ABSTRACT
Thirteen-valent pneumococcal conjugate vaccine (PCV13) was licensed in adults to address the unmet medical need of vaccine-type community acquired pneumonia (CAP) and the limitations of previous plain-polysaccharide vaccines. Since then, some have questioned the utility of adult PCV13 use, arguing that: i) high PCV13 uptake in young children would provide indirect effects that, by themselves, would sufficiently protect unvaccinated adults and ii) no data describing the real-world effectiveness of PCV13 use in adults, especially with immunocompromising conditions, exist. Even in countries like the United States where PCV13 has been routinely recommended for all adults aged ≥ 65 years, the recommendation is contingent on a re-evaluation to determine if continued use is needed in the context of a mature PCV13 pediatric immunization program. Emerging evidence, however, suggests that i) a meaningful burden of PCV13-type pneumococcal pneumonia still persists in adults at increased risk for pneumococcal disease, despite indirect effects from long-standing pediatric PCV13 use, ii) adult PCV13 use is effective and has reduced pneumococcal CAP, even in the elderly and those with chronic medical or immunocompromising conditions – and disease could come back if PCV13 were removed, and iii) ethical and pragmatic vaccine policy considerations support continued adult PCV13 use in countries that have already introduced the vaccine (eg, disparities in adult PCV13 uptake, confusion stemming from removing a previously-recommended vaccine for a non-safety-related concern, and the reality that next-generation PCVs are only a few years away). Together, these findings suggest that continued PCV13 vaccination in adults is needed to control vaccine-type CAP.
KEYWORDS: 13-valent pneumococcal conjugate vaccine (PCV13), adult, community-acquired pneumonia (CAP), policy, recommendation
Introduction
Thirteen-valent pneumococcal conjugate vaccine (PCV13) was licensed in adults to address the unmet medical need of vaccine-type community-acquired pneumonia (CAP) and the limitations of previous plain-polysaccharide vaccines against that outcome. Specifically, data describing the ability of 23-valent pneumococcal polysaccharide vaccine (PPSV23) to adequately prevent nonbacteremic pneumococcal pneumonia in older adults1−17 are inconsistent.14,17,18 In contrast, PCV13 demonstrated efficacy (vs placebo) against both overall and nonbacteremic vaccine-type CAP in the Community-Acquired Pneumonia Trial in Adults (CAPiTA) study,19 representing a potentially “meaningful therapeutic benefit over existing treatments.”20 Based on the findings from the CAPiTA trial19 various countries have since introduced PCV13 into their adult immunization programs, including the United States.14,18
Still, some have questioned the true public-health value of adult PCV13 use in the context of a mature PCV13 infant vaccination program. One of the primary questions regarding adult PCV13 use is whether a meaningful burden of PCV13-type pneumococcal pneumonia still persists in adults in the context of indirect (herd) effects stemming from long-standing pediatric PCV13 use. It is well accepted that high uptake of PCVs in infants and young children has led to profound reductions in vaccine-type pneumococcal disease for all age groups, including unvaccinated adults through indirect effects.21 This added benefit is the result of interrupting transmission in children, the primary reservoir for pneumococcal disease, thereby reducing PCV-type circulation among all age groups. These indirect effects are thought to be conferred primarily by high uptake in the toddler (“booster”) dose – the dose administered around age one year that is most responsible for eliminating or preventing acquisition of pneumococcal carriage, or both.22,23
Another question pertaining to adult PCV13 use is a perceived lack of real-world PCV13 effectiveness data in adults with chronic or immunocompromising conditions after the vaccine has been introduced into an adult immunization program. The large randomized controlled trial (RCT) that demonstrated PCV13 efficacy against vaccine-type CAP excluded immunocompromised patients and was conducted in a relatively unique and homogenous population (ie, the Netherlands).19 Many wondered whether the efficacy observed in the RCT setting of the Netherlands19 would be reflective of the real-life experience of more clinically–and demographically–diverse populations outside of the clinical setting.
Finally, there is the example of the United States, where PCV13 was introduced in adults aged ≥ 65 years in 2014,14 but the recommendation is contingent on a re-evaluation to determine if continued use is needed in the context of a mature PCV13 pediatric immunization program.14 The United States is evaluating whether the adult PCV13 immunization program could be implemented and then removed based on the assumption that levels of PCV13-type CAP in adults would be controlled over time by continued implementation of the pediatric PCV program.14 This decision is likely to have global consequences.
Our present commentary highlights that all of these questions regarding adult PCV13 use have now largely been answered by an accumulation of evidence over the last few years. Based on this new evidence, the arguments presented to date against adult PCV13 use seem largely overcome. Specifically, we present emerging evidence showing that i) a meaningful burden of PCV13-type pneumococcal pneumonia still persist in adults at increased risk for pneumococcal disease, despite indirect effects from long-standing pediatric PCV13 use, ii) adult PCV13 use is effective and has reduced pneumococcal CAP, even in the elderly and those with chronic medical and immunocompromising conditions – and disease could come back if PCV13 were removed, and iii) ethical and pragmatic vaccine policy considerations support continued adult PCV13 use in countries that have already introduced the vaccine (eg, current disparities in adult PCV13 uptake, confusion stemming from removing a previously-recommended vaccine for a non-safety-related concern, and the reality that next-generation PCVs are only a few years away). Taken together these data suggest that continued vaccination with PCV13 in adults is needed to control vaccine-type CAP.
A meaningful burden of PCV13-type pneumococcal pneumonia still persists in adults, despite indirect effects from pediatric PCV13 use
CAP makes up the vast majority of pneumococcal disease in adults.18,24 To characterize the PCV13-preventable fraction of CAP remaining in adults, a two-stage approach must be taken. First, the total burden of adult CAP must be estimated (typically as an annual incidence rate). Second, the proportion of CAP due to the serotypes contained in PCV13 must be determined. Multiplying these two estimates together yields the incidence of PCV13-type CAP (ie, the remaining burden of adult CAP that is potentially vaccine-preventable).
Three studies have estimated the incidence rate of hospitalized CAP in the United States using a prospective, population-based surveillance approach.25–27 Although all had similar clinical and radiographic criteria for defining CAP hospitalizations, they differed in their exclusion criteria. Two specific exclusion criteria proved to be critical for determining the incidence of hospitalized CAP in these three studies: i) the exclusion of immunocompromised patients (one25 of three studies) and ii) the exclusion of patients with what has historically been defined as healthcare-associated pneumonia (HCAP; two25,26 of three studies).
