Place and Kanneganti review the role of pyroptosis and necroptosis in driving inflammatory diseases, current cytokine blockade therapies, and the potential benefits of targeting the executioners of lytic cell death.
Abstract
Targeting apoptosis to treat diseases has seen tremendous success over the past decades. More recently, alternative forms of regulated cell death, including pyroptosis and necroptosis, have been described. Understanding the molecular cascades regulating both pyroptosis and necroptosis will yield even more targets to treat diseases. These lytic forms of cell death are distinct from apoptosis due to their characteristic lysis and release of cellular components that promote disease or direct a beneficial immune response. In this review, we focus on how pyroptosis and necroptosis, which release potent immune cytokines such as IL-1 and IL-18, contribute to various diseases. We also consider the important role that the executioners of these cell death pathways, GSDMD and MLKL, play in the progression of inflammatory diseases. Crosstalk between the different cell death pathways likely plays a major role physiologically. New therapeutic strategies targeting these specific molecules hold enormous potential for managing inflammatory diseases.
Introduction
Regulated cell death has been understood as a concept for decades, with apoptosis being the first well-defined process in which cells dismantle themselves in a process that is generally immunologically quiet (Kerr et al., 1972; Elmore, 2007). Apoptosis is induced homeostatically and upon exposure to a wide variety of insults, leading to the activation of initiator caspases (caspase-8, -9, -10) and effector caspases (caspase-3, -6, -7), resulting in a nonlytic cell death characterized by membrane blebbing, cell shrinkage, and chromosomal condensation (Elmore, 2007). While apoptosis facilitates the controlled degradation of intracellular proteins and organelles, pyroptosis and necroptosis lead to cell lysis and the release of a wide range of intracellular components and inflammatory cytokines. We focus in this review on the lytic forms of cell death (pyroptosis and necroptosis) and the consequences of their cytokine release, with an eye toward new ways of treating inflammatory diseases.
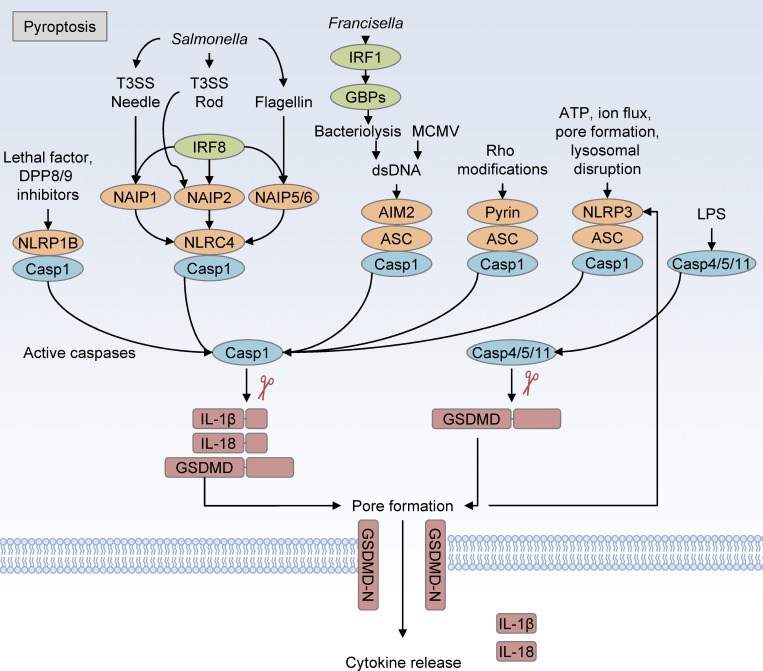
Unlike apoptotic cell death, in which plasma membrane integrity is maintained and intracellular components are sequestered, pyroptosis is a lytic form of cell death that, through a tightly regulated pathway, activates and releases the potent cytokines IL-1β and IL-18 (Fig. 1; Dinarello et al., 1974; Dinarello, 1998; Cookson and Brennan, 2001; Martinon et al., 2002). Pyroptosis plays a major role in the control of intracellular pathogens by destroying their replicative niche and driving immune responses through the release of matured cytokines (Man et al., 2017). Like apoptosis, pyroptosis is regulated by caspases, in particular caspase-1 and caspase-11 (in mice) or caspase-1/4/5 (in humans). Caspase-4/5/11 binds intracellular LPS directly, leading to cleavage of the substrate gasdermin D (GSDMD), the recently discovered executioner of pyroptosis (He et al., 2015; Kayagaki et al., 2015; Shi et al., 2015). Upon cleavage, the autoinhibitory C-terminal domain of GSDMD is released from the pore-forming N-terminal domain, leading to insertion of the GSDMD N-terminal domain into the plasma membrane and oligomerization with other GSDMD fragments, thereby generating a large pore 10–20 nm in diameter (Ding et al., 2016). Similar to caspase-4/5/11, caspase-1 activation by upstream sensor proteins leads to caspase-1–mediated cleavage of GSDMD. The upstream regulators of caspase-1 activation differ in their specificity to different ligands and cellular states and include the proteins NLRP3, NLRC4, AIM2, NLRP1, and pyrin, with other related proteins being suggested as additional sensors (Place and Kanneganti, 2018). A subset of these sensors (NLRP3, AIM2, and pyrin) require the adaptor protein ASC to activate caspase-1 after ligand sensing.
Figure 1.
Overview of pyroptosis. Pyroptosis is mediated by the inflammasome sensor proteins NLRC4, NLRP1, AIM2, Pyrin, and NLRP3, leading to activation of caspase-1. The NLRP1 and NLRC4 sensor proteins do not require the adaptor protein ASC, while the remaining sensors require ASC to oligomerize with caspase-1. Caspase-1 directly cleaves GSDMD to release the autoinhibitory C-terminal domain from its pore-forming N-terminal domain and also cleaves pro-IL-1β and pro-IL-18 into their active forms, which are released through the GSDMD pore. Caspase-11 binding to intracellular LPS activates caspase-11 to cleave GSDMD, which drives pore formation that leads to cell lysis and downstream NLRP3 inflammasome activation through the loss of ion homeostasis. dsDNA, double-stranded DNA.
A single specific trigger for NLRP3 activation is not known, but many cellular insults induce NLRP3 inflammasome activation and cleavage of caspase-1, including loss of cellular ion homeostasis and plasma membrane disruption (Kanneganti et al., 2006; He et al., 2016; Jo et al., 2016). NLRC4 is activated by a set of sensor proteins including human NAIP/mouse NAIP1, NAIP2, and NAIP5/6, which recognize the bacterial type 3 secretion system (T3SS) needle, T3SS rod, or flagellin proteins, respectively (Sharma and Kanneganti, 2016). The upstream NAIP proteins are also transcriptionally regulated by the transcription factor IRF8 (Karki et al., 2018). The AIM2 inflammasome is activated by cytosolic double-stranded DNA binding with AIM2, which is detected upon viral entry into the cytosol or downstream of IRF1-mediated expression of IFN-stimulated genes that lyse intracellular bacteria (Fernandes-Alnemri et al., 2009; Hornung et al., 2009; Man et al., 2015; Meunier et al., 2015). The mouse NLRP1b inflammasome is activated following proteolysis after exposure to Bacillus anthracis lethal toxin and DPP8/9 inhibitors (Chavarría-Smith and Vance, 2013; Okondo et al., 2017). Newer studies have shown that endogenous human DPP9 binds and negatively regulates NLRP1, and a mutation in this binding domain is associated with NLRP1-associated autoinflammation with arthritis and dyskeratosis (NAIAD; Zhong et al., 2018). DPP8/9 inhibitors activate murine NLRP1b by inducing proteasomal degradation of the autoinhibitory N-terminal fragment, while anthrax lethal toxin functions by induction of N-end rule–mediated proteolysis of the N-terminal fragment of NLRP1 (Okondo et al., 2018; Chui et al., 2019; de Vasconcelos et al., 2019b; Sandstrom et al., 2019). Pyrin inflammasome activation is triggered by RhoA-GTPase–modifying toxins (Xu et al., 2014). Concurrent with GSDMD cleavage, caspase-1 activation cleaves inactive pro-IL-1β and pro-IL-18 into their active forms. The formation of GSDMD pores in the plasma membrane facilitates the release of intracellular contents and mature IL-1β and IL-18, leading to inflammatory signaling in responding cells, which can have both beneficial and detrimental effects. Following pore formation by GSDMD, other inflammatory cytokines and alarmins that do not need processing by caspase-1, such as IL-1α, IL-33, HMGB1, ATP, and ASC complexes, are released, further contributing to the inflammatory response (Vande Walle et al., 2011; Basiorka et al., 2018; Frank and Vince, 2019). Together, the majority of these inflammasome complexes function to expel intracellular pathogens from the cell or halt viral replication in addition to releasing inflammatory cytokines, serving a critical role in host defense. Excessive or dysregulated activation of these complexes can also be detrimental to the host and is associated with inflammatory diseases, as discussed later in this review.
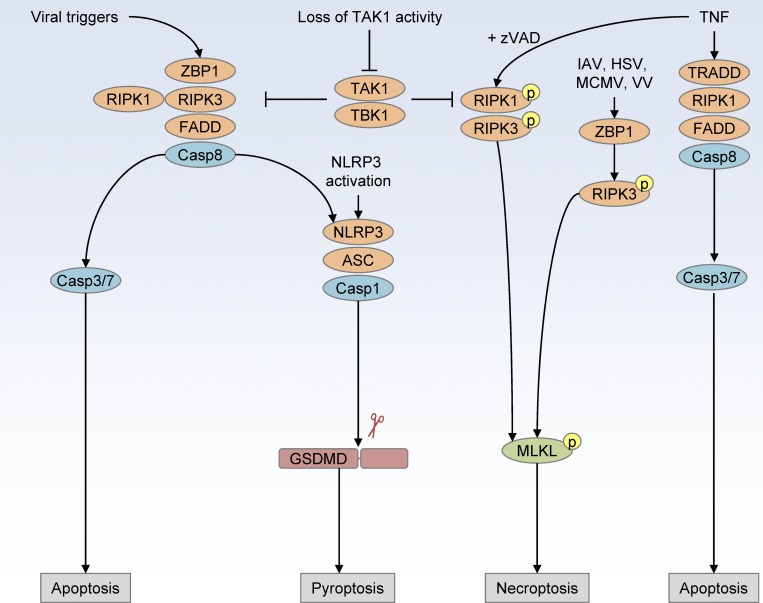
Similar to pyroptosis, necroptosis is a lytic form of cell death that is activated by a different pathway. In cells where caspase-8 is inhibited or missing, inflammatory signaling via TNF superfamily receptors, IFNs, TLR3, or TLR4 can lead to the phosphorylation of RIPK1, RIPK3, and ultimately mixed-lineage kinase domain-like pseudokinase (MLKL; Fig. 2; Holler et al., 2000; Cho et al., 2009; He et al., 2009; Linkermann and Green, 2014; Wang et al., 2014; Mocarski et al., 2015). Following phosphorylation, MLKL assembles into oligomers that create a channel in the plasma membrane, disrupting the ion homeostasis of the cell, similar to GSDMD-mediated lysis (Holler et al., 2000; Sun et al., 2012; Zhao et al., 2012; Linkermann and Green, 2014; Wang et al., 2014). The conditions for necroptosis are fairly strict in comparison to pyroptosis, but recent research has identified scenarios in which this pathway is engaged. Physiologically, it seems that necroptosis plays a backup role for cell death when the apoptotic pathway becomes inhibited by pathogens, mutations, changes in the regulators of apoptosis, or cancer (Pasparakis and Vandenabeele, 2015). Due to the complicated role of Fas-associated protein with death domain (FADD), caspase-8, RIPK1, RIPK3, and MLKL in regulating this form of cell death and also in regulating cytokine production, it is difficult to definitively attribute necroptosis to the progression of many diseases. New mouse models, chemical inhibitors, and assays that probe for phosphorylation of MLKL are helping to advance our understanding of the role of necroptosis in driving diseases (Pasparakis and Vandenabeele, 2015; Weinlich et al., 2017).
