Abstract
Leprosy is a chronic infectious, granulomatous disease caused by the intracellular bacillus Mycobacterium leprae that infects macrophages and Schwann cells. While relatively rare in the USA, there is about 200 new cases of leprosy every year with the majority occurring in the southern parts of the country. It is believed to be linked to the region of the nine-banned armadillo in patients with no significant travel history outside of the country. In this case report, we encountered a 58-year-old Central Florida man that had extensive exposure to armadillos and presented with the typical symptoms of large erythaematous patches, numbness and peripheral nerve hypertrophy. Once diagnosed properly, patients are then reported to the National Hansen’s Centre who provides the multidrug therapy for 12–24 months. Due to its rarity and its ability to mimic other more common ailments, leprosy should be included in the differential diagnosis in patients that have significant exposure to armadillos, live in the southern part of the country or have recently travelled to countries that have a high prevalence of leprosy.
Keywords: dermatology, infections, skin, general practice/family medicine, dermatological
Background
This case was written up because it shows the importance of gaining a full social history of the patient to gain a broader differential diagnosis, as well as offer an example of extended exposure to armadillos and leprosy. This case also presents how to approach diagnosing and treating leprosy.
Case presentation
A 58-year-old Central Florida man presented to clinic with a complaint of a 0.8-cm hyperpigmented plaque of the left arm located in the anterior cubital fossa that had been present for 5 months and had increased in size. He admitted to decreased sensation in the lesion as well as occasional sharp pain. The patient was seen previously at an urgent care centre where he tested negative for Lyme’s disease. Patient is a self-proclaimed out-doors man, so initial diagnosis was chronic arthropod bite hypersensitivity reaction and was treated with 0.2 cc of 5.0 mg/cc kenalog intralesional injection and he was instructed to try emollients and antihistamines for pruritus. Patient returned 8 months later with complaint of increasing lesion size, decreased sensation and 4-month history of increased erythaema (figure 1). On further history, it was discovered that the patient used to trap and sell armadillos for leprosy research approximately 30 years ago. The differential diagnosis was then expanded to include pseudodolymphoma, cutaneous B cell lymphoma, cutaneous T cell lymphoma, cutaneous toxoplasmosis, granuloma annulare, erythaema elavatum diutium, lichen amyloidosis, deep fungal infection, leprosy and sarcoidosis. A punch biopsy of the lesion was performed in clinic and sent to pathology for H&E stain. Tissue was also sent for bacterial culture, Mycobacterium culture, deep fungal culture, acid-fast bacilli (AFB) stain, Gram stain and tissue potassium hydroxide. Radiographic studies included a posterior-anterior and lateral chest X-ray.
Figure 1.
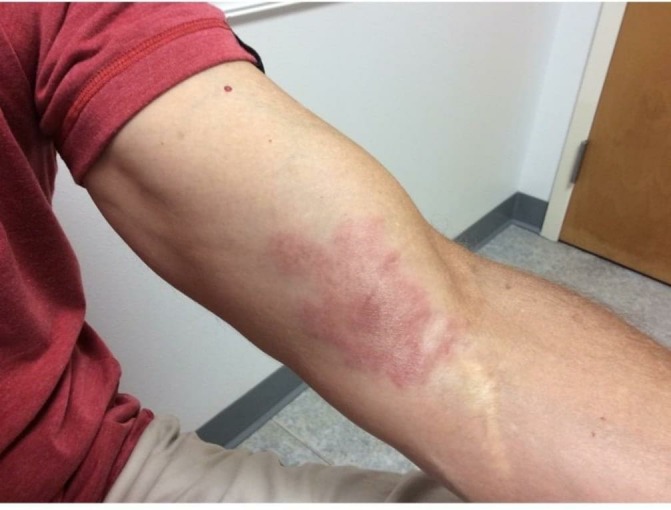
Initial photograph documentation at first follow-up appointment.
The patient returned a month later, with all tests returning negative including PCR for Mycobacterium leprae DNA and acid-fast bacterial cultures which were still pending. These results may have been due to the kenalog injection or a possibly low-bacterial load of the skin biopsy that can lead to negative PCR results.1 His symptoms continued to be unresolved with evidence of palpable peripheral nerve hypertrophy proximal to the plaque (figure 2). Skin exam showed a well-defined annular plaque with a hypopigmented centre which was anaesthetic with no sensation to pinprick which increased suspicion of turberculoid leprosy. A biopsy of possible hypertrophied nerve was then taken looking for sarcoid granulomas within the nerve to try to confirm possible tuberculoid leprosy. Patient was then sent for G6PD level and complete blood count (CBC) to prepare patient for possible start of dapsone for treatment. Labs showed no G6PD deficiency, but did show low white blood cell count, and biopsy showed evidence for granulomatous dermatitis. Despite the negative AFB culture and negative PCR for M. leprae, based on the highly suggestive pathology and clinical presentation including the nerve hypertrophy and anaesthesia of the lesion and after discussion with experts at the National Hansens Center, the patient was diagnosed clinically with tuberculoid leprosy.
