Watch a video presentation of this article
Watch the interview with the author
Abbreviations
- ACR
acute cellular rejection
- DDLT
deceased donor liver transplantation
- DSA
donor‐specific antibody
- IST
immunosuppressive therapy
- LDLT
living donor liver transplantation
- LT
liver transplantation
Since the 1980s, there have been substantial improvements in survival after liver transplantation (LT), and advances in immunosuppressive therapy (IST) are largely responsible for these successes. Despite these achievements, LT recipients are burdened with lifelong IST, thereby incurring health care costs and the risks of side effects, infections, de novo malignancy, cardiovascular events, and chronic kidney disease.1, 2 As such, outcomes for patients could be enhanced if IST exposure could be minimized or even eliminated after LT. Fortunately, the liver has immunoregulatory properties that can allow for select recipients to withdraw immunosuppression and require less IST in combined liver/other organ transplantation. This review discusses basic and clinical concepts of LT tolerance and situations in which the immunosuppressive advantage can potentially translate into improved outcomes.
Basic and Clinical Evidence for Hepatic Tolerance
The liver itself is the most immunoregulatory organ transplanted; it contains a high number of extramedullary hematopoietic cells and a large mass of nonimmunogenic cells, and secretes a variety of immunoregulatory proteins (Fig. 1). The abundance of resident regulatory T cells and immature antigen‐presenting cells appears to be protective of graft rejection. Clonal deletion of alloreactive immunocytes, inhibition of alloantibody effects, mixed donor‐recipient hematopoietic chimerism, and immunological senescence are also hypothesized components of liver immunoregulation. Moreover, the graft itself may be considered the source of persistent tolerogen over being a stimulus and target for immune destruction. Early animal studies revealed that most mouse strains and outbred pigs spontaneously accept liver grafts without the need for immunosuppression, although this is not the case in skin and other organs of higher immunogenicity.3 In addition, in basic studies, the addition of a liver in the transplant procedure provides immunological protection of other organs (skin, heart, kidney) in combined transplantation.4 Early reports from Calne et al.5, 6 of LT in pigs demonstrated an immunoprotective effect of the liver on rejection of a simultaneously transplanted non‐hepatic organ.
Figure 1.
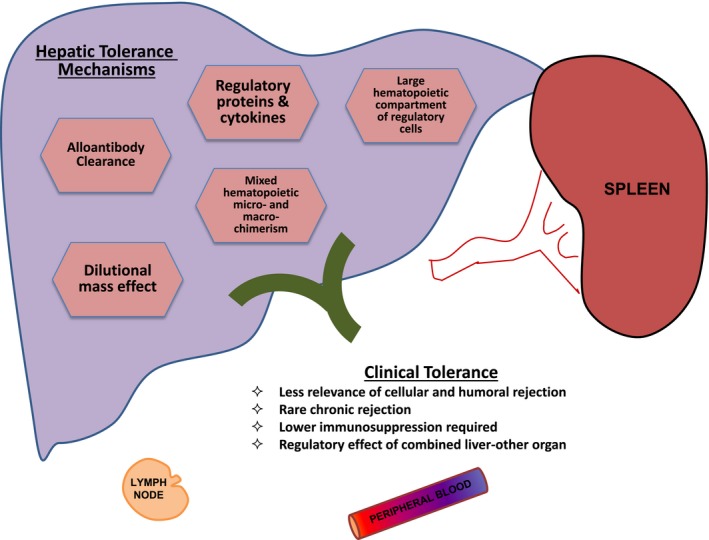
Mechanisms of and clinical evidence for hepatic tolerance.
