Abstract
Despite the recent onslaught of approved medications in oncology, acute myeloid leukemia (AML) has been a disease state bereft of pharmaceutical development for decades. The long-standing first-line regimen, 7 + 3, was developed in 1973. A group of four physicians at Roswell Park Memorial Institute built upon prior combinations of daunorubicin and cytarabine to find the optimal combination of 7 days of cytarabine and 3 days of daunorubicin (Lichtman, 2013). This regimen has undergone multiple modifications and patient performance status-based stratifications, but has remained the first-line therapy for AML for the past 45 years. In September 2017, gemtuzumab ozogamicin returned to market and shortly thereafter was added to the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines for AML, to be administered in combination with 7 + 3, and as monotherapy for both newly diagnosed and relapsed patients with acute myeloid leukemia (NCCN, 2018; US Food & Drug Administration, 2017). Gemtuzumab ozogamicin continues to be explored in various leukemia settings and is a welcomed addition to the currently available treatment options for AML.
Acute myeloid leukemia (AML) is a malignancy characterized by differentiation abnormalities combined with uncontrolled clonal proliferation that prevents normal bone marrow hematopoiesis. These immature, undifferentiated, malignant cells are often referred to as "blasts," as they are unable to progress past the myeloblast stage of the hematopoietic cascade.
The American Cancer Society predicts that almost 20,000 patients will be diagnosed with AML in 2018, with the majority of those patients greater than 60 years of age (National Cancer Institute [NCI], 2017). In spite of recent advances, 5-year overall survival (OS) rates are still a paltry 27.4% (NCI, 2017). Although this does represent a vast improvement from 6.3% in 1975 when 7 + 3 was adopted as the new standard of care for AML (NCI, 2017), there remains much room for improvement. When adjusting the 27.4% 5-year OS rate for age, 39% of patients younger than 65 years old were alive at 5 years compared to 8.5% for patients 65 to 74 years of age.
Potentially curative therapy hinges on aggressive induction, followed by multiple cycles of high-dose chemotherapy and/or an allogeneic stem cell transplant, which has often proven too toxic for elderly patients. Through off-label use, hypomethylating agents such as azacitidine and decitabine have increased treatment options for elderly patients with AML. However, these agents are typically reserved for those patients deemed unfit for high-intensity therapy (Schuh et al., 2017). This gap in therapy has created a need to target the disease in our aging populations in a way that provides both safe and effective treatment.
FDA APPROVAL
In May 2000, the US Food and Drug Administration (FDA) granted accelerated approval for gemtuzumab ozogamicin (Mylotarg) for patients aged 60 or greater with CD33-positive relapsed AML who were not candidates for conventional chemotherapy (Bross et al., 2001). Gemtuzumab ozogamicin is a humanized anti-CD33 monoclonal antibody covalently linked to a semisynthetic derivative of calicheamicin, a potent cytotoxic antibiotic (Bross et al., 2001). Accelerated approval was granted based on three early phase studies that demonstrated complete response (CR) or complete response with incomplete platelet recovery (CRp) in 30% of adult patients with an acceptable adverse event profile. Through the accelerated approval process, gemtuzumab ozogamicin was allowed admittance to the market based on its early phase successes with the requirement that phase III studies be completed as planned to prove benefit over existing therapy. Unfortunately, the phase III Southwest Oncology Group (SWOG) S0106 trial found no benefit of gemtuzumab ozogamicin combined with chemotherapy over chemotherapy alone, and an increase in treatment-related mortality (Petersdorf et al., 2013).
The most common causes of treatment-related fatal adverse events were hemorrhage, infection, and/or acute respiratory distress syndrome. This data combined with other negative phase III studies led to Pfizer voluntarily withdrawing gemtuzumab ozogamicin from the market, 10 years after its original approval (NCI, 2010). In September 2017, the FDA reapproved gemtuzumab ozogamicin for the indications of adults with newly diagnosed CD33-positive AML and for relapsed or refractory CD33-positive AML in patients aged 2 years and older (FDA, 2017). The FDA specifically cited changes to the dosing regimen that improved gemtuzumab ozogamicin’s safety and efficacy and led to its reapproval.
PHARMACOLOGY AND MECHANISM OF ACTION
The CD33 surface antigen is expressed on the majority of immature myeloblasts and is downregulated as the normal cell matures. However, in AML, this maturation process never occurs, meaning a plethora of binding sites for a CD33-specific agent are present to direct therapy. Mature hematopoietic stem cells, as a result of the aforementioned downregulation, and nonhematopoietic cells do not express this receptor (Linenberger, 2005). Multiple methodologies have been tested in determining the most efficacious use of a CD33 antibody, including radioimmunoconjugates and immunotoxin conjugates. These studies showed that the CD33-positive cells were quick to internalize these antibodies after binding.
Gemtuzumab ozogamicin is a humanized IgG4 monoclonal antibody-drug conjugate that binds to the CD33 surface receptor and is then rapidly internalized, as seen in Figure 1 (Bross et al., 2001; Jerjian, Glode, Thompson, & O’Bryant, 2016). The antibody is linked to its payload, calicheamicin, which is an extremely cytotoxic enediyne antibiotic (Figure 2). The peptide linker that connects the antibody portion to calicheamicin was designed to only release within an acidic environment, such as the lysosome of the myeloblast. The antibody-drug conjugate delivery method allows for clinical use of calicheamicin, as it was proved to be too cytotoxic for direct therapeutic use. Calicheamicin’s cytotoxicity is conveyed through its binding to the minor groove of DNA, leading to double-strand breaks and ultimately cell death. Due to the high level of potency and cytotoxicity demonstrated by calicheamicin, the specificity of antibody binding and the focused expression within the body of the antibody’s target are key to limiting toxicity from its administration (Appelbaum & Bernstein, 2017; Smith & Nicolaou, 1996).
Figure 1.
