ABSTRACT
Hepatitis A is a vaccine-preventable infection caused by the HA virus (HAV) with transitional to intermediate endemicity in China. An inactivated vaccine first licensed in China in 2010 (Avaxim® 80U Pediatric) is indicated for primary and booster vaccination in children from 12 months to 15 years of age. This Phase IV, open-label, single-arm trial supported licensure in pediatric age groups in China. A total of 355 healthy infants and toddlers (< 2 years of age), children (2 to 11 years of age), and adolescents (≥ 12 years of age) were enrolled to receive two doses of intramuscular HA vaccine, separated by 6 months. Participants were split into Group 1 (infants and toddlers: N = 270) and Group 2 (children and adolescents: N = 85). Safety was assessed by solicited injection site and systemic adverse events (AEs) for 7 days and unsolicited AEs for 30 days after each vaccination. Serious AEs (SAEs) were collected throughout. Immunogenicity was not assessed. Analyses were descriptive. Both vaccinations were very well tolerated in each group. The incidence of solicited injection site reactions was lower in Group 1 (17.9%) than Group 2 (33.3%) and for solicited systemic reactions was similar for each group. The incidence of unsolicited AEs in Group 1 was 6.3% and none in Group 2. For solicited and unsolicited AEs the incidence was slightly higher after the first vaccination. There were no SAEs. Overall, the good safety profile of this pediatric HA vaccine was confirmed in infants, toddlers, children, and adolescents aged 12 months to 15 years in China.
KEYWORDS: hepatitis A, inactivated vaccine, China, pediatric, safety
Introduction
Hepatitis A (HA) is a liver disease caused by the HA virus (HAV). In young children the infection is often asymptomatic but severity increases with age and over the age of 5 years HA is generally associated with symptoms such as fever, malaise, nausea, diarrhea, joint pain, abdominal pain, or vomiting being more common.1,2
The incidence of HA is closely related to hygiene and sanitation levels. China represents an area of transitional or intermediate HA endemicity3,4 although there are regional disparities in seroprevalence.5,6 Furthermore, an increasing adult population is susceptible to HA infection due to a reduction in HAV circulation over recent decades and associated lower rates of innate immunity resulting from lower rates of childhood infection.7
A range of vaccines is currently available globally that have been shown to provide effective and long-lasting protection against HA. Of these, the inactivated HA vaccine Avaxim® 80U Pediatric is formulated to include a reduced amount of active antigen (80 antigen units [U] per dose) and is indicated for primary and booster vaccination for children aged 12 months to 15 years.8,9 This vaccine has shown a good safety profile in more than 6200 children in clinical trials10,11,12,13 and Avaxim® 80U Pediatric vaccine was first licensed in China in February 2010. Even though the vaccine was licensed in China, it was not marketed in that country prior to the present study and so no post-marketing data were available from China. However, the national recommendations in China require that post-marketing safety data are submitted to support the renewal of the license. The present study was therefore conducted to generate safety data in China to support the renewal of the vaccine’s license for administration to toddlers, children, and adolescents aged 12 months to 15 years in a 2-dose schedule, as well as to collect further post-licensure data for its administration in these age groups in China. As immunogenicity data have recently been reported,8 this study focused only on vaccine safety.
Results
Participants studied
A total of 355 participants were enrolled on Day 1, of whom 353 participants received the first vaccination (2 participants withdrew consent after enrolment and prior to the first vaccination) (Group 1: N = 269; Group 2: N = 84). There were a further 21 participants who withdrew after the first vaccination and prior to the second vaccination (16 participants withdrew voluntarily [not due to an AE] and 5 participants were lost to follow-up), and 332 participants received the second vaccination (Group 1: N = 257; Group 2: N = 75). There were no further withdrawals between the second vaccination and safety follow-up 1 month later. There was no stratification by age group at enrolment and the number of participants in Group 1 was higher than Group 2 since hepatitis A vaccination is performed routinely at < 2 years of age in China. Participant disposition is presented in Figure 1.
Figure 1.
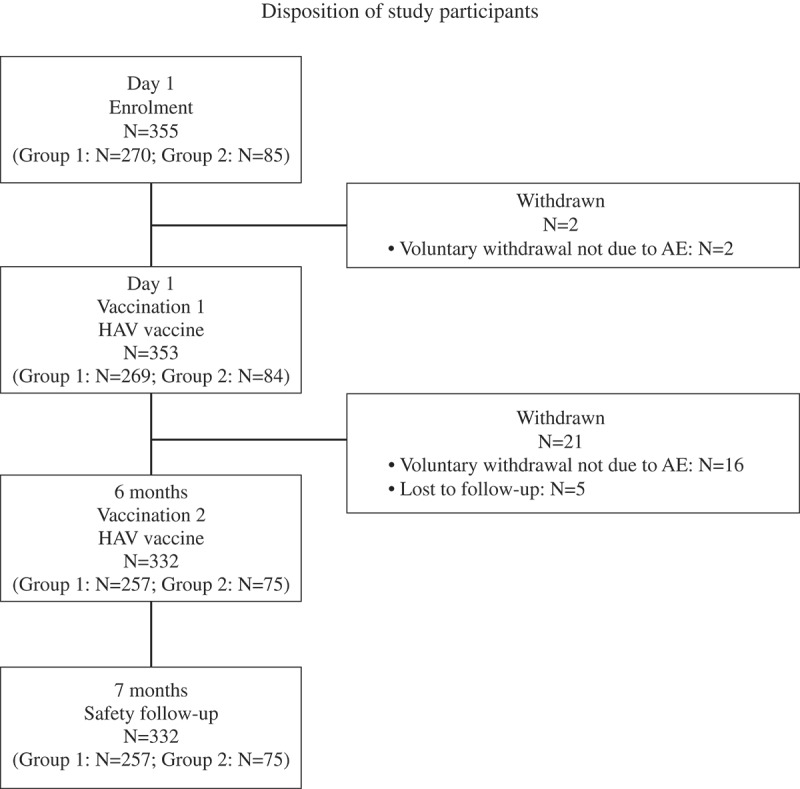
Group 1: infants and toddlers (<2 years) who received 2 injections of HAV vaccine; Group 2: children (aged 2 to 11 years) and adolescents (aged >12 years) who received 2 injections of HAV vaccine
Overall there was a similar number of male (51.5%) and female (48.5%) participants, although in Group 2 the incidence of males (58.5%) was slightly higher than for females (41.2%). The overall median age at inclusion was 1.6 years (medians of 1.5 years and 9.4 years in Groups 1 and 2, respectively). Participant demography is presented in Table 1. No health issues or immunity medications were reported.