In contrast with other solid organ recipients, LT recipients demonstrate clinical evidence supporting these immunoregulatory phenomena: the lower significance of human leukocyte antigen matching and rejection, incidence of chronic rejection, and amount of initial and maintenance IST required. Similar to animal studies, the liver appears to provide some level of immunological protection of other organs in combined human organ transplantation and is generally less adversely impacted by donor‐specific antibodies (DSAs).7, 8, 9
Immunosuppression Withdrawal in Clinical LT
Several studies, mostly single‐center studies, have demonstrated the feasibility of IST withdrawal in select LT recipients, further supporting the concept of the liver providing immunosuppressive advantage.10 Overall, withdrawal has been successful for about 20% to 30% of patients (range 5%‐70%), which is significantly higher than other organ transplants. At this point, the main predictors of success are absence of significant inflammation on initial biopsy before withdrawal, older age, and a longer time from transplantation.11 Fortunately, the development of acute cellular rejection (ACR) within the highly monitored clinical trial setting does not appear to negatively affect liver allografts, because most episodes have been diagnosed early when ACR is histologically mild and responsive to escalation of baseline IST. Overall, although of unclear clinical benefit, available literature strongly suggests that attempted IST withdrawal in appropriately selected and compliant patients in monitored research studies is reasonably safe.
Immunological Advantage of Living Donor LT
Although clinically less proven, the living donor liver transplantation (LDLT) procedure may provide an environment favoring tolerance over alloreactivity. This may be due to a less inflammatory operation because of significantly less ischemia in a more controlled setting, as well as biological similarity between donor and recipient. Evidence for LDLT providing further immunosuppressive advantage are as follows: (1) generally higher IST withdrawal rates in LDLT versus deceased donor liver transplantation (DDLT), although there are no head‐to‐head studies10; (2) lower impact (rejection, graft survival) of DSAs in LDLT versus DDLT12; and (3) lower rate of biopsy‐proven ACR in biologically related LDLT versus other donor‐recipient combinations.13, 14 Thus, there may be more opportunity in LDLT to conduct controlled tolerance studies with higher success, particularly when initiated at the time of or soon after LT.
Immunological Advantage of Combined Liver/Other Organ Transplant
Perhaps the best evidence for hepatic immunological protection is the lower rejection rate when a non‐hepatic organ (kidney, heart, lung) is combined with a liver transplant. Taner et al.15, 16, 17 performed a number of studies demonstrating that the simultaneous liver graft is a key predictive factor of lower cellular and antibody‐mediated rejection and renal function decline in kidney transplants, and that simultaneous liver‐kidney (SLK) recipients have lower circulating effector memory T cells, proliferative responses to donor cells, and frequency of interferon‐γ‐producing alloreactive T cells compared with kidney‐alone recipients. In addition, performing a kidney transplant from a different donor after LT results in higher rejection than if they were combined, supporting the importance of biological similarity.18 However, the liver is not completely protective against non‐hepatic transplant cellular and antibody‐mediated rejection. Single‐center data have shown higher antibody‐mediated kidney rejection rates in patients with class II DSAs undergoing SLK, and that the overall rate of kidney rejection (all types) is approximately 20% in SLK, which is surprisingly high.19, 20 The tolerability of each rejection may be higher than in kidney transplant alone in terms of renal graft loss, but data have shown that renal function in SLK recipients is somewhat worse over time for those with versus without history of rejection.19 Overall, the evidence supports at least a partial immunoprotective effect of the liver on other organs, supporting its “immunosuppressive advantage.”
Conclusion
It is clear that the liver provides a significant level of immunosuppressive benefit in basic studies and most clinical scenarios. Translating this to improvements in transplant outcomes (e.g., benefit of less immunosuppression, protection of other organs) needs to be further studied and tested in prospective clinical trials.
Potential conflict of interest: Nothing to report.
