Abstract
The management of head and neck cancers (HNC) and esophageal cancer (EC) is complex and often involves multiple modalities of treatment, including chemotherapy, radiation therapy, and surgery. The side effects associated with these therapies and disease processes are extensive. A literature review was performed to evaluate the use of botulinum toxin as an intervention for side-effect management in patients with HNC and EC. Specific adverse events reviewed included salivary function (hypersalivation, fistula, hyposalivation) and gastrointestinal motility (esophageal stricture, delayed gastric emptying after esophagectomy). Published results demonstrate an improvement in hypersalivation and, when botulinum toxin was used as an adjunct to treatment, a reduction in symptoms associated with salivary fistula, or an inappropriate communication between the salivary gland and the skin that causes the leakage of saliva through the skin. Positive effects were also demonstrated in regard to esophageal stricture and equivalent effects in the management of gastric emptying to prevent complications after esophagectomy when compared to currently available interventions. However, the potential for increased symptoms associated with botulinum toxin injection related to its use in the management of gastric secretions was noted in one of the studies reviewed.
Head and neck cancers (HNC) include those of the oral cavity and pharynx. Oral cavity and pharynx cancers have the eighth highest incidence rate for men in the United States, with an estimated 37,160 new cases in 2018 (Siegel, Miller, & Jemal, 2018). Although the incidence of esophageal cancer (EC) is not among the top ten cancers diagnosed in men, its aggressive nature makes it the seventh leading cause of cancer deaths in the United States for this population, with an estimated 12,850 deaths in 2018 (Siegel et al., 2018). For women, HNC and EC are not among the top ten cancers for cancer incidence or deaths, as rates are lower in this population.
Head and neck cancers and EC usually require a multidisciplinary approach to treatment that may include any combination of chemotherapy, radiation therapy, and surgery (National Comprehensive Cancer Network, 2016). The side-effect burden from treatment-induced changes in salivary function and complications from surgery can be substantial. Treatment-related side effects can have a long-term negative impact on quality of life.
In HNC, surgical manipulation of the salivary glands and damage from radiation therapy can cause salivary dysfunction, which can present as hypersalivation as well as hyposalivation (Steffen, Hasselbacher, Heinrichs, & Wollenberg, 2014; Teymoortash et al., 2016). Impaired saliva production can contribute to dysphagia, poor wound healing, fistula formation, and patient discomfort. Rates of wound dehiscence, infection, and fistula formation secondary to hypersalivation are as high as 40% (Corradino, Di Lorenzo, & Moschella, 2012). A fistula creates an inappropriate communication between the salivary gland and the skin, causing the leakage of saliva through the skin (Lazaridou et al., 2012). The use of botulinum toxin to dry salivary excretion and promote wound healing has been described by Laskawi, Winterhoff, Köhler, Kottwitz, and Matthias (2013) in a nononcology-specific population that underwent parotidectomy. Alternatively, maintaining saliva throughout treatment may aid in avoiding other downstream complications, such as dysphagia, infection, taste changes, and periodontal disease.
Complications following surgery for EC include esophageal strictures and delayed gastric emptying. A less invasive alternative to esophagectomy in patients with superficial EC is endoscopic submucosal dissection (ESD). Although ESD is less invasive, mucosal defects are common, with high rates of subsequent esophageal stricture development, especially in patients with larger mucosal defects (Wen et al., 2016). Esophageal dilation is a treatment option for esophageal strictures; however, in order for the procedure to be effective, it may need to be repeated more than once, and the procedure is not without risks. Oral or injected steroids are another treatment option used in the management of esophageal stricture, yet steroids may cause additional side effects requiring management (Neuhaus, 2016; Wen et al., 2016).
There are several approaches to esophagectomy, including transthoracic and transhiatal, based on the location of the tumor and indication for surgery (Flanagan et al., 2016). These procedures create a gastric conduit, with or without a procedure to manage pyloric drainage. A common complication of esophagectomy, whether open or through a minimally invasive procedure, is gastric outlet obstruction causing delayed gastric emptying, which can further complicate the clinical course by contributing to the aspiration of gastrointestinal contents (Eldaif et al., 2014). According to Antonoff and colleagues (2014), delayed gastric emptying occurs in approximately 15% of patients who undergo esophagectomy with gastric pull-up, but there is considerable variability in the reported rate of this complication, ranging from 4% to 50% of patients. Pyloric drainage is a potential intervention to prevent delayed gastric emptying. Both pyloroplasty and pyloromyotomy are surgical interventions in which an incision is made into the pylorus to facilitate the movement of gastric contents from the stomach to the small intestine. In two large meta-analyses, outcomes from pyloric drainage procedures showed no improvement in overall complication rates or mortality (Antonoff et al., 2014; Khan, Manners, Rengarajan, & Dunning, 2007; Urschel, Blewett, Young, Miller, & Bennett, 2002).