Figure 2.
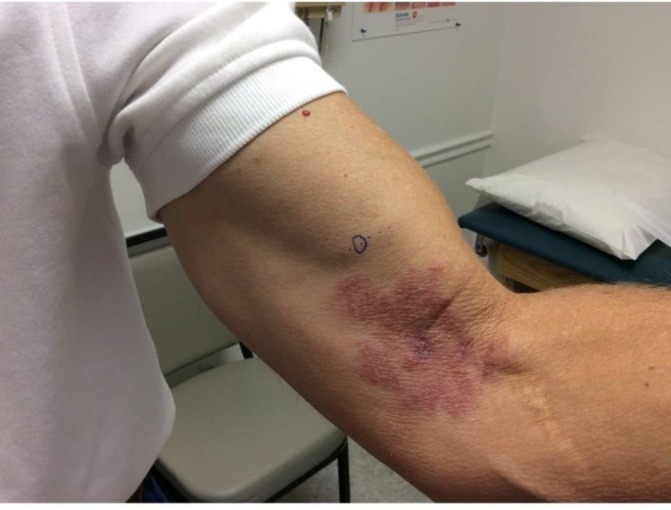
1-Month follow-up photograph with hypertrophied nerve indicated by marking.
Differential diagnosis
The differential diagnosis included chronic arthropod bite, pseudodolymphoma, cutaneous B cell lymphoma, cutaneous T cell lymphoma, cutaneous toxoplasmosis, granuloma annulare, erythaema elavatum diutium, lichen amyloidosis, deep fungal infection, leprosy and sarcoidosis.
Treatment, outcome and follow-up
The National Hansen’s Center began providing the patient with dapsone 100 and 300 mg of rifampin daily. Patient returned for follow-up 5 months after initial diagnosis with improvement in the size of the lesion and returning of sensation as well (figures 3 and 4). Patient will continue to follow-up every 3–4 months with CBC and comprehensive metabolic panels to look for possible adverse reactions to treatment, as recommended by the National Hansen’s Centre.
Figure 3.
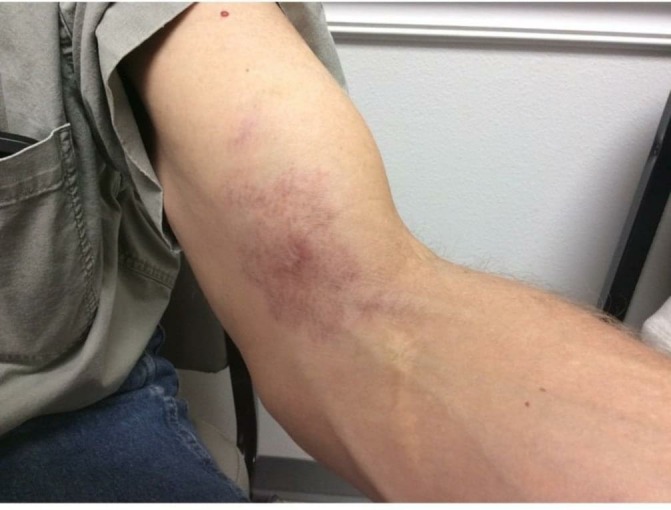
6 Month follow-up from initial photograph showing improvement with treatment.
Figure 4.
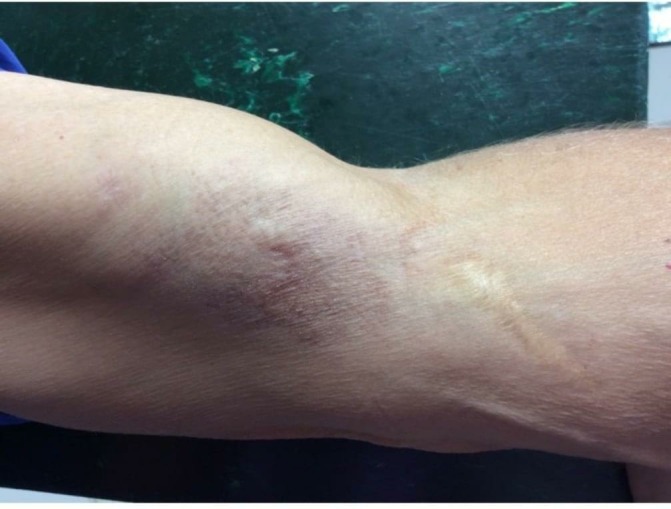
Final photograph that shows almost complete resolution of lesion.
