Abstract
Telomere length (TL) has long been associated with aging, as telomeres serve as protective caps of chromosomes, and are thus deeply involved in the preservation of genome integrity and are vital to cellular functions. Traditionally, a strong link connects aging and infertility in both sexes, with an earlier onset in females. Over the past decade, telomeres have attracted increasing attention due to the role they play in fertility. In this review, we investigated the potential positive or negative association between relative TL and different factors of female and male infertility. A systematic search of the PubMed database was conducted. Out of the 206 studies identified, 45 were reviewed as they fulfilled the criteria of validity and relevance. Following an analysis and a comparison of the study outcomes, several clear trends were observed. The majority of female infertility factors were associated with a shorter TL, with the exception of endometriosis, premature ovarian failure and clear cell carcinoma that were associated with a longer TL and polycystic ovary syndrome (PCOS), which revealed conflicting results among several studies, leading to ambiguous conclusions. Male infertility factors were associated with a shorter TL. Although this review can provide an outline of general trends in the association of TL with infertility factors, further epidemiological and original research studies are required to focus on investigating the basis of these varying lengths of telomeres.
Keywords: aging, female infertility, fertility, male infertility, telomere shortening
1. Introduction
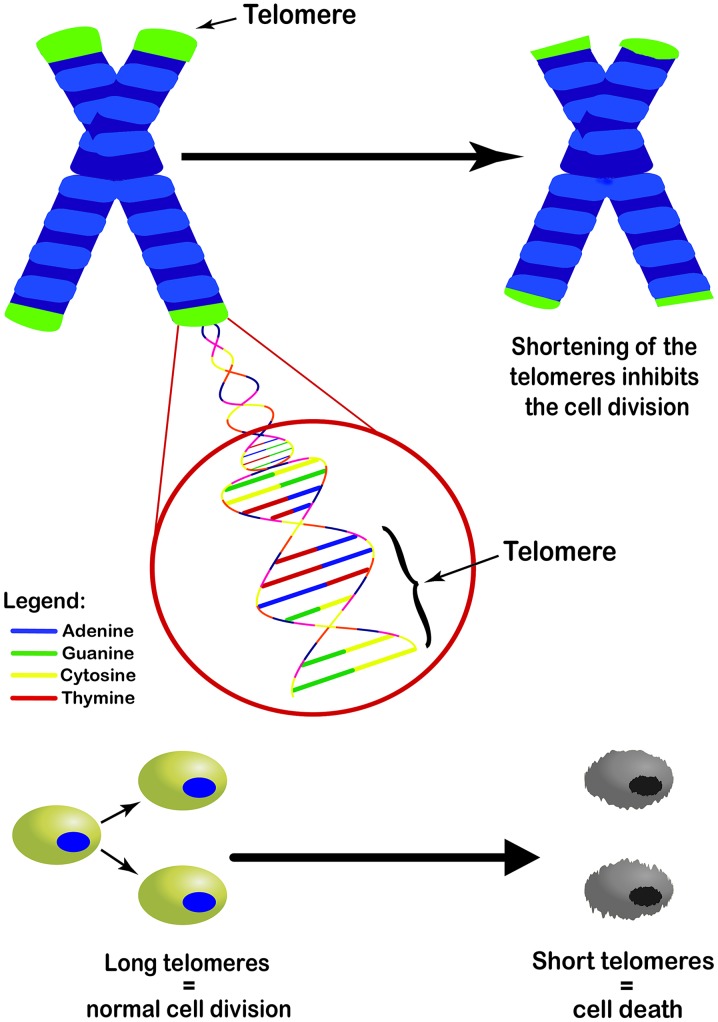
The irreversible process of aging is marked by a decline in the body's physiological functions and adaptation ability. One of the systems impaired by this process is the reproductive system. According to recent publications, infertility affects an estimated 15% of couples globally (1). In 2016, almost 66,000 births in the United States were achieved from the assisted reproduction techniques application (2). According to the statistics of the Society for Assisted Reproductive Technology (SART), 241,570 cycles for egg retrieval, frozen embryo transfer and frozen egg thawing were performed (3). Despite common belief that infertility factors are usually associated with only the female sex, male factors have been found to be solely responsible for 20-30% of infertility cases and to contribute to 50% of the cases overall (4). The deleterious effects of reproductive aging affect both males and females; however, an earlier onset is observed among females, as a sharp decrease in fertility is reported after the age of 35 (5). The process of aging is strongly influenced by genetics, as well as environmental and lifestyle factors. Currently, the aging process is divided into two major components, biological and chronological age, which can differ for the same individual. Biological aging can be calculated by telomere length (TL) and DNA methylation levels (6). Telomeres are the end parts of linear chromosomes and consist of many tandem repeats of 5′-TTAGGG-3′. When cells divide, they are unable to replicate approximately 50 base pairs to the end of each chromosome. This leads to the progressive shortening with each round of cell division (7) (Fig. 1), resulting in cell proliferation arrest and cell senescence. This mechanism is the leading cause of aging and age-related chronic diseases. In 2009, Blackburn, Greider and Szostak received the Nobel Prize for discovering the protective role of telomeres and the enzyme telomerase on chromosomes. These highly significant discoveries opened the way for researchers to further explore the role of telomere shortening in aging. By the construction of a biological age equation, the measuring of TL has come to be a calculating tool for the biological age of the body, including, but not limited to gonadal age (8,9) thus more accurately predicting the fertility status. Recently, a database was published named 'BIOTEL version 2.4', Telomere Length Database Project (TLDP), a semi-automated worksheet that calculates a wide range of TL statistics and it is a useful tool with applications in research on telomere biology, and in biological age estimation (10).
Figure 1.
Role of telomeres in cellular division. Every cycle of cell division leads to the progressive shortening of telomeres. Shortened telomeres ultimately are unable to divide further and are led to senescence and cell death.
Both genetic signature and external factors can decrease the gonadal pool and can thus subsequently increase male and female infertility, despite a favorable chronological age (11). Specifically, such external factors include human exposure to chemicals and pesticides used in agriculture and industry that act as endocrine disruptors (EDs) (11-15), dysregulating normal reproductive system functions (16-18), as well as drug abuse (19).
It has been claimed that epigenetic factors, such as nutrition, exercise and tobacco can also affect the rate at which telomeres shorten and the risk of developing chronic diseases. Notably, nutrition supplements have been shown to benefit the length of short telomeres (20).
A method of delaying the aging process has been proposed to be telomerase activation (21). Telomerase activation by natural molecules has been suggested to be potent in anti-aging and the treatment of related diseases. Telomerase activation can be achieved through natural molecules, synthetic molecules, and genetic manipulation and intervention. Recently, supplements and natural extracts were tested for their capacity to enhance telomerase activity (TA) in human peripheral blood mononuclear cells. It was demonstrated that formulations containing Astragalus extract activate telomerase to higher levels than the reported levels, indicating that the synergistic effects of nutrients and natural compounds can activate telomerase and can produce more potent formulations (22). One way in which telomerase can repair short or dysfunctional telomeres is by the addition of nucleotides at their ends, further stabilizing them. Indeed, TL and TA have been proposed as biomarkers of aging and studies have investigated their implications in other chronic diseases (23,24).
In an effort to identify the causes of age-related infertility, researchers have managed to link the aging process to genomic and epigenomic alterations, DNA damage and oxidative stress (25). However, several human studies, which will be further reviewed below, have attempted to investigate the role of telomeres in infertility on a wider spectrum, by indicating the relative TL in individuals with varying types of infertility.
The aim of this review was to elucidate the potential positive or negative association between relative TL and different factors of infertility, namely male factor, tubal factor, polycystic ovary syndrome (PCOS), ovarian reserve, endometriosis, anovulation, cancer related infertility and idiopathic infertility.
The Medline [Ovid MEDLINE(R) In-Process & Other Non- Indexed Citations and Ovid MEDLINE(R) (1946 to February, 2018)] electronic database was searched to detect all publications focusing on the association of TL with human female and male infertility. The comprehensive literature was searched in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement (26), using the following literature search strategies: i) Telomeres OR TL AND fertility OR infertility; ii) Male Factor: telomere AND (idiopathic male infertility OR oligospermia OR azoospermia OR hypospermia OR teratospermia OR aspermia OR asthenozoospermia OR necrozoospermia OR leukospermia); iii) Tubal Factor: Telomere AND (hydrosalpinx OR fallopian tube OR tubal factor); iv) PCOS: Telomere AND PCOS; v) Ovarian Reserve: Telomere AND (ovary OR ovarian reserve); vi) Endometriosis: Telomere AND endometriosis; vii) Anovulation: Telomere AND (anovulation OR ovulation); viii) Unknown: Telomere AND (unexplained infertility OR idiopathic infertility).
After excluding duplicates, titles and abstracts were screened for relevance, marked by a link between infertility cause and relative TL (longer or shorter). Two review authors (E. Vasilopoulos and C. Kalliora) independently scanned the title or the abstract content, or both, of every record retrieved to determine which studies should be assessed further and extracted all data. Citations in conference abstract form, review articles, animal studies, editorials, and non-English language articles were excluded.
