Lock et al. offer a perspective on how the discovery of a new class of cell adhesion complex drives convergence of the integrin and clathrin fields.
Abstract
An understanding of the mechanisms whereby cell adhesion complexes (ACs) relay signals bidirectionally across the plasma membrane is necessary to interpret the role of adhesion in regulating migration, differentiation, and growth. A range of AC types has been defined, but to date all have similar compositions and are dependent on a connection to the actin cytoskeleton. Recently, a new class of AC has been reported that normally lacks association with both the cytoskeleton and integrin-associated adhesome components, but is rich in components of the clathrin-mediated endocytosis machinery. The characterization of this new type of adhesion structure, which is emphasized by mitotic cells and cells in long-term culture, identifies a hitherto underappreciated link between the adhesion machinery and clathrin structures at the plasma membrane. While this discovery has implications for how ACs are assembled and disassembled, it raises many other issues. Consequently, to increase awareness within the field, and stimulate research, we explore a number of the most significant questions below.
Introduction
In metazoa, most cells are in permanent or transient contact with an ECM. This adhesion is enabled by thousands of dynamic contacts, which are largely mediated by members of the integrin family of receptors (Miranti and Brugge, 2002; Hynes, 2004; Campbell and Humphries, 2011). Integrins bridge the ECM to all three major cytoskeletal networks (actomyosin polymers, microtubules, and intermediate filaments) via a regulated network of adaptors and signaling proteins collectively termed the adhesome. These cell–ECM contact sites, or adhesion nexi, are focally distributed because they map onto the anisotropic structure of the ECM. Consequently, they enable spatial sensing of the chemistry, mechanical properties, and topology of the immediate cellular microenvironment. In turn, this information is integrated to regulate most aspects of cellular phenotype, including growth, movement, and differentiation.
There is strong evidence that adhesion nexi exist both in 3D culture and in vivo (Webb et al., 2003; Harunaga and Yamada, 2011; van Geemen et al., 2014), but their visualization and compositional interrogation are challenging because they are frequently small, heterogeneous, and labile. Consequently, much of our understanding is derived from studies in vitro. In keeping with the broad range of functional roles mediated by adhesion, it is unsurprising that a diverse array of adhesion complexes (ACs) has been defined. In fibroblastic cells, the formation of new adhesion sites involves the sequential creation of structures termed nascent adhesions/focal complexes, focal adhesions, and fibrillar adhesions (Geiger and Yamada, 2011). Although differences exist between the different types of complex, each has a relatively similar composition and, in this article, they are collectively termed canonical ACs (Geiger et al., 2001). The temporal maturation of canonical ACs is observed both in cells adhering from suspension and in migratory cells. In addition to these structures, other cells can exhibit more specialized ACs, including podosomes, hemidesmosomes, and cell–cell synapses that overlap in composition with canonical ACs (Bromley et al., 2001; Jurdic et al., 2006; Walko et al., 2015).
These various types of AC have a very wide range of half-lives. Some, such as rivet-like hemidesmosomes, are specialized to maintain long-term adhesion of epithelia to basement membranes. Others, such as nascent adhesions/focal complexes, turn over in minutes to enable membrane protrusion and migration. During these processes, integrin uptake and recycling are required to reposition receptors and deliver new membrane. Both clathrin- and caveolin-mediated endocytic mechanisms are involved, and it is now appreciated that receptor trafficking makes an essential contribution to both cell translocation and adhesion receptor signaling (Caswell et al., 2009; Moreno-Layseca et al., 2019).
Recently, our laboratories have described a new class of AC and, as a result, the integrin and clathrin fields have been brought even closer together (Baschieri et al., 2018; Lock et al., 2018). The classical scheme of clathrin-mediated endocytosis (CME) is viewed to regulate the uptake of a diverse range of cell surface receptors and their ligands, and thereby allow cells to acquire nutrients, to control the composition of the plasma membrane and to regulate signaling pathways (Kaksonen and Roux, 2018). In addition, a subset of large clathrin-containing structures (often termed clathrin-coated plaques, flat clathrin lattices, or clathrin sheets) has long been observed to remain at the cell surface for extended periods of time (Lampe et al., 2016). The stability of these clathrin plaques has brought into question their role as endocytic structures, and they have consequently received relatively little attention compared with the clathrin-coated pits that support receptor-mediated endocytosis.
Clathrin plaques were first observed several decades ago and shown to be both in close proximity to the substratum and associated with integrin receptors (Heuser, 1980; Maupin and Pollard, 1983; De Deyne et al., 1998; Lampe et al., 2016). While suggestive of a role in cell adhesion, no direct evidence was generated that plaques were adhesive, and they were still viewed as endocytic intermediates with a primary function of receptor redistribution (De Deyne et al., 1998). Recently, however, it has been reported that the αVβ5 integrin, which is the most commonly identified receptor within plaques, actually anchors the clathrin coat to the substrate, preventing clathrin budding and leading to the formation of extended plaques (Baschieri et al., 2018). In contemporaneous studies in the integrin field, αVβ5 was found to dominate the adhesion of cells in long-term culture and during mitosis (Lock et al., 2018). The ACs responsible, which were termed reticular adhesions (RAs), were unusual in that they lacked association with actin and most canonical AC components. Proteomic cataloging of RAs revealed many components of the CME machinery, and subsequent ultrastructural and kinetic analyses confirmed their similarity, if not identity, to clathrin plaques (Fig. 1; Lock et al., 2018). Although direct experimental comparisons of clathrin plaques and RAs have not yet been performed, the evidence from independently acquired datasets suggests that they are indeed equivalent structures. For the remainder of this article, we will refer to them as RAs/plaques. Much remains to be investigated with regard to the roles these structures play in vivo. RA/plaques have been observed extensively in cells in culture (Fig. 2), and clathrin plaques have been observed in some physiological contexts, such as at the surface of myocytes and at the contact sites between osteoclasts and bone (Akisaka, 2000; Vassilopoulos et al., 2014). Furthermore, αVβ5 is maintained at the apical surface of retinal pigment epithelial cells in clathrin structures (Nandrot et al., 2012), where it has been shown to play a role in the daily phagocytosis of spent photoreceptor outer segment fragments in vivo (Nandrot et al., 2004). Therefore, there are likely to be highly tissue-specific roles for RA/plaques in vivo.
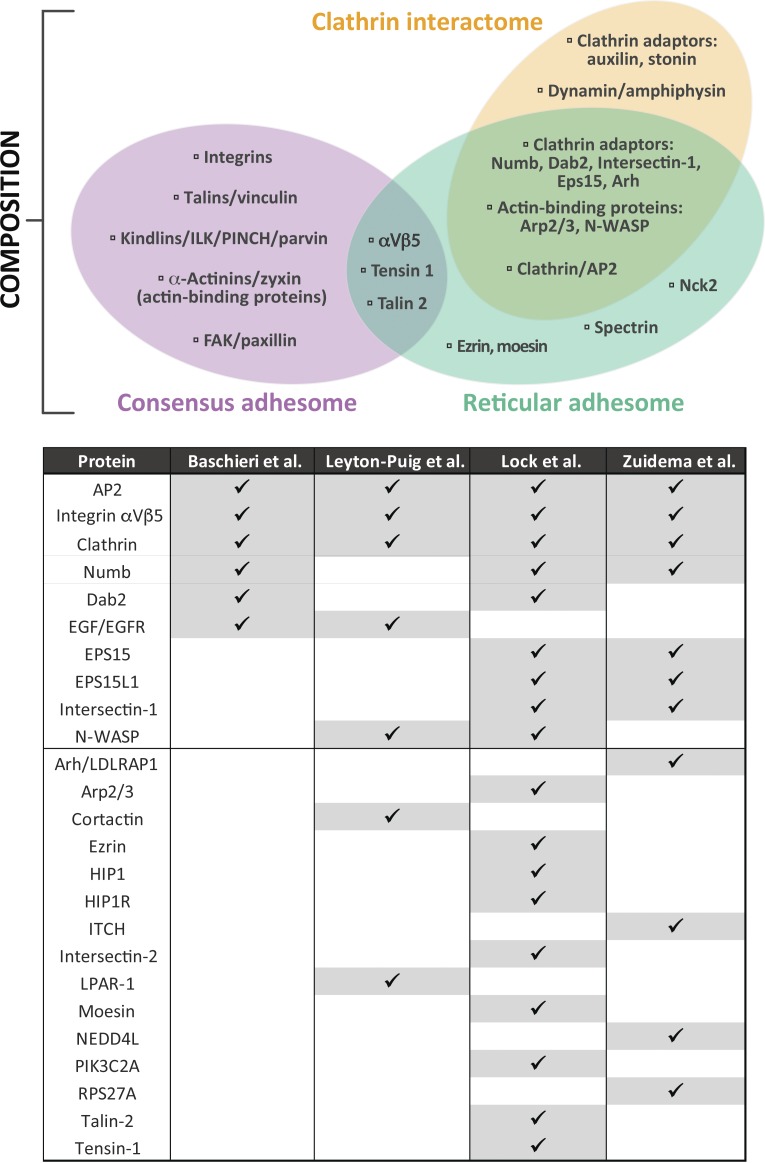
Figure 1.
