ABSTRACT
Objectives: Recommendations regarding the need to use alcohol prior to vaccine injections are inconsistent and based on low-level evidence. The objective was to assess the effectiveness of alcohol in reducing local skin reactions and infection post-vaccination.
Methods: Randomized controlled trial in a pediatric clinic. A research assistant cleansed the skin with alcohol at (swab group) or adjacent to (control group) the pre-defined injection site(s). Clinicians, parents and children were blinded to group allocation. Parents reported local skin reactions using paper diaries for 15 days post-vaccination (Day 0–14). Telephone interviews were conducted Day 1, 5, and 14. The Brighton Collaboration criteria were used to diagnose cellulitis and infectious abscess Day 5 and afterward.
Results: 170 children participated (May-November 2017). Baseline characteristics did not differ (p > 0.05) between groups. Children received 1–4 separate injections. There were no differences between swab and control groups in the incidence of any local skin reactions (58% vs. 54%), and specifically, pain (45% vs. 40%), redness (26% vs. 21%), swelling (20% vs. 13%), warmth (19% vs. 27%), and spontaneous drainage of pus (0% in both groups) over the post-vaccination follow-up period. Day 5 data was available for 99% of participants from diaries and telephone surveys; there were no cases of cellulitis or infectious abscess.
Conclusion: These findings are the first direct evidence for vaccine injections demonstrating that cleansing the skin with alcohol may not be needed. Our study is underpowered; however, to detect a difference in incidence of skin infection, future research is recommended.
KEYWORDS: Vaccines, infections, alcohol swabs, immunization, cellulitis, adverse events
Introduction
There are comprehensive guidelines by different health agencies around the world specifying optimal vaccine injection techniques.1-3 In some countries, such as Canada, this guidance includes cleansing the skin with alcohol prior to injection.4 Skin cleansing with alcohol is a common practice derived from the demonstrated effectiveness of alcohol in decreasing skin bacterial counts which has been extrapolated to imply a lower risk of skin infections.5-7 However, the World Health Organization (WHO) recommends against cleansing the skin with alcohol prior to vaccine injections.1
The WHO recommendation is based on a systematic review that found no evidence of infection when alcohol skin cleansing was omitted for subcutaneous insulin injections.8 Four additional studies including intramuscular, intradermal and subcutaneous injections of a variety of drugs, including vaccines, reported no benefit of alcohol.7,9-11 There are methodological shortcomings in all studies that prevent definite conclusions from being made, including lack of adequate randomization or blinding, assessment of skin reactions using non-validated tools, retrospective data collection, and passive reporting of adverse events. In addition, no study evaluated vaccine injections specifically. Separately, Cook recently summarized 1,010 cases of cellulitis and 360 cases of infectious abscess following vaccination reported in passive surveillance systems, vaccine studies, and published reports, and recommended that additional randomized trials are needed to investigate this issue.12
There is an increasing interest in our current health care environment to reduce unnecessary tests, treatments and procedures, as exemplified by the Choosing Wisely initiative of the American Board of Internal Medicine (ABIM).13 A recent systematic review examined pediatric medical overuse practices specifically and highlighted the associated costs and risks of patient harm.14 Omission of alcohol for skin cleansing may qualify as an unnecessary treatment as it has not been demonstrated to have a benefit on infection rate. There are potential benefits of removing alcohol swabs, including; 1) reducing resource use due to shorter procedure time and supplies,7,9 2) reducing pre-procedural anxiety due to alcohol serving as a cue to injection15 and 3) reducing pain from alcohol being tracked into the tissue during injection.16,17
We undertook a randomized controlled trial (RCT) to determine the impact of alcohol application at the site of injection before vaccination injections on the incidence of local skin reactions, including infection.
Results
Participant flow
Of 402 children assessed, 170 were recruited between May 8 and November 20, 2017 (Figure 1). The last telephone survey was conducted on December 12, 2017.
Figure 1.
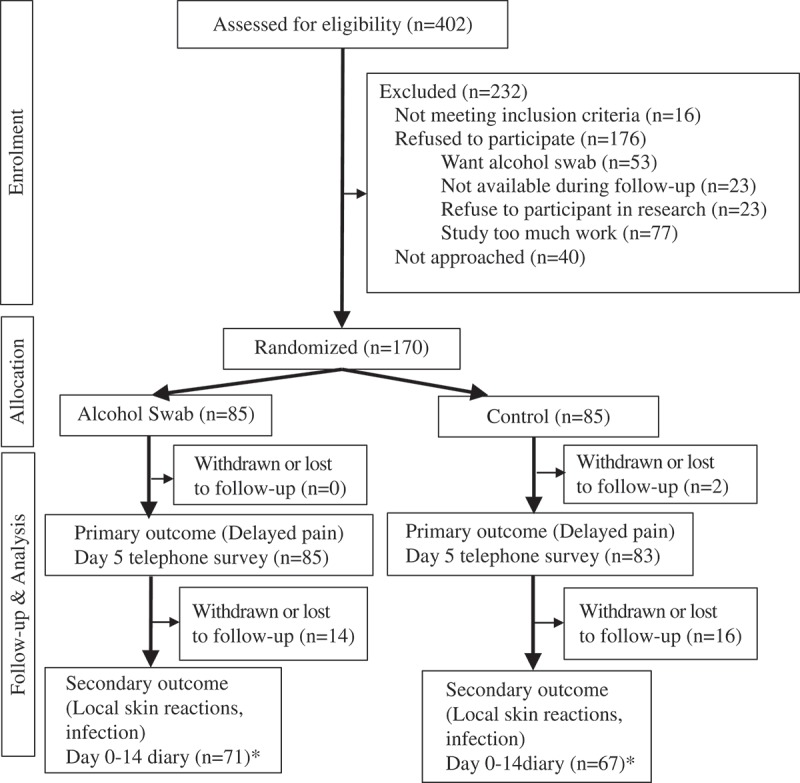
Participant flow diagram.
