ABSTRACT
The study was to evaluate the safety, immunogenicity and lot-to-lot consistency of live attenuated varicella vaccine in Chinese population aged 1–3 years. The double-blind, randomized phase III trial was conducted in Henan Province, China. In total, 1197 subjects were included in this study. Subjects were randomly assigned into four groups in a 2:2:2:1 ratio to receive one of the three lots of commercial scale (CS) vaccine or the licensed pilot scale (LPS) vaccine. Seroconversion rate and neutralizing antibody titers (NATb) were assessed at day 0 pre-vaccination and at day 30 post-vaccination. Safety data were recorded for 30 days post-vaccination. After vaccination, the geometric mean titers (GMTs) of the three CS groups were 25.04 (95% confidence interval [CI], 22.85 to 27.44), 24.47 (95% CI, 22.35 to 26.78) and 25.88 (95% CI, 23.61 to 28.36), respectively (P= 0.6928). The ratio of GMTs adjusted for covariates of each pair of lots were all between 0.67 to 1.50 in susceptible subjects. The difference of seroconversion rate between pooled CS group and LPS group was 3.82 (95% CI, 0.55 to 8.81). Meanwhile, the percentage of solicited local, systemic and unsolicited adverse reactions showed no difference across the four groups, and most of the adverse reactions were mild or moderate in intensity. The CS group was comparable to the LPS group in safety and immunogenicity. The consistency of three consecutive CS lots was reliable. Moreover, the CS group was non-inferior to the LPS group.
KEYWORDS: live attenuated varicella vaccine, immunogenicity, consistency, safety, phase III clinical trial
Introduction
Varicella (chickenpox) is caused by the infection of double-strand DNA virus varicella zoster virus (VZV),1,2 which belongs to the family of herpesvirus.1,3 The disease is highly contagious, spreading through direct contact or aerosol of respiratory droplets.4 Varicella often outbreaks in temperate and mostly in tropical areas. Winter and spring are seasons of high incidence.5 Varicella mainly affects people younger than 15 years old with a highest morbidity age of 1–9. The typical symptoms include topical vesicular exanthema on the skin or mucosa, fever and malaise, which are usually moderate intensity. Sometimes severe symptoms or complications occur after secondary infection, such as pneumonia, encephalitis, hepatitis and so on.6–10 In addition, the virus can enter latent state in dorsal root ganglia and be reactivated in adults or the elderly when their immunity wanes, causing shingles.11–13
To date, the most effective way to prevent varicella is immunization using the live attenuated vaccine produced from the Oka strain of VZV.14,15 Vaccines produced from different manufacturers have been proved to be safe and effective.16–18 At present, there are several marketed varicella vaccine products in China. However, the market demand of varicella vaccine remains far from saturation and the need of varicella vaccine is ever-increasing after the inclusion of varicella vaccine into the National Immunization Program management system (currently in discussion). Currently, a live attenuated varicella vaccine produced by Sinovac (Dalian) is in the stage of phase III clinical trial. The phase I and phase III efficacy clinical trials proved the safety of the vaccine and the immunogenicity of the test group was superior to the placebo group. However, the quality and consistency of the post-marketing varicella vaccines were still unclear. Therefore, we aimed to evaluate the safety, immunogenicity and lot consistency of the varicella vaccine produced by Sinovac (Dalian).
Results
Study population
A total of 1197 subjects were enrolled in this study, with 342 subjects in each CS group and 171 subjects in LPS group. Of the 1197 subjects, two subjects discontinued the study as one in CS3 group and the other one in LPS group failed to receive vaccine, leaving 1195 subjects included in safety analysis set (SAS). The number of subjects in full analysis set (FAS) was the same as the SAS. Next, there were 1145 subjects included in per protocol set (PPS) because 17 subjects in CS1 group, 13 subjects in CS2 group, 15 subjects in CS3 group and one subject in LPS group refused to be taken blood sample, and one subject in CS2 group failed in blood sample collection and three subjects in LPS group used other vaccines (Figure 1). The basic characteristics of subjects were shown in Table 1 and there was no statistical difference in age, gender, height and weight (Table 1).
Figure 1.
