Watch a video presentation of this article
Watch the interview with the author
Abbreviations
- 0‐ABDRmm
no mismatches for HLA A, B, and DR
- A2ALL
Adult‐to‐Adult Living Donor Liver Transplantation Cohort
- AR
acute rejection
- CCS
continued corticosteroid
- CI
confidence interval
- CNI
calcineurin inhibitor
- CSWD
corticosteroid withdrawal
- ECSWD
early corticosteroid withdrawal
- EPTS
estimated post transplant survival
- GFR
glomerular filtration rate
- KAL
kidney after liver
- KDPI
kidney donor profile index
- KTA
kidney transplant alone
- LTA
liver transplant alone
- mTORi
mammalian target of rapamycin inhibitor
- PTDM
posttransplant diabetes
- RR
relative risk
- SLK
simultaneous liver‐kidney transplant
- SRTR
Scientific Registry of Transplant Recipients
On August 10, 2017, a new allocation policy was introduced to define and standardize eligibility criteria and organ allocation priority for simultaneous liver‐kidney transplants (SLKs). Key components of this policy include not only the potential impact on SLK utilization, but also the potential for patients who undergo liver transplant alone (LTA) who have persistent severe kidney disease to be prioritized under a “safety net” (Fig. 1). This “safety net” permits LTA recipients with glomerular filtration rate (GFR) with continued dialysis dependency or GFR ≤ 20 mL/min in the period 2 to 12 months after liver transplant to receive priority for kidney allocation for kidneys with kidney donor profile index (KDPI) greater than 20% if deemed a kidney transplant candidate. In the first 8 months since implementation of the new SLK allocation policy, the SLK utilization has decreased by 16% (annualized rates from 2017 prepolicy of 785 transplants to 658 transplants after implementation). Concurrent with this decrease, kidney after liver (KAL) transplant increased from an average of 1.4 to 7.3 KAL/month (from 28 total KAL during the 20 months prior to the new SLK allocation policy to 64 in the 9 months after the new policy).
Figure 1.
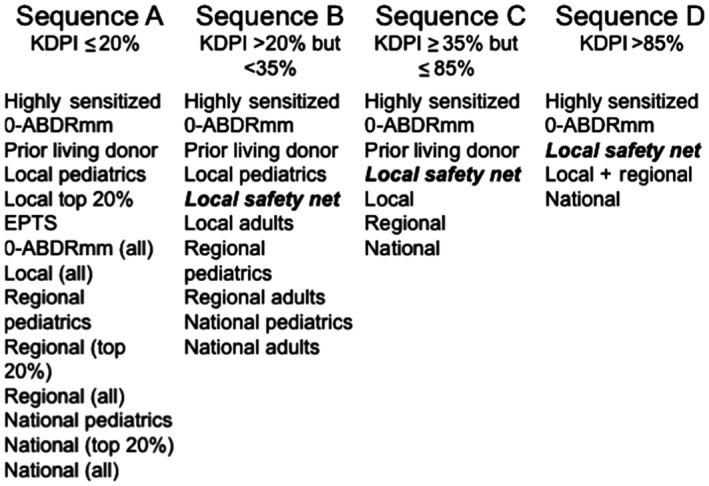
Allocation order for liver transplant recipients who qualify for the “safety net.” Liver transplant recipients with continued dialysis dependency or kidney dysfunction with GFR ≤ 20 mL/min in the period 2 to 12 months after liver transplant will receive priority for kidney allocation for kidneys with a KDPI greater than 20%.
With these changes now ensuring consistency in candidate and recipient characteristics, it is now reasonable to consider more consistent management strategies for both the SLK recipient and the LTA safety net candidate. For SLK, this includes harmonization of induction strategies (perhaps not needed in the SLK setting,1 yet a common practice in the kidney transplant alone (KTA) and thus the KAL setting) and immune monitoring strategies (e.g., screening for BK virus, donor‐specific antibodies, and surveillance biopsies), areas in which there are little data beyond small single‐center experiences. One area in need of harmonization is immunosuppression management, in particular early corticosteroid withdrawal (ECSWD; within 1 week from transplant) in the SLK population. The strategy of CSWD has traditionally been much more broadly applied in the LTA setting compared with the KTA setting with very little data available in the SLK population (Fig. 2). According to the most recent Organ Procurement and Transplantation Network/Scientific Registry of Transplant Recipients (SRTR) Annual Data Report, ~25% of LTAs undergo ECSWD, and an additional 15% undergo later withdrawal over the first year.2 This “later” withdrawal strategy has decreased in frequency from 40% to 15% from 2012 to 2016. This is in contrast with KTA recipients in whom ~33% undergo ECSWD with very few patients undergoing withdrawal thereafter.3
Figure 2.
