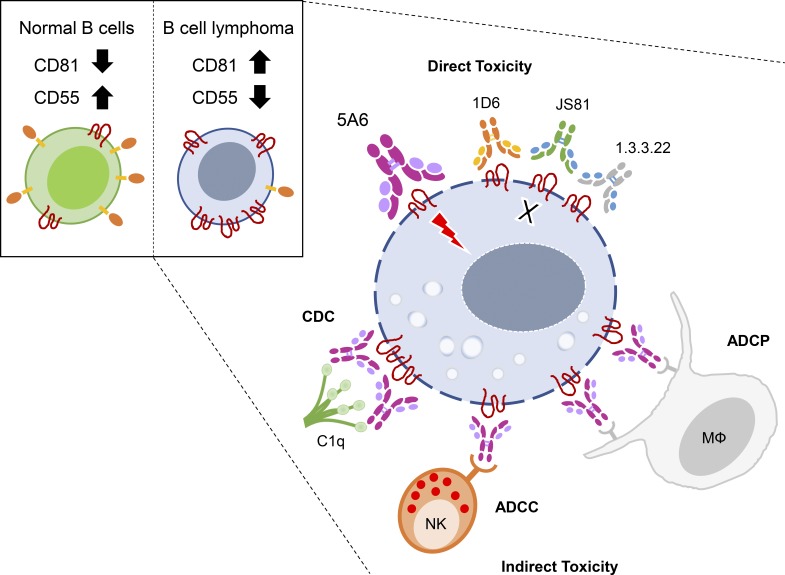
The anti-CD81 mAb (5A6) eliminates lymphoma tumor cells from patient follicular biopsy specimens while sparing the imbedded normal B and T lymphocytes. It has equivalent therapeutic effects as rituximab against a xenografted human B cell lymphoma.
Abstract
The tetraspanin CD81 was initially discovered by screening mAbs elicited against a human B cell lymphoma for their direct antiproliferative effects. We now show that 5A6, one of the mAbs that target CD81, has therapeutic potential. This antibody inhibits the growth of B cell lymphoma in a xenograft model as effectively as rituximab, which is a standard treatment for B cell lymphoma. Importantly, unlike rituximab, which depletes normal as well as malignant B cells, 5A6 selectively kills human lymphoma cells from fresh biopsy specimens while sparing the normal lymphoid cells in the tumor microenvironment. The 5A6 antibody showed a good safety profile when administered to a mouse transgenic for human CD81. Taken together, these data provide the rationale for the development of the 5A6 mAb and its humanized derivatives as a novel treatment against B cell lymphoma.
Graphical Abstract
Introduction
Rituximab, an antibody against CD20, was the first mAb approved by the US Food and Drug Administration for the treatment of cancer (Maloney et al., 1997). mAbs that target other tumor cell-surface proteins are now used to treat a variety of cancers (Scott et al., 2012). More recently, antibodies that target the immune system of the host rather than the cancer cells have become a mainstay of treatment for many types of solid tumors and for Hodgkin’s disease (Wei et al., 2018). Interestingly, these checkpoint antibodies have not shown similar efficacy against non-Hodgkin’s lymphomas (NHLs; Ansell et al., 2019). Rituximab remains part of the standard regimen for treatment of B cell lymphomas, alone or in combination with chemotherapy (Lim and Levy, 2014). This is the case even though rituximab targets normal as well as malignant B cells (Maloney et al., 1997). However, almost all patients with low-grade lymphoma relapse after treatment with rituximab and chemotherapy (Stolz and Schuler, 2009), and loss or reduced expression of CD20 has been reported in some relapsed patients (Hiraga et al., 2009; Stolz and Schuler, 2009). Additionally, almost half of the more aggressive types of lymphomas remain incurable (Feugier et al., 2005). Thus, there is a need for novel therapeutic agents in this disease.
The tetraspanin molecule CD81 plays an important role in B cell physiology. It is required for the intracellular trafficking of CD19 to the cell surface, where it forms the B cell coreceptor (CD19–CD21–CD81) complex that lowers the threshold of B cell activation via the B cell receptor (Bradbury et al., 1992; Maecker and Levy, 1997; Vences-Catalán et al., 2015a). Indeed, a homozygous crippling mutation in CD81 prevented intracellular trafficking of CD19 to the cell surface and was the cause of immune deficiency in a human patient (van Zelm et al., 2010). CD81 also has an important role in immuno-oncology. KO (Cd81KO) mice show a reduction in experimental tumor growth and metastases due to defects in their regulatory T cells and myeloid-derived suppressor cells (Vences-Catalán et al., 2015b). We originally discovered the CD81 protein as the target of a mAb (5A6) that inhibited the growth of human B lymphoma cell lines (Oren et al., 1990; Takahashi et al., 1990). These facts prompted us to interrogate the suitability of CD81 as a therapeutic target in B cell lymphoma. Here, we show that one particular anti-CD81 antibody, 5A6 (mouse IgG1), induces direct killing of human B lymphoma cell lines in vitro, and activates innate immune cytotoxic mechanisms in vivo. Moreover, a class-switched version of 5A6 (IgG2a) or a chimeric version containing the human IgG1 (HuIgG1) constant region were even better able to mediate indirect cytotoxicity against human lymphoma cell lines and patient-derived follicular lymphoma (FL) tumors. Surprisingly, in contrast to rituximab, which kills normal B cells, peripheral blood mononuclear cells (PBMCs) derived from healthy donors and normal B cells within the immune infiltrates of human FLs were less susceptible than the malignant B cells to killing by 5A6. Finally, the 5A6 mAb showed general safety in human CD81 transgenic mice. These lines of evidence provide the rationale for the development of 5A6 and its derivatives for the therapy of human lymphoma.
Results
Targeting human CD81 in a lymphoma xenograft model reduces tumor burden and improves survival
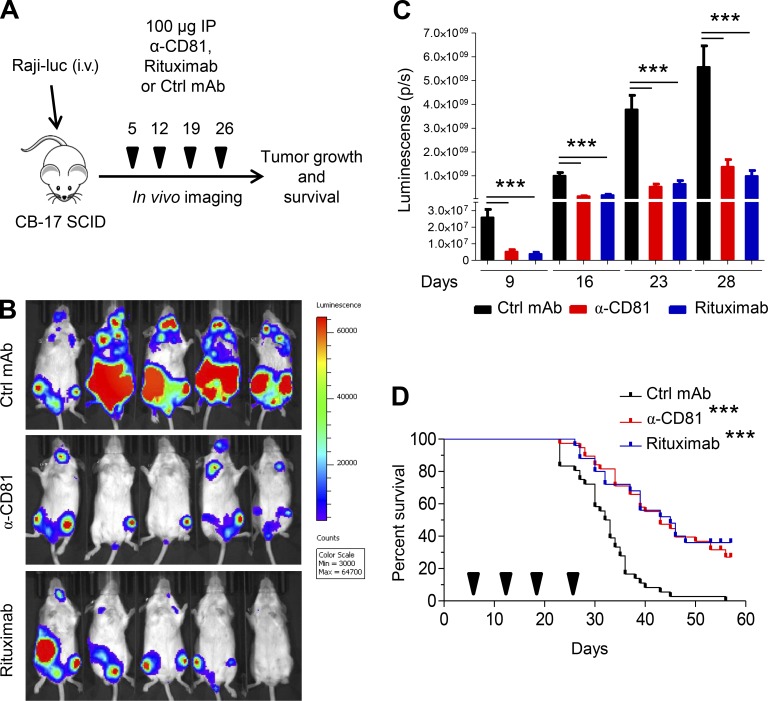
We set out to compare the 5A6 anti-human CD81 antibody to rituximab, the prototype B cell lymphoma therapeutic that is a current mainstay in the clinic. We employed a xenograft model in which SCID mice were injected i.v. with the human Raji-luciferase lymphoma cell line. To ensure tumor engraftment, mice were imaged on day 5 after tumor injection using an in vivo imaging system (IVIS), and then they were randomized to treatment groups (Fig. 1 A). Tumor load was monitored by IVIS 2–4 d after each treatment, and survival of mice was also recorded. The 5A6 antibody induced a major therapeutic effect in this model (Fig. 1, B and C) comparable to that of rituximab, both in retardation of tumor growth and in mouse survival. This was the case not only for the Raji tumor but also for SUP-B8, a cell line derived from a patient with diffuse large B cell lymphoma (Figs. 1 D and S1).
Figure 1.