Given the significant symptom burden following treatment for HNC and EC, interventions to address impaired salivary function and complications of esophageal surgery are needed. Botulinum toxin is one intervention that has been used clinically in both settings. This review was conducted to gather evidence on the effectiveness of botulinum toxin to manage treatment-related side effects in patients with HNC and EC.
METHODS
A comprehensive review of the literature was conducted to evaluate the use of botulinum toxin as an intervention to optimize salivary function and prevent esophageal stricture and delayed gastric emptying following surgical manipulation in patients with HNC and EC. Electronic databases searched included Cochrane, CINAHL, and PubMed, with the search terms "neoplas* OR cancer* OR malignancy OR tumor" AND "botulinum toxin OR botox" AND "radiation therapy OR radiotherapy OR chemotherapy OR surgery." All treatment modalities were included in the search; however, the articles identified regarding botulinum toxin use were in the postsurgery setting. Due to the differences in nomenclature for head and neck cancer, the specific cancer type was not included in the search, and inclusion criteria were applied to a broader yield of articles to avoid unintentionally eliminating articles. A research librarian assisted with the literature search.
Studies with adult human subjects with HNC or EC who had received radiation, chemotherapy, and/or surgery in which botulinum toxin injection was the intervention for side-effect management were included. Reviews, abstracts, case studies, and editorials, as well as articles that focused on the technique of botulinum toxin injection, were excluded. While important, trismus, pain, and pharyngoesophageal spasm disorders (including aphonia, spasticity, and dystonia) were outside the scope of this review and were excluded.
Inclusion criteria included peer-reviewed, original research studies published in English after January 1, 2012, and before December 31, 2016. Cochrane, CINAHL, and PubMed initially yielded 172 articles. A secondary review of references identified four additional articles. After excluding articles published outside of the date range and removing duplicates, there was a total yield of 126 articles for review. Of the 126 articles screened for eligibility, 100 were excluded based on abstract review. Full-text review of the remaining 26 articles identified seven articles that are included in this synthesis of available literature (Figure 1; Table 1). The level of evidence of the included articles was assessed using the Association of periOperative Nurses (AOPN) Revised Model for Evidence Appraisal and Rating (Spruce, Van Wicklin, & Wood, 2016).
Figure 1.
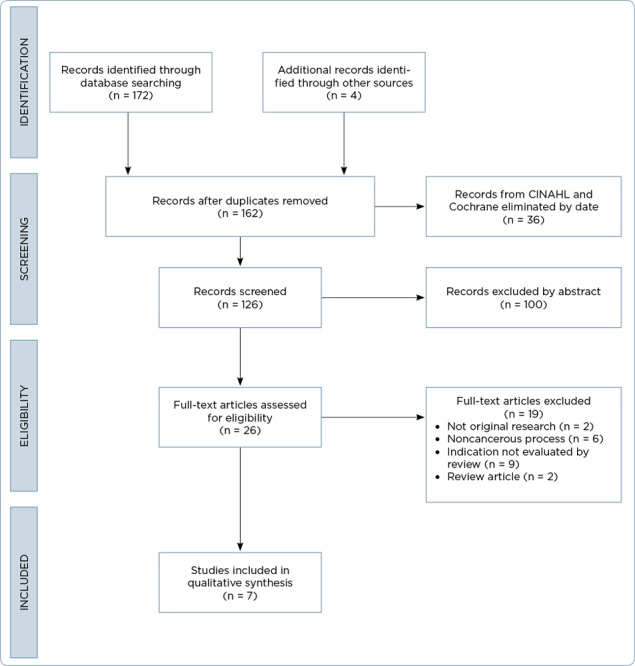
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) 2009 flow diagram adapted for this study. Adapted from Moher et al. (2009).
Table 1.
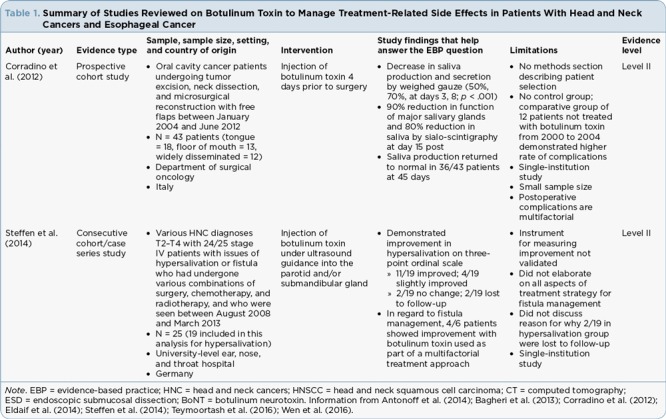
Summary of Studies Reviewed on Botulinum Toxin to Manage Treatment-Related Side Effects in Patients With Head and Neck Cancers and Esophageal Cancer
Table 1.
