Abstract
Purpose:
Six treatments have improved overall survival in men with metastatic castration-resistant prostate cancer (mCRPC), each differing in toxicities and cost. This study identified patient and provider factors associated with variation in treatment of men with mCRPC to identify potential barriers to treatments.
Methods:
A claims database of commercially insured patients was used to identify patients with prostate cancer treated with abiraterone, enzalutamide, docetaxel, cabazitaxel, sipuleucel-T, or radium-223 between 2010 and 2016. Multinomial and binomial logistic regressions were conducted to determine patient and provider factors associated with treatment patterns.
Results:
Among 5,575 patients identified, patients with a household income >$99,000 were less likely to receive an expensive oral androgen signaling inhibitor (abiraterone or enzalutamide) as first-line treatment versus docetaxel compared to patients with a household income <$50,000 (odds ratio, OR, 0.66, 95% confidence interval, CI, 0.48–0.92). Patients who are Black (OR 1.43, 95% CI 1.02–2.01), live in the Pacific region versus the South Atlantic (OR 2.68, 95% CI 1.74–4.11), received treatment from a urologist versus a medical oncologist (OR 16.05, 95% CI 6.01–42.86), or had pre-existing heart failure (OR 1.69, 95% CI 1.18–2.42) were more likely to receive first-line oral androgen signaling inhibitors over docetaxel, independent of other factors on multivariable analysis.
Conclusion:
Clinicians and policy makers should be aware of the potential barriers and provider factors that influence use of novel therapies among patients with advanced prostate cancer, including the paradoxical effect of income and the substantial effect of provider specialty on first-line treatment rendered to patients with mCRPC.
Keywords: prostate cancer, novel agents, variation, care delivery, claims data
Introduction
Several treatments have demonstrated an improvement in overall survival for men with metastatic castration-resistant prostate cancer (mCRPC). Since docetaxel was approved by the Food and Drug Administration (FDA) in 2004,1 five more therapies have demonstrated a survival benefit and are FDA-approved to treat mCRPC,2–8 each unique with respect to administration, cost, and side effects. Docetaxel and cabazitaxel are intravenous chemotherapies, each associated with neuropathy, fatigue, and the potential for life-threatening myelosuppression. Abiraterone and enzalutamide are secondary oral androgen signaling inhibitors with fewer and less life-threatening side effects such as hypertension and hypokalemia for abiraterone and fatigue for enzalutamide, but higher potential out-of-pocket costs. Radium-223 and sipuleucel-T are relatively well-tolerated but require specialized infrastructure for treatment delivery (nuclear medicine for radium-223 and a pheresis center for sipuleucel-T). Guidelines from specialty societies, the American Society of Clinical Oncology and the American Urologic Association, recommend starting with abiraterone or enzalutamide in treatment-naïve mCRPC.9,10 Beyond first-line treatment, sipuleucel-T can be considered in patients with minimal to no symptoms, radium-223 in patients with symptomatic bone-only metastatic disease, and docetaxel and cabazitaxel reserved for patients with a good performance status. However, despite these recommendations, there is still tremendous room for uncertainty and variation at the hands of the treating provider and little is known about optimal treatment sequences and whether providers are prescribing treatments concordant with guidelines. Supplementary Table 1 describes key factors that may affect prescribing patterns.
Considering that some of these treatments were approved before others, their use will vary over time as additional treatments enter the market, as we have demonstrated in previous work.11,12 There will also be warranted variation in treatment according to a patient’s site of disease (e.g. radium-223 used for patients with bone-only metastases), symptoms (e.g. sipuleucel-T for asymptomatic patients), and preference. However, there is still a potential for significant unwarranted variation due to non-clinical patient and physician factors, such as race, income, and place of residence, similar to other conditions.13–18
In this context, we sought to evaluate variation in prescription patterns at the level of the patient, provider, and region. We hypothesized that nonclinical patient factors such as race and income would affect receipt of expensive oral androgen signaling inhibitors (abiraterone or enzalutamide) versus a less expensive but more toxic therapy (docetaxel) as first-line treatment. We also expected to find geographic variability, and substantial variation in use of these treatments depending on whether a patient was treated by a urologist or a medical oncologist.
Materials and Methods
Data Source and Sample Selection
We used the Clinformatics TM Data Mart Database (OptumInsight, Eden Prairie, Minnesota) to identify a cohort of men over the age of 18 with prostate cancer. This database includes patients who are insured through a national private commercial payer and is representative of privately insured patients with prostate cancer in the general US population by age, and socioeconomic variables.19 We further restricted our study cohort to include patients with prostate cancer who received at least one of the six focus treatments (abiraterone, enzalutamide, sipuleucel-T, docetaxel, cabazitaxel, radium-223) between January 2010 and June 2016 and were enrolled continuously for 180 days before the first focus treatment claim.
We identified prescription records of the six focus treatments by national drug codes, brand names, and Healthcare Common Procedure Coding System codes and matched to respective fields within the medical claims and pharmacy claims data. (Supplementary Materials 2.1–2.2.)
Patient and Provider Variables
Sociodemographic variables were derived from a demographic-based analytical model using a major data syndicator, Knowledge-Based Marketing Solutions (KBM, Richardson, TX). Pre-existing comorbid diseases known to affect treatment decisions and common to patients with advanced prostate cancer (i.e. diabetes, hypertension, cardiac arrhythmias, congestive heart failure, and osteoporosis) were assessed for each patient (Supplementary Materials 2.1).