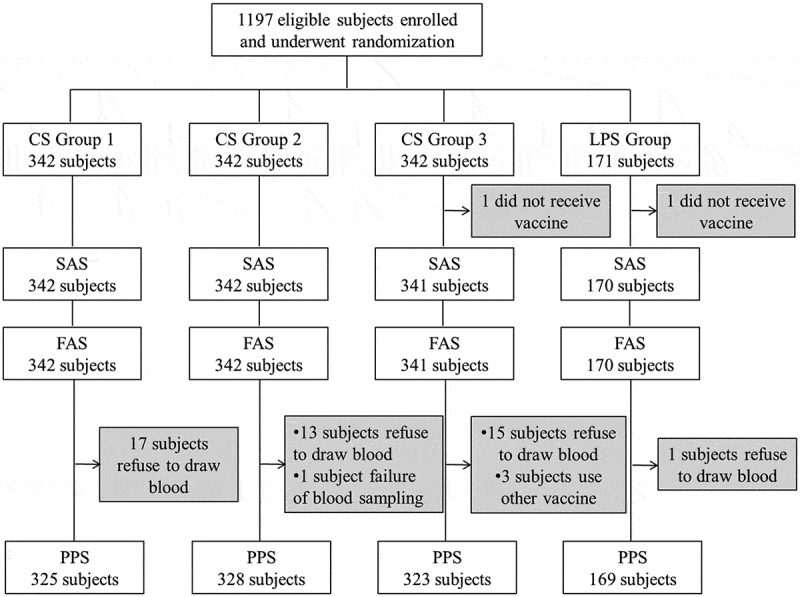
Subjects disposition of this study.
Table 1.
Demographic characteristics of study subjects.
| Variables | CS1 | CS2 | CS3 | LPS | P value | |
|---|---|---|---|---|---|---|
| SAS/FASa | N | 342 | 342 | 341 | 170 | - |
| Age (Year) | 2.04 ± 0.83 | 2.13 ± 0.82 | 2.04 ± 0.82 | 2.16 ± 0.77 | 0.1722 | |
| Gender Ratio (Male/Female) | 1:0.85 | 1:0.90 | 1:0.84 | 1:0.89 | 0.9692 | |
| Han ethnic, No.(%) | 342 (100.0) | 341 (99.71) | 341 (100.0) | 170 (100.0) | 1.0000 | |
| Height (cm) | 89.37 ± 8.69 | 90.14 ± 8.79 | 89.38 ± 8.66 | 90.74 ± 9.08 | 0.2552 | |
| Weight (kg) | 13.63 ± 2.56 | 13.94 ± 2.51 | 13.70 ± 2.56 | 13.87 ± 2.54 | 0.3949 | |
| PPS | N | 325 | 328 | 323 | 169 | - |
| Age (Year) | 2.06 ± 0.83 | 2.16 ± 0.82 | 2.04 ± 0.83 | 2.16 ± 0.77 | 0.1424 | |
| Gender Ratio (Male/Female) | 1:0.85 | 1:0.93 | 1:0.86 | 1:0.88 | 0.9356 | |
| Han ethnic, No.(%) | 325 (100.0) | 327 (99.70) | 323 (100.0) | 169 (100.0) | 1.0000 | |
| Height (cm) | 89.51 ± 8.65 | 90.36 ± 8.63 | 89.37 ± 8.54 | 90.68 ± 9.08 | 0.2481 | |
| Weight (kg) | 13.68 ± 2.55 | 14.00 ± 2.47 | 13.71 ± 2.53 | 13.85 ± 2.54 | 0.3486 |
aAll subjects in SAS set were enrolled into FAS set.
Immunogenicity
The results of immunogenicity were shown in Table 2. Before vaccination, the antibody levels of the four groups were balanced and comparable, with GMTs of the three CS groups between 2.54 and 2.63 and seropositive rate between 23.84% and 25.54%.
Table 2.
