Abstract
Eosinophilic granulomatosis with polyangiitis (EGPA) is a rare antineutrophil cytoplasmic antibody-associated vasculitis that can affect any organ system. It is most often characterised by chronic airway inflammation along with prominent peripheral blood eosinophilia, although the disease can affect the cardiovascular, gastrointestinal, renal or central nervous systems. Ocular manifestations are uncommon and when they do occur, are varied in their clinical presentations. To the best of our knowledge, this is the first case of corneal melt secondary to EGPA to have been reported.
Keywords: ophthalmology, respiratory medicine, eye
Background
Eosinophilic granulomatosis with polyangiitis (EGPA), or, as it was known previously, Churg-Strauss syndrome, is a rare condition with an estimated incidence of 1–3 per million.1 While EGPA can manifest at any age, the mean age of onset is 40 years and it is an uncommon cause of vasculitis in children and in the older people.2–4 It affects both sexes almost equally.2 3 5 It is classified as an antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV), sharing features with both granulomatosis with polyangiitis (previously known as Wegener’s granulomatosis) and microscopic polyangiitis. EGPA is a granulomatosis necrotising inflammation that affects the small and medium sized blood vessels, although this is often not evident in the initial stages of the disease. Instead, it is preceded by asthma or chronic allergic rhinosinusitis, followed by eosinophilia before manifesting as a systemic vasculitis years later.6–8
EGPA most commonly affects the lungs, with 90% of patients suffering from asthma.9 Often the condition is suspected when a patient’s asthma is poorly controlled despite moderate doses of inhaled glucocorticoids. However, the disease is capable of affecting any organ system, and as such, clinical presentations can vary. Skin involvement (palpable purpura, petechiae or cutaneous nodules) is present in up to 70% of cases.10 Ophthalmic manifestations are rarely the presenting feature, and overall ocular involvement is uncommon.11–13 There are only a few reported cases in the literature and presentations vary; peripheral ulcerative keratitis, conjunctival granulomas, episcleritis, uveitis, choroidal ischaemia, ischaemic retinal neuropathy and cranial nerve palsies.12 14–20 Cardiac involvement is the major cause of death in EGPA.10
Corneal melt has been linked to several autoimmune conditions and connective tissue disorders, most commonly rheumatoid arthritis.13 21 22 To the best of our knowledge, this is the first case of corneal melt secondary to EGPA to have been reported. Thus, we present a diagnostically challenging case of an older male patient who presented with corneal melt on a background history of a poorly defined respiratory illness that was later identified as EGPA.
Case presentation
A 72-year-old man presented to his community ophthalmologist with a 3-day history of loss of vision in his left eye associated with mild pain and redness. The loss of vision was sudden in onset and had not been preceded by other ocular symptoms. There was no history of injury or trauma to the eye. He had no significant past ocular history. On examination, an area of corneal ulceration was noted in his left eye with no evidence of active leakage. He was commenced on hourly g-ofloxacin and transferred to our emergency service for further assessment and treatment.
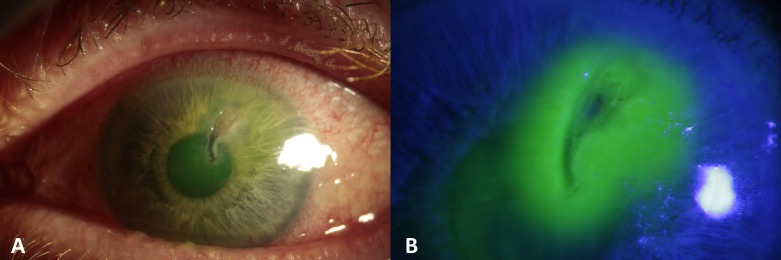
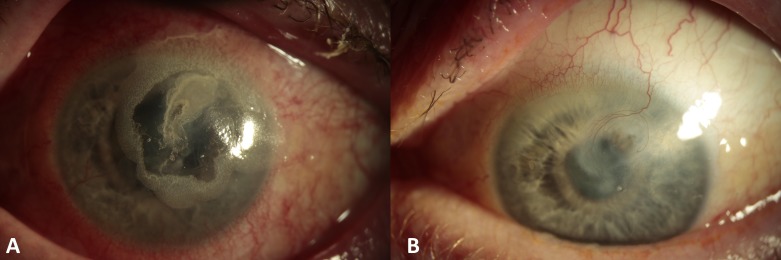
On arrival the patient’s visual acuity was 6/6 in the right eye and count fingers in the left. Examination revealed a 3.5×2.5 mm paracentral area of corneal ulceration (figure 1A) with a small seidel’s positive perforation near the superior border (figure 1B). The anterior chamber was shallow with conjunctival injection and peripheral pannus. The fundal view was hazy, but there were no apparent signs of vitritis or vasculitis. Corneal sensation was normal in both eyes. The patient was admitted to hospital, a protective shield was placed and he was commenced on oral ciprofloxacin. Corneal glueing was performed to seal the leaking corneal melt and a protective bandage contact lens (BCL) was placed in situ. Over the next 3 days, the patient required re-glueing on two further occasions due to repeated leakage and shallowing of the anterior chamber before the perforation was eventually stabilised (figure 2A).