The specialty of provider who prescribed patients their first-line treatment was determined by self-reported taxonomy and categorized as medical oncologist, urologist, radiation oncologist, or other (Supplementary Materials 2.3–2.4).
Outcomes
The first objective was to identify the temporal pattern of first-line treatment choices from January 2010 through June 2016. First-line treatment was defined as the first focus treatment for a patient during the study period with no receipt of any other focus treatments during the preceding 12 months. Our second objective was to determine the association of patient sociodemographic and provider variables with first-line treatments. To minimize the effect of market availability and insurance coverage on use of treatments, we restricted our analysis to January 2014 through June 2016 since the last of the six focus treatments (radium-223) was FDA-approved in 2013 (Supplementary Materials 2.2).
Statistical Analysis
Patients were classified into one of the three categories of treatment prescribed as first-line: oral therapy (abiraterone or enzalutamide), docetaxel, or other treatment (sipuleucel-T, radium-223, cabazitaxel) during the timeframe January 2014 through June 2016. Using overall tests for independence and unadjusted odds ratios (ORs) from a simple multinomial logistic regression (with three categories), patients across treatment categories were compared. Among those patients who received an oral therapy first-line during that same 30-month time period, patients who received abiraterone versus enzalutamide were compared using a simple binomial logistic regression model. We then used corresponding multivariable multinomial and binomial logistic regression models to analyze the adjusted associations between first-line treatment and patient and provider characteristics.
Missing data was assumed to be missing at random. Supplementary Materials 3.0–6.0 detail methods used to handle missing, sensitivity analyses, and the stability of the cohort.
Results
Table 1 demonstrates the characteristics of patients with prostate cancer and the characteristics of those in the final cohort (n=5,575) who received one of the six focus treatments.
Table 1.
Patient Characteristics: Total cohort includes all patients ≥18 years with prostate cancer (N=328,989) and study cohort includes patients with prostate cancer who received any of the six treatments and were continuously enrolled for ≥ 180 days before receipt of their first treatment (n = 5,575) over the study period years 2010 through mid-2016
| Total Cohort (N=328,989) | Final Cohort (n=5,575) | |||
|---|---|---|---|---|
| Variable | Mean [Range]/Count (%) | Mean range/Count (%) | ||
| Age | 71.44 | [18,90] | 73.62 | [32,90] |
| Race | ||||
| White | 223,035 | (67.8) | 3,888 | (69.7) |
| Black | 37,567 | (11.4) | 737 | (13.2) |
| Asian | 6,239 | (1.9) | 108 | (1.9) |
| Hispanic | 23,091 | (7.0) | 410 | (7.4) |
| Unknown | 39,057 | (11.9) | 432 | (7.8) |
| Education level | ||||
| Less than 12th Grade | 1,530 | (0.5) | 37 | (0.7) |
| High School Diploma | 89,140 | (27.1) | 1,621 | (29.1) |
| Less than Bachelor Degree | 162,252 | (49.3) | 2,858 | (51.3) |
| Bachelor Degree Plus | 45,880 | (13.9) | 785 | (14.1) |
| Unknown | 30,187 | (9.2) | 274 | (4.9) |
| Household income range | ||||
| <$50,000 | 98,244 | (29.9) | 1,985 | (35.6) |
| $50,000-$99,000 | 96,987 | (29.5) | 1,721 | (30.9) |
| >$99,000 | 76,551 | (23.3) | 1,091 | (19.6) |
| Unknown | 57,207 | (17.4) | 778 | (14.0) |
| Net worth | ||||
| <$25,000 | 18,347 | (5.6) | 334 | (6.0) |
| $25,000-$149,000 | 47,045 | (14.3) | 891 | (16.0) |
| $150,000-$249,000 | 42,972 | (13.1) | 773 | (13.9) |
| $250,000-$499,000 | 81,771 | (24.9) | 1,393 | (25.0) |
| >$500,000 | 85,002 | (25.8) | 1,454 | (26.1) |
| Unknown | 53,852 | (16.4) | 730 | (13.1) |
| Geographic Region* | ||||