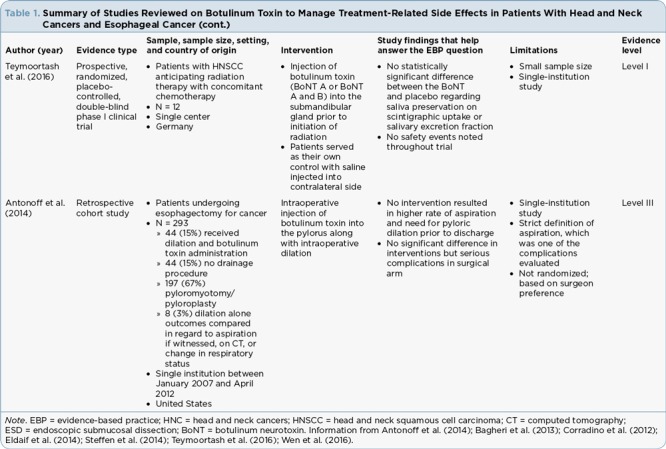
Summary of Studies Reviewed on Botulinum Toxin to Manage Treatment-Related Side Effects in Patients With Head and Neck Cancers and Esophageal Cancer (cont.)
Table 1.
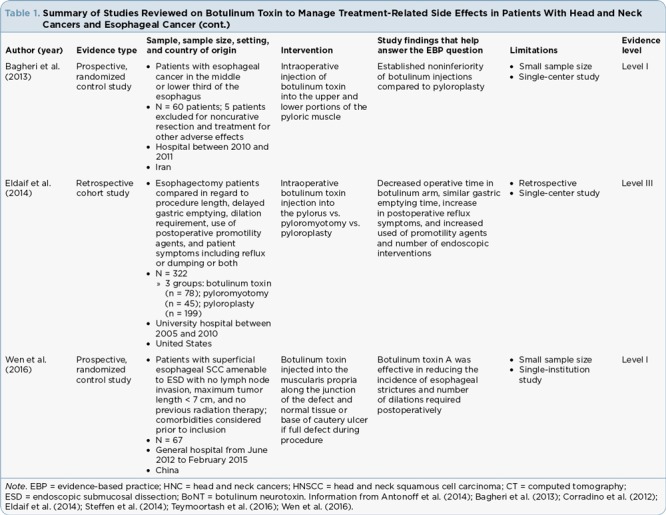
Summary of Studies Reviewed on Botulinum Toxin to Manage Treatment-Related Side Effects in Patients With Head and Neck Cancers and Esophageal Cancer (cont.)
RESULTS
The results of the studies are organized by botulinum toxin use in the management of salivary function and effect on surgical complications of ESD or esophagectomy associated with the treatment of HNC and EC. Patients within the seven studies identified underwent various combinations of surgery, chemotherapy, and radiotherapy. A summary of the study findings is shown in Table 2.
Table 2.
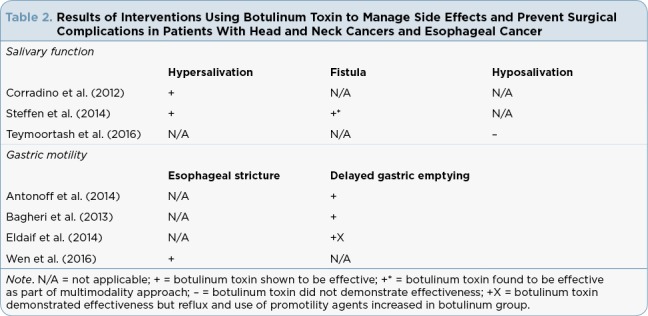
Results of Interventions Using Botulinum Toxin to Manage Side Effects and Prevent Surgical Complications in Patients With Head and Neck Cancers and Esophageal Cancer
Management of Salivary Function
Three studies addressed the management of salivary gland function. Both Corradino and colleagues (2012) and Steffen and colleagues (2014) assessed the use of botulinum toxin for hypersalivation, which may contribute to complications after treatment. Steffen and colleagues (2014) also assessed fistula management. Only one study, by Teymoortash and colleagues (2016), addressed saliva preservation to prevent hyposalivation through the use of botulinum toxin into the submandibular glands prior to initiation of chemotherapy and radiation therapy.
