Del Signore and Rodal discuss work from Mondin et al. revealing a surprising collaboration between PTEN and PLCXD to restrict endosomal PtdIns(4,5)P2 levels.
Abstract
Loss of the phosphoinositide 5-phosphatase OCRL causes accumulation of PtdIns(4,5)P2 on membranes and, ultimately, Lowe syndrome. In this issue, Mondin et al. (2019. J. Cell Biol. https://doi.org/10.1083/jcb.201805155) discover that a surprising partnership between PTEN and the phospholipase PLCXD can compensate for OCRL to suppress endosomal PtdIns(4,5)P2 accumulation.
Phosphoinositides are key membrane lipids that control many dynamic cell biological processes, from cell migration to mitosis (1). These functions require precise spatiotemporal control over the abundance of specific phosphoinositide species, which can be phosphorylated or dephosphorylated at any of the 3, 4, or 5 positions of the inositol headgroup by competing lipid-modifying enzymes (Fig. 1). In this issue of JCB, Mondin et al. (2) discover a surprising collaboration between two such enzymes that were previously shown to exert opposite effects on the phosphoinositide PtdIns(4,5)P2: PTEN, a 3-phosphatase that typically increases PtdIns(4,5)P2 abundance via the dephosphorylation of PtdIns(3,4,5)P3, and PLCXD, which decreases PtdIns(4,5)P2 abundance by cleaving off the inositol headgroup. This unexpected partnership normally prevents excess accumulation of PtdIns(4,5)P2 on endosomes, and is able to compensate for the loss of the 5-phosphatase OCRL, a critical enzyme that also normally limits PtdIns(4,5)P2 buildup.
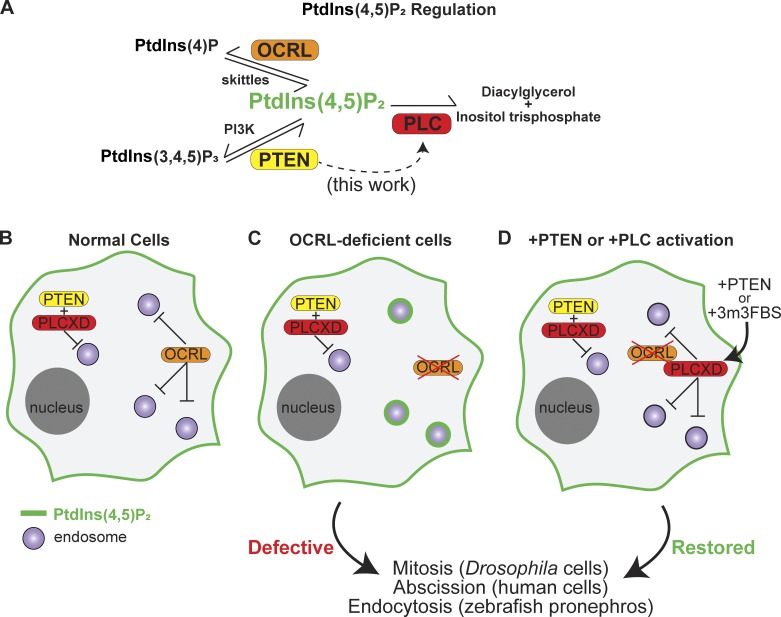
Figure 1.
PTEN and PLCXD counteract endosomal PIP2 accumulation in the absence of OCRL. (A) Schematic of PtdIns(4,5)P2 metabolism: OCRL and PLC directly decrease PtdIns(4,5)P2 levels, while PTEN canonically increases PtdIns(4,5)P2 by dephosphorylation of PtdIns(3,4,5)P3. (B) In healthy cells, OCRL and PTEN–PLCXD prevent PtdIns(4,5)P2 accumulation on endosomes. (C) OCRL-deficient cells accumulate PtdIns(4,5)P2 on endosomes. (D) Activation of the PTEN–PLCXD pathway by exogenous PTEN or PLCXD activation restores normal endosomal PtdIns(4,5)P2 levels.
OCRL itself regulates PtdIns(4,5)P2 levels in a number of cellular contexts. Loss of OCRL leads to aberrant PtdIns(4,5)P2 accumulation at cilia, the cytokinetic bridge, and most commonly on endosomes, thereby disrupting cell division, ciliogenesis, and other intracellular trafficking processes (3, 4). These defects are physiologically relevant, as mutations in OCRL cause Lowe syndrome, a congenital disorder with severe cognitive, renal, and ocular symptoms for which there are no effective treatments (3). One possible therapeutic strategy could be to coopt alternative or competing phosphoinositide-processing pathways to restore normal PtdIns(4,5)P2 homeostasis.