The Etiology of Pneumonia in the Community (EPIC) study, which was funded by the Centers for Disease Control and Prevention (CDC) and published in 2015, excluded both immunocompromised and HCAP patients. Using these exclusion criteria, EPIC found annual incidence rates of all-cause CAP of roughly 130 and 900 per 100,000 persons among adults aged 18 to 64 and ≥ 65 years, respectively.25 A study conducted in 1991 by Marston et al. included immunocompromised patients, but excluded HCAP patients, and found annual incidence rates of all-cause CAP of approximately 180 and 1000 per 100,000 persons among adults aged 18 to 64 and ≥ 65 years, respectively.26
Inclusion of immunocompromised and HCAP patient populations, however, is important given that i) immunocompromised patients have been shown to be at higher risk of pneumococcal disease28-30 and ii) treating HCAP patients as a separate clinical entity from CAP is a practice that is no longer recommended based on current guidelines from the Infectious Diseases Society of America and the American Thoracic Society.31 Consequently, the more recent Louisville Pneumonia Study27 included both immunocompromised and HCAP patients. As a result, the Louisville Pneumonia Study27 reported CAP incidence rates that were 2 to 3 times higher (annual incidence rates of all-cause CAP of roughly 360 and 2300 per 100,000 persons among adults aged 18 to 64 and ≥ 65 years, respectively) than the EPIC study and Marston et al.25,26
If similar exclusion criteria are applied to the Louisville Pneumonia Study27 as were applied to EPIC25 and Marston et al,26 results from all three studies are remarkably similar. Specifically, if HCAP patients are excluded from Louisville estimates (methodology similar to Marston et al.26) the annual incidence rate observed in Louisville27 among adults aged ≥ 65 years was reduced from approximately 2300 per 100,000 to roughly 1290 per 100,000, quite similar to the roughly 1000 per 100,000 persons per year seen in Marston et al.26 Likewise, if HCAP and immunocompromised patients are excluded from Louisville27 estimates (methodology similar to the EPIC study) the annual incidence rate observed in Louisville27 among adults aged ≥ 65 years was reduced even farther, to roughly 800 per 100,000, akin to the approximately 900 per 100,000 persons per year seen in EPIC (Figure 1). These findings confirmed that the higher incidence rates observed in the Louisville Pneumonia Study27 are explained by selection criteria (ie, not excluding HCAP and immunocompromised patients). Specifically, excluding HCAP and immunocompromising patients leads to unnecessarily eliminating one-half to two-thirds of all incident hospitalized CAP cases – a considerable amount of which, as we describe in detail in the next section of this article, is still caused by pneumococcus. Thus, when estimating the overall burden of hospitalized CAP, including these two patient populations is critical, and the recent Louisville Pneumonia Study27 provides a more contemporary CAP burden estimate from which to apply the proportion of remaining disease that is PCV13-type.
Figure 1.
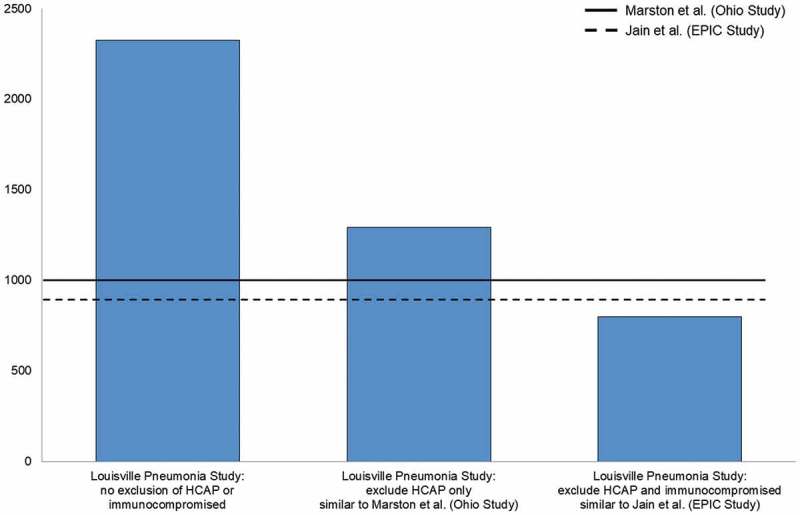
Impact of excluding patients with healthcare-associated community-acquired pneumonia (HCAP) and immunocompromising conditions on incidence rates of hospitalized community-acquired pneumonia (per 100,000 person-years) in previously-conducted prospective surveillance studies.25–27
EPIC = Etiology of Pneumonia in the Community; HCAP = healthcare-associated community-acquired pneumonia.
It is also worth noting that outpatient adult CAP burden is not included in any of these previously described estimates of hospitalized CAP but is equally substantial. Several publications have documented that for every one case of hospitalized CAP in adults aged ≥ 65 years, there is at least one outpatient CAP case as well.32–36 This suggests that the overall burden of CAP in older adults is at least double what is traditionally captured by hospital surveillance studies, and more data are needed to understand what proportion of this burden also remains vaccine-preventable. Similarly, many pneumonia surveillance studies (in both the in- and outpatient setting) rely primarily on chest x-ray opacity to identify incident cases, and many studies have shown this approach has low sensitivity and could lead to a further underestimation of overall clinical pneumonia burden.37–42
The gold standard for identifying invasive pneumococcal disease (IPD) has been culture from blood or other normally-sterile sites. Culture-based methods for IPD, however, do not detect nonbacteremic CAP, which makes up the vast majority of pneumococcal CAP burden in adults.24 Urine antigen detection assays were developed as an alternative method for detecting nonbacteremic pneumococcal pneumonia. The BinaxNOW S. pneumoniae assay (Alere Inc., Waltham, MA), introduced in 1999, was the first widely-used urine assay. Although BinaxNOW improves the detection of pneumococcal pneumonia, it has demonstrated only modest sensitivity43,44 and does not determine which specific pneumococcal serotype is causing disease. To overcome these limitations, a new serotype-specific urine antigen detection (SSAUD) assay capable of detecting the 13 pneumococcal serotypes included in PCV13 was developed by Pfizer45 and subsequently accepted by the US Food and Drug Administration for use in the CAPiTA trial. The SSUAD assay is ≥ 95% sensitive and specific for detecting PCV13 serotypes in patients with bacteremic or nonbacteremic radiographically-confirmed CAP when validated against typed, positive blood culture results from bacteremic pneumonia patients.45–47 Thus, this validated, limit assay demonstrated much higher sensitivity than culture or BinaxNOW.45–47
Early in 2018, estimates of the proportion of CAP caused by PCV13 serotypes in US adults from the CDC-funded EPIC study were published.47 The EPIC study prospectively enrolled immunocompetent adults hospitalized with CAP with the primary objective of determining pneumonia etiology. PCV13 serotypes were detected in 6.3% of all CAP between July 2010 and June 2012 in the EPIC study based on culture and SSUAD. SSUAD proved to be critical for identifying pneumococcal disease, and the study showed that without SSUAD, roughly half of all pneumococcal pneumonias would have gone undetected.47 While the most recent EPIC data made it clear that PCV13 serotypes were still causing adult CAP in the time frame early after PCV13 introduction in children (2010−2012), questions still remained about whether PCV13-type disease would persist in adults after further indirect effects from children were fully realized.