Figure 1.
(A) Paracentral corneal melt, a shallow anterior chamber, with diffuse conjunctival hyperaemia. (B) Corneal surface stained with 2% fluorescein and siedel’s positive area near the ulcer’s superior border.
Figure 2.
(A) Corneal surface sealed with cyanoacrylate glue. The surface is stable, and the anterior chamber is formed. (B) Corneal surface healed, with mild stromal scarring and inactive neovascularisation.
At presentation, the patient had reported his medical history as being significant for bowel cancer with a subsequent colostomy, as well as an ill-defined respiratory illness for which he was under the care of a local hospital respiratory physician. The patient had described it as a ‘viral pneumonia’ and had been taking 30 mg of prednisolone daily for over a year as the sole oral treating agent. He reported dyspnoea on climbing one flight of stairs when he reduced the prednisolone dose below 30 mg/day. He had also been prescribed formeterol/budesonide 200/6 two puffs two times per day, one time per day fluticasone/vilanterol 92/22 and salbutamol/ipratropium nebulisers as needed but was poorly-compliant with all inhalers.
Extensive testing was undertaken to establish a possible underlying cause of the corneal melt. Viral swabs were sent and numerous blood tests were undertaken, looking for possible inflammatory, autoimmune or infective causes: full blood count (FBC), urea and electrolytes (U&E), liver function tests (LFTs), C reactive protein, erythrocyte sedimentation rate (ESR), serum protein electrophoresis, complement C3, C4, antinuclear antibodies (ANA), ANCA, rheumatoid factor (RF), anti-cyclic citrullinated peptide antibody (anti-CCP), extractable nuclear antigen antibodies (ENA), ACE and venereal disease research laboratory test (VDRL).
After discussion with the patient’s respiratory physician over the following days, it was revealed that the he had in fact an underlying diagnosis of EGPA. This diagnosis was based on the clinical findings and characteristic changes on high-resolution CT thorax but had not been confirmed on tissue biopsy. During this time, the results of our investigations were also returned. The blood tests were remarkable for eosinophilia and ANCA positivity with myeloperoxidase (MPO) antibody (p-ANCA), in keeping with this diagnosis. All other results were negative or within normal ranges. Our patient had no cutaneous manifestations of EGPA. He had no cardiac findings and his dyspnoea was attributable to the lung disease. It is important to note that findings of EGPA may be obscured by systemic glucocorticoids used to control asthma: vasculitis may be unmasked when, for example, a patient’s steroids are tapered on commencing a leukotriene receptor-1 antagonist.10 23
One week following his initial presentation, the patient’s corneal perforation was well sealed with glue, the anterior chamber was fully formed and a BCL remained in situ. He was discharged on G. chloramphenicol (preservative free), G. cyclopentolate, doxycycline 100 mg one time per day orally, and a tapering course of prednisolone with a proton pump inhibitor. As he was not on any other immunosuppression, the ophthalmology team liaised with his respiratory physicians and it was planned for the patient to start intravenous cyclophosphamide at his local hospital.
Outcome and follow-up
Three months after initial presentation, the glue was removed revealing a healed corneal melt with corneal stromal scarring and superficial vascularisation but no active inflammation (figure 2B). At his most recent review, 12 months after presenting with the perforated corneal melt, the patient had a left unaided visual acuity of 6/30+1, which is a satisfactory outcome for this presentation.
Systemically, he is also doing well. Having received four doses of monthly intravenous cyclophosphamide he was switched to mycophenolate mofetil which he tolerates well. His oral prednisolone has been successfully tapered down to maintenance dose of 7.5 mg daily.