Comparison of molecular composition. The three-way Venn diagram illustrates a compositional comparison between the consensus (Horton et al., 2015), reticular (Lock et al., 2018), and clathrin interactomes (Schmid and McMahon, 2007). Proteins were chosen and grouped to illustrate typical functional modules, and the selection is not intended to indicate an exhaustive set of components. This analysis shows the limited overlap between canonical AC composition and the RA/clathrin interactomes. However, considerably more overlap is evident between the RA and clathrin interactomes. The table represents a direct comparison between clathrin plaque and RA datasets from the four indicated studies from proteomic and antibody-based localization data (Leyton-Puig et al., 2017; Baschieri et al., 2018; Lock et al., 2018; Zuidema et al., 2018). Proteins were ordered according to the number of datasets in which they were detected. These analyses indicate that while canonical ACs are distinct from RAs and conventional clathrin structures, RAs and plaques are highly compositionally similar and are likely the same.
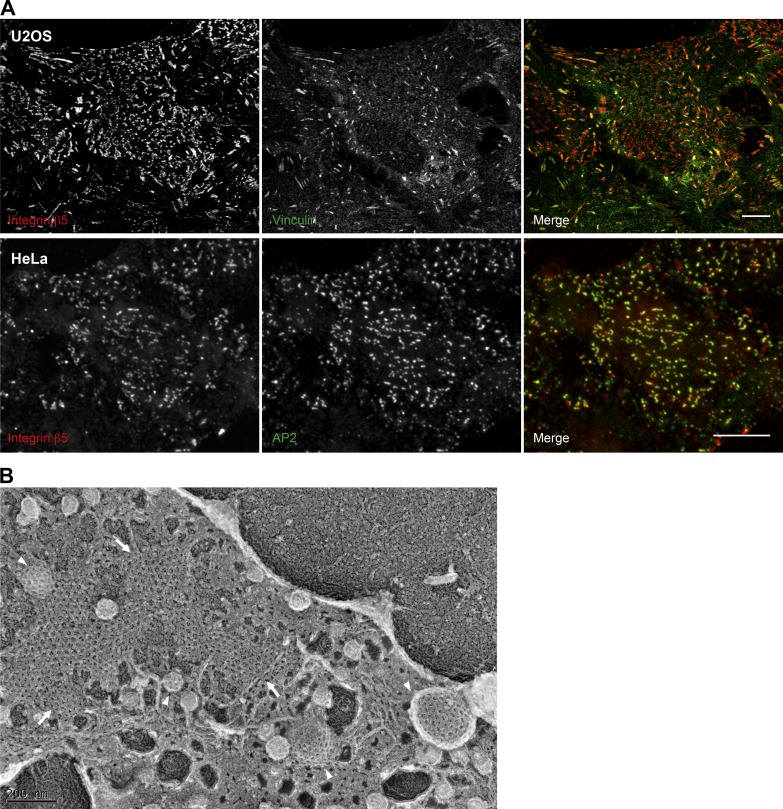
Figure 2.
Imaging of ACs. (A) U2OS and HeLa cells were plated on uncoated or collagen I–coated glass coverslips and cultured for 24 h. Cells were subsequently fixed and immunostained for integrin αVβ5, the canonical AC component vinculin, or the AP2 complex subunit α-adaptin. Scale bars: 10 µm. (B) HS578t cells were plated on collagen I–coated glass coverslips. 24 h later, cells were unroofed by sonication to generate a platinum replica of the inner leaflet of the adherent part of the plasma membrane as previously described (Elkhatib et al., 2017). Imaging was performed by transmission EM. Arrows point to plaques and arrowheads to clathrin-coated pits. Image in B was provided courtesy of Dr. Nadia Elkhatib.
How do the composition and dynamics of RA/plaques and canonical ACs compare?
The first comprehensive cataloging of canonical AC composition came through a literature curation exercise, led by Geiger and colleagues, which amalgamated information from studies of all types of canonical AC (Zaidel-Bar et al., 2007; Geiger and Yamada, 2011; Winograd-Katz et al., 2014). A theoretical network of >200 components was constructed (Winograd-Katz et al., 2014). Following the development of methods to isolate canonical ACs, mass spectrometry was used to determine their actual composition (Humphries et al., 2009; Kuo et al., 2011; Schiller et al., 2011, 2013). Seven of these mass spectrometry datasets were used to generate both a “meta-adhesome” database of >2,000 proteins that are enriched at fibronectin-induced canonical ACs and a “consensus adhesome,” which represents the 60 most frequently detected proteins (Horton et al., 2015). A limited proximity ligation-based proteomic study subsequently confirmed and complemented the original isolation strategies (Dong et al., 2016). The consensus adhesome has recently been merged with the literature-curated adhesome to provide a detailed view of the integrin-proximal connections responsible for adhesion via canonical ACs (Horton et al., 2016).
Comparison of the consensus adhesome of canonical ACs to the adhesome of RAs (Lock et al., 2018) and the composition of clathrin plaques (Leyton-Puig et al., 2017; Zuidema et al., 2018) demonstrate that canonical ACs are clearly distinct, while RAs and clathrin plaques are highly analogous (Fig. 1). For example, comparison of biological pathways (using Kyoto Encyclopedia of Genes and Genomes [KEGG]) shows strong similarities in pathway enrichment, with three out of the first five ranked pathways shared between plaques and RAs (endocytosis, endocrine and other factor-regulated calcium reabsorption, and synaptic vesicle cycle). RAs/plaques are distinctive in their expression of integrin αVβ5, together with a large number of clathrin- as well as phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2)–associated components (Leyton-Puig et al., 2017; Baschieri et al., 2018; Lock et al., 2018; Zuidema et al., 2018). Inspection of the broader canonical AC meta adhesome (Horton et al., 2015) reveals many components of the CME machinery, including clathrin itself and a range of adaptors (Dab2, Eps8, Eps15, AP2, GGA2, and epsin). The presence of CME components in the canonical AC meta-adhesome, but not the consensus adhesome, may reflect the heterogeneous nature of ACs in cultured cells. Alternatively, these differences may indicate context-specific recruitment of some CME and RA/plaque components to canonical ACs, suggesting a continuous spectrum of adhesion structures in cells.
In both canonical ACs and RAs, integrins organize into nanoclusters whose spatial arrangement is essentially indistinguishable between these types of complex (Fig. 3; Lock et al., 2018; Spiess et al., 2018). Nanoscale molecular organization is less fully characterized within structures explicitly defined as clathrin plaques, but current data also support the existence of punctate sub-structures (Leyton-Puig et al., 2017). Given that the composition of canonical ACs and RAs/plaques is distinct, and RAs/plaques tend to lack adhesome components, integrin nanoscale organization may depend on a range of directly binding proteins, including talin, as previously suggested (Klapholz and Brown, 2017). 3D analyses of canonical ACs and RAs/plaques have revealed very different nanoscale arrangements: In canonical ACs, signaling components, integrin–F-actin linkers, and F-actin regulators occupy specific strata (Kanchanawong et al., 2010), while in RAs/plaques, there is a dynamic partitioning of endocytic proteins whose location depends on the stage of endocytosis (Sochacki et al., 2017).