The answer to this question was provided by a similar study that also estimated the proportion of all-cause CAP that was due to PCV13 serotypes using SSUAD (and culture), but did so in a more recent time frame (2013−2016).48,49 The study, which recruited hospitalized CAP patients from the Louisville Pneumonia Study (described previously)27 and nine other geographically-disperse US sites (21 total enrolling hospitals), like EPIC, also estimated the proportion of adult CAP caused by PCV13 serotypes based on both traditional methods and SSUAD.48,49 Results from this multi-site surveillance study of CAP showed that PCV13 serotypes still cause at least 4% to 5% of all CAP in unvaccinated adults48,50 (including adults aged ≥ 65 years who have not yet received PCV1351). The study also showed that, in immunocompetent adults aged < 65 years (where PCV13 is not currently used) PCV13 serotypes plateaued at roughly 5% over the entire duration of the study.50 This plateau in disease rates has been seen recently for IPD in surveillance data from the United States52 and the United Kingdom53 as well. Importantly, this plateau in PCV13-type disease among unvaccinated adults illustrates that although indirect effects stemming from the pediatric vaccination program are profound (especially in the initial years of the pediatric program), they have stalled after a few years. Thus, PCV13 serotypes continue to circulate and cause illness in vulnerable adult populations (eg, the elderly and adults with immunocompromising or chronic medical conditions) who are not directly vaccinated. This remaining PCV13 disease in adults, while made up predominantly of serotypes included in PCV13 but not in PCV7, is not due to a single serotype as many vaccine serotypes all play a cumulative role.48
At first glance, 4% to 5% of all CAP seems negligible, and contrarians to adult PCV13 use have dismissed this remaining PCV13-type disease.54 However, when put into the context of the large burden of CAP in certain populations of adults, this percentage is still a meaningful persistent disease burden. For example, as described previously, the incidence of all-cause CAP among adults aged ≥ 65 years was recently shown to be roughly 2300 per 100,000 persons, per year in the Louisville Pneumonia Study.27 Applying 4% to 5% of CAP that is still PCV13-type in unvaccinated adults still translates to an annual incidence rate of hospitalized PCV13-type CAP of 90 to 115 per 100,000 persons, per year.
Further, data from the same CAP incidence study showed that adults who were aged < 65 years with underlying risk factors for pneumococcal disease (eg, diabetes mellitus, congestive heart failure, chronic obstructive pulmonary disease) have comparable, and in some cases even higher, CAP burden compared to all adults ≥ 65 years.27 This finding has been confirmed by other studies.29 Currently, adults aged < 65 years with these comorbidities are not recommended to receive PCV13 given the expectation that indirect effects from the pediatric PCV program would eliminate disease in this population. Yet, a persistent 4% to 5% of CAP still remains PCV13-type in this group. Given the range of CAP incidence rate estimates for younger adults with these comorbid conditions of at least 1000 to 2000 per 100,000 persons per year,27,29 this 4% to 5% translates to a meaningful PCV13-type CAP (ie, potentially-preventable) burden in this population as well (ie, an annual incidence rate of hospitalized PCV13-type CAP of at least 40 to 100 per 100,000 persons, per year).55
PCV13 is effective and provides a meaningful impact against pneumococcal pneumonia – even in those with chronic medical or immunocompromising conditions
In 2014, the CAPiTA study, a double-blind, placebo-controlled RCT conducted in the Netherlands, demonstrated efficacy of PCV13 against both overall and nonbacteremic vaccine-type CAP in adults aged ≥ 65 years.19 Soon after, PCV13 was routinely recommended for all US adults aged ≥ 65 years.14 Since the time of the recommendation (September 2014),14 more than 45% of US adults aged ≥ 65 years have received the vaccine.56–58 Following introduction of PCV13 in older US adults, a recent observational study51 confirmed that the efficacy observed in the RCT setting of the Netherlands19 was reflective of the real-life experience of the clinically- and demographically-diverse US adult population. This case-control study used a test-negative design and was nested within a large population-based study of adults hospitalized with CAP (ie, the Louisville Pneumonia Study described previously27). The test-negative design study showed that PCV13 was 72.8% (95% confidence interval [CI]: 12.8% to 91.5%) effective against hospitalized PCV13-type CAP and 70.1% (95%CI: 4.1% to 90.7%) effective against nonbacteremic CAP. Most notably, this high effectiveness was observed in a US population with a high prevalence of immunocompromising conditions (46%) and other chronic medical conditions including COPD (53%), congestive heart failure (32%), and diabetes mellitus (32%), and where PPV23 was used (21% in the last five years).51
The study also showed that directly vaccinating adults still represents a meaningful public-health impact.51 Specifically, the authors applied their observed vaccine-effectiveness estimates51 to the CAP burden seen in the Louisville Pneumonia Study27 using a simple mathematical model that assumed: i) approximately 49 million adults aged ≥ 65 years in the United States (based on US Census data), ii) the incidence rate of all-cause hospitalized CAP is roughly 2300 per 100,000 per year (based on the Louisville Pneumonia Study),27 iii) median hospital length of stay for CAP is 6 days,51 iv) mortality for patients hospitalized for CAP occurs in 6.5% to 12.7% of cases,51 v) at least 4% of all CAP still caused by PCV13 serotypes in unvaccinated older adults,51 vi) 73% (95%CI: 13% to 92%) effectiveness of PCV13 against vaccine-type CAP,51 and vii) five years is the minimum duration of protection for PCV13.59,60 Based on these assumptions, it was projected that as many as 137,000 (24,000 to 173,000) cumulative cases of hospitalized CAP, 824,000 (145,000 to 1,035,000) hospital days, and 17,440 (1570 to 21,920) deaths could potentially be averted over five years with adult PCV13 use in the United States (assuming 100% uptake; Figure 2).51 The study underscored that this potential impact of PCV13 use in adults aged ≥ 65 years is comparable to the number of hospitalizations potentially averted with the US seasonal influenza vaccination program in the same age group.51
Figure 2.
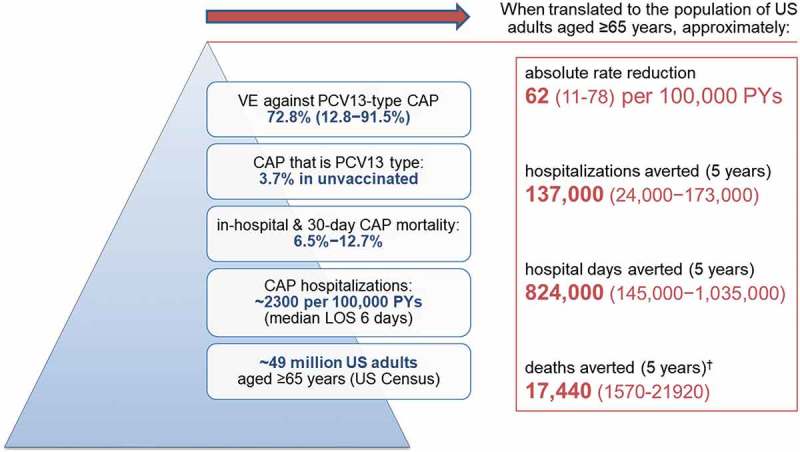
Potential impact* of PCV13 use in US adults aged ≥ 65 years51.