Discussion
The diagnosis and management of this patient’s corneal melt and the underlying disorder EGPA, presented a challenge. Corneal melting is clinically and pathologically distinct from peripheral ulcerative keratitis, the type of keratitis most commonly associated with systemic vasculitis. It commonly presents with sudden loss of vision due to perforation, as inflammatory symptoms like pain and redness are often absent until then as in this case. In contrast, patients with the more common manifestation of vasculitis in the anterior segment of the eye, peripheral ulcerative keratitis, typically present with pain and redness allowing corneal perforation to be prevented through medical management in the majority of cases.22 24 25
There is no specific laboratory test for EGPA and as such it can be difficult to definitively diagnose. Clinically, the condition should be suspected in patients who report chronic rhinosinusitis or asthma that is poorly controlled despite moderate doses of inhaled glucocorticoids and with an eosinophilia. ANCA prevalence is between 38% and 67%, with the majority of these patients also testing positive for antibodies against myeloperoxidase with a perinuclear staining pattern (called MPO or p-ANCA).26–28 Other non-specific laboratory findings include anaemia, increased ESR and increased serum IgE. This patient presented with a vague history of a long-standing respiratory illness for which he had been on oral steroids as well as various inhalers, raising the suspicion of an underlying condition that may have contributed to his corneal melt. EGPA was later confirmed by his treating hospital physician, and blood results taken during his admission correlated with this diagnosis.
EGPA is primarily treated using systemic glucocorticoids, as this man had been prescribed prior to admission. Additional immunosuppressive agents are added in the instance of patients with advanced or refractory disease or in patients who experience flares when systemic glucocorticoids are tapered and include cyclophosphamide, azathioprine and methotrexate.29 Messmer and Foster believe systemic immunosuppressive therapy with corticosteroids and cytotoxic agents to be ‘mandatory’ when treating EGPA and other multisystem disorders associated with vasculitic peripheral ulcerative keratitis.13 Studies have suggested that corneal melt can herald the early signs of a potentially lethal necrotising vasculitis.24 25 30 The Five-Factor Score (FFS) is used by clinicians to quantify the extent of EGPA and help guide therapy.31 32 The revised 2011 FFS includes: age >65 years, cardiac insufficiency, renal insufficiency, gastrointestinal involvement and absence of ear nose and throat (ENT) manifestations.33 Cyclophosphamide is recommended in patients with an FFS score of ≥2 as treatment with cyclophosphamide and glucocorticoids is associated with a lower mortality when compared with glucocorticoids alone.34 Patients with an FFS score of 1 may be treated with azathioprine or methotrexate instead of cyclophosphamide, though cyclophosphamide is the preferred agent in the presence of cardiac or CNS involvement. Our patient had an FFS score of 2 (age >65, absence of ENT manifestations) and as such warranted the addition of an immunosuppressive agent to his treatment regime.
Learning points.
To the best of our knowledge, this is the first case of corneal melt secondary to eosinophilic granulomatosis with polyangiitis (EGPA) to be reported.
EGPA can be difficult to diagnose and can have varying clinical manifestations. It should be suspected in patients with poorly controlled asthma or chronic rhinosinusitis despite moderate dose of steroids and prominent peripheral blood eosinophilia.
It is important for clinicians to correlate ocular manifestations with possible underlying systemic disease. This will allow for appropriate treatment to be initiated at an early stage and thus reduce the extent of disease related morbidity and mortality.