Immune response before and after varicella vaccination.
| Characteristics | CS1 | CS2 | CS3 | Pooled CS | LPS | P valueb | P valuec | Difference % (95%CI) |
|---|---|---|---|---|---|---|---|---|
| Pre-vaccination | ||||||||
| Seropositive (n) | 83 | 83 | 77 | 243 | 41 | - | - | - |
| Seronegative (n) | 242 | 245 | 246 | 733 | 128 | - | - | - |
| Seropositive rate, % | 25.54 | 25.30 | 23.84 | 24.90 | 24.26 | 0.8633 | 0.8594 | - |
| (95% CI) | (20.89–30.64) | (20.69–30.37) | (19.30–28.87) | (22.21–27.73) | (18.01–31.44) | |||
| GMT (95% CI) | 2.63 (2.48–2.79) | 2.59 (2.45–2.74) | 2.54 (2.41–2.67) | 2.59 (2.51–2.67) | 2.61 (2.40–2.84) | 0.6507 | 0.8380 | - |
| Post-vaccination | ||||||||
| Seroconversion rate, %(95% CI) | 97.54 (95.21–98.93) | 95.73 (92.94–97.65) | 96.90 (94.38–98.51) | 96.72 (95.40–97.75) | 92.90 (87.93–96.28) | 0.4208 | 0.0274 | 3.82 (0.55–8.81) |
| GMT (95% CI) | 25.04 (22.85–27.44) | 24.47 (22.35–26.78) | 25.88 (23.61–28.36) | 25.12 (23.83–26.47) | 23.43 (20.74–26.47) | 0.6928 | 0.3156 | - |
| GMI (95% CI) | 9.51 (8.72–10.37) | 9.43 (8.65–10.29) | 10.20 (9.36–11.10) | 9.70 (9.23–10.20) | 8.97 (7.93–10.15) | 0.3891 | 0.2372 | - |
bThe P values were calculated for comparisons of the three consecutive CS groups and LPS group by ANOVA.
cThe P values were calculated for comparison of the pooled CS group and LPS group by Fisher’ exact test.
After vaccination, the seroconversion rate of the three consecutive CS groups were 97.54% (95% CI, 95.21 to 98.93), 95.73% (95% CI, 92.94 to 97.65) and 96.90% (95% CI, 94.38 to 98.51). The GMTs were 25.04 (95% CI, 22.85 to 27.44), 24.47 (95% CI, 22.35 to 26.78) and 25.88 (95% CI, 23.61 to 28.36), respectively, and the corresponding GMI were 9.51 (95% CI, 8.72 to 10.37), 9.43 (95% CI, 8.65 to 10.29) and 10.20 (95% CI, 9.36 to 11.10). In addition, the seroconversion rate of LPS group was 92.90 % (95% CI, 87.93 to 96.28) and GMT was 23.43 (95% CI, 20.74 to 26.47) (Table 2).
Lot-to-lot consistency in CS groups
The equivalence of the day 30 immune responses to the three lots of the vaccines was demonstrated in Table 3. The ratio of GMTs adjusted for covariates in each pair of lots were between 0.67 to 1.50, with 95% CI of 0.89 to 1.23 between CS1 and CS2, 0.83 to 1.17 between CS1 and CS3, 0.89 to 1.26 between CS2 and CS3 in susceptible subjects (Table 3).
Table 3.
Immune response after vaccination in susceptible subjectsd.
| Characteristics | Criteria for equivalence | CS1 | CS2 | CS3 | P value |
|---|---|---|---|---|---|
| Post-vaccination | |||||
| GMT (95% CI) | 21.49 (19.44–23.76) | 20.52 (18.54–22.72) | 21.81 (19.76–24.08) | 0.6770 | |
| GMT adjusted covariates (95% CI) | 21.38 (19.50–23.99) | 20.42 (18.62–22.91) | 21.88 (12.59–23.99) | 0.6770 | |
| 95% CI of the ratio of GMT adjusted covariatesbetween: | |||||
| Lots CS1 and CS2 | (0.67–1.50) | (0.89–1.23) | (0.89–1.23) | - | - |
| Lots CS1 and CS3 | (0.67–1.50) | (0.83–1.17) | - | (0.83–1.17) | - |
| Lots CS2 and CS3 | (0.67–1.50) | - | (0.89–1.26) | (0.89–1.26) | - |
dLot consistency was studied only in children who had pre-vaccination NTAb titres <1:4.
Non-inferiority between CS and LPS group
The 95% CI of seroconversion rate difference was 0.55 to 8.81, indicating that the pooled CS group was non-inferior to the LPS group (Table 2).