Discussion
Introduction
Leprosy is a chronic, infectious, granulomatous disease caused by the intracellular bacillus M. leprae that infects macrophages and Schwann cells.2–6 These bacteria survive in cooler parts of the body leading to damage of the extremities, ears and face.6 The majority of cases are seen in tropical and sub-tropical regions of Southeast Asia, sub-Saharan Africa, Latin America and the Caribbean. In 2012, new cases of leprosy world-wide numbered 232 857 with 200 seen in the USA.6–8 Leprosy infection is difficult to diagnose due to its long incubation period of 8–12 years and lack of a diagnostic test able to identify infection before clinical symptoms present.7 9 Patients’ genetics appear to be a strong indicator of susceptibility to M. leprae with about 95% of the population being naturally resistant to leprosy.9 Transmission of the bacteria occurs often through close contact with infected persons by inspiration of aerosolised bacteria and through skin erosions.2 5 6 9 In the southern USA, M. leprae can be spread by armadillos shedding the bacilli into the environment through their bodily secretions. The bacteria are able to survive up to 8 months in the environment.9
Reservoir in the USA
Leprosy is uncommon in the USA, but around 200 new registered cases in the USA every year.6–8 About 60%–75% of new cases seen in the USA occur in immigrants, especially those from the Federated States of Micronesia or Marshall Islands, or in people who have close contact with these immigrants.6–8 10 This is thought to be the main form of leprosy transmission in areas such as New York City with high numbers of immigrants and international travellers but no armadillo exposure.11 12 In the last 20 years, the largest number of new leprosy cases has been reported in the Gulf Coast states.4 6 8 Evidence has shown that leprosy in these regions is most likely due to zoonotic transmission from the nine banded armadillo (Dasypus novemcinctus).9 The majority of cases in Louisiana, Texas and Florida are infected with the 3 l-2-v1 strain of M. leprae, the strain found most often in armadillos.9 13 The armadillo population has been expanding to the north and east since the 19th century which may explain why some leprosy cases have been reported in patients from Canada who have not travelled outside of North America.13 14 Exposure maybe not have to be through direct contact with armadillos but may instead occur via exposure to infected soil or sphagnum moss containing M. leprae shed by the armadillo.6 13
Presentation
Patients infected with M. leprae often present with non-distinct progressive hypopigmented or erythaematous plaques on their forehead, arms, back and legs. These non-specific signs can make initial diagnosis difficult.2 6 Additional symptoms such as progressive lesional numbness, burning pain, palpable peripheral nerve hypertrophy and muscle weakness are often pathognomonic for leprosy, but are only present in 30%–50% of patients.2 6 8 13 14 Chronic arthritis along with recurrent fever are also presenting symptoms in some leprosy patients. These can also be seen with lupus erythaematosus, rheumatoid arthritis and antiphospholipid syndrome but these symptoms usually abate with treatment for the underlying M. leprae infection.4 12 15 In tuberculoid leprosy, the patient often has a small number of hypopigmented lesions that are characterised by well-defined borders, possible numbness in lesions and alopecia in older lesions.2 15 In lepromatous leprosy, the patient will have many erythaematous to hypopigmented lesions with indistinct edges that increase in size and coalesce. Nerve damage is often more pronounced with bilateral loss of sensibility in the ‘boot’ or ‘glove’ distribution.2
Diagnosis of chronic infection
If the patient lacks the symptoms of numbness and palpable peripheral nerve hypertrophy, symptoms can mimic many other much more common diseases including sarcoidosis, lupus vulgaris, lymphoma, leishmaniosis, tertiary syphilis, mycosis fungoides, diabetic neuropathy and Lyme disease.6 8 Once other possible diagnoses are ruled out with blood work and imaging, a biopsy of the advancing border of the lesion and of the hypertrophic peripheral nerve should be taken.6 H&E staining shows granulomas as well as epithelioid histiocytes filled with intracellular bacilli.16 The intracellular organisms are acid-fast.11 14 The tissue should also treated with Anti-phenolic glycolipid antigen (PGL)-1 and PCR testing that targets the M. leprae-specific RLEP gene.2 13 Electroneuromyography, ultrasound or MRI of the nerve trunks can also be used to evaluate nerve damage in the neural forms.2 Accurate diagnosis of M. leprae infection is often the combination of high suspicion based on history, clinical presentation of granulomatous lesions and demonstration of acid-fast bacteria. The case is then reported to the National Hansen’s Centre that provides patients with treatment.6
Treatment
Patients are often started on a 12–24 month course of a combination of rifampin (600 mg/month for 6 months) and dapsone (100 mg/day) with some patients also taking clofazimine (100 mg/day) depending on the severity of disease.4 13 Rifampin, ofloxacin or minocycline may be used if the patient is unable to tolerate standard therapy.2 5 6 Multidrug therapy is often recommended to increase treatment effectiveness as well as decrease the likelihood of drug resistance developing.2 5 Increased release of mycobacterial antigens can occur during treatment. This can cause adverse reactions including oedema, exuberant erythaema, ulcerative skin lesions, erythaema nodosum, neuritis and constitutional symptoms.8 16 Steroids, thalidomide and methotrexate can be used to calm this inflammatory response.4 It is for these reasons that patients must receive blood work and follow-up every 3–4 months to monitor progress.