Hypersalivation. In a prospective cohort study of 43 patients, Corradino and colleagues (2012) injected botulinum toxin into the major salivary glands 4 days before surgery and used sialo-scintigraphy plus weighed gauze to quantify the amount of saliva production after injection. Scintigraphy is a noninvasive nuclear medicine technique used to evaluate the ability of the salivary glands to take up injected 99mTc pertechnetate by measuring the rate and density of uptake through imaging (dos Santos et al., 2010). In addition to sialo-scintigraphy measurement, the authors used preweighed gauze to absorb saliva for 2 minutes, at which point the gauze was weighed again to determine a quantifiable amount of saliva. Although there is no description of patient selection, the sample was comprised of patients with tongue cancer (n = 18), floor of mouth cancer (n = 13), and widely disseminated head and neck cancer (n = 12). The authors reported a statistically significant reduction in the amount of saliva produced using the weighed gauze (p < .01), with a 50% reduction on postinjection day 3 and a 70% reduction on postinjection day 8 when compared to pretreatment (Corradino et al., 2012). On postinjection day 15, scintigraphy showed a 90% reduction in gland function, with an 80% overall reduction in saliva production. The authors reported return to normal salivary production in the majority of patients 45 days following the injection of botulinum toxin (Corradino et al., 2012).
Steffen and colleagues (2014) assessed salivary symptoms over a 5-year period in a consecutive cohort of 25 patients with various stage T2 to T4 HNC at a single institution. At various points in their treatment, the patients were injected with botulinum toxin into the parotid and/or submandibular gland under ultrasound guidance to address salivary dysfunction. Nineteen patients were treated for hypersalivation. The authors used a nonvalidated 3-point ordinal scale that measured patient-rated perception of improvement approximately 10 days after treatment as "no change," "slightly improved," or "improved." In this study, 11 of the 19 patients presenting with hypersalivation had "improved," and four of the 19 were "slightly improved," with two patients experiencing "no change" and two lost to follow-up.
Fistula. In the same study, Steffen and colleagues (2014) also reported on six patients with salivary fistulas who received botulinum toxin injections as part of their management. Steffen and colleagues (2014) evaluated botulinum toxin administered as part of a multimodality approach for fistula treatment. The authors used the same 3-point ordinal scale described previously of patient perception of "no change," "slightly improved," and "improved." Four of the six patients demonstrated improvement and two showed slight improvement following botulinum toxin injection (Steffen et al., 2014).
Hyposalivation. Teymoortash et al. (2016) evaluated whether injection of botulinum toxin into the submandibular glands prior to initiation of concurrent chemoradiation would contribute positively to preserving gland function in patients with HNC. The submandibular glands contribute to the sensation of moisture in the mouth. In this prospective, randomized, placebo-controlled, double-blind phase I clinical trial, 12 patients were randomized to one of four intervention arms, with half of the participants receiving injection into the submandibular gland with one formulation of botulinum neurotoxin A (BoNT/A) and the other half receiving another formulation, which was a combination of botulinum neurotoxin (BoNT/A and B). Half of the participants in each group received injections into the right gland and half received injections into the left gland. Each patient served as their own control, with saline injected into the contralateral submandibular gland as a placebo to assess salivary function during treatment. Throughout the course of treatment, there was no significant difference when comparing placebo to either BoNT/A (p = .84) or BoNT/A and B (p = .56) for scintigraphic uptake or salivary excretion fraction (p = .44 for both formulations). Salivary excretion fraction was measured as the amount of saliva excreted radiographically after stimulation by drinking lemon juice (Teymoortash et al., 2016).
Gastrointestinal Motility After Esophagectomy
Studies evaluating the use of botulinum toxin to promote gastrointestinal motility include interventions using botulinum toxin to prevent esophageal stricture or delayed gastric emptying. Wen and colleagues (2016) conducted the only study to evaluate botulinum toxin to prevent esophageal stricture. Antonoff and colleagues (2014), Bagheri and colleagues (2013), and Eldaif and colleagues (2014) evaluated botulinum toxin for the promotion of gastric emptying after esophagectomy in order to prevent complications of excessive gastric secretions associated with delayed gastric emptying.