* Day 5 (n = 85) and Day 14 (n = 81) – includes diary and telephone survey* Day 5 (n = 83) and Day 14 (n = 82) – includes diary and telephone survey
Participant characteristics
Participants did not differ between the two groups with respect to age, sex and number of vaccine injections administered (Table 1). All participants received the allocated treatment.
Table 1.
Characteristics of participants.
| Alcohol Swab (n = 85) | Control (n = 85) | p-value* | |
|---|---|---|---|
| Age (years) | 5.6 (5.1) | 5.9 (5.6) | 0.71 |
| Male sex | 45 (53) | 51 (60) | 0.35 |
| Ethnicity | 0.84 | ||
| Caucasian | 36 (42) | 37 (44) | |
| Asian | 27 (32) | 29 (34) | |
| Other | 22 (26) | 19 (22) | |
| Only one | 44 (52) | 49 (58) | 0.23 |
| vaccination** | |||
| Pediacel® | 21 | 19 | - |
| Bexsero® | 17 | 13 | - |
| Prevnar® 13 | 13 | 17 | - |
| Gardasil® 9 | 13 | 14 | - |
| Flulaval® | 13 | 12 | - |
| Fluzone® | 12 | 13 | - |
| Twinrix® | 6 | 9 | - |
| Menjugate® | 6 | 8 | - |
| Priorix® | 7 | 7 | - |
| Adacel®-Polio | 9 | 4 | - |
| Priorix-TetraTM | 4 | 4 | - |
| Varivax® | 4 | 4 | - |
| Adacel® | 2 | 5 | - |
| ProQuad® | 6 | 1 | - |
| Varilrix® | 2 | 3 | - |
| Engerix®-B | 2 | 2 | - |
| Menactra® | 2 | 1 | - |
| Recombivax HB® | 2 | 1 | - |
| Boosterix®-polio | 1 | 1 | - |
| M-M-R®II | 0 | 1 | - |
Results are mean (standard deviation) or frequency (percent)
*Chi-squared test or t-test
**Participants received between 1 and 4 vaccinations. In total 279 vaccine doses were administered, 237 were administered intramuscularly, 119 swab vs. 118 control and 42 were administered subcutaneously, 23 swab vs. 19 control
Feasibility
Over the recruitment timeline, 402 patients were screened and 170/386 (44%) eligible patients were recruited. Eighty-one percent (138/170) of the diaries were returned by parents. Follow-up telephone surveys were available for 99% (168/170) on Day 1, 99% (168/170) on Day 5, and 94% (160/170) on Day 14 post-vaccination. The pattern of local skin reactions determined using the diary data is shown in Figure 2. No child experienced 3 or more adverse events on Day 4 and onwards, and none experienced pus leaking at the injection site. There were no new reactions reported after Day 3 post-vaccination. All reactions resolved by Day 10. Seven participants reported 1 or 2 local reactions on Day 5 and were invited for a consultation. Only 1 parent returned with their child; the diagnosis made by the pediatrician was injection site swelling. There were no comments or concerns raised regarding study procedures. No parents were asked about their acceptability with having their child undergo aspiration to determine the presence of bacteria in the tissue due to the low frequency of reactions at Day 5 and beyond.
Figure 2.