Targeting human CD81 by 5A6 reduces growth of B cell lymphoma (Raji) and improves survival. (A) Treatment schedule. SCID-Beige mice were injected i.v. with 1.5 × 106 Raji-luciferase cells. On day 5 after tumor challenge, mice were treated i.p. with 100 µg of anti-human CD81 MsIgG1, control MsIgG1, or rituximab and then weekly thereafter for a total of four doses. (B) Representative in vivo bioluminescence imaging (IVIS) on day 23 after lymphoma injection. (C) Bioluminescence quantification (Living Image) at days 9, 16, 23, and 28. (D) Survival plots. Arrowheads denote days of treatment with each mAb (n = 35 control [Ctrl] MsIgG1, 35 anti-CD81 MsIgG1, and 25 rituximab; four independent experiments). Mice were sacrificed after they exhibited hindleg paralysis. Error bars represent mean ± SEM. ***, P < 0.0001; Student’s t test (C) or log-rank (Mantel–Cox) test (D).
The cytotoxic effect was restricted to the epitope recognized by 5A6 and not shared by other antibodies against CD81
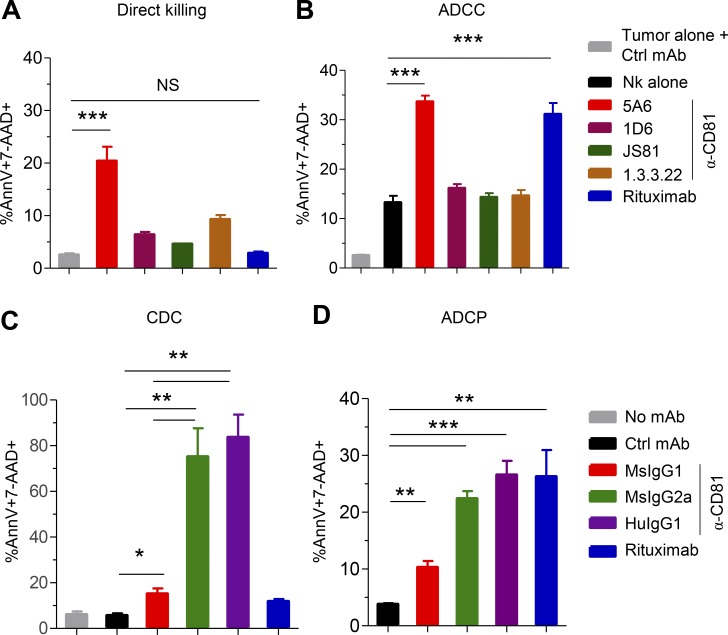
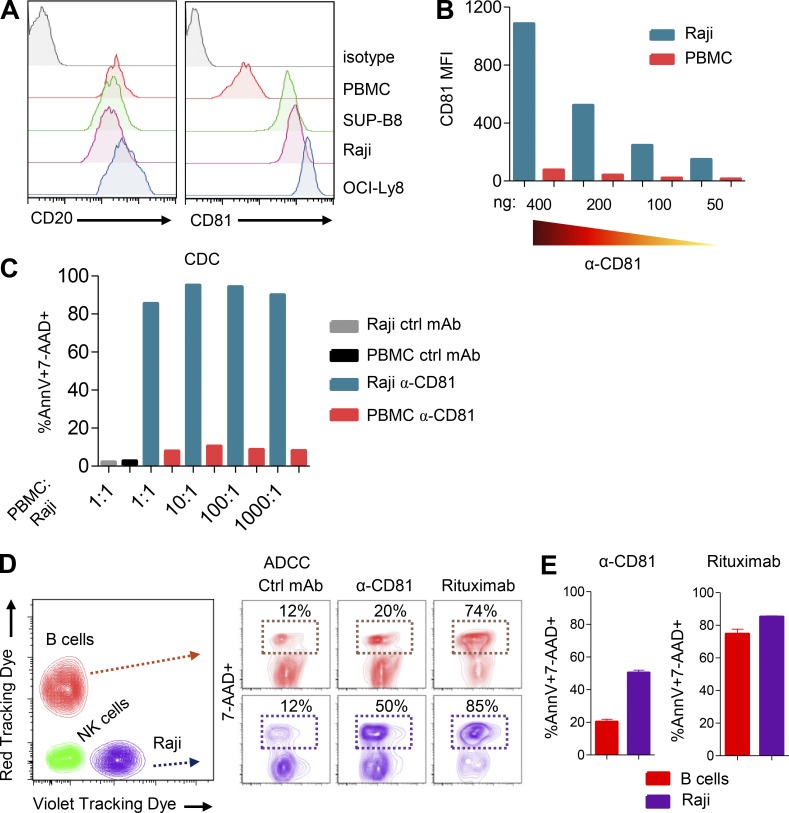
To determine the mechanisms by which 5A6 eliminated Raji cells in vivo, we performed an assay of direct cytotoxicity against the lymphoma tumor cells in vitro. We incubated Raji cells with 5A6 or rituximab for 24 h followed by enumeration of dead cells by flow cytometry as indicated by Annexin-V and 7-aminoactinomycin D (7-AAD). Interestingly, 5A6, but not rituximab, induced direct cell killing (Fig. 2 A) by activating caspase-3 and poly(ADP ribose) polymerase (PARP; Fig. S2 A). Next, we wondered if direct toxicity is a unique property of 5A6 or shared with other anti-human CD81 mAbs (1D6, JS81, or 1.3.3.22). Importantly, only 5A6 induced direct killing (Fig. 2 A). This unique property of 5A6 is likely due to its binding epitope that differs from the other anti-human CD81 mAbs. These antibodies cross-react with monkey CD81, whereas 5A6 binds weakly (Fig. S2 B; Higginbottom et al., 2000). In addition, 5A6 requires an intact 135VVD137 motif in the large extracellular loop of the CD81 molecule (Yalaoui et al., 2008).
Figure 2.
5A6 induces direct cytotoxicity, ADCC, CDC, and ADCP and is unique among anti-CD81 mAbs. (A and B) 106 Raji cells were incubated overnight with 1 µg/ml of mouse anti-CD81 (5A6), rituximab, or different anti-CD81 mAbs clones (5A6, 1D6, JS81, and 1.3.3.22, all MsIgG1 isotypes) in the absence (A) or presence (B) of purified human NK cells (5:1). Cell death was measured by Annexin-V and 7-AAD staining. Ctrl, control. (C) 106 Raji cells were incubated with 10 µg/ml of 5A6 (MsIgG1, MsIgG2a, or chimeric HuIgG1), rituximab, or control antibody for 1.5 h in the presence of fresh pooled human serum. (D) Raji cells labeled with pHrodo green AM (fluorescence increase in acidic pH denotes active phagocytosis) were opsonized for 15 min with the indicated mAbs. Cells were then incubated for 4 h at a 1:3 ratio with peritoneal mouse macrophages previously activated with 100 ng/ml of LPS for 24 h. Phagocytic events were defined as percentage of F4/80 and high MFI pHrodo green AM double-positive macrophages. All experiments were done in triplicate in at least two independent experiments. Error bars represent mean ± SEM. *, P < 0.0149; **, P < 0.0015; ***, P < 0.0001; Student’s t test (A–D).
Even though 5A6 showed direct cellular cytotoxicity, only 20% of Raji cells were killed in this assay (Fig. 2 A), suggesting additional mechanisms of tumor clearance in vivo. Antibodies used in immunotherapy such as rituximab can mediate antibody-dependent cell cytotoxicity (ADCC) against tumor cells (Clynes et al., 2000). We measured cell death of Raji or SUP-B8 cells incubated with purified human natural killer (NK) cells in the presence of the anti-human 5A6 mAbs or rituximab. 5A6, but not the other anti-CD81 mAbs, was as effective as rituximab in mediating ADCC (Figs. 2 B and S2 C).
Substituting constant regions in the 5A6 antibody enhance efficacy in vitro and in vivo
5A6 is an MsIgG1 isotype, whereas rituximab is a chimeric HuIgG1. To better compare their cytotoxic mechanisms, we generated a chimeric 5A6 with HuIgG1, as well as its corresponding homologue mouse IgG2a (MsIgG2a) isotype. We did this by cloning and expressing the 5A6 variable regions into an expression vector containing the human kappa and IgG1 constant region genes. Alternatively, we selected from the original MsIgG1-producing 5A6 hybridoma a rare class-switched variant cell producing the same antibody as MsIgG2a (Spira and Scharff, 1992).