Esophageal Stricture. Wen and colleagues (2016) evaluated patients with superficial esophageal squamous cell carcinoma who were candidates for ESD. The patients in the study had a mucosal defect that disseminated across at least half of the circumference of the esophagus. Esophageal stents were placed intraoperatively for patients with a completely circumferential mucosal defect. The 72 participants were divided into two groups: One group received an injection of botulinum immediately after ESD, and the other group served as a control to evaluate whether the injection of botulinum toxin was an effective intervention to prevent the development of esophageal stricture. Five patients were excluded due to surgical complications, including noncurative resection. Stricture formation was determined by endoscopy at follow-up and was defined as a less than 9.8-mm opening that did not allow passage of a standard endoscope through the stenotic area. A secondary outcome measure was the number of esophageal balloon dilations required after surgery. There was a lower incidence of stricture in the botulinum toxin group compared to the control group in both per protocol and intention-to-treat analyses (6.1% vs. 32.4%, p = .02; 11.4% vs. 37.8%, p = .02, respectively). The botulinum group also required fewer esophageal dilations as compared to the control group (mean = 1.5 vs. 2.8, p = .002; Wen et al., 2016).
Dysphagia grading using the Mellow-Pinkas dysphagia score (Mellow & Pinkas, 1985) and quality of life were also used as outcome measures in the study by Wen and colleagues (2016). The grade of dysphagia was lower in the botulinum-treated group, with a score of 0, compared to a score of 1 in the untreated group (p = .02). The botulinum group also had lower scores on the quality of life questionnaire (European Organisation for Research and Treatment of Cancer oesophageal cancer module; QLQ-OES18), with a mean score of 25.8 (standard deviation, 6.2) compared to the control group, with a score of 30.5 (standard deviation, 7.2; p = .01). The findings related to quality of life are difficult to interpret because the authors did not clarify whether scores pertained to symptoms or functional status. Furthermore, a low score on the quality of life questionnaire can be positive or negative based on the item being scored.
Delayed Gastric Emptying. Antonoff and colleagues (2014), Bagheri and colleagues (2013), and Eldaif and colleagues (2014) explored the efficacy of botulinum toxin as an appropriate intervention for the management of gastric emptying following esophagectomy compared to alternative strategies, such as pyloroplasty and pyloromyotomy. Bagheri and colleagues (2013) divided 60 patients into two equal groups; one group received intraoperative pyloroplasty, while the other group received an injection of botulinum toxin at the upper and lower portions of the pyloric muscle. Patients returned in 1 week to assess delayed gastric emptying using a barium swallow, with a follow-up evaluation utilizing an isotope scan 3 weeks after surgery. There were no significant differences noted between the two groups at either time point. At 1 week, 80% of the botulinum toxin group and 70% of the pyloroplasty group had normal gastric emptying; however, the difference was not statistically significant (p = .446). Three weeks following surgery, 93% of the botulinum toxin group and 76% of the pyloroplasty group had normal emptying of gastric contents based on isotope scan, but this difference was also not statistically significant (p = .355).
Antonoff et al. (2014) used outcome variables of witnessed aspiration, computed tomography changes, diagnosis of pneumonia, change in respiratory status, anastomotic leak, and presence of gastric outlet syndrome to evaluate four options for the prevention of delayed gastric emptying postoperatively in esophageal cancer patients. In this retrospective analysis of 361 patients, 68 were excluded due to benign disease or prior esophagogastric surgery, with the remaining 293 patients placed into four groups: no intervention, pyloromyotomy, dilation alone, and dilation plus onabotulinumtoxin A. The data were analyzed as any intervention vs. no intervention. The report of delayed gastric emptying was low overall (1.7%) and was not statistically significant between groups. In the intervention groups, 3.2% of patients required postoperative dilation, which was significantly lower than those who had no intervention (15.9%; p = .008), and lower rates of aspiration were observed in the intervention groups compared to the nonintervention group (2.4% vs. 11.4%; p = .030; Antonoff et al., 2014).
The authors also reported on time spent in the operating room for each group in the study. The group receiving dilation with botulinum spent significantly more time in the operating room—6 hours and 23 minutes—compared to 3 hours and 44 minutes for no intervention, 2 hours and 55 minutes for dilation alone, and 4 hours and 55 minutes for surgical intervention, not taking standard deviation into account (Antonoff et al., 2014). The significantly increased time in the operating room was correlated with procedure type and individual surgeon differences (Antonoff et al., 2014).