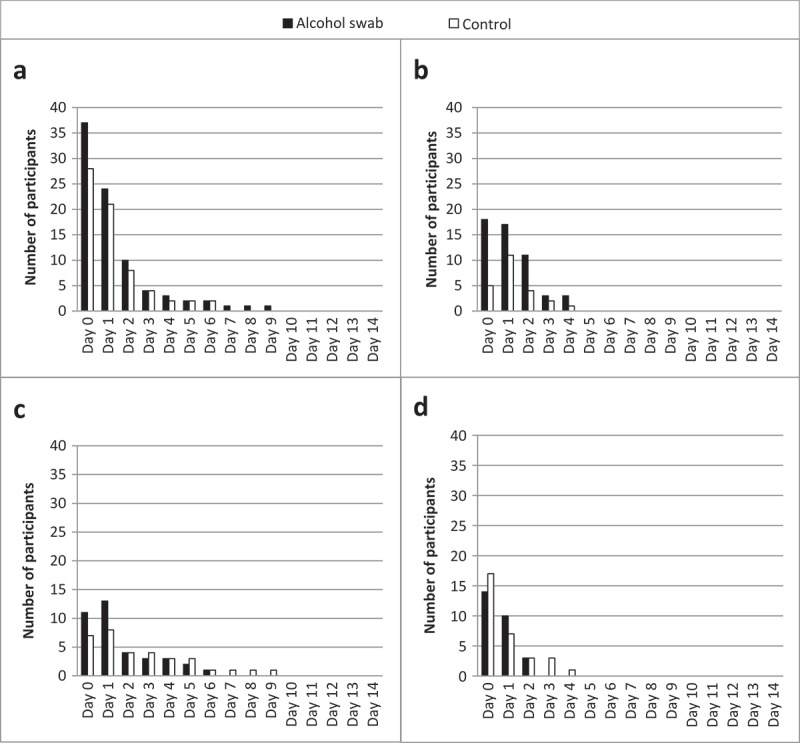
Number of participants reporting each local skin reaction post-vaccination.
a – pain; b – redness; c – swelling; d – warmth to touch. Data collected from both diaries and telephone surveys. No participants experienced more than 2 local skin reactions on Day 5 and onwards, precluding a diagnosis of cellulitis or infectious abscess.
One hundred and thirty-five of 170 (79%) participants had both diary and phone interview data for Days 1, 5, and 14 post-vaccination. The concordance between the two sources was 99% for pain, 99% for redness, 98% for swelling, 100% for warmth and 100% for spontaneous drainage of pus at injection site.
Incidence of local reactions and infection
The incidence of local skin reactions did not differ between groups (Table 2). The incidence of delayed pain was 45% (38/85) vs. 40% (33/83) for the swab and control groups, p = 0.47. The incidence of any local skin reaction was 58% (49/85) vs. 54% (45/83), p = 0.59, respectively. There were no cases of cellulitis or infectious abscess.
Table 2.
Local skin reactions day 0–14 post-vaccination.
| Alcohol Swab (n = 85)a | Control (n = 83)a | p-value* | |
|---|---|---|---|
| Number with skin reaction (percent) | |||
| Any local skin reactionb | 49 (58%) | 45 (54%) | 0.59 |
| Pain | 38 (45%) | 33 (40%) | 0.47 |
| Redness | 22 (26%) | 17 (21%) | 0.38 |
| Swelling | 17 (20%) | 11 (13%) | 0.23 |
| Warmth to touch | 16 (19%) | 22 (27%) | 0.25 |
| Pus leaking | 0 (0%) | 0 (0%) | - |
| Cellulitis | 0 (0%) | 0 (0%) | - |
| Infectious abscess | 0 (0%) | 0 (0%) | - |
| Mean duration of skin reaction in days (standard deviation)** | |||
| Pain | 0.6 (1.2) | 0.3 (0.7) | <0.001 |
| Redness | 0.4 (1.2) | 0.4 (1.4) | 0.91 |
| Swelling | 1.0 (1.6) | 0.8 (1.4) | 0.38 |
| Warmth to touch | 0.3 (0.7) | 0.3 (0.8) | 0.79 |
| Pus leaking | 0 (0) | 0 (0) | - |
| Cellulitis | 0 (0) | 0 (0) | - |
| Infectious abscess | 0 (0) | 0 (0) | - |
Values are frequency (percent) or mean (standard deviation), as specified above
a As reported on diary or follow-up telephone survey
b Number of participants reporting at least 1 local skin reaction
* Chi-squared test or t-test
** Post-hoc analysis
Post-hoc analysis revealed a longer duration of redness for the alcohol swab group; there were no other differences between groups (Table 2). Logistic regression revealed no significant impact (p > 0.05) on any local reactions according to group allocation, whether the vaccine was a live vaccine or not and whether the vaccine was a frequently administered one or not (data available on request).
Discussion
This is the first RCT to specifically examine the effect of alcohol swab skin cleansing on the incidence of local skin reactions and infection in children undergoing vaccine injections. We found no evidence of a difference in the rate of local skin reactions and no cases of cellulitis or infectious abscess when alcohol swabs were and were not used. Practicality of the study design and conduct was clearly demonstrated for 3 of 4 a-priori criteria: participant compliance, suitability of follow-up procedures and other procedural issues. Participant compliance was also high, with 94% of parents completing all 3 follow-up telephone interviews and 81% returning diaries. This exceeded our cut-off of 75%. No participant experienced 3 or more local adverse events on Day 4 and onwards. The recruitment rate was slightly lower than the target (44% vs. 50%). It should be noted; however, that not all eligible patients were approached due to unavailability of the clinic-based research assistant. If all eligible patients were approached, it is likely this criterion would have been met. The presence of more eligible children than anticipated allowed for a shorter recruitment period.