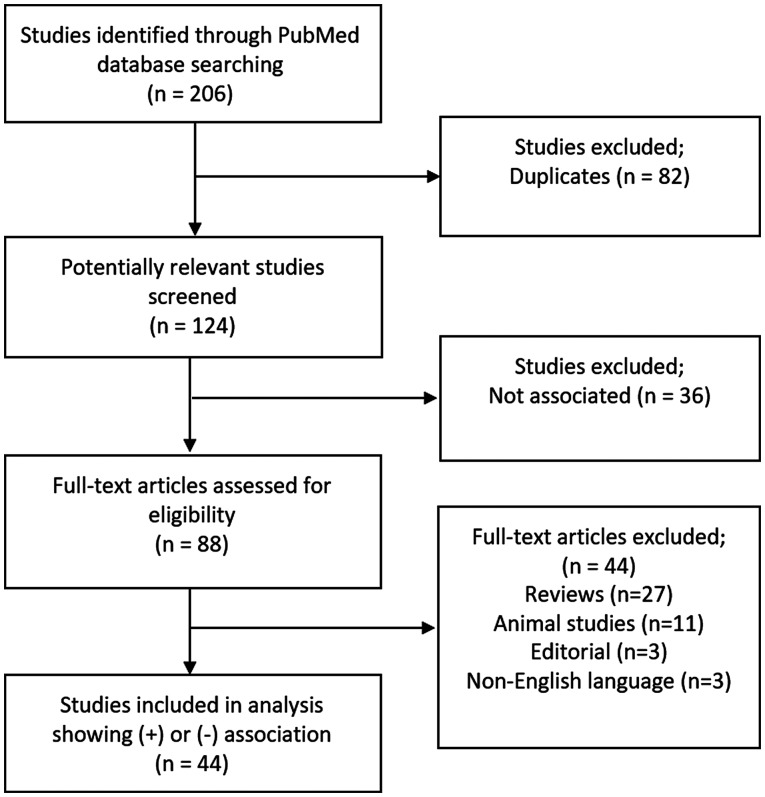
For each of these studies, it was determined whether a positive or negative association between the infertility factor they are focused on and TL is shown. This specific infertility factor was noted for each study, along with other important information (sex, relative TL and the method of measurement, population size, population age, population region, sample type and conclusion). The studies were further organized by infertility factor, allowing collective conclusions to be drawn. A total of 45 studies showing either a positive or negative association between male or female infertility and TL were included in the present review (Fig. 2).
Figure 2.
PRISMA flow diagram for the identification and selection of studies.
2. Telomere length and female infertility
It is known that shorter telomeres are associated with a range of chronic diseases, such as cardiovascular disease (CAD), stroke, cancer, arthritis, osteoporosis, diabetes type 2, hypertension, mental diseases, chronic obstructive pulmonary disease and dementia (27-31). Apart from natural, chronological aging, telomere shortening can be influenced by physical activity, body mass index (BMI), hormone replacement therapy, smoking, chronic inflammation, oxidative stress, dietary antioxidants and vitamins. Previous studies have found that women following a healthy lifestyle have longer telomeres (32).
A fundamental factor for the achievement of early pregnancy is the successful implantation of the embryo. A window of implantation is in the mid-secretory phase of the menstrual cycle, which involves the dominant action of progesterone with maximum cell differentiation. This period is also associated with the lowest endometrial telomerase activity (TA) and the shortest mean TL, suggesting a requirement of a low endometrial TA for the establishment of an early pregnancy (33,34). This suppression of TA in the endometrium of fertile women has been proposed as a necessary process in order to allow endometrial cells to undergo differentiation with cellular apoptosis/senescence required to make space for the invading embryo (33-35). The underlying mechanisms for this regulation and differential (dys)regulation are still under research.
Several studies have thoroughly investigated the association of female infertility with TL. The majority of studies exploring the association between female infertility factors and TL are outlined in Table I and have revealed a positive association, while only 3 have revealed negative association (36-38). Of the 21 positively associated studies, 11 studies demonstrated a shorter TL, while 10 demonstrated a longer TL. Specifically, a positive correlation was observed between a short TL and oocyte maturation, parity, a disrupted gap-junctional inter-cellular communication (GJIC) in stromal cells and oocytes of poor quality (39-42). Barha et al measured TL in a group of 75 Kaqchikel Mayan women over a 13-year study period. They demonstrated that women with fewer children exhibited shorter TLs than those who had more children. In the same study, Barha et al found that estradiol, the levels of which increase during pregnancy and is a potent antioxidant, protects TL (43). Czamanski-Cohen et al measured TL in lymphocytes of peripheral blood in women undergoing in vitro fertilization (IVF) techniques due to infertility; women undergoing IVF were found to have statistically significant shorter telomeres compared to the healthy controls in various phases of the menstrual cycle (44). In addition, lifestyle factors, such as employment and the work schedule are related to TL in women (45). Moreover, estradiol increases TA, maintains TL, and has been related to pregnancy complications (46).
Table I.
Studies associating telomere length with female infertility.
| Authors/(Refs.) | Sex | Association with infertility | Relative TL | Method | Population no. | Population age (years) | Population country | Sample type | Infertility factor | Conclusions |
|---|---|---|---|---|---|---|---|---|---|---|
| Hapangama et al, 2008 (51) | F | + | Ln | RT-PCR | -(n=29), endo -(n=27), non-endo |
18-46 25-44 |
UK | Endom Bx | Endo | • TL is longer in endometria of F with endo during the implantation window. Tissue TL and LTL are not correlated • LTL is (+) correlated with circulating estradiol |
| Butts et al, 2009 (52) | F | + | Sh | RT-PCR | -(n=12) OOI -(n=42), CNTL (IVF) |
30-37 23-37 |
US | G | DOR | • GTL shortening and diminished TA are associated with occult ovarian insufficiency |
| Hapangama et al, 2010 (35) | F | + | Ln | RT-PCR | -(n=38), endo -(n=35), non-endo |
18-45 25-38 |
UK | Endom Bx | Endo | • (+) correlation between endometrial TL and both glandular and stromal expression of nucleolin |
| Hanna et al, 2009 (63) | F | + | Ln | RT-PCR | -(n=34), POF -(n=95), SAB history -(n=108), HC -(n=46), fert |
21-50 24-45 17-55 37-54 |
CA | L | POF | • F with POF have longer LTL |
| Kuhn et al, 2010 (55) | F | + | Sh | Q-FISH | -(n=22), STIC -(n=134) HGSC |
N/A | US | Tubal epithelial cells | TFI/Ca | • Support of the proposal that STICs are precursors of HGSC potentially an early event in ovarian HGSC |
| Treff et al, 2011 (53) | F | + | Sh | RT-PCR | -(n=21) | 32-42 | US | Oocytes (polar bodies) | DOR | • Lower telomere DNA in aneuploid polar bodies compared to euploid polar bodies (-3.04-fold, P = 0.016) • Oocytes with telomere DNA deficiency are prone to aneuploidy development during meiosis |
| Kuhn et al, 2011 (64) | F | + | Ln | Q-FISH | -(n=219), ovarian Ca -(n=106), HGSC -(n=26), LGSC -(n=56), OCCC -(n=31), LGEC |
23-88 | US, TW | SC | Ca/DOR | • TL significantly differs by histologic type in ovarian Ca • OCCC has longer mean relative TL compared with the other histologic types |
| Valentijn et al, 2013 (62) | F | + | Ln | RT-PCR | -(n=60), pre-menopausal, normal -(n=15), post-menopausal -(n=20), endo |
23-49 46-78 18-49 |
UK | Endom cells | Endo | • Showed significantly higher expression of TA in F with endo • Longer mean TLs in F with endo |
| Turner and Hartshorne, 2013 (39) | M/F | + | Sh | Q-FISH | -(n=45), M -(n=32), F |
21-49 25-42 | UK | Sperm (semen), oocytes | C | • STL not crucial for M fert • TL reset in embryo after fertilization • TL shortening during human oocyte maturation • Oocyte-induced sperm telomere DNA modification, via a recombination-based TL increase towards the blastocyst stage |
| Cheng et al, 2013 (42) | F | + | Sh | RT-PCR | -(n=45), <38yo -(n=35), ≥38yo |
29.2+3.4 41.4+1.9 |
TW | Oocyte- cumulus complex | C | • Mature oocytes have longer TL than immature oocytes • Good-quality embryos have longer TL than poor-quality embryos (cut-off value of the T/S ratio on embryonic Day 3 was 4.235) |
| Dracxler et al, 2014 (91) | F | + | Ln | q-PCR | -(n=86), endo -(n=21), HC |
33 36 |
BZ | Lymphoc | Endo | • Lymphoc. TL is significantly longer in F with endo • High variety in lymphocyte telomere content in F with endo |
| Li et al, 2014 (58) | F | + | Sh | RT-PCR | -(n=698), PCOS -(n=611), HC |
26.42±0.19 28.77±0.27 |
CN | L | PCOS | • LTL is shorter in F with PCOS • LTL is not associated with biochemical traits in F with PCOS • (−) correlation between healthy CNTL LTL and serum DHEAS |
| Yu et al, 2014 (41) | F | + | Sh | RT-PCR | (n=6), no endo/PID) for laparoscopic tubal ligation | N/A | US | ET (Isolation of eMSC lines) | C | • Disrupted GJIC in endometrial SC reduced the mean TL by 45%. Disrupted GJIC causes ED, which is associated with abnormal uterine bleeding, failed embryonic implantation, infert, or endom Ca |
| Valentijn et al, 2015 (62) | F | + | Ln | RT-PCR, Q-FISH | -(n=70), fert (blood samples) -(n=74), HC (endom Bx) -(n=10), endo (ectopic and eutopic endom samples) -(n=8), HC in mid-secretory phase |
18-46 23-45 24-47 26-45 |
UK | L, ET | Endo | • Endom TL is longer than donor-matched LTL andis (−) correlated with serum progesterone levels • Short TL is observed in proliferating EECs in vivo and in vitro • Ectopic endom lesions have a higher epithelial telomeric signal than that in the uterine (eutopic) endom of the same individuals |
| Czamanski-Cohen et al, 2015 (44) | F | + | Sh | Q-PCR | -(n=20), IVF Tx -(n=10), HC |
29.3±4.3 28.6±3.4 |