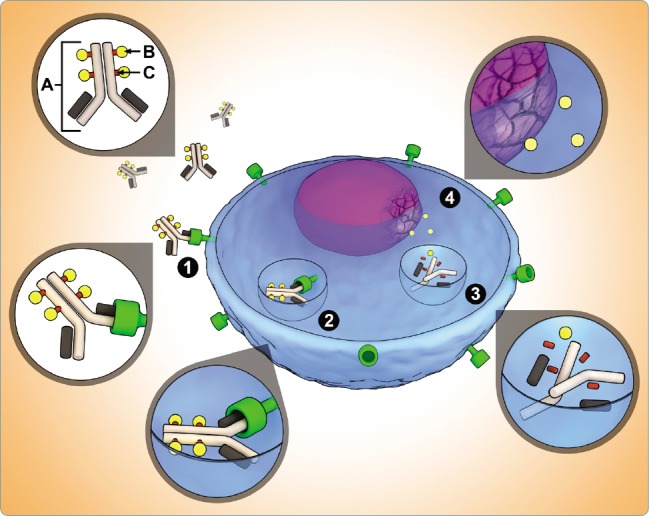
Antibody-drug conjugate (ADC) structure and mechanism of action. The ADC consists of three key components: (A) site-specific monoclonal antibody; (B) cytotoxic drug; and (C) functional linker that binds drug to antibody. ADC mechanism of action: (1) binding of ADC to antigen of cancer cell; (2) internalization of antigen-ADC complex; (3) lysosomal degradation releases drug from antibody; (4) drug causes DNA or microtubule disruption. From "Antibody-drug conjugates: A clinical pharmacy perspective on an emerging cancer therapy," by Jerjian et al., 2016, Pharmacotherapy, 36(1), 99–116. © 2016 Pharmacotherapy Publications, Inc. Reprinted with permission.
Figure 2.
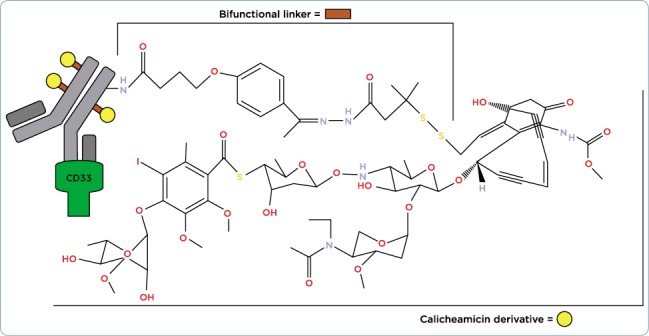
Gemtuzumab ozogamicin structure.
CLINICAL TRIALS
Gemtuzumab ozogamicin was first approved in 2000 by the FDA’s accelerated approval program for single-agent use in patients with CD33-positive AML in first relapse who were 60 years or older and not considered candidates for other cytotoxic treatment (Pfizer Inc., 2017). As part of the accelerated approval pathway, a confirmatory trial was required to be completed. As stated previously, this trial was negative, and 10 years later Pfizer voluntarily withdrew gemtuzumab ozogamicin from the market (Pfizer Inc., 2017).
Initial dosing of gemtuzumab ozogamicin was 9 mg/m² intravenously (IV) every 2 weeks based on phase I data showing complete or near-complete CD33-binding site saturation and lack of dose-limiting nonhematologic toxicity. However, further data showed that new CD33 proteins are continuously expressed on the cell surface, and antigen levels return to baseline 72 hours after gemtuzumab ozogamicin exposure (Godwin, Gale, & Walter, 2017). Therefore, subsequent studies sought to overcome this through the use of fractionated dosing, or lower doses given more frequently.
Gemtuzumab ozogamicin received FDA approval for a second time on September 1, 2017, for adults with newly diagnosed CD33-positive AML and adults and children 2 years and older with relapsed or refractory CD33-positive AML (Pfizer Inc., 2017). This reapproval was based upon several trials discussed below evaluating gemtuzumab ozogamicin as treatment of AML in various clinical scenarios using the fractionated dosing strategy.
DATA FOR INITIAL APPROVAL
Cumulative Results From Trials 201, 202, and 203
Gemtuzumab ozogamicin received accelerated approval based upon aggregate data from three open-label phase II studies (Trials 201, 202, 203). Collectively, 142 patients were enrolled who had CD33-positive AML in first relapse. Patients included in Trials 201 and 202 were ≥ 18 years of age, with a first remission duration of ≥ 6 months. Trial 203 only included patients ≥ 60 years, and their first remission had to have lasted at least 3 months. Patients with secondary leukemia or white blood cell count (WBC) ≥ 30,000/µL were excluded. Patients were treated with two 9 mg/m² IV infusions separated by 14 days and were followed up 28 days after the last dose. Patients received premedications of acetaminophen and diphenhydramine. The primary endpoint for all three trials was the rate of CRs, defined as leukemic blasts absent from the peripheral blood, ≤ 5% blasts in the bone marrow, hemoglobin ≥ 9 g/dL, platelets ≥ 100,000/µL, absolute neutrophil count ≥ 1,500/µL, and red cell and platelet transfusion independent (no red cell transfusions for 2 weeks and no platelet transfusions for 1 week) or CRp (meeting the definition of CR without platelet recovery to ≥ 100,000/µL; Bross et al., 2001; Wyeth Laboratories, n.d.).
The overall response rate for the three studies was 30% (42/142), with 16% (23/142) of patients with a CR and 13% (19/142) with a CRp. The median time to remission was 60 days regardless of response type. Patients < 60 years of age had a 34% response rate, with an overall response rate of 26% for those ≥ 60 years. The relapse-free survival (RFS) was 6.8 months for those with a response, 7.2 months for CR, and 4.4 months for CRp. Patients > 60 years had RFS of 2.3 months vs. 17 months for patients < 60 years of age. The median duration of overall survival for the entire study population was 5.9 months (Bross et al., 2001; Wyeth Laboratories, n.d.).
The data from these three phase II studies supported gemtuzumab ozogamicin’s accelerated approval based upon the overall response rate (CR and CRp) being a surrogate endpoint "reasonably likely" to predict clinical benefit. The FDA approved gemtuzumab ozogamicin for use in patients with CD33-positive AML in first relapse who were ≥ 60 years and not candidates for cytotoxic chemotherapy. The approved dosing regimen was 9 mg/m² IV over 4 hours repeated 14 days later. A requirement of the accelerated approval was confirmatory studies of clinical benefit (Bross et al., 2001).
Confirmatory Study SWOG S0106
Based upon the tolerability and efficacy of gemtuzumab ozogamicin in combination with daunorubicin and cytarabine in phase I/II trials, SWOG began enrolling patients in a prospective phase III randomized trial in a 1:1 ratio to evaluate the effects of adding gemtuzumab ozogamicin to standard induction chemotherapy (daunorubicin and cytarabine) in patients with newly diagnosed AML. Patients with secondary AML were excluded.
Patients who were randomized to receive all three drugs were given the following: daunorubicin at 45 mg/m² IV on days 1 to 3, cytarabine at 100 mg/m² continuous IV infusion on days 1 to 7, and gemtuzumab ozogamicin at 6 mg/m² IV over 2 hours on day 4. Those receiving standard induction therapy were administered daunorubicin at 60 mg/m² IV on days 1 to 3 and cytarabine at 100 mg/m² IV continuous infusion. A second 1:1 randomization was also part of the study design, in which patients who achieved a CR after 3 cycles of cytarabine consolidation therapy could be given 3 doses of gemtuzumab ozogamicin at 5 mg/m² IV at least 28 days apart in hopes of improving disease-free survival (DFS). With the double randomization scheme, investigators planned to evaluate if a benefit was seen with the addition of gemtuzumab ozogamicin during induction, postconsolidation, or both. The two primary objectives were to test whether the CR rate was higher in patients who received gemtuzumab ozogamicin as part of induction therapy and to see if the postconsolidation DFS rate was higher among patients randomized to the gemtuzumab ozogamicin arm (Petersdorf et al., 2013).
