b, showed strong prolonged desensitization to a degree similar to that observed for Mut3 (Fig. 4, B and D; and Fig. S2). To further identify the key amino acid residues within the mutated sequences in Mut4, we focused on T25, which has been shown to play a critical role in determining the ion selectivity of ASIC2a and its neighboring residues by introducing site-directed mutations at T23R, S24T, and T25K, as seen in Mut S3 (Coscoy et al., 1999). We found that those three mutations were sufficient to evoke a gradual reduction in acid-evoked currents over the course of the recordings (Fig. S1, D and F; and Fig. S2). Furthermore, a single mutation at T25K in ASIC2a led to a slight but significant reduction in acid-evoked currents during the consecutive pH-4.0 treatments, as seen in Mut5 (Fig. 4, C and D). To further identify additional amino acids that mediate the recovery from the desensitization of ASIC2a following activation, we generated a series of mutants and found that a combinatorial mutation at T25, T39, and I40 in ASIC2a to K65 and A79-Q84 in ASIC2b (Mut6); to K65, A79, and Q84 in ASIC2b (Mut S4); or to alanine (Mut7) was sufficient to induce prolonged desensitization (Fig. 4, E–G; Fig. S1, E and F; and Fig. S2). These results suggest that T25, T39, and I40 in ASIC2a are normally necessary for the rapid resensitization of homomeric channels of ASIC2a, and mutation of these amino acids leads to a prolonged desensitization. Furthermore, such desensitization shown by the ASIC2a mutants might be due to a loss-of-function mutation of ASIC2a rather than to a gain-of-function mutation originating from the ASIC2b sequences, because mutations to alanine also elicited such desensitization (Fig. 4, F and G). Finally, we examined whether switching of the respective residues in Mut2 to those three amino acids in ASIC2a was sufficient to relieve the prolonged desensitization exhibited by Mut2. We found that mutations of K65 and A79-Q84 in Mut2 to comparable sequences in ASIC2a, as seen in Mut8, were sufficient for a partial but significant rescue from the desensitization exhibited by Mut2 (Fig. 4, H and I; and Fig. S2).
Figure 4.
T25, T39, and I40 at the N terminus of ASIC2a are required for the rapid resensitization of the ion channel following the response to extracellular protons. (A) Alignment of ASIC2a (1–68) and ASIC2b (1–112) amino acid sequences. (B, C, E, F, and H) Schematic showing the structure of the indicated mutant and its current responses elicited by three successive applications of pH 4.0 delivered at 2-min intervals. Acidic stimuli were delivered by switching the bath solution from pH 7.4 to pH 4.0. (D, G, and I) Peak current densities normalized to those elicited by the first application of pH 4.0. n = 5–9 for each group. ***, P < 0.001; ****, P < 0.0001, two-way ANOVA followed by Tukey’s multiple comparisons test.