CAP = community-acquired pneumonia; US = United States; VE = vaccine effectiveness. *assumes 5% all-cause mortality each year and 100% vaccine uptake. †A significant effect on all-cause mortality was not demonstrated in Community Acquired Pneumonia Immunization Trial in Adults (CAPiTA).19
In addition to these vaccine effectiveness data, a recent ecological study suggested that adult PCV13 use has already been associated with reductions in PCV13-type hospitalized CAP over and above the indirect effects stemming from the pediatric PCV13 program. This ecological study, data from which were recently presented at the 2018 February US Advisory Committee on Immunization Practices (ACIP) meeting50 (full manuscript submitted for peer-review), compared the proportion of hospitalized CAP caused by PCV13 serotypes before and after the 2014 ACIP recommendation for universal use of PCV13 in adults aged ≥ 65 years14 (from October 2013 through September 2016) for two groups of adults. The first group had universal recommendation to receive the vaccine (adults aged ≥ 65 years beginning in September 2014), and the other (control) group did not have a recommendation for PCV13 use (immunocompetent adults aged < 65 years with underlying chronic conditions). Although both populations are currently recommended to receive PPSV23,14,61 PPSV23 uptake has remained stable for both populations over the last several years, and the effect of PPSV23 against vaccine-type pneumonia was assumed to be very limited.1–16 For these reasons, PPSV23 was assumed to have no impact on the study findings.50
Study results showed that before the 2014 ACIP recommendation, PCV13 uptake in adults aged ≥ 65 was very low (< 2%). After the recommendation, however, PCV13 uptake among older adults steadily increased to 32% through the end of September 2016. This post-recommendation increase in PCV13 coverage over time was correlated with a significant decline in the proportion of CAP caused by PCV13 serotypes in the same age group over the same time period (Figure 3). In contrast, among adults aged 18 to 64 years with underlying chronic conditions, a population at similar risk for developing pneumococcal disease as older adults27,29,62 but for whom PCV13 is not recommended, PCV13 uptake remained negligible (< 2%) both before and after the 2014 ACIP recommendation for PCV13 use in older adults. A decline in PCV13-type CAP was not observed in this population, and the proportion of CAP caused by PCV13 serotypes remained constant (around 5%) over the entire duration of our study (Figure 4). Given that both populations experienced indirect effects, but only adults aged ≥ 65 years experienced direct effects (and saw a reduction in PCV13-type CAP), these data provided early evidence that introducing PCV13 in US adults aged ≥ 65 years corresponded to a measurable reduction in the burden of hospitalized, PCV13-type CAP over and above the indirect effects of the pediatric vaccination program.50
Figure 3.
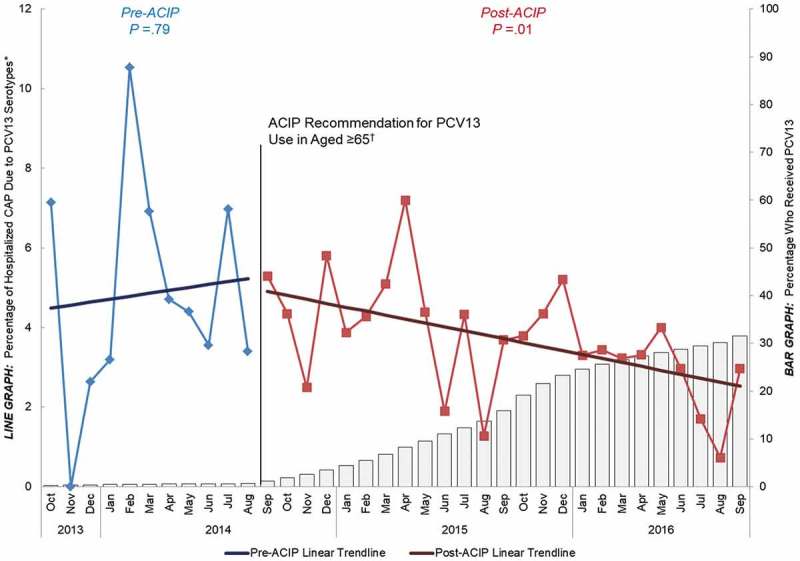
Percentage of hospitalized CAP caused by PCV13 serotypes and percentage that have received PCV13 over time among adults
aged ≥ 65 years, October 2013−September 2016 (n = 6347) ACIP = Advisory Committee on Immunization Practices. CAP = community-acquired pneumonia. PCV13 = 13-valent pneumococcal conjugate vaccine. *Y-axis does not go to 100%. †Recommendation for routine use of PCV13 for all adults aged ≥ 65 years.14
Figure 4.
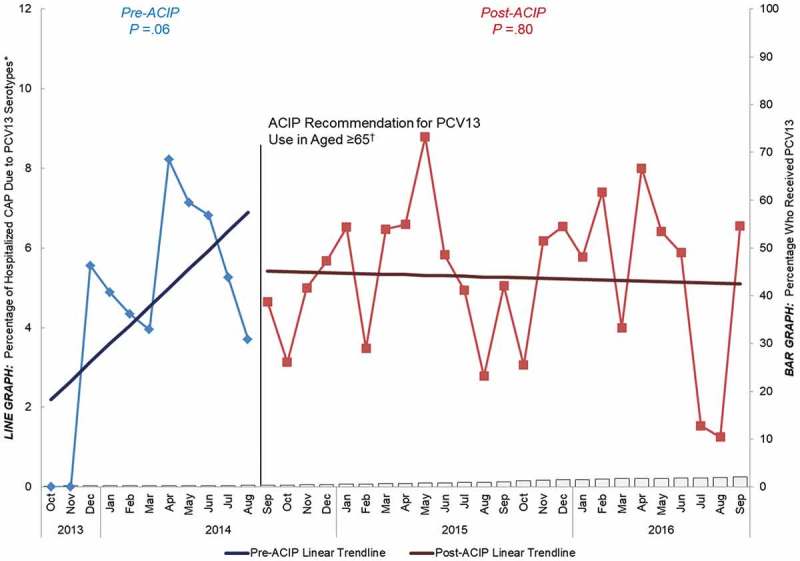
Percentage of hospitalized CAP caused by PCV13 serotypes and percentage that have received PCV13 over time among adults aged 18 to 64 years with “at-risk” conditions, October 2013 to September 2016 (n = 2976) ACIP = Advisory Committee on Immunization Practices. CAP = community-acquired pneumonia. PCV13 = 13-valent pneumococcal conjugate vaccine. “At-risk” patients were defined as the absence of immunocompromising conditions but the presence of ≥ 1 chronic medical condition including: congestive heart failure, diabetes mellitus, chronic obstructive pulmonary disease (COPD), asthma, liver disease, or current alcoholism or smoking. *Y-axis does not go to 100%. †Recommendation for routine use of PCV13 for all adults aged ≥ 65 years.14
Hence, the new evidence we have described thus far shows both a persistence of PCV13-type CAP in adults and real-world effectiveness51 plus public-health impact50 of the vaccine against this remaining vaccine-type pneumonia. These data support the direct use of PCV13 in adults. Further, if PCV13 use in adults was prematurely stopped (ie, direct vaccine pressure is removed) in countries that have already introduced the vaccine (eg, the United States), re-emergence of PCV13-type disease is likely.
Finally, there is a recent concern about the effectiveness of PCV13 against disease caused by one of its vaccine serotypes – serotype 3. Serotype 3 disease has behaved differently and has not meaningfully declined (in any age group) at the population level following the introduction of PCV13 into pediatric immunization programs across the globe. Nevertheless, recent evidence in older adults suggests that PCV13 provides some degree of direct protection against serotype 3 hospitalized CAP.19,51 The CAPiTA trial, although not powered for serotype-specific endpoints, showed point estimates that were consistent with individual-level efficacy against first episodes of vaccine-type hospitalized CAP caused by serotype 3 of 56.3% (95%CI: −12.4% to 84.8%; P =.09) in the per-protocol population, and of 60.0% (95%CI: 5.2% to 84.8%; P =.04) in the modified intent-to-treat population.19,63 In addition, the recently-published real-world PCV13 effectiveness study (described previously) found a similar point estimate against serotype 3 hospitalized CAP (VE = 52.8%, 95%CI: −100% to 88.9%) in adults of the same age,51,63 although this study was also underpowered for determining serotype-specific effectiveness.