Safety
The results of adverse reactions were shown in Table 4. There were 185 (15.48%) cases of adverse reactions in total and only 0.84% were grade III. The number of solicited and unsolicited adverse reactions were 179 (14.98%) and 6 (0.50%), respectively. The most common local reaction was pain, while the most common systemic reaction was fever. Most adverse reactions were mild and could recover within 30 days. Furthermore, no serious adverse reaction or death was reported. All of the local and systemic reactions had no significant difference among groups except cough (P= 0.0389), the incidence of which was a little higher in LPS group than the three CS groups (Table 4).
Table 4.
Adverse reactions after vaccination by study groupse.
| Type and Grade | CS1 | CS2 | CS3 | LPS | Total | P valuef |
|---|---|---|---|---|---|---|
| Overall | 61 (17.84) | 46 (13.45) | 51 (14.96) | 27 (15.88) | 185 (15.48) | 0.4569 |
| Grade 3 | 3 (0.88) | 5 (1.46) | 1 (0.29) | 1 (0.59) | 10 (0.84) | 0.4507 |
| Solicited | 59 (17.25) | 44 (12.87) | 51 (14.96) | 25 (14.71) | 179 (14.98) | 0.4596 |
| Local | ||||||
| Redness | 0 (0.00) | 1 (0.29) | 2 (0.59) | 0 (0.00) | 3 (0.25) | 0.5086 |
| Pain | 3 (0.88) | 1 (0.29) | 0 (0.00) | 2 (1.18) | 6 (0.50) | 0.1534 |
| Rash (injection site) | 1 (0.29) | 0 (0.00) | 0 (0.00) | 1 (0.59) | 2 (0.17) | 0.3456 |
| Swelling | 0 (0.00) | 1 (0.29) | 0 (0.00) | 0 (0.00) | 1 (0.08) | 1.0000 |
| Pruritus | 2 (0.58) | 1 (0.29) | 0 (0.00) | 1 (0.59) | 4 (0.33) | 0.6385 |
| Systemic | ||||||
| Fever | 50 (14.62) | 41 (11.99) | 49 (14.37) | 20 (11.76) | 160 (13.39) | 0.6508 |
| Cough | 4 (1.17) | 1 (0.29) | 0 (0.00) | 3 (1.76) | 8 (0.67) | 0.0389 |
| Nausea/Vomiting | 0 (0.00) | 0 (0.00) | 1 (0.29) | 0 (0.00) | 1 (0.08) | 0.4276 |
| Malaise | 1 (0.29) | 1 (0.29) | 0 (0.00) | 0 (0.00) | 2 (0.17) | 1.0000 |
| Allergy | 2 (0.58) | 1 (0.29) | 0 (0.00) | 0 (0.00) | 3 (0.25) | 0.8594 |
| Unsolicited | 2 (0.58) | 2 (0.58) | 0 (0.00) | 2 (1.18) | 6 (0.50) | 0.2877 |
eThe adverse reactions was presented as No. (%).
fThe P values were calculated for comparisons of the four groups by Pearson’s chi-square or Fisher’ exact test.
Discussion
Varicella attenuated vaccine has been on the market for many years at home and abroad and there have been many clinical trial studies conducted on it. The product in our study is a generic vaccine, so there is no need to conduct phase II trial to explore the dosage. We conducted phase III after its safety confirmed preliminarily in phase I trial. The existing studies about varicella vaccine mainly focused on its safety, effectiveness or immunogenicity. However, there were limited studies about lot-to-lot consistency, which is a significant indicator to evaluate the stability of the vaccine production process. Thus, the present phase III trial was to evaluate the safety, immunogenicity and lot consistency of the three lots of CS varicella vaccines.
The three consecutive lots of CS vaccines were shown to be highly immunogenic. The GMTs induced by the three CS lots vaccines at the day 30 post-vaccination were similar and comparable to the LPS lot. The seroconversion rates of the three CS lots were between 95.73% and 97.54%. The seroconversion rate of others in marketing were between 68.12% and 100.00%, and GMTs were between 4.36 and 1126.10.19 The vaccine in our study showed similar immunogenicity to those marketed domestic and imported varicella vaccine.20-23 Furthermore, the ratio of GMTs adjusted for covariates of each pair of lots were between 0.67 and 1.50, which satisfied the pre-defined equivalence criteria. The study also showed that the CS groups were non-inferior to the LPS group, manifesting that the manufacturing process and product quality of the CS vaccine are stable.