At the second planned interim analysis, the SWOG Data and Safety Monitoring Committee (DSMC) recommended closing the study. The CR rates at that time were 66% in the 227 patients who were treated with gemtuzumab ozogamicin induction, and 69% in the 229 patients treated with standard induction chemotherapy. The hypothesis that the combination regimen would increase the CR rate by 12% was rejected at the predefined significance level (p < .0025). In addition, RFS was also not significantly better in the gemtuzumab ozogamicin induction arm. The first planned analysis of the postconsolidation outcome also resulted in the DSMC recommending closure of this arm, as the hypothesis was rejected that gemtuzumab ozogamicin would improve DFS with a hazard ratio of 1.5 at the prespecified significance level (p < .001). The study team accepted the DSMC recommendations and SWOG closed the study to accrual. Based on the results from this study, Pfizer voluntarily withdrew gemtuzumab ozogamicin from the US market in June 2010, prior to the release of results from other randomized trials. The Acute Leukemia French Association (ALFA) 0701 trial was one of the trials ongoing at the time of this trial closure and was actually supportive of gemtuzumab ozogamicin’s reapproval at a lower dose (Petersdorf et al., 2013).
DATA FOR REAPPROVAL
Newly Diagnosed Acute Myeloid Leukemia
ALFA-0701 Trial. The ALFA-0701 trial was a phase III, open-label evaluation of the addition of low fractionated dose gemtuzumab ozogamicin to standard front-line chemotherapy to improve survival outcomes without excessive toxicity. The study was conducted at 26 centers in France and enrolled patients 50 to 70 years of age with previously untreated de novo AML. Patients were not required to have CD33 expression on leukemic blast cells to be enrolled. Patients were excluded if they had a previous myeloproliferative or myelodysplastic syndrome (MDS) or exposure to chemotherapy or radiation therapy.
Two hundred and eighty patients were randomized in a 1:1 ratio to receive standard treatment with or without gemtuzumab ozogamicin. Patients received methylprednisolone prior to each dose of gemtuzumab ozogamicin. Standard induction treatment included 7 + 3 induction: cytarabine at 200 mg/m² continuous IV infusion on days 1 to 7, and daunorubicin at 60 mg/m² IV on days 1 to 3. Patients randomized to gemtuzumab ozogamicin received 3 mg/m² (max of 5 mg) IV over 2 hours on days 1, 4, and 7 during induction in addition to 3 + 7. Patients with > 10% leukemic blasts in the day 15 bone marrow were given a second induction course of daunorubicin at 60 mg/m² IV for 2 days and cytarabine at 1,000 mg/m² IV over 2 hours every 12 hours for 3 days without gemtuzumab ozogamicin, followed by daily granulocyte colony-stimulating factor until neutrophil recovery. Clinical and hematologic responses were evaluated after induction therapy. Patients who did not respond to induction treatment were discontinued from treatment and those who did respond with a CR or CRp were given two consolidation courses of daunorubicin at 60 mg/m² IV for one day in course 1, and two days in course 2, in combination with cytarabine at 1,000 mg/m² IV over 2 hours every 12 hours on days 1 to 4 with or without gemtuzumab ozogamicin at 3 mg/m² on day 1. Evaluation of clinical and hematologic responses were conducted before the beginning of the second consolidation course and then every 3 months for 2 years (Castaigne et al., 2012).
The primary outcome of event-free survival (EFS) estimated at 2 years was superior in the gemtuzumab ozogamicin arm (40.8% [32.8%–50.8%] vs. 17.1% [10.8%–27.1%]; hazard ratio [HR], 0.58 [0.43–0.78]; p = .0003). The secondary endpoints of RFS and OS were also improved in the investigational arm. Relapse-free survival was 50.3% (41.0%–61.6%) in the investigational arm vs. 22.7% (14.5%–35.7%) in the standard care arm (HR, 0.52 [0.36–0.75]; p = .0003) and OS was 53.2% (44.6%–63.5%) vs. 41.9% (33.1%–53.1%), respectively (HR, 0.69 [0.49–0.98]; p = .0368). In general, patients in the gemtuzumab ozogamicin arm experienced an increased incidence of hematologic and nonhematologic adverse events, but patients did not experience an increase in the risk of death from toxicity (Castaigne et al., 2012).
In 2014, the final analysis of the ALFA-0701 study was reported with a longer median follow-up for patients alive at 43 months (30–63.5 months). The estimated 3-year EFS in the gemtuzumab ozogamicin arm was 31% vs. 19% (HR, 0.66 [0.50–0.87]; median 15.6 vs. 9.7 months; p = .0026). Three-year RFS was reported as 38% in the gemtuzumab ozogamicin arm vs. 25% in the standard treatment arm (p = .006). Overall survival at 3 years did not remain statistically significant (44% vs. 36%; HR, 0.82 [0.60–1.10]; median 25.4 vs. 20.8 months; p = .18). At the reference date, 132 patients had relapsed; 63 in the gemtuzumab ozogamicin arm and 69 in the control arm. The majority of patients post relapse received intensive salvage treatment with idarubicin and cytarabine or gemtuzumab ozogamicin as a single agent or in combination. Second CR rates were not significantly different based upon prior study treatment (p = .38). The ALFA-0701 trial supports the safe and effective use of fractionated lower doses of gemtuzumab ozogamicin in the newly diagnosed setting in combination with induction therapy (Castaigne et al., 2014).
AML-19 Trial. The purpose of the AML-19 sequential phase II/III trial was to compare gemtuzumab ozogamicin as a single agent with best supportive care (BSC) including hydroxyurea as first-line therapy in older adults with AML unsuitable for intensive chemotherapy. Two different gemtuzumab ozogamicin dosing strategies were evaluated in the phase II portion of the trial to determine the optimal dose for the sequential phase III portion. In the open-label phase III part of the trial, 237 patients > 60 years old were randomized 1:1 to receive a single induction course of gemtuzumab ozogamicin dosed at 6 mg/m² on days 1 and 3 and 3 mg/m² on day 8 with BSC, or BSC alone. Best supportive care included blood product transfusions, antimicrobials, and other symptomatic therapies per institutional policies. Patients in the BSC arm alone were allowed to receive hydroxyurea at doses to keep their WBC count < 20,000/ µL. Randomization was stratified by age (61–75, 76–80, ≥ 81 years), World Health Organization (WHO) performance score (0–1, 2, > 2), CD33 expression (< 20%, 20%–80%, > 80%, unknown), WBC count at diagnosis (< 30,00/µL, ≥ 30,000/µL), and treating center. This study was conducted at 35 centers in three European countries. Patients who did not progress after gemtuzumab ozogamicin induction were allowed to receive gemtuzumab ozogamicin at 2 mg/m² monthly for up to 8 doses (Amadori et al., 2016).