Table 1.
Demographic characteristics at baseline (all participants).
| Group 1 (N = 270) | Group 2 (N = 85) | All participants (N = 355) | |
|---|---|---|---|
| Sex (n [%]) | |||
| Male | 133 (49.3) | 50 (58.8) | 183 (51.5) |
| Female | 137 (50.7) | 35 (41.2) | 172 (48.5) |
| Sex ratio: Male/Female | 0.97 | 1.43 | 1.06 |
| Age at baseline (years) | |||
| Mean (SD) | 1.6 (0.1) | 9.8 (3.4) | 3.5 (3.9) |
| Minimum; Maximum | 1.2; 1.9 | 2.9; 15.0 | 1.2; 15.0 |
| Median | 1.5 | 9.4 | 1.6 |
| Q1; Q3 | 1.5; 1.6 | 6.8; 13.1 | 1.5; 1.9 |
N: number of participants in group
n: number of participants
SD: standard deviation
Q1; Q3: first quartile; third quartile
Group 1: infants and toddlers < 2 years) who received 2 injections of Avaxim® 80U Pediatric vaccine
Group 2: children (aged 2 to 11 years) and adolescents (aged > 12 years) who received 2 injections of Avaxim® 80U Pediatric vaccine
Safety and tolerability
One participant in Group 1 was the child of an employee of the study sponsor and was excluded from the safety analysis set; as such, in Groups 1 and 2, respectively, data from 268 and 84 participants were analyzed after the first vaccination and data from 256 and 75 participants were analyzed after the second vaccination (Tables 2–5).
Table 2.
Overview of safety for any vaccination and for the first and second vaccinations (safety analysis set).
| Group 1 |
Group 2 |
All participants |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vaccination | Participants experiencing at least one: | n/M | % | (95% CI) | n/M | % | (95% CI) | n/M | % | (95% CI) |
| Any | Immediate unsolicited systemic AE | 0/268 | 0.0 | (0.0; 1.4) | 0/84 | 0.0 | (0.0; 4.3) | 0/352 | 0.0 | (0.0; 1.0) |
| Immediate unsolicited systemic AR | 0/268 | 0.0 | (0.0; 1.4) | 0/84 | 0.0 | (0.0; 4.3) | 0/352 | 0.0 | (0.0; 1.0) | |
| Solicited reaction | 86/268 | 32.1 | (26.5; 38.0) | 34/84 | 40.5 | (29.9; 51.7) | 120/352 | 34.1 | (29.1; 39.3) | |
| Solicited injection site reaction | 46/268 | 17.2 | (12.8; 22.2) | 28/84 | 33.3 | (23.4; 44.5) | 74/352 | 21.0 | (16.9; 25.7) | |
| Solicited systemic reaction | 62/268 | 23.1 | (18.2; 28.7) | 21/84 | 25.0 | (16.2; 35.6) | 83/352 | 23.6 | (19.2; 28.4) | |
| Unsolicited AE | 17/268 | 6.3 | (3.7; 10.0) | 0/84 | 0.0 | (0.0; 4.3) | 17/352 | 4.8 | (2.8; 7.6) | |
| Unsolicited AR | 1/268 | 0.4 | (0.0; 2.1) | 0/84 | 0.0 | (0.0; 4.3) | 1/352 | 0.3 | (0.0; 1.6) | |
| Vaccination 1 | Immediate unsolicited systemic AE | 0/268 | 0.0 | (0.0; 1.4) | 0/84 | 0.0 | (0.0; 4.3) | 0/352 | 0.0 | (0.0; 1.0) |
| Immediate unsolicited systemic AR | 0/268 | 0.0 | (0.0; 1.4) | 0/84 | 0.0 | (0.0; 4.3) | 0/352 | 0.0 | (0.0; 1.0) | |
| Solicited reaction | 70/268 | 26.1 | (21.0; 31.8) | 28/84 | 33.3 | (23.4; 44.5) | 98/352 | 27.8 | (23.2; 32.8) | |
| Solicited injection site reaction | 32/268 | 11.9 | (8.3; 16.4) | 21/84 | 25.0 | (16.2; 35.6) | 53/352 | 15.1 | (11.5; 19.2) | |
| Solicited systemic reaction | 55/268 | 20.5 | (15.9; 25.9) | 15/84 | 17.9 | (10.4; 27.7) | 70/352 | 19.9 | (15.8; 24.4) | |
| Unsolicited AE | 16/268 | 6.0 | (3.5; 9.5) | 0/84 | 0.0 | (0.0; 4.3) | 16/352 | 4.5 | (2.6; 7.3) | |
| Unsolicited AR | 0/268 | 0.0 | (0.0; 1.4) | 0/84 | 0.0 | (0.0; 4.3) | 0/352 | 0.0 | (0.0; 1.0) | |
| Vaccination 2 | Immediate unsolicited systemic AE | 0/256 | 0.0 | (0.0; 1.4) | 0/75 | 0.0 | (0.0; 4.8) | 0/331 | 0.0 | (0.0; 1.1) |
| Immediate unsolicited systemic AR | 0/256 | 0.0 | (0.0; 1.4) | 0/75 | 0.0 | (0.0; 4.8) | 0/331 | 0.0 | (0.0; 1.1) | |
| Solicited reaction | 19/256 | 7.4 | (4.5; 11.3) | 16/75 | 21.3 | (12.7; 32.3) | 35/331 | 10.6 | (7.5; 14.4) | |
| Solicited injection site reaction | 15/256 | 5.9 | (3.3; 9.5) | 14/75 | 18.7 | (10.6; 29.3) | 29/331 | 8.8 | (5.9; 12.3) | |
| Solicited systemic reaction | 9/256 | 3.5 | (1.6; 6.6) | 8/75 | 10.7 | (4.7; 19.9) | 17/331 | 5.1 | (3.0; 8.1) | |
| Unsolicited AE | 1/256 | 0.4 | (0.0; 2.2) | 0/75 | 0.0 | (0.0; 4.8) | 1/331 | 0.3 | (0.0; 1.7) | |
| Unsolicited AR | 1/256 | 0.4 | (0.0; 2.2) | 0/75 | 0.0 | (0.0; 4.8) | 1/331 | 0.3 | (0.0; 1.7) | |
n: number of participants experiencing the endpoint; M: number of participants with available data
Severity scales based on CFDA scales for classification
Group 1: infants and toddlers < 2 years) who received 2 injections of Avaxim® 80U Pediatric vaccine
Group 2: children (aged 2 to 11 years) and adolescents (aged > 12 years) who received 2 injections of Avaxim® 80U Pediatric vaccine
Table 5.