A retrospective analysis of patients who had undergone open esophagectomy was completed by Eldaif and colleagues (2014). The patients (n = 322) received one of the three interventions: botulinum toxin injection into the pyloric muscle, pyloromyotomy, or pyloroplasty. There was no statistically significant difference in the percentage of participants experiencing delayed gastric emptying evaluated by a swallow study 5 to 7 days postoperatively in the botulinum arm, compared to the pyloromyotomy and pyloroplasty arms (18%, 5%, and 13%, respectively; p = .08; Eldaif et al., 2014). More patients in the botulinum intervention group required endoscopic dilation at 6 months compared to the pyloromyotomy and pyloroplasty arms (41% vs. 22% vs. 14%, respectively; p < .001). The authors also reported on measures not addressed previously in the literature, including reflux symptoms and use of promotility agents, which were higher in the botulinum intervention arm. In contrast to Antonoff and colleagues (2014), time in the operating room was less in the botulinum intervention group compared to the pyloromyotomy and pyloroplasty groups (239 minutes vs. 312 minutes vs. 373 minutes, respectively; p < .001).
DISCUSSION
Hypersalivation, a complication that can arise from the treatment of HNC and contribute to patient symptoms, decreased following injection of botulinum toxin into the salivary glands. Corradino and colleagues (2012) demonstrated this outcome with sialo-scintigraphy and weighed gauze, while Steffen and colleagues (2014) used a 3-point ordinal scale to show a decrease in hypersalivation and demonstrated patient-perceived improvement in hypersalivation. There was also patient-perceived improvement in fistula symptoms when botulinum toxin was used as an adjunct in fistula management (Steffen et al., 2014).
Although improved radiation techniques have reduced the development of hyposalivation, saliva preservation remains a consideration in patients receiving radiation for HNC. Teymoortash and colleagues (2016) evaluated the use of botulinum toxin injections to prevent loss of saliva during treatment with radiation and chemotherapy. Their study used similar outcome measures to those used by Corradino and colleagues (2012), including scintigraphic uptake and salivary excretion fraction; however, in contrast, they did not find a significant difference between treated and untreated salivary glands in their randomized control trial (Teymoortash et al., 2016).
The results for the use of botulinum toxin in the prevention of esophageal strictures following minimally invasive ESD were promising. Not only does ESD provide a less invasive alternative to open esophagectomy, but the use of intraoperative botulinum toxin was effective in preventing strictures (Wen et al., 2016). Additionally, the use of botulinum toxin could obviate the need for other interventions, such as repeated dilation and the use of steroids, reducing the risk and discomfort experienced by patients. Continued research to establish efficacy, best technique, and dosing is warranted.
There are mixed results among the studies addressing the use of botulinum toxin injection as an intervention to prevent delayed gastric emptying after esophagectomy. Bagheri and colleagues (2013) demonstrated the noninferiority of botulinum toxin when compared with pyloroplasty for the management of gastric emptying 1 week and 3 weeks following surgery. There were no complications reported from the use of botulinum toxin, establishing the safety of botulinum toxin in the population studied and pointing to the possibility that the less invasive procedure of botulinum toxin injection may have similar outcomes related to pyloric drainage.
Results from the study by Antonoff and colleagues (2014) demonstrated that an intervention to prevent delayed gastric emptying resulted in fewer complications compared to no intervention. Based on the incidence of aspiration and need for dilation prior to discharge from the hospital, the study demonstrated similar outcomes between the botulinum toxin group and the surgical management groups.
Serious adverse events were reported in two of the studies; however, there were no serious adverse outcomes in the botulinum groups. Antonoff and colleagues (2014) reported two serious outcomes in the surgical arm: one patient died on day 68 following a leak from the site of the pyloroplasty, and another patient required a second surgery on postoperative day 1, with conversion of a pyloromyotomy to pyloroplasty. Eldaif and colleagues (2014) found increased postoperative reflux, increased use of promotility agents, and increased need for anastomotic dilation at the 6-month follow-up in the botulinum intervention group compared to the pyloromyotomy and pyloroplasty groups, and noted that the adverse reactions to the treatment were enough to limit the use of botulinum toxin.
It is important to note that botulinum toxin is not currently approved by the US Food and Drug Administration (FDA) for the indications investigated. Currently approved uses include headache prophylaxis, treatment of upper and lower limb spasticity, and treatment of cervical dystonia, axillary hyperhidrosis, overactive bladder, blepharospasm, and strabismus (Allergen, Inc., 2011). Botulinum toxin treatments beyond the FDA indications for use are limited to "off-label" use and research protocols.
Lack of standardized outcomes and assessment tools were also limitations of the studies reviewed. The scale used to measure hypersalivation improvement in the study by Steffen and colleagues (2014) was not a validated assessment tool, and as a result is not used elsewhere in the literature. The authors discussed two questionnaires that address hypersalivation, including the Drooling Severity and Frequency Scale (DSFS) and the Bogenhausener Dysphagia Score (BODS-1 assesses the ability to swallow saliva for protection of the deep airways; BODS-2 assesses the ability to take in food), which have been used in other settings; however, the authors did not feel the questionnaires met the needs of their study. The generalizability of the studies is limited by the use of different outcome measurements to assess the management of pyloric drainage and promotion of gastric emptying, or prevention of gastric outlet obstruction, making it difficult to directly compare results. Also, in the study by Bagheri and colleagues (2013), the botulinum toxin intervention included intraoperative balloon dilation, making it difficult to elucidate which component contributed to the results. Similarly, the study by Steffen and colleagues (2014) used a variety of management strategies in their approach to fistula management, creating the same confounding issue.