The primary endpoint of OS was superior in the patients who received gemtuzumab ozogamicin; there was a median OS 4.9 (4.2–6.8) vs. 3.6 months (2.6–4.2) in those who received BSC (HR, 0.69 [0.53–0.90]; p = .005). At 1 year, the OS rate remained superior in the gemtuzumab ozogamicin arm, at 24.3% vs. 9.7%. In the exploratory subgroup analyses, there was no interaction between baseline patient characteristics and treatment effect for OS, with the exception of CD33 expression, sex, and cytogenetic profile. After induction, 24.3% of patients in the gemtuzumab ozogamicin group experienced CR (8.1% CR + 16.2% CRi [CR with incomplete recovery of peripheral blood counts]). Additionally, 6.3% of patients had a partial remission and 39.6% had stable disease. The median progression-free survival (PFS) for the patients who received gemtuzumab ozogamicin was 2.8 months (2.4–3.8).
The secondary endpoint of this phase III trial was safety. Adverse event rates were similar between the two arms, and patients did not experience excess mortality with gemtuzumab ozogamicin treatment. The overall incidence of any grade of adverse event was 87.3% in the gemtuzumab ozogamicin arm compared to 90.4% in the BSC arm, with the incidence of grade ≥ 3 adverse events being 61.2% vs. 67.5%, respectively. Death from any adverse event was 17.1% in the gemtuzumab ozogamicin arm and 20.2% in the BSC arm. AML-19 supports the use of low-dose gemtuzumab ozogamicin as first-line monotherapy in older patients with AML who are ineligible for intensive chemotherapy based on an improvement in OS over BSC (Amadori et al., 2016).
Relapsed Acute Myeloid Leukemia
MyloFrance-1 Trial. The MyloFrance-1 trial was a phase II multicenter uncontrolled sequential trial to assess the efficacy and safety of fractionated doses of gemtuzumab ozogamicin given to 57 adult patients with AML in first relapse. Patients were required to have CD33-positive AML as determined by bone marrow aspirate and immunophenotyping. Gemtuzumab ozogamicin was administered at 3 mg/m² IV over 2 hours on days 1, 4, and 7 as induction therapy. Patients who experienced CR or CRp were recommended to receive consolidation with high-dose cytarabine (< 55 years: cytarabine at 3 g/m² IV every 12 hours for 3 days; or > 55 years and/or creatinine clearance < 50 mL/min: cytarabine at 1 g/m² IV every 12 hours for 3 days). Eligible patients could go on to receive a hematopoietic stem cell transplant (HSCT) after a minimum delay of 90 days from gemtuzumab ozogamicin treatment (Taksin et al., 2007).
This study was planned to detect a 15% benefit in response rate from gemtuzumab ozogamicin compared to the referenced literature of 15%. At the second interim analysis, based upon 26 enrolled patients, the trial was stopped due to the upper boundary being crossed, therefore concluding gemtuzumab ozogamicin was beneficial. At the final analysis, 57 patients had been enrolled. The overall response rate was 33.3%, with 26% of patients achieving a CR and 7% CRp. The median RFS was 11 months. The median OS was 8.4 months (8.3 months for patients < 60 years vs. 8.9 months for those > 60 years; p = .15). Patients with a CR or CRp recovered their absolute neutrophil count to > 500/µL in a median of 23 days and platelet count to > 50,000/µL in a median of 20 days from the first dose of gemtuzumab ozogamicin. No grade 3 or 4 liver toxicity was reported and no episodes of veno-occlusive disease (VOD) occurred. The results of the MyloFrance-1 study supports the use of fractionated lower-dose gemtuzumab ozogamicin in the relapsed setting (Taksin et al., 2007).
TOXICITIES AND THEIR MANAGEMENT
Gemtuzumab ozogamicin has a black box warning for hepatotoxicity, including severe or fatal VOD. In the ALFA-0701 trial, VOD was reported to occur in 5% of patients during or following gemtuzumab ozogamicin treatment or following HSCT. Median time from dosing to onset of VOD was 9 days (2 to 298 days), with 83.3% of events occurring within 28 days of any gemtuzumab ozogamicin dose. In the MyloFrance-1 study, VOD was not reported in any of the 57 patients during or following treatment with gemtuzumab ozogamicin, or following HSCT. When analyzing trial data, the risk of VOD was higher in adults who received higher doses of gemtuzumab ozogamicin as monotherapy, in patients with moderate or severe hepatic impairment prior to gemtuzumab ozogamicin (8.7 times more likely compared with patients without moderate or severe hepatic impairment at baseline), in patients treated with gemtuzumab ozogamicin after HSCT (2.6 times more likely compared with those without prior HSCT), and in patients who had an HSCT after gemtuzumab ozogamicin treatment (2.9 times more likely compared with those without HSCT after gemtuzumab ozogamicin treatment). If a patient develops VOD, it is recommended that gemtuzumab ozogamicin be discontinued (Pfizer Inc., 2018).
Additional warnings and precautions in the product labeling include the risk of infusion-related reactions occurring during or within 24 hours following infusion (including anaphylaxis). Patients should be premedicated with a corticosteroid, acetaminophen, and diphenhydramine to minimize the risk of infusion-related reactions. They should also be monitored during the infusion, and for at least 1 hour after the end of the infusion. If a patient experiences an infusion reaction, the infusion may be interrupted, steroids or antihistamines given, or permanent discontinuation of the gemtuzumab ozogamicin may be necessary. Patients are also at risk for severe (including fatal) hemorrhage due to prolonged thrombocytopenia. Platelet counts should be monitored frequently and transfusions given as necessary. Patients may need to have gemtuzumab ozogamicin held or even permanently discontinued for severe bleeding, hemorrhage, or persistent thrombocytopenia. QT interval prolongation has been reported in patients receiving drugs containing calicheamicin, the cytotoxic payload. Patients who have a history of or predisposition for QTc prolongation, those who are taking medications known to prolong the QT interval, or those who have electrolyte abnormalities should have an electrocardiogram and receive electrolyte supplementation prior to the start of gemtuzumab ozogamicin and throughout treatment as needed (Pfizer Inc., 2018).
