Abstract
We present the case of a previously fit and well 30-year-old primiparous woman who developed Hamman’s syndrome during the second stage of labour. This is an unusual and peculiar condition, characterised by spontaneous pneumomediastinum and subcutaneous emphysema. The rarity of the condition means it can easily be misdiagnosed. Its exact aetiology is unknown, but it is believed that extreme Valsalva manoeuvre during prolonged or difficult labours may contribute to its development. Chest X-ray is the first investigation of choice in this presentation, followed by CT scanning. Fortunately, it is usually a benign condition that can be managed conservatively and resolved quickly, with no long-term effects and low risk of recurrence in future pregnancies. We are pleased to say that this case does not differ in this respect. It is, however, important to rule other more serious pathologies that present in a similar way, for example, Boerhaave syndrome, pneumothorax or pulmonary embolism.
Keywords: pregnancy, obstetrics and gynaecology
Background
The occurrence of spontaneous pneumomediastinum has been described in several populations, notably in young men, and fortunately it tends to follow a benign course.1 2 However, the rarity of the condition means it is easily misdiagnosed, which can lead to mismanagement of patients. Louis Hamman first reported this syndrome in 1945, documenting a case series.3 The incidence in the obstetric population is estimated to be around 1 in 100 000 vaginal deliveries, affecting nulliparous women in particular. Common features of its presentation, such as chest pain, dysphagia, dysphonia, shortness of breath and swelling may be confused with other more serious diseases. Treatment for Hamman’s syndrome is usually conservative.
Case presentation
The patient has given informed consent for the publication of this case report, which has been written in accordance with the Consensus-based Clinical Case Reporting Guidelines (http://www.equator-network.org/). A 30-year-old nulliparous woman was admitted at 41 weeks and 4 days for induction of labour due to mild pregnancy-induced hypertension. She had a raised body mass index of 32, with no other risk factors antenatally. She received 10 mg dinoprostone vaginally and 6 hours later, the patient began involuntary pushing. On vaginal examination, her cervix was found to be 9 cm dilated. Shortly after this, as she continued pushing, she showed clinical signs of full dilatation, and her midwife noticed that she had developed orbital swelling, accompanied by a change in the tone of her voice. She was reviewed by the obstetric team, and the initial impression was a drug reaction of unknown source. The facial swelling progressed, and the patient was reviewed by the anaesthetist on call, who also felt her symptoms could be secondary to an adverse reaction. There was no neurological, cardiovascular or respiratory compromise. She was given chlorphenamine, and observation of the patient continued. After approximately 2 hours of active pushing, delivery of her baby was successfully undertaken with Neville Barnes forceps for failure to progress. Her vital signs remained normal throughout labour and the puerperium. Following delivery, a further examination by a different member of the obstetric team revealed extensive swelling of the face and neck extending to the upper thorax, associated with crepitation on palpation, suggestive of subcutaneous emphysema. Only then was Hamman’s syndrome suspected. Medical and general surgical reviews were requested.
Investigations
A chest radiograph was performed which showed bilateral subcutaneous emphysema and some bronchial wall thickening, possibly related to inflammatory disease. The radiograph did not show evidence of pneumothorax, and no clear features of pneumomediastinum could be distinguished. Face, neck and chest CT were diagnostic: this showed the striking appearance of subcutaneous emphysema extending from the scalp to the upper abdomen and the presence of pneumomediastinum (figures 1–5). Again, no significant pneumothorax was seen.
Figure 1.
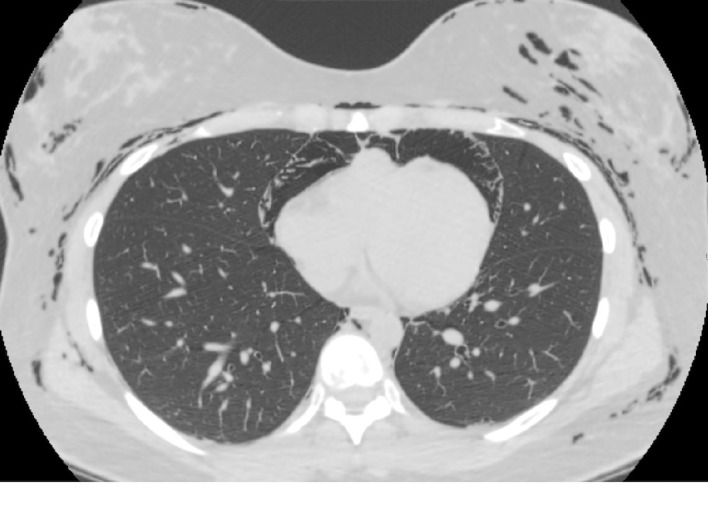
CT scan of the chest at cardiac level showing pneumomediastinum and subcutaneous emphysema.