IL | L | Infert | • Patients seeking IVF treatment exhibited shorter TL than healthy controls. |
| Pedroso et al, 2015 (38) | F | − | N/A | RT-PCR | -(n=150), PCOS -(n=124), HC |
29.36±5.18 | BZ | L | PCOS | • No difference in LTL between PCOS and healthy |
| Miranda- Furtado et al, 2016 (37) | F | − | N/A | RT-PCR | -(n=45), PCOS -(n=52), HC |
18-37 | BZ | L | PCOS | • No difference in telomere content between CNTL and PCOS • TL shortening after progressive resistance training intervention |
| Li et al, 2017 (59) | F | + | Sh | RT-PCR | -(n=65), PCOS (IVF) -(n=98), non-PCOS (IVF) |
30.3±4.3 31.7±3.8 |
CN | L, G | PCOS | • F with PCOS have shorter GTL • F with lower TA levels and shorter TL have an earlier onset of infert symptoms |
| Wei et al, 2017 (56) | F | + | Ln | RT-PCR | -75 F, PCOS -81 F, non-PCOS (IVF) |
28.36±2.55 28.09±2.26 |
CN | L, G | PCOS | • No difference in LTL between PCOS and non-PCOS • GTL is longer in women with PCOS |
| Wang et al, 2017 (57) | F | + | Ln | RT-PCR | -(n=40), PCOS -(n=35), HC |
24.78±3.54 26.33±2.15 |
CN | L | PCOS | • LTL is significantly longer in F with PCOS • (+) correlation between LTL and testosterone levels |
| Kalyan et al, 2017 (36) | F | - | N/A | RT-PCR | -(n=25), PCOS -(n=41), HC |
40.3±3.4 42.5±4.2 |
CA | L | PCOS | • No difference in LTL between PCOS and CNTL |
| Sofiyeva et al, 2017 (61) | F | + | Ln | (Telo TAGGG) PCR ELISA | -(n=14), IE -(n=17), FE -(n=16), HC |
30.42±1.36 40.82±1.36 47.9±0.96 |
TR | Eutopic endom cystic wall/ovarian cortex, venous blood | Endome- triosis | • High TA was found in the secretory phase of infertile endo women compared to that of healthy patients |
| Xu et al, 2017 (54) | F | + | Sh | RT-PCR | -(n=120), POI (IVF) -(n=279), HC (IVF) |
32.95±4.27 29.98±4.28 |
CN | L, G | Premature ovarian insufficiency | • Shorter LTL and GTL in women with POI • LTL decreases 0.067 T/S every year from age 21 up till 39 years • GTL decreases 0.089 T/S every year from age 23 to 39 years • Biochemical POI is associated with 0.69 T/S LTL reductions and 0.98T/S GTL reductions |
| Pollack et al, 2018 (40) | F | + | Sh | RT-PCR | -(n=1505), parous -(n=444), nulliparous |
20-44 | US | L | Combination | • Leukocyte T/S ratio 4.2% (95% CI: 0.9, 7.3) • TLparous < TLnulliparous • Parity: 116 fewer base pairs (95% CI: 26, 204) |
(+), positive; (−), negative; BR, Brazil; Bx, biopsy; C, combination; CA, Canada; CI, confidence interval; CN, China; CNTL, control; Ca, cancer; DHEAS, dehydroepiandrosterone sulfate; DK, Denmark; DOR, diminished ovarian reserve; ED, endometrial dysfunction; EEC, endometrial epithelial cells; ET, endometrial tissue; F, female; FE, fertile endometriosis; FISH, quantitative fluorescent in situ hybridization; G, granulosa cells; GJIC, gap-junctional intercellular communication; GTL, granulosa cell telomere length; HC, healthy control; HGSC, high-grade serous carcinoma; HGSC, high-grade serous carcinoma; IE, infertile endometriosis; IN, India; IT, Italy; IVF, in vitro fertilization; L, leukocytes; LGEC, low-grade endometrioid carcinoma; LGSC, low-grade serous carcinoma; LTL, leukocyte telomere length; Ln, longer; Lymphoc, lymphocytes; M, male; N/A, not applicable; OCCC, ovarian clear cell carcinoma; OOI, occult ovarian insufficiency; PCOS, polycystic ovary syndrome; PID, pelvic inflammatory disease; POI, primary ovarian insufficiency; RT-PCR, reverse transcription-polymerase chain reaction; SAB, spontaneous abortion; SC, stromal cells; STIC, serous tubal intraepithelial carcinoma; STL, sperm telomere length; Sh, shorter; T-T, telomere-telomere; TA, telomerase activity; TFI, tubal factor infertility; TL, telomere length; TR, Turkey; TW, Taiwan; Tx, treatment; UK, United Kingdom; US, United States; endo, endometriosis; endom, endometrium/al; fert, fertile/fertility; infert, infertile/infertility; yo, years old.
However, a late maternal age was reported in centenarians, supporting that a maternal age over 33 years is a marker of slow aging, predicting a longer life expectancy (47,48). Fagan et al and others have shown that the genetic basis between reproductive age, longevity and biological aging is strongly associated with TL (49,50).
In a nationally representative, cross-sectional study which included 1,954 women of reproductive age from the National Health and Nutrition Examination Survey, leukocyte TL (LTL) was measured by polymerase chain reaction (PCR). It was found that the women who had at least one live birth versus those that had biochemical pregnancies, spontaneous abortions, still births, or other pregnancy complications, had a shorter TL associated with accelerated cellular aging. The early decline observed in the reproductive potential of women was suggested to be associated with TL, which declines earlier in the reproductive system than it does in somatic cells (40).
According to Hapangama et al, endometrial telomerase exhibits specific expression patterns in various types of reproductive failure. Their study included endometrial biopsies during the window of implantation from women with idiopathic recurrent loss of empty gestational sacs, fetal loss after cardiac activity identification and recurrent implantation failure. No significant differences were observed in the mean TL between the groups. However, telomerase immunostaining was found to be elevated in endometrial samples from women with reproductive failure of all types, particularly those with recurrent implantation failure (51).
Additionally, Butts et al and others have revealed a short granulosa cell TL (GTL) and lower telomere DNA in patients with occult ovarian insufficiency (OOI) (52,53). Premature ovarian insufficiency (POI) has also been linked to both a shorter LTL and GTL (54). A short TL has been found in tubal epithelial cells in a study that supported the proposal that serous tubal intraepithelial carcinomas (STICs) are precursors of high-grade serous carcinoma (HGSC), potentially an early event in ovarian high-grade serous carcinogenesis (55). Another factor commonly leading to infertility is polycystic ovary syndrome (PCOS). Several studies have focused on the association between TL and PCOS. However, studies differ in the type of association and correlation. Of the 7 studies reviewed, 3 studies showed a negative association between LTL and PCOS patients (35-37) while Wei et al demonstrated significantly longer GTL but a negative association between LTL and PCOS patients (56). Further, 1 study revealed significantly longer LTL in patients with PCOS (57). Contrastingly, Li et al demonstrated that LTL was shorter in women with PCOS and that a short LTL increased the risk of disease (58). A different group demonstrated shorter GTL and an earlier onset of infertility symptoms in women with lower TA levels and PCOS (59).
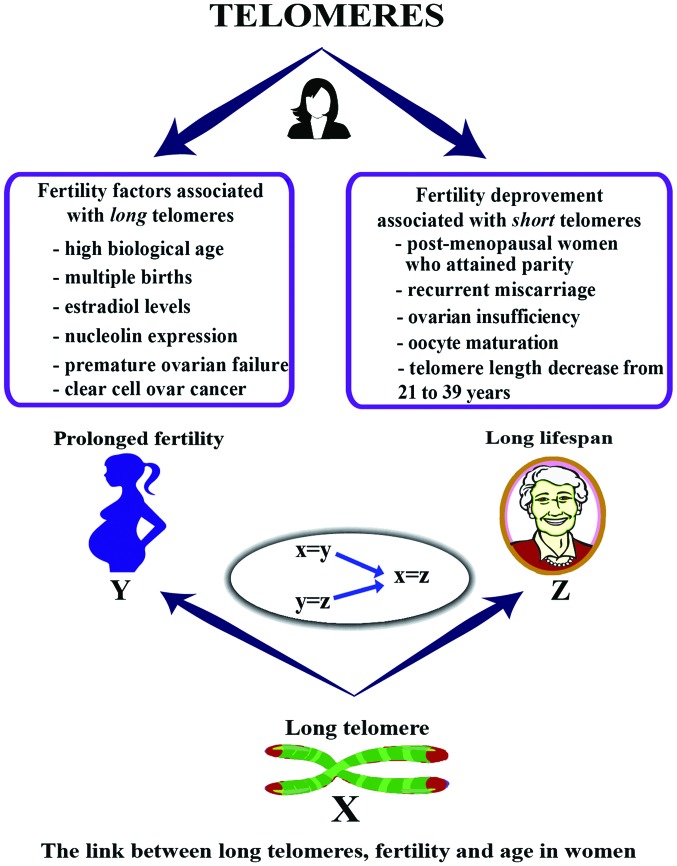
In addition, a longer TL is widely observed in women with endometriosis. The tissue-specific regulation of TL in patients with endometriosis has been suggested, as endometrial TL is significantly longer, but not is not associated with LTL, which correlates with circulating estradiol levels (33,60). Additionally, endometrial TL has been shown to exhibit a positive correlation with the glandular and stromal expression of nucleolin, involved in the exponential growth of eukaryotic cells (35). A high telomerase activity was seen in the secretory phase of infertile women with endometriosis when compared to healthy women or fertile women with endometriosis (61). Similarly, significantly higher expression of telomerase activity, longer mean TLs, lower expression of genes for steroid receptors was found in ectopic endometriosis lesions (62). Furthermore, Hanna et al demonstrated a long LTL in women with premature ovarian failure (POF) (63). Lastly, telomere lengths are varied with specific histologic types in ovarian carcinomas. Specifically, short TLs are observed in tubal epithelial cells of serus tubal intraepithelial carcinoma (STIC), an early event in ovarian high-grade serous carcinogenesis (55). However, stromal cells of clear cell carcinoma have longer mean relative TL compared to other histologic types (64). An overview of the link between TL, fertility and lifespan in women is presented in Fig. 3.