Adverse event rates varied by clinical trial due to differences in the patient population studied and additional therapies received. The most common (> 15%) adverse reactions reported were hemorrhage, infection, fever, nausea, vomiting, constipation, headache, increased aspartate aminotransferase (AST) and alanine aminotransferase (ALT), rash, and mucositis. Patients should have blood counts and chemistries monitored at least three times per week through recovery from treatment-related toxicities. Management of some adverse events may require doses of gemtuzumab ozogamicin to be held or therapy discontinued. There are no dose reductions recommended.
Gemtuzumab ozogamicin should be discontinued in patients receiving it in combination with chemotherapy if their platelet count does not recover to ≥ 100,000/µL or neutrophil count does not recover to ≥ 500/µL within 14 days following the planned start date for consolidation. If a patient has a total bilirubin ≥ 2 × upper limit of normal (ULN), or AST and/or ALT ≥ 2.5 × ULN, treatment should be delayed until the bilirubin recovers to ≤ 2 × ULN, and AST and ALT to ≤ 2.5 × ULN prior to each dose. If a dose is delayed more than 2 days between sequential infusions, then it should be omitted (Pfizer Inc., 2018).
Gemtuzumab ozogamicin is considered low emetic risk, and patients should receive nausea/vomiting prophylaxis according to institutional practice. Patients with AML are considered at high risk for infection and should receive appropriate antimicrobial prophylaxis. Refer to Tables 1 to 4 for reported trial-specific adverse effect incidence rates and severities. Tables 1 and 2 contain results from the ALFA-0701 trial of gemtuzumab ozogamicin in combination with chemotherapy given to patients with newly diagnosed de novo AML ages 50 to 70 years. Table 3 contains data from the AML-19 trial evaluating single-agent gemtuzumab ozogamicin in elderly patients with a performance status > 2 and newly diagnosed AML. Table 4 reports on the MyloFrance-1 trial that enrolled patients with relapsed or refractory CD33-positive AML in first relapse who were treated with single-agent gemtuzumab ozogamicin.
Table 1.
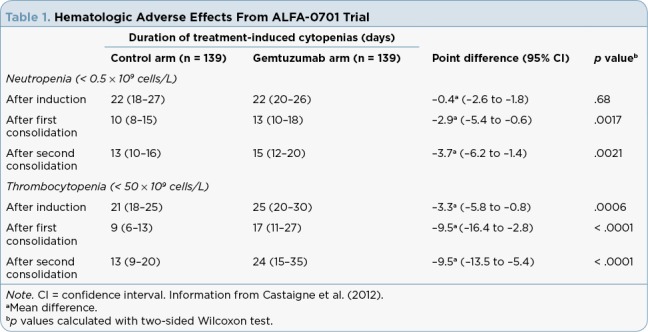
Hematologic Adverse Effects From ALFA-0701 Trial
Table 2.
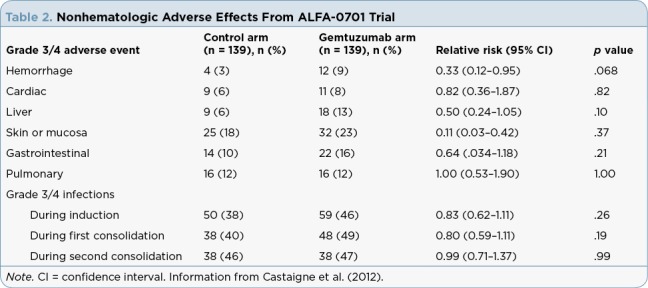
Nonhematologic Adverse Effects From ALFA-0701 Trial
Table 3.
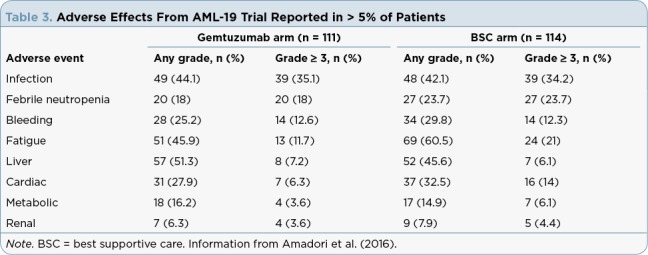
Adverse Effects From AML-19 Trial Reported in > 5% of Patients
Table 4.
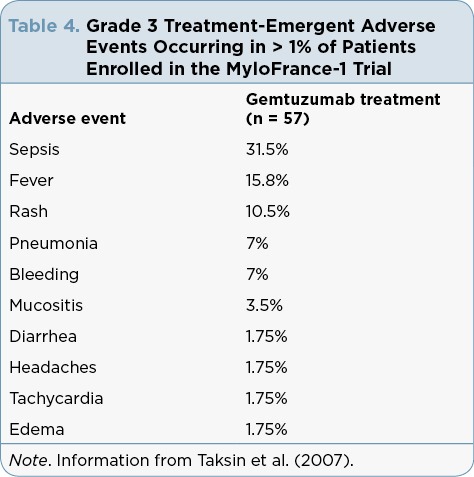
Grade 3 Treatment-Emergent Adverse Events Occurring in > 1% of Patients Enrolled in the MyloFrance-1 Trial
CURRENT PLACE IN THERAPY
Gemtuzumab ozogamicin is not considered intensive therapy, which makes it an attractive option for patients who cannot tolerate intensive therapy, such as frail, elderly patients or patients with significant comorbidities. Due to its tolerability, it can also be used in combination with intensive chemotherapy in patients who have favorable or intermediate-risk disease (e.g., core binding factor +/- KIT mutation, normal cytogenetics, etc.; National Comprehensive Cancer Network [NCCN], 2018). Gemtuzumab ozogamicin has a serious risk of hepatotoxicity and VOD, making it less than ideal in patients who are likely to proceed to allogeneic transplant.
FDA Indication
Gemtuzumab ozogamicin is indicated in adults with CD33-positive newly diagnosed AML and relapsed/refractory CD33-positive disease in adults and children ages 2 years and up (Pfizer Inc., 2018). Gemtuzumab ozogamicin is only contraindicated in a patient with a hypersensitivity to the drug or a component but should be used with extreme caution in patients who are stem cell transplant candidates, have a history of stem cell transplant, have AML with adverse-risk cytogenetics, WBC > 30,000/µL, hepatic impairment, electrolyte abnormalities, QT prolongation or family history of QT prolongation, history of torsades de pointes or ventricular arrhythmias, bradycardia, recent myocardial infarction, or congestive heart failure.
Acute Myeloid Leukemia
The NCCN Clinical Practice Guidelines for AML were updated February 2018 and now include recommendations for gemtuzumab ozogamicin in several clinical scenarios (NCCN, 2018).