The three CS groups were comparable to the LPS group in safety. The overall incidence of adverse reactions was between 13.45% and 17.84% in the three CS groups, 15.88% in LPS group. Most of the solicited local and systemic reactions with all the vaccine groups were mild or moderate, and subjects could recover within 30 days. A few grade III adverse reactions were reported, the incidence of which was about 1% in each group and below 1% in total. Frequencies of unsolicited and solicited adverse reactions were similar across the four groups. The symptoms of local solicited adverse reactions were mainly pain, pruritus and redness, and the systemic were mainly fever, cough and allergy. No adverse reactions of special interest or safety concerns had been reported. The published clinical studies of marketed varicella vaccines showed that the incidence of adverse reactions was 0.0%-35.0% in total, 0%-11.54% in local, with the main local symptoms of pain, redness and induration, the main systemic symptoms of fever (mainly mild) and rash.19 In general, the symptoms of adverse reactions in this study was basically consistent with previous studies. Therefore, the studied vaccine was good in safety.
The strength of this study is that the safety, immunogenicity and lot-to-lot consistency of the varicella vaccine was assessed comprehensively, which ensured the vaccine applied for the children in a large scale. Moreover, bridging design was adopted to assess the three consecutive CS vaccines non-inferior to the LPS vaccine, evaluating the quality of the varicella vaccine in a simple and feasible method. In conclusion, the clinical study demonstrated that the varicella vaccine of Sinovac (Dalian) was eligible according to the Chinese pharmacopoeia immunogenicity criteria. The vaccine was safe for 1–3 years old children. Its immunogenic consistency and lot-to-lot reproducibility was also well-characterized. Varicella vaccine offers the most efficient and cost-effective immunotherapy to varicella virus and helps to reduce chickenpox-related hospitalizations and costs in children.
Conclusions
The CS lot was comparable to the LPS lot in safety and immunogenicity. Moreover, the three lots of CS vaccine was consistent and the production process was stable. The live attenuated varicella vaccine produced by Sinovac (Dalian) was suitable for large scale use.
Materials and methods
Study design
The double-blind, randomized phase III trial was conducted in Xiangfu District, Kaifeng City, Henan Province, China in 2017.
Study population
A total of 1322 healthy children aged 1–3 years with proved legal identity were initially recruited. The sample size of each test group (CS lot) was 283, calculated according to the equivalent design using NCSS-PASS. The assessment indicator was GMT at the day 30 after immunization, and the equivalent threshold was equal to ±0.176. The two-side value of α was 0.05. The power of the overall test was 80% and each test after adjusted was 93.4% (1–20%/3 = 93.4%). The estimated value of σ was 0.6 according to results of our preliminary study and other similar studies.
The sample size of control group (LPS lot) was 147, calculated according to non-inferiority assessment using NCSS-PASS. The assessment indicator was seroconversion rate at the day 30 after immunization. The estimated value of seroconversion rate was 80% referred to our previous trail study. The ratio of the sample size of test group to control group was 6:1. The single-side value of α was 0.025. The non-inferior criterion was that the lower limit of 95% CI of the difference between test group and control group was greater or equal to −10%.
In consideration of 20% seropositive rate pre-vaccination and 10% follow-up rate of each group, we finally obtained the sample size 342 of each test group and 171 of control group. The ratio of sample size in the four groups was 2:2:2:1.
1197 subjects were enrolled in this study. The exclusion criteria included: (1) axillary temperature > 37°C (2) acute diseases or chronic diseases attack within 7 days before vaccination (3) priory vaccinated with varicella vaccine or having a history of varicella or herpes zoster infection (4) history of vaccine allergies or severe side effects (5) any known immunodeficiency (6) severe malnutrition, congenital malformations, developmental disorders or serious chronic diseases (7) received blood products, immunosuppressants, hormones and other research drugs within 30 days before vaccination (8) received live attenuated vaccines within 30 days, or received subunit vaccines or inactivated vaccines within 7 days before vaccination (9) having abnormal physical examination results (10) having any factors and was considered inappropriate for clinical trial.