Learning points.
Although rare leprosy should be included on the differential list of any patient residing in the southern USA with compatible clinical signs.
Patients with compatible clinical signs to leprosy should be quizzed extensively about their travel history and their exposure to wildlife especially armadillos.
Leprosy is very difficult to diagnose and treat so it is important if it is highly suspected to contact the National Hansen Center for expert advice.
Footnotes
Contributors: CML is the main author for the case write up, body of the article and research for this case. KBH: saw, diagnosed, treated and continues to follow-up patient’s progress. She also took the photographs of the lesion that are displayed in this article. She is responsible for gaining patient’s permission and waver for this case report.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Wichitwechkarn J, Karnjan S, Shuntawuttisettee S, et al. . Detection of Mycobacterium leprae Infection by PCR. Journal of Clincial Microbiology 1995;33:45–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cruz R, Bührer-Sékula S, Penna MLF, et al. . Leprosy: current situation, clinical and laboratory aspects, treatment history and perspective of the uniform multidrug therapy for all patients. An Bras Dermatol 2017;92:761–73. 10.1590/abd1806-4841.20176724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Silva CAM, Belisle JT. Host Lipid Mediators in Leprosy: The Hypothesized Contributions to Pathogenesis. Front Immunol 2018;9 10.3389/fimmu.2018.00134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Labuda SM, Schieffelin JS, Shaffer JG, et al. . Hansen’s Disease and Rheumatoid Arthritis Crossover of Clinical Symptoms: a case series of 18 patients in the United States. Am J Trop Med Hyg 2017;97:1726–30. 10.4269/ajtmh.17-0197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mohanty PS, Bansal AK, Naaz F, et al. . Ribonucleotide reductase as a drug target against drug resistance Mycobacterium leprae : A molecular docking study. Infection, Genetics and Evolution 2018;60:58–65. 10.1016/j.meegid.2018.02.013 [DOI] [PubMed] [Google Scholar]
- 6. Hundemer M, Wan CR. Disfiguring facial lesions and loss of sensation. JAAPA 2018;31:27–9. 10.1097/01.JAA.0000526775.98315.5a [DOI] [PubMed] [Google Scholar]
- 7. Maros LA. Indigenous Cases of Leprosy (Hansen’s Disease) in Southern Mississippi. Journal Mississippi State Medical Association 2015;56:188–91. [PubMed] [Google Scholar]
- 8. Leon KE, Jacob JT, Franco-Paredes C, et al. . Delayed Diagnosis, Leprosy Reactions, and Nerve Injury Among Individuals With Hansen’s Disease Seen at a United States Clinic. Open Forum Infect Dis 2016;3:ofw063–4. 10.1093/ofid/ofw063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sharma R, Singh P, Loughry WJ, et al. . Zoonotic Leprosy in the Southeastern United States. Emerg Infect Dis 2015;21:2127–34. 10.3201/eid2112.150501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cardenas VM, Orloff MS, Kaminaga J, et al. . Tuberculosis and leprosy infections in the Marshallese population of Arkansas, USA. Lepr Rev 2016;87:109–12. [PubMed] [Google Scholar]
- 11. Rendini T, Levis W. Autochthonous Leprosy without Armadillo Exposure, Eastern United States. Emerg Infect Dis 2017;23(11):1928 10.3201/eid2311.171145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rendini T, Levis W. Autochthonous leprosy in the eastern United States is from international migration, not from armadillos. JAAD Case Rep 2017;3:370 10.1016/j.jdcr.2017.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bonnar PE, Cunningham NP, Boggild AK, et al. . Leprosy in Nonimmigrant Canadian Man without Travel outside North America, 2014. Emerg Infect Dis 2018;24:165–6. 10.3201/eid2401.170547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Villada G, Zarei M, Romagosa R, et al. . Autochthonous borderline tuberculoid leprosy in a man from Florida. Lepr Rev 2016;87:101–3. [PubMed] [Google Scholar]
- 15. Virk A, Pritt B, Patel R, et al. . Mycobacterium lepromatosis Lepromatous Leprosy in US Citizen Who Traveled to Disease-Endemic Areas. Emerg Infect Dis 2017;23:1864–6. 10.3201/eid2311.171104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cuevas J, Rodríguez-Peralto JL, Carrillo R, et al. . Erythema nodosum leprosum: reactional leprosy. Semin Cutan Med Surg 2007;26:126–30. 10.1016/j.sder.2007.02.010 [DOI] [PubMed] [Google Scholar]