Figure 2.
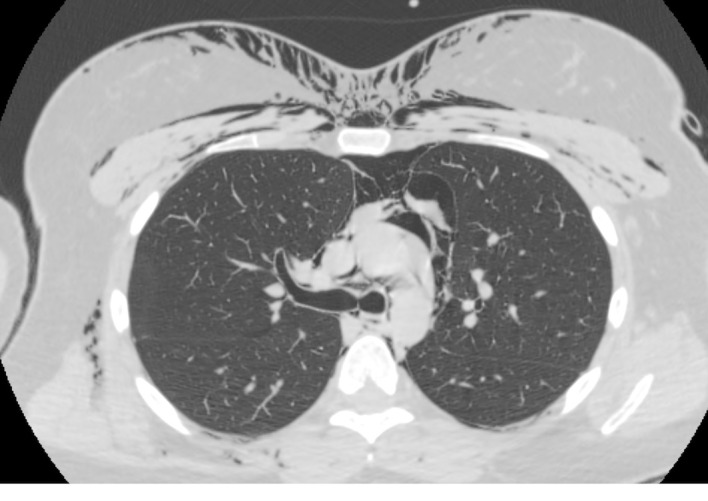
CT scan of the chest at the level of the carina showing pneumomediastinum and subcutaneous emphysema.
Figure 3.
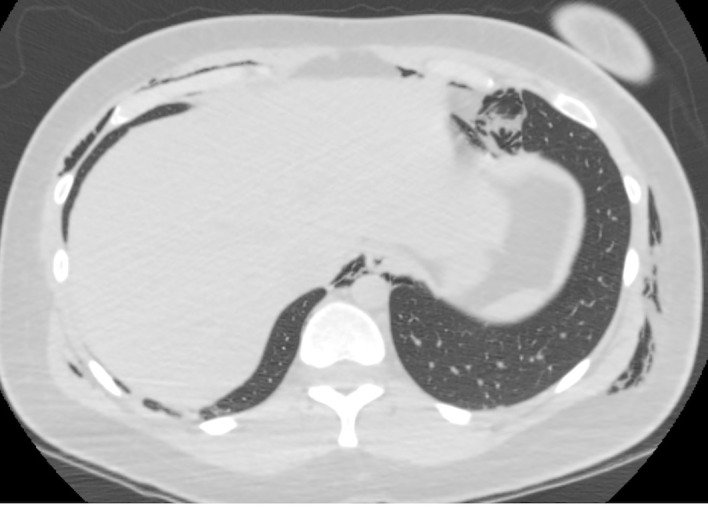
CT scan showing pneumomediastinum and subcutaneous emphysema at the level of the lower chest.
Figure 4.
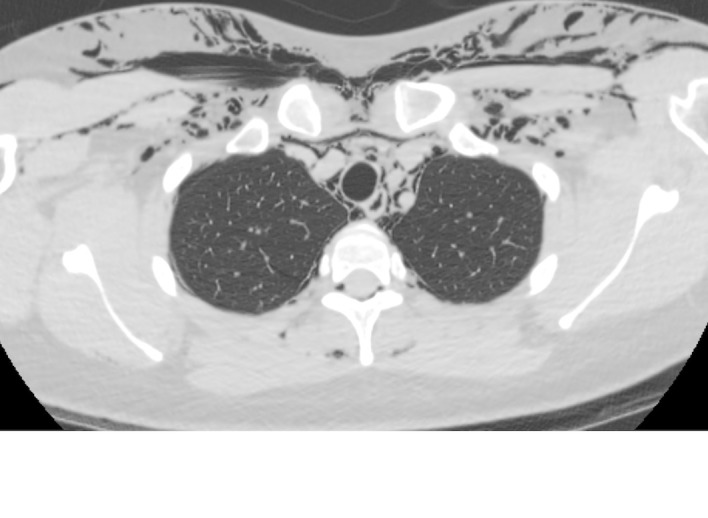
CT scan showing pneumomediastinum and subcutaneous emphysema and the level of the upper chest.
Figure 5.