Our findings are consistent with prior studies demonstrating a lack of impact of alcohol skin cleansing on intradermal, intramuscular and subcutaneous injections.7-11 Previous studies included >700 patients with >90,700 injections without skin preparation and >8,700 injections with skin preparation primarily focused on insulin injections. Other injections included botulinum toxin A and other drugs and vaccines; the number of each, however, were not specified. Furthermore, these studies had some methodological shortcomings. We used a more rigorous design including an accepted global case definition for infection. Our findings are consistent with these published data, and are the first results to specifically target vaccine injections.
There are potential benefits to omitting alcohol swabs from vaccine injections not assessed in the present study. Alcohol can serve as a cue to impending acute injection pain and increase pre-procedural anticipatory anxiety. It can also contribute to vaccine administration pain by being tracked into the tissue along with the vaccine.16,17 Foregoing this step can therefore have a positive effect on the vaccination experience for children. There are also costs associated with alcohol. According to various sources, the swab itself costs between 1 and 18 cents. Extrapolating to the province of Ontario alone where approximately 9 million doses of public vaccines were distributed in 2015, the cost amounts to $90,000 to $1,620,000.18 Adding an estimated 1 minute for its administration per injection, the amount of time administering alcohol swabs for 9 million doses of vaccines is 150,000 hours, which could be allocated to other health care services.
There are some limitations that are worthy of discussion. Firstly, local skin reactions were parent-reported and not validated by researchers. While this may lead to errors in the true incidence of local reactions, it is not expected to lead to a systematic bias between groups. This is also an acceptable and usual method for obtaining adverse event data following immunization (AEFIs).19 Secondly, we were powered to detect differences in the incidence of local reactions, specifically, delayed pain. The clinically important and primary outcome; however, was skin infection. Local skin reactions represent surrogate markers for infection as their presence precedes a diagnosis of infection. To this end, our results are not conclusive and do not answer our primary research question. A much larger sample size would be required to rule out an increase in the risk of infection, the clinically important outcome. At present, the rate of cellulitis and infectious abscess is believed to be very low – Ontario AEFI data suggests a risk of <0.001% (1/100,000).18 Approximately 4,710,000 participants would be needed to detect a 2-fold increase in infection rate, 0.002% (2/100,000), with power = 80% and alpha = 0.05.20 Applying our own recruitment rate and timeline as an estimate, 27,706 clinics would be required to recruit the requisite total number of participants. The large sample size makes it unlikely that such a trial will ever be undertaken. We cannot conclude with certainty that our schedule for data collection (i.e., telephone surveys on Days 5 and 14) is adequate to capture the occurrence of infection. Based on accepted guidelines for adverse event monitoring, however, a 14-day window appears to be a reasonable timeline for the collection of information about infection.21 In addition, follow-up periods between 5 and 14 days have been adequate in previous studies to identify cases of cellulitis.22-24 Post-hoc analysis identified a longer duration of redness for the alcohol group. The clinical importance of this isolated finding is unknown and future research is recommended that systematically investigates vaccine and patient factors associated with vaccine-associated adverse events. Finally, while we expect similar results to be obtained in other outpatient clinics with similar types of patients and vaccination administration practices, we cannot generalize the results to other settings whereby hygienic practices may differ.
There are numerous strengths of the study. Firstly, participants were randomized to the groups, reducing selection bias. Secondly, clinic staff, parents, children and researchers obtaining outcome data were blinded to the treatment allocation, reducing measurement bias. Thirdly, the participants were followed-up for 15 days post-vaccination and we used globally accepted definitions for cellulitis and infectious abscess. Fourthly, we included all vaccines being administered, enhancing the generalizability of the results.
Despite the finding that alcohol skin cleansing may not be needed, immunizers may find it difficult to part with this step of the vaccine administration process.25 A similar phenomenon has been observed with not aspirating before vaccine injection.26 In addition, some patients may not readily accept omitting alcohol swabs either.27 This is exemplified by 30% of excluded parents reporting that they were uncomfortable with not using alcohol swabs. However, initiatives such as Choosing Wisely by the ABIM foundation are advocating for reducing unnecessary procedures in health care with evidence-based recommendations.13 These initiatives demonstrate that with evidence and support from health care providers, clinical practice can change.
Conclusion
This study demonstrated no evidence of a difference in the incidence of delayed pain post-vaccination when skin cleansing at the injection site was and was not done. There were no cases of cellulitis or infectious abscess. These data should be taken into consideration in vaccination administration policies.
Methods
Study design and setting
This was a partially blinded RCT conducted in a private outpatient pediatric primary care clinic in Toronto, Canada.