So, how is it that ecological IPD surveillance trends (which include both direct and indirect effects of vaccination as well as environmental and epidemiological factors) show little or no overall population-level reductions in serotype 3, but individual-level efficacy and effectiveness estimates from the clinical-trial and real-world settings suggest some level of direct protection against serotype 3? The most likely explanation is that although some-level of direct effectiveness of PCV13 against serotype 3 disease exists, PCV13 likely has more limited impact against serotype 3 carriage.64 Thus, continued transmission of serotype 3 may be occurring (ie, more than other PCV13 serotypes). As a result, even if large population-level decreases in serotype 3 disease have not been observed following routine PCV13 introduction into pediatric immunization programs to-date, direct protection (in children65 and especially in adults) may be important to prevent large increases in (ie, serotype replacement with) serotype 3 disease. Indeed, notable increases in serotype 3 disease have been observed in some countries following the routine pediatric introduction of PCV10, which does not include serotype 3 in its formulation. In the same vein, in the United Kingdom and Germany, where PCV13 is used in children but not in adults, serotype 3 disease has increased in older adults in recent years.53,66 Thus, one of the effects of vaccinating adults directly with PCV13 may be to keep serotype 3 disease at bay, even if large population-level declines are not ultimately observed.
Removing existing adult recommendations has additional ethical and pragmatic and vaccine policy considerations
While the most recent epidemiological and vaccine effectiveness data (outlined previously) seem to support continued, long-term adult PCV13 use in countries that have already introduced the vaccine, there are other programmatic considerations for these countries as well. Using first the example of the United States, in the short time since PCV13 was routinely recommended for all adults aged ≥ 65 years in September 2014,14 PCV13 uptake in this population has increased steadily but is plateauing at modest levels.56,58 More importantly, black and Hispanic adults, adults with low socioeconomic or educational status, and those living in rural communities or urban/inner-city areas have had significantly lower levels of PCV13 uptake.56,58 Removing the current recommendation for use of PCV13 in older US adults would essentially cement these disparities in PCV13 utilization in the very communities at increased risk for pneumococcal disease.67–71 This potential unintended consequence should be carefully evaluated by any country considering only short-term adult PCV13 use.
Secondly, removing a safe, effective, and currently-recommended vaccine from a national adult program will likely be misinterpreted by the general public. Recent evidence suggests that levels of vaccine hesitancy may be on the rise,72–75 fueled, in part, by recent enhancements of the anti-vaccine platform on the Internet and social media.76 Removing a proven vaccine for a non-safety-related concern could intensify anti-vaccine sentiment for all vaccines. This concern should be carefully considered by policy-makers and clinicians alike.
Finally, expanded-valency PCVs are on the horizon, and are expected to be available in only a few years. Adult vaccine recommendations take time to implement and to gain traction.77 A decision to prematurely remove the recommendation for adult PCV13 use and then re-evaluate and potentially re-instate recommendations for expanded-valency adult PCVs only a few short years later may create unnecessary confusion and slow the current momentum for building a successful adult vaccination platform.
Conclusions
Emerging evidence suggests that directly protecting adults with PCV13 is necessary. First, PCV13-type pneumococcal pneumonia persists in adults, despite indirect effects from pediatric PCV13 use. The overall burden of hospitalized CAP in adults has recently been shown to be much higher than previously estimated,27 driven by the fact that previous studies have long used selection criteria that excluded groups at known risk for pneumococcal disease (ie, HCAP and immunocompromised patients).25,26 In addition, outpatient CAP burden is not well-studied and is often overlooked. Most importantly, PCV13 serotypes still cause adult disease. Although it is unquestionable that use of PCV13 in children has led to indirect effects in adults, recent data show that PCV13 serotypes still make up at least 4% to 5% of all-cause CAP in unvaccinated adults.50,51 Based on CAP incidence data from the Louisville Pneumonia Study,27 this 4% to 5% still translates to thousands of pneumonia cases each year, in both adults aged ≥ 65 years who have not yet received the vaccine (despite ACIP recommendation) and in adults aged < 65 with comorbidities or are not currently recommended to receive PCV13.27,50,51
Second, adult PCV13 use is working. In addition to efficacy data from a large RCT,19 PCV13 was recently shown to be 73% effective against vaccine-type CAP (including HCAP) in adults, even in a population that included older adults with a high prevalence of immunocompromising and chronic medical conditions.51 Additionally, recent data have shown a measurable impact of PCV13 use in older adults over and above the indirect effects from the pediatric program,50 and disease could come back if vaccine pressure from direct PCV13 use was removed.
Third, in countries that have already introduced the vaccine, ethical and pragmatic considerations support continued use of PCV13 in adults. For example, in the United States, where the adult PCV13 program is still in its relative infancy, disparities in PCV13 coverage still exist56,58 in the very communities at increased risk for pneumococcal disease.67-71 Removing the current recommendation for PCV13 use would cement these disparities, and could also inject unwarranted concerns about vaccine safety into the general public. These potential unintended consequences should be carefully evaluated by any country considering only short-term adult PCV13 use. In addition, expanded-valency PCVs are on the horizon, and maintaining the current momentum for building a successful adult vaccination platform, both in the United States and globally, would likely be stalled by a decision to remove a new adult vaccine recommendation only a few years after it was implemented.
Together, these findings suggest that vaccinating only children with PCV13 (and future PCVs) – or only children and immunocompromised adults, an adult subpopulation that is notoriously difficult to immunize77 – and then hoping that indirect effects take care of everyone else, may no longer be enough.
Funding Statement
This study was sponsored by Pfizer Inc.
Disclosure of potential conflicts of interest
All authors are employees and shareholders of Pfizer Inc. The sponsor was involved with study concept and design, conduct, analysis, and interpretation of the data; drafting of the manuscript; and the decision to submit the manuscript for publication.
