Watch a video presentation of this article
Answer questions and earn CME
Abbreviations
- ADH
antidiuretic hormone
- BP
blood pressure
- CI
cardic index
- CPAP
continuous positive airway pressure
- CT
computed tomography
- CXR
chest X‐ray
- ECG
echocardiogram
- FiO2
fraction of inspired oxygen
- GCS
Glasgow coma scale
- G‐I
gastro‐intestinal
- Hb
hemoglobin
- HCO3
bicarbonate
- HRS
hepatorenal syndrome
- IAP
intra‐abdominal pressure
- ICU
intensive care unit
- IJ
internal jugular
- MAP
mean arterial pressure
- PaO2
arterial partial pressure of oxygen
- PCO2
partial pressure carbon dioxide
- PPS
portopulmonary syndrome
- PVH
pulmonary venous hypertension
- RAAS
renin‐angiotensin‐aldosterone system
- RAP
right atrial pressure
- RRT
renal replacement therapy
- Rx
treatment
- SCVO2
central venous oxygen saturation
- SNS
sympathetic nervous system
- SVV
stroke volume variation
CASE
You are performing an inpatient consult for a 55‐year‐old man with nonalcoholic steatohepatitis cirrhosis who is admitted to the hospital for hepatic encephalopathy, which has since resolved. Although he is asymptomatic, the primary service is concerned about the patient’s low blood pressure of 96/40 mm Hg and would like your input on whether he can be safely discharged with the low blood pressure.
INTRODUCTION AND PATHOPHYSIOLOGY OF BASELINE HYPOTENSION IN CIRRHOSIS
Circulatory and cardiac compromise in cirrhosis has been well studied. The primary pathophysiology stems from portal hypertension, which is induced from an increased resistance to flow secondary to distorted sinusoidal architecture and is further sustained from an increase in portal venous flow.1 Portal hypertension induces both progressive splanchnic and systemic vasodilation mediated via nitric oxide and other vasoactive molecules secondary to endothelial stretching and sheer stress.2 A hyperdynamic state then ensues with an increase in cardiac output, increase in heart rate, and a low systemic vascular resistance secondary to systemic vasodilatation. The decreased effective arterial blood volume activates neurohormonal systems such as the renin‐angiotensin‐aldosterone system (RAAS), leading to further volume expansion via sodium and water retention. However, because of portosystemic shunting and splanchnic vasodilatation, effective central blood volume remains low and the hyperdynamic state continues, eventually leading to high‐output heart failure (Fig. 1). A lower mean arterial pressure (MAP) of 60 to 65 mm Hg is often tolerated in compensated and stable patients through these compensatory mechanisms, which allow end‐organ perfusion to be maintained.1, 2 Any degree of insult to this system can result in significant hypotension and decompensation.
Figure 1.
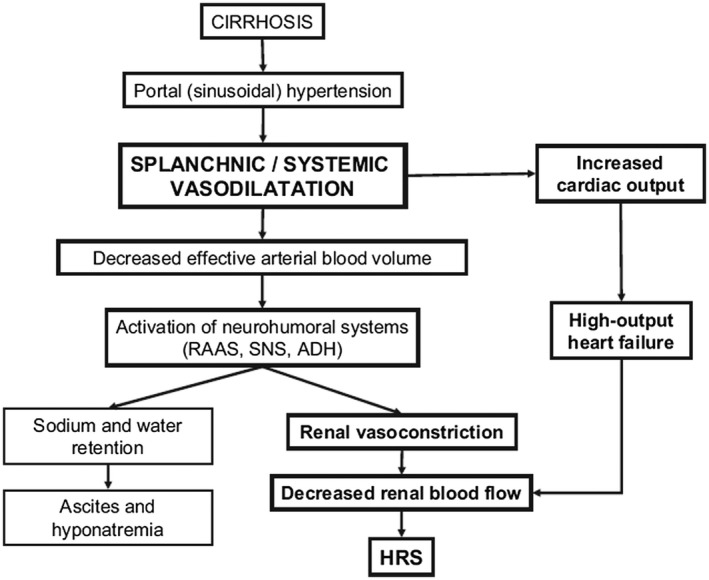
Pathophysiology of hypotension in cirrhosis.