Gemtuzumab ozogamicin is recommended for the front-line treatment of AML in patients < 60 years of age in combination with standard induction regimens when fluorescence in situ hybridization analysis shows CD33 positivity (NCCN, 2018). If CR is achieved in this population, postremission therapy includes gemtuzumab ozogamicin in combination with standard maintenance therapy. This is the recommendation for core binding factor translocations or other favorable-risk molecular abnormalities, as well as intermediate-risk cytogenetics and/or molecular abnormalities. Note that there is currently no recommendation for repeat gemtuzumab ozogamicin dosing in the reinduction setting in the event that complete remission is not achieved with primary induction therapy. Poor-risk populations do not have a recommendation for gemtuzumab ozogamicin treatment at this time (NCCN, 2018).
For the AML population that is ≥ 60 years, gemtuzumab ozogamicin can be used in combination with standard intensive induction regimens if the patient is appropriately fit and lacks unfavorable cytogenetics/molecular markers. It can also be used in maintenance therapy combined with chemotherapy in these patients if they achieved CR with induction. For a patient > 60 years who is not a candidate for intensive induction, gemtuzumab ozogamicin is recommended as a single agent for induction therapy.
Acute Promyelocytic Leukemia
Acute promyelocytic leukemia (APL) is considered highly curable with contemporary treatment consisting of all-trans retinoic acid (ATRA), arsenic trioxide (ATO), and anthracycline-based chemotherapy. However, these agents come with risks of toxicity and long-term sequelae, especially in patients with preexisting comorbid conditions. In the NCCN Guidelines, gemtuzumab ozogamicin is a recommended treatment option for the front-line treatment of APL in the high-risk setting (presentation with WBC > 10,000/µL) in combination with ATRA and ATO (NCCN, 2018). It is important to note that the dose for this population is typically 9 mg/m² IV on day 1 of therapy. Gemtuzumab ozogamicin is recommended for APL consolidation therapy as a single agent if the patient cannot continue their ATRA and ATO consolidation due to toxicities (NCCN, 2018).
In the APL patient who is high risk with cardiac issues (i.e., low ejection fraction or established QTc prolongation), gemtuzumab ozogamicin is recommended both in combination with ATRA and ATO (at varying doses), as well as with ATRA alone in both the induction and consolidation settings (NCCN, 2018).
Gemtuzumab ozogamicin is also recommended in APL in the relapsed setting if the patient has had no prior exposure to ATO (induction with only ATRA +/- anthracycline), or the patient has had a late relapse (≥ 6 months after an ATO-containing regimen) in combination with ATO and ATRA until count recovery with confirmation of remission (NCCN, 2018).
FUTURE DIRECTIONS
Despite the array of data available for gemtuzumab ozogamicin from its previous approval, studies are ongoing to determine its role in additional patient populations and therapy combinations. Currently, enrolling studies are looking at gemtuzumab ozogamicin in elderly patient populations and in combination with hypomethylating agents and other unique combinations (see Table 5).
Table 5.
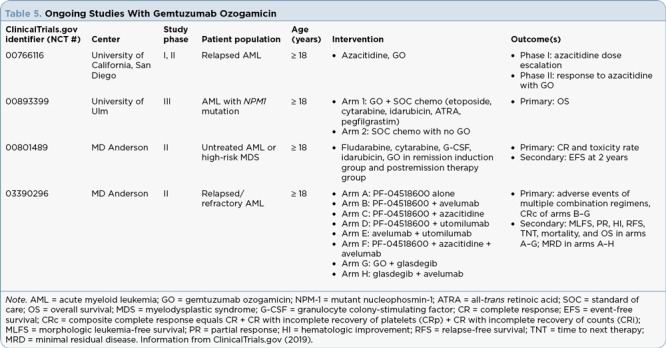
Ongoing Studies With Gemtuzumab Ozogamicin
Acute Promyelocytic Leukemia
As previously mentioned, gemtuzumab ozogamicin is now an NCCN category 2A recommendation for the treatment of APL in combination with ATRA and ATO or as a single agent in the event ATRA and/or ATO is not tolerated. This is partly based on the study by Abaza and colleagues published in 2017. This trial examined long-term outcomes of patients with newly diagnosed APL treated at the University of Texas MD Anderson Cancer Center using ATRA and ATO, with or without gemtuzumab ozogamicin. Induction consisted of ATRA (45 mg/m² IV daily) and ATO (0.15 mg/kg IV daily) with gemtuzumab ozogamicin (9 mg/m² IV on day 1) added to induction of high-risk patients (WBC > 10,000/µL), as well as low-risk patients with leukocytosis. The study concluded that the combination of ATRA, ATO, and gemtuzumab ozogamicin was a safe and effective treatment option for APL that maintained durable responses and spared high-risk patients the exposure of chemotherapy that has historically been the treatment of choice. It remains to be seen if the FDA-approved indication for gemtuzumab ozogamicin is expanded to include APL at the 9 mg/m² dose.
Hypomethylating Agents
Azacitidine and decitabine remain the backbone of AML treatment in the elderly, frail, and relapsed population (NCCN, 2018). Hypomethylating agents tend to be well tolerated and achieve some level of response, though it is rarely durable (Tessoulin et al., 2014). A phase II study by Daver and colleagues published in Leukemia in 2016 studied the combination of gemtuzumab ozogamicin with decitabine in 110 patients at the University of Texas MD Anderson Cancer Center (Daver et al., 2016). Included were patients who had relapsed/refractory AML, had newly diagnosed AML but had been determined unfit for intense chemotherapy-based induction by their treating physician, or had an MDS or myelofibrosis diagnosis. This study concluded that the combination had improved response rates but did not show improved OS when compared to historical analysis in the AML group, and would therefore be an acceptable treatment regimen. However, the MDS group had inferior outcomes, and more research is needed for that population.
Ongoing Studies
Studies with gemtuzumab ozogamicin in alternate diseases and with varying combinations are ongoing. See Table 5 for more details.
IMPLICATIONS FOR THE ADVANCED PRACTITIONER
Like most medications for AML, gemtuzumab ozogamicin is dosed slightly differently based on the indication and place in therapy. In a patient with CD33-positive AML who is newly diagnosed (de novo) and appropriate for intensive therapy, gemtuzumab ozogamicin is given in combination with institution-specific 7 + 3 induction regimens. The recommended dose when used in this combination is 3 mg/m² IV (capped at 4.5 mg) on days 1, 4, and 7. As previously mentioned, gemtuzumab ozogamicin is not recommended in the second induction cycle if one is needed. For the consolidation/maintenance cycles for this population, the recommended dose of gemtuzumab ozogamicin is 3 mg/m² IV on day 1 only (up to one 4.5-mg vial) in combination with daunorubicin and cytarabine (based on institution practice). The average wholesale price per vial of gemtuzumab ozogamicin is around $10,000 (Castaigne et al., 2012; NCCN, 2018; Pfizer Inc., 2018).