References
- 1.Centers for Disease Control and Prevention Advisory committee on immunization practices. Updated recommendations for prevention of invasive pneumococcal disease among adults using the 23-valent pneumococcal polysaccharide vaccine (PPSV23). MMWR Morb Mortal Wkly Rep. 2010;59:1102–1106. [PubMed] [Google Scholar]
- 2.Metersky ML, Dransfield MT, Jackson LA.. Determining the optimal pneumococcal vaccination strategy for adults: is there a role for the pneumococcal conjugate vaccine? Chest. 2010;138:486–490. doi: 10.1378/chest.10-0738. [DOI] [PubMed] [Google Scholar]
- 3.Moberley SA, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev. 2013; (1):CD000422.doi:10.1002/14651858.CD000422.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Macleod CM, Hodges RG, Heidelberger M, Bernhard WG. Prevention of pneumococcal pneumonia by immunization with specific capsular polysaccharides. J Exp Med. 1945;82:445–465. doi: 10.1084/jem.82.6.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nichol KL, Baken L, Wuorenma J, Nelson A. The health and economic benefits associated with pneumococcal vaccination of elderly persons with chronic lung disease. Arch Intern Med. 1999;159:2437–2442. [DOI] [PubMed] [Google Scholar]
- 6.Simberkoff MS, Cross AP, Al-Ibrahim M, Baltch AL, Geiseler PJ, Nadler J, Richmond AS, Smith RP, Schiffman G, Shepard DS. Efficacy of pneumococcal vaccine in high-risk patients. Results of a veterans administration cooperative study. N Engl J Med. 1986;315:1318–1327. doi: 10.1056/NEJM198611203152104. [DOI] [PubMed] [Google Scholar]
- 7.Koivula I, Sten M, Leinonen M, Makela PH. Clinical efficacy of pneumococcal vaccine in the elderly: a randomized, single-blind population-based trial. Am J Med. 1997;103:281–290. [DOI] [PubMed] [Google Scholar]
- 8.Ortqvist A, Hedlund J, Burman LA, Elbel E, Hofer M, Leinonen M, Lindblad I, Sundelöf B, Kalin M. Randomised trial of 23-valent pneumococcal capsular polysaccharide vaccine in prevention of pneumonia in middle-aged and elderly people. Swedish pneumococcal vaccination study group. Lancet. 1998;351:399–403. [DOI] [PubMed] [Google Scholar]
- 9.Honkanen PO, Keistinen T, Miettinen L, Herva E, Sankilampi U, Laara E, Leinonen M, Kivelä SL, Mäkelä PH. Incremental effectiveness of pneumococcal vaccine on simultaneously administered influenza vaccine in preventing pneumonia and pneumococcal pneumonia among persons aged 65 years or older. Vaccine. 1999;17:2493–2500. [DOI] [PubMed] [Google Scholar]
- 10.Davis AL, Aranda CP, Schiffman G, Christianson LC. Pneumococcal infection and immunologic response to pneumococcal vaccine in chronic obstructive pulmonary disease. A pilot study. Chest. 1987;92:204–212. [DOI] [PubMed] [Google Scholar]
- 11.Huss A, Scott P, Stuck AE, Trotter C, Egger M. Efficacy of pneumococcal vaccination in adults: a meta-analysis. Cmaj. 2009;180:48–58. doi: 10.1503/cmaj.080734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dear K, Holden J, Andrews R, Tatham D. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev. 2003; (4):CD000422. [DOI] [PubMed] [Google Scholar]
- 13.Musher DM, Rueda-Jaimes AM, Graviss EA, Rodriguez-Barradas MC. Effect of pneumococcal vaccination: a comparison of vaccination rates in patients with bacteremic and nonbacteremic pneumococcal pneumonia. Clin Infect Dis. 2006;43:1004–1008. doi: 10.1086/507699. [DOI] [PubMed] [Google Scholar]
- 14.Tomczyk S, Bennett NM, Stoecker C, Gierke R, Moore MR, Whitney CG,Hadler S, Pilishvili T. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the advisory committee on immunization practices (ACIP). Mmwr. 2014;63:822–825. [PMC free article] [PubMed] [Google Scholar]
- 15.Vila-Corcoles A, Ochoa-Gondar O, Rodriguez-Blanco T, Raga-Luria X, Gomez-Bertomeu F. Epivac study group. Epidemiology of community-acquired pneumonia in older adults: a population-based study. Respir Med. 2009;103:309–316. doi: 10.1016/j.rmed.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Jackson LA, Neuzil KM, Yu O, Benson P, Barlow WE, Adams AL, Hanson CA, Mahoney LD, Shay DK, Thompson WW. Effectiveness of pneumococcal polysaccharide vaccine in older adults. N Engl J Med. 2003;348:1747–1755. doi: 10.1056/NEJMoa022678. [DOI] [PubMed] [Google Scholar]
- 17.Latifi-Navid H, Latifi-Navid S, Mostafaiy B, Jamalkandi SA, Ahmadi A. Pneumococcal disease and the effectiveness of the PPV23 vaccine in adults: A two-stage bayesian meta-analysis of observational and RCT reports. Sci Rep. 2018;8:11051. doi: 10.1038/s41598-018-29280-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schuchat A. Pneumococcal prevention gets older and wiser. JAMA Intern Med. 2015;175:1897–1898. doi: 10.1001/jamainternmed.2015.6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonten MJ, Huijts SM, Bolkenbaas M, Webber C, Patterson S, Gault S, van Werkhoven CH, van Deursen AMM, Sanders EAM, Verheij TJM, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372:1114–1125. doi: 10.1056/NEJMoa1408544. [DOI] [PubMed] [Google Scholar]
- 20.Khoie T, Tiernan R, deVore N.. FDA briefing document. Prevnar 13 (PCV13): pneumococcal 13-valent conjugate vaccine (Diphtheria CRM197 Protein). Applicant. Silver Spring, Maryland: Wyeth Pharmaceuticals Inc; 2011. [Google Scholar]
- 21.Shiri T, Datta S, Madan J, Tsertsvadze A, Royle P, Keeling MJ, et al. Indirect effects of childhood pneumococcal conjugate vaccination on invasive pneumococcal disease: A systematic review and meta-analysis. Lancet Glob Health. 2017;5:e51–e9. [DOI] [PubMed] [Google Scholar]
- 22.Deloria Knoll M, Park DE, Johnson TS, Chandir S, Nonyane BA, Conklin L, Fleming-Dutra KE, Loo JD, Goldblatt D, Whitney CG, et al. Systematic review of the effect of pneumococcal conjugate vaccine dosing schedules on immunogenicity. Pediatr Infect Dis J. 2014;33(Suppl 2):S119–29. doi: 10.1097/INF.0000000000000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whitney CG. Examining duration of protection: should a booster dose be part of all infant pneumococcal conjugate vaccine programs? Clin Infect Dis. 2018;67:375–377. doi: 10.1093/cid/ciy135. [DOI] [PubMed] [Google Scholar]
- 24.Said MA, Johnson HL, Nonyane BA, et al. Estimating the burden of pneumococcal pneumonia among adults: a systematic review and meta-analysis of diagnostic techniques. PLoS One. 2013;8:e60273. doi: 10.1371/journal.pone.0060273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jain S, Self WH, Wunderink RG, Fakhran S, Balk R, Bramley AM, Reed C, Grijalva CG, Anderson EJ, Courtney DM, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373:415–427. doi: 10.1056/NEJMoa1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marston BJ, Plouffe JF, File TM Jr., Hackman BA, Salstrom SJ, Lipman HB, Kolczak MS, Breiman RF. Incidence of community-acquired pneumonia requiring hospitalization. Results of a population-based active surveillance study in Ohio. The community-based pneumonia incidence study group. Arch Intern Med. 1997;157:1709–1718. [PubMed] [Google Scholar]
- 27.Ramirez JA, Wiemken TL, Peyrani P, Arnold FW, Kelley R, Mattingly WA, Nakamatsu R, Pena S, Guinn BE, Furmanek SP, et al. Adults hospitalized with pneumonia in the United States: incidence, epidemiology, and mortality. Clin Infect Dis. 2017;65:1806–1812. doi: 10.1093/cid/cix647. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention. . Use of13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the advisory committee on immunization practices (ACIP). MMWR Morb Mortal Wkly Rep. 2012;61:816–819. [PubMed] [Google Scholar]