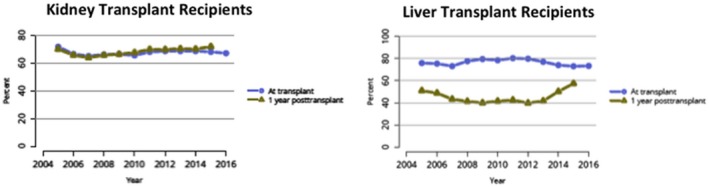
Steroid utilization after kidney and liver transplant in the United States. Reproduced with permission from American Journal of Transplantation.2, 3 Copyright 2018, American Journal of Transplantation.
One potential reason for the decreased enthusiasm for (or lack of greater adoption of) CSWD over time is that rejection rates are demonstrably higher with ECSWD compared with continued corticosteroid (CCS) use. In KTA, a landmark multicenter, randomized, prospective, double‐blind trial examining 5‐year outcomes of CCS versus ECSWD demonstrated similar graft and patient survival at 5 years, with acute rejection (AR) rates in the CCS arm of 10.8% versus 17.8% in the ECSWD arm.4 No differences in weight gain, infections (including cytomegalovirus, BK virus), hypertension, cholesterol, Framingham risk score, or cataract formation were noted, with some evidence that bone disease fractures or avascular necrosis were more common in the CCS cohort. In contrast, another multicenter randomized trial in 615 KTA recipients showed no differences in AR rates in the CCS versus ECSWD arms, although the immunological risk factors were less than the previously mentioned study.5 Further, using the American Diabetes Association definition of posttransplant diabetes (PTDM), PTDM rates were significantly lower in the ECSWD arms (22.7%‐23.9%) than the CCS arm (39.2%; P = 0.0004).
In the LTA population, the experience with ECSWD has recently been summarized in a meta‐analysis of 16 studies with 1347 participants.6 The main findings of this metaanalysis were consistent with the KTA experience in that although graft loss and mortality were similar, the adjusted relative risk (RR) for rejection was 1.33 (95% confidence interval [CI], 1.08‐1.64) with ECSWD compared with CCS (Fig. 3). Although rejection has not been as feared an outcome in LTA versus KTA given the regenerative capacity of the liver, a recent analysis of the A2ALL study and SRTR data demonstrated a detrimental association of AR with graft failure and mortality, raising concerns that the increased rejection risk of ECSWD may not be balanced by any theoretical (and as yet unproven) advantages in reducing cardiovascular or infection‐related outcomes (Fig. 4).7 Taking the current LTA and KTA data together to create recommendations for steroid use in SLK, it appears that equipoise may favor steroid utilization rather than ECSWD, acknowledging the lack of data and discounting the purported protective effects of liver transplant on kidney transplant.
Figure 3.
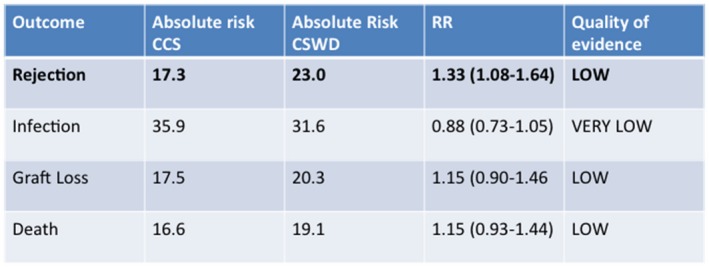
Meta‐analysis of the risk for rejection, infection, graft loss, and death by treatment regimen in liver transplant, CCS versus CSWD.
Figure 4.
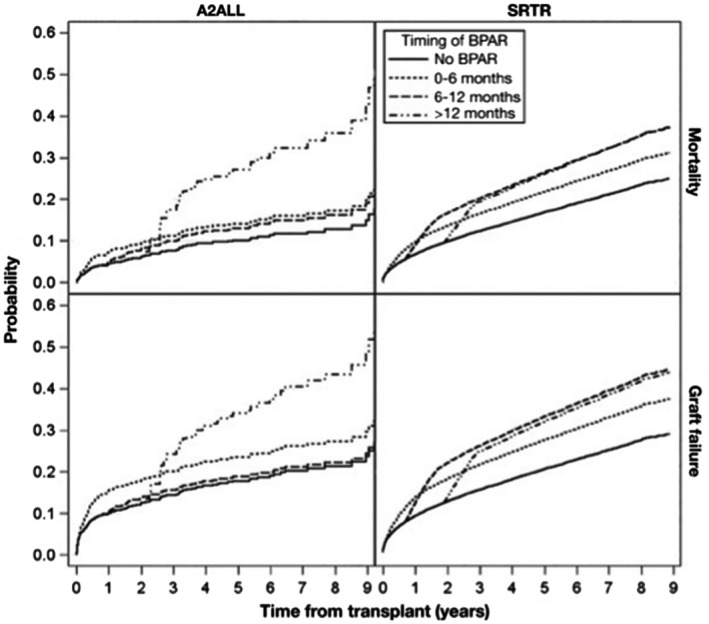
Acute liver rejection: impact on graft loss and mortality in the A2ALL study (2003‐2014) and registry analysis from the SRTR 2005‐2013. Reproduced with permission from Clinical Gastroenterology and Hepatology.7 Copyright 2017.