Unsolicited adverse events occurring within 30 days for the first and second vaccinations by preferred term and system organ class (safety analysis set).
| Group 1 (N=268a) |
Group 2 (N=84a) |
All participants (N=352a) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vaccination | System Organ ClassPreferred Term | n | % | 95% CI | n AEs | n | % | 95% CI | n AEs | n | % | 95% CI | n AEs |
| Vaccination 1 | Gastrointestinal disorders | ||||||||||||
| Diarrhea | 2 | 0.7 | (0.1; 2.7) | 2 | 0 | 0.0 | (0.0; 4.3) | 0 | 2 | 0.6 | (0.1;2.0) | 2 | |
| General disorders and administration site conditions | |||||||||||||
| Pyrexia | 4 | 1.5 | (0.4; 3.8) | 4 | 0 | 0.0 | (0.0; 4.3) | 0 | 4 | 1.1 | (0.3; 2.9) | 4 | |
| Infections and infestations | |||||||||||||
| Nasopharyngitis | 7 | 2.6 | (1.1; 5.3) | 7 | 0 | 0.0 | (0.0; 4.3) | 0 | 7 | 2.0 | (0.8;4.1) | 7 | |
| Pneumonia | 1 | 0.4 | (0.0; 2.1) | 1 | 0 | 0.0 | (0.0; 4.3) | 0 | 1 | 0.3 | (0.0;1.6) | 1 | |
| Tonsillitis | 1 | 0.4 | (0.0; 2.1) | 1 | 0 | 0.0 | (0.0; 4.3) | 0 | 1 | 0.3 | (0.0;1.6) | 1 | |
| Respiratory, thoracic, and mediastinal disorders | |||||||||||||
| Cough | 1 | 0.4 | (0.0; 2.1) | 1 | 0 | 0.0 | (0.0; 4.3) | 0 | 1 | 0.3 | (0.0;1.6) | 1 | |
| Rhinorrhea | 1 | 0.4 | (0.0; 2.1) | 1 | 0 | 0.0 | (0.0; 4.3) | 0 | 1 | 0.3 | (0.0;1.6) | 1 | |
| Vaccination 2 | Skin and subcutaneous tissue disorders | ||||||||||||
| Rash | 1 | 0.4 | (0.0; 2.1) | 1 | 0 | 0.0 | (0.0; 4.3) | 0 | 1 | 0.3 | (0.0;1.6) | 1 | |
n: number of participants experiencing the endpoint; N: number of participants with available data
aN is for Vaccination 1; for Vaccination 2, N = 256, 75, and 331 for Group 1, Group 2, and All participants, respectively
Group 1: infants and toddlers < 2 years) who received 2 injections of Avaxim® 80U Pediatric vaccine
Group 2: children (aged 2 to 11 years) and adolescents (aged > 12 years) who received 2 injections of Avaxim® 80U Pediatric vaccine
Immediate unsolicited AEs (in the 30 minutes after either vaccination), solicited injection site and systemic ARs (in the 7 days after vaccination), and unsolicited AEs and ARs (in the 30 days after vaccination) are summarized in Table 2. There were no unsolicited immediate AEs in either group. The overall incidence of solicited injection site reactions was slightly lower in Group 1 (17.2%) than Group 2 (33.3%) and was similar in each group for solicited systemic reactions (23.1% and 25.0% in Group 1 and Group 2, respectively). There were no unsolicited AEs in Group 2, and the overall incidence in Group 1 was 6.3%, of which only 1 participant (0.4%) reported an AE (which occurred after the second vaccination) that was considered to be related to the vaccination (i.e. an AR); this was a Grade 1 systemic rash that occurred the day after the second vaccination and resolved with treatment.
The incidence of solicited ARs and unsolicited AEs was higher after the first vaccination than after the second vaccination. For solicited injection site ARs the respective incidence for each vaccination was 11.9% and 5.9% (Group 1) and 25.0% and 18.7% (Group 2); for solicited systemic ARs the respective incidence was 20.5% and 3.5% (Group 1) and 17.9% and 10.7% (Group 2); for unsolicited AEs and ARs in Group 1 the respective incidence was 6.0% and 0.4% (no unsolicited AEs were reported in Group 2).
Table 3 summarizes solicited injection site ARs by type and intensity (for any and Grade 3 intensities). In Group 1, injection site tenderness was the most frequently reported solicited injection site AR and was the only one to be reported after the second vaccination (11.2% and 5.9% after the first and second vaccinations, respectively). In Group 2, injection site pain was the most frequently reported solicited injection site AR and was the only one to be reported after the second vaccination (25.0% and 18.7% after the first and second vaccinations, respectively). Most solicited injection site ARs in each group were Grade 1 in intensity; all occurred between Day 0 and Day 3 and most lasted 0 to 3 days.
Table 3.