Figure 2.
Cell death crosstalk overview. Activation of ZBP1 recruits RIPK3, FADD, and caspase-8 to drive parallel cell death pathways of apoptosis and pyroptosis. NLRP3 activation leads to ASC and caspase-1 inflammasome assembly and cleavage of the pyroptosis executioner GSDMD. TNF-mediated cell death induces apoptosis via TRADD, RIPK1, RIPK3, caspase-8, caspase-3, and caspase-7, but, upon inhibition of caspase-8 (by zVAD), TNF drives RIPK1, RIPK3, and MLKL phosphorylation to drive necroptotic cell death. The nucleic acid binding protein ZBP1 can also be directly activated by viral infection (influenza A virus [IAV], HSV, mouse cytomegalovirus [MCMV], and vaccinia virus [VV]) to induce cell death through parallel pathways of necroptosis, apoptosis, and pyroptosis.
Crosstalk between apoptosis, necroptosis, and pyroptosis makes it difficult to determine how each pathway contributes to complex inflammatory diseases (Fig. 2). For example, apoptosis signaling through FADD and caspase-8 has been shown to link signaling between TLRs and priming of the NLRP3 inflammasome, and many of the proteins of the apoptosis pathway also regulate necroptosis (Elmore, 2007; Gurung et al., 2014). In addition to caspase-8–mediated priming of the inflammasome, others have observed that caspase-8 can localize to caspase-1– and ASC-containing specks, facilitating inflammasome activation and downstream cytokine maturation (Gringhuis et al., 2012; Man et al., 2013; Karki et al., 2015). Treatment of colorectal cancer cells with the platinum-based, DNA-damaging chemotherapy drug lobaplatin resulted in caspase-3–dependent cleavage of GSDME, a second gasdermin family pore-forming protein (Yu et al., 2019). Infection with Yersinia spp. has also revealed a role for caspase-8–mediated cleavage of GSDMD, suggesting this plays a previously understudied role in regulating crosstalk between apoptosis and pyroptosis (Orning et al., 2018; Sarhan et al., 2018a). While recent studies have shown that crosstalk between apoptotic signaling pathways with necroptosis and pyroptosis occurs, we focus here on the lytic forms of cell death as the final outcome that drives inflammatory pathology. In human cell lines, others have found that MLKL ion channel formation can lead to NLRP3 inflammasome activation in a cell-intrinsic manner, suggesting that this crosstalk between necroptosis and pyroptosis plays a larger role than previously appreciated (Conos et al., 2017; Gutierrez et al., 2017). Other recent studies have described key new players that regulate crosstalk between cell death pathways. Inhibition or loss of TAK1 leads to autocrine TNF-mediated RIPK1-dependent cell death through combined apoptosis, pyroptosis, and necroptosis pathways (Malireddi et al., 2018). In some viral infections, it also appears that the protein ZBP1 can act as a master regulator of all three cell death pathways, which requires further study in other contexts (Kuriakose et al., 2016). Importantly, these lytic forms of cell death also regulate the release of inflammatory cytokines that have been targeted successfully in many disease treatments. New studies examining the executioners of pyroptosis and necroptosis and their crosstalk in different contexts will shed more light on their physiological relevance.
Monogenic inflammatory diseases
Due to the potent inflammatory signaling role of IL-1 and IL-18, the dysregulated activation and release of these cytokines contribute to many diseases (Table 1). Well-characterized gain-of-function mutations in inflammasome sensors lead to excessive production of these cytokines and cell death, underlying the pathogenesis of a number of monogenic diseases. Gain-of-function mutations in pyrin are associated with the autoinflammatory disease familial Mediterranean fever (FMF) and drive the excessive release of IL-1β via GSDMD-mediated pyroptosis in a mouse model of FMF (Sharma et al., 2017; Kanneganti et al., 2018). While commonly treated with colchicine, recent work has shown that inhibition of IL-1 with anakinra (recombinant IL-1R antagonist), canakinumab (anti–IL-1β monoclonal antibody), or rilonacept (decoy IL-1R/IL1-RA/IgG1 fusion protein) can improve outcomes in colchicine-resistant FMF and that GSDMD inhibitors may also play a future role (Varan et al., 2019). In addition to the role of pyroptosis-mediated IL-1 release, a recent study showed that TNF signaling promoted the expression of pyrin in a positive feedback loop (Sharma et al., 2019). Patients with FMF also exhibit elevated serum IL-18, but whether this promotes disease and is a promising target for inhibition is unclear (Wada et al., 2018).
Table 1. Monogenic autoinflammatory diseases.
| Disease | Key regulator | Treatment target | Reference |
|---|---|---|---|
| CAPS | NLRP3 IL-1β GSDMD |
IL-1β (canakinumab) IL-1R (anakinra) IL-1 decoy receptor (rilonacept) NLRP3 (MCC950)? GSDMD? |
Goldbach-Mansky et al., 2006; Gattorno et al., 2007; Lachmann et al., 2009; Kitamura et al., 2014; Coll et al., 2015; Xiao et al., 2018 |
| FMF and PAAND | Pyrin IL-1β TNF IL-18? GSDMD |
IL-1R (anakinra) IL-1β (canakinumab) IL-1 decoy receptor (rilonacept) IL-18? GSDMD? |
Hashkes et al., 2012; Sharma et al., 2017, 2019; Varan et al., 2019 |
| DIRA | IL-1RA | IL-1R (anakinra) | Jesus and Goldbach-Mansky, 2014 |
| Blau syndrome/PGA | NOD2 IL-1β |
IL-1R (anakinra) IL-1β (canakinumab) TNF |
Aróstegui et al., 2007; Simonini et al., 2013 |
| MKD/HIDS | MVK Pyrin IL-1β |
IL-1R (anakinra) IL-1β (canakinumab) |
Manthiram et al., 2017 |
| TRAPS | TNFR IL-1β GSDMD? MLKL? |
IL-1R (anakinra) IL-1β (canakinumab) |
Jesus and Goldbach-Mansky, 2014 |
| Majeed syndrome | LPIN2-NLRP3 IL-1β |
IL-1R (anakinra) IL-1β (canakinumab) |
Jesus and Goldbach-Mansky, 2014 |
| PAPA syndrome | PSTPIP1-Pyrin IL-1β IL-18? |
IL-1R (anakinra) IL-1β (canakinumab) TNF |
Kanameishi et al., 2017; Manthiram et al., 2017 |
| PFIT | WDR1-Pyrin IL-18 GSDMD? |
IL-18? | Standing et al., 2017 |
| AIFEC | NLRC4 IL-18 IL-1? IFNγ? |
IL-18? | Romberg et al., 2017 |
| NAIAD | NLRP1 DPP9 |
IL-1? IL-18? NLRP1? GSDMD? |
Grandemange et al., 2017; Zhong et al., 2018 |
| NLRP12AD | NLRP12 IL-1 GSDMD? TNF? |
IL-1R (anakinra)? | Jéru et al., 2011 |
| Chronic proliferative dermatitis | Sharpin TNF RIPK1 RIPK3 MLKL NLRP3 IL-1R IL-1β |
TNF? RIPK1 (Necrostatin-1)? RIPK3 (GSK-872)? MLKL (necrosulfonamide) IL-1R (anakinra)? NLRP3 (MCC950)? GSDMD? |
HogenEsch et al., 1993; Gijbels et al., 1996; Gerlach et al., 2011; Kumari et al., 2014; Rickard et al., 2014; Gurung et al., 2016b |
AIFEC, autoinflammation with infantile enterocolitis; DIRA, deficiency of IL-1R antagonist; HIDS, hyper IgD syndrome; MKD, mevalonate kinase deficiency; NLRP12AD, NLRP12-associated autoinflammatory disease; PAAND, Pyrin-associated autoinflammation with neutrophilic dermatoses; PAPA, pyogenic arthritis, pyoderma gangrenosum, and acne; PFIT, periodic fever, immunodeficiency, and thrombocytopenia; PGA, pediatric granulomatous arthritis; TRAPS, TNF receptor–associated periodic fever.
Autoinflammatory diseases associated with NLRP3 mutations, collectively described as cryopyrin-associated periodic syndromes (CAPS), include familial cold autoinflammatory syndrome, Muckle-Wells syndrome, and neonatal-onset multisystem inflammatory disorder/chronic infantile neurological, cutaneous, and articular syndrome (Manthiram et al., 2017). Together, these diseases are associated with systemic inflammation, neutrophilia, fever, rashes, and joint pain and are successfully treated with anakinra, rilonacept, and canakinumab and may be treated with NLRP3 or GSDMD inhibitors in the future (Lachmann et al., 2009; Gillespie et al., 2010; Coll et al., 2015; Xiao et al., 2018). In addition to FMF and CAPS, other well-characterized diseases driven by IL-1, including deficiency of IL-1R antagonist; Blau syndrome; mevalonate kinase deficiency; Majeed syndrome; and PAPA (pyogenic arthritis, pyoderma gangrenosum, and acne) syndrome, are effectively treated with IL-1 blockade, but may eventually also be treated by inhibiting GSDMD or IL-18.