MsIgG2a and HuIgG1 isotypes are strong mediators of complement-dependent cytotoxicity (CDC) and ADCC (Irani et al., 2015). Indeed, when Raji cells were incubated in the presence of fresh human serum and each of the individual mAbs, only 5A6 MsIgG2a and the chimeric HuIgG1 isotypes induced potent CDC-mediated killing (Fig. 2 C). By contrast, 5A6 MsIgG1 mediated only low levels of CDC, and rituximab was even lower in this ability (Fig. 2 C). Additional lymphoma cell lines, OCI-Ly8 and SUP-B8, were also sensitive to CDC mediated by 5A6 MsIgG2a and HuIgG1 (Fig. S2 D). We went on to test if 5A6 would mediate antibody-dependent cell phagocytosis (ADCP). We found that the original 5A6 MsIgG1 induced a low level of ADCP, but the MsIgG2a and HuIgG1 versions were more efficient in eliminating Raji cells by ADCP (Fig. 2 D). Moreover, all versions of 5A6 mAbs retained their ability to mediate ADCC (Fig. S2 E) and induce direct killing against tumor cells (Fig. S2 F).
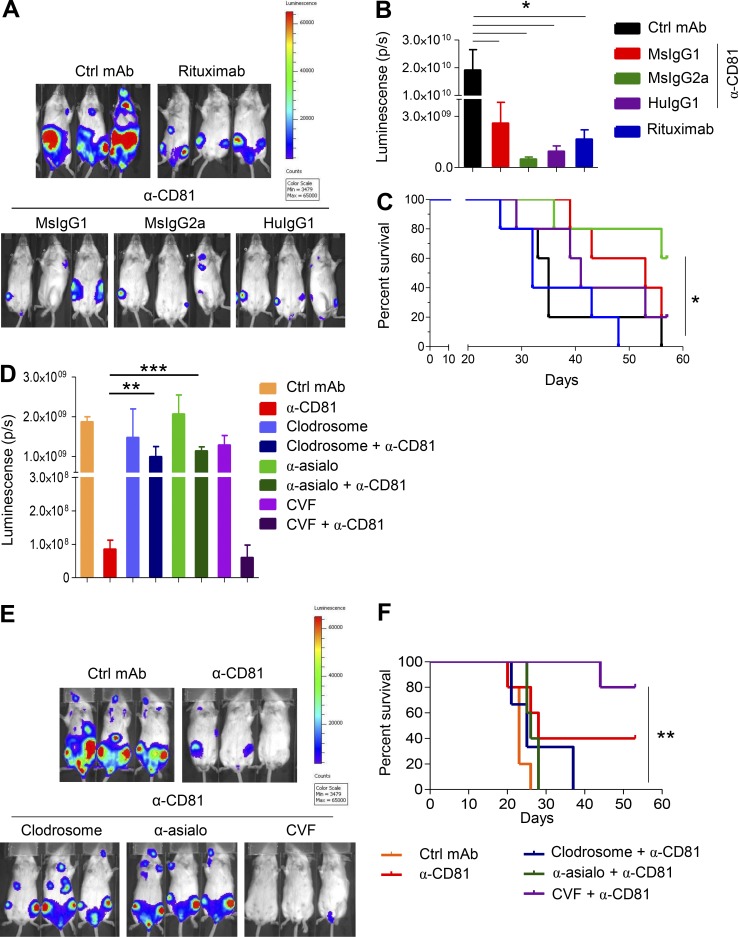
Next, we compared the therapeutic potency of the various versions of 5A6 mAbs (MsIgG1, MsIgG2a, and HuIgG1) in the xenograft model against Raji-luc cells. The versions of the 5A6 mAbs that enabled better cytotoxicity, namely MsIgG2a and HuIgG1, were significantly more effective in reducing lymphoma growth and improving survival than 5A6 MsIgG1 and even superior to rituximab (Fig. 3, A–C).
Figure 3.
5A6 eliminates Raji cells in vivo in a macrophage- and NK cell–dependent manner. (A–C) 1.5 × 106 Raji-luciferase cells were injected i.v. into SCID-Beige mice. Mice were treated on day 5 after tumor challenge i.p. with 100 µg and weekly thereafter (four doses) with the indicated 5A6-based mAbs (MsIgG1, MsIgG2a, and chimeric HuIgG1); rituximab served as a positive control, and a nonspecific MsIgG1 served as a negative control. Ctrl, control. (A) Representative in vivo bioluminescence imaging (IVIS) on day 22 after challenge. (B) Bioluminescence quantification (Living Image) on day 29 (n = 5 mice in each group). (C) Survival was quantified when mice exhibited hindleg paralysis. (D–F) Macrophages from SCID-Beige mice were depleted with 200 µl of clodrosome liposomes (5 mg/ml) i.p. 4 h after tumor challenge and on day 5. NK cells were depleted by i.p. injection of 50 µg of anti-asialo GM1 on days −1 and 0 of tumor challenge and every 5 d thereafter for 3 wk. Complement was depleted by i.p. injection of 25 U CVF on the day of tumor challenge and on days 3, 6, and 9 after challenge. Mice (five per group) were injected with Raji-luc cells as in Figs. 1 A and 3 A and then treated with 100 µg of 5A6 MsIgG2a or isotype control on day 5 and every week thereafter for a total of four doses. (D) Bioluminescence quantification (Living Image) on day 20 after tumor challenge. (E) Representative in vivo bioluminescence imaging (IVIS) on day 20 after lymphoma injection. (F) Survival was quantified as in C. Error bars represent mean ± SEM. *, P < 0.0149; **, P < 0.0036; ***, P < 0.0001; Student’s t test (B and D; n = 5 mice in each group). *, P < 0.0154; **, P < 0.0022; log-rank (Mantel–Cox) test (C and F).
The in vivo activity of 5A6 is mediated by macrophages and NK cells
To determine which arms of the innate immunity system contribute to the 5A6-mediated therapeutic effects, we depleted macrophages using clodronate liposomes, NK cells using anti-Asialo GM1 mAb, and complement using cobra venom factor (CVF). We then challenged mice with Raji-luc cells, followed by treatment with 5A6 MsIgG2a as in Fig. 3 A. Depletion of each of the components was confirmed by quantification of F4/80 macrophages, NK.1.1 NK cells, and C3a complement in serum (Fig. S3). The therapeutic potency of 5A6 was significantly reduced in mice depleted of macrophages or NK cells (Fig. 3, D–F), suggesting ADCP and ADCC as likely mechanism of action in vivo. By contrast, depleting mouse complement had no effect on 5A6 in vivo efficacy but significantly improved survival (Fig. 3 F). Interestingly, CVF showed some tumor toxicity (data not shown), which could explain the improved survival when mice were treated with 5A6 and CVF.
5A6 selectively eliminates lymphoma cells when mixed with normal lymphocytes
CD81 is not expressed on human red blood cells, granulocytes, or platelets, although it is expressed on a variety of other normal tissues, thus creating a potential safety challenge. Tumor-specific antigens, such as idiotypes that are solely expressed on B cell lymphoma are ideal targets (Houot and Levy, 2009). Other antigens expressed at higher levels on tumor cells than on normal tissues have served as targets for antibodies and other immunotherapies (van de Donk et al., 2016). We compared the expression of CD81 on lymphoma cell lines to that of normal human B cells. All the lymphoma cell lines tested expressed higher levels of CD81 than do normal B cells; by contrast, CD20 is expressed at similar levels on normal and on tumor B cells (Fig. 4 A). Next, we asked if this higher expression on tumor cells could outcompete normal PBMCs for antibody binding. We mixed Raji cells with PBMCs from a healthy individual at a 1:1 ratio, followed by incubation of the mixture with decreasing amounts of 5A6. We found that Raji cells outcompeted antibody binding at all concentrations (Fig. 4 B). To determine if 5A6 leads to selective killing of B cell lymphomas versus normal cells, we incubated PBMCs with decreasing ratios of Raji cells in the presence of saturating amounts of the antibody. PBMCs were resistant to 5A6 MsIgG2a–mediated CDC, whereas Raji cells were preferentially killed even when present in a 1:1,000 ratio (Fig. 4 C). Next, we focused on possible selective sensitivity of Raji cells versus normal B cells to 5A6-mediated ADCC. We incubated a 1:1 mixture of red tracking dye (RTD)–labeled purified normal B cells with violet tracking dye (VTD)–labeled Raji cells in the presence of NK cells. While rituximab failed to distinguish between lymphoma and normal B cells, 5A6 preferentially eliminated Raji cells (Fig. 4, D and E).
Figure 4.
