Abstract
A 72-year-old woman was admitted to the hospital because of dorsal, lumbar and lower abdomen pain that had started 4 days before. She had a history of age-related macular degeneration (treated with intraocular bevacizumab). Blood tests showed anaemia, thrombocytopaenia, acute kidney injury, elevated liver enzymes and total bilirubin (mainly because of the indirect fraction). Viral serologies and ADAMTS13 activity levels were normal, and stool testing was negative for Escherichia coli-producing Shiga toxins. E. coli was isolated in urine. Atypical haemolytic uremic syndrome triggered by a urinary tract infection or by the vascular endothelial growth factor-inhibitor bevacizumab were the most likely hypothesis. The patient started urgent plasmapheresis and dialysis that lasted for a total of 18 days. There was complete remission and recovery of kidney function allowing for treatment discontinuation, and she was discharged home. After 6 months of follow-up, she shows no signs of relapse.
Keywords: haematology (incl blood transfusion), dialysis, fluid electrolyte and acid-base disturbances, acute renal failure
Background
The aetiological differential diagnosis of an acute thrombotic microangiopathy (TMA) is among the most difficult challenges a doctor must face.1 Clinically, acute TMA is characterised by the association of microangiopathic haemolytic anaemia and consumptive thrombocytopaenia leading to platelet-mediated microvascular occlusion and organ failure.1 Its prompt recognition and of its key differential diagnosis is critical to initiate supportive/specific therapy within the first 24–48 hours, thus improving the prognosis.2 Atypical haemolytic uremic syndrome (aHUS) is one of the main forms of TMA. It is mediated by complement alternative pathway dysregulation caused by a combination of genetic factors and environmental triggers.3 Although considered to be an ultrarare disease (European prevalence estimated in approximately 2 cases per 1 million people) affecting mainly children and young adults it can manifest in any age.1 Traditionally, the prognosis has been poor, with death rates of up to 25% and development of end-stage renal disease in about 50% of patients.2 3
We present the case of an aHUS that manifested at a later age and was triggered by an unusual association of a urinary tract infection and, possibly, therapy with vascular endothelial growth factor (VEGF) inhibitor, in a patient with a genetic vulnerability in a key alternate complement pathway regulatory protein. The immediate recognition of acute TMA and prompt initiation of therapy were critical in improving the patient`s outcome.
Case presentation
A 72-year-old Caucasian woman presented to the emergency department with complaints of continuous, incessant bilateral lumbar pain, associated with pain in the lower abdomen; these symptoms had started 7 days before. She mentioned foul-smelling urine but did not have any other urinary symptoms. Her general practitioner had already evaluated her, and paracetamol was started for pain relief with no effect.
The patient denied having fever, any cardiorespiratory or gastrointestinal symptoms, particularly nausea, vomiting or diarrhoea.
She was a British national, residing in Portugal for the past 7 years. She had a long-time history of hypertension (well controlled with 30 mg/day of nifedipine) and age-related macular degeneration treated regularly for the past 4 years with intraocular bevacizumab on a bimonthly basis. Other relevant medical history includes dyslipidaemia and osteoporosis for which she was treated with atorvastatin 20 mg/day and alendronic acid 70 mg/week with vitamin D 5600 IU/week. There was no prior history of kidney disease, and previous laboratory results were available showing a serum creatinine of 0.9 mg/dL, 3 months before.
She smoked (50 pack-years) and drank regularly (estimated alcohol intake 12 g/day). There was no history of drug abuse or of taking any sort of natural drugs/herbal teas.
On physical examination, the patient appeared well. The temperature was 36.8°C, the pulse 91 beats/min, the blood pressure 160/64 mm Hg, the respiratory rate 18 breaths/min and peripheral oxygen saturation 99% while breathing ambient air. She had scleral icterus and multiple haematomas on her legs. The remaining of her physical examination was normal.
Investigations
The initial laboratory evaluation (table 1) revealed normal haemoglobin (129 g/L) and white cells count (0.0083×109/L) and severe thrombocytopaenia (0.014×109/L). Coagulation function tests were within normal range. Evaluation of peripheral blood smear revealed the presence of schizocytes (85/high power field). Coombs test was negative. There was an increased serum creatinine level (6.3 mg/dL), an increased lactate dehydrogenase (>5000 U/L), elevated alanine aminotransferase (1200 U/L), aspartate aminotransferase (1000 U/L), alkaline phosphatase (334 U/L) and bilirubin (4.1 mg/dL—mainly because of the indirect fraction); C reactive protein was high (28 mg/dL).