Solicited injection site reactions occurring within 7 days after any vaccination and for the first and second vaccinations.
| Group 1 |
Group 2 |
All participants |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Vaccination | Participants experiencing at least one: | Maximum intensity | n/M | % | (95% CI) | n/M | % | (95% CI) | n/M | % | (95% CI) |
| Any | Injection site tenderness | Any | 44/268 | 16.4 | (12.2; 21.4) | NA | NA | NA | 44/268 | 16.4 | (12.2; 21.4) |
| Grade 3 | 0/268 | 0.0 | (0.0; 1.4) | NA | NA | NA | 0/268 | 0.0 | (0.0; 1.4) | ||
| Injection site pain | Any | NA | NA | NA | 28/84 | 33.3 | (23.4; 44.5) | 28/84 | 33.3 | (23.4; 44.5) | |
| Grade 3 | NA | NA | NA | 0/84 | 0.0 | (0.0; 4.3) | 0/84 | 0.0 | (0.0; 4.3) | ||
| Injection site erythema | Any | 9/268 | 3.4 | (1.5; 6.3) | 3/84 | 3.6 | (0.7; 10.1) | 12/352 | 3.4 | (1.8; 5.9) | |
| Grade 3 | 0/268 | 0.0 | (0.0; 1.4) | 0/84 | 0.0 | (0.0; 4.3) | 0/352 | 0.0 | (0.0; 1.0) | ||
| Injection site swelling | Any | 5/268 | 1.9 | (0.6; 4.3) | 3/84 | 3.6 | (0.7; 10.1) | 8/352 | 2.3 | (1.0; 4.4) | |
| Grade 3 | 0/268 | 0.0 | (0.0; 1.4) | 1/84 | 1.2 | (0.0; 6.5) | 1/352 | 0.3 | (0.0; 1.6) | ||
| Vaccination 1 | Injection site tenderness | Any | 30/268 | 11.2 | (7.7; 15.6) | NA | NA | NA | 30/268 | 11.2 | (7.7; 15.6) |
| Grade 3 | 0/268 | 0.0 | (0.0; 1.4) | NA | NA | NA | 0/268 | 0.0 | (0.0; 1.4) | ||
| Injection site pain | Any | NA | NA | NA | 21/84 | 25.0 | (16.2; 35.6) | 21/84 | 25.0 | (16.2; 35.6) | |
| Grade 3 | NA | NA | NA | 0/84 | 0.0 | (0.0; 4.3) | 0/84 | 0.0 | (0.0; 4.3) | ||
| Injection site erythema | Any | 9/268 | 3.4 | (1.5; 6.3) | 3/84 | 3.6 | (0.7; 10.1) | 12/352 | 3.4 | (1.8; 5.9) | |
| Grade 3 | 0/268 | 0.0 | (0.0; 1.4) | 0/84 | 0.0 | (0.0; 4.3) | 0/352 | 0.0 | (0.0; 1.0) | ||
| Injection site swelling | Any | 5/268 | 1.9 | (0.6; 4.3) | 3/84 | 3.6 | (0.7; 10.1) | 8/352 | 2.3 | (1.0; 4.4) | |
| Grade 3 | 0/268 | 0.0 | (0.0; 1.4) | 1/84 | 1.2 | (0.0; 6.5) | 1/352 | 0.3 | (0.0; 1.6) | ||
| Vaccination 2 | Injection site tenderness | Any | 15/256 | 5.9 | (3.3; 9.5) | NA | NA | NA | 15/256 | 5.9 | (3.3; 9.5) |
| Grade 3 | 0/256 | 0.0 | (0.0; 1.4) | NA | NA | NA | 0/256 | 0.0 | (0.0; 1.4) | ||
| Injection site pain | Any | NA | NA | NA | 14/75 | 18.7 | (10.6; 29.3) | 14/75 | 18.7 | (10.6; 29.3) | |
| Grade 3 | NA | NA | NA | 0/75 | 0.0 | (0.0; 4.8) | 0/75 | 0.0 | (0.0; 4.8) | ||
| Injection site erythema | Any | 0/256 | 0.0 | (0.0; 1.4) | 0/75 | 0.0 | (0.0; 4.8) | 0/331 | 0.0 | (0.0; 1.1) | |
| Grade 3 | 0/256 | 0.0 | (0.0; 1.4) | 0/75 | 0.0 | (0.0; 4.8) | 0/331 | 0.0 | (0.0; 1.1) | ||
| Injection site swelling | Any | 0/256 | 0.0 | (0.0; 1.4) | 0/75 | 0.0 | (0.0; 4.8) | 0/331 | 0.0 | (0.0; 1.1) | |
| Grade 3 | 0/256 | 0.0 | (0.0; 1.4) | 0/75 | 0.0 | (0.0; 4.8) | 0/331 | 0.0 | (0.0; 1.1) | ||
n: number of participants experiencing the endpoint; M: number of participants with available data
NA = not applicable (symptom not recorded based on age range)
Severity scales based on CFDA scales for classification
Group 1: infants and toddlers < 2 years) who received 2 injections of Avaxim® 80U Pediatric vaccine
Group 2: children (aged 2 to 11 years) and adolescents (aged > 12 years) who received 2 injections of Avaxim® 80U Pediatric vaccine
Table 4 summarizes solicited systemic ARs by type and intensity (for any and Grade 3 intensities). Following the first vaccination, fever was the most frequently reported in Group 1 (11.9%) and malaise was the most frequently reported in Group 2 (10.7%). The only solicited systemic AR reported after the second vaccination was a single episode of fever in Group 1. In both groups, most episodes were Grade 1 in intensity; most occurred between Day 0 and Day 3 and lasted 0 to 3 days.
Table 4.