In a patient with newly diagnosed CD33-positive AML who is not appropriate for intensive induction chemotherapy, gemtuzumab ozogamicin can be used for induction and up to 8 cycles of continuation therapy. For single-agent induction, the recommended dose is 6 mg/m² IV on day 1, and 3 mg/m² (not capped at 4.5 mg) IV on day 8. For continuation, the recommended dose is 2 mg/m² (not capped at 4.5 mg) IV as a single agent on day 1 every 4 weeks (NCCN, 2018; Pfizer Inc., 2018).
In relapsed/refractory AML, when using gemtuzumab ozogamicin as a single agent, the dose is 3 mg/m² (up to 4.5 mg) IV on days 1, 4, and 7 (NCCN, 2018; Pfizer Inc., 2018).
Like all antibodies, there is a significant risk of infusion-related reactions with IV administration of gemtuzumab ozogamicin. When administering gemtuzumab ozogamicin, practitioners should premedicate patients appropriately with acetaminophen and diphenhydramine 1 hour prior to infusion and methylprednisolone at 1 mg/kg orally or IV, or an equivalent dose of an alternative corticosteroid 30 minutes prior to infusion. Adult patients should receive 650 mg of acetaminophen orally and 50 mg of diphenhydramine orally or IV. Pediatric patients should receive acetaminophen at 15 mg/kg (maximum of 650 mg) and diphenhydramine at 1 mg/kg (maximum of 50 mg). Additional doses of acetaminophen and diphenhydramine may be administered every 4 hours after the initial pretreatment doses. Additional corticosteroid doses may be needed for an infusion reaction during the infusion or within 4 hours afterwards (Pfizer Inc., 2018).
Gemtuzumab ozogamicin should be reconstituted appropriately according to the package insert, and then diluted to a final concentration of 0.075 mg/mL to 0.234 mg/mL and infused over 2 hours with an inline 0.2 micron polyethersulfone filter. The IV bag should be protected from light during infusion. Practitioners should monitor vital signs frequently (per institutional practice) during the infusion. If signs of an infusion reaction occur (fever, chills, dyspnea, bronchospasm, or hypotension), practitioners should interrupt the infusion immediately and manage the reaction by repeating doses of acetaminophen, diphenhydramine, and the corticosteroid as described above. The infusion should be held until symptoms resolve, and then restarting the infusion can be considered at no more than half of the previous rate. Gemtuzumab should be permanently discontinued in the event of severe or life-threatening reactions (anaphylaxis, severe respiratory symptoms, or clinically significant hypotension; Pfizer Inc., 2018).
Prophylaxis and management of tumor lysis syndrome should always be considered when treating patients with AML, and supportive medications should be given as appropriate and dictated by institutional practice. Cytoreduction should be considered in patients with a leukocyte count ≥ 30,000/µL (Pfizer Inc., 2018).
SUMMARY
Gemtuzumab ozogamicin, much like 7 + 3 and many other treatment regimens that have come before it, has been examined, modified, and tested numerous times in an attempt to maximize both its safety and efficacy. Despite its recent FDA approval, gemtuzumab ozogamicin is quickly climbing up the therapeutic ladder for both AML and APL. This includes options for first-line therapy for select patients, as well as multiple options in the relapsed and refractory settings for both disease states. Furthermore, within AML, regimens exist featuring gemtuzumab ozogamicin as monotherapy or as combination therapy with traditional chemotherapy. This should allow for more patients to receive induction therapy for their leukemia, where previously few options existed.
It is important to remember that the new package insert still carries a black box warning for hepatotoxicity (Pfizer Inc., 2018). Overall, toxicity is less than what was previously seen with the original FDA approval; however, this is by no means a benign medication and does carry the risk for adverse events that require close clinical monitoring. The toxicity profile and efficacy data demonstrated by gemtuzumab ozogamicin make it an attractive option for the treatment of AML and APL going forward.
Footnotes
Dr. Yacko is Regional Medical Liaison, Oncology, for Amgen US Medical Affairs. The other authors have no conflicts of interest to disclose.
References
- 1.Abaza Yasmin, Kantarjian Hagop, Garcia-Manero Guillermo, Estey Elihu, Borthakur Gautam, Jabbour Elias, Faderl Stefan, O’Brien Susan, Wierda William, Pierce Sherry, Brandt Mark, McCue Deborah, Luthra Rajyalakshmi, Patel Keyur, Kornblau Steven, Kadia Tapan, Daver Naval, DiNardo Courtney, Jain Nitin, Verstovsek Srdan, Ferrajoli Alessandra, Andreeff Michael, Konopleva Marina, Estrov Zeev, Foudray Maria, McCue David, Cortes Jorge, Ravandi Farhad. Long-term outcome of acute promyelocytic leukemia treated with all-trans-retinoic acid, arsenic trioxide, and gemtuzumab. Blood. 2017;129:1275–1283. doi: 10.1182/blood-2016-09-736686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amadori Sergio, Suciu Stefan, Selleslag Dominik, Aversa Franco, Gaidano Gianluca, Musso Maurizio, Annino Luciana, Venditti Adriano, Voso Maria Teresa, Mazzone Carla, Magro Domenico, De Fabritiis Paolo, Muus Petra, Alimena Giuliana, Mancini Marco, Hagemeijer Anne, Paoloni Francesca, Vignetti Marco, Fazi Paola, Meert Liv, Ramadan Safaa Mahmoud, Willemze Roel, de Witte Theo, Baron Frédéric. Gemtuzumab Ozogamicin Versus Best Supportive Care in Older Patients With Newly Diagnosed Acute Myeloid Leukemia Unsuitable for Intensive Chemotherapy: Results of the Randomized Phase III EORTC-GIMEMA AML-19 Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016;34:972–979. doi: 10.1200/JCO.2015.64.0060. [DOI] [PubMed] [Google Scholar]
- 3.Appelbaum Frederick R, Bernstein Irwin D. Gemtuzumab ozogamicin for acute myeloid leukemia. Blood. 2017;130:2373–2376. doi: 10.1182/blood-2017-09-797712. [DOI] [PubMed] [Google Scholar]
- 4.Bross P F, Beitz J, Chen G, Chen X H, Duffy E, Kieffer L, Roy S, Sridhara R, Rahman A, Williams G, Pazdur R. Approval summary: gemtuzumab ozogamicin in relapsed acute myeloid leukemia. Clinical cancer research : an official journal of the American Association for Cancer Research. 2001;7:1490–1496. [PubMed] [Google Scholar]
- 5.Castaigne Sylvie, Pautas Cécile, Terré Christine, Raffoux Emmanuel, Bordessoule Dominique, Bastie Jean-Noel, Legrand Ollivier, Thomas Xavier, Turlure Pascal, Reman Oumedaly, de Revel Thierry, Gastaud Lauris, de Gunzburg Noémie, Contentin Nathalie, Henry Estelle, Marolleau Jean-Pierre, Aljijakli Ahmad, Rousselot Philippe, Fenaux Pierre, Preudhomme Claude, Chevret Sylvie, Dombret Hervé. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): a randomised, open-label, phase 3 study. Lancet (London, England) 2012;379:1508–1516. doi: 10.1016/S0140-6736(12)60485-1. [DOI] [PubMed] [Google Scholar]
- 6.Castaigne S, Pautas C, Terré C, Renneville A, Gardin C, Suarez F, Dombret H. Final analysis of the ALFA 0701 study [Abstract 376]. Blood (ASH Annual Meeting Abstracts) 2014;124(21):376. Retrieved from http://www.bloodjournal.org/content/124/21/376. [Google Scholar]
- 7.ClinicalTrials.gov. ClinicalTrials.gov. 2019 Retrieved from https://clinicaltrials.gov.