Understanding the mechanism behind other monogenic diseases associated with excessive IL-1 or TNF production has led to successful treatment with neutralizing antibodies against these cytokines, summarized in Table 1. In TNF receptor-associated periodic syndrome, which is mediated by a mutation that causes hyperactivation of TNFR1, IL-1 blockade but not TNF blockade was shown to be effective in treating patients, suggesting that treatments targeting the downstream TNF-mediated cell death, possibly via inhibition of MLKL and GSDMD, may be effective alternatives (Jesus and Goldbach-Mansky, 2014; Gattorno et al., 2017). In mice, a mutation in Sharpin (Sharpincpdm) causes mice to develop chronic proliferative dermatitis, which is driven by excessive TNF production, which leads to excessive epithelial cell apoptosis and inflammation that is promoted by RIPK1, RIPK3, MLKL, caspase-1/11, NLRP3, and IL-1β, suggesting that inhibition of cell death in this model may protect from similar skin disorders in humans (Gijbels et al., 1996; Kumari et al., 2014; Rickard et al., 2014; Gurung et al., 2016b). In humans, the NLRP1 P1214R mutation, adjacent to the autocleavage site F1212-S1213, is associated with NAIAD, and molecular studies have found that DPP9 binding to the NLRP1 FIIND domain is disrupted by this mutation, suggesting that the binding of DPP9 and possibly DPP9-mediated degradation of the autoinhibitory C-terminal fragment of NLRP1 is lost in this disease (Grandemange et al., 2017; Zhong et al., 2018). Loss of the executioner of pyroptotic cell death, GSDMD, in mouse models of FMF and CAPS protects mice from disease, suggesting that inhibitors against GSDMD or other steps of the pyroptosis signaling cascade may also show therapeutic potential in other inflammatory disorders.
IL-1–mediated diseases
Mutations in key regulators of the pyroptosis pathway are known to drive autoinflammatory diseases. In addition to monogenic diseases, other complex inflammatory diseases can be successfully treated with IL-1 blockade. These inflammatory diseases include Behcet’s disease, Sweet’s syndrome, Schnitzler’s syndrome, adult-onset Still’s disease, and juvenile idiopathic disease, summarized in Table 2. Because IL-1 is elevated in multiple inflammatory diseases, it is suspected to contribute to the progression of gout, rheumatoid arthritis, kidney disease, lung disease, cancer, and many others reviewed elsewhere (Jesus and Goldbach-Mansky, 2014; Cavalli and Dinarello, 2018).
Table 2. Cell death–mediated inflammatory cytokine-driven diseases.
| Disease | Key regulator | Treatment target | Reference |
|---|---|---|---|
| Behcet’s disease | IL-1 IL-18? |
IL-1R (anakinra) IL-1β (canakinumab) |
Masters, 2013; Vitale et al., 2016 |
| Sweet’s syndrome (including neutrophilic dermatoses) | PTPN6? RIPK1 IL-1α MLKL? |
IL-1α? GSDMD? MLKL? |
Nesterovitch et al., 2011; Lukens et al., 2013 |
| Schnitzler syndrome | IL-1β | IL-1R (anakinra) IL-1β (canakinumab) IL-1 decoy receptor (rilonacept) |
de Koning et al., 2015 |
| AOSD | IL-1β IL-18? |
IL-1R (anakinra) IL-1β (canakinumab) IL-1 decoy receptor (rilonacept) |
Lee et al., 2015; Junge et al., 2017 |
| JIA/soJIA | IL-1 | IL-1R (anakinra) IL-1β (canakinumab) IL-1 decoy (rilonacept) |
Pascual et al., 2005; Cimaz, 2016 |
| HLH | IL-1? IL-18? |
IL-1R (anakinra)? IL-18? |
Schulert and Grom, 2015 |
| Osteomyelitis | IL-1β NLRP3 Caspase-8 MLKL? |
IL-1R (anakinra)? MLKL? |
Lukens et al., 2014; Gurung et al., 2016a |
| Lung cancer | IL-1β | IL-1β (canakinumab) | Ridker et al., 2017b |
| Cardiovascular diseases | IL-1α?, IL-1β | IL-1β (canakinumab) | Ridker et al., 2017a |
| Gout | NLRP3 MSU crystals IL-1 GSDMD MLKL? |
IL-1R (anakinra)? IL-1β (canakinumab)? IL-1 decoy (rilonacept)? |
Dayer et al., 2017 |
| Kidney diseases | IL-18 IL-1β RIPK3 MLKL? GSDMD? |
IL-18? MLKL (necrosulfonamide)? GSDMD? |
Wu et al., 2008; Chen et al., 2018a; Sarhan et al., 2018b |
| Alzheimer’s disease | NLRP3 ASC |
NLRP3 (MCC950)? ASC? GSDMD? |
Venegas et al., 2017 |
| Ischemic brain injury | NLRP3 NLRC4? GSDMD RIPK1 RIPK3? MLKL? |
RIPK1 (Necrostatin-1) RIPK3 (GSK-872) |
Degterev et al., 2005; Chen et al., 2018b; Xu et al., 2018b; Poh et al., 2019; Zhang et al., 2019 |
| Rheumatoid arthritis | TNF IL-1? GSDMD? MLKL? |
TNF | Yamanaka, 2015; Dayer et al., 2017 |
| Septic shock | Caspase-11 GSDMD Caspase-8 |
GSDMD? | Kayagaki et al., 2015; Kang et al., 2018; Mandal et al., 2018 |
| Inflammatory bowel diseases | MLKL? NLRP3 IL-1α |
NLRP3 (MCC950)? IL-1α (MABp1)? MLKL (necrosulfonamide)? |
Malik et al., 2016; Li et al., 2018 |
AOSD, adult-onset Still’s disease; HLH, hemophagocytic lympho-histiocytosis; JIA, juvenile idiopathic arthritis; soJIA, systemic-onset juvenile idiopathic arthritis.
The recently completed Canakinumab Antiinflammatory Thrombosis Outcomes Study (CANTOS), in which a large cohort of 10,061 patients was treated with canakinumab following myocardial infarction, found that neutralizing IL-1β–mediated inflammation reduced recurrent cardiovascular events (Ridker et al., 2017a). In atherosclerotic disease, it is thought that cell death, pyroptosis, and necroptosis lead to instability of plaques, which further suggests that blockade of these cell death pathways or their cytokine release may provide protection (Leeper, 2016; Xu et al., 2018c). Following the completion of the study, a second analysis of the CANTOS data found that canakinumab also had a significant effect on reducing lung cancer incidence and mortality, which was an unexpected finding from the trial (Ridker et al., 2017b). In the tumor microenvironment, IL-1–driven inflammation is thought to increase tumor invasiveness, metastasis, and growth (Apte et al., 2006; Gottschlich et al., 2018; Karki and Kanneganti, 2019). Together, these data provide compelling evidence that inhibiting IL-1β can have profound benefits in humans. While the protection from lung cancer was observed in patients with atherosclerosis, future studies in other patient populations will determine whether IL-1β inhibition can broadly protect from this devastating disease.
While IL-1β is often implicated in IL-1–driven diseases, the IL-1 family of proteins contains many other members including IL-1α, IL-18, IL-33, IL-36α/β/γ, IL-37, and IL-38 (Dinarello, 2018). Importantly, IL-1α and IL-1β both bind the IL-1R, but the role for IL-1α is poorly understood in most disease conditions. Treatments targeting the IL-1R or both IL-1α and IL-1β (as with IL-1R–blocking anakinra or the decoy IL-1R drug rilonacept) have shown great success, but future trials will examine whether targeting either IL-1α or IL-1β alone may provide additional benefits in specific disease models or reduce side effects of dual inhibition. An IL-1α–specific neutralizing antibody (bermekimab, MABp1) is currently in clinical trials to determine its effectiveness in multiple inflammatory diseases (Dinarello, 2018). Unlike IL-1β, IL-1α does not require caspase-1–mediated cleavage for its activation and can be released from pyroptotic or necroptotic cells. In a mouse model of neutrophilic dermatosis (via mutation in PTPN6), both RIPK1-driven up-regulation of inflammatory cytokines and IL-1α promote disease independently of inflammasome signaling and RIPK3, raising the question of whether MLKL or GSDMD may cooperate in some way to facilitate the release of IL-1α (Lukens et al., 2013). In patients with colorectal cancer, bermekimab-mediated neutralization of IL-1α appears to protect against chemotherapy-induced weight loss (Hickish et al., 2017; O’Sullivan Coyne and Burotto, 2017). In patients with TNF blockade–resistant severe hidradenitis suppurativa, bermekimab also seems to reduce symptoms (Kanni et al., 2018). Blockade of IL-1α may also protect patients from psoriasis and is being studied (Coleman et al., 2015). More studies will likely reveal an important role for IL-1α in driving many other diseases independent from inflammasome-driven production of IL-1β.
IL-18–driven diseases
The second key cytokine processed by caspase-1 and released by pyroptosis is IL-18, a key costimulatory cytokine of NK and T cell IFNγ responses (Dinarello, 2018). Interestingly, a subset of autoinflammatory diseases associated with mutations in the pyroptosis machinery are poorly responsive to IL-1 blockade and better treated with IL-18 blockade. Excessive release of IL-18 in diseases, including Behcet’s disease, hemophagocytic histiocytosis (HLH) with macrophage activation syndrome, and NLRC4-mediated autoinflammation with infantile enterocolitis is thought to contribute to their pathology, suggesting that IL-18 blockade may be beneficial in treating these diseases (Masters, 2013; Schulert and Grom, 2015; Vitale et al., 2016; Romberg et al., 2017). Blockade of IL-18 may be beneficial in the context of colitis, inflammatory bowel disease, or cardiac disease, but conflicting findings on harmful and beneficial roles for IL-18 require more study (Zaki et al., 2010; Dinarello et al., 2013; Kanai et al., 2013; Toldo et al., 2014). IL-18 is also thought to contribute to the pathogenesis of a number of skin diseases, including psoriasis, atopic dermatitis, and lupus, but more definitive studies are needed to determine whether inhibition with IL-18 binding protein (IL-18BP) will be beneficial (Lee et al., 2015). Blockade strategies include recombinant IL-18BP or neutralizing antibodies targeting IL-18. To date, it is not clear where IL-18 blockade or supplementation will be useful clinically, but ongoing clinical trials and mouse studies should reveal important contributions for this caspase-1–regulated cytokine.
Pyroptosis-driven diseases
While it has been understood for years that caspase-1 regulates pyroptosis and inflammasome-dependent cytokine release upon activation of specific inflammasome complexes, the recent discovery of GSDMD as the executioner of pyroptosis provides a new way of studying the relative contributions of both pyroptotic cell death and functional cytokine release. This is particularly important in light of many recent studies showing that the apoptotic, necroptotic, and pyroptotic pathways interact. Therapeutically, inhibition of GSDMD directly may prevent the release of cytokines as well as other alarmins released as a consequence of pyroptotic cell death that also contribute to disease progression, such as IL-1α, IL-33, HMGB1, or ATP. This is of particular interest in the treatment of sepsis, which in mouse models is critically dependent on caspase-11 and GSDMD, suggesting inhibitors may be able to effectively treat patients and block the release of many of the inflammatory mediators of septic shock (Kayagaki et al., 2015; Shi et al., 2015; Rathkey et al., 2018). Recent studies have identified two potential inhibitors of GSDMD that may hold therapeutic value. A GSDMD-derived peptide inhibitor, Ac-FLTD-CMK, inhibits pyroptosis by acting as an uncleavable substrate (Yang et al., 2018). Necrosulfonamide, an inhibitor that disrupts disulfide bonds in both GSDMD (Cys191) and human (but not murine) MLKL (Cys86), inhibits both pyroptosis and necroptosis, suggesting it may be useful to inhibit both cell death modalities in patients (Rathkey et al., 2018). In the brain, microglia are proposed to release ASC specks, which can seed amyloid-β plaques, suggesting that pyroptosis may promote Alzheimer’s disease (Venegas et al., 2017). Downstream inflammatory consequences of this release are likely to promote an inflammatory positive feedback loop. In mouse models of CAPS, FMF, and septic shock, GSDMD deficiency alone protects from disease, suggesting that cytokine inhibition may not be necessary in some diseases (Kayagaki et al., 2015; Kanneganti et al., 2018; Xiao et al., 2018). Interestingly, in the absence of GSDMD, apoptotic pathways are sometimes activated, which likely has important implications for negative regulation of cytokine release (Taabazuing et al., 2017). Future research will likely reveal other diseases that are promoted specifically by GSDMD-mediated pyroptosis and may lead to better treatments than specific cytokine blockade.