Solicited systemic reactions occurring within 7 days after any vaccination and for the first and second vaccinations (safety analysis set).
| Group 1 |
Group 2 |
All participants |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Vaccination | Participants experiencing at least one: | Maximum intensity | n/M | % | (95% CI) | n/M | % | (95% CI) | n/M | % | (95% CI) |
| Any | Fever | Any | 34/268 | 12.7 | (8.9; 17.3) | 2/84 | 2.4 | (0.3; 8.3) | 36/352 | 10.2 | (7.3; 13.9) |
| Grade 3 | 4/268 | 1.5 | (0.4; 3.8) | 0/84 | 0.0 | (0.0; 4.3) | 4/352 | 1.1 | (0.3; 2.9) | ||
| Vomiting | Any | 9/268 | 3.4 | (1.5; 6.3) | NA | NA | NA | 9/268 | 3.4 | (1.5; 6.3) | |
| Grade 3 | 1/268 | 0.4 | (0.0; 2.1) | NA | NA | NA | 1/268 | 0.4 | (0.0; 2.1) | ||
| Crying abnormal | Any | 22/268 | 8.2 | (5.2; 12.2) | NA | NA | NA | 22/268 | 8.2 | (5.2; 12.2) | |
| Grade 3 | 1/268 | 0.4 | (0.0; 2.1) | NA | NA | NA | 1/268 | 0.4 | (0.0; 2.1) | ||
| Drowsiness | Any | 19/268 | 7.1 | (4.3; 10.8) | NA | NA | NA | 19/268 | 7.1 | (4.3; 10.8) | |
| Grade 3 | 0/268 | 0.0 | (0.0; 1.4) | NA | NA | NA | 0/268 | 0.0 | (0.0; 1.4) | ||
| Appetite lost | Any | 22/268 | 8.2 | (5.2; 12.2) | NA | NA | NA | 22/268 | 8.2 | (5.2; 12.2) | |
| Grade 3 | 2/268 | 0.7 | (0.1; 2.7) | NA | NA | NA | 2/268 | 0.7 | (0.1; 2.7) | ||
| Irritability | Any | 27/268 | 10.1 | (6.7; 14.3) | NA | NA | NA | 27/268 | 10.1 | (6.7; 14.3) | |
| Grade 3 | 1/268 | 0.4 | (0.0; 2.1) | NA | NA | NA | 1/268 | 0.4 | (0.0; 2.1) | ||
| Headache | Any | NA | NA | NA | 7/84 | 8.3 | (3.4; 16.4) | 7/84 | 8.3 | (3.4; 16.4) | |
| Grade 3 | NA | NA | NA | 0/84 | 0.0 | (0.0; 4.3) | 0/84 | 0.0 | (0.0; 4.3) | ||
| Malaise | Any | NA | NA | NA | 13/84 | 15.5 | (8.5; 25.0) | 13/84 | 15.5 | (8.5; 25.0) | |
| Grade 3 | NA | NA | NA | 0/84 | 0.0 | (0.0; 4.3) | 0/84 | 0.0 | (0.0; 4.3) | ||
| Myalgia | Any | NA | NA | NA | 13/84 | 15.5 | (8.5; 25.0) | 13/84 | 15.5 | (8.5; 25.0) | |
| Grade 3 | NA | NA | NA | 0/84 | 0.0 | (0.0; 4.3) | 0/84 | 0.0 | (0.0; 4.3) | ||
| Vaccination 1 | Fever | Any | 32/268 | 11.9 | (8.3; 16.4) | 2/84 | 2.4 | (0.3; 8.3) | 34/352 | 9.7 | (6.8; 13.2) |
| Grade 3 | 4/268 | 1.5 | (0.4; 3.8) | 0/84 | 0.0 | (0.0; 4.3) | 4/352 | 1.1 | (0.3; 2.9) | ||
| Vomiting | Any | 7/268 | 2.6 | (1.1; 5.3) | NA | NA | NA | 7/268 | 2.6 | (1.1; 5.3) | |
| Grade 3 | 1/268 | 0.4 | (0.0; 2.1) | NA | NA | NA | 1/268 | 0.4 | (0.0; 2.1) | ||
| Crying abnormal | Any | 22/268 | 8.2 | (5.2; 12.2) | NA | NA | NA | 22/268 | 8.2 | (5.2; 12.2) | |
| Grade 3 | 1/268 | 0.4 | (0.0; 2.1) | NA | NA | NA | 1/268 | 0.4 | (0.0; 2.1) | ||
| Drowsiness | Any | 17/268 | 6.3 | (3.7; 10.0) | NA | NA | NA | 17/268 | 6.3 | (3.7; 10.0) | |
| Grade 3 | 0/268 | 0.0 | (0.0; 1.4) | NA | NA | NA | 0/268 | 0.0 | (0.0; 1.4) | ||
| Appetite lost | Any | 21/268 | 7.8 | (4.9; 11.7) | NA | NA | NA | 21/268 | 7.8 | (4.9; 11.7) | |
| Grade 3 | 2/268 | 0.7 | (0.1; 2.7) | NA | NA | NA | 2/268 | 0.7 | (0.1; 2.7) | ||
| Irritability | Any | 25/268 | 9.3 | (6.1; 13.5) | NA | NA | NA | 25/268 | 9.3 | (6.1; 13.5) | |
| Grade 3 | 1/268 | 0.4 | (0.0; 2.1) | NA | NA | NA | 1/268 | 0.4 | (0.0; 2.1) | ||
| Headache | Any | NA | NA | NA | 5/84 | 6.0 | (2.0; 13.3) | 5/84 | 6.0 | (2.0; 13.3) | |
| Grade 3 | NA | NA | NA | 0/84 | 0.0 | (0.0; 4.3) | 0/84 | 0.0 | (0.0; 4.3) | ||
| Malaise | Any | NA | NA | NA | 9/84 | 10.7 | (5.0; 19.4) | 9/84 | 10.7 | (5.0; 19.4) | |
| Grade 3 | NA | NA | NA | 0/84 | 0.0 | (0.0; 4.3) | 0/84 | 0.0 | (0.0; 4.3) | ||
| Myalgia | Any | NA | NA | NA | 7/84 | 8.3 | (3.4; 16.4) | 7/84 | 8.3 | (3.4; 16.4) | |
| Grade 3 | NA | NA | NA | 0/84 | 0.0 | (0.0; 4.3) | 0/84 | 0.0 | (0.0; 4.3) | ||
| Vaccination 2 | Fever | Any | 3/256 | 1.2 | (0.2; 3.4) | 0/75 | 0.0 | (0.0; 4.8) | 3/331 | 0.9 | (0.2; 2.6) |
| Grade 3 | 0/256 | 0.0 | (0.0; 1.4) | 0/75 | 0.0 | (0.0; 4.8) | 0/331 | 0.0 | (0.0; 1.1) | ||
| Vomiting | Any | 2/256 | 0.8 | (0.1; 2.8) | NA | NA | NA | 2/256 | 0.8 | (0.1; 2.8) | |
| Grade 3 | 0/256 | 0.0 | (0.0; 1.4) | NA | NA | NA | 0/256 | 0.0 | (0.0; 1.4) | ||
| Crying abnormal | Any | 1/256 | 0.4 | (0.0; 2.2) | NA | NA | NA | 1/256 | 0.4 | (0.0; 2.2) | |
| Grade 3 | 0/256 | 0.0 | (0.0; 1.4) | NA | NA | NA | 0/256 | 0.0 | (0.0; 1.4) | ||
| Drowsiness | Any | 2/256 | 0.8 | (0.1; 2.8) | NA | NA | NA | 2/256 | 0.8 | (0.1; 2.8) | |
| Grade 3 | 0/256 | 0.0 | (0.0; 1.4) | NA | NA | NA | 0/256 | 0.0 | (0.0; 1.4) | ||
| Appetite lost | Any | 1/256 | 0.4 | (0.0; 2.2) | NA | NA | NA | 1/256 | 0.4 | (0.0; 2.2) | |
| Grade 3 | 0/256 | 0.0 | (0.0; 1.4) | NA | NA | NA | 0/256 | 0.0 | (0.0; 1.4) | ||
| Irritability | Any | 2/256 | 0.8 | (0.1; 2.8) | NA | NA | NA | 2/256 | 0.8 | (0.1; 2.8) | |
| Grade 3 | 0/256 | 0.0 | (0.0; 1.4) | NA | NA | NA | 0/256 | 0.0 | (0.0; 1.4) | ||
| Headache | Any | NA | NA | NA | 2/75 | 2.7 | (0.3; 9.3) | 2/75 | 2.7 | (0.3; 9.3) | |
| Grade 3 | NA | NA | NA | 0/75 | 0.0 | (0.0; 4.8) | 0/75 | 0.0 | (0.0; 4.8) | ||