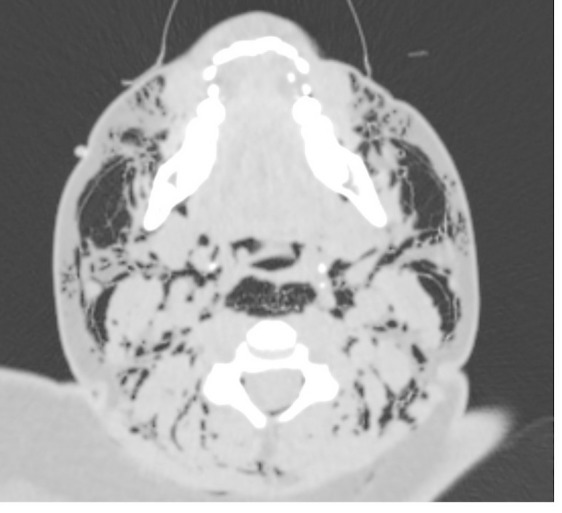
CT scan showing subcutaneous emphysema at the level of the neck.
Differential diagnosis
This patient’s symptoms were first thought to be secondary to an adverse reaction due to the facial swelling. The most common presenting symptom in Hamman’s syndrome is chest pain, therefore, it is important to rule out potentially lethal conditions, such as pulmonary embolism, amniotic fluid embolism, aortic dissection, myocardial infarction or pneumothorax. Another cause of pneumomediastium is oesophageal rupture (also known as Boerhaave syndrome) and if diagnosed requires prompt surgical referral.
Treatment
The patient was treated conservatively with analgesia and followed up in partnership with the thoracic surgery team. She remained clinically stable and was discharged home 3 days after delivery once symptoms had improved.
Outcome and follow-up
She was seen to have made a complete recovery at a 6-week postnatal follow-up appointment with the thoracic team.
Discussion
Hamman’s syndrome is considered to be an idiopathic condition, however, some theories have been formulated to explain its occurrence. Inevitably, some Valsalva pressure is employed during labour when coughing, vomiting, screaming or pushing. Intra-alveolar pressure is raised, altering the pressure gradient between the alveoli and the lung interstitium, which can lead to alveolar rupture. Air tracks along the bronchovascular connective tissue into the mediastinum and then onwards through the fascial planes into subcutaneous and retroperitoneal tissues. This is known as the Macklin effect.4 It follows that labours which are prolonged or difficult, due to nulliparity among other factors, would involve greater physical effort and pressure changes, placing these women at higher risk. Other precipitants of spontaneous pneumomediastinum have also been described, including diabetic ketoacidosis,5 oral sex,6 asthma, illicit drug use, ethanol intoxication7 and self-induced oral injury.8 These conditions also produce symptoms that involve Valsalva pressure, such as chronic cough or vomiting. Thoracic endometriosis is known to place women at risk of catamenial pneumothorax,9–11 which may occur through alveolar rupture. Theoretically, these patients may then be at a higher risk of Hamman’s syndrome. However, alternative mechanisms for the creation of pneumothoraxes with thoracic endometriosis have been proposed, such as the passage of air through diaphragmatic fenestrations created by endometriosis. This would make the condition an entirely different entity. Very often a specific trigger cannot be found. Macia et al looked at a series of 41 cases of spontaneous pneumomediastinum and could not identify a precipitating factor in 51%.2
We identified 14 case reports of pregnancy-related Hamman’s syndrome available through Pubmed over the past 20 years.12–25 The age range of the patients was 21 to 32 years old. Thirteen of these women were nulliparous; there was one case reported by Khurram et al involving a patient during her second labour. All of the patients reached the second stage of labour. The duration of second stage was documented in 11 cases, and out of these, 10 lasted 80 min or more. Symptoms were noted in all but three cases, including chest pain (79%), facial or neck swelling (57%), dyspnoea (38%), crepitus (93%), tachycardia (29%), dysphonia (29%). This is in line with a case review by Macia et al, which reports a wide variety of symptoms, such as finger numbness and sore throat but with chest pain as the most common symptom found in 85% of patients and crepitus found in 71%.2 Of the cases we looked at, only one patient was documented to be undergoing induction and one other patient was documented to have received an epidural, eliminating these as possible risk factors. None of the patients or their babies suffered any long-term complications.
Diagnosis was delayed in our case as the patient’s first symptom of facial swelling was attributed to an adverse reaction, despite normal observations and the absence of other features of hypersensitivity. Subcutaneous crepitation was the crucial examination finding that finally guided us to the correct diagnosis. The dysphonia our patient experienced was most likely caused by emphysema reaching the larynx, affecting the voice’s quality. This is not a very common symptom in Hamman’s syndrome; it was seen in 4 out of the 14 cases we reviewed.9–12 Overall, we believe that there may be many cases of mild Hamman’s syndrome which are undiagnosed, as it is associated with non-specific symptoms and generally does not present a threat to the patient.