Table 1.
Initial laboratory results
| Blood tests on arrival | Normal values | |
| Haemoglobin (g/L) | 129 | 125–175 |
| Leukocytes (109/L) | 8.3 | 4.0–10.0 |
| Platelets (109/L) | 14 | 150–400 |
| Serum creatinine (mg/dL) | 6.27 | 0.72–1.18 |
| LDH (U/L) | 5299 | 125–220 |
| Total bilirubin (mg/dL) | 4.1 | 0.3–1.2 |
| Aspartate aminotransferase (U/L) | 1046 | <35 |
| Alanine aminotransferase (U/L) | 1255 | <45 |
| Alkaline phosphatase | 334 | 40–150 |
| C reactive protein (mg/dL) | 27.9 | 0–0.5 |
| D-dimers (ng/mL) | 61 886 | <230 |
LDH, lactate dehydrogenase.
Chest X-ray and abdominal and kidney ultrasound were performed and reported as normal.
Due to the complaints of pain and very high D-dimers (~62 000 ng/mL), there was concern for a possible pulmonary thromboembolism/aortic dissection, so a thoracic and abdominal CT scan was performed and reported normal.
Additional blood tests performed in the next hours, revealed a haemoglobin drop to 75 g/L, with increased reticulocyte levels (3%) and undetectable haptoglobin levels (<0.08 gr/L).
ADAMTS13 activity levels were within normal range and stool testing was negative for Escherichia coli-producing Shiga toxins (table 2).
Table 2.
Full immune evaluation and further blood and stool tests
| Additional blood tests performed | |
| Toxoplasma serology | Negative |
| Cytomegalovirus serology | Negative |
| Herpes simplex serology | Negative |
| Epstein-Barr serology | Negative |
| Rickettsia serology | Negative |
| Coxiella serology | Negative |
| Borrelia serology | Negative |
| Leptospira serology | Negative |
| Faecal PCR testing for bacterial pathogens | Negative |
| Urinary culture | Positive for Escherichia coli |
| C3 | 1.03 g/L (normal) |
| C4 | 0.28 g/L (normal) |
| Antinuclear antibodies | Titre 1:160 |
| Anti C and P ANCA antibodies | Negative |
| ADAMTS13 activity | 87% (normal) |
| Haptoglobin | <0.08 g/L |
| Ratio of kappa and lambda light chains | Normal |
| Immunofixation | Normal |
| Coombs test | Negative |
ANCA, antineutrophil cytoplasmic antibodies; PANCA, perinuclear antineutrophil cytoplasmic antibodies.
Urine sample culture resulted positive for E. coli. Serologies for multiple infectious agents were performed (table 2) and were negative. A comprehensive immune evaluation (table 2) was performed, all tests being within normal range, including normal serum C3 and C4 levels.
Since we were in the presence of an acute, Coombs negative, haemolytic anaemia, with schizocytes in the peripheral blood smear (the hallmarks of microangiopathic haemolytic anaemia), with serious thrombocytopaenia and acute renal failure, the possibility of aHUS was a relevant one.