| South Atlantic | 89,340 | (27.2) | 1,291 | (23.1) |
| New England | 17,999 | (5.5) | 285 | (5.1) |
| Middle Atlantic | 32,045 | (9.7) | 440 | (7.9) |
| East North Central | 46,568 | (14.2) | 806 | (14.5) |
| East South Central | 11,747 | (3.6) | 202 | (3.6) |
| West North Central | 32,548 | (9.9) | 604 | (10.8) |
| West South Central | 32,775 | (10.0) | 539 | (9.7) |
| Mountain | 31,429 | (9.6) | 609 | (10.9) |
| Pacific | 33,078 | (10.1) | 771 | (13.8) |
| Unknown | 1,460 | (0.4) | 28 | (0.5) |
| Metastatic | ||||
| Yes | 28,451 | (8.6) | 4,890 | (87.7) |
| No | 300,538 | (91.4) | 685 | (12.3) |
| Year | ||||
| 2010 | 97,410 | (29.6) | 2,365 | (42.4) |
| 2011 | 101,968 | (31.0) | 2,743 | (49.2) |
| 2012 | 110,243 | (33.5) | 3,071 | (55.1) |
| 2013 | 117,544 | (35.7) | 3,237 | (58.1) |
| 2014 | 115,297 | (35.0) | 3,132 | (56.2) |
| 2015 | 122,555 | (37.3) | 2,872 | (51.6) |
| 2016 | 99,292 | (30.2) | 1,992 | (35.7) |
| Comorbid condition | ||||
| Hypertension | 223,777 | (68.0) | 3,842 | (68.9) |
| Diabetes | 94,275 | (28.7) | 1,584 | (28.4) |
| Arrhythmia | 71,897 | (21.9) | 1,151 | (20.4) |
| Congestive heart failure | 39,342 | (12.0) | 597 | (10.7) |
| Osteoporosis | 13,374 | (4.1) | 385 | (6.9) |
OR, Odds Ratio; CI, Confidence Interval
Geographic region:
New England: Connecticut, Maine, Massachusetts, New Hampshire, Rhode Island, Vermont
Middle Atlantic: New Jersey, New York, Pennsylvania
East North Central: Illinois, Indiana, Michigan, Ohio, Wisconsin
West North Central: Iowa, Kansas, Minnesota, Missouri, Nebraska, North Dakota, South Dakota
South Atlantic: Delaware, Washington D.C., Florida, Georgia, Maryland, North Carolina, South Carolina, Virginia, West Virginia
East South Central: Alabama, Kentucky, Mississippi, Tennessee
West South Central: Arkansas, Louisiana, Oklahoma, and Texas
Mountain: Arizona, Colorado, Idaho, Montana, Nevada, New Mexico, Utah, Wyoming
Pacific: Alaska, California, Hawaii, Oregon, Washington
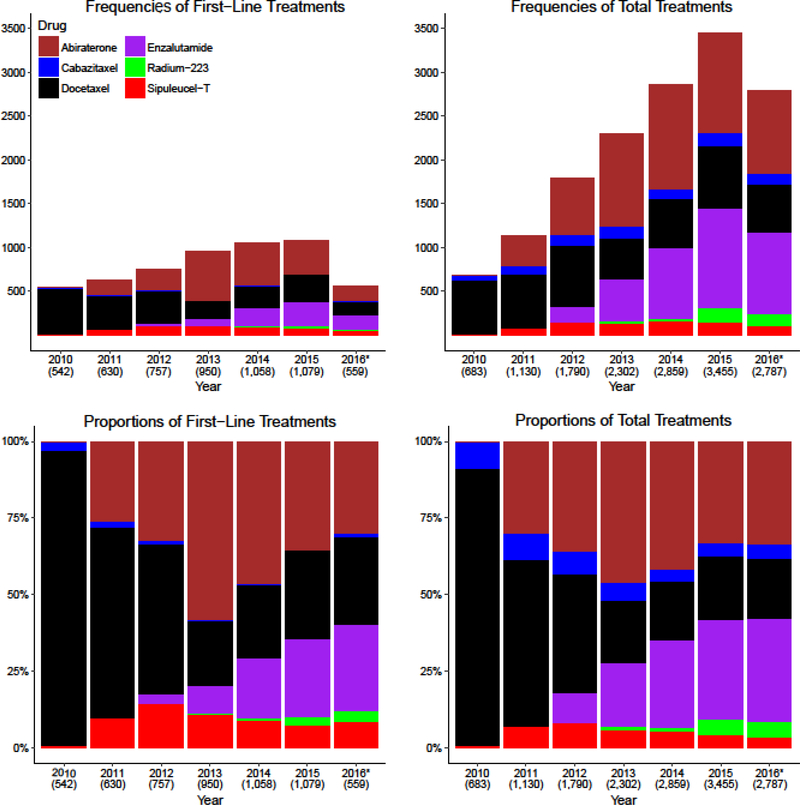
Figure 1 illustrates the frequencies and proportions of the different treatments used over time with the top panel demonstrating an increasing total number of patients treated each year as newer agents came onto the market. The number of patients in the cohort was relatively similar each year (Table 1), so the increased number of treated patients cannot be explained by an increasing insured cohort. The number of patients treated with first-line docetaxel declined initially, but then stayed relatively stable while the use of other therapies increased, indicating both a substitution effect of the newer therapies for docetaxel and an expansion of treatment for patients with mCRPC. Furthermore, as the number of patients prescribed enzalutamide first-line increased from 2013 to 2015, there was an apparent substitution effect of enzalutamide for abiraterone as the use of first-line abiraterone declined at a similar rate. The use of sipuleucel-T and cabazitaxel did not substantially rise throughout the entire study period. Supplementary Table 2 includes the corresponding values for Figure 1.
Figure 1. Frequencies and Rates of Therapy Use.
The top panels show the frequency of treatments used: the top left panel shows the frequency of patients who were started on a treatment as first-line therapy each year, and the top right panel shows the total use of treatment each year. The bottom panels show proportions of treatments used in relation to the other available treatments: the bottom left panel shows proportions of patients who started a first-line therapy each year and the bottom right panel shows the total proportions of the different therapies used each year. The proportions in the bottom panels sum to 100%. *2016 only includes data from the first six months of the year. The number of patients included in each column is included beneath the year on the X-axis of each bar graph.