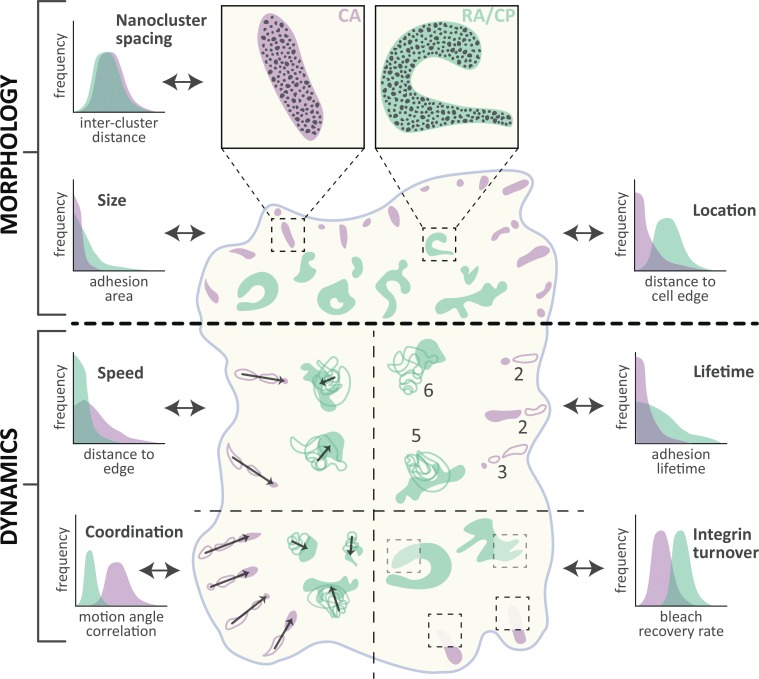
Figure 3.
Comparison of morphology and dynamics. Schematized view of a cell containing canonical ACs (CA; purple) and RAs/plaques (RA/CP; green). Specific morphological (above thick dotted line) and dynamic (below thick dotted line) properties are compared both visually and with illustrative graphs indicating quantitative differences measured in referenced publications. Morphology: Integrin αVβ5 nanoclusters are similar in appearance and arrangement (Nanocluster spacing) in CA and RA/CP (Lock et al., 2018). Visibly more convoluted in shape, RA/CP also achieve larger areas (Size) and are commonly located further from the cell edge (Location) than CA (Lock et al., 2018). Dynamics: CA slide rapidly (Speed; Ballestrem et al., 2001; Lock et al., 2018), linearly, and in a locally anisotropic fashion (Coordination) due to F-actin–derived forces (Besser and Safran, 2006). In contrast, RA/CP grow isotropically and show little directionality (Lock et al., 2018). Dynamics of individual complexes depicted with increasing opacity over time; arrows indicate net motion direction and distance. RA/CP complexes as a whole are highly stable (Lifetime) relative to CA (Lock et al., 2018; numbers indicate total time points observed per structure depicted in schematic). Conversely, the association of integrin αVβ5 proteins (Integrin turnover) with RA/CP is more dynamic than in CA, as revealed by higher recovery rates after photobleaching (Lock et al., 2018; transparency level within dotted boxed depicts relative fluorescence recovery).
A hallmark of canonical ACs is their strong link to F-actin. This can be mediated through several molecular modules, such as talin-vinculin, kindlin-ILK-PINCH-parvin, tensin, filamin, and α-actinin-zyxin-VASP (Horton et al., 2015; Humphries et al., 2019). These physical F-actin connections and associated mechanical forces shape canonical AC characteristics during their entire life cycle, including initiation, maturation, mobility, and disassembly (Vicente-Manzanares and Horwitz, 2011). Indeed, actin-derived mechanical forces induce clear adaptive changes (Lock et al., 2008), including the modification of composition and alterations in morphological anisotropy (linearization) and dynamics (sliding; Fig. 3; Goldyn et al., 2009; Lock et al., 2018). Actin filaments have been reported to be associated with plaques, and to organize desmin filaments in muscle cells (Franck et al., 2019), but this association is not at the same level of enrichment as in canonical ACs and rarely involves actomyosin fibers. Consistent with this finding, compositional analyses found that RAs/plaques lack almost all integrin–F-actin–linking modules (Lock et al., 2018; Zuidema et al., 2018). Accordingly, F-actin fiber/bundle association with RAs/plaques appears much less enriched or even absent (Leyton-Puig et al., 2017; Lock et al., 2018; Zuidema et al., 2018), and RA/plaque morphology and dynamics are isotropic (Lock et al., 2018), since without F-actin–directed forces, there is no clear polarizing influence. The lack of actomyosin-derived forces, together with the absence of microtubules, may also explain the higher stability of RAs than canonical ACs (Lock et al., 2018). Moreover, though both canonical ACs and RAs can support cell attachment to the ECM, the more robust attachment mediated by canonical ACs is likely due to their strong connection with the actin cytoskeleton (Lock et al., 2018). Having said this, RAs/plaques are not independent of actin regulation, since WASP and Arp2/3 activity down-regulates clathrin plaques, likely through their conversion into endocytic pits (Leyton-Puig et al., 2017). Such conversion would mirror well-established transitions in the maturation of CME structures, and may be an indication that actin association only occurs at particular stages of maturation (Lu et al., 2016). Regardless, even before WASP and Arp2/3 (and thus actin) recruitment, RA/plaque formation is sensitive to the mechanical properties of the environment, although not to actomyosin contractility (Leyton-Puig et al., 2017; Baschieri et al., 2018; Lock et al., 2018). Thus, both canonical ACs and RAs/plaques are mechanosensitive structures, though with different underlying mechanisms. As a consequence, the formation of RAs/plaques provides cells not only with an alternative means of mediating attachment to ECM ligands, but also with an alternative mechanism by which mechanical forces can be distributed.
How is integrin αVβ5 activated at RAs/plaques?
Why RAs/plaques appear to use integrin αVβ5 exclusively is not yet clear. One speculative possibility is that αVβ5 is more potent than other integrins in frustrating the clathrin machinery. The degree of endocytic frustration could depend on the affinity of the integrin extracellular domain for its ECM substrate and/or the affinity of the integrin cytoplasmic domains for clathrin and its adaptors, or it could be the result of a receptor-specific signal. It is possible that any integrin located in clathrin patches and engaged with the substrate may mechanically impair clathrin pit formation and thereby promote formation of RAs/plaques; however, to date, there is only limited evidence for integrins being localized to these sites. β1 integrin has been localized to plaques adherent to collagen fibers in 3D culture (Elkhatib et al., 2017), and recently α6β4 has been reported in structures resembling plaques in normal human glandular epithelial cells, where it has been proposed to increase the resistance to mechanical stretch (Wang et al., 2019).
The activation status of integrin αVβ5 at RAs/plaques has not been directly tested, but a significant portion is likely to be in an active conformation given that RAs/plaques are found only at cell–ECM substrate contact sites (Baschieri et al., 2018; Bucher et al., 2018 Preprint; Lock et al., 2018) and RA/plaque formation requires the αVβ5 high-affinity ligand vitronectin (Baschieri et al., 2018; Lock et al., 2018; Zuidema et al., 2018). Given the dogma that talin binding to a conserved integrin β-chain NPXY motif is necessary for integrin activation (Tadokoro et al., 2003), and at least some talin-2 is present in RAs (Lock et al., 2018), the presence of activated integrin αVβ5 in RAs is surprising since RAs/plaques can form independently of talin (Baschieri et al., 2018; Lock et al., 2018). However, it has been reported that some integrin β-chains, including β5, interact through their NPXY motifs with phosphotyrosine binding domain–containing clathrin adaptors such as Dab2 and Numb (Calderwood et al., 2003; Yu et al., 2015), which are components of RAs/plaques (Mettlen et al., 2010; Baschieri et al., 2018; Lock et al., 2018; Zuidema et al., 2018). These interactions may be sufficient to maintain integrin activity, though this remains to be examined. Another possibility is that the membrane-bending property of clathrin could be the origin of forces that may participate in αVβ5 activation and/or reinforcement of its affinity for the ECM. Besides clathrin itself, several clathrin plaque–associated proteins are endowed with the capacity to bend membranes through different mechanisms (Sochacki and Taraska, 2019), and it is already known that clathrin plaques exert forces on substrate-anchored cargoes (Stabley et al., 2011). Further investigations will be required to assess whether such forces participate in the activation of αVβ5 and the formation of RAs/plaques. If this were the case, cells would be able to disperse forces through the plasma membrane and into the cortical actin cytoskeleton in a uniform manner, rather than through intracellular actin fibers in localized sites of traction force. This may reflect fundamentally different roles for canonical ACs compared with RAs/plaques, whereby traction forces generated by canonical ACs facilitate cell motility (Elkhatib et al., 2017), whereas RAs/plaques mediate mechano-responsiveness in a different manner. The role of RAs/plaques in motile cells and how these structures interact with highly dynamic canonical ACs in this context require investigation.