- 29.Shea KM, Edelsberg J, Weycker D, Farkouh RA, Strutton DR, Pelton SI. Rates of pneumococcal disease in adults with chronic medical conditions. Open Forum Infect Dis. 2014;1:ofu024. doi: 10.1093/ofid/ofu088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Aalst M, Lotsch F, Spijker R, van der Meer JTM, Langendam MW, Goorhuis A, Grobusch MP, de Bree GJ. Incidence of invasive pneumococcal disease in immunocompromised patients: A systematic review and meta-analysis. Travel Med Infect Dis. 2018;24:89–100. doi: 10.1016/j.tmaid.2018.05.016. [DOI] [PubMed] [Google Scholar]
- 31.Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, Napolitano LM, O’Grady NP, Bartlett JG, Carratalà J, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the infectious diseases society of America and the American thoracic society. Clin Infect Dis. 2016;63:e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jackson ML, Neuzil KM, Thompson WW, Shay DK, Yu O, Hanson CA, Jackson LA. The burden of community-acquired pneumonia in seniors: results of a population-based study. Clin Infect Dis. 2004;39:1642–1650. doi: 10.1086/425615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sato R, Gomez Rey G, Nelson S, Pinsky B. Community-acquired pneumonia episode costs by age and risk in commercially insured US adults aged >/=50 years. Appl Health Econ Health Policy. 2013;11:251–258. doi: 10.1007/s40258-013-0026-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas CP, Ryan M, Chapman JD, Stason WB, Tompkins CP, Suaya JA, Polsky D, Mannino DM, Shepard DS. Incidence and cost of pneumonia in medicare beneficiaries. Chest. 2012;142:973–981. doi: 10.1378/chest.11-1160. [DOI] [PubMed] [Google Scholar]
- 35.Yu H, Rubin J, Dunning S, Li S, Sato R. Clinical and economic burden of community-acquired pneumonia in the medicare fee-for-service population. J Am Geriatr Soc. 2012;60:2137–2143. doi: 10.1111/j.1532-5415.2012.04208.x. [DOI] [PubMed] [Google Scholar]
- 36.Nelson JC, Jackson M, Yu O, Whitney CG, Bounds L, Bittner R, Zavitkovsky A, Jackson LA. Impact of the introduction of pneumococcal conjugate vaccine on rates of community acquired pneumonia in children and adults. Vaccine. 2008;26:4947–4954. doi: 10.1016/j.vaccine.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 37.Self WH, Courtney DM, McNaughton CD, Wunderink RG, Kline JA. High discordance of chest x-ray and computed tomography for detection of pulmonary opacities in ED patients: implications for diagnosing pneumonia. Am J Emerg Med. 2013;31:401–405. doi: 10.1016/j.ajem.2012.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hayden GE, Wrenn KW. Chest radiograph vs. computed tomography scan in the evaluation for pneumonia. J Emerg Med. 2009;36:266–270. doi: 10.1016/j.jemermed.2007.11.042. [DOI] [PubMed] [Google Scholar]
- 39.Esayag Y, Nikitin I, Bar-Ziv J, Cytter R, Hadas-Halpern I, Zalut T, Yinnon AM. Diagnostic value of chest radiographs in bedridden patients suspected of having pneumonia. Am J Med. 2010;123:88 e1–5. doi: 10.1016/j.amjmed.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 40.Cortellaro F, Colombo S, Coen D, Duca PG. Lung ultrasound is an accurate diagnostic tool for the diagnosis of pneumonia in the emergency department. Emerg Med J. 2012;29:19–23. doi: 10.1136/emj.2010.101584. [DOI] [PubMed] [Google Scholar]
- 41.Kea B, Gamarallage R, Vairamuthu H, Fortman J, Lunney K, Hendey GW, Rodriguez RM. What is the clinical significance of chest CT when the chest x-ray result is normal in patients with blunt trauma? Am J Emerg Med. 2013;31:1268–1273. doi: 10.1016/j.ajem.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gessner BD, Jiang Q, Van Werkhoven CH, Sings HL, Webber C, Scott D, Neuzil KM, O’Brien KL, Wunderink RG, Grobbee DE, et al. A public health evaluation of 13-valent pneumococcal conjugate vaccine impact on adult disease outcomes from a randomized clinical trial in the Netherlands. Vaccine. 2018. doi: 10.1016/j.vaccine.2018.05.097. [DOI] [PubMed] [Google Scholar]
- 43.Leeming JP, Cartwright K, Morris R, Martin SA, Smith MD. South-west pneumococcus study G. Diagnosis of invasive pneumococcal infection by serotype-specific urinary antigen detection. J Clin Microbiol. 2005;43:4972–4976. doi: 10.1128/JCM.43.10.4972-4976.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith MD, Derrington P, Evans R, Creek M, Morris R, Dance DA, Cartwright K. Rapid diagnosis of bacteremic pneumococcal infections in adults by using the binax NOW streptococcus pneumoniae urinary antigen test: a prospective, controlled clinical evaluation. J Clin Microbiol. 2003;41:2810–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pride MW, Huijts SM, Wu K, Souza V, Passador S, Tinder C, Song E, Elfassy A, McNeil L, Menton R, et al. Validation of an immunodiagnostic assay for detection of 13 streptococcus pneumoniae serotype-specific polysaccharides in human urine. CVI. 2012;19:1131–1141. doi: 10.1128/CVI.00064-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pride MW, Jansen KU. Reevaluation of positivity cutoff values for the pneumococcal urinary antigen detection assay. CVI. 2017;24:e00239–17. (in press). doi: 10.1128/CVI.00239-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wunderink RG, Self WH, Anderson EJ, Balk R, Fakhran S, Courtney DM, Qi C, Williams DJ, Zhu Y, Whitney CG, et al. Pneumococcal community-acquired pneumonia detected by serotype-specific urinary antigen detection assays. Clin Infect Dis. 2018. doi: 10.1093/cid/cix1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramirez J, Alexander R, Carrico R, Ford K, Gray S, Pride M, Sebastian S, Jiang Q, Peyrani P, Isturiz R. Distribution of PCV13 pneumococcal serotypes in patients with community-acquired pneumonia presenting at 20 United States hospitals. Open Forum Infect Dis. 2015;2:1582. doi: 10.1093/ofid/ofv133.1135. [DOI] [Google Scholar]
- 49.Alexander RM, Peyrani P, Ramirez J, Self WH, Grijalva CG, Counselman F, Volturo GA, Kabler H, Ostrosky-Zeichner L, Wunderink R, et al. Rationale and methods of the study protocol: streptococcus pneumoniae serotypes in adults 18 years and older with radiographically-confirmed community-acquired pneumonia (CAP). JRI. 2017;1:35–39. doi: 10.18297/JRI/. [DOI] [Google Scholar]
- 50.McLaughlin JM. Presentation at the advisory committee on immunization practices (ACIP): Effectiveness of PCV13 in US adults. Atlanta (GA): Centers for Disease Control and Prevention; 2018. February 22.
- 51.McLaughlin JM, Jiang Q, Isturiz RE, Sings HL, Swerdlow DL, Gessner BD, Carrico RM, Peyrani P, Wiemken TL, Mattingly WA, et al. Effectiveness of 13-valent pneumococcal conjugate vaccine against hospitalization for community-acquired pneumonia in older US adults: a test-negative design. Clin Infect Dis. 2018 Oct 30;67(10):1498–1506. doi: 10.1093/cid/ciy312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Centers for Disease Control and Prevention Pneumococcal disease surveillance and reporting. National center for immunization and respiratory diseases division of bacterial diseases; 2018. February 16 https://www.cdc.gov/pneumococcal/surveillance.html.