Participants
Healthy children aged 0–18 years who qualified for vaccination in accordance with the Ontario Immunization Schedule were eligible.28 Children were excluded if a parent was not fluent in English or was unavailable for the 15 days post-vaccination follow-up period, if they previously participated in the study, had a documented allergy to isopropyl alcohol, were taking antibiotics, or had a contraindication to vaccination according to national guidelines.4
Randomization procedures
Randomization was achieved offsite by a researcher not involved in other aspects of the trial. A computer-generated block randomization code was used to allocate each participant in a 1:1 ratio to 1 of 2 treatments: 1) alcohol swab at injection site (alcohol swab), or 2) alcohol swab adjacent to injection site (control). The randomization code was kept in a secure location that could not be accessed by study personnel. Treatment allocation was concealed using sequentially numbered opaque sealed envelopes (SNOSE) maintained at the clinic.
Study procedures
When children scheduled for vaccination arrived at the clinic, parents (and children, if applicable) were asked if they were agreeable to discussing the study. Those that agreed were directed to a clinic-based research assistant that described the study, answered questions and obtained signed informed consent. Additionally, assent was obtained from children over 6 years of age. The study was approved by the University of Toronto Research Ethics Board. The study was registered on www.clinicaltrials.gov (NCT03131843).
Following recruitment, the clinic-based research assistant obtained the next consecutive SNOSE and opened it to reveal the treatment allocation; hence, the clinic-based research assistant was not blinded. All other individuals were blinded, including the pediatrician administering the vaccinations, clinic staff, off-site research assistants, parents, and children.
The clinic-based research assistant was trained by one of the study investigators (SM) on the anatomical location for vaccination injections for children of different ages receiving different vaccines prior to the commencement of the trial. After obtaining consent and confirming the vaccines being administered with the attending physician, the clinic-based research assistant opened a commercial 70% isopropyl alcohol swab packet (Antiseptic Isopropyl Alcohol Pad, LorisTM, Lernapharma Inc., Montreal, Québec, Canada) and swabbed the allocated site – either the vaccine injection site (i.e., swab group) or adjacent to the vaccine injection site (i.e., control group) (Figure 3) in a spiral motion starting from the center outwards covering a circular area 2 inches in diameter for 30 seconds, followed by a drying time of at least 30 seconds.1 The research assistant physically obstructed the view of any other individual in the room, including the child, using his body and the allocation envelope. A wristwatch was used to time alcohol application and drying phases. For children receiving more than 1 separate vaccine injection, the same treatment was used for all sites sequentially using the same swab.
Figure 3.
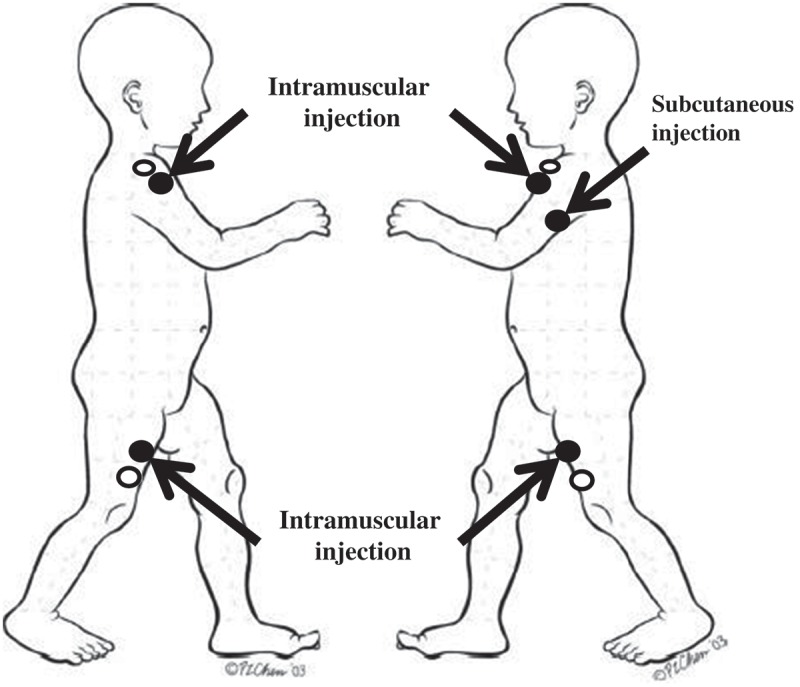
Diagram depicting injection sites and cleansing locations.
The arrows indicate where the vaccines were injected. Swabbing of alcohol occurred in filled circles (alcohol swab) or empty circles (control). Modified from Vaccine, Vol.25/31, Halperin S, Kohl KS, Gidudu J, Ball L, Hammer SJ, Heath P, Hennig R, Labadie J, Rothstein E, Schuind A, Varricchio F, Walop W. Cellulitis at injection site: case definition and guidelines for collection, analysis, and presentation of immunization safety data, 5818, Copyright (2018), with permission from Elsevier.