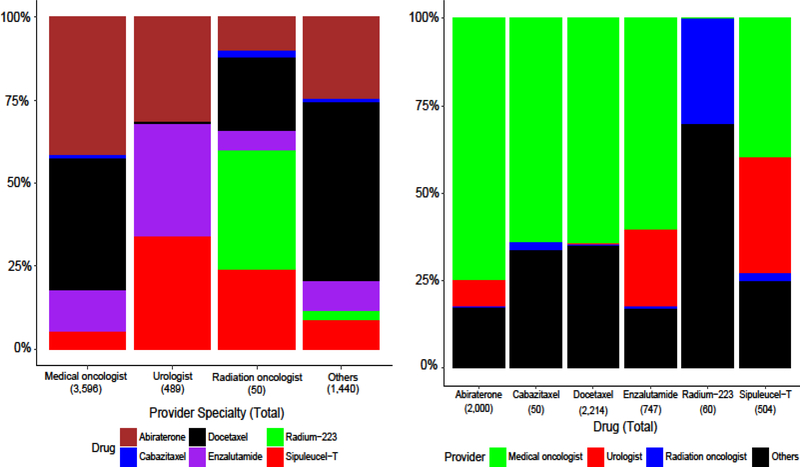
The frequency of and proportion of different treatments prescribed also varied by provider type (Figure 2). Medical oncologists prescribed the majority (64.5%) of first-line treatments for patients, abiraterone in 41.5%, docetaxel in 39.6%, enzalutamide in 12.5%, and sipuleucel-T in 5.5% of patients. In contrast, urologists prescribed 8.8% of the first-line treatments, using sipuleucel-T, enzalutamide, and abiraterone, each in a third of patients.
Figure 2. Provider Type and First-Line Treatment.
The top panel shows the proportions of first-line treatments prescribed by different provider specialties and the bottom panel shows the proportions of providers that prescribe the different studied treatments. These proportions include all patients started on a treatment between January 2010 and June 2016. “Others” includes other providers such as advanced practice providers, primary care providers, pharmacies, facilities such as hospitals, and other taxonomies for which we could not confirm that the provider was a medical oncologist, urologist, or radiation oncologist. Details for the classification of providers are in Sections 2.3–2.4 of the Supplementary Materials.
Table 2 shows the results of a multinomial logistic regression model demonstrating the odds of receiving an oral therapy versus docetaxel. The odds of receiving an “other” treatment versus docetaxel as first-line was also calculated and is reported in Supplementary Table 4. Race, income, geography, and provider type were factors significantly associated with choice of first-line therapy in multivariable analysis. Black patients had greater odds of receiving an oral therapy over docetaxel first-line (OR 1.43, 95% CI 1.02–2.01) compared to white patients. Paradoxically, those with household income >$99,000 had a lower odds of receiving the more expensive oral agents over docetaxel (OR 0.66, 95% CI 0.48–0.92) compared to those with a household income <$50,000, a finding that was independent of other factors such as education, provider type, and year of treatment. In addition, patients who received first-line treatment by a urologist versus a medical oncologist had a substantially greater odds of receiving an oral therapy over docetaxel (OR 16.05, 95% CI 6.01–42.86). The results from the regressions that were done treating the “unknown” category as a separate category and the complete case analysis where unknowns were excluded are numerically similar to the findings that we report using multiple imputed dataset and combined inference (Supplementary Tables 5 and 6).
Table 2.
Multinomial Logistic Regression of First-Line Treatment Among Patients Treated 2014–2016: Multinomial Logistic Regression of first-line drugs among patients prescribed one of the three categories of treatments: oral (abiraterone or enzalutamide), docetaxel, or other (sipuleucel-T, radium-223, cabazitaxel) from 2014 to mid-2016. Multivariable analyses also adjusted for net worth, insurance product, and whether the insurance plan was administrative services only. The regression results for the category of other (sipuleucel-T, cabazitaxel, and radium-223) were not included in this Table, but are in Supplementary Materials, Section 8.3.
| Total (n=2,696) |
Docetaxel (n=728) |
Abiraterone or Enzalutamide (n=1,678) |
Abiraterone or Enzalutamide vs Docetaxel |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted OR |
Multivariable Analysis |
||||||||||
| Variable | Count (%) | Count (%) | Count (%) | P-value | OR | 95% CI | OR | 95% CI | |||
| Age | <0.01 | ||||||||||
| <55 | 70 | (2.6) | 35 | (4.8) | 31 | (1.8) | 1.00 | 1.00 | |||
| 55–64 | 310 | (11.5) | 94 | (12.9) | 178 | (10.6) | 2.14 | (1.24,3.68) | 2.12 | (1.18,3.80) | |
| 65–74 | 906 | (33.6) | 355 | (48.8) | 451 | (26.9) | 1.43 | (0.87,2.37) | 1.37 | (0.78,2.41) | |
| ≥75 | 1,410 | (52.3) | 244 | (33.5) | 1,018 | (60.7) | 4.71 | (2.85,7.79) | 4.06 | (2.28,7.22) | |
| Race | <0.01 | ||||||||||
| White | 1,830 | (67.9) | 505 | (69.4) | 1,129 | (67.3) | 1.00 | 1.00 | |||
| Black | 329 | (12.2) | 69 | (9.5) | 219 | (13.1) | 1.40 | (1.05,1.87) | 1.43 | (1.02,2.01) | |
| Asian | 60 | (2.2) | 13 | (1.8) | 44 | (2.6) | 1.40 | (0.74,2.63) | 1.38 | (0.68,2.82) | |
| Hispanic | 231 | (8.6) | 51 | (7.0) | 166 | (9.9) | 1.39 | (0.99,1.95) | 0.95 | (0.65,1.40) | |
| Unknown | 246 | (9.1) | 90 | (12.4) | 120 | (7.2) | |||||
| Education level | 0.72 | ||||||||||
| Less than 12th Grade | 17 | (0.6) | 2 | (0.3) | 12 | (0.7) | 1.00 | 1.00 | |||