Figure 3.
Telomere length is associated with aging and several infertility factors in females. Analysis of the present literature showed certain fertility/infertility characteristics and factors are associated with long TL and others are associated with short TL. Additionally, long TL has shown to lead to prolonged fertility which in turn leads to a long lifespan. Therefore, long telomeres can lead to a longer lifespan.
The female reproductive system poses certain paradoxes. Although somatic tissues of the uterus remain well-functioning and receptive throughout the reproductive years, oocytes exhibit precocious profound aging. Meiotic dysfunction increasingly afflicts women as they age, resulting in infertility, miscarriage, and offspring with congenital abnormalities (65). For normal reproduction, the biological state of the ovum is of high importance. Apart from TL in lymphocytes, another factor that appears to be associated with fertility is the TL of oocytes.
According to the study by Kalmbach et al, reproductive aging involves declines in both oocyte number and developmental capacity. In women, the effects of reproductive aging on oocyte quality are largely explained by telomere shortening (66). Also paradoxical, is that despite the reduction of telomeres with aging, their length resets across generations via the novel mechanism involving recombination and sister chromatid exchange in the early cell cycles, and with telomerase activity after the blastocyst stage (67).
3. Telomere length and male infertility
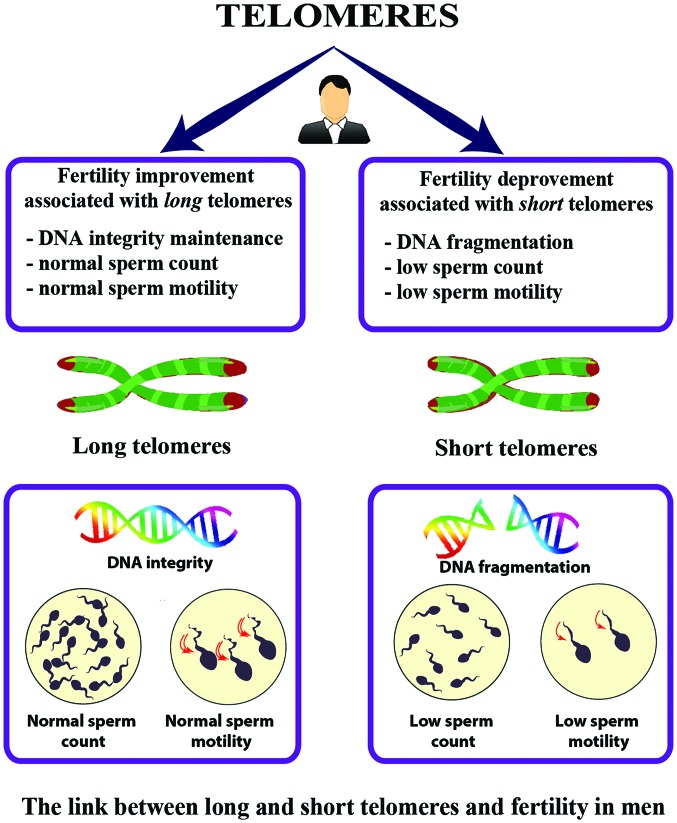
A positive association between male infertility factors and TL was observed in 19 studies (68-86) and a negative association in 2 studies (39,87) (Table II). In total, 15 of the positively associated studies detected a sperm TL (STL) association (68-70,72,74-84), while 3 studies detected an LTL association (73,85,86), and one study detected both STL and LTL (71). In several studies, shorter STL was reported in association to infertility (72,76,78,80) while Vecoli et al reported an increase in STL in areas of high environmental exposure (77). Although Turner and Hartshorne found an association between female fertility and STL, they did not find a significant association for male fertility (39). Further, a study focusing on paternal age at the time of conception reported no significant change in TL between younger and older males (87). On the other hand, paternal age at birth has been shown to be positively correlated with LTL (73) and with offspring LTL in the nurses' health study (8). An overview of the link between TL, and fertility in men is presented in Fig. 4.
Table II.
Studies associating telomere length with male infertility.
| Authors/(Refs.) | Sex | Association with infert | Relative TL | Method | Population no. | Population age (years) | Population country | Sample type | Infertility factor | Conclusions |
|---|---|---|---|---|---|---|---|---|---|---|
| Baird et al, 2006 (75) | M | + | Sh | RT-PCR, (TRF) (STELA) | (n=54), 10 samples analyzed | ~35 | UK | Sperm (semen) | C | • Human telomere truncation events occur with frequency of 3.6%, while 96.4% of telomeres at any one chromosomal end are normal • Telomere truncation in the male germline may contribute to the observed levels of aneuploidy (up to 1.55%) in human sperm range • Telomere truncation can limit replicative potential, and the subsequent loss of end capping can result in genomic instability |
| Moskovtsev et al, 2010 (74) | M | + | Disrupted T-T ixns | Q-F1SH | -(n=20), DNA damaged sperm | N/A | CA | Sperm (semen) | C | • Sperm DNA damage is correlated with disruption of the normal T-T interactions leading to possible loss of the looped chromosome configuration |
| Prescott et al, 2012 (73) | M | + | Ln | RT-PCR | N/A | N/A | US | Genomic DNA | Age | • + correlation for participant's relative LTL with paternal age at birth • Inverse relationship between maternal age at birth and relative LTL |
| Turner and Hartshorne, 2013 (39) | M/F | NIA, M | Q-FISH Sh, F | -(n=45), M -(n=32), F |
21-49 25-42 |
UK | Sperm (semen), oocytes | C | • STL not crucial for male fert • TL reset in embryo after fertilization • TL shortening during human oocyte maturation • Oocyte-induced sperm telomere DNA modification, via a recombination-based TL increase towards the blastocyst stage |
|
| Ferlin et al, 2013 (71) | M | + | Sh | RT-PCR | -(n=20), oligozoosp | 18-19 | IT | Sperm (DGC), leukocytes | Oligo zoosp | • + correlation between STL and LTL • Shorter STL in oligozoosp M • + correlation between STL and total SC • + correlation between STL and paternal age at conception |
| Thilagavathi et al, 2013 (72) | M | + | Sh | RT-PCR | -(n=32), idiopathic infert -(n=25), fert |
N/A | IN | Sperm (semen) | I MI | • Infert men had shorter STL • STL is not correlated with semen parameters |
| J0rgensen et al, 2013 (87) | M | − | N/A | Q-FISH | -(n=6), younger -(n=6), older |
31-40 43-60 |
DK | Testicular Bx | Age | • There is no difference in TL during spermatogenesis between older and younger men |
| Yan et al, 2014 (70) | M | + | Sh | RT-PCR | -(n=137), infert, non-OA -(n=125), infert, oligozoosp -(n=318), infert, normal SC |
24-42 | CN | Sperm (semen) | Azoosp | • Genetic variants such as polymorphisms in telomere- associated pathway genes affect TL and chromosomal stability • The polymorphism TERT rs2736100 was associated with male infert risk (adjusted odds ratio (OR)=0.66, 95% Cl: 0.47-0.92; Ptrend=0.011) • The polymorphism TEP1 rsl713449 was positively associated with risk of male infert (adjusted OR=1.39, 95% Cl: 1.20-1.62; Ptrend<0.001) |
| Reigh-Viader et al, 2014 (69) | M | + | Sh | TERRA and telomerase distribution | -(n=4), NO deficiency of spermatozoa due to unknown causes, seeking fert treatment -(n=l), fert, undergoing vasectomy at the time of tissue retrieval -CNTL, HeLa cells with known TL |
ES | Testicular Bx | Azoosp | • Telomere homeostasis is impaired in infert patients, shown by a decrease in TERRA levels and an alteration of the TERRA-protein component of telomerase telomeric association in primary spermatocytes. • Telomere structure and homeostasis in germ cells is compromised in infert individuals |
|
| Yang et al, 2015 (68) | M | + | Sh | RT-PCR | -(n=418), couples, idiopathic infert (IVF) | 30.3±4.0 | CN | Sperm (DGC) | C | • No significant correlation between TL and paternal age at the time of conception (rp=0.18) • + correlation between STL and total SC • + correlation between STL and paternal/maternal ages at conception • + correlation between STL and embryo quality |
| Yang et al, 2015 (84) | M | + | Sh | RT-PCR | -105 M undergoing IVF | 31.2±6.1 | CN | Sperm (DGC) | Oligo zoosp | • + correlated between STL and SC • Longer TL sperm selected for ART Tx |
| Liu et al, 2015 (82) | M | + | Sh | RT-PCR | -(n=126), idiopathic infert -(n=138), fert |
23-57 | CN | Sperm (semen) | SM/SC | • The relative sperm mean TL of infertile men was significantly shorter than the relative TL of fertile men (2.894±0.115 vs. 4.016±0.603, P=5.097×10 5) • TL is associated with SC and SM |
| Antunes et al, 2015 (83) | M | + | Lg | SCT-qPCR | -(n=10), IVF | 32-47 | US | Sperm (SU) | Age | • Longer and more variable STL associated with older age |
| Rocca et al, 2016 (81) | M | + | Sh | RT-PCR | -(n=100), normozoosp | 34.0±8.6 | IT | Sperm (DGC) | SM | • Positive association between STL and progressive motility, vitality, and protamination in normozoosp men • Shorter STL is associated with lower SM |
| Cariati et al, 2016 (79) | M | + | Sh | RT-PCR | -(n=19), oligozoosp -(n=54), normozoosp |
39.4±5.5 | IT | Sperm (DGC) | Oligo zoosp | • STL is negatively correlated with sperm diploidy. but positively correlated with SC • Oligozoosp men have shorter STL |
| Mishra et al, 2016 (80) | M | + | Sh | RT-PCR | -(n=112), infert -(n=102), fert |
31.71±4.45 32.22±4.0 |
IN | Sperm (semen) | C | • STL is shorter in infert males • Oxidative stress reduces STL |
| Biron-Shental et al, 2017 (76) | M | + | Sh | Q-FISH | -(n=16), sub-fert, ICSI -(n=10), fert |
37.4±5.0 36.5±7.0 |
IT | Sperm (semen) | C | • Shorter STL in sub-fertile men. Sperm from sub-fertile men has a higher percentage of telomere aggregates and lower TERT expressions |
| Vecoli et al, 2017 (77) | M | + | Ln | RT-PCR | -(n=112), normosp -(n=112), residents of unexposed areas |
18-42 | IT | Sperm (semen) | C | • Young male residents in areas with high environmental exposure had a significant increase in TL of their sperm |
| Lafuente et al, 2017 (78) | M | + | Sh | Q-FISH | (n=30) | N/A | ES | Sperm (pre/post DGC and SU) | C | • Exposure of sperm to increasing concentrations of H2O2 is associated with telomere shortening • STL of male partners of couples with primary infertility is lower than that of male partners of fertile couples • STL is the same pre/post sperm selection (either DGC and SU) • STL is the same in DGC and SU-selected sperm with respect to unselected sperm • STL is negatively correlated with SM and sperm concentration |