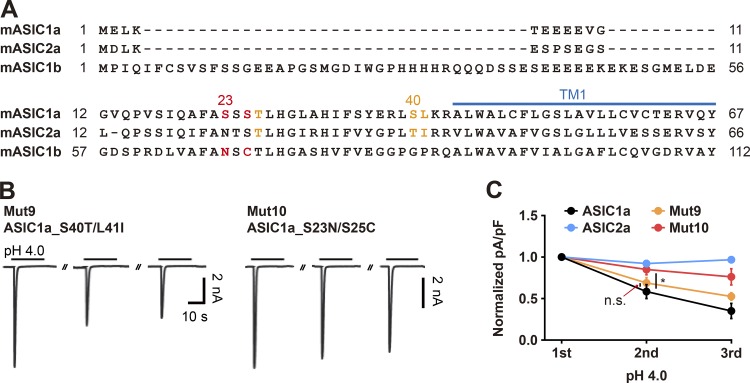
Distinct amino acids in the N termini of ASIC1a and ASIC2a are crucial for desensitization and the recovery from desensitization
Since ASIC2a mutants exhibit gating kinetics that resemble those of ASIC1a, we then asked whether the desensitization and recovery of ASIC1a and ASIC2a are mediated by amino acid residues at the same positions in their respective N termini. By aligning sequences of ASIC1a and ASIC2a, we found that T26, S40, and L41 in ASIC1a are matched to T25, T39, and I40 in ASIC2a (Fig. 5 A). Thus, we mutated S40 and L41 in ASIC1a to threonine and isoleucine, respectively. In response to three consecutive treatments with acidic stimuli, Mut9 (ASIC1a_S40T/L41I) exhibited a gradual reduction in peak currents, similar to that observed with wild-type ASIC1a (Fig. 5, B and C). Since S23 and S25 have been suggested to be the key residues in the prolonged desensitization of ASIC1a (Chen and Gründer, 2007), we switched S23 and S25 in ASIC1a to N68 and C70 in ASIC1b, as seen in Mut10. This mutation resulted in significant attenuation of the prolonged desensitization compared with that exhibited by ASIC1a (Fig. 5, B and C). These results suggest that the desensitization and recovery of ASIC1a and ASIC2a are mediated by distinct amino acids in the N termini.
Figure 5.
The desensitization and resensitization of ASIC1a and ASIC2a are mediated by distinct amino acids in the N termini. (A) Alignment of ASIC1a (1–67), ASIC2a (1–66), and ASIC1b (1–112) amino acid sequences. (B) Currents elicited by three successive applications of pH 4.0 delivered at 2-min intervals in cells transfected with the indicated ASIC1a mutant. Acidic stimuli were delivered by switching the bath solution from pH 7.4 to pH 4.0. (C) Peak current densities normalized to those evoked by the first stimulus. n = 5 for each group. *, P < 0.05, two-way ANOVA followed by Tukey’s multiple comparisons test.
Heteromeric channels containing a mutant ASIC2a subunit exhibit prolonged desensitization following activation by extracellular protons
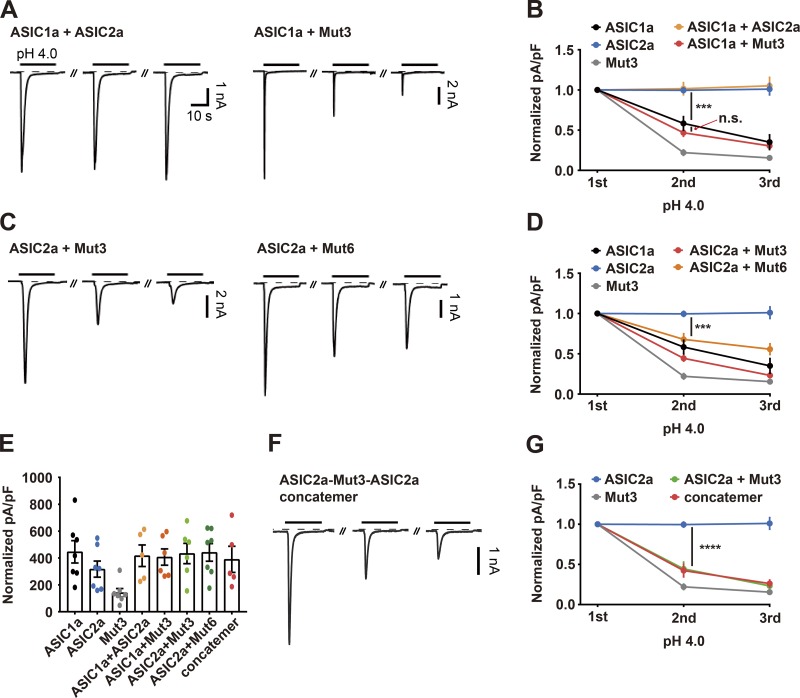
It has been shown that heteromeric channels consisting of ASIC1a and ASIC2a do not exhibit prolonged desensitization when responding to repetitive acidic stimuli (Chen and Gründer, 2007). To examine whether heteromeric ASICs containing an ASIC2a mutant as a subunit exhibit prolonged desensitization, we transfected HEK293T cells with a mixture of cDNAs expressing wild-type and mutant versions of ASICs. Consistent with previous findings, coexpression of wild-type ASIC1a and wild-type ASIC2a resulted in the absence of the desensitized peak currents upon repeated application of protons. In contrast, cells transfected with a mixture of wild-type ASIC1a and Mut3 displayed prolonged desensitization that was as severe as that observed in cells transfected with wild-type ASIC1a alone (Fig. 6, A and B). Coexpression of wild-type ASIC2a and a mutant of ASIC2a (Mut3 or Mut6) resulted in prolonged desensitization, although the extent of the desensitization was milder than that observed with homomeric channels of either Mut3 or Mut6 (Fig. 6, C and D). Since acidic stimuli elicited amounts of currents comparable to those elicited in wild-type ASIC1a and ASIC2a, but the desensitization properties seen were similar to those of Mut3 and Mut6, it is unlikely