The fragility of this system may not only be secondary to circulatory abnormalities but also due to underlying structural, electrical, and mechanical cardiac abnormalities termed cirrhotic cardiomyopathy. This condition can be seen in 40% to 50% of patients with cirrhosis, leading to reduced cardiac contractility with systolic and diastolic dysfunction, and electrophysiological abnormalities such as prolongation of the QT interval.3 As opposed to patients with cirrhosis without cardiomyopathy in whom cardiac output is increased, in patients with cirrhosis with cardiomyopathy there is a blunted cardiac response to the activated neurohormonal compensatory mechanisms secondary to impaired cardiac function, leading to a further reduction in MAP.4 Thus, cirrhotic cardiomyopathy further impairs the ability of the patient to tolerate any alteration to his or her hemodynamics. It is prudent to recognize the development of new‐onset hypotension in cirrhosis, because this signals the presence of an insult that has the capability to induce multiorgan involvement and acute on chronic liver failure.
ETIOLOGIES AND PATHOPHYSIOLOGY OF NEW‐ONSET HYPOTENSION
A systematic approach in evaluating new‐onset hypotension includes classifying the different etiologies into these broad categories of shock: distributive, hypovolemic, and cardiogenic shock. Although adrenal insufficiency can be classified under distributive shock, recognizing adrenal insufficiency is paramount in patients with cirrhosis, so we will discuss this as a separate entity.
Distributive
Of the multiple etiologies that can precipitate distributive shock, infections are by far the most common in patients with cirrhosis. Patients are at risk for both bacterial and fungal infections largely as a result of dysbiosis, small bacterial overgrowth, bacterial translocation, an impaired immunity, and increased rate of organism colonization.5, 6 Both sepsis and systemic inflammation can result in further deterioration of liver function via activation of tumor necrosis factor α, interlukin‐6, and other markers of the innate immune system. These factors contribute to hepatocellular microcirculatory dysfunction, leading to worsening portal hypertension and reduced hepatic blood flow, which further worsens liver function.7
Hypovolemia
Hypervolemia is often seen in this population due to third spacing of volume, over‐diuresis, and variceal bleeding, a severe complication of portal hypertension. Hemorrhagic shock secondary to variceal bleeding is more likely to occur when the hepatic venous pressure gradient is more than 12 mm Hg. Over‐transfusion of volume in patients with hypotension should be done carefully because it can increase the portal pressures, and thus place patients at greater risk for variceal rupture.8
Cardiogenic
As discussed previously, patients with cirrhosis are at risk for development of cirrhotic cardiomyopathy, which includes decreased contractility, impaired relaxation, and electrophysiological abnormalities causing cardiac dysfunction. Signs and symptoms of this condition are not readily identifiable until changes in demand occur, that is, exercise, infection, hemorrhagic shock, transjugular portosystemic shunt, and transplantation. QT prolongation can be an early indicator of this condition, which when unmasked can further renal hypoperfusion and lead to the development of hepatorenal syndrome (HRS).9
Adrenal Insufficiency
Adrenal insufficiency shares similar hemodynamic features with those that occur in cirrhosis and septic shock, namely, increased cardiac output, decreased peripheral vascular resistance, decreased MAP, and a blunted response to vasopressors.10 Although adrenal insufficiency is seen in conjecture with stressed states such as sepsis, patients with cirrhosis may have a relative adrenal insufficiency that is more pronounced as the disease severity worsens. Several mechanisms likely contribute to impaired adrenal function in cirrhosis. An increase in inflammatory markers such as cytokines has been shown to blunt the cortisol response to corticotropin. Impaired synthesis of adrenal steroid hormone and synthesis of cholesterol, a major precursor of steroid, in the liver may suppress the activation of the hypothalamic‐pituitary‐adrenal axis during periods of stress.10
OVERALL MANAGEMENT OF HYPOTENSION
The first step is recognition of new‐onset hypotension and responsiveness to volume challenge. In patients who are not responsive to a simple volume challenge, it is necessary to then systematically evaluate their volume status, with either invasive or noninvasive measures, and for an etiology of new‐onset hypotension. Transfer to an intensive care unit (ICU) is often necessary for further hemodynamic monitoring and treatment, and should be completed in a timely manner in patients who are showing signs of end‐organ dysfunction, such as encephalopathy, acute renal failure, and respiratory distress.