Whereas canonical AC formation depends on integrin interactions with talin and kindlin (Tadokoro et al., 2003; Moser et al., 2008; Ussar et al., 2008; Ye et al., 2014; Sun et al., 2019), integrin αVβ5 recruitment to, and/or maintenance at, RA/plaques requires interactions with clathrin plaque components (Baschieri et al., 2018; Zuidema et al., 2018). This is further complicated by the fact that some integrin α-chains interact with the major clathrin adaptor AP2 (De Franceschi et al., 2016). Furthermore, the clathrin plaque components ARH and Eps15L1 may contribute to αVβ5 recruitment and/or maintenance through direct interactions (Zuidema et al., 2018). Strikingly, mutation of the membrane-proximal NPXY motif of β5, or swapping the β5 intracellular domain to that of β1 or β3, shifts αVβ5 accumulation from clathrin plaques to canonical ACs in the context of expression in keratinocytes (Zuidema et al., 2018). Injection of a synthetic peptide containing the β5 NPXY sequence also depleted clathrin from plaque structures (De Deyne et al., 1998). These findings imply that β5 tail affinity for clathrin adaptors is instrumental in RA/plaque recruitment. By contrast, a similar chimeric β5/β3 mutant expressed in U2OS osteosarcoma cells accumulated in RAs/plaques like WT β5 (Lock et al., 2018), while integrin β3 itself underwent Dab2-dependent accumulation in RA/plaque-like structures in mouse fibroblasts (Yu et al., 2015). Together, these data suggest context-dependent influences of integrin cytoplasmic tails on their recruitment to RA/plaques.
As with integrin regulation in canonical ACs, inhibitory proteins such as ICAP-1 and SHARPIN (Bouvard et al., 2003; Rantala et al., 2011) may also modulate integrin activity and localization at RAs/plaques. Another candidate is Pak4, which shows selective negative regulation of integrin αVβ5 (relative to αVβ3 and β1 integrins) through phosphorylation of two serine residues in a β5-specific cytoplasmic SERS-motif (Zhang et al., 2002; Li et al., 2010a,b). It remains unclear how such negative regulation may influence RAs/plaques versus canonical ACs.
Do RA/plaques and canonical ACs interchange?
While canonical ACs appear distinct across a number of important biological dimensions, they are functionally coupled to RAs/plaques through a regulatory interplay/relationship that balances these different types of molecular complex. There are precedents for other integrins switching between adhesive structures: For example, α6β4 is found in hemidesmosomes and canonical ACs, with the switch to use in canonical ACs believed to contribute to a pro-migratory and invasive phenotype in cancer cells (Ramovs et al., 2017). It has been suggested that canonical AC-dependent modification of the ECM marks the sites for plaque formation, and that this process is regulated by Eps15 and Eps15R (Bucher et al., 2018 Preprint). Integrin αVβ5 locates to both canonical ACs and RAs/plaques, yet the integrin tropism for one structure or the other can be modulated. Indeed, perturbation of one structure tends to promote the other through redistribution of αVβ5. This implies a common reservoir of integrin αVβ5 that shuttles between canonical ACs and RAs/plaques, thereby determining the relative abundance of these structures. For example, canonical AC depletion through perturbation of actin polymerization, myosin II activity, or talin expression increases RAs/plaques and their αVβ5 pool (Baschieri et al., 2018; Lock et al., 2018). Conversely, canonical AC promotion, through a RhoA-driven increase in actomyosin contractility, depletes RAs/plaques and rebalances integrin αVβ5 toward canonical ACs (Zuidema et al., 2018). Furthermore, RA/plaque depletion, through knockdown of AP2, Numb, Eps15L1, or ARH, drives an increase in canonical AC size and αVβ5 content in a manner dependent on β5 expression levels (Baschieri et al., 2018; Zuidema et al., 2018). This recurring balance between canonical ACs and RAs/plaques indicates that they may actually compete for integrin αVβ5 from a collective pool. In support of this hypothesis, overexpression of β5 promotes RAs/plaques by enlarging the αVβ5 reservoir (Lock et al., 2018), while β5 depletion reduces RAs/plaques but does not reduce canonical ACs (Baschieri et al., 2018).
Interestingly, the phosphatidylinositols PI(4,5)P2 and phosphatidylinositol (3,4,5)-trisphosphate (PIP3) also impact the balance of the canonical AC–RA/plaque axis. Specifically, PI(4,5)P2 favors RAs/plaques, while PIP3 favors canonical ACs (Lock et al., 2018). The dependence of RAs/plaques on PI(4,5)P2 is reflected in the large number of PI(4,5)P2-binding proteins that comprise these structures, including the AP2 components α-adaptin and β2-adaptin, as well as Dab2, Numb, N-WASP, and Eps15 (Lock et al., 2018). Consensus adhesome components such as talin and vinculin also bind PI(4,5)P2, and kindlin and ILK bind PIP3. It is well established that the PIP3-generating phosphoinositide 3-kinase plays a role in force-dependent strengthening of canonical ACs (Katsumi et al., 2004), hence the preference for high PIP3 conditions is consistent with previous findings.
While the details above provide multiple lines of evidence supporting some form of exchange or communication between canonical ACs and RAs/plaques, it remains unclear how αVβ5 physically transfers between canonical ACs and RA/plaques. This likely involves integrin endocytosis and recycling (Moreno-Layseca et al., 2019), yet FRAP analyses also indicate rapid αVβ5 diffusion within the plasma membrane, providing a second avenue for integrin movement (Li et al., 2010a). Identifying factors that regulate the balancing of this emerging canonical AC–RA/plaque axis is now necessary to understand how these structures collectively enable cell interactions with, and responses to, the extracellular environment.
What are the implications for adhesion signaling?
Canonical ACs are well known to mediate cell attachment and to serve as signaling hubs where integrins orchestrate an array of chemical- and force-induced signaling events (Green and Brown, 2019; Humphries et al., 2019). Given their distinct composition, it is remarkable that canonical ACs and RAs/plaques share similar functional roles. First, RAs/plaques can also support cell adhesion to the ECM (Lock et al., 2018). Second, clathrin plaques are signaling platforms whose formation is sensitive to the mechanical properties of the environment (Baschieri et al., 2018).
Currently, most evidence for clathrin plaques as adhesive structures is circumstantial rather than directly measured, with EM images showing the membranes of cells in close association with ECM (Maupin and Pollard, 1983; Lampe et al., 2016). Furthermore, as discussed in the Introduction, integrins such as αVβ5 localize to RA/plaques together with clathrin adaptors, suggesting an adhesive function. Intriguingly, integrin β1 and clathrin adaptors have also been observed to wrap around collagen fibers in 3D collagen gels in structures termed tubular clathrin/AP2 lattices (Elkhatib et al., 2017). These structures, which resemble RAs/plaques, were important for cell adhesion and migration in a manner dependent on integrin, Dab2, and AP2, but independent of clathrin. Perhaps the most direct evidence for an adhesive role of RA/plaques has come from assays measuring cell attachment to vitronectin, an αVβ5 ECM ligand, where RAs facilitated cell attachment in the absence of F-actin and this attachment was blocked by competitive inhibition of αVβ5 using cyclic RGDfV or cilengitide (Baschieri et al., 2018; Lock et al., 2018).