that acid-evoked currents were measured from singly transfected, rather than cotransfected, cells (Fig. 6 E). To further examine whether the prolonged desensitization observed in a cotransfected cell was mediated solely by heteromeric channels or contributed by mixed currents of homomeric and heteromeric channels, we constructed a concatemer by linking individual subunits of wild-type ASIC2a, Mut3, and wild-type ASIC2a with short peptides. Cells expressing the concatemer exhibited a substantial reduction in peak currents in response to the repetitive acidic stimuli to an extent similar to that observed in cells coexpressing wild-type ASIC2a and Mut3 (Fig. 6, F and G). Taken together, these results suggest that the N terminus of ASIC2a plays an important role in the recovery of heteromeric channels containing an ASIC2a subunit.
Figure 6.
The N terminus of ASIC2a is involved in the rapid resensitization of heteromeric ASIC channels following the response to extracellular protons. (A, C, and F) Currents elicited by three successive applications of pH 4.0 in HEK293T cells transiently transfected with the indicated ASIC cDNAs. Acidic stimuli were delivered by switching the bath solution from pH 7.4 to pH 4.0 at 2-min intervals. The dashed line indicates the zero-current level. (B, D, and G) Peak current densities normalized to those elicited by the initial acidic stimulus. n = 5–8 for each group. ***, P < 0.001; ****, P < 0.0001, two-way ANOVA followed by Tukey’s multiple comparisons corrections. (E) Peak current densities elicited by the initial acidic stimulus.
Discussion
In this study, we have investigated the biophysical properties underlying desensitization and resensitization of homomeric and heteromeric ASIC2a upon activation by extracellular protons, using ASIC2a mutants generated by domain-swapping between two splicing variants, ASIC2a and ASIC2b, and site-directed mutagenesis of targeted amino acids. First, we found that domain-swapping mutations of the N terminus in ASIC2a result in prolonged desensitization upon response to repetitive acidic stimuli. These data are consistent with the previous finding that the N terminus of ASIC2a is normally necessary for rapid recovery from the desensitization of homomeric ASIC2a channels following an acidic stimulus, and absence of this sequence causes prolonged desensitization (Schuhmacher et al., 2015; Kweon et al., 2016). Second, we have further narrowed down the N terminus of ASIC2a to identify the key amino acid residues mediating the rapid recovery of the channels. We demonstrated that mutations of three residues in the N terminus, T25, T39, and I40, to either comparable amino acids in ASIC2b or alanine caused de novo prolonged desensitization. This finding suggests that the three amino acids normally play an important role in the rapid resensitization of wild-type ASIC2a following desensitization of homomeric channels by extracellular protons. Third, our data revealed that the prolonged-desensitization phenotypes shown by ASIC2a mutants were not the result of a decrease in the amount of channel proteins in the plasma membrane with repetitive acidic stimulation. Fourth, we found that prolonged desensitization displayed by ASIC2a mutants can be partially recovered after a 5-min resting period. The recovery is unlikely to result from the translocation of newly