| Heidary et al, 2018 (86) | M | + | Sh | RT-PCR | -(n=30) idiopathic NOA -(n=30), HC, fert | 35.4±4.52 | IR | L | Azoosp | • Association between LTL shortening in a population of Iranian infert men affected by idiopathic azoosp |
| Yang et al, 2018 (85) | Male | + | Sh | RT-PCR | -(n=270), normosp -(n=247), OA -(n=349), non-OA |
25-38 | CN | L | Azoosp | • Significantly shorter relative LTL in men with NOA compared to those with OA or to the normozoosp controls (odds ratio [OR] 0.81,95% [Cl] 0.64-0.98 vs. OR0.92,95% Cl 0.70-1.24 vs. OR 0.99, 95% Cl 0.83-1.22), respectively) • Shorter telomeres are significantly associated with higher risk for NOA • Shorter LTL is strongly associated with NOA and defective spermatogenesis |
(+), positive; (−), negative; Assoc, association; ART, assisted reproductive techniques; Bx, biopsy; C, combination; CA, Canada; CI, confidence interval; CN, China; CNTL, control; DGC, density gradient centrifugation; DK, Denmark; ES, Spain; F, female; G, granulosa cells; ICSI, intracytoplasmic sperm injection; IMI, idiopathic male infertility; IN, India; IR, Iran; IT, Italy; IVF, in vitro fertilization; L, leukocytes; LTL, leukocyte telomere length; Ln, longer; M, male; N/A, not applicable; NO, non-obstructive; NOA, non-obstructive azoospermia; OA, obstructive azoospermia; OR, odds ratio; Q-FISH, quantitative fluorescent in situ hybridization; RT-PCR, reverse transcription-polymerase chain reaction; SC, sperm count; SCT-qPCR, stem cell transplantation quantitative polymerase chain reaction; SM, sperm motility; STL, sperm telomere length; SU, swim up; Sh, shorter; TEP1, telomerase-associated protein 1; TERRA, telomere repeat-containing RNA; TERT, telomerase reverse transcriptase; TL, telomere length; TRF, terminal restriction fragments; Tx, treatment; UK, United Kingdom; US, United States; azoosp, azoospermia; fert, fertile/fertility; infert, infertile/infertility; oligo(zoo)sp, oligo(zoo)spermia.
Figure 4.
Telomere length is associated with infertility factors in males. Long telomeres are linked to characteristics associated with increased fertility in males, such as DNA integrity, normal sperm count/motility. Short telomeres are linked to characteristics such as DNA fragmentation, and low sperm count/motility, and other male infertility factors.
Antunes et al reported a longer and more variable STL with an older age (83), and Mishra et al reported the disruption of normal telomere interactions, leading to the loss of the looped chromosomal configuration (80). In the latter study on 112 infertile men, seminal ROS and 8-isoprostane levels were reported to be increased compared to the controls, demonstrating that the infertile men experienced some form of oxidative stress. When TL was measured using reverse transcription-polymerase chain reaction (RT-PCR) and correlated with reactive oxygen species (ROS) levels, it was suggested that mild oxidative stress caused the lengthening of telomeres, whereas in severe oxidative stress, TL was shorter in comparison to TL when normal ROS levels. Therefore, mild oxidative stress and the progression of meiosis in infertile men is beneficial to TL as it correlates with their increased lengths, whereas severe oxidative stress has harmful effects (80). In a previous study, in sperm samples with high DNA damage based on the DNA fragmentation index (DFI) that underwent fluorescence in situ hybridization analysis (FISH) there was an increased number of telomere signals compared to those with a low DFI. This could be due to abnormal telomere-telomere interactions in the case of DNA damage, evident by the fact that in low DFI, almost 71% of the samples had normal telomere distribution, whereas in high DFI, this number dropped to 42% (74).
A high expression of telomerase has been reported in undifferentiated spermatogonia and the complete depletion of telomerase produces telomere shortening and the eventual loss of germ line cells, since undifferentiated spermatogonia reduce significantly (88). It has also been shown that telomerase association at telomeres is reduced in testes cells of patients with idiopathic infertility. Telomere repeat-containing RNA (TERRA) was also found to be reduced in the same study, although TL was not affected. However, the study pointed out that telomere integrity is thought to be affected with aberrant telomerase association, leading to reduced fertility (69).
Additionally, men with oligospermia have been found to have a shorter STL and a positive correlation has been found between a shorter STL and total sperm count (68,71,79), LTL and paternal age (71), and a positive correlation between a shorter STL and sperm diploidy has also been found (79). Moreover, samples that were used for IVF and had abnormal STLs did not produce a successful pregnancy, whereas samples that had STLs within the normal range had a 35.7% success rate (79). Likewise, males with azoospermia exhibited a shortened STL (69,70), as well as a shorter LTL (85,86).
Baird et al performed terminal restricted fragment (TRF) length and single TL (STELA) analysis in 54 human semen samples. The results from STELA on XpYp coincided with results from the TRF analysis and revealed that only 19% of germ cells would at any time have a full set of chromosomes with genome-wide TL and would not be truncated. Truncated telomeres may result in abnormal synapsis during meiotic cell division or in aneuploids observed in human sperm which may explain the miscarriages and abortions observed in couples trying to have a baby and the reduced fecundity of humans (75). Previous studies have shown that men with genotypes containing 2 specific genetic variants of telomerase reverse transcriptase (TERT) and telomerase-associated protein 1 (TEP1) have the chance of being infertile increased by 100%. In particular, TERT rs2736100 is negatively associated with male infertility risk, whereas TEP1 rs1713449 is positively associated with male infertility. Individuals with the TEP1 rs1713449 variant also exhibit an increased DFI. Therefore, these genetic variations play a role in the risk of male infertility (70).
In patients with decreased sperm motility, a shorter STL has been found and has also been associated with a lower sperm count, vitality and protamination (81,82), but negatively associated with DNA fragmentation in normozoospermic individuals (81). Abnormal semen quality, according to the WHO criteria, has been associated with a shorter TL than that of semen with normal parameters. Lastly, men with idiopathic male infertility have been shown to have a short STL (72).
STL is significantly affected by environmental factors, such as pollution. In a study carried out in Southern Italy, it was found that STL was higher in individuals that resided in a highly polluted area (77). The effect of polycyclic aromatic hydrocarbons (PAHs) on STL was assessed in a large study with 666 participants. The results revealed that a high concentration of urinary PAH metabolites was associated with shorter STLs. However, semen quality or sperm apoptosis did not appear to be influenced by PAHs. Moreover, benzo(α)pyrene administration also caused the shortening of STL and reduced telomerase expression in the germline in a dose-dependent manner (89). Anticancer agents have also been investigated for their effects on TL. Of the 4 agents tested, 2 alkylating agents, cisplatin and 4-hydroperoxycyclophosphamide resulted in a shorter TL, a reduced TA, reduced telomere specific fluorescence in FISH experiments, and the mRNA expression of two components of the telomerase. All of the above can lead to reduced fertility and developmental issues in offspring (90).
Another study demonstrated that aberrant fertilization and embryo cleavage were the result of fertilization with either one or both of the gametes being telomerase deficient. In these telomerase-null gametes, a small subset of telomeres was not present in some metaphase I chromosomes, suggesting that the absence of telomerase causes telomere shortening and eventually loss (84). In cases of idiopathic recurrent pregnancy loss, LTL was measured in suffering couples and compared to the controls. A statistically significantly shorter LTL was found in both males and females of the suffering couples in addition to the present TL decrease observed in males due to age. A positive correlation between the LTL and sperm DNA fragmentation index was also found, but it was not statistically significant (72).
Collectively, a prominent trend can be observed towards a shorter TL in both sperm and leukocytes, associated with the male factor in infertility. An overview of the link between TL and fertility in males is presented in Fig. 4.