Footnotes
Contributors: All authors made substantial contributions in the drafting and revising of the manuscript. CCM: conception of idea for case report, review of drafts, generation of correction and edits. EG: drafting of manuscript, incorporating revisions, background research of subject area. EF: drafting of manuscript, incorporating revisions, background research of subject area. All authors gave their final approval for the version submitted for publication.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Churg J, Strauss L. Allergic granulomatosis, allergic angiitis, and periarteritis nodosa. Am J Pathol 1951;27:277–301. [PMC free article] [PubMed] [Google Scholar]
- 2. Conron M. Rare diseases bullet 11: Churg-Strauss syndrome. Thorax 2000;55:870–7. 10.1136/thorax.55.10.870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zwerina J, Eger G, Englbrecht M, et al. Churg-Strauss syndrome in childhood: a systematic literature review and clinical comparison with adult patients. Semin Arthritis Rheum 2009;39:108–15. 10.1016/j.semarthrit.2008.05.004 [DOI] [PubMed] [Google Scholar]
- 4. Gendelman S, Zeft A, Spalding SJ. Childhood-onset eosinophilic granulomatosis with polyangiitis (formerly Churg-Strauss syndrome): a contemporary single-center cohort. J Rheumatol 2013;40:929–35. 10.3899/jrheum.120808 [DOI] [PubMed] [Google Scholar]
- 5. Fauci A. Harrison’s Rheumatology. 2nd Edition McGraw Hill, 2010. [Google Scholar]
- 6. Ameli F, Phang KS, Masir N. Churg-Strauss syndrome presenting with conjunctival and eyelid masses: a case report. Med J Malaysia 2011;66:517–9. [PubMed] [Google Scholar]
- 7. Guillevin L, Cohen P, Gayraud M, et al. Churg-Strauss syndrome. Clinical study and long-term follow-up of 96 patients. Medicine 1999;78:26–37. 10.1097/00005792-199901000-00003 [DOI] [PubMed] [Google Scholar]
- 8. Cottin V, Khouatra C, Dubost R, et al. Persistent airflow obstruction in asthma of patients with Churg-Strauss syndrome and long-term follow-up. Allergy 2009;64:589–95. 10.1111/j.1398-9995.2008.01854.x [DOI] [PubMed] [Google Scholar]
- 9. Comarmond C, Pagnoux C, Khellaf M, et al. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): clinical characteristics and long-term followup of the 383 patients enrolled in the French Vasculitis Study Group cohort. Arthritis Rheum 2013;65:270–81. 10.1002/art.37721 [DOI] [PubMed] [Google Scholar]
- 10. Abril A, Calamia KT, Cohen MD. The Churg Strauss syndrome (allergic granulomatous angiitis): review and update. Semin Arthritis Rheum 2003;33:106–14. 10.1016/S0049-0172(03)00083-0 [DOI] [PubMed] [Google Scholar]
- 11. Robin JB, Schanzlin DJ, Meisler DM, et al. Ocular involvement in the respiratory vasculitides. Surv Ophthalmol 1985;30:127–40. 10.1016/0039-6257(85)90081-5 [DOI] [PubMed] [Google Scholar]
- 12. Bawazeer AM, Jackson WB. Marginal infiltrative ulcerative keratitis secondary to Churg-Strauss syndrome: a case report. Cornea 2000;19:402–4. 10.1097/00003226-200005000-00030 [DOI] [PubMed] [Google Scholar]
- 13. Messmer EM, Foster CS. Vasculitic peripheral ulcerative keratitis. Surv Ophthalmol 1999;43:379–96. 10.1016/S0039-6257(98)00051-4 [DOI] [PubMed] [Google Scholar]
- 14. Margolis R, Kosmorsky GS, Lowder CY, et al. Conjunctival involvement in Churg-Strauss syndrome. Ocul Immunol Inflamm 2007;15:113–5. 10.1080/09273940701299388 [DOI] [PubMed] [Google Scholar]
- 15. Chumbley LC, Harrison EG, DeRemee RA. Allergic granulomatosis and angiitis (Churg-Strauss syndrome). Report and analysis of 30 cases. Mayo Clin Proc 1977;52:477–84. [PubMed] [Google Scholar]
- 16. Cury D, Breakey AS, Payne BF. Allergic granulomatous angiitis associated with uveoscleritis and papilledema. AMA Arch Ophthalmol 1956;55:261–6. 10.1001/archopht.1956.00930030265013 [DOI] [PubMed] [Google Scholar]
- 17. Vitali C, Genovesi-Ebert F, Romani A, et al. Ophthalmological and neuro-ophthalmological involvement in Churg-Strauss syndrome: a case report. Graefes Arch Clin Exp Ophthalmol 1996;234:404–8. 10.1007/BF00190718 [DOI] [PubMed] [Google Scholar]