synthesized ion channels to the plasma membrane, since a longer (10-min) rest did not further increase the acid-evoked currents, suggesting that the recovery of currents is caused by slow resensitization of a subset of ASIC2a channels in the plasma membrane. Fifth, we have provided experimental evidence that the desensitization and recovery of ASIC1a and ASIC2a are mediated by distinct amino acid residues in the N terminus. Previous studies have shown the importance of specific residues in the N terminus or TM1 of ASIC1a for stabilizing the desensitized state (Chen and Gründer, 2007; Li et al., 2012). We found here that three key residues, T25, T39, and I40, in ASIC2a are located in close proximity to those previously reported residues in ASIC1a in the sequence alignment (S23/S25 in the N terminus and A44-L45 in the TM1 of ASIC1a). However, we confirmed that replacing the respective amino acids in ASIC1a with the three key residues in ASIC2a caused no detectable changes in the prolonged desensitization phenomenon compared with that exhibited by wild-type ASIC1a. In contrast, switching S23 and S25 in ASIC1a to the respective residues in ASIC1b relieved such desensitization (Chen and Gründer, 2007). Finally, by recording cells transiently transfected with either a mixture or a concatemer of wild-type and mutant versions of ASICs, we found that the N terminus and, in particular, T25, T39, and I40, of ASIC2a are critical for the resensitization of heteromeric channels containing ASIC2a.
It has been suggested that homomeric ASIC1a channels make a transition from the closed to open state upon binding to extracellular protons, and then rapidly shift into the long-lasting desensitized state before returning to the closed state (Li et al., 2012). Using x-ray crystallography and single particle cryo-electron microscopy, researchers have provided the structural mechanisms to account for the state transitions of ASIC1a (Baconguis and Gouaux, 2012; Yoder et al., 2018). The binding of an extracellular proton triggers a transition from the closed to open state by causing a conformational change in the extracellular loop of ASIC1a, in particular in the acid pocket as well as the upper and lower palm domains, which is followed by the movement of TMs. The β11-β12 linkers that connect the upper and lower palm domains in the extracellular loop play a critical role in this process. Two TMs then switch back to a conformation similar to that of the closed state, leading to a channel transition from the open to the desensitized state. When the proton is released from the channel, the extracellular domains then return to the closed-state conformation, which shifts the channel into the resensitized state. Since these structural analyses were performed on an ASIC1a mutant that is partially truncated in both the N and C termini, the contribution of the intracellular domains of ASIC1a to the state transitions was not determined. Interestingly, the truncated mutant exhibited a higher stability in the desensitized state than did the full-length wild-type channel, raising the intriguing possibility that the intracellular domains of ASICs may be involved in state transitions (Yoder et al., 2018). Further structural analyses using wild-type ASICs are necessary to clearly determine the role of the N terminus in the regulation of ASIC channel gating.