| High School Diploma | 770 | (28.6) | 200 | (27.5) | 499 | (29.7) | 0.43 | (0.10,1.90) | 0.66 | (0.13,3.46) | |
| Less than Bachelor Degree | 1,349 | (50.0) | 356 | (48.9) | 851 | (50.7) | 0.42 | (0.09,1.85) | 0.68 | (0.13,3.58) | |
| Bachelor Degree Plus | 381 | (14.1) | 101 | (13.9) | 239 | (14.2) | 0.41 | (0.09,1.85) | 0.79 | (0.15,4.28) | |
| Unknown | 179 | (6.6) | 69 | (9.5) | 77 | (4.6) | |||||
| Household income range | <0.01 | ||||||||||
| <$50,000 | 895 | (33.2) | 197 | (27.1) | 613 | (36.5) | 1.00 | 1.00 | |||
| $50,000-$99,000 | 868 | (32.2) | 216 | (29.7) | 555 | (33.1) | 0.82 | (0.66,1.03) | 0.94 | (0.72,1.23) | |
| >$99,000 | 537 | (19.9) | 187 | (25.7) | 292 | (17.4) | 0.51 | (0.41,0.65) | 0.66 | (0.48,0.92) | |
| Unknown | 396 | (14.7) | 128 | (17.6) | 218 | (13.0) | |||||
| Geographic Region* | <0.01 | ||||||||||
| South Atlantic | 495 | (18.4) | 138 | (19.0) | 302 | (18.0) | 1.00 | 1.00 | |||
| New England | 130 | (4.8) | 42 | (5.8) | 80 | (4.8) | 0.87 | (0.57,1.33) | 1.06 | (0.65,1.70) | |
| Middle Atlantic | 279 | (10.3) | 72 | (9.9) | 178 | (10.6) | 1.13 | (0.80,1.58) | 0.96 | (0.65,1.41) | |
| East North Central | 451 | (16.7) | 113 | (15.5) | 279 | (16.6) | 1.13 | (0.84,1.52) | 1.09 | (0.78,1.53) | |
| East South Central | 101 | (3.7) | 27 | (3.7) | 58 | (3.5) | 0.98 | (0.59,1.61) | 0.81 | (0.46,1.41) | |
| West North Central | 249 | (9.2) | 135 | (18.5) | 83 | (4.9) | 0.28 | (0.20,0.39) | 0.30 | (0.20,0.45) | |
| West South Central | 263 | (9.8) | 66 | (9.1) | 167 | (10.0) | 1.15 | (0.81,1.63) | 1.04 | (0.70,1.55) | |
| Mountain | 324 | (12.0) | 76 | (10.4) | 203 | (12.1) | 1.22 | (0.87,1.70) | 1.08 | (0.73,1.62) | |
| Pacific | 400 | (14.8) | 57 | (7.8) | 326 | (19.4) | 2.60 | (1.84,3.67) | 2.68 | (1.74,4.11) | |
| Unknown | 4 | (0.1) | 2 | (0.3) | 2 | (0.1) | |||||
| Metastatic | <0.01 | ||||||||||
| Yes | 2,189 | (81.2) | 598 | (82.1) | 1,335 | (79.6) | 1.00 | 1.00 | |||
| No | 507 | (18.8) | 130 | (17.9) | 343 | (20.4) | 1.18 | (0.94,1.48) | 0.85 | (0.65,1.11) | |
| Year | 0.03 | ||||||||||
| 2014 | 1,058 | (39.2) | 254 | (34.9) | 698 | (41.6) | 1.00 | 1.00 | |||
| 2015 | 1,079 | (40.0) | 313 | (43.0) | 655 | (39.0) | 0.76 | (0.63,0.93) | 0.77 | (0.62,0.97) | |
| 2016 | 559 | (20.7) | 161 | (22.1) | 325 | (19.4) | 0.73 | (0.58,0.93) | 0.77 | (0.58,1.02) | |
| Comorbid Conditions | |||||||||||
| Hypertension | 1,965 | (72.9) | 503 | (69.1) | 1,237 | (73.7) | <0.01 | 1.25 | (1.04,1.52) | 1.11 | (0.88,1.40) |
| Diabetes | 792 | (29.4) | 187 | (25.7) | 512 | (30.5) | 0.03 | 1.27 | (1.04,1.55) | 1.12 | (0.89,1.41) |
| Arrhythmia | 658 | (24.4) | 159 | (21.8) | 437 | (26.0) | 0.04 | 1.26 | (1.02,1.55) | 0.97 | (0.76,1.25) |
| Congestive heart failure | 332 | (12.3) | 54 | (7.4) | 244 | (14.5) | <0.01 | 2.12 | (1.56,2.89) | 1.69 | (1.18,2.42) |
| Osteoporosis | 234 | (8.7) | 41 | (5.6) | 144 | (8.6) | <0.01 | 1.57 | (1.10,2.25) | 1.44 | (0.97,2.14) |
| Provider Type | <0.01 | ||||||||||
| Medical oncologist | 1,613 | (59.8) | 417 | (57.3) | 1,127 | (67.2) | 1.00 | 1.00 | |||
| Urologist | 363 | (13.5) | 2 | (0.3) | 264 | (15.7) | 16.66 | (6.34,43.79) | 16.05 | (6.01,42.86) | |
| Others | 613 | (22.7) | 275 | (37.8) | 220 | (13.1) | 0.31 | (0.25,0.38) | 0.35 | (0.28,0.44) | |
| Unknown | 107 | (4.0) | 34 | (4.7) | 67 | (4.0) | |||||
OR, Odds Ratio; CI, Confidence Interval
Geographic region:
New England: Connecticut, Maine, Massachusetts, New Hampshire, Rhode Island, Vermont
Middle Atlantic: New Jersey, New York, Pennsylvania
East North Central: Illinois, Indiana, Michigan, Ohio, Wisconsin
West North Central: Iowa, Kansas, Minnesota, Missouri, Nebraska, North Dakota, South Dakota
South Atlantic: Delaware, Washington D.C., Florida, Georgia, Maryland, North Carolina, South Carolina, Virginia, West Virginia
East South Central: Alabama, Kentucky, Mississippi, Tennessee
West South Central: Arkansas, Louisiana, Oklahoma, and Texas
Mountain: Arizona, Colorado, Idaho, Montana, Nevada, New Mexico, Utah, Wyoming
Pacific: Alaska, California, Hawaii, Oregon, Washington
Standard binomial logistic regression was also conducted to demonstrate variables associated with the odds of receiving abiraterone versus enzalutamide (Supplementary Tables 7 and 8). Compared to 2014, patients prescribed first-line treatment in 2015 (OR 1.66, 95% CI 1.31–2.11) and 2016 (OR 2.01, 95% CI 1.50–2.68) had greater odds of being prescribed enzalutamide than abiraterone. In addition, patients treated by a urologist versus a medical oncologist were more likely to receive enzalutamide than abiraterone (OR 2.64, 95% CI 1.97–3.52), independent of other variables.