As such, a full genetic study of the genes encoding the alternate complement pathway regulatory proteins was ordered. This study, that was only available at a later date, analysed by NGS 11 genes: CFH, CD46 (MCP), CFI, C3, THBD, CFB, CFHR5, CFHR1, CFHR3, CFHR4 and DGKE. Two heterozygous benign missense variants were identified in the CFH gene (c.3172 T>C, p.Tyr1058His and c.3178 G>C, p.Val1060Leu). Another rare heterozygous missense variant was also found in CFH (c.3226 C>G, p.Gln1076Glu) that was analysed with five in silico algorithms and had a score >=3 and thus considered to be likely pathogenic.4 5
Differential diagnosis
Our patient presented herself with an association of Coombs negative haemolytic anaemia, with schizocytes in the peripheral blood smear, severe thrombocytopaenia and acute organ injury (renal failure and acute hepatitis). These are the key clinical findings that must evoke the diagnosis of acute TMA.1–3 Additionally, our patient had the diagnosis of acute pyelonephritis by E. coli. In the context of an infectious process, the key differential diagnosis to immediately exclude is that of disseminated intravascular coagulation; the presence of normal coagulation tests rules out this possibility.1–3
The presence of such severe thrombocytopaenia raises the possibility of thrombocytopaenic thrombotic purpura. It is probably the main primary TMA to be considered in an adult patient with a reported incidence of six cases per million per year in the UK and a mortality rate of 90% if prompt therapy with plasma exchange is delayed.1 It is defined by severe ADAMTS13 deficiency (<10%) leading to the formation of very high molecular weight multimers of the von Willebrand factor that lead to platelet aggregation in the microvasculature.1–3 In our centre, we are lucky to be able to assess the ADAMTS13 activity usually within the first 24 hours, and its normal level excluded this possibility.
Another major TMA to be considered is that of HUS-associated enterohaemorrhagic Shiga toxin-producing E. coli infection; usually serotype O157:H7.1–3 In most patients, there is a clinical history of bloody diarrhoea in the previous 5–10 days.1 This was not the case in our patient and the stool analysis, being negative for Shiga toxin, ruled out this possibility.
Thus, the diagnosis of aHUS although extremely unusual at this age comes to the forefront, especially after ruling out autoimmune and infectious pathology that could be causes of secondary TMA.
This hypothesis was later reinforced by the results of the study of the genes encoding alternate complement pathway regulatory proteins and the identification of a likely pathogenic variant in the CFH gene. In association, this patient had an acute pyelonephritis that could have acted as a trigger for alternate complement pathway activation and was also doing intraocular therapy with bevacizumab, a VEGF inhibitor, known to serve as a trigger for aHUS when administered systemically and topically.6 7
Treatment
The patient was initially admitted to the internal medicine ward and empiric antibiotic therapy with ceftriaxone was started; blood pressure was easily controlled with amlodipine 5 mg/day and carvedilol 12.5 mg/day.
In the face of acute TMA with severe thrombocytopaenia, dropping haemoglobin and severe acute kidney injury nephrology collaboration was sought; plasma exchange was started 48 hours after admission (replacement of 1.5 plasma volume/session using fresh frozen plasma). The next day, due to hyperkalaemia, metabolic acidosis and oliguria with hypervolaemia, urgent haemodialysis was started, and the patient was transferred to the nephrology ward.
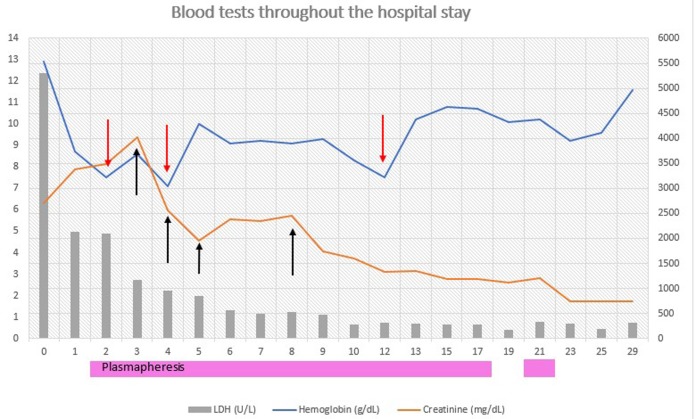
For the first 3 days, haemodialysis was performed daily. After that, the patient had a recovery of renal function with increased urinary output, therefore ceasing haemodialysis after only four sessions. Plasmapheresis on the other hand was performed daily for the first 12 days and, since the patient went into complete remission of haemolysis, was spaced out to every other day sessions; a total of 15 sessions were performed (figure 1). In the meantime, the patient had been preemptively vaccinated against pneumococcus and meningococcus in preparation for the eventual need to use eculizumab.
Figure 1.
Evolution during the patient’s hospital stay. Red arrow represents every blood transfusion administered. Black arrow represents every time the patient underwent haemodialysis. LDH, lactate dehrydrogenase.