The chest radiograph is accepted as the primary investigation which detects pneumomediastinum and subcutaneous emphysema. Lateral views increase the visibility of the anterior mediastinum. However, chest CT remains the gold standard for detecting pneumomediastinum.26 In our case, the chest radiograph showed bilateral subcutaneous emphysema and some bronchial wall thickening, but there was no clear evidence of pneumomediastinum. CT of the face, neck and chest was eventually diagnostic (figures 1–5).
Currently, there is no guidance for recommended management of cases of Hamman’s syndrome or prevention in future pregnancies. The overall general opinion suggests conservative management for uncomplicated cases, which are the majority, and supportive measures with oxygen and analgesics.2 In the rare event of complications with these patients, we believe it is important to request early involvement of the local thoracic team for joint assessment and management. While recurrence is considered unlikely (we have not found any reported in the literature over the past two decades), in future pregnancies expectant management and limiting the duration of the second stage with instrumental delivery may be suggested, aiming to minimise barotrauma; however, this is not evidence based. Systematic studies are needed to identify factors that may increase the risk of developing Hamman’s syndrome, for example birth weight, position during delivery or analgesia used during labour. The possibility of such studies occurring is, of course, limited by the low incidence of the condition.
As expected, our patient and her baby were both well and discharged home 3 days after delivery. The same outcome was seen for all 14 patients we reviewed; there were no complications and hospital stay ranged from 1 to 6 days.
We believe it is important to raise awareness of this syndrome among professionals involved in the care of women in labour, with the hope of improving diagnosis and determining the real incidence of the syndrome.
Learning points.
Hamman’s syndrome is a rare but potentially underdiagnosed complication of the second-stage of labour.
More cases may be identified with raised awareness, allowing clinicians to have a high index of suspicion when patients present with non-specific symptoms during the second stage. These patients should be examined for surgical emphysema, as this is present in most cases.
Serious conditions, such as Boerhaave syndrome, pulmonary or amniotic fluid embolus and aortic dissection should be excluded.
Treatment is usually conservative and symptoms improve in less than 1 week; however, liaison with the thoracic surgical team may be necessary if symptoms are significant or in the rare event of complications.
Footnotes
Contributors: REJ is primary author and collected images. CB primarily performed literature search. SH proofread and assisted in revision of text. ELC identified the case, supervised, assisted in literature search and proofread the text.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Mondello B, Pavia R, Ruggeri P, et al. Spontaneous pneumomediastinum: experience in 18 adult patients. Lung 2007;185:9–14. 10.1007/s00408-006-0002-7 [DOI] [PubMed] [Google Scholar]
- 2. Macia I, Moya J, Ramos R, et al. Spontaneous pneumomediastinum: 41 cases. Eur J Cardiothorac Surg 2007;31:1110–4. 10.1016/j.ejcts.2007.03.008 [DOI] [PubMed] [Google Scholar]
- 3. Hamman LV. Spontaneous mediastinal emphysema. 64 Baltimore: Bulletin of the Johns Hopkins Hospital, 1939:1–21. [Google Scholar]
- 4. Macklin MT, Macklin- CC. Malignant interstitial emphysema of the lungs and mediastinum as an important occult complication in many respiratory diseases and other conditions: an interpretation of the clinical literature in light of laboratory experiment. Medicine 1944;23:281–358. [Google Scholar]
- 5. Weathers LS, Brooks WG, DeClue TJ. Spontaneous pneumomediastinum in a patient with diabetic ketoacidosis: a potentially hidden complication. South Med J 1995;88:483–4. [DOI] [PubMed] [Google Scholar]