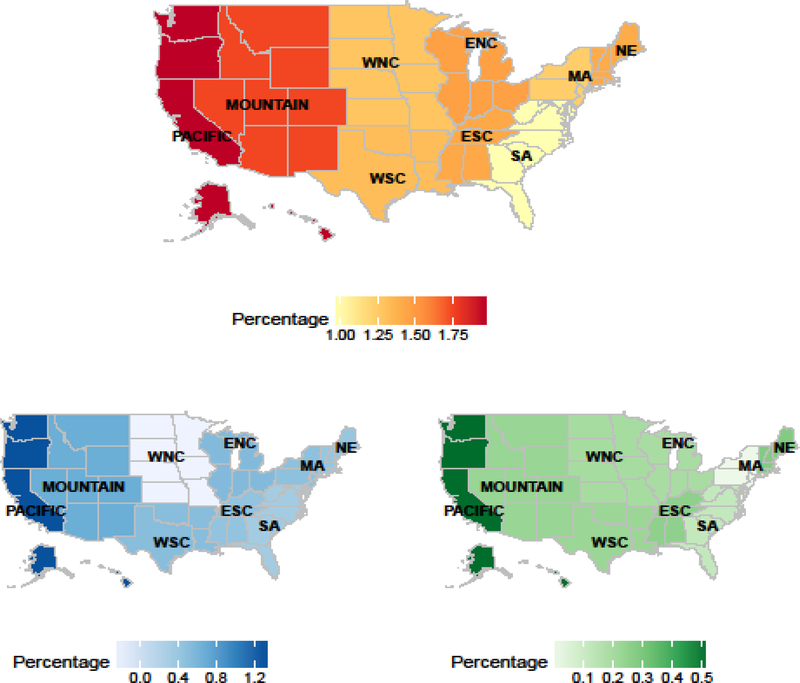
For all regression analyses, we observed geographic variability. Considering patients in the South Atlantic as a reference, patients in the West North Central region had substantially lower odds of being prescribed an oral therapy as first-line versus docetaxel (OR 0.30, 95% CI 0.20–0.45), and patients in the Pacific region had a greater odds of being prescribed an oral therapy as first-line (OR 2.68, 95% CI 1.74–4.11). Among patients who received an oral first-line therapy, patients in the West North Central region had lower odds of receiving enzalutamide than abiraterone (OR 0.51, 95% CI 0.29–0.90). These findings were independent of other factors such as age, race, education, income, and provider specialty. Using methods described in Supplementary Materials 6.1, Figure 3 demonstrates the observed geographic variability.
Figure 3. Geographic variation of drug patterns by region.
The top panel shows the proportion of patients who received one of the six treatments as first-line therapy among all patients with prostate cancer during the years 2014 through mid-2016 in each region. The bottom left panel shows the difference of proportions between oral drug (abiraterone or enzalutamide) and docetaxel for first-line treatment among all patients with prostate cancer during the years 2014 through mid-2016 in each region. The darker blue indicates more prescriptions for an oral therapy than docetaxel. The bottom right panel shows the difference of proportion between abiraterone and enzalutamide for first-line treatment among all patients with prostate cancer during the years 2014 through mid-2016 in each region. The darker green indicates more prescriptions for abiraterone than enzalutamide. Patients with prostate cancer were defined as patients with at least one diagnostic code as a medical claim during the years 2014 and mid-2016. Methods to generate this figure are in Supplementary Materials, Section 6.1.