Since the patient reacted so well to supportive care treatment and daily plasmapheresis and had complete remission of haemolysis that was sustained even after stopping plasmapheresis, eculizumab was not used.
After stopping plasmapheresis, the haematological parameters were stable (haemoglobin was 104 g/L and increasing, platelets were 180 ×109/L), lactate dehydrogenase and haptoglobin had reached normal levels and serum creatinine had improved and stabilised at 1.7 mg/dL (glomerular filtration rate of 30 mL/min/1.73 m2 Chronic Kidney Disease Epidemiology Collaboration (CKD -EPI)).
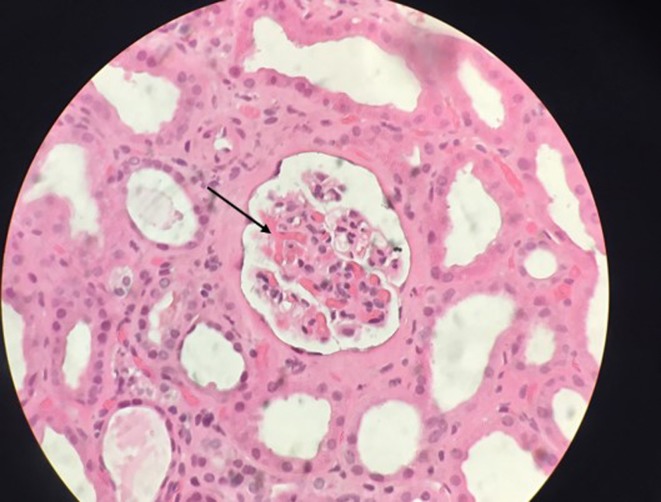
After normalisation of platelet counts, the patient was submitted to a kidney biopsy that confirmed the clinical diagnosis of a thrombotic microangiopathy (figure 2).
Figure 2.
Kidney biopsy (H&E stain) with arteriolar thrombosis (black arrow).
Outcome and follow-up
The patient was discharged after a 24-day long admission. Close ambulatory follow-up was kept with blood tests twice per week during the first month. One month after discharge, there was no anaemia or thrombocytopaenia, LDH levels were normal and serum creatinine had stabilised at about 1.4 mg/dL (glomerular filtration rate of 38 mL/min/1.73 m2).
She continues to be followed monthly and shows no signs of relapse 6 months after being discharged. Regular analyses include serum creatinine, haemoglobin, platelet count, lactate dehydrogenase, haptoglobin and protein/creatinine ratio. Regular blood pressure monitoring is also recommended, and the patient is instructed to contact in case of any clinical intercurrence (particularly an infectious event). The case was discussed with the patient ophthalmology specialist, and the decision was made to permanently stop treatment with bevacizumab.
Discussion
The association of coombs negative haemolytic anaemia, with schizocytes in the peripheral blood smear, severe thrombocytopaenia and acute organ injury are the clinical hallmarks of acute TMA. It is a quickly evolving, life-threatening condition, and its prompt recognition is critical to initiate treatment in the first 24–48 hours to improve prognosis.1 2
Although TMA should be promptly identified in clinical practice, the determination of its cause is usually difficult and time consuming. Indeed, TMA is an extremely complex process that results from an imbalance between immunity, clotting and complement.8 Often there is a complex interplay between different precipitating factors (that are the key in secondary TMA) and genetic determinants of complement regulation (that are the key in aHUS), making the differential diagnosis between aHUS and secondary TMA difficult.8 In approximately two-thirds of aHUS cases, a complement activating condition is present, such as infections, malignancy, drug therapy (for example, immunosuppressive agents) and autoimmune diseases.2
This quasicontinuum in the pathogenesis, where predisposing factors acting as complement amplifying conditions and genetic predisposition interact, is, in our opinion, clearly illustrated by our case. Our patient had an acute pyelonephritis caused by E. coli infection that is a common trigger for aHUS. Even more interestingly, she was under intraocular therapy with the VEGF inhibitor bevacizumab.