Additional limitations of the studies reviewed include small sample sizes and difference in tumor type. Sample sizes for the studies reviewed ranged from 12 to 322. Small sample sizes decrease the statistical power of the study, increasing the risk of a type II error, also known as a "false negative" finding. In the study by Eldaif and colleagues (2014), although the majority of patients (86%) underwent surgery for a cancerous process, the sample included patients in each of the groups who had surgery for noncancer causes. Bagheri and colleagues (2013) reported differences between the intervention and control group in regard to tumor type, with all six patients diagnosed with adenocarcinoma in the intervention group, vs. squamous cell carcinoma in the pyloroplasty control group (p = .021). The significance of this difference is unclear in regard to a comparison of the interventions and generalizability of the findings.
There are varying levels of evidence presented in this review, with level 1 evidence from only three of the studies: Bagheri and colleagues (2013), Wen and colleagues (2016), and Teymoortash and colleagues (2016). The studies were also all single-center trials, which lack the external validity needed to support practice change.
CONCLUSION
There is limited evidence in the literature about the use of botulinum toxin for the management of symptoms associated with treatment for HNC and EC as it relates to salivary function and gastric motility. As research moves forward, standardizing the assessment of symptoms (hypersalivation, those associated with fistula, hyposalivation, esophageal stricture, and delayed gastric emptying) will be important to allow the data collected within small cohorts to be more universally translated. Large randomized control trials would contribute to the strength of the currently available research in regard to the safety and efficacy of using botulinum toxin in this population, as some of the results are conflicting and leave doubt about the appropriateness of the botulinum toxin interventions.
Treatment-related side effects for HNC and EC patients are difficult to manage and may negatively impact quality of life and patients’ ability to complete a treatment regimen (Mason et al., 2016). It is essential that advanced practice providers (APPs) be knowledgeable of the complications that occur as a result of complex treatment and monitor patients closely for treatment side effects. Standardizing assessment tools for side-effect management and adopting common language to report side effects are important in order to better understand best approaches to care. Given the morbidity and significant negative impact on quality of life related to treatment side effects, APPs should continue to optimize outcomes through evidence-based practice and contribute to the body of knowledge related to side-effect management.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Allergan, Inc. Botox (onabotulinumtoxinA) package insert. 2011 Retrieved from http://www.allergan.com/assets/pdf/botox_pi.pdf.
- 2.Antonoff M B, Puri V, Meyers B F, Baumgartner K, Bell J M, Broderick S, Crabtree T D. Comparison of pyloric intervention strategies at the time of esophagectomy: Is more better? Annals of Thoracic Surgery. 2014;97(6):1950–1958. doi: 10.1016/j.athoracsur.2014.02.046. Retrieved from . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagheri Reza, Fattahi Seyed Hossein, Haghi Seyed Ziaollah, Aryana Kamran, Aryanniya Ali, Akhlaghi Saeed, Riyabi Fateme Naghavi, Sheibani Shima. Botulinum toxin for prevention of delayed gastric emptying after esophagectomy. Asian cardiovascular & thoracic annals. 2013;21:689–692. doi: 10.1177/0218492312468438. [DOI] [PubMed] [Google Scholar]
- 4.Corradino Bartolo, Di Lorenzo Sara, Moschella Francesco. Botulinum toxin A for oral cavity cancer patients: in microsurgical patients BTX injections in major salivary glands temporarily reduce salivary production and the risk of local complications related to saliva stagnation. Toxins. 2012;4:956–961. doi: 10.3390/toxins4110956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.dos Santos L A N, Neto F H, Boscolo F N, Campos P S F, Martelli, Jr H, Laranjeira A L, Santos A E S. Scintigraphy of the salivary gland in patients with Sjögren’s syndrome treated with pilocarpine. Revista de Clínica e Pesquisa Odontológica. 2010;6(1):101–106. [Google Scholar]