It has been shown that individual subunits of ASIC1a and ASIC2a in heteromeric channels play distinct roles in determining the electrophysiological properties of the ion channels (Askwith et al., 2004). For example, ASIC1a mainly affects the amplitude of acid-evoked currents, whereas ASIC2a has little effect on the amplitude but contributes to other functional aspects of the ion channel, including desensitization, recovery after desensitization, pH sensitivity, and potentiation by FRRFamide and zinc (Joeres et al., 2016). Interestingly, hippocampal neurons prepared from mice lacking ASIC2 exhibit prolonged desensitization in response to two consecutive acidic stimuli, whereas those prepared from wild-type mice recover rapidly after the initial acidic stimulus (Askwith et al., 2004). Since acid-evoked currents in the hippocampal neurons are mainly associated with heteromeric channels composed of ASIC1a and ASIC2a, ASIC2a is likely necessary for rapid resensitization of the acid-evoked currents, forming heteromeric channels with ASIC1a in these cells. Organotypic hippocampal slices prepared from ASIC2a-deficient mice are more resistant to neuronal cell death evoked by either acidosis or deprivation of oxygen and glycogen than are those prepared from wild-type mice (Jiang et al., 2017). Similarly, mice lacking ASIC2a exhibit a significant reduction in infarcted brain regions following focal ischemia. These results suggest that ASIC2a is responsible for the exacerbation of neuronal cell death under such pathological conditions, probably through the formation of heteromeric channels together with ASIC1a and the generation of nondesensitized current responses (Jiang et al., 2017). Thus, further investigation into the key amino acid residues of ASIC2a identified in this study, T25, T39, and I40, should not only reveal novel insights concerning the biophysical mechanism by which homomeric and heteromeric channels of ASIC2a make the transition from the open to the desensitized and then to the closed state in response to an acidic stimulus but should also provide clues for developing novel therapeutic strategies to alleviate a number of disease conditions mediated by homomeric and heteromeric channels involving ASIC2a.
Acknowledgments
We thank many laboratories for providing the plasmids. We also thank Dr. Seung-Ryoung Jung at the University of Washington for helpful discussion and simulational analysis on ASIC1a and ASIC2a structure and Dr. Deborah McClellan for editorial assistance.
This work was supported by the Basic Science Research Program (2017R1A2B4003351 to H. Lee and 2016R1A2B4014253 to B.-C. Suh) and the Brain Research Program (2017R1A4A1015534 to B.-C. Suh) of the National Research Foundation of Korea, funded by the Ministry of Sciences and ICT, and the basic research program through the Korea Brain Research Institute, the Ministry of Sciences and ICT (19-BR-04-03 to H. Lee and 19-BR-04-01 to B.-C. Suh) and the Daegu Gyeongbuk Institute of Science and Technology Research and Development Program of the Ministry of Science, ICT and Future Planning (18-BD-06 to B.-C. Suh).
The authors declare no competing financial interests.
Author contributions: H. Lee and B.-C. Suh designed the research; J.S. Lee and H.-J. Kweon performed the biochemical and electrophysiological research; J.S. Lee, H. Lee, and B.-C. Suh wrote the paper.
Kenton J. Swartz served as editor.
References
- Askwith C.C., Wemmie J.A., Price M.P., Rokhlina T., and Welsh M.J.. 2004. Acid-sensing ion channel 2 (ASIC2) modulates ASIC1 H+-activated currents in hippocampal neurons. J. Biol. Chem. 279:18296–18305. 10.1074/jbc.M312145200 [DOI] [PubMed] [Google Scholar]
- Baconguis I., and Gouaux E.. 2012. Structural plasticity and dynamic selectivity of acid-sensing ion channel-spider toxin complexes. Nature. 489:400–405. 10.1038/nature11375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron A., Schaefer L., Lingueglia E., Champigny G., and Lazdunski M.. 2001. Zn2+ and H+ are coactivators of acid-sensing ion channels. J. Biol. Chem. 276:35361–35367. 10.1074/jbc.M105208200 [DOI] [PubMed] [Google Scholar]