The fact that RAs/plaques persist following disruption of the actin cytoskeleton or inhibition of actomyosin contractility suggests these structures may act to facilitate cell adhesion in environments unfavorable for formation of canonical ACs. Indeed, loss of physical forces on ligand-bound β3 integrin promotes association of endocytic adaptors such as Dab2 and Numb and exclusion of talin from β3-associated complexes (Yu et al., 2015). This is particularly relevant in the context of mitosis, where canonical ACs must be disassembled to allow cell rounding and the reuse of canonical AC components and the actomyosin machinery during cytokinesis, and will be discussed in depth later. However, formation of RAs/plaques is in itself mechano-responsive, with an increase in structures being observed on stiffer substrates (Baschieri et al., 2018). Therefore, the balance between canonical AC- and RA/plaque-mediated adhesion is likely to be determined by a number of factors beyond simply ECM rigidity. The tight interplay between the different types of structures and the ability of cells to switch the balance between canonical ACs and RAs/plaques suggest that the use of these structures is likely to be highly context specific.
In addition to mechanosensing, there is now evidence that RAs/plaques are also able to transduce chemical signals, although the full complement of signaling pathways that are directly activated remains to be determined. In addition to integrins, many classes of receptors accumulate at clathrin plaques, including the epidermal growth factor receptor (EGFR), c-Met, CCR5, and others (Grove et al., 2014; Baschieri et al., 2018). Recruitment of the EGFR to clathrin plaques is required for optimal signal transduction, independently of endocytosis (Garay et al., 2015), and localization at clathrin plaques and subsequent activation of ERK requires both the EGFR and anchorage to the substrate (Baschieri et al., 2018). Thus, a new model progressively emerges in which RAs/plaques serve as sorting stations (Grove et al., 2014) as well as signaling platforms for different receptors (Baschieri et al., 2018), and the generation of these platforms is dependent on integrin–ligand engagement. The influence of clathrin plaques on receptor signaling may also be receptor specific; for example, lysophosphatidic acid receptor-1 triggers plaque disassembly and receptor endocytosis, resulting in down-regulation of Akt activation (Leyton-Puig et al., 2017). In contrast, stimulation of the EGFR did not promote clathrin plaque disassembly (Baschieri et al., 2018). Therefore, understanding how clathrin plaques interact with canonical clathrin pits and how the formation of these two structures is determined by receptors is key to determining how RAs/plaques are able to impact upon signaling networks.
What are the implications for endocytosis?
CME relies on the formation of clathrin-coated structures on the internal leaflet of the plasma membrane. These structures recruit receptors and progressively bend the plasma membrane to form clathrin-coated pits that further mature into receptor-containing vesicles that bud off in the cytosol. Although the core of clathrin plaques is not considered competent for endocytosis at steady-state, these structures may participate in a spatial and temporal regulation of endocytic events depending on the conditions of the environment. For example, RAs/plaques only assemble at regions of the plasma membrane contacting the ECM (Baschieri et al., 2018; Bucher et al., 2018 Preprint), stressing the need for αVβ5 engagement with the substrate to support their formation.
Clathrin pit dynamics are reduced, although not stalled, in the vicinity of canonical ACs (Batchelder and Yarar, 2010), as well as on collagen fibers (Elkhatib et al., 2017), in a β1 integrin–dependent manner, demonstrating that ECM engagement is able to influence endocytosis. These observations are consistent with a model in which AC disassembly normally leads to integrin endocytosis, but in the case of αVβ5, persistent integrin–ligand binding can act to frustrate endocytosis, leading to accumulation of RAs/plaques at the plasma membrane. However, these RAs/plaques also act as hotspots for endocytosis with discrete scission events being found to cluster at the edges of RAs/plaques (Lampe et al., 2016). This suggests that clathrin pits are able to form via a process that involves remodeling of RAs/plaques, consistent with the observation that it is bending of a preexisting clathrin lattice that induces formation of clathrin pits, rather than formation de novo of curved clathrin structures (Sochacki and Taraska, 2019). This transition from a flat to curved clathrin structure requires that physical forces mediated by integrin binding to the ECM, which prevent spontaneous bending of the membrane by clathrin, are surmounted and may therefore require specific factors. In the case of lysophosphatidic acid–driven disassembly of clathrin plaques and formation of CCPs, polymerization of actin by N-WASP and Arp2/3 is required (Leyton-Puig et al., 2017), and this is consistent with actin playing a role in facilitating endocytosis at the edges of clathrin plaques. Clathrin pit formation may also require rearrangements of plaque components (Sochacki et al., 2017) as well as resolution of the αVβ5 plaque attachment (Baschieri et al., 2018). Though it remains unclear how αVβ5 inhibition may occur and if there might be any commonality among the regulation of αVβ5 and N-WASP–Arp2/3 in this context, it is interesting that Pak4 can both inhibit αVβ5 attachment (as noted above; Zhang et al., 2002; Li et al., 2010a,b) and promote Arp2/3-mediated actin polymerization through phosphorylation of N-WASP (Zhao et al., 2017).
In general, beyond their roles as adhesive structures and signaling hubs, it is unclear how RAs/plaques influence endocytic trafficking. In particular, while endocytosis and subsequent trafficking of β3 and β1 integrins is well established and contributes to a wide range of cellular functions (Moreno-Layseca et al., 2019), whether endocytosis of αVβ5 from RAs/plaques can be observed and how this process might be regulated have not been determined.
Why are RA/plaques used at mitosis?
When considering the balance of the canonical AC–RA/plaque axis, an intriguing case is that of mitosis, where canonical ACs are completely depleted (Dix et al., 2018), while RAs are maintained to enable effective mitosis and daughter cell respreading (Lock et al., 2018; Zaidel-Bar, 2018; Li and Burridge, 2019). This dramatic yet naturally occurring perturbation of the canonical AC–RA/plaque equilibrium now provides an instructive setting in which to interrogate mechanisms controlling this balance, though it remains unclear if the RAs that attach mitotic cells are identical to clathrin plaques or maintain the same molecular composition as during interphase.
Other fundamental questions may also be tractable in this context. For instance, it is unclear how the integrin αVβ5–ECM connection in RAs/plaques is mechanically coupled to the cell as a whole. Though the molecular modules typically linking integrins to F-actin are absent and large-scale F-actin fiber bundles are not recruited, a more subtle or indirect actin connection may nonetheless exist. Candidates for such actin coupling include RA/plaque components ezrin and moesin (Lock et al., 2018), which link actin and the plasma membrane in other situations (Fehon et al., 2010). Given the exquisite colocalization of mitotic retraction fibers and RAs (Lock et al., 2018), and the potentially force-driven remodeling of these adhesive structures during mitosis (Lock et al., 2018), an understanding of how F-actin may be coupled to RAs/clathrin plaques is required.
By providing adhesion, RAs/plaques orientate the mitotic process (Lock et al., 2018), which is critical for cell fate determination (Théry et al., 2005). It would be therefore of interest to investigate the possibility that RAs/plaques influence cellular differentiation. In agreement with this putative role, Numb, a known antagonist of the cell fate determinant Notch (Hutterer and Knoblich, 2005), is found in RAs (Lock et al., 2018). It could be speculated that variations in the distribution of Numb in RAs might contribute to the cell fate decisions of differentiating cells.
Another intriguing research question relates to potential roles of RAs/plaques in the substantial shutdown of CME that occurs during mitosis (Fielding and Royle, 2013), a process dependent on the RA/plaque component Dab2 (Chetrit et al., 2011; Lock et al., 2018). Notably, this shutdown appears to involve blockade at the invagination stage of CME (Pypaert et al., 1987), potentially stalling the maturation of endocytic structures at the prior stage that, as we have already noted, corresponds both morphologically and compositionally with RAs/plaques (Lu et al., 2016). If RA/plaque-mediated adhesion is prioritized during mitosis (given the loss of other adhesion mechanisms), this may exacerbate the adhesion-mediated frustration of endocytosis recently described (Baschieri et al., 2018) and thus unmask a pivotal interplay between RA/plaque functions in adhesive and endocytic regulation during mitosis.
Conclusions
Emerging evidence demonstrates that RAs/plaques are bona fide cellular structures that are distinct from canonical ACs and control multiple key cellular events, including adhesion, receptor-mediated signaling, mechanosensing, endocytosis, and cell division. Given the recent nature of these findings, it is likely that RAs/plaques will prove to be important in additional processes. The intriguing composition of RAs/plaques, which contain both adhesion- and clathrin-associated components, may guide us to gain a better future understanding of these events and how they contribute to physiology and disease.