- 18. Acheson JF, Cockerell OC, Bentley CR, et al. Churg-Strauss vasculitis presenting with severe visual loss due to bilateral sequential optic neuropathy. Br J Ophthalmol 1993;77:118–9. 10.1136/bjo.77.2.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kattah JC, Chrousos GA, Katz PA, et al. Anterior ischemic optic neuropathy in Churg-Strauss syndrome. Neurology 1994;44:2200–2. 10.1212/WNL.44.11.2200 [DOI] [PubMed] [Google Scholar]
- 20. Perez VL, Chavala SH, Ahmed M, et al. Ocular manifestations and concepts of systemic vasculitides. Surv Ophthalmol 2004;49:399–418. 10.1016/j.survophthal.2004.04.008 [DOI] [PubMed] [Google Scholar]
- 21. Pfister RR, Murphy GE. Corneal ulceration and perforation associated with Sjögren’s syndrome. Arch Ophthalmol 1980;98:89–94. 10.1001/archopht.1980.01020030091006 [DOI] [PubMed] [Google Scholar]
- 22. McKibbin M, Isaacs JD, Morrell AJ. Incidence of corneal melting in association with systemic disease in the Yorkshire Region, 1995-7. Br J Ophthalmol 1999;83:941–3. 10.1136/bjo.83.8.941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Churg A, Churg J. Steroids and Churg-Strauss syndrome. The Lancet 1998;352:32–3. 10.1016/S0140-6736(05)79511-8 [DOI] [PubMed] [Google Scholar]
- 24. Squirrell DM, Winfield J, Amos RS. Peripheral ulcerative keratitis ’corneal melt' and rheumatoid arthritis: a case series. Rheumatology 1999;38:1245–8. 10.1093/rheumatology/38.12.1245 [DOI] [PubMed] [Google Scholar]
- 25. Jifi-Bahlool H, Saadeh C, O’Connor J. Peripheral ulcerative keratitis in the setting of rheumatoid arthritis: treatment with immunosuppressive therapy. Semin Arthritis Rheum 1995;25(1):67–73. 10.1016/S0049-0172(95)80019-0 [DOI] [PubMed] [Google Scholar]
- 26. Lhote F, Guillevin L. Polyarteritis nodosa, microscopic polyangiitis, and Churg-Strauss syndrome. Clinical aspects and treatment. Rheum Dis Clin North Am 1995;21:911–47. [PubMed] [Google Scholar]
- 27. Sablé-Fourtassou R, Cohen P, Mahr A, et al. Antineutrophil cytoplasmic antibodies and the Churg-Strauss syndrome. Ann Intern Med 2005;143:632–8. 10.7326/0003-4819-143-9-200511010-00006 [DOI] [PubMed] [Google Scholar]
- 28. Cottin V, Bel E, Bottero P, et al. Revisiting the systemic vasculitis in eosinophilic granulomatosis with polyangiitis (Churg-Strauss): A study of 157 patients by the Groupe d’Etudes et de Recherche sur les Maladies Orphelines Pulmonaires and the European Respiratory Society Taskforce on eosinophilic granulomatosis with polyangiitis (Churg-Strauss). Autoimmun Rev 2017;16:1–9. 10.1016/j.autrev.2016.09.018 [DOI] [PubMed] [Google Scholar]
- 29. Bosch X, Guilabert A, Espinosa G, et al. Treatment of antineutrophil cytoplasmic antibody-associated vasculitis: A systematic review. J Am Med Assoc 2007;298:655–69. [DOI] [PubMed] [Google Scholar]
- 30. Foster CS, Forstot SL, Wilson LA. Mortality rate in rheumatoid arthritis patients developing necrotizing scleritis or peripheral ulcerative keratitis. Effects of systemic immunosuppression. Ophthalmology 1984;91:1253–63. 10.1016/S0161-6420(84)34160-4 [DOI] [PubMed] [Google Scholar]
- 31. Moosig F, Bremer JP, Hellmich B, et al. A vasculitis centre based management strategy leads to improved outcome in eosinophilic granulomatosis and polyangiitis (Churg-Strauss, EGPA): monocentric experiences in 150 patients. Ann Rheum Dis 2013;72:1011–7. 10.1136/annrheumdis-2012-201531 [DOI] [PubMed] [Google Scholar]
- 32. Samson M, Puéchal X, Devilliers H, et al. Long-term outcomes of 118 patients with eosinophilic granulomatosis with polyangiitis (Churg-Strauss syndrome) enrolled in two prospective trials. J Autoimmun 2013;43:60–9. 10.1016/j.jaut.2013.03.003 [DOI] [PubMed] [Google Scholar]
- 33. Guillevin L, Pagnoux C, Seror R, et al. The Five-Factor Score revisited: assessment of prognoses of systemic necrotizing vasculitides based on the French Vasculitis Study Group (FVSG) cohort. Medicine 2011;90:19–27. 10.1097/MD.0b013e318205a4c6 [DOI] [PubMed] [Google Scholar]
- 34. Gayraud M, Guillevin L, le Toumelin P, et al. Long-term followup of polyarteritis nodosa, microscopic polyangiitis, and Churg-Strauss syndrome: analysis of four prospective trials including 278 patients. Arthritis Rheum 2001;44:666–75. [DOI] [PubMed] [Google Scholar]