4. Conclusions and future perspectives
In light of the increasing life expectancies of individuals worldwide, age-related diseases, such as infertility have progressively become a greater medical and social concern. The standard laboratory markers for age-related diseases or infertility have been insufficient. TL has long been a marker of cellular aging. The question posed is whether TL can be used to predict not only the biological, but also the reproductive age. Multiple studies have focused on elucidating this question and have demonstrated that infertility is in fact most often associated with shorter telomeres. Specifically, a trend associating female infertility factors, such as PCOS, DOR, ovarian insufficiency and tubal factor with shorter TLs was observed in oocyte granulosa cells, endometrial tissue and leukocytes. On the contrary, longer TLs were observed in endometrial biopsy, the eutopic endometrium, cyst and lymphocyte tissue in cases of endometriosis. As regards studies focusing on male infertility factors, including azoospermia, oligospermia, abnormal sperm motility and idiopathic male infertility, these demonstrated a shorter TL in either sperm or leukocyte samples obtained.
Although this study was able to identify major trends in the association of several factors of infertility and TL, limitations apply due to the relatively low number of relevant studies for each infertility factor published to date. Additionally, TL in a number of established factors of infertility has not yet been studied. Specifically, no published literature on oligospermia, hypospermia, teratosperimia, aspermia, asthenozospermia, necrozospermia, leukospermia and hydrosalpinx was found. Although this serves as a limitation, it indicates a knowledge gap where further research could be undertaken. Regardless, TL is associated with a sufficient amount of infertility factors, thus not compromising the external validity of the review.
As regards female infertility factors, ovarian HGSC, mature oocytes, low-quality embryos, multiform endometrial dysfunction, premature ovarian insufficiency, DOR, and nulliparity were all linked to a shorter TL. Conversely, all studies on endometriosis and TL agreed that TLs are longer. Additionally, one study noted that clear cell carcinoma stromal cells exhibit longer TL that other histological types. A noticeable disagreement is evident in studies exploring the TL of females with PCOS, as TL has been negatively and positively associated, and further correlated to both a shorter and longer TL in different studies. Thus, although for all other above-mentioned infertility factors a clear conclusion is drawn, for PCOS, the present evidence is inconclusive. Of the 21 studies reviewed focusing on male infertility factors, 1 study showed no association between TL in the sperm of older versus younger males, while 2 studies showed that older males had a longer relative TL and one study found STL not crucial for male fertility. Furthermore, the remaining studies were in agreement, indicating a shorter TL in infertile patients.
With recent advances in reproductive technology, we need powerful predictive biomarkers that will improve the clinical strategy in individuals with infertility. TL can be a marker used to identify the reproductive capacity. Additionally, premature senescence can be avoided by the use of drugs preventing telomere shortening, thereby increasing the reproductive capacity of patients. However, many questions must first be answered before the efficient use of such methods in a clinical setting is possible. It is critical that further studies are conducted to understand the bases of TL associations with biological aging and reproductive capacity.
The strength of this review is that, at least to the best of our knowledge, this is the first collection of the results from human epidemiological studies exploring the association of different factors of infertility and TL. Despite the present limitations, this review may be a useful aid, and the studies mentioned, may function as building blocks for further research needed to establish further associations and to determine how this knowledge can be used in medical applications.
Acknowledgments
Not applicable.
Abbreviations
- ART
assisted reproductive techniques
- GJIC
gap-junctional intercellular communication
- GTL
granulosa cell telomere length
- HGSC
high-grade serous carcinoma
- IVF
in vitro fertilization
- LTL
leukocyte telomere length
- OOI
occult ovarian insufficiency
- PCOS
polycystic ovary syndrome
- POI
primary ovarian insufficiency
- Q-FISH
quantitative fluorescent in situ hybridization
- RT-PCR
reverse transcription-polymerase chain reaction
- STIC
serous tubal intraepithelial carcinoma
- STL
sperm telomere length
- TA
telomerase activity
- TEP1
telomerase-associated protein 1
- TERT
telomerase reverse transcriptase
- TL
telomere length
- DOR
diminished ovarian reserve
Funding
This study was funded by Metabolomic Medicine S.A. and Spin-Off Toxplus S.A. and supported by the Special Research Account of University of Crete (ELKE nos. 4602, 4920 and 3963).
Availability of data and materials
Not applicable.
Authors' contributions
All the authors (EVasilopoulos, PF, CK, DF, AOD, EVakonaki, DT, DC, AMP, GG, CM, AM, DAS and AT) contributed to conceiving and designing the study. EVasilopoulos and CK searched the literature for inclusion in the study that was then checked and reviewed by AOD, EV, DT, DC, AMB, GG and CM. EVasilopoulos, PF, CK, DF, AOD, EV, DT, DC, AMB and GG drafted and wrote the manuscript. AM, DAS, AT, CM provided advice on the experimental design, interpreted the results and critically revised the manuscript. AOD, DC, AMB, DT, EV, GG designed the figures. CK and EV designed the tables. All authors have read and approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had no personal involvement in the reviewing process, or any influence in terms of adjudicating on the final decision, for this article. The other authors declare that they have no competing interests.
References
- 1.Aghajanova L, Hoffman J, Mok-Lin E, Herndon CN. Obstetrics and gynecology residency and fertility needs. Reprod Sci. 2017;24:428–434. doi: 10.1177/1933719116657193. [DOI] [PubMed] [Google Scholar]
- 2.Center for Disease Control and Prevention Assisted reproductive technology success rates: National summary and fertility clinic reports. 2016 www.cdc.gov/art/pdf/2016-report/ART-2016-National-Summary-Report.pdf.
- 3.Society for Assisted Reproductive Technology . National Summary Report. Society for Assisted Reproductive Technology; 2016. www.sartcorsonline.com/rptCSR_PublicMultYear.aspx?reportingYear=2016. [Google Scholar]
- 4.Agarwal A, Mulgund A, Hamada A, Chyatte MR. A unique view on male infertility around the globe. Reprod Biol Endocrinol. 2015;13:37. doi: 10.1186/s12958-015-0032-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Artini PG, Obino ME, Vergine F, Sergiampietri C, Papini F, Cela V. Assisted reproductive technique in women of advanced fertility age. Minerva Ginecol. 2018;70:738–749. doi: 10.23736/S0026-4784.18.04247-8. [DOI] [PubMed] [Google Scholar]
- 6.Zhang WG, Zhu SY, Bai XJ, Zhao DL, Jian SM, Li J, Li ZX, Fu B, Cai GY, Sun XF, et al. Select aging biomarkers based on telomere length and chronological age to build a biological age equation. Age (Dordr) 2014;36:9639. doi: 10.1007/s11357-014-9639-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pfeiffer V, Lingner J. Replication of telomeres and the regulation of telomerase. Cold Spring Harb Perspect Biol. 2013;5:a010405. doi: 10.1101/cshperspect.a010405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones MJ, Goodman SJ, Kobor MS. DNA methylation and healthy human aging. Aging Cell. 2015;14:924–932. doi: 10.1111/acel.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rizvi S, Raza ST, Mahdi F. Telomere length variations in aging and age-related diseases. Curr Aging Sci. 2014;7:161–167. doi: 10.2174/1874609808666150122153151. [DOI] [PubMed] [Google Scholar]
- 10.Tsatsakis A, Tsoukalas D, Fragkiadaki P, Vakonaki E, Tzatzarakis M, Sarandi E, Nikitovic D, Tsilimidos G, Alegakis AK. Developing BIOTEL: A semi-automated spreadsheet for estimating telomere length and biological age. Front Genet. 2019;10:84. doi: 10.3389/fgene.2019.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petrakis D, Vassilopoulou L, Mamoulakis C, Psycharakis C, Anifantaki A, Sifakis S, Docea AO, Tsiaoussis J, Makrigiannakis A, Tsatsakis AM. Endocrine disruptors leading to obesity and related diseases. Int J Environ Res Public Health. 2017;14:E1282. doi: 10.3390/ijerph14101282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehrpour O, Karrari P, Zamani N, Tsatsakis AM, Abdollahi M. Occupational exposure to pesticides and consequences on male semen and fertility: A review. Toxicol Lett. 2014;230:146–156. doi: 10.1016/j.toxlet.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 13.Kalliora C, Mamoulakis C, Vasilopoulos E, Stamatiades GA, Kalafati L, Barouni R, Karakousi T, Abdollahi M, Tsatsakis A. Association of pesticide exposure with human congenital abnormalities. Toxicol Appl Pharmacol. 2018;346:58–75. doi: 10.1016/j.taap.2018.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sifakis S, Androutsopoulos VP, Tsatsakis AM, Spandidos DA. Human exposure to endocrine disrupting chemicals: Effects on the male and female reproductive systems. Environ Toxicol Pharmacol. 2017;51:56–70. doi: 10.1016/j.etap.2017.02.024. [DOI] [PubMed] [Google Scholar]