Acknowledgments
Work from the authors that contributed to this perspective was supported by Cancer Research UK (grant C13329/A21671) to M.J. Humphries; the Gustave Roussy Institute (core funding), the Institut National de la Santé et de la Recherche Médicale (core funding), the ATIP (Action thématique incitative sur programme)/Avenir Program, la Fondation ARC pour la Recherche sur le Cancer, and l’Agence Nationale de la Recherche (grants ANR-15-CE15-0005-03 and ANR-17-CE13-0020-03), all to G. Montagnac; and the Swedish Research Council (grant 2016-02380) and the Swedish Foundation for Strategic Research (grant SB16-0046) to S. Strömblad. F. Baschieri was supported by a fellowship from La Ligue Nationale Contre le Cancer.
The authors declare no competing financial interests.
References
- Akisaka T. 2000. [Ultrastructure of clathrin sheets and cytoskeleton of podosomes on the cytoplasmic side of ventral membranes of cultured osteoclasts]. Kaibogaku Zasshi. 75:381–386. [PubMed] [Google Scholar]
- Ballestrem C., Hinz B., Imhof B.A., and Wehrle-Haller B.. 2001. Marching at the front and dragging behind: differential alphaVbeta3-integrin turnover regulates focal adhesion behavior. J. Cell Biol. 155:1319–1332. 10.1083/jcb.200107107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baschieri F., Dayot S., Elkhatib N., Ly N., Capmany A., Schauer K., Betz T., Vignjevic D.M., Poincloux R., and Montagnac G.. 2018. Frustrated endocytosis controls contractility-independent mechanotransduction at clathrin-coated structures. Nat. Commun. 9:3825 10.1038/s41467-018-06367-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchelder E.M., and Yarar D.. 2010. Differential requirements for clathrin-dependent endocytosis at sites of cell-substrate adhesion. Mol. Biol. Cell. 21:3070–3079. 10.1091/mbc.e09-12-1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besser A., and Safran S.A.. 2006. Force-induced adsorption and anisotropic growth of focal adhesions. Biophys. J. 90:3469–3484. 10.1529/biophysj.105.074377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvard D., Vignoud L., Dupé-Manet S., Abed N., Fournier H.N., Vincent-Monegat C., Retta S.F., Fassler R., and Block M.R.. 2003. Disruption of focal adhesions by integrin cytoplasmic domain-associated protein-1 alpha. J. Biol. Chem. 278:6567–6574. 10.1074/jbc.M211258200 [DOI] [PubMed] [Google Scholar]
- Bromley S.K., Burack W.R., Johnson K.G., Somersalo K., Sims T.N., Sumen C., Davis M.M., Shaw A.S., Allen P.M., and Dustin M.L.. 2001. The immunological synapse. Annu. Rev. Immunol. 19:375–396. 10.1146/annurev.immunol.19.1.375 [DOI] [PubMed] [Google Scholar]
- Bucher D., Mukenhirn M., Sochacki K.A., Saharuka V., Huck C., Zambarda C., Taraska J., Cavalcanti-Adam E.A., and Boulant S.. 2018. Focal adhesion-generated cues in extracellular matrix regulate cell migration by local induction of clathrin-coated plaques. bioRxiv. doi:. (Preprint posted December 11, 2018) 10.1101/493114 [DOI] [Google Scholar]
- Calderwood D.A., Fujioka Y., de Pereda J.M., García-Alvarez B., Nakamoto T., Margolis B., McGlade C.J., Liddington R.C., and Ginsberg M.H.. 2003. Integrin beta cytoplasmic domain interactions with phosphotyrosine-binding domains: a structural prototype for diversity in integrin signaling. Proc. Natl. Acad. Sci. USA. 100:2272–2277. 10.1073/pnas.262791999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell I.D., and Humphries M.J.. 2011. Integrin structure, activation, and interactions. Cold Spring Harb. Perspect. Biol. 3:a004994 10.1101/cshperspect.a004994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caswell P.T., Vadrevu S., and Norman J.C.. 2009. Integrins: masters and slaves of endocytic transport. Nat. Rev. Mol. Cell Biol. 10:843–853. 10.1038/nrm2799 [DOI] [PubMed] [Google Scholar]
- Chetrit D., Barzilay L., Horn G., Bielik T., Smorodinsky N.I., and Ehrlich M.. 2011. Negative regulation of the endocytic adaptor disabled-2 (Dab2) in mitosis. J. Biol. Chem. 286:5392–5403. 10.1074/jbc.M110.161851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Deyne P.G., O’Neill A., Resneck W.G., Dmytrenko G.M., Pumplin D.W., and Bloch R.J.. 1998. The vitronectin receptor associates with clathrin-coated membrane domains via the cytoplasmic domain of its beta5 subunit. J. Cell Sci. 111:2729–2740. [DOI] [PubMed] [Google Scholar]
- De Franceschi N., Arjonen A., Elkhatib N., Denessiouk K., Wrobel A.G., Wilson T.A., Pouwels J., Montagnac G., Owen D.J., and Ivaska J.. 2016. Selective integrin endocytosis is driven by interactions between the integrin α-chain and AP2. Nat. Struct. Mol. Biol. 23:172–179. 10.1038/nsmb.3161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix C.L., Matthews H.K., Uroz M., McLaren S., Wolf L., Heatley N., Win Z., Almada P., Henriques R., Boutros M., et al. 2018. The Role of Mitotic Cell-Substrate Adhesion Re-modeling in Animal Cell Division. Dev. Cell. 45:132–145.e3. 10.1016/j.devcel.2018.03.009 [DOI] [PubMed] [Google Scholar]
- Dong J.M., Tay F.P., Swa H.L., Gunaratne J., Leung T., Burke B., and Manser E.. 2016. Proximity biotinylation provides insight into the molecular composition of focal adhesions at the nanometer scale. Sci. Signal. 9:rs4 10.1126/scisignal.aaf3572 [DOI] [PubMed] [Google Scholar]
- Elkhatib N., Bresteau E., Baschieri F., Rioja A.L., van Niel G., Vassilopoulos S., and Montagnac G.. 2017. Tubular clathrin/AP-2 lattices pinch collagen fibers to support 3D cell migration. Science. 356:eaal4713 10.1126/science.aal4713 [DOI] [PubMed] [Google Scholar]
- Fehon R.G., McClatchey A.I., and Bretscher A.. 2010. Organizing the cell cortex: the role of ERM proteins. Nat. Rev. Mol. Cell Biol. 11:276–287. 10.1038/nrm2866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding A.B., and Royle S.J.. 2013. Mitotic inhibition of clathrin-mediated endocytosis. Cell. Mol. Life Sci. 70:3423–3433. 10.1007/s00018-012-1250-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franck A., Lainé J., Moulay G., Lemerle E., Trichet M., Gentil C., Benkhelifa-Ziyyat S., Lacène E., Bui M.T., Brochier G., et al. 2019. Clathrin plaques and associated actin anchor intermediate filaments in skeletal muscle. Mol. Biol. Cell. 30:579–590. 10.1091/mbc.E18-11-0718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garay C., Judge G., Lucarelli S., Bautista S., Pandey R., Singh T., and Antonescu C.N.. 2015. Epidermal growth factor-stimulated Akt phosphorylation requires clathrin or ErbB2 but not receptor endocytosis. Mol. Biol. Cell. 26:3504–3519. 10.1091/mbc.E14-09-1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B., and Yamada K.M.. 2011. Molecular architecture and function of matrix adhesions. Cold Spring Harb. Perspect. Biol. 3:a005033 10.1101/cshperspect.a005033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B., Bershadsky A., Pankov R., and Yamada K.M.. 2001. Transmembrane crosstalk between the extracellular matrix--cytoskeleton crosstalk. Nat. Rev. Mol. Cell Biol. 2:793–805. 10.1038/35099066 [DOI] [PubMed] [Google Scholar]
- Goldyn A.M., Rioja B.A., Spatz J.P., Ballestrem C., and Kemkemer R.. 2009. Force-induced cell polarisation is linked to RhoA-driven microtubule-independent focal-adhesion sliding. J. Cell Sci. 122:3644–3651. 10.1242/jcs.054866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green H.J., and Brown N.H.. 2019. Integrin intracellular machinery in action. Exp. Cell Res. 378:226–231. 10.1016/j.yexcr.2019.03.011 [DOI] [PubMed] [Google Scholar]