| Malaise | Any | NA | NA | NA | 4/75 | 5.3 | (1.5; 13.1) | 4/75 | 5.3 | (1.5; 13.1) | |
| Grade 3 | NA | NA | NA | 0/75 | 0.0 | (0.0; 4.8) | 0/75 | 0.0 | (0.0; 4.8) | ||
| Myalgia | Any | NA | NA | NA | 6/75 | 8.0 | (3.0; 16.6) | 6/75 | 8.0 | (3.0; 16.6) | |
| Grade 3 | NA | NA | NA | 0/75 | 0.0 | (0.0; 4.8) | 0/75 | 0.0 | (0.0; 4.8) | ||
n: number of participants experiencing the endpoint; M: number of participants with available data
NA = not applicable (symptom not recorded based on age range)
Severity scales based on CFDA scales for classification
Group 1: infants and toddlers < 2 years) who received 2 injections of Avaxim® 80U Pediatric vaccine
Group 2: children (aged 2 to 11 years) and adolescents (aged > 12 years) who received 2 injections of Avaxim® 80U Pediatric vaccine
Unsolicited AEs are presented by Preferred Term (PT) and System Organ Class (SOC) in Table 5. The overall incidence of unsolicited AEs was low and the most frequently reported was nasopharyngitis (2.6%) (Group 1); none was reported in Group 2 and there were no SAEs in either group.
The sub-analysis that excluded data from 22 subjects is presented as supplementary material. The results of the sub-analysis were comparable to those of the full analysis described above.
Discussion
This clinical study was conducted primarily to support the renewal of licensure for Avaxim® 80U Pediatric in a two-dose (complete) vaccination schedule for infants, toddlers, children, and adolescents aged 12 months to 15 years residing in China. As such, per the requirements in China and since immunogenicity data are reported elsewhere,8 only safety was evaluated. The vaccine was very well tolerated, as expected from previous studies with the same vaccine.8 There were no unsolicited AEs reported in the 30 minutes after either vaccination, and the overall incidence of solicited injection site and systemic ARs and of unsolicited AEs was lower than in previous studies using the same vaccine in this population.8 This difference was particularly apparent for children and adolescents. The incidence of both solicited and unsolicited AEs following the first vaccination was slightly higher than after the second vaccination; this is as expected based on previous clinical studies using a two-dose regimen.10,11,12,13
A limitation of the study was a potential problem regarding the recording of information in the diary cards. During routine study monitoring, it was observed that the diary cards for 22 participants were completed by the investigator retrospectively after following up with the participant. As diary cards should have been completed by the study participant, this was raised as a potential GCP non-compliance concern and the appropriate ethics committee was notified. Despite the resolution of this potential issue, a sub-analysis was performed excluding these data and is presented as supplementary material. This sub-analysis showed a good safety profile for the Avaxim® 80U Pediatric vaccine, supporting the findings from the overall analysis. A further potential limitation is that most participants were < 2 years of age (i.e. Group 1) although this reflects routine practice for hepatitis A vaccination in China.
Overall, this study confirmed the good safety profile of the Avaxim® 80U Pediatric vaccine, administered in a two-dose schedule 6 months apart to infants, toddlers, children, and adolescents aged 12 months to 15 years in China, supporting its continued licensure.
Patients and methods
Study design and participants
This was a Phase IV, open-label, single-arm clinical trial conducted in one site in China (WHO Universal Trial Number: U1111-1127–7652).14 Before the inclusion of the first study participant, an Independent Ethics Committee (IEC) reviewed and approved the study protocol; one protocol amendment (to document the removal of an interim analysis) was approved by the IEC during the study. The study was consistent with the standards established by the Declaration of Helsinki and complied with the International Conference on Harmonization guidelines for Good Clinical Practice, as well as with all local and national regulations and directives. Informed consent was obtained either from the study participant and his/her parent(s) or legally acceptable representative (for participants aged 12 years and above), or from the parent(s) or legally acceptable representative alone (for participants aged less than 12 years). If the participant’s mother and father were, respectively, less than 20 and 22 years of age, the participant’s grandparent(s) were required to sign the informed consent form (ICF). A literate impartial witness also signed the ICF if the parent(s), grandparent(s), or legally acceptable representative were illiterate, to attest that the information contained in the ICF had been accurately explained and understood. The study took place between December 2013 and October 2014.