Randomization and double-blind
We randomized all vaccines and enrolled the subjects in sequence. First, we used SAS software to generate two same sets of random numbers. Then, all vaccines were relabeled with a random number. The random number of the vaccine was corresponding to the group of vaccines, which was double-blind. Finally, each subject was assigned a random number in the order they enrolled and given the corresponding vaccine.
Vaccine
CS vaccine is the post-marketing varicella vaccine produced in a large scale, while LPS vaccine refers that the varicella vaccine produced in a small scale which was only used for pre-clinical trails. There had no difference between the CS vaccine and the LPS vaccine in quality, production process and other aspects. We used bridging design and took the LPS vaccine as control to evaluate the safety and immunogenicity of the CS vaccine. In addition, we had no other vaccines as comparators.
All vaccines were lyophilized products contain live attenuated Oka strain. Each vial contained 0.5ml of no less than 3.3 lg PFU (plaque forming unit) antigens. All the chickenpox vaccines were produced under industrial production conditions in accordance with good manufacturing practices (GMPs) requirements.24 The three consecutive lots of CS vaccine (Lot 201609001, Lot 201609002 and Lot 201609003) and the single lot of LPS vaccine (Lot 201510003) were tested by the National Institutes for Food and Drug Control, China (NIFDC) and confirmed to adhere to the necessary specifications. All subjects received one subcutaneous injection of varicella vaccine at deltoid region.
Compared with other available varicella vaccines, vaccines produced by Sinovac (Dalian) adopted a new human diploid cell (SV-1 cell) to culture Oka strain. The source and donor of SV-1 cell were clear, which were not only compliance with the legal and ethical requirements, but also meet the demands of Chinese pharmacopoeia, ICH, WHO and FDA. Moreover, the acquisition of the strain did not introduce chemicals, which improves the safety of the vaccine.
Immunogenicity assessment
The main immunogenic endpoints of the study were GMT, seroconversion rate and geometric mean increase (GMI).
Consistency assessment
The endpoints of consistency was GMT adjusted covariates. Under the premise of the consistency, the three CS lots were merged into one pooled CS lot, and then non-inferiority test was used to evaluate if the pooled CS lot was non-inferior to the LPS lot.
Safety assessment
Active surveillance was carried on for 30 days after vaccination. Subjects were observed for at least 30 minutes to monitor immediate adverse reactions after vaccination. Solicited injection-site reactions (pain, mucocutaneous disease, induration, redness, swelling, rash, pruritus) and systemic reactions (fever, allergy, headache, malaise, nausea/vomiting, diarrhea, myalgia, cough) were recorded for 14 days after vaccination by the guardians and/or investigators. Unsolicited adverse reactions were collected for 30 days after vaccination.
The grading standard of adverse reactions was based on the Guidelines for grading standard of adverse reactions in clinical trials of preventive vaccines produced by Chinese food and drug administration (CFDA).
Laboratory testing
Blood samples were collected pre-vaccination (day 0) and at day 30 (~30 + 12 days) post-vaccination to detect antibodies. All serum samples were tested by National Institutes for Food and Drug Control, which was the legal organization in China to test the quality of pharmaceutical and biological products. The serum samples were sent simultaneously and tested in blind state using fluorescent antibody to membrane antigen (FAMA) method, which has become a preferred method to definite the serodiagnosis infection of VZN. There was no testing kits using in the process.
The detection fluorescent plates were prepared with Oka virus-diploid cell suspensions and stored at −70°C. Serum samples were separated from blood samples, inactivated in water bath at 56°C for 30min and stored at −20°C. Diluted in 2-fold serials, samples were dropped into fluorescent plate wells with positive and negative controls. The results were observed under the fluorescence microscopes after incubating with detection antibodies and Evan Blue.
Statistical analysis
We used SAS version 9.4 software (SAS Institute, Cary, NC) to conduct statistical analysis. All analyses were run on the PPS. Safety and immunogenicity analyses were assessed in all subjects who received a dose of vaccine. Lot consistency analysis was conducted in susceptible subjects (pre-vaccination NTAb titers < 1:4).