- Grove J., Metcalf D.J., Knight A.E., Wavre-Shapton S.T., Sun T., Protonotarios E.D., Griffin L.D., Lippincott-Schwartz J., and Marsh M.. 2014. Flat clathrin lattices: stable features of the plasma membrane. Mol. Biol. Cell. 25:3581–3594. 10.1091/mbc.e14-06-1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harunaga J.S., and Yamada K.M.. 2011. Cell-matrix adhesions in 3D. Matrix Biol. 30:363–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. 1980. Three-dimensional visualization of coated vesicle formation in fibroblasts. J. Cell Biol. 84:560–583. 10.1083/jcb.84.3.560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton E.R., Byron A., Askari J.A., Ng D.H.J., Millon-Frémillon A., Robertson J., Koper E.J., Paul N.R., Warwood S., Knight D., et al. 2015. Definition of a consensus integrin adhesome and its dynamics during adhesion complex assembly and disassembly. Nat. Cell Biol. 17:1577–1587. 10.1038/ncb3257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton E.R., Humphries J.D., James J., Jones M.C., Askari J.A., and Humphries M.J.. 2016. The integrin adhesome network at a glance. J. Cell Sci. 129:4159–4163. 10.1242/jcs.192054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries J.D., Byron A., Bass M.D., Craig S.E., Pinney J.W., Knight D., and Humphries M.J.. 2009. Proteomic analysis of integrin-associated complexes identifies RCC2 as a dual regulator of Rac1 and Arf6. Sci. Signal. 2:ra51 10.1126/scisignal.2000396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries J.D., Chastney M.R., Askari J.A., and Humphries M.J.. 2019. Signal transduction via integrin adhesion complexes. Curr. Opin. Cell Biol. 56:14–21. 10.1016/j.ceb.2018.08.004 [DOI] [PubMed] [Google Scholar]
- Hutterer A., and Knoblich J.A.. 2005. Numb and alpha-Adaptin regulate Sanpodo endocytosis to specify cell fate in Drosophila external sensory organs. EMBO Rep. 6:836–842. 10.1038/sj.embor.7400500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R.O. 2004. The emergence of integrins: a personal and historical perspective. Matrix Biol. 23:333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurdic P., Saltel F., Chabadel A., and Destaing O.. 2006. Podosome and sealing zone: specificity of the osteoclast model. Eur. J. Cell Biol. 85:195–202. 10.1016/j.ejcb.2005.09.008 [DOI] [PubMed] [Google Scholar]
- Kaksonen M., and Roux A.. 2018. Mechanisms of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 19:313–326. 10.1038/nrm.2017.132 [DOI] [PubMed] [Google Scholar]
- Kanchanawong P., Shtengel G., Pasapera A.M., Ramko E.B., Davidson M.W., Hess H.F., and Waterman C.M.. 2010. Nanoscale architecture of integrin-based cell adhesions. Nature. 468:580–584. 10.1038/nature09621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsumi A., Orr A.W., Tzima E., and Schwartz M.A.. 2004. Integrins in mechanotransduction. J. Biol. Chem. 279:12001–12004. 10.1074/jbc.R300038200 [DOI] [PubMed] [Google Scholar]
- Klapholz B., and Brown N.H.. 2017. Talin - the master of integrin adhesions. J. Cell Sci. 130:2435–2446. 10.1242/jcs.190991 [DOI] [PubMed] [Google Scholar]
- Kuo J.C., Han X., Hsiao C.T., Yates J.R. III, and Waterman C.M.. 2011. Analysis of the myosin-II-responsive focal adhesion proteome reveals a role for β-Pix in negative regulation of focal adhesion maturation. Nat. Cell Biol. 13:383–393. 10.1038/ncb2216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe M., Vassilopoulos S., and Merrifield C.. 2016. Clathrin coated pits, plaques and adhesion. J. Struct. Biol. 196:48–56. 10.1016/j.jsb.2016.07.009 [DOI] [PubMed] [Google Scholar]
- Leyton-Puig D., Isogai T., Argenzio E., van den Broek B., Klarenbeek J., Janssen H., Jalink K., and Innocenti M.. 2017. Flat clathrin lattices are dynamic actin-controlled hubs for clathrin-mediated endocytosis and signalling of specific receptors. Nat. Commun. 8:16068 10.1038/ncomms16068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., and Burridge K.. 2019. Cell-Cycle-Dependent Regulation of Cell Adhesions: Adhering to the Schedule: Three papers reveal unexpected properties of adhesion structures as cells progress through the cell cycle. BioEssays. 41:e1800165 10.1002/bies.201800165 [DOI] [PubMed] [Google Scholar]
- Li Z., Lock J.G., Olofsson H., Kowalewski J.M., Teller S., Liu Y., Zhang H., and Strömblad S.. 2010a Integrin-mediated cell attachment induces a PAK4-dependent feedback loop regulating cell adhesion through modified integrin alpha v beta 5 clustering and turnover. Mol. Biol. Cell. 21:3317–3329. 10.1091/mbc.e10-03-0245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Zhang H., Lundin L., Thullberg M., Liu Y., Wang Y., Claesson-Welsh L., and Strömblad S.. 2010b p21-activated kinase 4 phosphorylation of integrin beta5 Ser-759 and Ser-762 regulates cell migration. J. Biol. Chem. 285:23699–23710. 10.1074/jbc.M110.123497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock J.G., Wehrle-Haller B., and Strömblad S.. 2008. Cell-matrix adhesion complexes: master control machinery of cell migration. Semin. Cancer Biol. 18:65–76. 10.1016/j.semcancer.2007.10.001 [DOI] [PubMed] [Google Scholar]
- Lock J.G., Jones M.C., Askari J.A., Gong X., Oddone A., Olofsson H., Göransson S., Lakadamyali M., Humphries M.J., and Strömblad S.. 2018. Reticular adhesions are a distinct class of cell-matrix adhesions that mediate attachment during mitosis. Nat. Cell Biol. 20:1290–1302. 10.1038/s41556-018-0220-2 [DOI] [PubMed] [Google Scholar]
- Lu R., Drubin D.G., and Sun Y.. 2016. Clathrin-mediated endocytosis in budding yeast at a glance. J. Cell Sci. 129:1531–1536. 10.1242/jcs.182303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maupin P., and Pollard T.D.. 1983. Improved preservation and staining of HeLa cell actin filaments, clathrin-coated membranes, and other cytoplasmic structures by tannic acid-glutaraldehyde-saponin fixation. J. Cell Biol. 96:51–62. 10.1083/jcb.96.1.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettlen M., Loerke D., Yarar D., Danuser G., and Schmid S.L.. 2010. Cargo- and adaptor-specific mechanisms regulate clathrin-mediated endocytosis. J. Cell Biol. 188:919–933. 10.1083/jcb.200908078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranti C.K., and Brugge J.S.. 2002. Sensing the environment: a historical perspective on integrin signal transduction. Nat. Cell Biol. 4:E83–E90. 10.1038/ncb0402-e83 [DOI] [PubMed] [Google Scholar]
- Moreno-Layseca P., Icha J., Hamidi H., and Ivaska J.. 2019. Integrin trafficking in cells and tissues. Nat. Cell Biol. 21:122–132. 10.1038/s41556-018-0223-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser M., Nieswandt B., Ussar S., Pozgajova M., and Fässler R.. 2008. Kindlin-3 is essential for integrin activation and platelet aggregation. Nat. Med. 14:325–330. 10.1038/nm1722 [DOI] [PubMed] [Google Scholar]