Abbreviations: New England (NE): Connecticut (CT), Maine (ME), Massachusetts (MA), New Hampshire (NH), Rhode Island (RI), Vermont (VT), Middle Atlantic (MA): New Jersey (NJ), New York (NY), Pennsylvania (PA), East North Central (ENC): Illinois (IL), Indiana (IN), Michigan (MI), Ohio (OH), Wisconsin (WI), West North Central (WNC): Iowa (IA), Kansas (KS), Minnesota (MN), Missouri (MO), Nebraska (NE), North Dakota (ND), South Dakota (SD), South Atlantic (SA): Delaware (DE), Washington D.C. (DC), Florida (FL), Georgia (GA), Maryland (MD), North Carolina (NC), South Carolina (SC), Virginia (VA), West Virginia (WV), East South Central (ESC): Alabama (AL), Kentucky (KY), Mississippi (MS), Tennessee (TN), West South Central (WSC): Arkansas (AR), Louisiana (LA), Oklahoma (OK), and Texas (TX), Mountain (M): Arizona (AZ), Colorado (CO), Idaho (ID), Montana (MT), Nevada (NV), New Mexico (NM), Utah (UT), Wyoming (WY), Pacific (PAC): Alaska (AK), California (CA), Hawaii (HI), Oregon (OR), Washington (WA)
Discussion
Throughout the study period, we observed both an expansion of total treatment use to more patients than would otherwise have been treated and a substitution effect of oral therapies for docetaxel and enzalutamide for abiraterone. Not surprisingly, patients treated by urologists were more likely to receive an oral therapy first-line than docetaxel, and more likely to receive enzalutamide. In addition, urologists prescribed a disproportionate amount of sipuleucel-T and enzalutamide for the number of patients they treated. An unexpected finding was the paradoxical effect that patients with higher incomes were more likely to receive the less expensive docetaxel than secondary androgen signaling inhibitors, independent of other factors.
Similar to studies in other clinical conditions, we were not surprised to find race and geographic region to influence prescribing.13–18 The association of Black race with receiving first-line oral androgen signaling inhibitors over docetaxel could be due to patient preference or other factors we were unable to measure, such as rural/urban distribution. To explain the unexpected association of patients with higher income being more likely to receive docetaxel first-line over an oral androgen signaling inhibitor, we considered a landmark study (CHAARTED) published in August 2015, as a possible explanation. CHAARTED demonstrated a benefit to using docetaxel in an earlier phase of disease (hormone-naïve setting).20 With our methods, patients treated with docetaxel per CHAARTED would be identified as receiving docetaxel first-line, and one would expect that patients with higher income might be the patients to seek out groundbreaking care concordant with the latest evidence. However, the paradoxical income effect we observed did not extinguish in multivariable analysis. Thus, one can infer that there must be another explanation for the association of higher income and receipt of docetaxel first-line than the guideline changes as a result of CHAARTED. Another possible explanation for this paradoxical effect of income on receipt of docetaxel may be due to the high out-of-pocket costs faced by some patients for oral medications since oral therapies are typically covered through a different insurance benefit than intravenous medications. Patients with lower incomes are commonly eligible to receive patient assistance through pharmaceutical companies or through other grant mechanisms, while patients with higher incomes, who may still have prohibitive cost sharing requirements, may not qualify for patient assistance.21,22
To explain why urologists were responsible for a disproportionate number of sipuleucel-T treatments, we considered that patients treated for mCRPC by urologists may have less aggressive and asymptomatic disease. There is also increasing interest among urologists in treating patients with advanced prostate cancer since therapies that do not involve cytotoxic chemotherapy have become available.23–25 Investing in the infrastructure to be able to offer sipuleucel-T could potentially give patients treated by urologists an additional treatment option. We expected patients treated by urologists to be more likely to receive an oral agent over docetaxel, since urologists are not typically trained to deliver cytotoxic chemotherapy, but were not expecting urologists to have a preference for enzalutamide over abiraterone. It is possible this preference may stem from the fact that abiraterone, while on the market longer and arguably better tolerated in patients, requires concurrent use of prednisone and frequent blood pressure and laboratory monitoring that may not be feasible for some urology practices.
There are limitations to this study. First, regarding cohort identification, we included all patients with a claim for one of the focus treatments, regardless of having a diagnosis code for metastatic disease, since methods to identify metastatic disease through claims are known to be insensitive.26 We were also not able to identify whether a patient had castration-resistant disease. Since the objective was to compare patterns and preferences of treatments among treated patients, this limitation is not germane to the present study. Fortunately, all of the focus treatments, except docetaxel, were FDA-approved for use only in mCRPC during the proposed years of analysis, so we were confident that treatments prescribed were being used for mCRPC and not another cancer. Among patients who received docetaxel, very few patients (3.9%) had a diagnosis of a co-occurring cancer for which docetaxel is used. Another limitation to this study is the generalizability of our findings since all patients in this study had private insurance. Thus, greater disparities are likely to exist in treatment patterns with a more inclusive cohort of patients. Finally, there were limitations inherent to the claims database for identifying sociodemographic variables, several of which were generated from a composite of public records, purchase transactions, census data, and consumer surveys, and for identifying provider specialty. However, our algorithm for identifying providers was conservative and our “other” category that was primarily associated with docetaxel was large, suggesting that our findings may be underestimating the difference between medical oncologists and urologists.
Conclusion
A patient’s race, income level, geographic area of residence, and specialty of provider caring for them all significantly influence the treatment they will receive for mCRPC. In particular, whether a patient follows with medical oncology or urology may substantially influence the treatment that a patient receives. Once we have more evidence on sequences of therapy that lead to best outcomes for patients, the discrepancy between the treatments patients receive depending on their provider specialty could have tremendous implications for patients. In addition, the finding that higher income may be a barrier to novel oral therapies has implications for patient care that mandate further consideration regarding assistance programs, which may very well account for our findings.27 Effectively improving access to tolerable and available treatments hinges on the understanding of current practices. Future work will use knowledge of drivers of variation in treatment to develop targeted implementation strategies for current and future therapies.