Antibody levels of each group after vaccination was described as GMT and GMI using geometric mean and 95% CI, while comparisons were performed using ANOVA after logarithmic transformation. Seroconversion rate and the difference between each two groups were analyzed using chi-square test or Fisher’s exact test.
The positive standard was defined as the GMT ≥ 1:4. Seroconversion was defined as the positive change of subjects who were initially seronegative or at least 4-fold increase of NTAb titers in those who were initially seropositive.
The consistency of the three consecutive lots of CS vaccine was assessed as following: (1) GMT equivalence: Covariance analysis model was conducted, with GMT pre-vaccination after natural logarithm as covariate and GMT post-vaccination after natural logarithm as dependent variable. According to the model, the ratio of the GMT after inverse logarithmic transformation in each two groups was calculated. If the ratio of GMTs of each pair of lots were between 0.67 and 1.50, the equivalence of the three lots was considered to be identified. (2) Non-inferiority of seroconversion rate: The two-sided 95% CI of seroconversion rate was calculated to evaluate the difference between test group and control group. If the lower limit was greater than −10%, the test group was considered to be non-inferior to the control group.
For the safety study, the number and incidence of vaccination-related solicited adverse reactions and unsolicited adverse reactions were calculated. Pearson chi-square test and Fisher’ exact test were used to calculate the difference of the rate among the groups, and ANOVA was used to calculate the difference of the means. All the test statistics and corresponding P values were analyzed using two-sided tests, and P≤ 0.05 were considered to be statistically significant.
Funding Statement
This study was funded by Sinovac (Dalian) Vaccine Technology Co., LTD.
Disclosure of potential conflicts of interest
All other authors: no conflicts.
Ethical statements
This study was conducted in compliance with Guidelines of Good Clinical Practice (GCP) [25] and “Varicella and herpes zoster vaccines: WHO position paper, June 2014” [4], and the study protocol was approved by the independent ethics committee of Henan CDC. Written-informed consent was obtained from the parent(s) or guardian(s) of each subject before enrollment.
References
- 1.Chiu SS, Lau YL.. Review of the VarilrixTM varicella vaccine. Expert Rev Vaccines. 2005;4(5):629 https://www.tandfonline.com/doi/full/10.1586/14760584.4.5.629 [DOI] [PubMed] [Google Scholar]
- 2.Mitra M, Faridi M, Ghosh A, Shah N, Shah R, Chaterjee S, Narang M, Bhattacharya N, Bhat G, Choudhury H, et al. Safetyand immunogenicity of single dose live attenuated varicella vaccine (VR795 Oka strain) in healthy Indian children: a randomized controlled study. Hum Vaccin Immunother. 2015;11 PMID:25692656 (2):443–449. doi: 10.1080/21645515.2014.1004031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takahashi M, Asano Y, Kamiya H, Baba K, Ozaki T, Otsuka T, Yamanishi K. Development of varicella vaccine. J Infect Dis. 2008;197(Suppl 2):S41–4. PMID:18419406. [DOI] [PubMed] [Google Scholar]
- 4.Varicella and herpes zoster vaccines: WHO position paper. 2014.