- Nandrot E.F., Kim Y., Brodie S.E., Huang X., Sheppard D., and Finnemann S.C.. 2004. Loss of synchronized retinal phagocytosis and age-related blindness in mice lacking alphavbeta5 integrin. J. Exp. Med. 200:1539–1545. 10.1084/jem.20041447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandrot E.F., Silva K.E., Scelfo C., and Finnemann S.C.. 2012. Retinal pigment epithelial cells use a MerTK-dependent mechanism to limit the phagocytic particle binding activity of αvβ5 integrin. Biol. Cell. 104:326–341. 10.1111/boc.201100076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pypaert M., Lucocq J.M., and Warren G.. 1987. Coated pits in interphase and mitotic A431 cells. Eur. J. Cell Biol. 45:23–29. [PubMed] [Google Scholar]
- Ramovs V., Te Molder L., and Sonnenberg A.. 2017. The opposing roles of laminin-binding integrins in cancer. Matrix Biol. 57–58:213–243. [DOI] [PubMed] [Google Scholar]
- Rantala J.K., Pouwels J., Pellinen T., Veltel S., Laasola P., Mattila E., Potter C.S., Duffy T., Sundberg J.P., Kallioniemi O., et al. 2011. SHARPIN is an endogenous inhibitor of β1-integrin activation. Nat. Cell Biol. 13:1315–1324. 10.1038/ncb2340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller H.B., Friedel C.C., Boulegue C., and Fässler R.. 2011. Quantitative proteomics of the integrin adhesome show a myosin II-dependent recruitment of LIM domain proteins. EMBO Rep. 12:259–266. 10.1038/embor.2011.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller H.B., Hermann M.R., Polleux J., Vignaud T., Zanivan S., Friedel C.C., Sun Z., Raducanu A., Gottschalk K.E., Théry M., et al. 2013. β1- and αv-class integrins cooperate to regulate myosin II during rigidity sensing of fibronectin-based microenvironments. Nat. Cell Biol. 15:625–636. 10.1038/ncb2747 [DOI] [PubMed] [Google Scholar]
- Schmid E.M., and McMahon H.T.. 2007. Integrating molecular and network biology to decode endocytosis. Nature. 448:883–888. 10.1038/nature06031 [DOI] [PubMed] [Google Scholar]
- Sochacki K.A., and Taraska J.W.. 2019. From Flat to Curved Clathrin: Controlling a Plastic Ratchet. Trends Cell Biol. 29:241–256. 10.1016/j.tcb.2018.12.002 [DOI] [PubMed] [Google Scholar]
- Sochacki K.A., Dickey A.M., Strub M.P., and Taraska J.W.. 2017. Endocytic proteins are partitioned at the edge of the clathrin lattice in mammalian cells. Nat. Cell Biol. 19:352–361. 10.1038/ncb3498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiess M., Hernandez-Varas P., Oddone A., Olofsson H., Blom H., Waithe D., Lock J.G., Lakadamyali M., and Strömblad S.. 2018. Active and inactive β1 integrins segregate into distinct nanoclusters in focal adhesions. J. Cell Biol. 217:1929–1940. 10.1083/jcb.201707075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabley D.R., Jurchenko C., Marshall S.S., and Salaita K.S.. 2011. Visualizing mechanical tension across membrane receptors with a fluorescent sensor. Nat. Methods. 9:64–67. 10.1038/nmeth.1747 [DOI] [PubMed] [Google Scholar]
- Sun Z., Costell M., and Fässler R.. 2019. Integrin activation by talin, kindlin and mechanical forces. Nat. Cell Biol. 21:25–31. 10.1038/s41556-018-0234-9 [DOI] [PubMed] [Google Scholar]
- Tadokoro S., Shattil S.J., Eto K., Tai V., Liddington R.C., de Pereda J.M., Ginsberg M.H., and Calderwood D.A.. 2003. Talin binding to integrin beta tails: a final common step in integrin activation. Science. 302:103–106. 10.1126/science.1086652 [DOI] [PubMed] [Google Scholar]
- Théry M., Racine V., Pépin A., Piel M., Chen Y., Sibarita J.B., and Bornens M.. 2005. The extracellular matrix guides the orientation of the cell division axis. Nat. Cell Biol. 7:947–953. 10.1038/ncb1307 [DOI] [PubMed] [Google Scholar]
- Ussar S., Moser M., Widmaier M., Rognoni E., Harrer C., Genzel-Boroviczeny O., and Fässler R.. 2008. Loss of Kindlin-1 causes skin atrophy and lethal neonatal intestinal epithelial dysfunction. PLoS Genet. 4:e1000289 10.1371/journal.pgen.1000289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Geemen D., Smeets M.W., van Stalborch A.M., Woerdeman L.A., Daemen M.J., Hordijk P.L., and Huveneers S.. 2014. F-actin-anchored focal adhesions distinguish endothelial phenotypes of human arteries and veins. Arterioscler. Thromb. Vasc. Biol. 34:2059–2067. 10.1161/ATVBAHA.114.304180 [DOI] [PubMed] [Google Scholar]
- Vassilopoulos S., Gentil C., Lainé J., Buclez P.O., Franck A., Ferry A., Précigout G., Roth R., Heuser J.E., Brodsky F.M., et al. 2014. Actin scaffolding by clathrin heavy chain is required for skeletal muscle sarcomere organization. J. Cell Biol. 205:377–393. 10.1083/jcb.201309096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente-Manzanares M., and Horwitz A.R.. 2011. Adhesion dynamics at a glance. J. Cell Sci. 124:3923–3927. 10.1242/jcs.095653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walko G., Castañón M.J., and Wiche G.. 2015. Molecular architecture and function of the hemidesmosome. Cell Tissue Res. 360:529–544. 10.1007/s00441-015-2216-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Hinton J.P., Gard J.M.C., Garcia J.G.N., Knudsen B.S., Nagle R.B., and Cress A.E.. 2019. Integrin α6β4E variant is associated with actin and CD9 structures and modifies the biophysical properties of cell-cell and cell-extracellular matrix interactions. Mol. Biol. Cell. 30:838–850. 10.1091/mbc.E18-10-0652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb D.J., Brown C.M., and Horwitz A.F.. 2003. Illuminating adhesion complexes in migrating cells: moving toward a bright future. Curr. Opin. Cell Biol. 15:614–620. 10.1016/S0955-0674(03)00105-4 [DOI] [PubMed] [Google Scholar]
- Winograd-Katz S.E., Fässler R., Geiger B., and Legate K.R.. 2014. The integrin adhesome: from genes and proteins to human disease. Nat. Rev. Mol. Cell Biol. 15:273–288. 10.1038/nrm3769 [DOI] [PubMed] [Google Scholar]
- Ye F., Snider A.K., and Ginsberg M.H.. 2014. Talin and kindlin: the one-two punch in integrin activation. Front. Med. 8:6–16. 10.1007/s11684-014-0317-3 [DOI] [PubMed] [Google Scholar]
- Yu C.H., Rafiq N.B., Cao F., Zhou Y., Krishnasamy A., Biswas K.H., Ravasio A., Chen Z., Wang Y.H., Kawauchi K., et al. 2015. Integrin-beta3 clusters recruit clathrin-mediated endocytic machinery in the absence of traction force. Nat. Commun. 6:8672 10.1038/ncomms9672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidel-Bar R. 2018. Atypical matrix adhesions guide cell division. Nat. Cell Biol. 20:1233–1235. 10.1038/s41556-018-0226-9 [DOI] [PubMed] [Google Scholar]
- Zaidel-Bar R., Itzkovitz S., Ma’ayan A., Iyengar R., and Geiger B.. 2007. Functional atlas of the integrin adhesome. Nat. Cell Biol. 9:858–867. 10.1038/ncb0807-858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Li Z., Viklund E.K., and Strömblad S.. 2002. P21-activated kinase 4 interacts with integrin alpha v beta 5 and regulates alpha v beta 5-mediated cell migration. J. Cell Biol. 158:1287–1297. 10.1083/jcb.200207008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M., Spiess M., Johansson H.J., Olofsson H., Hu J., Lehtiö J., and Strömblad S.. 2017. Identification of the PAK4 interactome reveals PAK4 phosphorylation of N-WASP and promotion of Arp2/3-dependent actin polymerization. Oncotarget. 8:77061–77074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuidema A., Wang W., Kreft M., Te Molder L., Hoekman L., Bleijerveld O.B., Nahidiazar L., Janssen H., and Sonnenberg A.. 2018. Mechanisms of integrin αVβ5 clustering in flat clathrin lattices. J. Cell Sci. 131:jcs221317 10.1242/jcs.221317 [DOI] [PubMed] [Google Scholar]