Supplementary Material
Acknowledgments
Acknowledgement of Research Support: Dr. Mukherjee is funded by the University of Michigan Comprehensive Cancer Center Core Grant - P30 CA 046592.
Dr. Hollenbeck is funded by an Agency for Healthcare Research and Quality R01 HS 025707.
Footnotes
Conflicts of interest: The authors have no competing interests and nothing to disclose.
Contributor Information
Megan E.V. Caram, Email: mveresh@med.umich.edu.
Shikun Wang, Email: shikunw@umich.edu.
Phoebe Tsao, Email: chengpa@med.umich.edu.
Jennifer J. Griggs, Email: jengrigg@med.umich.edu.
David C. Miller, Email: dcmiller@med.umich.edu.
Brent K. Hollenbeck, Email: bhollen@med.umich.edu.
Paul Lin, Email: linpaul@med.umich.edu.
Bhramar Mukherjee, Email: bhramar@umich.edu.
References
- 1.Tannock IF, de Wit R, Berry WR, et al. : Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 351:1502–12, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Kantoff PW, Higano CS, Shore ND, et al. : Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 363:411–22, 2010 [DOI] [PubMed] [Google Scholar]
- 3.de Bono JS, Oudard S, Ozguroglu M, et al. : Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 376:1147–54, 2010 [DOI] [PubMed] [Google Scholar]
- 4.de Bono JS, Logothetis CJ, Molina A, et al. : Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 364:1995–2005, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryan CJ, Smith MR, de Bono JS, et al. : Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 368:138–48, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scher HI, Fizazi K, Saad F, et al. : Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 367:1187–97, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Beer TM, Armstrong AJ, Rathkopf DE, et al. : Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 371:424–33, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parker C, Nilsson S, Heinrich D, et al. : Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 369:213–23, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Basch E, Loblaw DA, Oliver TK, et al. : Systemic therapy in men with metastatic castration-resistant prostate cancer:American Society of Clinical Oncology and Cancer Care Ontario clinical practice guideline. J Clin Oncol 32:3436–48, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lowrance WT, Roth BJ, Kirkby E, et al. : Castration-Resistant Prostate Cancer: AUA Guideline Amendment 2015. J Urol 195:1444–52, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Caram M, Estes J, Griggs J, et al. : Temporal & Geographic Variation in Systemic Treatment of Advanced Prostate Cancer, 2010–2015. BMC Cancer, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flaig TW, Potluri RC, Ng Y, et al. : Treatment evolution for metastatic castration-resistant prostate cancer with recent introduction of novel agents: retrospective analysis of real-world data. Cancer Med 5:182–91, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wennberg DE, Kellett MA, Dickens JD, et al. : The association between local diagnostic testing intensity and invasive cardiac procedures. JAMA 275:1161–4, 1996 [PubMed] [Google Scholar]
- 14.Wennberg DE, Dickens JD Jr., Biener L, et al. : Do physicians do what they say? The inclination to test and its association with coronary angiography rates. J Gen Intern Med 12:172–6, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisher ES, Wennberg DE, Stukel TA, et al. : Variations in the longitudinal efficiency of academic medical centers. Health Aff (Millwood) Suppl Variation:VAR 19–32, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Wennberg JE: Practice variations and health care reform: connecting the dots. Health Aff (Millwood) Suppl Variation:VAR 140–4, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Baicker K, Chandra A, Skinner JS: Geographic variation in health care and the problem of measuring racial disparities. Perspect Biol Med 48:S42–53, 2005 [PubMed] [Google Scholar]
- 18.Baicker K, Chandra A, Skinner JS, et al. : Who you are and where you live: how race and geography affect the treatment of medicare beneficiaries. Health Aff (Millwood) Suppl Variation:VAR 33–44, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Clinformatics DataMart User Manual, in OptumInsight (ed): (ed Version 7.1), 2017
- 20.Sweeney CJ, Chen YH, Carducci M, et al. : Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N Engl J Med 373:737–46, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choudhry NK, Lee JL, Agnew-Blais J, et al. : Drug company-sponsored patient assistance programs: a viable safety net? Health Aff (Millwood) 28:827–34, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao L, Joseph J, Santoro-Levy M, et al. : Utilization of Pharmaceutical Patient and Prescription Assistance Programs via a Pharmacy Department Patient Assistance Program for Indigent Cancer Patients. Hosp Pharm 51:572–6, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cookson MS, Roth BJ, Dahm P, et al. : Castration-resistant prostate cancer: AUA Guideline. J Urol 190:429–38, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Cookson MS, Lowrance WT, Murad MH, et al. : Castration-resistant prostate cancer: AUA guideline amendment. J Urol 193:491–9, 2015 [DOI] [PubMed] [Google Scholar]
- 25.Geynisman DM, Doyle J, Kutikov A, et al. : Urologists’ experiences with treatment of castration-resistant prostate cancer. Journal of Clinical Oncology 34:315–315, 2016 [Google Scholar]
- 26.Chawla N, Yabroff KR, Mariotto A, et al. : Limited validity of diagnosis codes in Medicare claims for identifying cancer metastases and inferring stage. Ann Epidemiol 24:666–72, 672.e1–2, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ubel PA, Bach PB: Copay Assistance for Expensive Drugs: A Helping Hand That Raises Costs. Ann Intern Med 165:878–879, 2016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.