The association between systemic use of anti-VEGF agents (including bevacizumab, usually as an anticancer agent-blocking angiogenesis) and kidney injury is well documented.9–11 Usually, kidney involvement consists of renal limited TMA in relation to microvascular endothelial damage (clinically ranging from mild hypertension/proteinuria to acute renal failure), many patients not expressing significant haematological manifestations of TMA.10 Postulated pathophysiological mechanisms include the loss of healthy fenestrated glomerular endothelium secondary to disruption of VEGF function, leading to and promoting the development of microvascular injury.9
Although perhaps not as well known, the association between local, intraocular therapy with bevacizumab and systemic TMA is also documented, and cases have been described where intraocular bevacizumab administration is associated with systemic aHUS.7 To reduce angiogenesis in the eye, VEGF inhibitors are injected directly in the vitreous and, although that should not have any effect systemically, it has been shown that intravitreal injection of anti-VEGF antibodies can lead to systemic absorption, with the potential to affect non-ocular VEGF-dependent pathways with potential to induce systemic adverse effects.12 Perhaps in patients with diseases that alter the retina blood barrier (as is the example with age-related macular degeneration) due to neovascularisation, the drug’s systemic release could be facilitated.7 13
In association to the above-discussed triggers, our patient had several missense variants in the factor H gene that codifies a key complement alternate pathway regulatory protein. One of the variants after in silico analysis was considered to be likely pathogenic; the other two, although considered benign and not having a marked pathogenic effect on their own, may contribute to an increased risk for a thrombotic microangiopathy when together.4 5 This genetic background made our patient more susceptible to environmental triggers like the ones stated beforehand.
Another curious association in our patient is that aHUS is not the only disease affected by the polymorphisms in complement factor H, since age-related macular degeneration initiation and progression has also been associated with such genetic changes.9 In our case, these three variants in the CFH gene (p.Tyr1058His, p.Val1060Leu and p.Gln1076Glu) are also associated with age-related macular degeneration and p.Gln1076Glu has been found in multiple cases within an AMD family.14 15 This further demonstrates a correlation between both aHUS and AMD as these variants may have had an impact in both pathologies.
All patients with a clinical diagnosis of aHUS are eligible for treatment with the complement inhibitor eculizumab.16 This drug has significantly improved the prognosis of patients with aHUS.1 2 This drug increases the risk of infections by encapsulated organism (particularly meningococcal infection) and, as such, all patients should receive vaccination against meningococcus.16 This was the reason for performing vaccination in our patient.
Despite this, if eculizumab therapy is not immediately available (as is the case in our centre), plasma therapy can still be used, this being particularly relevant in the emergency setting, with critically ill patients with severe TMA, as was the case with our patient.16
We followed standard recommendations, using plasma exchange with fresh frozen plasma, replacing 1.5 plasma volume per treatment.1 2 This therapy was performed daily until complete remission and spaced thereafter with every other day treatments. The complete response and the sustained clinical remission even after stopping plasma exchange made unnecessary the use of eculizumab. Despite her age, the fact that our patient was not diabetic and had no prior kidney disease, made her more more prone to recover from the initial aggression and less likely to need renal substitution therapy chronically.
Due to the risk of relapse inherent to aHUS, the patient was followed closely. We followed the recommendations published by Fakhouri in his analysis of the French aHUS registry experience with eculizumab discontinuation, with regular urine and blood tests performed weekly for the first month and monthly thereafter.17
Learning points.
In all patients with coombs-negative haemolytic anaemia (with schizocytes in the peripheral blood smear), severe thrombocytopaenia and acute organ injury the hypothesis of acutethrombotic microangiopathy (TMA)must be considered.
Atypical haemolytic uremic syndrome is a rare disease but must always be considered in a patient with acute TMA.
Treatment with plasmapheresis (or eculizumab if possible) should be started as soon as possible, preferably before the first 48 hours of the initial diagnosis to improve the odds of renal recovery.
Need for maintaining treatment should be evaluated on a case-by-case basis, and it should be spaced out according to the patient’s clinical features and analytical values.
Close clinical follow-up is recommended after treatment suspension due to the potential risk of relapse.