- 6. Sam Flatman,1 Edwin Morrison,1 and Maqsood Elahi1 J. Spontaneous Pneumomediastinum Associated with Sex. J Radiol Case Rep 2010;4:25–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kelly S, Hughes S, Nixon S, et al. Spontaneous pneumomediastinum (Hamman’s syndrome). Surgeon 2010;8:63–6. 10.1016/j.surge.2009.10.007 [DOI] [PubMed] [Google Scholar]
- 8. López-Peláez MF, Roldán J, Mateo S. Cervical emphysema, pneumomediastinum, and pneumothorax following self-induced oral injury: report of four cases and review of the literature. Chest 2001;120:306–9. 10.1378/chest.120.1.306 [DOI] [PubMed] [Google Scholar]
- 9. Maniglio P, Ricciardi E, Meli F, et al. Catamenial pneumothorax caused by thoracic endometriosis. Radiol Case Rep 2018;13:81–5. 10.1016/j.radcr.2017.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Visouli AN, Darwiche K, Mpakas A, et al. Catamenial pneumothorax: a rare entity? Report of 5 cases and review of the literature. J Thorac Dis 2012;4 Suppl 1(Suppl 1):17–31. 10.3978/j.issn.2072-1439.2012.s006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marjański T, Sowa K, Czapla A, et al. Catamenial pneumothorax - a review of the literature. Kardiochir Torakochirurgia Pol 2016;13:117–21. 10.5114/kitp.2016.61044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kouki S, Fares AA. Postpartum spontaneous pneumomediastinum ’Hamman’s syndrome'. BMJ Case Rep 2013;2013:bcr2013010354 10.1136/bcr-2013-010354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yadav Y, Ramesh L, Davies JA, et al. Gross spontaneous pneumomediastinum (Hamman’s syndrome) in a labouring patient. J Obstet Gynaecol 2008;28:651–2. 10.1080/01443610802378058 [DOI] [PubMed] [Google Scholar]
- 14. Zapardiel I, Delafuente-Valero J, Diaz-Miguel V, et al. Pneumomediastinum during the fourth stage of labor. Gynecol Obstet Invest 2009;67:70–2. 10.1159/000162103 [DOI] [PubMed] [Google Scholar]
- 15. Majer S, Graber P. Postpartum pneumomediastinum (Hamman’s syndrome). CMAJ 2007;177:32 10.1503/cmaj.061581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wijesuriya J, Van Hoogstraten R. Postpartum Hamman’s syndrome presenting with facial asymmetry. BMJ Case Rep 2015;2015:bcr2015213397 10.1136/bcr-2015-213397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Elshirif A, Tyagi-Bhatia J. Postpartum pneumomediastinum and subcutaneous emphysema (Hamman’s syndrome). J Obstet Gynaecol 2016;36:281–2. 10.3109/01443615.2015.1060205 [DOI] [PubMed] [Google Scholar]
- 18. Cho C, Parratt JR, Smith S, et al. Spontaneous pneumomediastinum (Hamman’s syndrome): a rare cause of postpartum chest pain. BMJ Case Rep 2015;2015:bcr1220103603 10.1136/bcr-12-2010-3603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Khurram D, Patel B, Farra MW. Hamman’s Syndrome: A Rare Cause of Chest Pain in a Postpartum Patient. Case Rep Pulmonol 2015;2015:201051 10.1155/2015/201051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kandiah S, Iswariah H, Elgey S. Postpartum pneumomediastinum and subcutaneous emphysema: two case reports. Case Rep Obstet Gynecol 2013;2013:1–3. 10.1155/2013/735154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kuruba N, Hla TT. Postpartum spontaneous pneumomediastinum and subcutaneous emphysema: Hamman’s syndrome. Obstet Med 2011;4:127–8. 10.1258/om.2011.110038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dilley JW. Postpartum hearing loss: an unusual presentation of Hamman’s syndrome. J Obstet Gynaecol 2011;31:268–9. 10.3109/01443615.2011.552745 [DOI] [PubMed] [Google Scholar]
- 23. Tixier H, Rattin C, Dunand A, et al. Hamman’s syndrome associated with pharyngeal rupture occurring during childbirth. Acta Obstet Gynecol Scand 2010;89:407–8. 10.3109/00016340903410881 [DOI] [PubMed] [Google Scholar]
- 24. Mahboob A, Eckford SD. Hamman’s syndrome: an atypical cause of postpartum chest pain. J Obstet Gynaecol 2008;28:652–3. 10.1080/01443610802378066 [DOI] [PubMed] [Google Scholar]
- 25. Bonin MM. Hamman’s syndrome (spontaneous pneumomediastinum) in a parturient: a case report. J Obstet Gynaecol Can 2006;28:128–31. 10.1016/S1701-2163(16)32056-4 [DOI] [PubMed] [Google Scholar]
- 26. Kaneki T, Kubo K, Kawashima A, et al. Spontaneous pneumomediastinum in 33 patients: yield of chest computed tomography for the diagnosis of the mild type. Respiration 2000;67:408–11. 10.1159/000029539 [DOI] [PubMed] [Google Scholar]