Two pediatricians performed all vaccine injections. Upon confirmation by the physician that a child was going to be vaccinated, the vaccines were prepared by clinic staff as per usual practice. The rubber stoppers of the vials were swabbed with 70% isopropyl alcohol before the vaccines were drawn, as recommended by national guidelines.4 They were labeled and were given to the pediatrician for administration. Vaccines were administered in a standardized manner according to national guidelines other than the skin cleansing step.4 The clinic-based research assistant observed the vaccination and verified that the vaccine was administered according to the allocated treatment.
Follow-up procedures
Parents assessed the injection sites and recorded local skin reactions (pain, redness, swelling, warmth and spontaneous drainage of pus from the injection site) daily using a paper diary for 15 days post vaccination (Day 0–14).19 Written and oral instructions were provided. Cellulitis and infectious abscess were diagnosed by a pediatrician blinded to group allocation using the Brighton Collaboration criteria (Table 3).29,30 Criteria for Level 2 of diagnostic certainty were accepted for both cellulitis and infectious abscess as aspiration of the injection site was not part of routine practice at the study site. If infectious abscess was suspected, however, parents would be asked permission for an aspirate. Parents returned the diaries by mail, fax, scan, email or drop-off at the clinic.
Table 3.
Brighton collaboration case definitions of cellulitis and infectious abscess.
| Cellulitis1 | Infectious abscess2 |
|---|---|
|
Level 1a of diagnostic certainty At least three of the following four signs/symptoms:
AND
If known, exclusion criteria are:
Level 1b of diagnostic certainty
If known, exclusion criteria are:
|
Level 1 of diagnostic certainty
AND
|
|
Level 2 of diagnostic certainty At least three of the following four signs/symptoms:
AND
|
Level 2 of diagnostic certainty
OR
AND
Abscesses of infectious etiology may be accompanied by fever and/or regional lymphadenopathy. |
1Halperin S, Kohl KS, Gidudu J, et al. Cellulitis at injection site: Case definition and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25:5803–5820.
2Kohl KS, Ball L, Gidudu J, et al. Abscess at injection site: Case definition and guidelines for collection, analysis and presentation of immunization safety data. Vaccine. 2007;25:5821–5838.
Off-site research assistants blinded to group allocation telephoned parents 24 hours (Day 1), 5 days (Day 5), and 14 days (Day 14) post-vaccination and inquired about the presence of local skin reactions. If any symptoms were present on Days 5 or 14, parents were asked to return to the clinic with their child for a consultation. Parents could contact the clinic or research staff if they had any questions or concerns at any time.
A safety committee comprised of an academic pediatrician, clinical pharmacologist and statistician was established to review safety data and provide recommendations.
Study outcomes
Feasibility
Feasibility of the trial was determined using four a-priori criteria: 1) recruitment, 2) compliance, 3) suitability of follow-up procedures, 4) other procedural issues. Recruitment was considered successful if completed within 12 months and 50% or more of the eligible population was recruited. Compliance with protocol procedures was defined as 75% or more of participants with complete outcome data (i.e., 3 follow-up phone calls and the diary returned). The natural time course for local skin reactions was used to assess whether the current approach of calling parents on Days 1, 5, and 14 was acceptable. Day 5 was determined a-priori to be the time when local skin reactions should resolve, and infection symptoms commence.29 Hence, any parent reporting local skin reactions on Day 5 was asked to return to the clinic with their child for a consultation. The number of individuals who returned to the clinic was recorded. Qualitative comments were obtained regarding the study procedures. Parents were also asked to comment on the burden of the study procedures. In the event that cellulitis or infectious abscess was suspected, parents were asked about acceptability for their children to undergo an invasive procedure (aspiration of the injection site) to aid in the diagnosis.
Incidence of local reactions and infection
The incidence of local skin reactions (i.e., delayed pain, redness, swelling, warmth to touch, spontaneous drainage of pus) and infection post-vaccination was calculated using data from the returned diaries and follow-up telephone interviews.19 Data missing from diaries was supplemented with telephone diary data for Days 5 and 14. Follow-up telephone interviews were used to identify any adverse events that required a consultation and to corroborate diary data.
The percent agreement between diary and interview data was calculated as a measure of reliability of parent reporting.
Sample size calculation and statistical analysis
The sample size calculation was based on a secondary (surrogate) outcome, incidence of delayed pain, as it was not feasible to recruit a sufficient number of participants to answer our primary outcome of skin infection. We recruited 85 participants per group (170 altogether) to allow us to detect a difference in the rate of delayed pain (i.e., pain in the hours to days post-vaccination) over the time course of the follow-up of 43% vs. 65% in the swab vs. control groups (i.e., 50% increase, considered a clinically significant difference), with power = 80% and alpha = 0.05 and accounting for drop-outs and missing data. The incidence of local skin reactions was compared between groups. Infection was considered for local skin reactions present on or after Day 5 only. Post-hoc analysis included examining the duration of local skin reactions between groups. In addition, we examined the association between incidence of local skin reactions and the following three factors: group allocation (swab vs. no swab), type of vaccine (live vs. other), and vaccine frequency (frequently administered vs. other). Demographic and outcome data were compared using t-test, χ2 test, or logistic regression, as appropriate. SPSS version 24 (IBM, Armonk, New York, United States) was used to analyze the data. A p-value <0.05 was considered significant.