Necroptosis-driven diseases
Compared with pyroptosis, where upstream caspase-1–mediated cleavage of its substrates IL-1β and IL-18 is required for their activity, necroptosis is not known to be associated with any specific processing of inflammatory mediators before cell lysis. Necroptosis is also generally considered a backup form of cell death when parts of the apoptotic pathway are inhibited by pathogens or inhibitors (Pasparakis and Vandenabeele, 2015). Interestingly, recent studies have shown that necroptosis (often determined by phospho-MLKL) plays a role in the pathogenesis of a number of diseases. Recent studies have shown that necroptosis promotes ischemic tissue damage. In kidneys, ischemia-reperfusion injury is promoted by RIPK3 and MLKL, apparently by regulating the cell death of renal proximal tubular cells, with subsequent recruitment of macrophages and NLRP3 inflammasome activation. Inhibitors targeting RIPK1, RIPK3, and MLKL appear to reduce this kidney damage, but MLKL deficiency alone does not necessarily protect from kidney reperfusion injury, suggesting there may be other interacting forms of cell death that drive injury (Linkermann et al., 2013; Newton et al., 2016; Anders, 2018; Chen et al., 2018a). In the liver, alcohol-induced damage was reduced in the absence of RIPK3 and MLKL, suggesting that necroptosis promotes disease (Roychowdhury et al., 2013; Wang et al., 2016). During spinal injury, it has also been proposed that microglia undergo necroptosis and that treatment with necrosulfonamide can reduce phospho-MLKL and pathology (Fan et al., 2015; Huang et al., 2018; Wang et al., 2018). In the mouse Sharpincpdm model, skin inflammation is driven by necroptosis via TNF, RIPK1, RIPK3, MLKL, and downstream inflammasome-mediated IL-1β (Kumari et al., 2014; Rickard et al., 2014; Gurung et al., 2016b). Together, these studies suggest that necroptosis can initiate and drive multiple inflammatory diseases and may be an effective target for future therapies.
Implications of cell death crosstalk
Pyroptosis and necroptosis both lead to lytic cell death after stimulation with inflammatory mediators such as pathogens, alarmins, and cytokines such as TNF. Genetic studies have shown that necroptotic signaling via RIPK3, MLKL, NLRP3, and caspase-1 leads to release of mature IL-1β independent of GSDMD (Moriwaki et al., 2015; Conos et al., 2017; Gutierrez et al., 2017). Mechanistically, this occurs through MLKL-mediated plasma membrane disruption, efflux of potassium ions, and subsequent NLRP3 inflammasome-mediated maturation of IL-1β that can be released by necroptosis. In inflamed tissues, this likely contributes significantly to the development of inflammatory diseases. Earlier studies also hinted at crosstalk between apoptotic and pyroptotic pathways, finding that caspase-8 can drive inflammasome activation (Man et al., 2013; Gurung et al., 2014, 2016a). During infections or under the condition of TAK1 inactivation, multiple cell death pathways can be engaged to prevent pathogen-mediated inhibition of cell death and facilitate effective immune responses (Kuriakose et al., 2016; Malireddi et al., 2018; Orning et al., 2018; Sarhan et al., 2018a; Xu et al., 2018a). Similarly, loss of TBK1 results in spontaneous cell death that is driven by RIPK1 (Xu et al., 2018a).
During multiple viral infections, the sensor ZBP1 coordinates parallel cell death pathways of necroptosis, apoptosis, and pyroptosis with potential to regulate cell death during other currently unknown diseases (Kuriakose et al., 2016; Koehler et al., 2017; Maelfait et al., 2017; Guo et al., 2018). In physiological conditions, it is not surprising that RIPK1- and RIPK3-mediated expression of inflammatory cytokines or induction of necroptosis indirectly promote further downstream apoptosis, necroptosis, or pyroptosis in other nearby cells. In cancer, for example, changes in the apoptotic death pathways likely change the form of cell death that occurs downstream of TNF or other inflammatory signals. A recent study showed that in colorectal cancer cells, lobaplatin treatment induces a unique caspase-3–mediated cleavage of GSDME, a pore-forming protein related to GSDMD, inducing a pyroptosis-like cell death (Yu et al., 2019). In endotoxic shock, recent work has shown that the combined action of caspase-11 and caspase-8, independent of RIPK1 kinase activity and RIPK3, drive endotoxin-mediated pathology in the small intestine and spleen. In addition to these key molecules, production of TNF and type I IFNs also promoted caspase-8–dependent apoptosis in the endotoxic shock model, highlighting an important role for both pyroptosis and apoptosis (Mandal et al., 2018). This highlights the importance of understanding which particular cell death pathways are involved in each unique disease scenario (Yu et al., 2019).
A key problem in treating multifactorial diseases such as chronic inflammatory bowel disease, rheumatoid arthritis, psoriasis, and many others is identifying which steps of the inflammatory process initiate and promote disease. The relative success of TNF blockade in treating many of these diseases suggests that TNF-driven inflammation and likely TNF-mediated apoptosis help to drive the disease, but some patients eventually need to discontinue TNF blockade to avoid serious infectious complications (Ko et al., 2009; Ali et al., 2013; Yamanaka, 2015; Katsanos et al., 2018). Understanding whether TNF promotes increased apoptosis, necroptosis, pyroptosis, and cytokine release to promote disease may suggest alternative treatments. In ischemic brain injury, NLRC4, NLRP3, GSDMD, RIPK1, RIPK3, and MLKL have all been proposed to promote pyroptosis and necroptosis in microglia or neuronal cells, and necrostatin-1 (an inhibitor of RIPK1) has been shown to ameliorate injury, but the role of each death pathway needs closer study (Degterev et al., 2005; Xu et al., 2018b; Poh et al., 2019; Zhang et al., 2019). In a model of osteomyelitis, mutations in PSTPIP2 lead to excessive TNF, osteoclastogenesis, and neutrophil recruitment that destroys bone in a caspase-8–, NLRP3-, and IL-1β–dependent manner, suggesting that crosstalk between the apoptotic, necroptotic, and pyroptotic pathways is occurring (Lukens et al., 2014; Gurung et al., 2016a). Whether the executioner molecules GSDMD or MLKL also play a role in promoting PSTPIP2-mediated bone disease remains to be seen. These cytokines and pathways also likely promote inflammation associated with rheumatoid arthritis of the joints (Jung et al., 2014; Yamanaka, 2015). Cancers are often driven by proinflammatory cytokine production, so future work may reveal interesting roles for the executioners MLKL and GSDMD in promoting tumor growth, invasiveness, and metastasis (Apte et al., 2006; Ridker et al., 2017b). Identifying the most upstream pathway in each of these diseases will help determine which treatments may be best for preventing or treating them.
Future perspectives
Better understanding of the molecular mechanisms regulating apoptosis, pyroptosis, necroptosis, and the executioner molecules of each pathway may lead to better treatment of inflammatory diseases driven by the release of inflammatory cytokines and alarmins. The extensive crosstalk that researchers have observed between each of these pathways also suggests that it will be difficult to specifically target the appropriate cell death pathway without first understanding the critical regulators of individual diseases. Indeed, when cells receive a lethal trigger, they will often set off multiple overlapping signal cascades that lead to a switch in cell death when one key molecule is inhibited, as exemplified by necroptosis. In addition to understanding which forms of cell death promote a particular disease, it will be important to understand how the release of inflammatory cytokines and alarmins also contributes. A major concern with any immunosuppressive approach targeting necroptosis or pyroptosis is that patients may become susceptible to opportunistic infections, so carefully balancing the immunosuppression with this risk is critical.
Emerging work on sublethal pyroptosis and necroptosis, where membrane repair through ESCRT negatively regulates cell lysis and cytokine release, presents an interesting new mechanism by which inflammatory diseases may be treated, though whether this pathway plays a major role is unclear (Gong et al., 2017; Rühl et al., 2018). Other recent studies have hinted at new mechanisms of inflammatory cytokine release that are GSDMD independent or GSDMD dependent but cell lysis independent, although other studies show that GSDMD-dependent rupture is required for cytokine release (Heilig et al., 2018; Monteleone et al., 2018; de Vasconcelos et al., 2019a). The physiological relevance of these alternative pathways is unclear and requires further study. Armed with our current understanding of these pathways, novel therapeutics specifically targeting pyroptosis- and necroptosis-mediated cell death, their upstream regulators, the apoptosis pathway, and the availability of cytokine-neutralizing antibodies hold great promise to treat a wide range of inflammatory diseases.
Acknowledgments
The authors acknowledge many investigators in the field whose primary data could not be cited in this review because of space limitations.
This work was supported by funding from American Lebanese Syrian Associated Charities to T.-D. Kanneganti.
The authors declare no competing financial interests.