Footnotes
Contributors: FSM wrote the article, proof read it and approved the final version of the article. ALN aided in the acquisition of the data, conceived the article, revised it and approved the final version. ARE interpreted the data, proofread the article and approved the final version. NO designed the article’s orientation, proofread it, provided critical intellectual content and approved the final version.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Azevedo A, Faria B, Teixeira C, et al. Portuguese consensus document statement in diagnosis and management of atypical hemolytic uremic syndrome. PJNH 2018;32:211–32. [Google Scholar]
- 2. Campistol JM, Arias M, Ariceta G, et al. Actualización en síndrome hemolítico urémico atípico: diagnóstico y tratamiento. Documento de consenso Nefrología 2013;33:27–45. [Google Scholar]
- 3. Brocklebank V, Wood KM, Kavanagh D, et al. Thrombotic microangiopathy and the Kidney. Clin J Am Soc Nephrol 2018;13:300–17. 10.2215/CJN.00620117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Matsumoto T, Fan X, Ishikawa E, et al. Analysis of patients with atypical hemolytic uremic syndrome treated at the Mie University Hospital: concentration of C3 p.I1157T mutation. Int J Hematol 2014;100:437–42. 10.1007/s12185-014-1655-2 [DOI] [PubMed] [Google Scholar]
- 5. Fidalgo T, Martinho P, Pinto CS, et al. Combined study of ADAMTS13 and complement genes in the diagnosis of thrombotic microangiopathies using next-generation sequencing. Res Pract Thromb Haemost 2017;1:69–80. 10.1002/rth2.12016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Campistol JM. “An update for atypical haemolytic uraemic syndrome: Diagnosis and treatment. A consensus document”. Nefrología 2015;35:421–47. [DOI] [PubMed] [Google Scholar]
- 7. Pellé G, Shweke N, Duong Van Huyen JP, Huyen JPD, et al. Systemic and kidney toxicity of intraocular administration of vascular endothelial growth factor inhibitors. Am J Kidney Dis 2011;57:756–9. 10.1053/j.ajkd.2010.11.030 [DOI] [PubMed] [Google Scholar]
- 8. Román E, Mendizábal S, Jarque I, et al. Secondary thrombotic microangiopathy and eculizumab: a reasonable therapeutic option. Nefrologia 2017;37:478–91. 10.1016/j.nefroe.2017.08.001 [DOI] [PubMed] [Google Scholar]
- 9. Eremina V, Jefferson JA, Kowalewska J, et al. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med 2008;358:1129–36. 10.1056/NEJMoa0707330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Izzedine H, Escudier B, Lhomme C, et al. Kidney diseases associated with anti-vascular endothelial growth factor (VEGF): an 8-year observational study at a single center. Medicine 2014;93:333–9. 10.1097/MD.0000000000000207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Uy AL, Simper NB, Champeaux AL, et al. Progressive bevacizumab-associated renal thrombotic microangiopathy. NDT Plus 2009;2:36–9. 10.1093/ndtplus/sfn168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Csaky K, Do DV, Dv D. Safety implications of vascular endothelial growth factor blockade for subjects receiving intravitreal anti-vascular endothelial growth factor therapies. Am J Ophthalmol 2009;148:647–56. 10.1016/j.ajo.2009.06.014 [DOI] [PubMed] [Google Scholar]
- 13. Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science 2005;308:385–9. 10.1126/science.1109557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Geerlings MJ, de Jong EK, den Hollander AI. The complement system in age-related macular degeneration: a review of rare genetic variants and implications for personalized treatment. Mol Immunol 2017;84:65–76. 10.1016/j.molimm.2016.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Triebwasser MP, Roberson ED, Yu Y, et al. Rare variants in the functional domains of complement factor H are associated with age-related macular degeneration. Invest Ophthalmol Vis Sci 2015;56:6873–8. 10.1167/iovs.15-17432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goodship TH, Cook HT, Fakhouri F, et al. Atypical hemolytic uremic syndrome and C3 glomerulopathy: conclusions from a "Kidney Disease: Improving Global Outcomes" (KDIGO) Controversies Conference. Kidney Int 2017;91:539–51. 10.1016/j.kint.2016.10.005 [DOI] [PubMed] [Google Scholar]
- 17. Fakhouri F, Hourmant M, Campistol JM, et al. Terminal complement inhibitor eculizumab in adult patients with atypical hemolytic uremic syndrome: a single-arm, open-label trial. Am J Kidney Dis 2016;68:84–93. 10.1053/j.ajkd.2015.12.034 [DOI] [PubMed] [Google Scholar]