- 8.Daver N, Kantarjian H, Ravandi F, Estey E, Wang X, Garcia-Manero G, Jabbour E, Konopleva M, O’Brien S, Verstovsek S, Kadia T, Dinardo C, Pierce S, Huang X, Pemmaraju N, Diaz-Pines-Mateo M, Cortes J, Borthakur G. A phase II study of decitabine and gemtuzumab ozogamicin in newly diagnosed and relapsed acute myeloid leukemia and high-risk myelodysplastic syndrome. Leukemia. 2016;30:268–273. doi: 10.1038/leu.2015.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Godwin C D, Gale R P, Walter R B. Gemtuzumab ozogamicin in acute myeloid leukemia. Leukemia. 2017;31:1855–1868. doi: 10.1038/leu.2017.187. [DOI] [PubMed] [Google Scholar]
- 10.Jerjian Taleen V, Glode Ashley E, Thompson Lisa A, O’Bryant Cindy L. Antibody-Drug Conjugates: A Clinical Pharmacy Perspective on an Emerging Cancer Therapy. Pharmacotherapy. 2016;36:99–116. doi: 10.1002/phar.1687. [DOI] [PubMed] [Google Scholar]
- 11.Lichtman Marshall A. A historical perspective on the development of the cytarabine (7days) and daunorubicin (3days) treatment regimen for acute myelogenous leukemia: 2013 the 40th anniversary of 7+3. Blood cells, molecules & diseases. 2013;50:119–130. doi: 10.1016/j.bcmd.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Linenberger M L. CD33-directed therapy with gemtuzumab ozogamicin in acute myeloid leukemia: progress in understanding cytotoxicity and potential mechanisms of drug resistance. Leukemia. 2005;19:176–182. doi: 10.1038/sj.leu.2403598. [DOI] [PubMed] [Google Scholar]
- 13.National Cancer Institute. Gemtuzumab ozogamicin voluntarily withdrawn from U.S. Markets: National Cancer Institute. 2010 Retrieved from https://www.cancer.gov/about-cancer/treatment/drugs/fda-gemtuzumab-ozogamicin.
- 14.National Cancer Institute. SEER Cancer Stat Facts: Acute myeloid leukemia (AML). 2017 Retrieved from https://seer.cancer.gov/statfacts/html/amyl.html.
- 15.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Acute myeloid leukemia. v1.2018. 2018 Retrieved from https://www.nccn.org/professionals/physician_gls/pdf/aml.pdf.
- 16.Petersdorf Stephen H, Kopecky Kenneth J, Slovak Marilyn, Willman Cheryl, Nevill Thomas, Brandwein Joseph, Larson Richard A, Erba Harry P, Stiff Patrick J, Stuart Robert K, Walter Roland B, Tallman Martin S, Stenke Leif, Appelbaum Frederick R. A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood. 2013;121:4854–4860. doi: 10.1182/blood-2013-01-466706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pfizer Inc. Pfizer receives FDA approval for Mylotarg™ (gemtuzumab ozogamicin). 2017 Retrieved from https://www.pfizer.com/news/press-release/press-release-detail/pfizer_receives_fda_approval_for_mylotarg_gemtuzumab_ozogamicin.
- 18.Pfizer Inc. Mylotarg (gemtuzumab ozogamicin) package insert. 2018 Retrieved from http://labeling.pfizer.com/ShowLabeling.aspx?id=9548.
- 19.Schuh Andre C, Döhner Hartmut, Pleyer Lisa, Seymour John F, Fenaux Pierre, Dombret Hervé. Azacitidine in adult patients with acute myeloid leukemia. Critical reviews in oncology/hematology. 2017;116:159–177. doi: 10.1016/j.critrevonc.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 20.Smith A L, Nicolaou K C. The enediyne antibiotics. Journal of medicinal chemistry. 1996;39:2103–2117. doi: 10.1021/jm9600398. [DOI] [PubMed] [Google Scholar]
- 21.Taksin A-L, Legrand O, Raffoux E, de Revel T, Thomas X, Contentin N, Bouabdallah R, Pautas C, Turlure P, Reman O, Gardin C, Varet B, de Botton S, Pousset F, Farhat H, Chevret S, Dombret H, Castaigne S. High efficacy and safety profile of fractionated doses of Mylotarg as induction therapy in patients with relapsed acute myeloblastic leukemia: a prospective study of the alfa group. Leukemia. 2007;21:66–71. doi: 10.1038/sj.leu.2404434. [DOI] [PubMed] [Google Scholar]
- 22.Tessoulin B, Delaunay J, Chevallier P, Loirat M, Ayari S, Peterlin P, Le Gouill S, Gastinne T, Moreau P, Mohty M, Guillaume T. Azacitidine salvage therapy for relapse of myeloid malignancies following allogeneic hematopoietic SCT. Bone marrow transplantation. 2014;49:567–571. doi: 10.1038/bmt.2013.233. [DOI] [PubMed] [Google Scholar]
- 23.US Food and Drug Administration. FDA news release: FDA approves Mylotarg for treatment of acute myeloid leukemia. 2017 Retrieved from https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm574507.htm.
- 24.Wyeth Laboratories. Mylotarg (gemtuzumab ozogamicin) package insert. n d Retrieved from https://www.accaessdata.fda.gov/drugsatfda_docs/label/2001/21174s2lbl.pdf.