References
- Ali T., Kaitha S., Mahmood S., Ftesi A., Stone J., and Bronze M.S.. 2013. Clinical use of anti-TNF therapy and increased risk of infections. Drug Healthc. Patient Saf. 5:79–99. 10.2147/DHPS.S28801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders H.-J. 2018. Necroptosis in Acute Kidney Injury. Nephron. 139:342–348. 10.1159/000489940 [DOI] [PubMed] [Google Scholar]
- Apte R.N., Dotan S., Elkabets M., White M.R., Reich E., Carmi Y., Song X., Dvozkin T., Krelin Y., and Voronov E.. 2006. The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis and tumor-host interactions. Cancer Metastasis Rev. 25:387–408. 10.1007/s10555-006-9004-4 [DOI] [PubMed] [Google Scholar]
- Aróstegui J.I., Arnal C., Merino R., Modesto C., Antonia Carballo M., Moreno P., García-Consuegra J., Naranjo A., Ramos E., de Paz P., et al. . 2007. NOD2 gene-associated pediatric granulomatous arthritis: clinical diversity, novel and recurrent mutations, and evidence of clinical improvement with interleukin-1 blockade in a Spanish cohort. Arthritis Rheum. 56:3805–3813. 10.1002/art.22966 [DOI] [PubMed] [Google Scholar]
- Basiorka A.A., McGraw K.L., Abbas-Aghababazadeh F., McLemore A.F., Vincelette N.D., Ward G.A., Eksioglu E.A., Sallman D.A., Ali N.A., Padron E., et al. . 2018. Assessment of ASC specks as a putative biomarker of pyroptosis in myelodysplastic syndromes: an observational cohort study. Lancet Haematol. 5:e393–e402. 10.1016/S2352-3026(18)30109-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalli G., and Dinarello C.A.. 2018. Anakinra Therapy for Non-cancer Inflammatory Diseases. Front. Pharmacol. 9:1157 10.3389/fphar.2018.01157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavarría-Smith J., and Vance R.E.. 2013. Direct proteolytic cleavage of NLRP1B is necessary and sufficient for inflammasome activation by anthrax lethal factor. PLoS Pathog. 9:e1003452 10.1371/journal.ppat.1003452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Fang Y., Wu J., Chen H., Zou Z., Zhang X., Shao J., and Xu Y.. 2018a RIPK3-MLKL-mediated necroinflammation contributes to AKI progression to CKD. Cell Death Dis. 9:878 10.1038/s41419-018-0936-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T., Pan H., Li J., Xu H., Jin H., Qian C., Yan F., Chen J., Wang C., Chen J., et al. . 2018b Inhibiting of RIPK3 attenuates early brain injury following subarachnoid hemorrhage: Possibly through alleviating necroptosis. Biomed. Pharmacother. 107:563–570. 10.1016/j.biopha.2018.08.056 [DOI] [PubMed] [Google Scholar]
- Cho Y.S., Challa S., Moquin D., Genga R., Ray T.D., Guildford M., and Chan F.K.-M.. 2009. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 137:1112–1123. 10.1016/j.cell.2009.05.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chui A.J., Okondo M.C., Rao S.D., Gai K., Griswold A.R., Johnson D.C., Ball D.P., Taabazuing C.Y., Orth E.L., Vittimberga B.A., and Bachovchin D.A.. 2019. N-terminal degradation activates the NLRP1B inflammasome. Science. 364:82–85. 10.1126/science.aau1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimaz R. 2016. Systemic-onset juvenile idiopathic arthritis. Autoimmun. Rev. 15:931–934. 10.1016/j.autrev.2016.07.004 [DOI] [PubMed] [Google Scholar]
- Coleman K.M., Gudjonsson J.E., and Stecher M.. 2015. Open-Label Trial of MABp1, a True Human Monoclonal Antibody Targeting Interleukin 1α, for the Treatment of Psoriasis. JAMA Dermatol. 151:555–556. 10.1001/jamadermatol.2014.5391 [DOI] [PubMed] [Google Scholar]
- Coll R.C., Robertson A.A.B., Chae J.J., Higgins S.C., Muñoz-Planillo R., Inserra M.C., Vetter I., Dungan L.S., Monks B.G., Stutz A., et al. . 2015. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat. Med. 21:248–255. 10.1038/nm.3806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conos S.A., Chen K.W., De Nardo D., Hara H., Whitehead L., Núñez G., Masters S.L., Murphy J.M., Schroder K., Vaux D.L., et al. . 2017. Active MLKL triggers the NLRP3 inflammasome in a cell-intrinsic manner. Proc. Natl. Acad. Sci. USA. 114:E961–E969. 10.1073/pnas.1613305114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cookson B.T., and Brennan M.A.. 2001. Pro-inflammatory programmed cell death. Trends Microbiol. 9:113–114. 10.1016/S0966-842X(00)01936-3 [DOI] [PubMed] [Google Scholar]
- Dayer J.-M., Oliviero F., and Punzi L.. 2017. A Brief History of IL-1 and IL-1 Ra in Rheumatology. Front. Pharmacol. 8:293 10.3389/fphar.2017.00293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degterev A., Huang Z., Boyce M., Li Y., Jagtap P., Mizushima N., Cuny G.D., Mitchison T.J., Moskowitz M.A., and Yuan J.. 2005. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 1:112–119. 10.1038/nchembio711 [DOI] [PubMed] [Google Scholar]
- de Koning H.D., Schalkwijk J., Stoffels M., Jongekrijg J., Jacobs J.F.M., Verwiel E., Koenen H.J.P.M., Preijers F., Holzinger D., Joosten I., et al. . 2015. The role of interleukin-1 beta in the pathophysiology of Schnitzler’s syndrome. Arthritis Res. Ther. 17:187 10.1186/s13075-015-0696-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vasconcelos N.M., Opdenbosch N.V., Gorp H.V., Parthoens E., and Lamkanfi M.. 2019a Single-cell analysis of pyroptosis dynamics reveals conserved GSDMD-mediated subcellular events that precede plasma membrane rupture. Cell Death Differ. 26:146–161. 10.1038/s41418-018-0106-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vasconcelos N.M., Vliegen G., Gonçalves A., Hert E.D., Martín-Pérez R., Opdenbosch N.V., Jallapally A., Geiss-Friedlander R., Lambeir A.-M., Augustyns K., et al. . 2019b DPP8/DPP9 inhibition elicits canonical Nlrp1b inflammasome hallmarks in murine macrophages. Life Sci. Alliance. 2:e201900313 10.26508/lsa.201900313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C.A. 1998. Interleukin-1 beta, interleukin-18, and the interleukin-1 beta converting enzyme. Ann. N. Y. Acad. Sci. 856:1–11. 10.1111/j.1749-6632.1998.tb08307.x [DOI] [PubMed] [Google Scholar]
- Dinarello C.A. 2018. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 281:8–27. 10.1111/imr.12621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C.A., Goldin N.P., and Wolff S.M.. 1974. Demonstration and characterization of two distinct human leukocytic pyrogens. J. Exp. Med. 139:1369–1381. 10.1084/jem.139.6.1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C.A., Novick D., Kim S., and Kaplanski G.. 2013. Interleukin-18 and IL-18 binding protein. Front. Immunol. 4:289 10.3389/fimmu.2013.00289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J., Wang K., Liu W., She Y., Sun Q., Shi J., Sun H., Wang D.-C., and Shao F.. 2016. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 535:111–116. 10.1038/nature18590 [DOI] [PubMed] [Google Scholar]
- Elmore S. 2007. Apoptosis: a review of programmed cell death. Toxicol. Pathol. 35:495–516. 10.1080/01926230701320337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H., Tang H.-B., Kang J., Shan L., Song H., Zhu K., Wang J., Ju G., and Wang Y.-Z.. 2015. Involvement of endoplasmic reticulum stress in the necroptosis of microglia/macrophages after spinal cord injury. Neuroscience. 311:362–373. 10.1016/j.neuroscience.2015.10.049 [DOI] [PubMed] [Google Scholar]
- Fernandes-Alnemri T., Yu J.-W., Datta P., Wu J., and Alnemri E.S.. 2009. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 458:509–513. 10.1038/nature07710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank D., and Vince J.E.. 2019. Pyroptosis versus necroptosis: similarities, differences, and crosstalk. Cell Death Differ. 26:99–114. 10.1038/s41418-018-0212-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattorno M., Tassi S., Carta S., Delfino L., Ferlito F., Pelagatti M.A., D’Osualdo A., Buoncompagni A., Alpigiani M.G., Alessio M., et al. . 2007. Pattern of Interleukin-1B Secretion in Response to Lipopolysaccharide and ATP Before and After Interleukin-1 Blockade in Patients With CIAS1 Mutations. Arthritis and Rheumatism. 56:3138-3148. doi:10.1002/art.22842 [DOI] [PubMed]
- Gattorno M., Obici L., Cattalini M., Tormey V., Abrams K., Davis N., Speziale A., Bhansali S.G., Martini A., and Lachmann H.J.. 2017. Canakinumab treatment for patients with active recurrent or chronic TNF receptor-associated periodic syndrome (TRAPS): an open-label, phase II study. Ann. Rheum. Dis. 76:173–178. 10.1136/annrheumdis-2015-209031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach B., Cordier S.M., Schmukle A.C., Emmerich C.H., Rieser E., Haas T.L., Webb A.I., Rickard J.A., Anderton H., Wong W.W.-L., et al. . 2011. Linear ubiquitination prevents inflammation and regulates immune signalling. Nature. 471:591–596. 10.1038/nature09816 [DOI] [PubMed] [Google Scholar]
- Gijbels M.J., Zurcher C., Kraal G., Elliott G.R., HogenEsch H., Schijff G., Savelkoul H.F., and Bruijnzeel P.L.. 1996. Pathogenesis of skin lesions in mice with chronic proliferative dermatitis (cpdm/cpdm). Am. J. Pathol. 148:941–950. [PMC free article] [PubMed] [Google Scholar]
- Gillespie J., Mathews R., and McDermott M.F.. 2010. Rilonacept in the management of cryopyrin-associated periodic syndromes (CAPS). J. Inflamm. Res. 3:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldbach-Mansky R., Dailey N.J., Canna S.W., Gelabert A., Jones J., Rubin B.I., Kim H.J., Brewer C., Zalewski C., Wiggs E., et al. . 2006. Neonatal-onset multisystem inflammatory disease responsive to interleukin-1beta inhibition. N. Engl. J. Med. 355:581–592. 10.1056/NEJMoa055137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y.-N., Guy C., Olauson H., Becker J.U., Yang M., Fitzgerald P., Linkermann A., and Green D.R.. 2017. ESCRT-III Acts Downstream of MLKL to Regulate Necroptotic Cell Death and Its Consequences. Cell. 169:286–300.e16. 10.1016/j.cell.2017.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschlich A., Endres S., and Kobold S.. 2018. Can we use interleukin-1β blockade for lung cancer treatment? Transl. Lung Cancer Res. 7(S2, Suppl 2):S160–S164. 10.21037/tlcr.2018.03.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandemange S., Sanchez E., Louis-Plence P., Tran Mau-Them F., Bessis D., Coubes C., Frouin E., Seyger M., Girard M., Puechberty J., et al. . 2017. A new autoinflammatory and autoimmune syndrome associated with NLRP1 mutations: NAIAD (NLRP1-associated autoinflammation with arthritis and dyskeratosis). Ann. Rheum. Dis. 76:1191–1198. 10.1136/annrheumdis-2016-210021 [DOI] [PubMed] [Google Scholar]