Funding Statement
Funding was provided by a Dean’s award to Dr. Taddio and miscellaneous funds by Dr. Taddio.
Abbreviations
- RCT
Randomized Controlled Trial
- WHO
World Health Organization
- SNOSE
Sequentially Numbered Opaque Sealed Envelopes
- ABIM
American Board of Internal Medicine
Acknowledgments
We wish to thank all of the parents and children who participated in the study, the staff at the study clinic and our safety committee members, Dr. Eugene Ng, Dr. Irena Nulman, and Mr. Philip Ye.
Contributors’ Statement Page
Horace Wong conceptualized, designed and, coordinated the study, assisted in the data acquisition, carried out the analysis and interpretation of the data, and drafted the initial manuscript and approved the final manuscript as submitted.
Corinne Moss and Steven M. Moss conceptualized and designed the study, assisted in the acquisition and interpretation of the data, reviewed and revised the manuscript, and approved the final manuscript as submitted.
Vibhuti Shah, Scott Halperin, and Shinya Ito conceptualized and designed the study, assisted in the interpretation of the data, reviewed and revised the manuscript, and approved the final manuscript as submitted.
Angie Qu and Priyanjali Mithal assisted in the acquisition and interpretation of the data, reviewed and revised the manuscript, and approved the final manuscript as submitted.
Anna Taddio conceptualized and designed the study, provided administrative assistance and supervision for the study, assisted in carrying out the analysis and interpretation of the data and drafted the initial manuscript and approved the final manuscript as submitted.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.World Health Organization WHO best practices for injections and related procedures toolkit. Geneva (Switzerland): WHO Document Production Services; 2010. [PubMed] [Google Scholar]
- 2.Ramsay M, ed. Immunisation against infectious disease. London (England): Crown; 2013. [Google Scholar]
- 3.Hamborsky J, Kroger A, Wolfe S, editors. Epidemiology and prevention of vaccine-preventable diseases. 13th ed. Washington (DC): Public Health Foundation; 2015. [Google Scholar]
- 4.Public Health Agency of Canada Canadian immunization guide. Ottawa (Ontario): Public Health Agency of Canada; 2018. https://www.canada.ca/en/public-health/services/canadian-immunization-guide.html. [Google Scholar]
- 5.Polishchuk D, Gehrmann R, Tan V.. Skin sterility after application of ethyl chloride spray. J Bone Joint Surg Am. 2012;94(2):118–120. doi: 10.2106/JBJS.K.00229. [DOI] [PubMed] [Google Scholar]
- 6.Koivisto VA, Felig P. Is skin preparation necessary before insulin injection? Lancet. 1978;1(8073):1072–1075. doi: 10.1016/S0140-6736(78)90916-9. [DOI] [PubMed] [Google Scholar]
- 7.Khawaja RA, Sikandar R, Qureshi R, Jareno RJM. Routine skin preparation with 70% Isopropyl alcohol swab: is it necessary before an injection? Quasi Study. Jlumhs. 2013;12(2):109–114. [Google Scholar]
- 8.Hutin Y, Hauri A, Chiarello L, Catlin M, Stilwell B, Ghebrehiwet T, Garner J, the Members of the Injection Safety Best Practices Development Group . Injection safety best practices development group. best infection control practices for intradermal, subcutaneous and intramuscular needle injections. Bull WHO. 2003;81:491–500. [PMC free article] [PubMed] [Google Scholar]
- 9.Kshanti IA, Suyono S. Infection at insulin injection sites in diabetic patients with or without alcohol swab. Maj Kedokt Indon. 2008;58(9):323–326. [Google Scholar]
- 10.Pham T, Perry JD. Botulinum toxin type A injection without isopropyl alcohol antisepsis. Ophthalmic Plast Reconstr Surg. 2009;25(3):178–179. doi: 10.1097/IOP.0b013e3181a145e5. [DOI] [PubMed] [Google Scholar]
- 11.Dann TC. Routine skin preparation before injection an unnecessary procedure. Lancet. 1969;96–97. doi: 10.1016/S0140-6736(69)92405-2. [DOI] [PubMed] [Google Scholar]
- 12.Cook IF. Sepsis, parenteral vaccination and skin disinfection. Hum Vaccin Immunother. 2016;12(10):2546–2559. doi: 10.1080/21645515.2016.1190489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choosingwisely.org. Wisely C. Promoting conversations between providers and patients. 2018. [accessed 2018 March 27]. http://www.choosingwisely.org.