Healthy participants aged 12 months to 15 years 5 months, inclusive, at the start of the study were eligible for inclusion (those aged < 2 years were born at full term (> 37 weeks) and/or had a birth weight of ≥ 2.5 kg). Participants aged 12 to 23 months, inclusive were classed as infants and toddlers, 2 to 11 years were children, and 12 to 15 years were adolescents.
The main exclusion criteria were: current or planned participation in another clinical study in the 4 weeks prior to the first study vaccination until the end of the study or non-study vaccination in the 4 weeks preceding or following any study vaccination; previous vaccination against HA; history of HAV infection or at high risk of HAV infection; receipt of blood products in the 3 months prior to the start of the study or any immune-modifying treatment in the 6 months prior to the start of the study or for more than two consecutive weeks in three months prior to the start of the study; known hypersensitivity to any component of Avaxim® 80U Pediatric vaccine; history of seizures; bleeding disorder contraindicating intramuscular (IM) injection; chronic or acute illness that could interfere with study conduct or completion; an employee of the study site or an immediate family member of any non-participant directly involved in the study; for post-menarche females, pregnant, lactating, or of child-bearing potential.
Enrolled participants were to receive two doses of Avaxim® 80U Pediatric vaccine administered 6 months apart and by the intramuscular route into the deltoid region of the upper arm. As this was a safety study, no blood samples for immunogenicity evaluation were taken, and the duration of the study for each participant was to be approximately 7 months.
Vaccine
The vaccine used was an inactivated, absorbed HA virus vaccine (Avaxim 80U Pediatric; batch number J0279-1), supplied in pre-filled, 0.5 mL-dose syringes. Each 0.5 mL dose contained 80 U inactivated HA virus, 0.15 mg aluminum hydroxide, 2.5 µL 2-phenoxyethanol, 12.5 µg formaldehyde, and ≤ 0.5 mL water for injection.
Reactogenicity and safety
Participants were kept under observation at the study site for 30 minutes after each vaccination to record any immediate unsolicited systemic adverse events (AEs). For 7 days after each vaccination, the participant and/or the parent(s) or legal representative recorded the intensity and duration of pre-defined (solicited) injection site (tenderness [for participants aged < 2 years] or pain [for participants aged ≥ 2 years], erythema, and swelling) and systemic (fever, vomiting, crying abnormal, drowsiness, appetite lost, and irritability for participants aged < 2 years; fever, headache, malaise, and myalgia for participants aged ≥ 2 years) reactions using diary cards. All immediate and solicited (injection site and systemic) adverse events were considered by definition to be related to the vaccination, and referred to as adverse reactions (ARs). The axillary route was used for measurement of body temperature.
The China Food and Drug Administration (CFDA) scales were used for the assessment of intensity for local reactions of erythema and injection site swelling, and systemic reactions of fever and vomiting. The definitions of intensity for all solicited reactions for infants/toddlers, children, and adolescents are as follows:
For infants and toddlers (aged below 2 years) Grade 1, 2, and 3 injection site tenderness were defined as ‘minor reaction when injection site is touched’, ‘cries or protests when injection site is touched’, and ‘cries when injected limb is moved or the movement of the injected limb is reduced’; Grade 1, 2, and 3 vomiting were 1, 2–5, and ≥ 6 episodes (or requiring parenteral hydration) per 24 hours; Grade 1, 2, and 3 crying abnormal were < 1 hour, 1–3 hours, and > 3 hours; Grade 1, 2, and 3 drowsiness were sleepier than usual or less interested in surroundings, not interested in surroundings or did not wake up for a meal, and sleeping most of the time or difficult to wake up; Grade 1, 2, and 3 appetite lost were eating less than normal, missed 1 to 2 meals, refused ≥ 3/most meals; and Grade 1, 2, and 3 irritability were easily consolable, requiring increased attention, inconsolable.
For children (aged 2 to 11 years): Grade 1, 2, and 3 pain were defined as ‘easily tolerated’, ‘sufficiently discomforting to interfere with normal behavior or activities’, and ‘incapacitating, unable to perform usual activities’.
For adolescents (aged ≥ 12 years): Grade 1, 2, and 3 pain were defined as ‘no interference with activity’, ‘some interference with activity’, and ‘significant, prevents daily activity’.
For children (aged 2 to 11 years) and adolescents (aged ≥ 12 years): Grade 1, 2, and 3 headache, malaise, and myalgia were defined as ‘no interference with activity’, ‘some interference with activity’, and ‘significant, prevents daily activity’.
For all ages: Grade 1, 2, and 3 erythema and swelling were defined as a diameter of < 15 mm, 15 to 30 mm, > 30 mm; Grade 1, 2, and 3 fever were temperature ≥ 37.1°C to 37.5°C, > 37.5°C to 39.0°C, and > 39.0°C.
Unsolicited AEs were recorded using diary cards for 30 days after each vaccination. Unsolicited injection site AEs were considered to be related to the vaccination (as for solicited injection site and systemic AEs). For unsolicited systemic AEs the Investigator assessed the relationship to the vaccination. Serious adverse events (SAEs) were collected throughout the study and until 30 days after the second vaccination; their relationship to the vaccination was assessed by the Investigator.
Statistical analyses
The study objective was to describe the safety of the Avaxim® 80U Pediatric vaccine after each dose, and the data were analysed overall and for two separate age groups: Group 1, infants and toddlers (< 2 years of age); Group 2, children (2–11 years of age) and adolescents (≥ 12 years).