- Gringhuis S.I., Kaptein T.M., Wevers B.A., Theelen B., van der Vlist M., Boekhout T., and Geijtenbeek T.B.H.. 2012. Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1β via a noncanonical caspase-8 inflammasome. Nat. Immunol. 13:246–254. 10.1038/ni.2222 [DOI] [PubMed] [Google Scholar]
- Guo H., Gilley R.P., Fisher A., Lane R., Landsteiner V.J., Ragan K.B., Dovey C.M., Carette J.E., Upton J.W., Mocarski E.S., and Kaiser W.J.. 2018. Species-independent contribution of ZBP1/DAI/DLM-1-triggered necroptosis in host defense against HSV1. Cell Death Dis. 9:816 10.1038/s41419-018-0868-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurung P., Anand P.K., Malireddi R.K.S., Vande Walle L., Van Opdenbosch N., Dillon C.P., Weinlich R., Green D.R., Lamkanfi M., and Kanneganti T.-D.. 2014. FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes. J. Immunol. 192:1835–1846. 10.4049/jimmunol.1302839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurung P., Burton A., and Kanneganti T.-D.. 2016a NLRP3 inflammasome plays a redundant role with caspase 8 to promote IL-1β-mediated osteomyelitis. Proc. Natl. Acad. Sci. USA. 113:4452–4457. 10.1073/pnas.1601636113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurung P., Sharma B.R., and Kanneganti T.-D.. 2016b Distinct role of IL-1β in instigating disease in Sharpincpdm mice. Sci. Rep. 6:36634 10.1038/srep36634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez K.D., Davis M.A., Daniels B.P., Olsen T.M., Ralli-Jain P., Tait S.W.G., Gale M. Jr., and Oberst A.. 2017. MLKL Activation Triggers NLRP3-Mediated Processing and Release of IL-1β Independently of Gasdermin-D. J. Immunol. 198:2156–2164. 10.4049/jimmunol.1601757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashkes P.J., Spalding S.J., Giannini E.H., Huang B., Johnson A., Park G., Barron K.S., Weisman M.H., Pashinian N., Reiff A.O., et al. . 2012. Rilonacept for colchicine-resistant or -intolerant familial Mediterranean fever: a randomized trial. Ann. Intern. Med. 157:533–541. 10.7326/0003-4819-157-8-201210160-00003 [DOI] [PubMed] [Google Scholar]
- He S., Wang L., Miao L., Wang T., Du F., Zhao L., and Wang X.. 2009. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 137:1100–1111. 10.1016/j.cell.2009.05.021 [DOI] [PubMed] [Google Scholar]
- He W.-T., Wan H., Hu L., Chen P., Wang X., Huang Z., Yang Z.-H., Zhong C.-Q., and Han J.. 2015. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 25:1285–1298. 10.1038/cr.2015.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y., Hara H., and Núñez G.. 2016. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem. Sci. 41:1012–1021. 10.1016/j.tibs.2016.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig R., Dick M.S., Sborgi L., Meunier E., Hiller S., and Broz P.. 2018. The Gasdermin-D pore acts as a conduit for IL-1β secretion in mice. Eur. J. Immunol. 48:584–592. 10.1002/eji.201747404 [DOI] [PubMed] [Google Scholar]
- Hickish T., Andre T., Wyrwicz L., Saunders M., Sarosiek T., Kocsis J., Nemecek R., Rogowski W., Lesniewski-Kmak K., Petruzelka L., et al. . 2017. MABp1 as a novel antibody treatment for advanced colorectal cancer: a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 18:192–201. 10.1016/S1470-2045(17)30006-2 [DOI] [PubMed] [Google Scholar]
- HogenEsch H., Gijbels M.J., Offerman E., van Hooft J., van Bekkum D.W., and Zurcher C.. 1993. A spontaneous mutation characterized by chronic proliferative dermatitis in C57BL mice. Am. J. Pathol. 143:972–982. [PMC free article] [PubMed] [Google Scholar]
- Holler N., Zaru R., Micheau O., Thome M., Attinger A., Valitutti S., Bodmer J.-L., Schneider P., Seed B., and Tschopp J.. 2000. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat. Immunol. 1:489–495. 10.1038/82732 [DOI] [PubMed] [Google Scholar]
- Hornung V., Ablasser A., Charrel-Dennis M., Bauernfeind F., Horvath G., Caffrey D.R., Latz E., and Fitzgerald K.A.. 2009. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 458:514–518. 10.1038/nature07725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z., Zhou T., Sun X., Zheng Y., Cheng B., Li M., Liu X., and He C.. 2018. Necroptosis in microglia contributes to neuroinflammation and retinal degeneration through TLR4 activation. Cell Death Differ. 25:180–189. 10.1038/cdd.2017.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jéru I., Hentgen V., Normand S., Duquesnoy P., Cochet E., Delwail A., Grateau G., Marlin S., Amselem S., and Lecron J.-C.. 2011. Role of interleukin-1β in NLRP12-associated autoinflammatory disorders and resistance to anti-interleukin-1 therapy. Arthritis Rheum. 63:2142–2148. 10.1002/art.30378 [DOI] [PubMed] [Google Scholar]
- Jesus A.A., and Goldbach-Mansky R.. 2014. IL-1 blockade in autoinflammatory syndromes. Annu. Rev. Med. 65:223–244. 10.1146/annurev-med-061512-150641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo E.-K., Kim J.K., Shin D.-M., and Sasakawa C.. 2016. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell. Mol. Immunol. 13:148–159. 10.1038/cmi.2015.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S.M., Kim K.W., Yang C.-W., Park S.-H., and Ju J.H.. 2014. Cytokine-mediated bone destruction in rheumatoid arthritis. J. Immunol. Res. 2014 10.1155/2014/263625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junge G., Mason J., and Feist E.. 2017. Adult onset Still’s disease-The evidence that anti-interleukin-1 treatment is effective and well-tolerated (a comprehensive literature review). Semin. Arthritis Rheum. 47:295–302. 10.1016/j.semarthrit.2017.06.006 [DOI] [PubMed] [Google Scholar]
- Kanai T., Kamada N., and Hisamatsu T.. 2013. Clinical strategies for the blockade of IL-18 in inflammatory bowel diseases. Curr. Drug Targets. 14:1392–1399. 10.2174/13894501113149990006 [DOI] [PubMed] [Google Scholar]
- Kanameishi S., Nakamizo S., Endo Y., Fujisawa A., Dainichi T., Tanaka T., Izawa K., Nishikomori R., and Kabashima K.. 2017. High level of serum human interleukin-18 in a patient with pyogenic arthritis, pyoderma gangrenosum and acne syndrome. J. Eur. Acad. Dermatol. Venereol. 31:e115–e116. 10.1111/jdv.13856 [DOI] [PubMed] [Google Scholar]
- Kang R., Zeng L., Zhu S., Xie Y., Liu J., Wen Q., Cao L., Xie M., Ran Q., Kroemer G., et al. . 2018. Lipid Peroxidation Drives Gasdermin D-Mediated Pyroptosis in Lethal Polymicrobial Sepsis. Cell Host Microbe. 24:97–108.e4. 10.1016/j.chom.2018.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanneganti A., Malireddi R.K.S., Saavedra P.H.V., Vande Walle L., Van Gorp H., Kambara H., Tillman H., Vogel P., Luo H.R., Xavier R.J., et al. . 2018. GSDMD is critical for autoinflammatory pathology in a mouse model of Familial Mediterranean Fever. J. Exp. Med. 215:1519–1529. 10.1084/jem.20172060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanneganti T.-D., Ozören N., Body-Malapel M., Amer A., Park J.-H., Franchi L., Whitfield J., Barchet W., Colonna M., Vandenabeele P., et al. . 2006. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 440:233–236. 10.1038/nature04517 [DOI] [PubMed] [Google Scholar]
- Kanni T., Argyropoulou M., Spyridopoulos T., Pistiki A., Stecher M., Dinarello C.A., Simard J., and Giamarellos-Bourboulis E.J.. 2018. MABp1 Targeting IL-1α for Moderate to Severe Hidradenitis Suppurativa Not Eligible for Adalimumab: A Randomized Study. J. Invest. Dermatol. 138:795–801. 10.1016/j.jid.2017.10.030 [DOI] [PubMed] [Google Scholar]
- Karki R., and Kanneganti T.-D.. 2019. Diverging inflammasome signals in tumorigenesis and potential targeting. Nat. Rev. Cancer. 19:197–214. 10.1038/s41568-019-0123-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki R., Man S.M., Malireddi R.K.S., Gurung P., Vogel P., Lamkanfi M., and Kanneganti T.-D.. 2015. Concerted activation of the AIM2 and NLRP3 inflammasomes orchestrates host protection against Aspergillus infection. Cell Host Microbe. 17:357–368. 10.1016/j.chom.2015.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki R., Lee E., Place D., Samir P., Mavuluri J., Sharma B.R., Balakrishnan A., Malireddi R.K.S., Geiger R., Zhu Q., et al. . 2018. IRF8 Regulates Transcription of Naips for NLRC4 Inflammasome Activation. Cell. 173:920–933.e13. 10.1016/j.cell.2018.02.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsanos K.H., Papamichael K., Feuerstein J.D., Christodoulou D.K., and Cheifetz A.S.. 2018. Biological therapies in inflammatory bowel disease: Beyond anti-TNF therapies. Clin. Immunol. 10.1016/j.clim.2018.03.004 [DOI] [PubMed] [Google Scholar]
- Kayagaki N., Stowe I.B., Lee B.L., O’Rourke K., Anderson K., Warming S., Cuellar T., Haley B., Roose-Girma M., Phung Q.T., et al. . 2015. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 526:666–671. 10.1038/nature15541 [DOI] [PubMed] [Google Scholar]
- Kerr J.F., Wyllie A.H., and Currie A.R.. 1972. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer. 26:239–257. 10.1038/bjc.1972.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura A., Sasaki Y., Abe T., Kano H., and Yasutomo K.. 2014. An inherited mutation in NLRC4 causes autoinflammation in human and mice. J. Exp. Med. 211:2385–2396. 10.1084/jem.20141091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko J.M., Gottlieb A.B., and Kerbleski J.F.. 2009. Induction and exacerbation of psoriasis with TNF-blockade therapy: a review and analysis of 127 cases. J. Dermatolog. Treat. 20:100–108. 10.1080/09546630802441234 [DOI] [PubMed] [Google Scholar]