- 14.Coon ER, Young PC, Quinonez RA, Morgan DJ, Dhruva SS, Schroeder AR. 2017 Update on pediatric medical overuse: a review. JAMA. 2018;172(5):482–486. [DOI] [PubMed] [Google Scholar]
- 15.Kikuta A, Gardezi F, Dubey V, Taddio A. Practices and perceptions regarding pain and pain management during routine childhood immunizations: findings from a focus-group study with nurses working at Toronto Public Health, Ontario. Can J Infect Dis Med Microbiol. 2011;22(2):43–48. doi: 10.1155/2011/381864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trevisani M, Smart D, Gunthorpe MJ, Tognetto M, Barbieri M, Campi B, Amadesi S, Gray J, Jerman SJ, Brough SJ, et al. Ethanol elicits and potentiates nociceptor responses via the vanilloid reveptor-1. Nat Neurosci. 2002;5(6):546–551. doi: 10.1038/nn852. [DOI] [PubMed] [Google Scholar]
- 17.McCarthy JA, Covarrubis B, Sink P. Is the traditional alcohol wipe necessary before an insulin injection? Diabetes Care. 1993;16(1):402. doi: 10.2337/diacare.16.1.402a. [DOI] [PubMed] [Google Scholar]
- 18.Ontario Agency for Health Protection and Promotion (Public Health Ontario) Annual Report on Vaccine Safety in Ontario, 2015. Toronto (Ontario): Queen’s Printer for Ontario; 2016. [Google Scholar]
- 19.Bonhoeffer J, Imoukhuede EB, Aldrovandi G, Bachtiar NS, Chan E, Chang S, Chen RT, Gernandopulle R, Goldenthal KL, Heffelfinger JD, et al. Template protocol for clinical trials investigating vaccines – focus on safety elements. Vaccine. 2013;31(47):5602–5620. doi: 10.1016/j.vaccine.2013.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.SAS software. version 9.4.. Cary (NC): SAS Institute Inc; 2013. [Google Scholar]
- 21.Ontario Agency for Health Protection and Promotion (Public Health Ontario) Adverse event following immunization reporting for health care providers in Ontario. 2017. https://www.publichealthontario.ca/en/eRepository/AEFI_factsheet_healthcare_providers.pdf
- 22.Jackson LA, Yu O, Nelson JC, Dominguez C, Peterson D, Baxter R, Hambidge SJ, Naleway AL, Belongia EA, Nordin JD, et al. Injection site and risk of medically attended local reactions to acellular pertussis vaccine. Pediatrics. 2011;127:e581–e587. doi: 10.1542/peds.2010-1886. [DOI] [PubMed] [Google Scholar]
- 23.Klein NP, Hansen J, Chao C, Belicer C, Emery M, Slezak J, Lewis N, Deosaransingh K, Sy L, Ackerson B, et al. Safety of quadrivalent human papillomavirus vaccine administered routinely to females. Arch Pediatr Adolesc Med. 2012;166(12):1140–1148. doi: 10.1001/archpediatrics.2012.1451. [DOI] [PubMed] [Google Scholar]
- 24.Agnandji ST, Asante KP, Lyimo J, Vekemans J, Soulanoudjingar SS, Owusu R, Shomari M, Leach A, Fernandes J, Dosoo D, et al. Evaluation of the safety and immunogenicity of the RTS,S/AS01E malaria candidate vaccine when integrated in the expanded program of immunization. J Infect Dis. 2010;202(7):1076–1087. doi: 10.1086/656190. [DOI] [PubMed] [Google Scholar]
- 25.Prasad V, Ioannidis JPA. Evidence-based de-implementation for contradicted, unproven, and aspiring healthcare practices. Implement Sci. 2014;9:1. doi: 10.1186/1748-5908-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pacheco SR, Abadesso C, Monteiro A, Taddio A. Pain management during childhood vaccination injections in Portugal – a call to action. Acta Paediatr. 2016;e338. doi: 10.1111/apa.13434. [DOI] [PubMed] [Google Scholar]
- 27.Montini T, Graham ID. “Entrenched practices and other biases”: unpacking the historical, economic, professional, and social resistance to de-implementation. Implement Sci. 2015;10(24). doi: 10.1186/s13012-015-0211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Government of Ontario Publicly funded immunization schedules for Ontario. December 2016. http://www.health.gov.on.ca/en/pro/programs/immunization/docs/immunization_schedule.pdf
- 29.Halperin S, Kohl KS, Gidudu J, Ball L, Hammer SJ, Heath P, Hennig R, Labadie J, Rothstein E, Schuind A, et al. Cellulitis at injection site: case definition and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25:5803–5820. doi: 10.1016/j.vaccine.2007.04.059. [DOI] [PubMed] [Google Scholar]
- 30.Kohl KS, Ball L, Gidudu J, Hammer SJ, Halperin S, Heath P, Hennig R, Labadie J, Rothstein E, Schuind A, et al. Abscess at injection site: case definition and guidelines for collection, analysis and presentation of immunization safety data. Vaccine. 2007;25:5821–5838. doi: 10.1016/j.vaccine.2007.04.059. [DOI] [PubMed] [Google Scholar]