5A6 selectively kills lymphoma cells while sparing PBMCs. (A) Flow cytometry analysis of CD81 and CD20 expression on the indicated lymphoma cell lines and human B cells compared with an isotype control mAb. (B) APC-conjugated 5A6 at decreasing concentration binds preferentially to CFSE-labeled Raji cells when mixed in a 1:1 ratio with VTD-labeled PBMCs. (C) CFSE-labeled Raji cells were mixed at the indicated ratios with VTD-labeled PBMCs, followed by incubation in the presence of 10 µg/ml of 5A6 MsIgG2a and fresh human serum for 1.5 h. Ctrl, control. (D and E) VTD-labeled Raji cells were mixed at a 1:1 ratio with RTD-labeled human purified B cells and incubated with human purified NK cells at a 5:1 ratio in the presence of 2.5 µg of 5A6-MsIgG2a, rituximab, or isotype control for 24 h All experiments were done in triplicate, except in B and C. (C–E) Cell death was measured by Annexin-V and 7-AAD staining. Error bars represent mean ± SEM.
5A6 mAb is not cytotoxic to normal PBMCs in vitro or to human CD81 transgenic mouse PBMCS in vivo
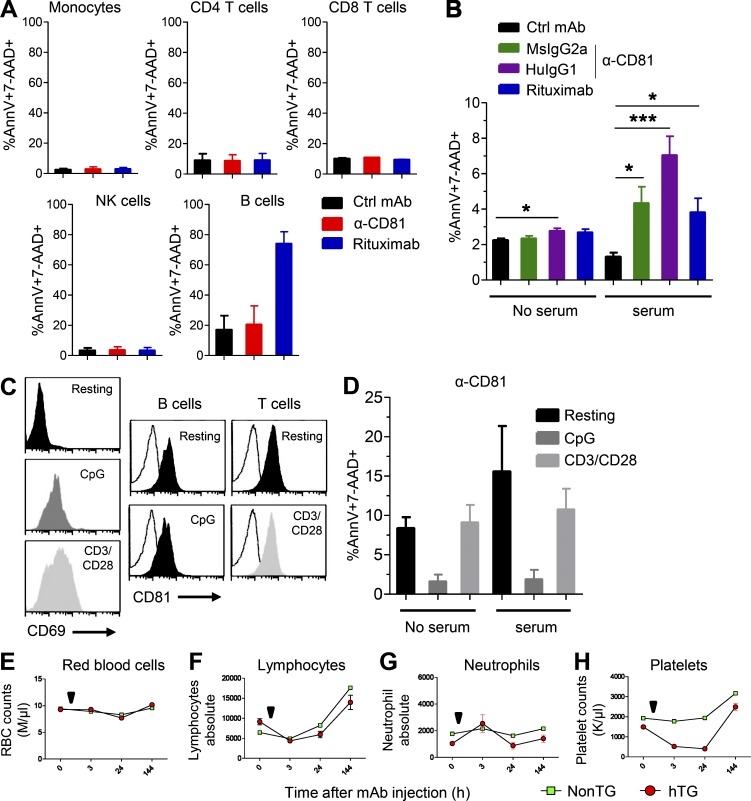
Unlike CD20, CD81 expression is not restricted to B cells; CD4+, CD8+ T cells, NK cells, and monocytes all express CD81 (Fig. S4 A). We therefore incubated PBMCs from a healthy donor with 5A6 MsIgG1, rituximab, or a control MsIgG1 for 24 h followed by enumeration of dead cells. 5A6 did not induce cell death of any of these cell types (Fig. 5 A). By contrast, rituximab depleted almost 80% of the normal B cells (Fig. 5 A). Next, we tested the susceptibility of normal PBMCs to CDC induced by 5A6 MsIgG2a and HuIgG1. We found that only 5–8% of lymphocytes were depleted in the presence of human serum by these 5A6-based mAbs (Fig. 5 B).
Figure 5.
Effect of 5A6 on normal human and CD81Tg mice PBMCs. (A) PBMCs from a healthy donor were incubated in triplicate with 2.5 µg/ml of 5A6 MsIgG1, rituximab, or control (Ctrl) MsIgG1 for 24 h. (B) Normal resting PBMCs from four healthy donors were incubated for 1.5 h in the absence or presence of fresh pooled human serum and 5A6-based MsIgG2a, chimeric HuIgG1, rituximab, or control MsIgG1. (C) Normal resting PBMCs from a healthy donor were left untreated (resting) or activated with CpG or anti-CD3/CD28 for 48 h, followed by staining by anti-CD69 and anti-CD81 mAbs and flow cytometric analysis. (D) Resting PBMCs from four healthy donors were activated as in C and then incubated for the CDC assay as in B. (A, B, and D) Cell death was measured by Annexin-V and 7-AAD staining; all experiments were done in triplicate. (E–H) HuCD81Tg (hTG, red symbols) and nontransgenic (non-TG, green symbols) mice were injected i.p. with a single dose (500 µg) of anti-CD81 MsIgG2a (arrow indicates time of mAb injection; n = 5 mice in each group). Shown are counts at the indicated times of RBCs (E), lymphocytes (F), neutrophils (G), or platelets (H). Error bars represent mean ± SEM. *, P < 0.014; ***, P < 0.0008; Student’s t test (B). M, millions per cubic millimeter; K, thousands per cubic millimeter.
To determine if the preferential susceptibility of tumor to normal lymphoid cells was due to their state of activation, we stimulated PBMCs from healthy donors with CpG, a Toll-like receptor agonist, or with antibodies against CD3 and CD28 and retested their sensitivity to the anti CD81 antibodies. The activated B and T lymphocytes up-regulated CD69+, whereas their CD81 expression did not change upon activation (Fig. 5 C). Moreover, activation of the normal B and T cells did not make them susceptible to 5A6 mAb (Fig. 5 D).
CD81 is widely expressed, hence the need for extensive safety studies to evaluate targeting by a therapeutic antibody. To test the in vivo effect of 5A6, we conducted a preliminary safety test in a transgenic mouse that lacks endogenous mouse CD81 but expresses human CD81 (Masciopinto et al., 2002). These transgenic mice express human CD81 protein on their B and T cell subsets (CD4 and CD8), NK cells, and monocytes in a pattern similar to that on human PBMCs (Fig. S4 B). However, because CD81 expression is driven by the actin promoter in these mice, their platelets and neutrophils also express human CD81. Importantly, human platelets and neutrophils do not express CD81 (Fig. S4 A). When these transgenic mice and their control Cd81KO littermates (non-Tg) were injected with single doses of 5A6 (MsIgG1, MsIgG2a, and chimeric HuIgG1), they maintained their healthy appearance and their body weight (Fig. S4 C). However, 5A6 did induce a transient decrement in blood platelet numbers in the transgenic mice, but not in non-Tg littermates that recovered after 6 d and remained unchanged thereafter (Fig. 5 H). Interestingly, neutrophils, that express CD81 in these transgenic mice were unaffected by the administration of the 5A6 antibodies (Fig. 5 G). As expected, red blood cells were unaffected (Fig. 5 E), and although we observed a transient drop in lymphocyte counts, the effect was not specific to expression of CD81 since, non-Tg control mice showed a similar transient fall and recovery (Fig. 5 F). Taken together, these data speak to the potential safety profile of the 5A6 antibody and its Ig subclass derivatives.