Assessment of volume status is difficult in patients with cirrhosis due to the presence of ascites and edema. In critically ill patients, arterial line catheters and central venous access are helpful to guide resuscitative efforts. Change in central venous pressure after resuscitation is more instructive than a single measurement alone.7 In addition, echocardiography is relatively inexpensive and can provide dynamic measurements of ventricular function and response to volume resuscitation.6 Other minimally invasive tools to assess hemodynamic parameters have gained popularity in the ICU, such as stroke volume variation and pulse pressure variation; however, they have limited utility in spontaneously breathing patients and furthermore have failed to demonstrate accuracy in patients with cirrhosis who are undergoing liver transplantation.1, 6
Endpoints of resuscitative efforts have not been well studied in patients with cirrhosis; however, a goal mean arterial blood pressure of 60 to 65 mm Hg is recommended in critically ill patients with cirrhosis with additional organ failures (i.e., septic shock) to ensure organ perfusion (Fig. 2).1 For patients who are volume depleted secondary to distributive shock, the use of crystalloid solutions (0.9% saline) at a rate of 10 to 20 mL/kg is recommended. Other balanced salt solutions are preferred in patients with hyperchloremic acidosis and those with relative hyperchloremia. If a volume overload state develops, fluids should be discontinued. Albumin should be used for specific indications such as in suspected type 1 HRS, acute kidney injury, after large‐volume paracentesis (>5 L), and in patients with confirmed or suspected bacterial peritonitis.6 In patients who are unresponsive to appropriate repletion of volume, the use of pharmacological management is indicated with norepinephrine as the first‐line vasopressor. Increasing pressor requirements should signal the use of stress‐dose steroids because adrenal insufficiency is common in critically ill patients with cirrhosis.1
Figure 2.
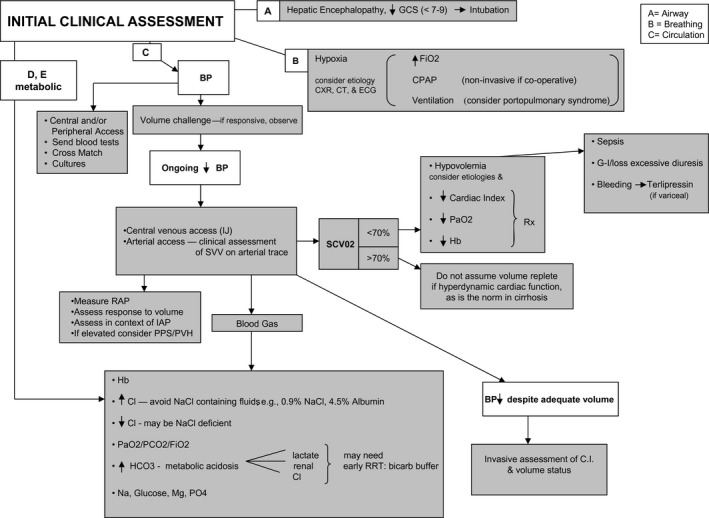
Management approaches for patients with cirrhosis who are admitted to the ICU. Reprinted with permission from Hepatology.7 Copyright 2011, American Association for the Study of Liver Diseases.
Specific management of insult leading to hypotension should be treated according to current practice guidelines. Broadly accepted management strategies of septic shock, such as tailoring antimicrobial therapy to a known pathogen after clinical improvement, apply to patients with cirrhosis as well. Because patients with cirrhosis are at higher risk for fungal infections, if there is no clinical improvement, empiric coverage with antifungal therapy and further evaluation for a source of infection with computed topography scans are indicated.1
CONCLUSION
As it pertains to the aforementioned case of a patient with cirrhosis who was admitted for hepatic encephalopathy, now with a low blood pressure of 96/40 mm Hg (MAP of 59 mm Hg), albeit asymptomatic, the next step should be to further investigate whether this is a change in his baseline blood pressure. Because he was admitted for an episode of hepatic encephalopathy, precipitating factors such as over‐diuresis, infection, and gastrointestinal bleeding should be considered in the differential because they can also lead to hypotension. Furthermore, a simple volume challenge with 0.9% saline can be undertaken to determine whether his blood pressure is responsive. If the initial workup is negative with no evidence of end‐organ damage, and he remains asymptomatic or responds to a volume challenge, he can be safely discharged home with close follow‐up. Discontinuation of antihypertensives, beta blockers, and diuretics should also be strongly considered.