References
- 1. Watt KD, Pedersen RA, Kremers WK, et al. Evolution of causes and risk factors for mortality post‐liver transplant: Results of the NIDDK long‐term follow‐up study. Am J Transplant 2010;10:1420‐1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Levitsky J, O’Leary JG, Asrani S, et al. Protecting the kidney in liver transplant recipients: Practice‐based recommendations from the American Society of Transplantation Liver and Intestine Community of Practice. Am J Transplant 2016;16:2532‐2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Calne RY, Sells RA, Pena JR, et al. Induction of immunological tolerance by porcine liver allografts. Nature 1969;223:472‐476. [DOI] [PubMed] [Google Scholar]
- 4. Kamada N, Davies HS, Roser B. Reversal of transplantation immunity by liver grafting. Nature 1981;292:840‐842. [DOI] [PubMed] [Google Scholar]
- 5. Calne RY, Sells RA, Marshall VC, et al. Multiple organ grafts in the pig. Techniques and results of pancreatic, hepatic, cardiac, and renal allografts. Br J Surg 1972;59:969‐977. [DOI] [PubMed] [Google Scholar]
- 6. Calne RY, Yoffa DE, White HJ, et al. A technique of orthotopic liver translantation in the pig. Br J Surg 1968;55:203‐206. [DOI] [PubMed] [Google Scholar]
- 7. O’Leary JG, Demetris AJ, Friedman LS, et al. The role of donor‐specific HLA alloantibodies in liver transplantation. Am J Transplant 2014;14:779‐787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kaneku H, O’Leary JG, Banuelos N, et al. De novo donor‐specific HLA antibodies decrease patient and graft survival in liver transplant recipients. Am J Transplant 2013;13:1541‐1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Taner T, Gandhi MJ, Sanderson SO, et al. Prevalence, course and impact of HLA donor‐specific antibodies in liver transplantation in the first year. Am J Transplant 2012;12:1504‐1510. [DOI] [PubMed] [Google Scholar]
- 10. Levitsky J, Feng S. Tolerance in clinical liver transplantation. Hum Immunol 2018;79:283‐287. [DOI] [PubMed] [Google Scholar]
- 11. Banff Working Group on Liver Allograft P . Importance of liver biopsy findings in immunosuppression management: Biopsy monitoring and working criteria for patients with operational tolerance. Liver Transpl 2012;18:1154‐1170. [DOI] [PubMed] [Google Scholar]
- 12. Levitsky J, Kaneku H, Jie C, et al. Donor‐specific HLA antibodies in living versus deceased donor liver transplant recipients. Am J Transplant 2016;16:2437‐2444. [DOI] [PubMed] [Google Scholar]
- 13. Liu LU, Bodian CA, Gondolesi GE, et al. Marked differences in acute cellular rejection rates between living‐donor and deceased‐donor liver transplant recipients. Transplantation 2005;80:1072‐1080. [DOI] [PubMed] [Google Scholar]
- 14. Levitsky J, Goldberg D, Smith AR, et al. Acute rejection increases risk of graft failure and death in recent liver transplant recipients. Clin Gastroenter Hepatol 2017;15:584‐593.e582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Taner T, Park WD, Stegall MD. Unique molecular changes in kidney allografts after simultaneous liver‐kidney compared with solitary kidney transplantation. Kidney Int 2017;91:1193‐1202. [DOI] [PubMed] [Google Scholar]
- 16. Taner T, Heimbach JK, Rosen CB, et al. Decreased chronic cellular and antibody‐mediated injury in the kidney following simultaneous liver‐kidney transplantation. Kidney Int 2016;89:909‐917. [DOI] [PubMed] [Google Scholar]
- 17. Taner T, Gustafson MP, Hansen MJ, et al. Donor‐specific hypo‐responsiveness occurs in simultaneous liver‐kidney transplant recipients after the first year. Kidney Int 2018;93:1465‐1474. [DOI] [PubMed] [Google Scholar]
- 18. Simpson N, Cho YW, Cicciarelli JC, et al. Comparison of renal allograft outcomes in combined liver‐kidney transplantation versus subsequent kidney transplantation in liver transplant recipients: Analysis of UNOS Database. Transplantation 2006;82:1298‐1303. [DOI] [PubMed] [Google Scholar]
- 19. Nilles KM, Krupp J, Lapin B, et al. Incidence and impact of rejection following simultaneous liver‐kidney transplantation. J Hepatol 2015;62:340‐345. [DOI] [PubMed] [Google Scholar]
- 20. O’Leary JG, Gebel HM, Ruiz R, et al. Class II alloantibody and mortality in simultaneous liver‐kidney transplantation. Am J Transplant 2013;13:954‐960. [DOI] [PMC free article] [PubMed] [Google Scholar]