5A6 is effective in eliminating B cell lymphoma while sparing normal lymphocytes in the tumor microenvironment
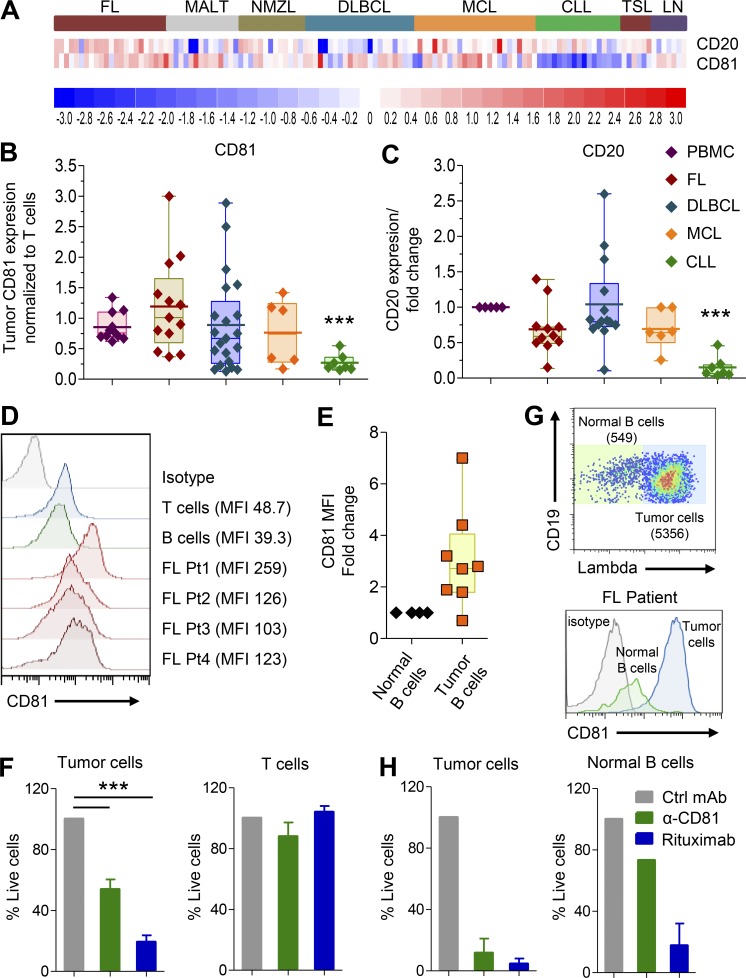
Gene expression was previously reported in 114 B cell NHL samples, normal tonsils, and lymph nodes (Gómez-Abad et al., 2011). Unlike high CD81 expression on B cell lymphoma lines, analysis of this publicly available data revealed heterogeneous expression of CD81 comparable to those expressed by normal B cells in many cases of FL, diffuse large B cell lymphoma, mantle cell lymphoma, marginal zone lymphoma of mucosa-associated lymphoid tissue, and nodal marginal zone lymphoma. Interestingly, the cases of lymphoma that expressed CD81 were not necessarily the same cases that expressed CD20, and therefore, the CD81 target may be an alternative to CD20 or even add lymphoma coverage to antibodies that target CD20. Interestingly, both CD81 and CD20 expression was significantly lower in chronic lymphocytic leukemia (CLL; Fig. 6 A). To validate the microarray data, we analyzed CD81 expression by flow cytometry in additional NHL samples taken at diagnosis, before treatment (6 mantle cell lymphoma, 13 FL, 7 CLL, and 21 diffuse large B cell lymphoma). CD81 and CD20 expression were also measured in healthy B and T cells within each sample. The fold change between CD81 and CD20 expression in tumor cells versus normal T cells within each individual sample is shown (Fig. 6, B and C). CD81 was heterogeneously expressed in all NHL samples, and in corroboration of the gene expression data, both CD81 and CD20 were expressed at low levels in all CLL samples.
Figure 6.
Malignant B cells in NHL biopsies express higher levels of CD81 and are more sensitive to 5A6 than normal T and B cells within the patients’ tumors. (A) Heatmap showing CD20 and CD81 expression on a series of 114 NHL patients and 14 control tissues (Gene Expression Omnibus accession number GSE32018). (B and C) Single-cell suspensions from lymphoma patients’ diagnosis biopsy specimens or PBMCs of healthy donors were thawed and rested for 90 min before staining with a combination of lineage marker–specific antibodies, followed by flow cytometry measurements. Tumor CD81 expression is shown as the MFI ratio to T cells within each sample; likewise, the MFI ratio between CD81 expression on B and T cells is shown for healthy donors’ PBMCs. The CD20 expression ratio was normalized to CD20 expression on normal B cells. ***, P < 0.0001; Student’s t test. (D) Representative histogram from four FL patient biopsy specimens analyzed for CD81 expression on malignant B and normal T and B cells. (E) CD81 MFI fold change expression on eight FL tumors relative to their own healthy normal B cells. (F) Single-cell suspension of six FL patient samples incubated with 5A6-MsIgG2a, rituximab, or mAb control in the presence of human serum for 1.5 h. Samples were analyzed by flow cytometry; shown is the percentage of remaining live tumor and normal T cells. (G and H) Tumor (CD19+lambda+) and normal B cells (CD19+lambda−) from two FL patient samples were incubated with rabbit serum for 1.5 h and then analyzed by flow cytometry; shown is dot plot showing tumor versus normal B cells (G, top), a histogram showing CD81 expression (G, bottom), and the percentage of remaining live tumor (H, left) and normal B cells (H, right). ***, P < 0.0001; Student’s t test (B, C, and F). Ctrl, control; DLBCL, diffuse large B cell lymphoma; MALT, marginal zone lymphoma of mucosa-associated lymphoid tissue; NMZL, nodal marginal zone lymphoma; TSL, tonsils. Error bars represent mean ± SEM.
We then determined the sensitivity of malignant B cells in FL biopsy specimens to the effects of 5A6 mAb. These biopsies also contain normal T and B cells that also express CD81 (Fig. 6, D and E), but in contrast FL tumor cells, they have on average two- to sixfold higher CD81 expression (Fig. 6 E). We incubated single-cell suspensions of six FL biopsies with 5A6 MsIgG2a, rituximab, or a control mAb in the presence of human serum as source of complement. Malignant FL B cells of all patients were sensitive to the cytotoxic effects of both 5A6 and rituximab (Fig. 6 F, left). By contrast, T cells were resistant to killing by 5A6 mAbs despite expressing CD81 (Fig. 6 F, right). Next, we examined two FL biopsies in which we were able to identify normal B cells by their expression of an Ig light chain that differed from that of their tumor (Fig. 6 G). This analysis revealed that the lymphoma B cells were eliminated (Fig. 6 H, left) but that normal B cells were relatively insensitive to the cytotoxic effects of 5A6 (Fig. 6 H, right), and by contrast, and as expected, rituximab failed to distinguish between tumor and normal B cells, killing both populations (Fig. 6 H). Finally, the selectivity of 5A6 toward neoplastic B cells is likely dependent on differential expression of CD81 in tumor versus normal B cells, whereas CD20 does not change (Fig. 6). In addition, CD81 exists in a complex with the complement receptor, CD21, and therefore, higher expression of CD81 would be expected to confer sensitivity to complement killing. Indeed, a reanalysis of a public gene expression dataset establishes a major difference between normal B cells and several different types of B cell malignancies. This analysis confirms the overexpression of CD81 and importantly a major underexpression of CD55, the complement inhibitor, by lymphoma compared with normal B cells (Fig. S5 A). Moreover, an orthogonal dataset of single-cell mRNA sequencing confirms that expression of CD81 is higher in lymphoma cells than in their own imbedded normal B cells, and conversely, expression of the complement inhibitor, CD55, is lower in tumor cells than in their normal B cells (Fig. S5 B).
Discussion
Tetraspanins are a family of proteins that have recently gained attention due to their role in infectious diseases and in cancer biology (Yang et al., 2016; Fast et al., 2017; Vences-Catalán et al., 2017). Several tetraspanins have been characterized as key players in tumor growth, invasion, and metastasis in both solid tumors and hematological malignancies (Yang et al., 2016). This has supported the notion that targeting particular tetraspanins could have potential therapeutic applications in cancer (Hemler, 2008).
Here, we initially tested the possibility of targeting CD81 by 5A6; this mAb was identified by screening hybridomas that induced an antiproliferative effect on B cell lymphomas (Oren et al., 1990). Indeed, we found that 5A6 effectively reduced tumor burden of human B cell lymphomas in xenograft models, improved survival, and was comparable to the therapeutic effects of rituximab. Tumor-targeting mAbs depend on their intrinsic capability to elicit direct cell death and recruitment of immune-mediated effector mechanisms. Interestingly, 5A6, but no other anti-CD81 mAbs, mediated direct killing and ADCC of lymphoma cell lines, suggesting unique properties of the 5A6 clone. Our ongoing studies indicate that 5A6 directly activates caspase-3 and its downstream PARP target, whereas other anti-human CD81 mAbs, such as 1D6, which targets a different epitope (van Zelm et al., 2010), do not trigger direct killing on Raji cells. These anti-CD81 mAbs cross-react with monkey CD81 (Higginbottom et al., 2000), whereas 5A6 binds weakly to monkey cells. In addition, it has been shown that 5A6 requires the 135VVD137 motif in contrast to other anti-CD81 mAbs (Yalaoui et al., 2008).
Therapeutic mAbs can be altered to better activate and recruit immune mechanisms that eliminate tumor cells (Weiner, 2015). Here, we demonstrated that class-switching 5A6 MsIgG1 to MsIgG2a or chimeric HuIgG1 enhanced ADCC, CDC, and ADCP. Importantly, 5A6 MsIgG2a and HuIgG1 eliminated lymphoma cells in vivo better than rituximab in an NK cell– and macrophage-dependent manner. Although mouse complement was not relevant in vitro and in vivo, 5A6 activated human complement effectively in vitro, suggesting that complement could contribute to elimination of lymphoma in humans. Altogether, 5A6 mediates lymphoma killing by a combination of different mechanisms.