- 15.Katsikantami I, Sifakis S, Tzatzarakis MN, Vakonaki E, Kalantzi OI, Tsatsakis AM, Rizos AK. A global assessment of phthalates burden and related links to health effects. Environ Int. 2016;97:212–236. doi: 10.1016/j.envint.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 16.Yawson Emmanuel O, Obasi KK, Lawal I. Spermatogenic and spermatotoxic effects of Telfairia occidentalis (Ugu) aqueous leaves extract in adult male Wistar rats (Rattus novergicus) Toxicol Rep. 2018;5:954–958. doi: 10.1016/j.toxrep.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Acosta IB, Junior ASV, E Silva EF, Cardoso TF, Caldas JS, Jardim RD, Corcini CD. Effects of exposure to cadmium in sperm cells of zebrafish, Danio rerio. Toxicol Rep. 2016;3:696–700. doi: 10.1016/j.toxrep.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mello MSC, Delgado IF, Favareto APA, Lopes CMT, Batista MM, Kempinas WD, Paumgartten FJR. Sexual maturation and fertility of mice exposed to triphenyltin during prepubertal and pubertal periods. Toxicol Rep. 2014;2:405–414. doi: 10.1016/j.toxrep.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vakonaki E, Tzatzarakis M, Tsiminikaki K, Nathena D, Fragkiadaki P, Kalliantasi K, Kanaki K, Vaki G, Plaitis S, Tsoukalas D, et al. Effect of chronic and heavy drug abuse on biological aging. World Acad J Sci. 2019;1:67–73. [Google Scholar]
- 20.Tsoukalas D, Fragkiadaki P, Docea AO, Alegakis AK, Sarandi E, Vakonaki E, Salataj E, Kouvidi E, Nikitovic D, Kovatsi L, et al. Association of nutraceutical supplements with longer telomere length. Int J Mol Med. 2019;44:218–226. doi: 10.3892/ijmm.2019.4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shammas MA. Telomeres, lifestyle, cancer, and aging. Curr Opin Clin Nutr Metab Care. 2011;14:28–34. doi: 10.1097/MCO.0b013e32834121b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valassi E, Crespo I, Santos A, Webb SM. Clinical consequences of Cushing's syndrome. Pituitary. 2012;15:319–329. doi: 10.1007/s11102-012-0394-8. [DOI] [PubMed] [Google Scholar]
- 23.Tedone E, Huang E, O'Hara R, Batten K, Ludlow AT, Lai TP, Arosio B, Mari D, Wright WE, Shay JW. Telomere length and telomerase activity in T cells are biomarkers of high-performing centenarians. Aging Cell. 2019;18:e12859. doi: 10.1111/acel.12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kordinas V, Ioannidis A, Chatzipanagiotou S. The telomere/telomerase system in chronic inflammatory Diseases Cause or effect? Genes (Basel) 2016;7:E60. doi: 10.3390/genes7090060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chakravarthi BV, Nepal S, Varambally S. Genomic and epigenomic alterations in cancer. Am J Pathol. 2016;186:1724–1735. doi: 10.1016/j.ajpath.2016.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma H, Zhou Z, Wei S, Liu Z, Pooley KA, Dunning AM, Svenson U, Roos G, Hosgood HD, III, Shen M, et al. Shortened telomere length is associated with increased risk of cancer: A meta-analysis. PLoS One. 2011;6:e20466. doi: 10.1371/journal.pone.0020466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haycock PC, Heydon EE, Kaptoge S, Butterworth AS, Thompson A, Willeit P. Leucocyte telomere length and risk of cardiovascular disease: Systematic review and meta-analysis. BMJ. 2014;349:g4227. doi: 10.1136/bmj.g4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Willeit P, Raschenberger J, Heydon EE, Tsimikas S, Haun M, Mayr A, Weger S, Witztum JL, Butterworth AS, Willeit J, et al. Leucocyte telomere length and risk of type 2 diabetes mellitus: New prospective cohort study and literature-based meta-analysis. PLoS One. 2014;9:e112483. doi: 10.1371/journal.pone.0112483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aviv A, Kark JD, Susser E. Telomeres, atherosclerosis, and human longevity: A causal hypothesis. Epidemiology. 2015;26:295–299. doi: 10.1097/EDE.0000000000000280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J, Rane G, Dai X, Shanmugam MK, Arfuso F, Samy RP, Lai MK, Kappei D, Kumar AP, Sethi G. Ageing and the telomere connection: An intimate relationship with inflammation. Ageing Res Rev. 2016;25:55–69. doi: 10.1016/j.arr.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 32.Parks CG, DeRoo LA, Miller DB, McCanlies EC, Cawthon RM, Sandler DP. Employment and work schedule are related to telomere length in women. Occup Environ Med. 2011;68:582–589. doi: 10.1136/oem.2010.063214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valentijn AJ, Saretzki G, Tempest N, Critchley HO, Hapangama DK. Human endometrial epithelial telomerase is important for epithelial proliferation and glandular formation with potential implications in endometriosis. Hum Reprod. 2015;30:2816–2828. doi: 10.1093/humrep/dev267. [DOI] [PubMed] [Google Scholar]
- 34.Williams CD, Boggess JF, LaMarque LR, Meyer WR, Murray MJ, Fritz MA, Lessey BA. A prospective, randomized study of endometrial telomerase during the menstrual cycle. J Clin Endocrinol Metab. 2001;86:3912–3917. doi: 10.1210/jcem.86.8.7729. [DOI] [PubMed] [Google Scholar]
- 35.Hapangama DK, Turner MA, Drury J, Heathcote L, Afshar Y, Mavrogianis PA, Fazleabas AT. Aberrant expression of regulators of cell-fate found in eutopic endometrium is found in matched ectopic endometrium among women and in a baboon model of endometriosis. Hum Reprod. 2010;25:2840–2850. doi: 10.1093/humrep/deq248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kalyan S, Patel MS, Kingwell E, Côté HCF, Liu D, Prior JC. Competing factors link to bone health in polycystic ovary syndrome: Chronic low-grade inflammation takes a toll. Sci Rep. 2017;7:3432. doi: 10.1038/s41598-017-03685-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miranda-Furtado CL, Ramos FK, Kogure GS, Santana-Lemos BA, Ferriani RA, Calado RT, Dos Reis RM. A nonrandomized trial of progressive resistance training intervention in women with polycystic ovary syndrome and its implications in telomere content. Reprod Sci. 2016;23:644–654. doi: 10.1177/1933719115611753. [DOI] [PubMed] [Google Scholar]
- 38.Pedroso DC, Miranda-Furtado CL, Kogure GS, Meola J, Okuka M, Silva C, Calado RT, Ferriani RA, Keefe DL, dos Reis RM. Inflammatory biomarkers and telomere length in women with polycystic ovary syndrome. Fertil Steril. 2015;103:542–547.e2. doi: 10.1016/j.fertnstert.2014.10.035. [DOI] [PubMed] [Google Scholar]
- 39.Turner S, Hartshorne GM. Telomere lengths in human pronuclei, oocytes and spermatozoa. Mol Hum Reprod. 2013;19:510–518. doi: 10.1093/molehr/gat021. [DOI] [PubMed] [Google Scholar]
- 40.Pollack AZ, Rivers K, Ahrens KA. Parity associated with telomere length among US reproductive age women. Hum Reprod. 2018;33:736–744. doi: 10.1093/humrep/dey024. [DOI] [PubMed] [Google Scholar]
- 41.Yu J, Berga SL, Zou W, Sun HY, Johnston-MacAnanny E, Yalcinkaya T, Sidell N, Bagchi IC, Bagchi MK, Taylor RN. Gap junction blockade induces apoptosis in human endometrial stromal cells. Mol Reprod Dev. 2014;81:666–675. doi: 10.1002/mrd.22334. [DOI] [PubMed] [Google Scholar]
- 42.Cheng EH, Chen SU, Lee TH, Pai YP, Huang LS, Huang CC, Lee MS. Evaluation of telomere length in cumulus cells as a potential biomarker of oocyte and embryo quality. Hum Reprod. 2013;28:929–936. doi: 10.1093/humrep/det004. [DOI] [PubMed] [Google Scholar]
- 43.Barha CK, Hanna CW, Salvante KG, Wilson SL, Robinson WP, Altman RM, Nepomnaschy PA. Number of children and telomere length in women: A prospective, longitudinal evaluation. PLoS One. 2016;11:e0146424. doi: 10.1371/journal.pone.0146424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Czamanski-Cohen J, Sarid O, Cwikel J, Douvdevani A, Levitas E, Lunenfeld E, Har-Vardi I. Cell-free DNA and telomere length among women undergoing in vitro fertilization treatment. J Assist Reprod Genet. 2015;32:1697–1703. doi: 10.1007/s10815-015-0581-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shalev I, Entringer S, Wadhwa PD, Wolkowitz OM, Puterman E, Lin J, Epel ES. Stress and telomere biology: A lifespan perspective. Psychoneuroendocrinology. 2013;38:1835–1842. doi: 10.1016/j.psyneuen.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fragkiadaki P, Tsoukalas D, Fragkiadoulaki I, Psycharakis C, Nikitovic D, Spandidos DA, Tsatsakis AM. Telomerase activity in pregnancy complications (Review) Mol Med Rep. 2016;14:16–21. doi: 10.3892/mmr.2016.5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perls TT, Alpert L, Fretts RC. Middle-aged mothers live longer. Nature. 1997;389:133. doi: 10.1038/38148. [DOI] [PubMed] [Google Scholar]