- 6.Eldaif S M, Lee R, Adams K N, Kilgo P D, Gruszynski M A, Force S D, Miller D. Intrapyloric botulinum injection increases postoperative esophagectomy complications. Annals of Thoracic Surgery. 2014;97(6):1959–1965. doi: 10.1016/j.athoracsur.2013.11.026. [DOI] [PubMed] [Google Scholar]
- 7.Flanagan J C, Batz R, Saboo S S, Nordeck S M, Abbara S, Kernstine K, Vasan V. Esophagectomy and gastric pull-through procedures: Surgical techniques, imaging features, and potential complications. Radiographics. 2016;36(1):107–121. doi: 10.1148/rg.2016150126. [DOI] [PubMed] [Google Scholar]
- 8.Khan Omar A, Manners James, Rengarajan Arvind, Dunning Joel. Does pyloroplasty following esophagectomy improve early clinical outcomes? Interactive cardiovascular and thoracic surgery. 2007;6:247–250. doi: 10.1510/icvts.2006.149500. [DOI] [PubMed] [Google Scholar]
- 9.Laskawi Rainer, Winterhoff Jan, Köhler Sabrina, Kottwitz Laura, Matthias Christoph. Botulinum toxin treatment of salivary fistulas following parotidectomy: follow-up results. Oral and maxillofacial surgery. 2013;17:281–285. doi: 10.1007/s10006-012-0375-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lazaridou Maria, Iliopoulos Christos, Antoniades Kostas, Tilaveridis Ioannis, Dimitrakopoulos Ioannis, Lazaridis Nicolas. Salivary gland trauma: a review of diagnosis and treatment. Craniomaxillofacial trauma & reconstruction. 2012;5:189–196. doi: 10.1055/s-0032-1313356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mason Heidi, DeRubeis Mary Beth, Burke Nancy, Shannon Melissa, Karsies Danielle, Wolf Gregory, Eisbruch Avi, Worden Francis. Symptom management during and after treatment with concurrent chemoradiotherapy for oropharyngeal cancer: A review of the literature and areas for future research. World journal of clinical oncology. 2016;7:220–226. doi: 10.5306/wjco.v7.i2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mellow M H, Pinkas H. Endoscopic laser therapy for malignancies affecting the esophagus and gastroesophageal junction. Analysis of technical and functional efficacy. Archives of internal medicine. 1985;145:1443–1446. [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman D G, The PRISMA group. Preferred reporting items for systematic reviews and meta-analysis: The PRISMA statement. PLoS Medicine. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Esophageal and esophagogastric junction cancers. v2.2016. 2016 Retrieved from https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf.
- 15.Neuhaus Horst. Prevention of strictures after endoscopic resection of esophageal neoplasia. Gastrointestinal endoscopy. 2016;84:614–617. doi: 10.1016/j.gie.2016.05.045. [DOI] [PubMed] [Google Scholar]
- 16.Siegel Rebecca L, Miller Kimberly D, Jemal Ahmedin. Cancer statistics, 2018. CA: a cancer journal for clinicians. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 17.Spruce Lisa, Van Wicklin Sharon A, Wood Amber. AORN’s Revised Model for Evidence Appraisal and Rating. AORN journal. 2016;103:60–72. doi: 10.1016/j.aorn.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 18.Steffen Armin, Hasselbacher Katrin, Heinrichs Sabrina, Wollenberg Barbara. Botulinum toxin for salivary disorders in the treatment of head and neck cancer. Anticancer research. 2014;34:6627–6632. [PubMed] [Google Scholar]
- 19.Teymoortash A, Pfestroff A, Wittig A, Franke N, Hoch S, Harnisch S, Strauch K. Safety and efficacy of botulinum toxin to preserve gland function after radiotherapy in patients with head and neck cancer: A prospective, randomized, placebo-controlled, double-blinded phase I clinical trial. PloS One. 2016;11(3):e0151316. doi: 10.1371/journal.pone.0151316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Urschel John D, Blewett Chris J, Young J Edward M, Miller John D, Bennett W Frederick. Pyloric drainage (pyloroplasty) or no drainage in gastric reconstruction after esophagectomy: a meta-analysis of randomized controlled trials. Digestive surgery. 2002;19:160–164. doi: 10.1159/000064206. [DOI] [PubMed] [Google Scholar]
- 21.Wen Jing, Lu Zhongsheng, Linghu Enqiang, Yang Yunsheng, Yang Jing, Wang Shufang, Yan Bin, Song Jie, Zhou Xiaodong, Wang Xiangdong, Meng Ke, Dou Yan, Liu Qingsen. Prevention of esophageal strictures after endoscopic submucosal dissection with the injection of botulinum toxin type A. Gastrointestinal endoscopy. 2016;84:606–613. doi: 10.1016/j.gie.2016.03.1484. [DOI] [PubMed] [Google Scholar]