- Chen X., and Gründer S.. 2007. Permeating protons contribute to tachyphylaxis of the acid-sensing ion channel (ASIC) 1a. J. Physiol. 579:657–670. 10.1113/jphysiol.2006.120733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coscoy S., de Weille J.R., Lingueglia E., and Lazdunski M.. 1999. The pre-transmembrane 1 domain of acid-sensing ion channels participates in the ion pore. J. Biol. Chem. 274:10129–10132. 10.1074/jbc.274.15.10129 [DOI] [PubMed] [Google Scholar]
- Gitterman D.P., Wilson J., and Randall A.D.. 2005. Functional properties and pharmacological inhibition of ASIC channels in the human SJ-RH30 skeletal muscle cell line. J. Physiol. 562:759–769. 10.1113/jphysiol.2004.075069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales E.B., Kawate T., and Gouaux E.. 2009. Pore architecture and ion sites in acid-sensing ion channels and P2X receptors. Nature. 460:599–604. 10.1038/nature08218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselager M., Timmermann D.B., and Ahring P.K.. 2004. pH Dependency and desensitization kinetics of heterologously expressed combinations of acid-sensing ion channel subunits. J. Biol. Chem. 279:11006–11015. 10.1074/jbc.M313507200 [DOI] [PubMed] [Google Scholar]
- Hiebsch R.R., Raub T.J., and Wattenberg B.W.. 1991. Primaquine blocks transport by inhibiting the formation of functional transport vesicles. Studies in a cell-free assay of protein transport through the Golgi apparatus. J. Biol. Chem. 266:20323–20328. [PubMed] [Google Scholar]
- Jasti J., Furukawa H., Gonzales E.B., and Gouaux E.. 2007. Structure of acid-sensing ion channel 1 at 1.9 A resolution and low pH. Nature. 449:316–323. 10.1038/nature06163 [DOI] [PubMed] [Google Scholar]
- Jiang N., Wu J., Leng T., Yang T., Zhou Y., Jiang Q., Wang B., Hu Y., Ji Y.H., Simon R.P., et al. . 2017. Region specific contribution of ASIC2 to acidosis-and ischemia-induced neuronal injury. J. Cereb. Blood Flow Metab. 37:528–540. 10.1177/0271678X16630558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joeres N., Augustinowski K., Neuhof A., Assmann M., and Gründer S.. 2016. Functional and pharmacological characterization of two different ASIC1a/2a heteromers reveals their sensitivity to the spider toxin PcTx1. Sci. Rep. 6:27647 10.1038/srep27647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kweon H.J., and Suh B.C.. 2013. Acid-sensing ion channels (ASICs): therapeutic targets for neurological diseases and their regulation. BMB Rep. 46:295–304. 10.5483/BMBRep.2013.46.6.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kweon H.J., Yu S.Y., Kim D.I., and Suh B.C.. 2015. Differential regulation of proton-sensitive ion channels by phospholipids: a comparative study between ASICs and TRPV1. PLoS One. 10:e0122014 10.1371/journal.pone.0122014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kweon H.J., Kim D.I., Bae Y., Park J.Y., and Suh B.C.. 2016. Acid-sensing ion channel 2a (ASIC2a) promotes Surface trafficking of ASIC2b via heteromeric assembly. Sci. Rep. 6:30684 10.1038/srep30684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Shin M.K., Ryu D.K., Kim S., and Ryu W.S.. 2010. Insertion and deletion mutagenesis by overlap extension PCR. Methods Mol. Biol. 634:137–146. 10.1007/978-1-60761-652-8_10 [DOI] [PubMed] [Google Scholar]
- Li T., Yang Y., and Canessa C.M.. 2012. Impact of recovery from desensitization on acid-sensing ion channel-1a (ASIC1a) current and response to high frequency stimulation. J. Biol. Chem. 287:40680–40689. 10.1074/jbc.M112.418400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neaga E., Amuzescu B., Dinu C., Macri B., Pena F., and Flonta M.L.. 2005. Extracellular trypsin increases ASIC1a selectivity for monovalent versus divalent cations. J. Neurosci. Methods. 144:241–248. 10.1016/j.jneumeth.2004.11.012 [DOI] [PubMed] [Google Scholar]