- Koehler H., Cotsmire S., Langland J., Kibler K.V., Kalman D., Upton J.W., Mocarski E.S., and Jacobs B.L.. 2017. Inhibition of DAI-dependent necroptosis by the Z-DNA binding domain of the vaccinia virus innate immune evasion protein, E3. Proc. Natl. Acad. Sci. USA. 114:11506–11511. 10.1073/pnas.1700999114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari S., Redouane Y., Lopez-Mosqueda J., Shiraishi R., Romanowska M., Lutzmayer S., Kuiper J., Martinez C., Dikic I., Pasparakis M., and Ikeda F.. 2014. Sharpin prevents skin inflammation by inhibiting TNFR1-induced keratinocyte apoptosis. eLife. 3:e03422 10.7554/eLife.03422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriakose T., Man S.M., Malireddi R.K.S., Karki R., Kesavardhana S., Place D.E., Neale G., Vogel P., and Kanneganti T.-D.. 2016. ZBP1/DAI is an innate sensor of influenza virus triggering the NLRP3 inflammasome and programmed cell death pathways. Sci. Immunol. 1:aag2045 10.1126/sciimmunol.aag2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachmann H.J., Kone-Paut I., Kuemmerle-Deschner J.B., Leslie K.S., Hachulla E., Quartier P., Gitton X., Widmer A., Patel N., and Hawkins P.N.. Canakinumab in CAPS Study Group . 2009. Use of canakinumab in the cryopyrin-associated periodic syndrome. N. Engl. J. Med. 360:2416–2425. 10.1056/NEJMoa0810787 [DOI] [PubMed] [Google Scholar]
- Lee J.H., Cho D.H., and Park H.J.. 2015. IL-18 and Cutaneous Inflammatory Diseases. Int. J. Mol. Sci. 16:29357–29369. 10.3390/ijms161226172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeper N.J. 2016. The role of necroptosis in atherosclerotic disease. JACC Basic Transl. Sci. 1:548–550. 10.1016/j.jacbts.2016.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Ning L.-G., Lou X.-H., and Xu G.-Q.. 2018. Necroptosis in inflammatory bowel disease and other intestinal diseases. World J. Clin. Cases. 6:745–752. 10.12998/wjcc.v6.i14.745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkermann A., and Green D.R.. 2014. Necroptosis. N. Engl. J. Med. 370:455–465. 10.1056/NEJMra1310050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkermann A., Hackl M.J., Kunzendorf U., Walczak H., Krautwald S., and Jevnikar A.M.. 2013. Necroptosis in immunity and ischemia-reperfusion injury. Am. J. Transplant. 13:2797–2804. 10.1111/ajt.12448 [DOI] [PubMed] [Google Scholar]
- Lukens J.R., Vogel P., Johnson G.R., Kelliher M.A., Iwakura Y., Lamkanfi M., and Kanneganti T.-D.. 2013. RIP1-driven autoinflammation targets IL-1α independently of inflammasomes and RIP3. Nature. 498:224–227. 10.1038/nature12174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukens J.R., Gross J.M., Calabrese C., Iwakura Y., Lamkanfi M., Vogel P., and Kanneganti T.-D.. 2014. Critical role for inflammasome-independent IL-1β production in osteomyelitis. Proc. Natl. Acad. Sci. USA. 111:1066–1071. 10.1073/pnas.1318688111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maelfait J., Liverpool L., Bridgeman A., Ragan K.B., Upton J.W., and Rehwinkel J.. 2017. Sensing of viral and endogenous RNA by ZBP1/DAI induces necroptosis. EMBO J. 36:2529–2543. 10.15252/embj.201796476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik A., Sharma D., Zhu Q., Karki R., Guy C.S., Vogel P., and Kanneganti T.-D.. 2016. IL-33 regulates the IgA-microbiota axis to restrain IL-1α-dependent colitis and tumorigenesis. J. Clin. Invest. 126:4469–4481. 10.1172/JCI88625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malireddi R.K.S., Gurung P., Mavuluri J., Dasari T.K., Klco J.M., Chi H., and Kanneganti T.-D.. 2018. TAK1 restricts spontaneous NLRP3 activation and cell death to control myeloid proliferation. J. Exp. Med. 215:1023–1034. 10.1084/jem.20171922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man S.M., Tourlomousis P., Hopkins L., Monie T.P., Fitzgerald K.A., and Bryant C.E.. 2013. Salmonella infection induces recruitment of Caspase-8 to the inflammasome to modulate IL-1β production. J. Immunol. 191:5239–5246. 10.4049/jimmunol.1301581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man S.M., Karki R., Malireddi R.K.S., Neale G., Vogel P., Yamamoto M., Lamkanfi M., and Kanneganti T.-D.. 2015. The transcription factor IRF1 and guanylate-binding proteins target activation of the AIM2 inflammasome by Francisella infection. Nat. Immunol. 16:467–475. 10.1038/ni.3118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man S.M., Karki R., and Kanneganti T.-D.. 2017. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol. Rev. 277:61–75. 10.1111/imr.12534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal P., Feng Y., Lyons J.D., Berger S.B., Otani S., DeLaney A., Tharp G.K., Maner-Smith K., Burd E.M., Schaeffer M., et al. . 2018. Caspase-8 Collaborates with Caspase-11 to Drive Tissue Damage and Execution of Endotoxic Shock. Immunity. 49:42–55.e6. 10.1016/j.immuni.2018.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manthiram K., Zhou Q., Aksentijevich I., and Kastner D.L.. 2017. The monogenic autoinflammatory diseases define new pathways in human innate immunity and inflammation. Nat. Immunol. 18:832–842. 10.1038/ni.3777 [DOI] [PubMed] [Google Scholar]
- Martinon F., Burns K., and Tschopp J.. 2002. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell. 10:417–426. 10.1016/S1097-2765(02)00599-3 [DOI] [PubMed] [Google Scholar]
- Masters S.L. 2013. Specific inflammasomes in complex diseases. Clin. Immunol. 147:223–228. 10.1016/j.clim.2012.12.006 [DOI] [PubMed] [Google Scholar]
- Meunier E., Wallet P., Dreier R.F., Costanzo S., Anton L., Rühl S., Dussurgey S., Dick M.S., Kistner A., Rigard M., et al. . 2015. Guanylate-binding proteins promote activation of the AIM2 inflammasome during infection with Francisella novicida. Nat. Immunol. 16:476–484. 10.1038/ni.3119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocarski E.S., Guo H., and Kaiser W.J.. 2015. Necroptosis: The Trojan horse in cell autonomous antiviral host defense. Virology. 479-480:160–166. 10.1016/j.virol.2015.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteleone M., Stanley A.C., Chen K.W., Brown D.L., Bezbradica J.S., von Pein J.B., Holley C.L., Boucher D., Shakespear M.R., Kapetanovic R., et al. . 2018. Interleukin-1β Maturation Triggers Its Relocation to the Plasma Membrane for Gasdermin-D-Dependent and -Independent Secretion. Cell Reports. 24:1425–1433. 10.1016/j.celrep.2018.07.027 [DOI] [PubMed] [Google Scholar]
- Moriwaki K., Bertin J., Gough P.J., and Chan F.K.-M.. 2015. A RIPK3-caspase 8 complex mediates atypical pro-IL-1β processing. J. Immunol. 194:1938–1944. 10.4049/jimmunol.1402167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesterovitch A.B., Gyorfy Z., Hoffman M.D., Moore E.C., Elbuluk N., Tryniszewska B., Rauch T.A., Simon M., Kang S., Fisher G.J., et al. . 2011. Alteration in the gene encoding protein tyrosine phosphatase nonreceptor type 6 (PTPN6/SHP1) may contribute to neutrophilic dermatoses. Am. J. Pathol. 178:1434–1441. 10.1016/j.ajpath.2010.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton K., Dugger D.L., Maltzman A., Greve J.M., Hedehus M., Martin-McNulty B., Carano R.A., Cao T.C., van Bruggen N., Bernstein L., et al. . 2016. RIPK3 deficiency or catalytically inactive RIPK1 provides greater benefit than MLKL deficiency in mouse models of inflammation and tissue injury. Cell Death Differ. 23:1565–1576. 10.1038/cdd.2016.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan Coyne G., and Burotto M.. 2017. MABp1 for the treatment of colorectal cancer. Expert Opin. Biol. Ther. 17:1155–1161. 10.1080/14712598.2017.1347631 [DOI] [PubMed] [Google Scholar]
- Okondo M.C., Johnson D.C., Sridharan R., Go E.B., Chui A.J., Wang M.S., Poplawski S.E., Wu W., Liu Y., Lai J.H., et al. . 2017. DPP8 and DPP9 inhibition induces pro-caspase-1-dependent monocyte and macrophage pyroptosis. Nat. Chem. Biol. 13:46–53. 10.1038/nchembio.2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okondo M.C., Rao S.D., Taabazuing C.Y., Chui A.J., Poplawski S.E., Johnson D.C., and Bachovchin D.A.. 2018. Inhibition of Dpp8/9 Activates the Nlrp1b Inflammasome. Cell Chem. Biol. 25:262–267.e5. 10.1016/j.chembiol.2017.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orning P., Weng D., Starheim K., Ratner D., Best Z., Lee B., Brooks A., Xia S., Wu H., Kelliher M.A., et al. . 2018. Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death. Science. 362:1064–1069. 10.1126/science.aau2818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual V., Allantaz F., Arce E., Punaro M., and Banchereau J.. 2005. Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. J. Exp. Med. 201:1479–1486. 10.1084/jem.20050473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasparakis M., and Vandenabeele P.. 2015. Necroptosis and its role in inflammation. Nature. 517:311–320. 10.1038/nature14191 [DOI] [PubMed] [Google Scholar]
- Place D.E., and Kanneganti T.-D.. 2018. Recent advances in inflammasome biology. Curr. Opin. Immunol. 50:32–38. 10.1016/j.coi.2017.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poh L., Kang S.-W., Baik S.-H., Ng G.Y.Q., She D.T., Balaganapathy P., Dheen S.T., Magnus T., Gelderblom M., Sobey C.G., et al. . 2019. Evidence that NLRC4 inflammasome mediates apoptotic and pyroptotic microglial death following ischemic stroke. Brain Behav. Immun. 75:34–47. 10.1016/j.bbi.2018.09.001 [DOI] [PubMed] [Google Scholar]
- Rathkey J.K., Zhao J., Liu Z., Chen Y., Yang J., Kondolf H.C., Benson B.L., Chirieleison S.M., Huang A.Y., Dubyak G.R., et al. . 2018. Chemical disruption of the pyroptotic pore-forming protein gasdermin D inhibits inflammatory cell death and sepsis. Sci. Immunol. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickard J.A., Anderton H., Etemadi N., Nachbur U., Darding M., Peltzer N., Lalaoui N., Lawlor K.E., Vanyai H., Hall C., et al. . 2014. TNFR1-dependent cell death drives inflammation in Sharpin-deficient mice. eLife. 3:e03464 10.7554/eLife.03464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker P.M., Everett B.M., Thuren T., MacFadyen J.G., Chang W.H., Ballantyne C., Fonseca F., Nicolau J., Koenig W., Anker S.D., et al. CANTOS Trial Group . 2017a Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 377:1119–1131. 10.1056/NEJMoa1707914 [DOI] [PubMed] [Google Scholar]
- Ridker P.M., MacFadyen J.G., Thuren T., Everett B.M., Libby P., Glynn R.J., Ridker P., Lorenzatti A., Krum H., Varigos J., et al. CANTOS Trial Group . 2017b Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet. 390:1833–1842. 10.1016/S0140-6736(17)32247-X [DOI] [PubMed] [Google Scholar]