- 5.Salle A, Ccv G. SAGE meeting of April 2014 WHO; accessed April. [Google Scholar]
- 6.Macartney K, Heywood A, McIntyre P. Vaccines for postexposure prophylaxis against varicella (chickenpox) in children and adults. Cochrane Database Syst Rev. 2014;(6):CD001833 PMID:24954057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skull SA, Wang EE. Varicella vaccination–a critical review of the evidence. Arch Dis Child. 2001;85:83–90. PMID:11466178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heininger U, Seward JF. Varicella. Lancet. 2006;368(9544):1365–1376. PMID:17046469. [DOI] [PubMed] [Google Scholar]
- 9.Sartori AM. A review of the varicella vaccine in immunocompromised individuals. Int J Infect Dis. 2004;8(5):259–270. PMID:15325594. [DOI] [PubMed] [Google Scholar]
- 10.Gershon AA, Gershon MD, Breuer J, Levin MJ, Oaklander AL, Griffiths PD. Advances in the understanding of the pathogenesis and epidemiology of herpes zoster. J Clin Virol. 2010;48(Suppl 1):S2–7. PMID:20510263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Listed N. Prevention of Varicella: recommendations of the advisory committee on immunization practices (ACIP). Centers for Disease Control and Prevention (CDC). 1996;45(RR–11):1–37. PMID:8668119. [PubMed] [Google Scholar]
- 12.Anon Varicella vaccines. WHO position paper. Wkly Epidem Rec; 1998; 73: 241–248. PMID:9715106 [PubMed] [Google Scholar]
- 13.Statement on recommended use of varicella virus vaccine National advisory committee on immunization (NACI). Can Commun Dis Rep. 1999;25(ACS–1);PMID:18217293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takahashi M, Otsuka T, Okuno Y, Asano Y, Yazaki T. Live vaccine used to prevent the spread of varicella in children in hospital. Lancet. 1974;2(7892):1288–1290. PMID:4139526. [DOI] [PubMed] [Google Scholar]
- 15.Arvin AM. Varicella Vaccine: genesis, Efficacy, and Attenuation. Virology. 2001;284:153–158. PMID:11384215. doi: 10.1006/viro.2001.0918. [DOI] [PubMed] [Google Scholar]
- 16.Tan AY, Connett CJ, Connett GJ, Quek SC, Yap HK, Meurice F, Lee BW. Use of a reformulated Oka strain varicella vaccine (Smith Kline Beecham Biologicals/Oka) in healthy children. Eur J Pediatr. 1996;155(8):706–711. PMID:8839730. [DOI] [PubMed] [Google Scholar]
- 17.Lau YL, Vessey SJ, Chan IS, Lee TL, Huang LM, Lee CY, Lin TY, Lee BW, Kwan K, Kasim SM, et al. A comparison of safety, tolerability and immunogenicity of Oka/Merck varic ella vaccine and VARILRIX in healthy children. Vaccine. 2002;20(23–24):2942–2949. PMID: 12126906. [DOI] [PubMed] [Google Scholar]
- 18.Kuter B, Matthews H, Shinefield H, Black S, Dennehy P, Watson B, Reisinger K, Kim LL, Lupinacci L, Hartzel J, et al. Group for Varivax. Ten year follow-up of healthy children who received one or two injections of varicella vaccine. Pediatr Infect Dis J. 2004;23(2):132–137. PMID:14872179. [DOI] [PubMed] [Google Scholar]
- 19.Fan R, Zhang J, Tang Y, Su J. Research progress of chicken pox and its vaccine. S China J Prev Med. 2016;42(4):390–396. In Chinese. [Google Scholar]
- 20.Bian G, Tang X, Shi H, Xu G, Li X, Gu Y, Ma R, Jiang Z, Chen E. Study on safety and immunogenicity of imported and domestic varicella attenuated live vaccine (Freeze-dried) for children. Chinese Journal of Vaccines and Immunization. 2012;18(5):435–437. In Chinese. [Google Scholar]
- 21.Tang Y, Su J, Xia Y, Feng S, Huang Y, Lin W, Yang J, Qiu Z, Liu Y, Wen W, et al. Safety and immunogenicity of domestic gelatin-free freeze-dried live attenuated varicella vaccine. Chin J Biologicals. 2012;25(11):1516–1519. In Chinese. [Google Scholar]
- 22.Wang S, Li C, Li Y, Yuan J, Tao H, Wang Z, Lv G, Wang X, Chen Z, Xie G. Study on safety and immunogenicity of lyophilized live atlenuated domestic varicella vaccine. Chinese Journal of Vaccines and Immunization. 2000;6(6):334–337. In Chinese. [Google Scholar]
- 23.Zhu C, Zhao Z, Tao H, Xu N, Wu J. Safety and immunogenicity of freeze-dried live attenuated varicella vaccine in India. Chin J Biologicals. 2015;28(7):711–714. In Chinese. [Google Scholar]
- 24.Chinese good manufacturing practices (GMPs) requirements. World Health Organization (WHO). 2010. edition. [Google Scholar]
- 25.Chinese guidelines of good clinical practice (GCP). China Food and Drug Administration (CFDA). 2004. edition. [Google Scholar]