CD37, a validated immunotherapy target, is expressed ≥15 times higher in normal B lymphocytes and the majority of CLL cases compared with non-B leukocytes (Beckwith et al., 2014), and CD38, another immunotherapy target, is also expressed at a high level in multiple myeloma tumor cells compared with normal B cells (Nijhof et al., 2015). Here, we show that the expression of CD81 is much higher in human B cell lymphoma cell lines than in normal lymphocytes. These malignant B cells were preferentially killed by the anti-CD81 5A6 mAb, even when mixed with normal PBMCs at extremely low ratios. CD81 is expressed on all other subpopulations of PBMCs, yet 5A6 did not induce killing of these subpopulations.
Remarkably, in FL biopsies that include both malignant lymphoma B cells and normal T and B cells, 5A6 eliminated tumor cells, but not normal lymphocytes. Previous studies reported expression of CD81 in most NHLs and variably in Hodgkin’s lymphoma, multiple myeloma, and acute myeloid leukemia (Luo et al., 2010; Gómez-Abad et al., 2011; Paiva et al., 2012; Boyer et al., 2016). Here, both mRNA microarray (Alizadeh et al., 2000), single-cell RNA sequencing (Andor et al., 2019), and flow cytometry analyses confirmed a higher CD81 expression in FL when compared with their own normal B cells. 5A6 preferentially eliminated FL tumor B cells. Moreover, higher expression of the complement inhibitor, CD55, in normal cells could confer CDC resistance to killing mediated by 5A6. Thus, we postulate that lymphomas that express relatively high levels of CD81 compared with their normal lymphocytes would be candidates for therapy with 5A6 antibodies.
We have begun to evaluate the safety of 5A6. It is noteworthy that a different anti-human CD81 mAb that reacts with cynomolgus monkey showed no toxicity in this primate species (Ji et al., 2015). These results are reassuring regarding the safety of CD81 as a target, but the fact that 5A6 is unique among anti-CD81 antibodies makes it necessary to test its safety as well. To address this need, we have generated a mouse model that express human, but not mouse, CD81 (Masciopinto et al., 2002). These mice express CD81 on neutrophils and platelets, whereas CD81 is not expressed in humans in these two populations. We found that injection of a single dose of 5A6 resulted in transient drop in platelets, but not in neutrophils. Future experiments will be aimed at testing not only the safety but also the efficacy of 5A6 in this transgenic mouse model when challenged with a syngeneic tumor that expresses human CD81.
Taken together, we have shown as proof of concept that CD81 represents a potential tumor target for the treatment of B cell lymphomas. The unique properties of 5A6 among other anti-CD81 antibodies make this antibody especially suitable for immunotherapy. Our results motivate further studies that will test the efficacy and safety of 5A6 in relevant animal models and, depending on the safety and efficacy profiles, eventually in human clinical trials.
Material and methods
Cell lines, PBMCs, and lymphoma patient samples
Cell lines were grown in complete Roswell Park Memorial Institute medium or IMDM containing 10% (vol/vol) FCS (HyClone), 1% sodium pyruvate, 1% L-glutamine (Cellgro), 100 U/ml penicillin, and 100 µg/ml streptomycin (Gibco). PBMCs from healthy donors were obtained from the Stanford Blood Center. FL patient and tonsil samples were obtained with informed consent in accordance to the declaration of Helsinki from Stanford University Medical Center (Stanford University Institutional Review Board Certification of Human Subject Approvals “Clinical and Pathologic Studies in Non-Hodgkin’s Lymphoma and Hodgkin’s Disease”; Stanford Institutional Review Board eProtocol 13500).
Mouse models
For the xenograft model, 1.5 × 106 Raji-luc cells were injected i.v. in the lateral tail vein of SCID-Beige mice. On postchallenge day 5, mice were treated i.p. with four weekly doses of 100 µg of the indicated antibodies. Bioluminescence was done by IVIS (Living Image) on day 5 to ensure tumor uptake and then after each antibody injection. Mice were housed at the pathogen-free animal facility of the Stanford University Medical Center, and all animal experiments were approved by the Stanford Administrative Panel on Laboratory Animal Care.
Direct antibody toxicity, CDC, ADCC, and ADCP
For direct killing assays, 106 lymphoma cell lines, normal PBMCs, tonsil, or lymphoma patient samples (FL) were incubated with 2.5–10 µg/ml of control, anti-CD81 isotypes or rituximab mAbs for 60 min, 90 min, 4 h, or 24 h (or as otherwise specified in the figures). For all CDC assays except when specified, cells were incubated for 90 min in the presence of 50% pooled human serum with indicated mAbs. In CDC assays aimed at distinguishing biopsy tumor B cells from normal B cells by kappa versus lambda staining, 50% of rabbit serum was used instead of human serum. For ADCC assays, human NK cells were purified using MACS kit (130–092-657) and then co-cultured at different E/T ratios with CFSE-labeled Raji or SUP-B8 cells for 24 h in the presence of 1 µg/ml of the indicated mAbs. Cell death was measured with Annexin-V and 7-AAD staining by flow cytometry (BD LRS II). For ADCP assays, mouse peritoneal macrophages were harvested and activated with LPS (100 ng/ml) for 24 h. pHrodo green AM–labeled Raji cells were then opsonized with the indicated mAbs (10 µg/ml) and coincubated with macrophages 1:3 ratio for 4 h. Phagocytic events were defined as percentage of F4/80 and high mean fluorescence intensity (MFI) pHrodo green AM double-positive macrophages by flow cytometry.
In vivo depletion of macrophages, NK cells, and complement
6–8-wk-old female SCID-beige mice were injected i.p. with 200 µl of clodrosome liposomes (5 mg/ml; Encapsula NanoSciences) to deplete macrophages 4 h after tumor inoculation and at the same dose on day 5. For depleting NK cells, mice were injected i.p. with 50 µl of anti-asialo GM1 mAb (1 mg/ml; Wako) on days −1 and 0 of tumor inoculation and every 5 d thereafter for 3 wk. For complement depletion, mice were injected i.p. with 25 U CVF (Quidel) on the day of tumor challenge and at days 3, 6, and 9 after tumor challenge. Depletion was corroborated on day 5 after the second dose of clodrosome injection and on day 4 after the last dose of anti-asialo by flow cytometry in the blood and spleen using F4/80 to identify macrophages and NK1.1 for NK cells. Complement depletion was determined on days −1, 1, and 8 by assessing C3a in mouse serum by ELISA.
Class-switched antibodies
We adapted the protocol of “sib selection” (Spira and Scharff, 1992) to obtain our class-switched hybridomas. Briefly, 5 × 104 5A6 MsIgG1–producing hybridoma cells were plated in 96-well plates and cultured for 5 d. Screening of 5A6 MsIgG2a–positive wells was done by ELISA using glutathione S-transferase–recombinant human CD81-large extracellular loop. After several rounds of selection and limited dilution, we cloned a single-cell 5A6 MsIgG2a high-producing hybridoma. 5A6 MsIgG2a bound to cell surface–expressed human CD81 when tested by flow cytometry.
Engineering chimeric antibodies
Total RNA was extracted from our mouse 5A6 hybridoma cell line using an RNeasy mini kit (Qiagen) and converted into cDNA using a SMART cDNA synthesis kit (Takara) with Ig-specific oligonucleotides; heavy and light chains encoding cDNA fragments were isolated and sequenced. These consecutive oligonucleotides that were used for γ1 heavy chain are 5′-CGGGGTTGCGTCTGAGCTGTGTGCACCT-3′ and 5′-GGTGAGCACATCCTTGGGCTTTGGGGGG-3′, while the consecutive oligonucleotides used for κ light chain are 5′-CGTCCTTGGTCAACGTGAGGGTGCTGC-3′ and 5′-CCTGATCAGTCCAACTGTTCAGGACGCC-3′.
Construction and generation of 5A6 chimeric antibody
Mouse V region of γ1 gene was amplified to include a 5′ PmeI site with oligo 5′-AGCTTTGTTTAAACCGGGCCACCATGGCTTGGGTGGGGACCTTGCTATTCCTGCTGGCAGCTGCCCAAAG-3′ and a 3′ NheI site with oligo 5′-GCCCTTGGTGCTAGCTGCAGAGACAGTGACCAGCGTCCC-3′. Mouse V region of κ gene was amplified to include a 5′ PmeI site with oligo 5′-AGCTTTGTTTAAACGGCGCGCCGGGCCACCATGGATTCACAGGCCCAGTTCTTATATTGCTGC-3′ and a 3′ BsiWI site with oligo 5′-TGCAGCCACCGTACGTCTCATTTCCAGCTTGGTCCCCCCTC-3′. Chimeric genes were then constructed by linking the amplified murine V regions into corresponding sites of modified pCEP plasmids containing either HuIgG1 or κ constant regions. The secreted supernatants of 5A6 chimeric antibody were produced by transiently cotransfecting both chimeric γ1 and κ DNA into 293f cells (plasmids and 293f cells were kindly provided by Dr. Jie Liu from R. Majeti Lab, Stanford University, Stanford, CA) with 293fectin transfection reagent from ThermoFisher. Antibody was purified from supernatant with HiTrap protein A column (GE Healthcare Life Sciences).