In this vein, Mondin et al. (2) set out to understand how rival processing pathways might interact to control endosomal PtdIns(4,5)P2, focusing initially on the 3-phosphatase PTEN. While best known for its roles in antagonizing PI3K and PtdIns(3,4,5)P3 signaling at the plasma membrane, PTEN has recently been shown to also localize to endosomes via its C2 domain (5, 6) and to promote accumulation of PtdIns(4,5)P2 at the plasma membrane as a byproduct of PtdIns(3,4,5)P3 dephosphorylation. Given this, the authors expected PTEN to exacerbate PtdIns(4,5)P2 accumulation in cells lacking OCRL. Quite surprisingly, they found precisely the opposite, as PTEN overexpression instead restored endosomal PtdIns(4,5)P2 to normal levels. Conversely, loss of PTEN increased endosomal PtdIns(4,5)P2 accumulation, suggesting that PTEN regulates endosomal PtdIns(4,5)P2 even in otherwise wild-type cells. Even more surprisingly, these effects did not involve the phosphatase activity of PTEN, as both a catalytically inactive version and a minimal PBD-C2 fragment (lacking the phosphatase domain) were able to keep PtdIns(4,5)P2 levels in check, both in normal cells and in cells lacking OCRL. This work adds to a small number of studies that have identified phosphatase-independent functions for PTEN in cancer cell migration (7) and membrane trafficking (8).
What could cause PTEN to behave so unexpectedly? The authors tested whether PTEN might regulate an alternative pathway to eliminate PtdIns(4,5)P2. One prime candidate is the PLC family of enzymes, which can cleave and thereby decrease PtdIns(4,5)P2. Indeed, by treating their PTEN-rescued, OCRL-deficient cells with inhibitors of PLC, they prevented the rescue of endosomal PtdIns(4,5)P2 accumulation. This effect was due specifically to one atypical PLC: PLCXD. Humans express three catalytically active PLCXD genes, a subset of which localizes to endosomal compartments (9). However, nothing is known of their cellular functions or regulation. Thus, this study provides the first insight into where and how PLCXD family members act and identifies an unexpected potential regulator in PTEN.
Several sets of experiments suggested that PTEN works by activating PLCXD: (1) PTEN and PLCXD appear together at a subset of endosomes, suggesting they act together locally to control PtdIns(4,5)P2 levels; (2) a catalytically inactive PLCXD is unable to rescue OCRL-deficient cells; (3) PTEN is required for the ability of PLCXD to rescue OCRL-deficient cells; and (4) chemical activation of PLCXD with the agonist m-3M3FBS bypasses the requirement of PTEN. Though the authors did not detect a direct interaction between PTEN and PLCXD, these experiments together suggest that PTEN works in a pathway to promote PLCXD phospholipase activity.
Notably, this pathway appears to be conserved from flies to vertebrates and operates in multiple cell biological contexts. The authors previously showed that loss of OCRL disrupts cell division in Drosophila melanogaster, endocytosis in the zebrafish pronephros (kidney), and the final step of mitosis in human Lowe syndrome patient cells. In each of these model systems, treatment with the PLC activator m-3M3FBS corrected the defects caused by loss of OCRL. The finding that pharmacological activation of PLCXD rescues OCRL deficiency to the same extent as PTEN overexpression is particularly exciting, as it suggests a potential therapy for Lowe syndrome patients.
Beyond the exciting therapeutic implications of this study, these experiments identify a completely unexpected interaction that reveals a novel pathway of endosomal PtdIns(4,5)P2 homeostasis and membrane trafficking, and raise many exciting questions. What is the mechanism of PTEN–PLCXD interaction and activation? What are the normal cell biological functions of this pathway, and in what trafficking events might it participate? Recent studies suggest two complementary ways that PTEN might regulate endosomal trafficking: by direct dephosphorylation of Rab7 to promote late endosome maturation (6), and by phosphatase-independent inhibition of retromer-mediated recycling (8). It will be particularly interesting to consider how PTEN–PLCXD regulation of phosphoinositides interacts with these other PTEN-dependent trafficking mechanisms to coordinate endosomal maturation and/or cargo sorting.
Overall, the surprising partnership between PTEN and PLCXD identified by this work provides a novel way (in addition to its better-characterized activities at the plasma membrane and in the nucleus) to consider how PTEN might control cell cycle, growth, migration, and other processes (10).
Acknowledgments
The authors declare no competing financial interests.
Work in the Rodal laboratory is supported by National Institutes of Health grant NS103967.
References
- 1.Schink K.O., et al. Annu. Rev. Cell Dev. Biol. 2016 doi: 10.1146/annurev-cellbio-111315-125349. [DOI] [PubMed] [Google Scholar]
- 2.Mondin V.E. J. Cell Biol. 2019 doi: 10.1083/jcb.201805155. [DOI] [Google Scholar]
- 3.Mehta Z.B., et al. Traffic. 2014 doi: 10.1111/tra.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Matteis M.A., et al. Nat. Rev. Nephrol. 2017 doi: 10.1038/nrneph.2017.83. [DOI] [Google Scholar]
- 5.Naguib A., et al. Mol. Cell. 2015 doi: 10.1016/j.molcel.2015.03.011. [DOI] [Google Scholar]
- 6.Shinde S.R., and Maddika S. Nat. Commun. 2016 doi: 10.1038/ncomms10689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raftopoulou M., et al. Science. 2004 doi: 10.1126/science.1092089. [DOI] [Google Scholar]
- 8.Shinde S.R., and Maddika S. Cell Reports. 2017 doi: 10.1016/j.celrep.2017.10.053. [DOI] [Google Scholar]
- 9.Gellatly S.A., et al. Biochem. Biophys. Res. Commun. 2012 doi: 10.1016/j.bbrc.2012.06.079. [DOI] [Google Scholar]
- 10.Hopkins B.D., et al. Trends Biochem. Sci. 2014 doi: 10.1016/j.tibs.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]