- 53.Ladhani SN, Collins S, Djennad A, Sheppard CL, Borrow R, Fry NK, Andrews NJ, Miller E, Ramsay ME. Rapid increase in non-vaccine serotypes causing invasive pneumococcal disease in England and Wales, 2000-17: a prospective national observational cohort study. Lancet Infect Dis. 2018;18:441–451. doi: 10.1016/S1473-3099(18)30052-5. [DOI] [PubMed] [Google Scholar]
- 54.Musher DM, Rodriguez-Barradas MB. Why the recent ACIP recommendations regarding conjugate pneumococcal vaccine in adults may be irrelevant. Hum Vaccin Immunother. 2016;12:331–335. doi: 10.1080/21645515.2015.1098794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Isturiz RE, Hall-Murray C, McLaughlin JM, Snow V, Schmoele-Thoma B, Webber C, Thompson A, Scott DA. Pneumococcal conjugate vaccine use for the prevention of pneumococcal disease in adults < 50 years of age. Expert Rev Vaccines. 2018;17:45–55. doi: 10.1080/14760584.2018.1411196. [DOI] [PubMed] [Google Scholar]
- 56.Black CL, Williams WW, Warnock R, Pilishvili T, Kim D, Kelman JA. Pneumococcal vaccination among medicare beneficiaries occurring after the advisory committee on immunization practices recommendation for routine use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults aged ≥ 65 years. MMWR Morb Mortal Wkly Rep. 2017;66:728–733. doi: 10.15585/mmwr.mm6627a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pfizer data on file provided by IQVIA. Durham, NC. [Google Scholar]
- 58.McLaughlin JM, Khan F, Curry A, Snow V, Isturiz RE, Swerdlow DL. Disparities in uptake of 13-valent pneumococcal conjugate vaccine among older adults following routine recommendation in the United States. Open Forum Infect Dis. 2017. Fall;2017:S468–S9. doi: 10.1093/ofid/ofx163.1197. [DOI] [Google Scholar]
- 59.McLaughlin JM, Swerdlow DL, Isturiz RE, Jodar L. Rethinking number-needed-to-vaccinate for pneumococcal conjugate vaccines in older adults: current and future implications. Vaccine. 2017;35:5360–5365. doi: 10.1016/j.vaccine.2017.08.028. [DOI] [PubMed] [Google Scholar]
- 60.Patterson S, Webber C, Patton M, Drews W, Huijts SM, Bolkenbaas M, Gruber WC, Scott DA, Bonten MJM. A post hoc assessment of duration of protection in CAPiTA (community acquired pneumonia immunization trial in adults). Trials Vaccinol. 2016;5:92–96. doi: 10.1016/j.trivac.2016.04.004. [DOI] [Google Scholar]
- 61.Centers for Disease Control and Prevention Prevention of pneumococcal disease: recommendations of the advisory committee on immunization practices (ACIP). MMWR Recomm Rep. 1997;46:1–24. [PubMed] [Google Scholar]
- 62.Weycker D, Strutton D, Edelsberg J, Sato R, Jackson LA. Clinical and economic burden of pneumococcal disease in older US adults. Vaccine. 2010;28:4955–4960. doi: 10.1016/j.vaccine.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 63.Pfizer data on file Collegeville, PA. [Google Scholar]
- 64.Dagan R, Patterson S, Juergens C, Greenberg D, Givon-Lavi N, Porat N, Gurtman A, Gruber WC, Scott DA. Comparative immunogenicity and efficacy of 13-valent and 7-valent pneumococcal conjugate vaccines in reducing nasopharyngeal colonization: a randomized double-blind trial. Clin Infect Dis. 2013;57:952–962. doi: 10.1093/cid/cit428. [DOI] [PubMed] [Google Scholar]
- 65.Sings HL, De Wals P, Gessner BD, Isturiz R, Laferriere C, McLaughlin JM, Pelton S, Schmitt HJ, Suaya J.A, Jodar L. Effectiveness of 13-valent pneumococcal conjugate vaccine against invasive disease caused by serotype 3 in children: a systematic review and meta-analysis of observational studies. Clin Infect Dis. 2018. doi: 10.1093/cid/ciy920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Perniciaro S, Van Der Linden M, Imöhl M. Reduced effect of the pediatric pneumococcal conjugate vaccination on invasive pneumococcal disease in adults in Germany. 28th ECCMID, Madrid, Spain. 2018;P0586. [Google Scholar]
- 67.Burton DC, Flannery B, Bennett NM, Farley MM, Gershman K, Harrison LH, Lynfield R, Petit S, Reingold AL, Schaffner W, et al. Socioeconomic and racial/ethnic disparities in the incidence of bacteremic pneumonia among US adults. Am J Public Health. 2010;100:1904–1911. doi: 10.2105/AJPH.2009.181313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kyaw MH, Rose CE Jr., Fry AM, Singleton JA, Moore Z, Zell ER, Whitney CG. The influence of chronic illnesses on the incidence of invasive pneumococcal disease in adults. J Infect Dis. 2005;192:377–386. doi: 10.1086/431521. [DOI] [PubMed] [Google Scholar]
- 69.Soto K, Petit S, Hadler JL. Changing disparities in invasive pneumococcal disease by socioeconomic status and race/ethnicity in Connecticut, 1998-2008. Public Health Rep. 2011;126(Suppl 3):81–88. doi: 10.1177/00333549111260S313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McLaughlin JM, Utt EA, Hill NM, Welch VL, Power E, Sylvester GC. A current and historical perspective on disparities in US childhood pneumococcal conjugate vaccine adherence and in rates of invasive pneumococcal disease: considerations for the routinely-recommended, pediatric PCV dosing schedule in the United States. Hum Vaccin Immunother. 2016;12:206–212. doi: 10.1080/21645515.2015.1069452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Warren JL, Pingali SC, Weinberger DM. Spatial variability in the persistence of pneumococcal conjugate vaccine-targeted pneumococcal serotypes among adults. Epidemiology. 2017;28:119–126. doi: 10.1097/EDE.0000000000000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dube E, Gagnon D, Nickels E, Jeram S, Schuster M. Mapping vaccine hesitancy–country-specific characteristics of a global phenomenon. Vaccine. 2014;32:6649–6654. doi: 10.1016/j.vaccine.2014.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dube E, Vivion M, MacDonald NE. Vaccine hesitancy, vaccine refusal and the anti-vaccine movement: influence, impact and implications. Expert Rev Vaccines. 2015;14:99–117. doi: 10.1586/14760584.2015.964212. [DOI] [PubMed] [Google Scholar]
- 74.Larson HJ, Jarrett C, Eckersberger E, Smith DM, Paterson P. Understanding vaccine hesitancy around vaccines and vaccination from a global perspective: a systematic review of published literature, 2007-2012. Vaccine. 2014;32:2150–2159. doi: 10.1016/j.vaccine.2014.01.081. [DOI] [PubMed] [Google Scholar]
- 75.Siddiqui M, Salmon DA, Omer SB. Epidemiology of vaccine hesitancy in the United States. Hum Vaccin Immunother. 2013;9:2643–2648. doi: 10.4161/hv.27243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chatterjee A, O’Keefe C. Current controversies in the USA regarding vaccine safety. Expert Rev Vaccines. 2010;9:497–502. doi: 10.1586/erv.10.36. [DOI] [PubMed] [Google Scholar]
- 77.Williams WW, Lu PJ, O’Halloran A, Kim DK, Grohskopf LA, Pilishvili T, Skoff TH, Nelson NP, Harpaz R, Markowitz LE, et al. Surveillance of vaccination coverage among adult populations - United States, 2015. MMWR Surveill Summ. 2017;66:1–28. doi: 10.15585/mmwr.ss6611a1. [DOI] [PMC free article] [PubMed] [Google Scholar]