For those patients who are transplanted with an LTA and fall under the safety net with persistent renal insufficiency, immunosuppression considerations not only must take into account the risk to the liver allograft but also the GFR of the recipient. One may consider renoprotective strategies such as calcineurin inhibitor (CNI) minimization or CNI withdrawal/transition to other agents in a patient with resolving and improving GFR during months 2 to 12 after transplant. For example, in a large randomized controlled trial, reduction of CNI (tacrolimus trough 3‐5 ng/mL) and use of the mammalian target of rapamycin inhibitor (mTORi) everolimus (trough 3‐8 ng/mL) 1 month after transplant were associated with less rejection episodes and improved GFR at 12, 24, and 36 months, despite higher discontinuation rates.8 A meta‐analysis of 10 randomized controlled trials with a total of 1927 patients that examined the impact of CNI conversion to mTORi found improved GFR of ~8 mL/min (when conversion occurred in the first year after transplant) at the expense of higher AR rates (RR 1.8, 95% CI: 1.3‐2.3) and higher discontinuation rates (RR 2.2, 95% CI: 1.4‐3.4), tempering enthusiasm for this nephroprotective strategy.9
Ultimately, immunosuppressive considerations in the safety net patient become more complicated as one attempts to improve renal function in real time while still keeping the kidney transplant prioritization available in case renal function does not recover adequately. A general approach may be to continue to optimize renal function in LTA patients with GFR that is approaching and exceeding 30 mL/min including immunosuppressive medication interventions, and for those patients with persistent GFR less than 30 mL/min that is fluctuating or declining, consideration should be placed on optimizing liver function and functional status as a priority without a specific focus on nephroprotective strategies. In any case of LTA with GFR less than 30 mL/min in the 2‐ to 12‐month window after transplant, prompt and timely referral for kidney transplant evaluation should be performed to ensure that patients remain candidates for another major surgery and increased immunosuppression, and to avoid any administrative or regulatory delays in determining eligibility.
Potential conflict of interest: Nothing to report.
References
- 1. AbdulRahim N, Anderson L, Kotla S, et al. Lack of benefit and potential harm of induction therapy in simultaneous liver‐kidney transplants. Liver Transpl 2019;25:411‐424. [DOI] [PubMed] [Google Scholar]
- 2. Kim WR, Lake JR, Smith JM, et al. OPTN/SRTR 2016 Annual Data Report: liver. Am J Transplant 2018;18(Suppl. 1):172‐253. [DOI] [PubMed] [Google Scholar]
- 3. Hart A, Smith JM, Skeans MA, et al. OPTN/SRTR 2016 Annual Data Report: kidney. Am J Transplant 2018;18(Suppl. 1):18‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Woodle ES, First MR, Pirsch J, et al. A prospective, randomized, double‐blind, placebo‐controlled multicenter trial comparing early (7 day) corticosteroid cessation versus long‐term, low‐dose corticosteroid therapy. Ann Surg 2008;248:564‐577. [DOI] [PubMed] [Google Scholar]
- 5. Thomusch O, Wiesener M, Opgenoorth M, et al. Rabbit‐ATG or basiliximab induction for rapid steroid withdrawal after renal transplantation (Harmony): an open‐label, multicentre, randomised controlled trial. Lancet 2016;388:3006‐3016. [DOI] [PubMed] [Google Scholar]
- 6. Fairfield C, Penninga L, Powell J, et al. Glucocorticosteroid‐free versus glucocorticosteroid‐containing immunosuppression for liver transplanted patients. Cochrane Database Syst Rev 2018;4:CD007606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Levitsky J, Goldberg D, Smith AR, et al. Acute rejection increases risk of graft failure and death in recent liver transplant recipients. Clin Gastroenterol Hepatol 2017;15:584‐593.e582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. De Simone P, Nevens F, De Carlis L, et al. Everolimus with reduced tacrolimus improves renal function in de novo liver transplant recipients: a randomized controlled trial. Am J Transplant 2012;12:3008‐3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Glover TE, Watson CJ, Gibbs P, et al. Conversion from calcineurin to mammalian target of rapamycin inhibitors in liver transplantation: a meta‐analysis of randomized controlled trials. Transplantation 2016;100:621‐629. [DOI] [PubMed] [Google Scholar]