Competitive binding and selective killing of tumor cells
CFSE-labeled Raji cells were mixed in a 1:1 ratio with normal VTD-labeled PBMCs then incubated with decreasing concentrations of anti-CD81. Similarly, CFSE-Raji or VTD-labeled PBMCs were incubated at decreasing ratios and then incubated with 5A6 MsIgG2a with or without human serum for the CDC assay. For ADCC, RTD-labeled purified human B cells or VTD-labeled Raji cells were incubated with 5A6 MsIgG2a, rituximab, or isotype control 1:1 ratio and with human purified NK cells 5:1. Cell death was measured as above.
Safety studies in Cd81KO mice expressing human CD81 (HuCD81Tg)
HuCD81Tg/Cd81KO and control Cd81KO non-Tg mice received a single i.p. dose of anti-CD81 (5A6 MsIgG1 or MsIgG2a as indicated) antibody in a range of 0–500 µg/mouse (0–20 mg/kg). Mice were weighed weekly. Flow cytometry was performed to quantify various cell types in peripheral blood samples collected at different time points following administration of 5A6. Complete blood count was done at the Veterinary Service Center at Stanford University. Peripheral blood samples collected from these mice prior and post treatments were lysed with 1× ammonium-chloride-potassium lysis buffer (Quality Biological) to remove red blood cells, washed, and then cells were incubated in PBS 1% BSA with Fc-blocking reagent for 15 min at room temperature. Staining of cell subsets was performed by incubating cells on ice for 30 min with fluorochrome-conjugated antibodies for the cell-surface markers CD4 (RM4-5), CD8 (Ly-2), B220 (RA3.6B2), CD11b (NKP15), CD49b (DX5), Ly6C (AL-21), Ly6G (1A8), NK1.1 (PK136), and human CD81 (JS-81) and corresponding isotype controls from BD Biosciences or eBioscience. Cells were washed twice in PBS/BSA and fixed in 2% paraformaldehyde, followed by analysis using the FACS Calibur or LSRII system (BD Biosciences). Data analysis was performed using FlowJo software (Tree Star).
Gene expression analysis
We analyzed the expression of CD20 and CD81 from a microarray database (Gene Expression Omnibus accession number GSE32018) of 114 NHL patient samples and 14 normal tissues using DNA-CHIP analyzer software (DCHIP). Data were plotted as a heatmap.
Statistical analysis
Data were analyzed using Prism 6.0 (GraphPad Software) by either an unpaired t test or one-way ANOVA. A P value of <0.05 was considered statistically significant.
Online supplemental material
Fig. S1 shows that 5A6, the anti-human CD81 mAb, improves survival of SUP-B8–bearing mice. Fig. S2 demonstrates that anti-CD81, 5A6 induces direct killing by activating caspase-3 and PARP and shows binding of anti-CD81 mAbs to monkey cells and in vitro sensitivity of B lymphoma cell lines to direct and indirect cytotoxicity induced by 5A6 mAbs. Fig. S3 confirms the depletion of macrophages, NK cells, and complement. Fig. S4 shows the expression of human CD81 in PBMCs from humans and human CD81 transgenic mice. Fig. S5 shows reanalysis of CD81 and CD55 mRNA expression in two separate lymphoma datasets.
Supplementary Material
Acknowledgments
We thank Dr. Ravi Majeti and Dr. Jie Liu (Stanford University, Stanford, CA) for providing plasmids and cell lines and for their technical assistance in developing chimeric antibodies, and Dr. Michael R. Green for his assistance in microarray data analysis.
This work was supported by the Translational Cancer Award from the Stanford Cancer Institute, Stanford’s SPARK and Coulter programs, the Breast Cancer Research program from the Department of Defense grant W81XWH-14-1-0397, and the National Institutes of Health grant 5 R35 CA197353-04. F. Vences-Catalán was supported by the American Society of Immunology through a Careers in Immunology fellowship.
The authors declare no competing financial interests.
Author contributions: F. Vences-Catalán, C-C. Kuo, R. Rajapaksa, C. Duault, D.K. Czerwinski, R. Levy, and S. Levy designed, performed, and analyzed the experiments and edited the manuscript. C-C. Kuo generated chimeric antibodies and performed ADCC assays. C. Duault class-switched 5A6 to MsIgG2a and analyzed PBMC toxicity. R. Rajapaksa performed toxicity studies in HuCD81Tg mice and the SUP-B8 in vivo experiment. N. Andor reanalyzed single-cell mRNA expression in malignant versus embedded normal B cells. D.K. Czerwinski analyzed expression of CD81 on FL patient samples. F. Vences-Catalán and S. Levy wrote the manuscript.
References
- Alizadeh A.A., Eisen M.B., Davis R.E., Ma C., Lossos I.S., Rosenwald A., Boldrick J.C., Sabet H., Tran T., Yu X., et al. 2000. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 403:503–511. 10.1038/35000501 [DOI] [PubMed] [Google Scholar]
- Andor N., Simonds E.F., Czerwinski D.K., Chen J., Grimes S.M., Wood-Bouwens C., Zheng G.X.Y., Kubit M.A., Greer S., Weiss W.A., et al. 2019. Single-cell RNA-Seq of follicular lymphoma reveals malignant B-cell types and coexpression of T-cell immune checkpoints. Blood. 133:1119–1129. 10.1182/blood-2018-08-862292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansell S.M., Minnema M.C., Johnson P., Timmerman J.M., Armand P., Shipp M.A., Rodig S.J., Ligon A.H., Roemer M.G.M., Reddy N., et al. 2019. Nivolumab for Relapsed/Refractory Diffuse Large B-Cell Lymphoma in Patients Ineligible for or Having Failed Autologous Transplantation: A Single-Arm, Phase II Study. J. Clin. Oncol. 37:481–489. 10.1200/JCO.18.00766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckwith K.A., Frissora F.W., Stefanovski M.R., Towns W.H., Cheney C., Mo X., Deckert J., Croce C.M., Flynn J.M., Andritsos L.A., et al. 2014. The CD37-targeted antibody-drug conjugate IMGN529 is highly active against human CLL and in a novel CD37 transgenic murine leukemia model. Leukemia. 28:1501–1510. 10.1038/leu.2014.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer T., Guihard S., Roumier C., Peyrouze P., Gonzales F., Berthon C., Quesnel B., Preudhomme C., Behal H., Duhamel A., et al. 2016. Tetraspanin CD81 is an adverse prognostic marker in acute myeloid leukemia. Oncotarget. 7:62377–62385. 10.18632/oncotarget.11481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury L.E., Kansas G.S., Levy S., Evans R.L., and Tedder T.F.. 1992. The CD19/CD21 signal transducing complex of human B lymphocytes includes the target of antiproliferative antibody-1 and Leu-13 molecules. J. Immunol. 149:2841–2850. [PubMed] [Google Scholar]
- Clynes R.A., Towers T.L., Presta L.G., and Ravetch J.V.. 2000. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat. Med. 6:443–446. 10.1038/74704 [DOI] [PubMed] [Google Scholar]
- Fast L.A., Lieber D., Lang T., and Florin L.. 2017. Tetraspanins in infections by human cytomegalo- and papillomaviruses. Biochem. Soc. Trans. 45:489–497. 10.1042/BST20160295 [DOI] [PubMed] [Google Scholar]
- Feugier P., Van Hoof A., Sebban C., Solal-Celigny P., Bouabdallah R., Fermé C., Christian B., Lepage E., Tilly H., Morschhauser F., et al. 2005. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d’Etude des Lymphomes de l’Adulte. J. Clin. Oncol. 23:4117–4126. 10.1200/JCO.2005.09.131 [DOI] [PubMed] [Google Scholar]
- Gómez-Abad C., Pisonero H., Blanco-Aparicio C., Roncador G., González-Menchén A., Martinez-Climent J.A., Mata E., Rodríguez M.E., Muñoz-González G., Sánchez-Beato M., et al. 2011. PIM2 inhibition as a rational therapeutic approach in B-cell lymphoma. Blood. 118:5517–5527. 10.1182/blood-2011-03-344374 [DOI] [PubMed] [Google Scholar]