- Romberg N., Vogel T.P., and Canna S.W.. 2017. NLRC4 inflammasomopathies. Curr. Opin. Allergy Clin. Immunol. 17:398–404. 10.1097/ACI.0000000000000396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roychowdhury S., McMullen M.R., Pisano S.G., Liu X., and Nagy L.E.. 2013. Absence of receptor interacting protein kinase 3 prevents ethanol-induced liver injury. Hepatology. 57:1773–1783. 10.1002/hep.26200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rühl S., Shkarina K., Demarco B., Heilig R., Santos J.C., and Broz P.. 2018. ESCRT-dependent membrane repair negatively regulates pyroptosis downstream of GSDMD activation. Science. 362:956–960. 10.1126/science.aar7607 [DOI] [PubMed] [Google Scholar]
- Sandstrom A., Mitchell P.S., Goers L., Mu E.W., Lesser C.F., and Vance R.E.. 2019. Functional degradation: A mechanism of NLRP1 inflammasome activation by diverse pathogen enzymes. Science. 364:eaau1330 10.1126/science.aau1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarhan J., Liu B.C., Muendlein H.I., Li P., Nilson R., Tang A.Y., Rongvaux A., Bunnell S.C., Shao F., Green D.R., and Poltorak A.. 2018a Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc. Natl. Acad. Sci. USA. 115:E10888–E10897. 10.1073/pnas.1809548115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarhan M., von Mässenhausen A., Hugo C., Oberbauer R., and Linkermann A.. 2018b Immunological consequences of kidney cell death. Cell Death Dis. 9:114 10.1038/s41419-017-0057-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulert G.S., and Grom A.A.. 2015. Pathogenesis of macrophage activation syndrome and potential for cytokine- directed therapies. Annu. Rev. Med. 66:145–159. 10.1146/annurev-med-061813-012806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma D., and Kanneganti T.-D.. 2016. The cell biology of inflammasomes: Mechanisms of inflammasome activation and regulation. J. Cell Biol. 213:617–629. 10.1083/jcb.201602089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma D., Sharma B.R., Vogel P., and Kanneganti T.-D.. 2017. IL-1β and Caspase-1 Drive Autoinflammatory Disease Independently of IL-1α or Caspase-8 in a Mouse Model of Familial Mediterranean Fever. Am. J. Pathol. 187:236–244. 10.1016/j.ajpath.2016.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma D., Malik A., Guy C., Vogel P., and Kanneganti T.-D.. 2019. TNF/TNFR axis promotes pyrin inflammasome activation and distinctly modulates pyrin inflammasomopathy. J. Clin. Invest. 129:150–162. 10.1172/JCI121372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J., Zhao Y., Wang K., Shi X., Wang Y., Huang H., Zhuang Y., Cai T., Wang F., and Shao F.. 2015. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 526:660–665. 10.1038/nature15514 [DOI] [PubMed] [Google Scholar]
- Simonini G., Xu Z., Caputo R., De Libero C., Pagnini I., Pascual V., and Cimaz R.. 2013. Clinical and transcriptional response to the long-acting interleukin-1 blocker canakinumab in Blau syndrome-related uveitis. Arthritis Rheum. 65:513–518. 10.1002/art.37776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standing A.S.I., Malinova D., Hong Y., Record J., Moulding D., Blundell M.P., Nowak K., Jones H., Omoyinmi E., Gilmour K.C., et al. . 2017. Autoinflammatory periodic fever, immunodeficiency, and thrombocytopenia (PFIT) caused by mutation in actin-regulatory gene WDR1. J. Exp. Med. 214:59–71. 10.1084/jem.20161228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Wang H., Wang Z., He S., Chen S., Liao D., Wang L., Yan J., Liu W., Lei X., and Wang X.. 2012. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 148:213–227. 10.1016/j.cell.2011.11.031 [DOI] [PubMed] [Google Scholar]
- Taabazuing C.Y., Okondo M.C., and Bachovchin D.A.. 2017. Pyroptosis and Apoptosis Pathways Engage in Bidirectional Crosstalk in Monocytes and Macrophages. Cell Chem. Biol. 24:507–514.e4. 10.1016/j.chembiol.2017.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toldo S., Mezzaroma E., O’Brien L., Marchetti C., Seropian I.M., Voelkel N.F., Van Tassell B.W., Dinarello C.A., and Abbate A.. 2014. Interleukin-18 mediates interleukin-1-induced cardiac dysfunction. Am. J. Physiol. Heart Circ. Physiol. 306:H1025–H1031. 10.1152/ajpheart.00795.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vande Walle L., Kanneganti T.-D., and Lamkanfi M.. 2011. HMGB1 release by inflammasomes. Virulence. 2:162–165. 10.4161/viru.2.2.15480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varan Ö., Kucuk H., Babaoglu H., Guven S.C., Ozturk M.A., Haznedaroglu S., Goker B., and Tufan A.. 2019. Efficacy and safety of interleukin-1 inhibitors in familial Mediterranean fever patients complicated with amyloidosis. Mod. Rheumatol. 29:363–366. 10.1080/14397595.2018.1457469 [DOI] [PubMed] [Google Scholar]
- Venegas C., Kumar S., Franklin B.S., Dierkes T., Brinkschulte R., Tejera D., Vieira-Saecker A., Schwartz S., Santarelli F., Kummer M.P., et al. . 2017. Microglia-derived ASC specks cross-seed amyloid-β in Alzheimer’s disease. Nature. 552:355–361. 10.1038/nature25158 [DOI] [PubMed] [Google Scholar]
- Vitale A., Rigante D., Lopalco G., Selmi C., Galeazzi M., Iannone F., and Cantarini L.. 2016. Interleukin-1 Inhibition in Behçet’s disease. Isr. Med. Assoc. J. 18:171–176. [PubMed] [Google Scholar]
- Wada T., Toma T., Miyazawa H., Koizumi E., Shirahashi T., Matsuda Y., and Yachie A.. 2018. Longitudinal analysis of serum interleukin-18 in patients with familial Mediterranean fever carrying MEFV mutations in exon 10. Cytokine. 104:143–146. 10.1016/j.cyto.2017.10.007 [DOI] [PubMed] [Google Scholar]
- Wang H., Sun L., Su L., Rizo J., Liu L., Wang L.-F., Wang F.-S., and Wang X.. 2014. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol. Cell. 54:133–146. 10.1016/j.molcel.2014.03.003 [DOI] [PubMed] [Google Scholar]
- Wang S., Ni H.-M., Dorko K., Kumer S.C., Schmitt T.M., Nawabi A., Komatsu M., Huang H., and Ding W.-X.. 2016. Increased hepatic receptor interacting protein kinase 3 expression due to impaired proteasomal functions contributes to alcohol-induced steatosis and liver injury. Oncotarget. 7:17681–17698. 10.18632/oncotarget.6893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wang J., Wang H., Feng X., Tao Y., Yang J., and Cai J.. 2018. Necrosulfonamide Attenuates Spinal Cord Injury via Necroptosis Inhibition. World Neurosurg. 114:e1186–e1191. 10.1016/j.wneu.2018.03.174 [DOI] [PubMed] [Google Scholar]
- Weinlich R., Oberst A., Beere H.M., and Green D.R.. 2017. Necroptosis in development, inflammation and disease. Nat. Rev. Mol. Cell Biol. 18:127–136. 10.1038/nrm.2016.149 [DOI] [PubMed] [Google Scholar]
- Wu H., Craft M.L., Wang P., Wyburn K.R., Chen G., Ma J., Hambly B., and Chadban S.J.. 2008. IL-18 contributes to renal damage after ischemia-reperfusion. J. Am. Soc. Nephrol. 19:2331–2341. 10.1681/ASN.2008020170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J., Wang C., Yao J.-C., Alippe Y., Xu C., Kress D., Civitelli R., Abu-Amer Y., Kanneganti T.-D., Link D.C., and Mbalaviele G.. 2018. Gasdermin D mediates the pathogenesis of neonatal-onset multisystem inflammatory disease in mice. PLoS Biol. 16:e3000047 10.1371/journal.pbio.3000047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D., Jin T., Zhu H., Chen H., Ofengeim D., Zou C., Mifflin L., Pan L., Amin P., Li W., et al. . 2018a TBK1 Suppresses RIPK1-Driven Apoptosis and Inflammation during Development and in Aging. Cell. 174:1477–1491.e19. 10.1016/j.cell.2018.07.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Yang J., Gao W., Li L., Li P., Zhang L., Gong Y.-N., Peng X., Xi J.J., Chen S., et al. . 2014. Innate immune sensing of bacterial modifications of Rho GTPases by the Pyrin inflammasome. Nature. 513:237–241. 10.1038/nature13449 [DOI] [PubMed] [Google Scholar]
- Xu Y., Zhang J., Ma L., Zhao S., Li S., Huang T., and Chu Z.. 2018b The Pathogenesis of Necroptosis-Dependent Signaling Pathway in Cerebral Ischemic Disease. Behav. Neurol. 2018:6814393 10.1155/2018/6814393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y.-J., Zheng L., Hu Y.-W., and Wang Q.. 2018c Pyroptosis and its relationship to atherosclerosis. Clin. Chim. Acta. 476:28–37. 10.1016/j.cca.2017.11.005 [DOI] [PubMed] [Google Scholar]
- Yamanaka H. 2015. TNF as a Target of Inflammation in Rheumatoid Arthritis. Endocr. Metab. Immune Disord. Drug Targets. 15:129–134. 10.2174/1871530315666150316121808 [DOI] [PubMed] [Google Scholar]
- Yang J., Liu Z., Wang C., Yang R., Rathkey J.K., Pinkard O.W., Shi W., Chen Y., Dubyak G.R., Abbott D.W., and Xiao T.S.. 2018. Mechanism of gasdermin D recognition by inflammatory caspases and their inhibition by a gasdermin D-derived peptide inhibitor. Proc. Natl. Acad. Sci. USA. 115:6792–6797. 10.1073/pnas.1800562115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Li S., Qi J., Chen Z., Wu Y., Guo J., Wang K., Sun X., and Zheng J.. 2019. Cleavage of GSDME by caspase-3 determines lobaplatin-induced pyroptosis in colon cancer cells. Cell Death Dis. 10:193 10.1038/s41419-019-1441-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki M.H., Vogel P., Body-Malapel M., Lamkanfi M., and Kanneganti T.-D.. 2010. IL-18 production downstream of the Nlrp3 inflammasome confers protection against colorectal tumor formation. J. Immunol. 185:4912–4920. 10.4049/jimmunol.1002046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Qian J., Zhang P., Li H., Shen H., Li X., and Chen G.. 2019. Gasdermin D serves as a key executioner of pyroptosis in experimental cerebral ischemia and reperfusion model both in vivo and in vitro. J. Neurosci. Res. 97:645–660. 10.1002/jnr.24385 [DOI] [PubMed] [Google Scholar]
- Zhao J., Jitkaew S., Cai Z., Choksi S., Li Q., Luo J., and Liu Z.-G.. 2012. Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc. Natl. Acad. Sci. USA. 109:5322–5327. 10.1073/pnas.1200012109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong F.L., Robinson K., Teo D.E.T., Tan K.-Y., Lim C., Harapas C.R., Yu C.-H., Xie W.H., Sobota R.M., Au V.B., et al. . 2018. Human DPP9 represses NLRP1 inflammasome and protects against auto-inflammatory diseases via both peptidase activity and FIIND domain binding. J. Biol. Chem. 293:18864–18878. 10.1074/jbc.RA118.004350 [DOI] [PMC free article] [PubMed] [Google Scholar]