No statistical hypotheses were tested and all analyses were descriptive. The number and percentage (with corresponding 95% CI) of participants with injection site or systemic AEs until 30 days following each injection (solicited injection site and systemic reactions from Day 0 to Day 7 and unsolicited AEs to Day 30). The 95% CIs of percentages were calculated using the exact binomial distribution according to the Clopper-Pearson method for proportions.15
According to Chinese Health Authorities’ requirements for safety surveillance, the planned sample size was 300 evaluable participants. Assuming that 15% of participants would not be evaluable, 353 participants were to be enrolled overall, with no stratification by age at enrolment. With 300 evaluable participants there was a 95% probability of observing an event that had a true incidence of at least 1.0% in the overall group of participants enrolled.
Only one population was defined for this study, the Safety Analysis Set, comprising all participants who received at least one dose of Avaxim® 80U Pediatric vaccine, and for the analysis of each dose comprising all participants who received that dose.
Due to a potential non-compliance issue relating to the management of diary cards (see Discussion for further detail) a sub-analysis excluding data from 22 participants was performed.
The statistical analysis was done under the responsibility of the study sponsor using SAS® software, at least Version 9.2 (SAS Institute, Cary, NC, USA).
Funding Statement
This study was supported by Sanofi Pasteur, Lyon, France
Acknowledgments
The authors would like to thank the study personnel for the conduct of the study and also all enrolled study participants, and parents/legal guardian(s) where applicable, for their participation. The authors also thank Sarah (Xioaling) Li (an employee of Sanofi Pasteur) for clinical project management.
This manuscript was prepared with the assistance of a professional medical writer, Dr Andrew Lane (Lane Medical Writing), in accordance with the European Medical Writers Association guidelines and Good Publication Practice.
Disclosure of potential conflicts of interest
NS did not receive any direct payment from Sanofi Pasteur with regard to his contribution to this manuscript. AR and YT are employees of Sanofi Pasteur.
References
- 1.Jeong SH, Lee HS, Hepatitis A.. clinical manifestations and management. Intervirology. 2010;53(1):15–19. doi: 10.1159/000252779. [DOI] [PubMed] [Google Scholar]
- 2.WHO Position paper on hepatitis A vaccines. Weekly Epidemiol Rec. 2012;87(28/29):261–276. [Google Scholar]
- 3.Jacobsen KH, Wiersma ST. Hepatitis A virus seroprevalence by age and world region, 1990 and 2005. Vaccine. 2010;28(41):6653–6657. doi: 10.1016/j.vaccine.2010.08.037. [DOI] [PubMed] [Google Scholar]
- 4.Mohd Hanafiah K, Jacobsen KH, Wiersma ST. Challenges to mapping the health risk of hepatitis A virus infection. Int J Health Geogr. 2011;10:57. doi: 10.1186/1476-072X-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO Meeting of the strategic advisory group of experts on immunization, November 2011 - conclusions and recommendations. Weekly Epidemiol Rec. 2012;87(1):1–16. [PubMed] [Google Scholar]
- 6.Zhuang GH, Pan XJ, Wang XL. A cost-effectiveness analysis of universal childhood hepatitis A vaccination in China. Vaccine. 2008;26(35):4608–4616. doi: 10.1016/j.vaccine.2008.05.086. [DOI] [PubMed] [Google Scholar]
- 7.Barzaga BN. Hepatitis A shifting epidemiology in South-East Asia and China. Vaccine. 2000;18(Suppl 1):S61–4. [DOI] [PubMed] [Google Scholar]
- 8.Li RC, Li Y, Yi N, Huang L, Wan Z, Zhang Y, Rasuli A. An open, prospective, randomized study comparing the immunogenicity and safety of two inactivated hepatitis A pediatric vaccines in toddlers, children and adolescents in China. Pediatr Infect Dis J. 2013;32(2):e77–81. https://www.ncbi.nlm.nih.gov/pubmed/23334341. [DOI] [PubMed] [Google Scholar]
- 9.Vidor E, Dumas R, Porteret V, Bailleux F, Veitch K. Aventis pasteur vaccines containing inactivated hepatitis A virus: a compilation of immunogenicity data. European J Clin Microbiol & Infect Dis. 2004;23(4):300–309. doi: 10.1007/s10096-003-1094-0. [DOI] [PubMed] [Google Scholar]
- 10.Abarca K, Ibanez I, Perret C, Vial P, Zinsou JA. Immunogenicity, safety, and interchangeability of two inactivated hepatitis A vaccines in Chilean children. Int J Infect Dis. 2008;12(3):270–277. doi: 10.1016/j.ijid.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Castillo de Febres O, Chacon de Petrola M, Casanova de Escalona L, Naveda O, Naveda M, Estopinan M, Bordones G, Zambrano B, Garcia A, Dumas R. Safety, immunogenicity and antibody persistence of an inactivated hepatitis A vaccine in 4 to 15 year old children. Vaccine. 1999;18(7–8):656–664. [DOI] [PubMed] [Google Scholar]
- 12.Dagan R, Greenberg D, Goldenbertg-Gehtman P, Vidor E, Briantais P, Pinsk V, Athias O, Dumas R. Safety and immunogenicity of a new formulation of an inactivated hepatitis A vaccine. Vaccine. 1999;17(15–16):1919–1925. [DOI] [PubMed] [Google Scholar]
- 13.Lopez EL, Del Carmen Xifro M, Torrado LE, De Rosa MF, Gomez R, Dumas R, Wood SC, Contrini MM. Safety and immunogenicity of a pediatric formulation of inactivated hepatitis A vaccine in Argentinean children. Pediatr Infect Dis J. 2001;20(1):48–52. [DOI] [PubMed] [Google Scholar]
- 14.EU Clinical Trials Register ECT Safety of two doses of avaxim® 80U pediatric (inactivated hepatitis A vaccine) administered 6 months apart in healthy toddlers, children and adolescents aged 12 months to 15 years in China 2016. [accessed 2018. September 24]. https://www.clinicaltrialsregister.eu/ctr-search/trial/2015-003190-14/results#trialInformationSection.
- 15.Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;17(8):857–872. [DOI] [PubMed] [Google Scholar]