- Price M.P., Snyder P.M., and Welsh M.J.. 1996. Cloning and expression of a novel human brain Na+ channel. J. Biol. Chem. 271:7879–7882. 10.1074/jbc.271.14.7879 [DOI] [PubMed] [Google Scholar]
- Qiang M., Dong X., Zha Z., Zuo X.K., Song X.L., Zhao L., Yuan C., Huang C., Tao P., Hu Q., et al. . 2018. Selection of an ASIC1a-blocking combinatorial antibody that protects cells from ischemic death. Proc. Natl. Acad. Sci. USA. 115:E7469–E7477. 10.1073/pnas.1807233115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuhmacher L.N., Srivats S., and Smith E.S.. 2015. Structural domains underlying the activation of acid-sensing ion channel 2a. Mol. Pharmacol. 87:561–571. 10.1124/mol.114.096909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood T.W., Lee K.G., Gormley M.G., and Askwith C.C.. 2011. Heteromeric acid-sensing ion channels (ASICs) composed of ASIC2b and ASIC1a display novel channel properties and contribute to acidosis-induced neuronal death. J. Neurosci. 31:9723–9734. 10.1523/JNEUROSCI.1665-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E.S., Zhang X., Cadiou H., and McNaughton P.A.. 2007. Proton binding sites involved in the activation of acid-sensing ion channel ASIC2a. Neurosci. Lett. 426:12–17. 10.1016/j.neulet.2007.07.047 [DOI] [PubMed] [Google Scholar]
- van Bemmelen M.X., Huser D., Gautschi I., and Schild L.. 2015. The human acid-sensing ion channel ASIC1a: Evidence for a homotetrameric assembly state at the cell surface. PLoS One. 10:e0135191 10.1371/journal.pone.0135191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk T., Konstas A.A., Bassalaý P., Ehmke H., and Korbmacher C.. 2004. Extracellular Na+ removal attenuates rundown of the epithelial Na+-channel (ENaC) by reducing the rate of channel retrieval. Pflugers Arch. 447:884–894. 10.1007/s00424-003-1193-x [DOI] [PubMed] [Google Scholar]
- Waldmann R., Champigny G., Bassilana F., Heurteaux C., and Lazdunski M.. 1997. A proton-gated cation channel involved in acid-sensing. Nature. 386:173–177. 10.1038/386173a0 [DOI] [PubMed] [Google Scholar]
- Wemmie J.A., Taugher R.J., and Kreple C.J.. 2013. Acid-sensing ion channels in pain and disease. Nat. Rev. Neurosci. 14:461–471. 10.1038/nrn3529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Z.G., Pignataro G., Li M., Chang S.Y., and Simon R.P.. 2008. Acid-sensing ion channels (ASICs) as pharmacological targets for neurodegenerative diseases. Curr. Opin. Pharmacol. 8:25–32. 10.1016/j.coph.2007.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yermolaieva O., Leonard A.S., Schnizler M.K., Abboud F.M., and Welsh M.J.. 2004. Extracellular acidosis increases neuronal cell calcium by activating acid-sensing ion channel 1a. Proc. Natl. Acad. Sci. USA. 101:6752–6757. 10.1073/pnas.0308636100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder N., Yoshioka C., and Gouaux E.. 2018. Gating mechanisms of acid-sensing ion channels. Nature. 555:397–401. 10.1038/nature25782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Wu S., Zhu J., Chai D., and Gan H.. 2017. Down-regulation of ASIC1 suppressed gastric cancer via inhibiting autophagy. Gene. 608:79–85. 10.1016/j.gene.2017.01.014 [DOI] [PubMed] [Google Scholar]
- Zhou R.P., Wu X.S., Wang Z.S., Xie Y.Y., Ge J.F., and Chen F.H.. 2015. Novel insights into acid-sensing ion channels: implications for degenerative diseases. Aging Dis. 7:491–501. 10.14336/AD.2015.1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 shows the acid-evoked currents exhibited by additional ASIC2a mutants that are used to identify the amino acid residues responsible for desensitization and resensitization of the channel. Fig. S2 shows the sequences of ASIC mutants used in the study in comparison with those of wild-type ASIC2a and ASIC2b. Tables S1, S2, and S3 list the information of primers and templates used to generate ASIC mutants.