Hypotension is not an uncommon occurrence in patients with cirrhosis and is to be expected secondary to the circulatory and cardiac compromise seen in this population. Compensatory mechanisms such as a hyperdynamic circulation and a neurohormonal response to low effective blood volume allow end‐organ perfusion to be maintained in the stable, compensated patient. Any protuberance to this fragile system can cause new‐onset hypotension and organ dysfunction; thus, it is imperative to both recognize and treat expeditiously. Of the multiple etiologies that can cause new‐onset hypotension, sepsis and hypovolemia are the most common. Initial management strategies include a volume challenge. If unresponsive or there is evidence of end‐organ injury, closer monitoring in an ICU with both invasive and noninvasive measurements is necessary. Treatment of the underlying etiology should commence with current practice guidelines.
Take‐Home Points
Hypotension is a well‐known complication in patients with cirrhosis, mainly stemming from portal hypertension, which leads to splanchnic and systemic vasodilatation.
Compensatory mechanisms allow adequate end‐organ perfusion in the stable, compensated patient.
Sepsis and hypovolemia are common etiologies of new‐onset hypotension and organ dysfunction, and thus should be promptly recognized and treated.
Initial management of new‐onset hypotension includes evaluation for an etiology and a volume challenge. In patients who are nonresponsive, further evaluation of volume status with noninvasive and invasive measures in an ICU setting may be necessary.
A goal mean arterial blood pressure of 60 mm Hg is recommended to ensure organ perfusion.
Pharmacological management is indicated in those who are unresponsive to appropriate repletion of volume.
Specific management of the insult leading to hypotension should be treated according to current practice guidelines.
Potential conflict of interest: Nothing to report.
References
- 1. Olson JC, Karvellas CJ. Critical care management of the patient with cirrhosis awaiting liver transplant in the intensive care unit. Liver Transpl 2017;23:1465‐1476. [DOI] [PubMed] [Google Scholar]
- 2. Iwakiri Y, Groszmann RJ. The hyperdynamic circulation of chronic liver diseases: from the patient to the molecule. Hepatology 2006;43:S121‐S131. [DOI] [PubMed] [Google Scholar]
- 3. Møller S, Hove JD, Dixen U, et al. New insights into cirrhotic cardiomyopathy. Int J Cardiol 2013;167:1101‐1108. [DOI] [PubMed] [Google Scholar]
- 4. Zardi EM, Abbate A, Zardi DM, et al. Cirrhotic cardiomyopathy. J Am Coll Cardiol 2010;56:539‐549. [DOI] [PubMed] [Google Scholar]
- 5. Bonnel AR, Bunchorntavakul C, Reddy KR. Immune dysfunction and infections in patients with cirrhosis. Clin Gastroenterol Hepatol 2011;9:727‐738. [DOI] [PubMed] [Google Scholar]
- 6. Nadim MK, Durand F, Kellum JA, et al. Management of the critically ill patient with cirrhosis: a multidisciplinary perspective. J Hepatol 2016;64:717‐735. [DOI] [PubMed] [Google Scholar]
- 7. Olson JC, Wendon JA, Kramer DJ, et al. Intensive care of the patient with cirrhosis. Hepatology 2011;54:1864‐1872. [DOI] [PubMed] [Google Scholar]
- 8. Ginès P, Fernández J, Durand F, et al. Management of critically‐ill cirrhotic patients. J Hepatol 2012;56(Suppl. 1):S13‐S24. [DOI] [PubMed] [Google Scholar]
- 9. Zardi EM, Zardi DM, Chin D, et al. Cirrhotic cardiomyopathy in the pre‐ and post‐liver transplantation phase. J Cardiol 2016;67:125‐130. [DOI] [PubMed] [Google Scholar]
- 10. Tsai MH, Peng YS, Chen YC, et al. Adrenal insufficiency in patients with cirrhosis, severe sepsis and septic shock. Hepatology 2006;43:673‐681. [DOI] [PubMed] [Google Scholar]