- Hemler M.E. 2008. Targeting of tetraspanin proteins--potential benefits and strategies. Nat. Rev. Drug Discov. 7:747–758. 10.1038/nrd2659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higginbottom A., Quinn E.R., Kuo C.C., Flint M., Wilson L.H., Bianchi E., Nicosia A., Monk P.N., McKeating J.A., and Levy S.. 2000. Identification of amino acid residues in CD81 critical for interaction with hepatitis C virus envelope glycoprotein E2. J. Virol. 74:3642–3649. 10.1128/JVI.74.8.3642-3649.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraga J., Tomita A., Sugimoto T., Shimada K., Ito M., Nakamura S., Kiyoi H., Kinoshita T., and Naoe T.. 2009. Down-regulation of CD20 expression in B-cell lymphoma cells after treatment with rituximab-containing combination chemotherapies: its prevalence and clinical significance. Blood. 113:4885–4893. 10.1182/blood-2008-08-175208 [DOI] [PubMed] [Google Scholar]
- Houot R., and Levy R.. 2009. Vaccines for lymphomas: idiotype vaccines and beyond. Blood Rev. 23:137–142. 10.1016/j.blre.2008.09.001 [DOI] [PubMed] [Google Scholar]
- Irani V., Guy A.J., Andrew D., Beeson J.G., Ramsland P.A., and Richards J.S.. 2015. Molecular properties of human IgG subclasses and their implications for designing therapeutic monoclonal antibodies against infectious diseases. Mol. Immunol. 67(2, 2 Pt A):171–182. 10.1016/j.molimm.2015.03.255 [DOI] [PubMed] [Google Scholar]
- Ji C., Liu Y., Pamulapati C., Bohini S., Fertig G., Schraeml M., Rubas W., Brandt M., Ries S., Ma H., and Klumpp K.. 2015. Prevention of hepatitis C virus infection and spread in human liver chimeric mice by an anti-CD81 monoclonal antibody. Hepatology. 61:1136–1144. 10.1002/hep.27603 [DOI] [PubMed] [Google Scholar]
- Lim S.H., and Levy R.. 2014. Translational medicine in action: anti-CD20 therapy in lymphoma. J. Immunol. 193:1519–1524. 10.4049/jimmunol.1490027 [DOI] [PubMed] [Google Scholar]
- Luo R.F., Zhao S., Tibshirani R., Myklebust J.H., Sanyal M., Fernandez R., Gratzinger D., Marinelli R.J., Lu Z.S., Wong A., et al. 2010. CD81 protein is expressed at high levels in normal germinal center B cells and in subtypes of human lymphomas. Hum. Pathol. 41:271–280. 10.1016/j.humpath.2009.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maecker H.T., and Levy S.. 1997. Normal lymphocyte development but delayed humoral immune response in CD81-null mice. J. Exp. Med. 185:1505–1510. 10.1084/jem.185.8.1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney D.G., Grillo-López A.J., White C.A., Bodkin D., Schilder R.J., Neidhart J.A., Janakiraman N., Foon K.A., Liles T.M., Dallaire B.K., et al. 1997. IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin’s lymphoma. Blood. 90:2188–2195. [PubMed] [Google Scholar]
- Masciopinto F., Freer G., Burgio V.L., Levy S., Galli-Stampino L., Bendinelli M., Houghton M., Abrignani S., and Uematsu Y.. 2002. Expression of human CD81 in transgenic mice does not confer susceptibility to hepatitis C virus infection. Virology. 304:187–196. 10.1006/viro.2002.1631 [DOI] [PubMed] [Google Scholar]
- Nijhof I.S., Groen R.W., Lokhorst H.M., van Kessel B., Bloem A.C., van Velzen J., de Jong-Korlaar R., Yuan H., Noort W.A., Klein S.K., et al. 2015. Upregulation of CD38 expression on multiple myeloma cells by all-trans retinoic acid improves the efficacy of daratumumab. Leukemia. 29:2039–2049. 10.1038/leu.2015.123 [DOI] [PubMed] [Google Scholar]
- Oren R., Takahashi S., Doss C., Levy R., and Levy S.. 1990. TAPA-1, the target of an antiproliferative antibody, defines a new family of transmembrane proteins. Mol. Cell. Biol. 10:4007–4015. 10.1128/MCB.10.8.4007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paiva B., Gutiérrez N.C., Chen X., Vídriales M.B., Montalbán M.A., Rosiñol L., Oriol A., Martínez-López J., Mateos M.V., López-Corral L., et al. GEM (Grupo Español de Mieloma)/PETHEMA (Programa para el Estudio de la Terapéutica en Hemopatías Malignas) cooperative . 2012. Clinical significance of CD81 expression by clonal plasma cells in high-risk smoldering and symptomatic multiple myeloma patients. Leukemia. 26:1862–1869. 10.1038/leu.2012.42 [DOI] [PubMed] [Google Scholar]
- Scott A.M., Wolchok J.D., and Old L.J.. 2012. Antibody therapy of cancer. Nat. Rev. Cancer. 12:278–287. 10.1038/nrc3236 [DOI] [PubMed] [Google Scholar]
- Spira G., and Scharff M.D.. 1992. Identification of rare immunoglobulin switch variants using the ELISA spot assay. J. Immunol. Methods. 148:121–129. 10.1016/0022-1759(92)90165-P [DOI] [PubMed] [Google Scholar]
- Stolz C., and Schuler M.. 2009. Molecular mechanisms of resistance to Rituximab and pharmacologic strategies for its circumvention. Leuk. Lymphoma. 50:873–885. 10.1080/10428190902878471 [DOI] [PubMed] [Google Scholar]
- Takahashi S., Doss C., Levy S., and Levy R.. 1990. TAPA-1, the target of an antiproliferative antibody, is associated on the cell surface with the Leu-13 antigen. J. Immunol. 145:2207–2213. [PubMed] [Google Scholar]
- van de Donk N.W., Janmaat M.L., Mutis T., Lammerts van Bueren J.J., Ahmadi T., Sasser A.K., Lokhorst H.M., and Parren P.W.. 2016. Monoclonal antibodies targeting CD38 in hematological malignancies and beyond. Immunol. Rev. 270:95–112. 10.1111/imr.12389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zelm M.C., Smet J., Adams B., Mascart F., Schandené L., Janssen F., Ferster A., Kuo C.C., Levy S., van Dongen J.J., and van der Burg M.. 2010. CD81 gene defect in humans disrupts CD19 complex formation and leads to antibody deficiency. J. Clin. Invest. 120:1265–1274. 10.1172/JCI39748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vences-Catalán F., Kuo C.C., Sagi Y., Chen H., Kela-Madar N., van Zelm M.C., van Dongen J.J., and Levy S.. 2015a A mutation in the human tetraspanin CD81 gene is expressed as a truncated protein but does not enable CD19 maturation and cell surface expression. J. Clin. Immunol. 35:254–263. 10.1007/s10875-015-0148-2 [DOI] [PubMed] [Google Scholar]
- Vences-Catalán F., Rajapaksa R., Srivastava M.K., Marabelle A., Kuo C.C., Levy R., and Levy S.. 2015b Tetraspanin CD81 promotes tumor growth and metastasis by modulating the functions of T regulatory and myeloid-derived suppressor cells. Cancer Res. 75:4517–4526. 10.1158/0008-5472.CAN-15-1021 [DOI] [PubMed] [Google Scholar]
- Vences-Catalán F., Duault C., Kuo C.C., Rajapaksa R., Levy R., and Levy S.. 2017. CD81 as a tumor target. Biochem. Soc. Trans. 45:531–535. 10.1042/BST20160478 [DOI] [PubMed] [Google Scholar]
- Wei S.C., Duffy C.R., and Allison J.P.. 2018. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 8:1069–1086. 10.1158/2159-8290.CD-18-0367 [DOI] [PubMed] [Google Scholar]
- Weiner G.J. 2015. Building better monoclonal antibody-based therapeutics. Nat. Rev. Cancer. 15:361–370. 10.1038/nrc3930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalaoui S., Zougbédé S., Charrin S., Silvie O., Arduise C., Farhati K., Boucheix C., Mazier D., Rubinstein E., and Froissard P.. 2008. Hepatocyte permissiveness to Plasmodium infection is conveyed by a short and structurally conserved region of the CD81 large extracellular domain. PLoS Pathog. 4:e1000010 10.1371/journal.ppat.1000010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.G., Sari I.N., Zia M.F., Lee S.R., Song S.J., and Kwon H.Y.. 2016. Tetraspanins: Spanning from solid tumors to hematologic malignancies. Exp. Hematol. 44:322–328. 10.1016/j.exphem.2016.02.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.