- 48.Sun F, Sebastiani P, Schupf N, Bae H, Andersen SL, McIntosh A, Abel H, Elo IT, Perls TT. Extended maternal age at birth of last child and women's longevity in the Long Life Family Study. Menopause. 2015;22:26–31. doi: 10.1097/GME.0000000000000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fagan E, Sun F, Bae H, Elo I, Andersen SL, Lee J, Christensen K, Thyagarajan B, Sebastiani P, Perls T, et al. Long Life Family Study Telomere length is longer in women with late maternal age. Menopause. 2017;24:497–501. doi: 10.1097/GME.0000000000000795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gray KE, Schiff MA, Fitzpatrick AL, Kimura M, Aviv A, Starr JR. Leukocyte telomere length and age at menopause. Epidemiology. 2014;25:139–146. doi: 10.1097/EDE.0000000000000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hapangama DK, Turner MA, Drury JA, Martin-Ruiz C, Von Zglinicki T, Farquharson RG, Quenby S. Endometrial telomerase shows specific expression patterns in different types of reproductive failure. Reprod Biomed Online. 2008;17:416–424. doi: 10.1016/S1472-6483(10)60227-1. [DOI] [PubMed] [Google Scholar]
- 52.Butts S, Riethman H, Ratcliffe S, Shaunik A, Coutifaris C, Barnhart K. Correlation of telomere length and telomerase activity with occult ovarian insufficiency. J Clin Endocrinol Metab. 2009;94:4835–4843. doi: 10.1210/jc.2008-2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Treff NR, Su J, Taylor D, Scott RT., Jr Telomere DNA deficiency is associated with development of human embryonic aneuploidy. PLoS Genet. 2011;7:e1002161. doi: 10.1371/journal.pgen.1002161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu X, Chen X, Zhang X, Liu Y, Wang Z, Wang P, Du Y, Qin Y, Chen ZJ. Impaired telomere length and telomerase activity in peripheral blood leukocytes and granulosa cells in patients with biochemical primary ovarian insufficiency. Hum Reprod. 2017;32:201–207. doi: 10.1093/humrep/dew283. [DOI] [PubMed] [Google Scholar]
- 55.Kuhn E, Meeker A, Wang TL, Sehdev AS, Kurman RJ, Shih IeM. Shortened telomeres in serous tubal intraepithelial carcinoma: An early event in ovarian high-grade serous carcinogenesis. Am J Surg Pathol. 2010;34:829–836. doi: 10.1097/PAS.0b013e3181dcede7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wei D, Xie J, Yin B, Hao H, Song X, Liu Q, Zhang C, Sun Y. Significantly lengthened telomere in granulosa cells from women with polycystic ovarian syndrome (PCOS) J Assist Reprod Genet. 2017;34:861–866. doi: 10.1007/s10815-017-0945-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang C, Shen F, Zhu Y, Fang Y, Lu S. Telomeric repeat-containing RNA (TERRA) related to polycystic ovary syndrome (PCOS) Clin Endocrinol (Oxf) 2017;86:552–559. doi: 10.1111/cen.13283. [DOI] [PubMed] [Google Scholar]
- 58.Li Q, Du J, Feng R, Xu Y, Wang H, Sang Q, Xing Q, Zhao X, Jin L, He L, et al. A possible new mechanism in the pathophysiology of polycystic ovary syndrome (PCOS): The discovery that leukocyte telomere length is strongly associated with PCOS. J Clin Endocrinol Metab. 2014;99:E234–E240. doi: 10.1210/jc.2013-3685. [DOI] [PubMed] [Google Scholar]
- 59.Li Y, Deng B, Ouyang N, Yuan P, Zheng L, Wang W. Telomere length is short in PCOS and oral contraceptive does not affect the telomerase activity in granulosa cells of patients with PCOS. J Assist Reprod Genet. 2017;34:849–859. doi: 10.1007/s10815-017-0929-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hapangama DK, Turner MA, Drury JA, Quenby S, Saretzki G, Martin-Ruiz C, Von Zglinicki T. Endometriosis is associated with aberrant endometrial expression of telomerase and increased telomere length. Hum Reprod. 2008;23:1511–1519. doi: 10.1093/humrep/den172. [DOI] [PubMed] [Google Scholar]
- 61.Sofiyeva N, Ekizoglu S, Gezer A, Yilmaz H, Kolomuc Gayretli T, Buyru N, Oral E. Does telomerase activity have an effect on infertility in patients with endometriosis? Eur J Obstet Gynecol Reprod Biol. 2017;213:116–122. doi: 10.1016/j.ejogrb.2017.04.027. [DOI] [PubMed] [Google Scholar]
- 62.Valentijn AJ, Palial K, Al-Lamee H, Tempest N, Drury J, Von Zglinicki T, Saretzki G, Murray P, Gargett CE, Hapangama DK. SSEA-1 isolates human endometrial basal glandular epithelial cells: Phenotypic and functional characterization and implications in the pathogenesis of endometriosis. Hum Reprod. 2013;28:2695–2708. doi: 10.1093/humrep/det285. [DOI] [PubMed] [Google Scholar]
- 63.Hanna CW, Bretherick KL, Gair JL, Fluker MR, Stephenson MD, Robinson WP. Telomere length and reproductive aging. Hum Reprod. 2009;24:1206–1211. doi: 10.1093/humrep/dep007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kuhn E, Meeker AK, Visvanathan K, Gross AL, Wang TL, Kurman RJ, Shih IeM. Telomere length in different histologic types of ovarian carcinoma with emphasis on clear cell carcinoma. Mod Pathol. 2011;24:1139–1145. doi: 10.1038/modpathol.2011.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Keefe DL, Liu L, Marquard K. Telomeres and aging-related meiotic dysfunction in women. Cell Mol Life Sci. 2007;64:139–143. doi: 10.1007/s00018-006-6466-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kalmbach KH, Antunes DM, Kohlrausch F, Keefe DL. Telomeres and female reproductive aging. Semin Reprod Med. 2015;33:389–395. doi: 10.1055/s-0035-1567823. [DOI] [PubMed] [Google Scholar]
- 67.Keefe DL, Liu L. Telomeres and reproductive aging. Reprod Fertil Dev. 2009;21:10–14. doi: 10.1071/RD08229. [DOI] [PubMed] [Google Scholar]
- 68.Yang Q, Zhao F, Dai S, Zhang N, Zhao W, Bai R, Sun Y. Sperm telomere length is positively associated with the quality of early embryonic development. Hum Reprod. 2015;30:1876–1881. doi: 10.1093/humrep/dev144. [DOI] [PubMed] [Google Scholar]
- 69.Reig-Viader R, Capilla L, Vila-Cejudo M, Garcia F, Anguita B, Garcia-Caldés M, Ruiz-Herrera A. Telomere homeostasis is compromised in spermatocytes from patients with idiopathic infertility. Fertil Steril. 2014;102:728–738.e1. doi: 10.1016/j.fertnstert.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 70.Yan L, Wu S, Zhang S, Ji G, Gu A. Genetic variants in telomerase reverse transcriptase (TERT) and telomerase-associated protein 1 (TEP1) and the risk of male infertility. Gene. 2014;534:139–143. doi: 10.1016/j.gene.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 71.Ferlin A, Rampazzo E, Rocca MS, Keppel S, Frigo AC, De Rossi A, Foresta C. In young men sperm telomere length is related to sperm number and parental age. Hum Reprod. 2013;28:3370–3376. doi: 10.1093/humrep/det392. [DOI] [PubMed] [Google Scholar]
- 72.Thilagavathi J, Kumar M, Mishra SS, Venkatesh S, Kumar R, Dada R. Analysis of sperm telomere length in men with idiopathic infertility. Arch Gynecol Obstet. 2013;287:803–807. doi: 10.1007/s00404-012-2632-8. [DOI] [PubMed] [Google Scholar]
- 73.Prescott J, Du M, Wong JY, Han J, De Vivo I. Paternal age at birth is associated with offspring leukocyte telomere length in the nurses' health study. Hum Reprod. 2012;27:3622–3631. doi: 10.1093/humrep/des314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moskovtsev SI, Willis J, White J, Mullen JB. Disruption of telomere-telomere interactions associated with DNA damage in human spermatozoa. Syst Biol Reprod Med. 2010;56:407–412. doi: 10.3109/19396368.2010.502587. [DOI] [PubMed] [Google Scholar]
- 75.Baird DM, Britt-Compton B, Rowson J, Amso NN, Gregory L, Kipling D. Telomere instability in the male germline. Hum Mol Genet. 2006;15:45–51. doi: 10.1093/hmg/ddi424. [DOI] [PubMed] [Google Scholar]
- 76.Biron-Shental T, Wiser A, Hershko-Klement A, Markovitch O, Amiel A, Berkovitch A. Sub-fertile sperm cells exemplify telomere dysfunction. J Assist Reprod Genet. 2018;35:143–148. doi: 10.1007/s10815-017-1029-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vecoli C, Montano L, Borghini A, Notari T, Guglielmino A, Mercuri A, Turchi S, Andreassi MG. Effects of highly polluted environment on sperm telomere length: A Pilot Study. Int J Mol Sci. 2017;18:E1703. doi: 10.3390/ijms18081703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lafuente R, Bosch-Rue E, Ribas-Maynou J, Alvarez J, Brassesco C, Amengual MJ, Benet J, Garcia-Peiró A, Brassesco M. Sperm telomere length in motile sperm selection techniques: A qFISH approach. Andrologia. 2018;50:e12840. doi: 10.1111/and.12840. [DOI] [PubMed] [Google Scholar]
- 79.Cariati F, Jaroudi S, Alfarawati S, Raberi A, Alviggi C, Pivonello R, Wells D. Investigation of sperm telomere length as a potential marker of paternal genome integrity and semen quality. Reprod Biomed Online. 2016;33:404–411. doi: 10.1016/j.rbmo.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 80.Mishra S, Kumar R, Malhotra N, Singh N, Dada R. Mild oxidative stress is beneficial for sperm telomere length maintenance. World J Methodol. 2016;6:163–170. doi: 10.5662/wjm.v6.i2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rocca MS, Speltra E, Menegazzo M, Garolla A, Foresta C, Ferlin A. Sperm telomere length as a parameter of sperm quality in normozoospermic men. Hum Reprod. 2016;31:1158–1163. doi: 10.1093/humrep/dew061. [DOI] [PubMed] [Google Scholar]
- 82.Liu SY, Zhang CJ, Peng HY, Huang XQ, Sun H, Lin KQ, Huang K, Chu JY, Yang ZQ. Association study of telomere length with idiopathic male infertility. Yi Chuan. 2015;37:1137–1142. doi: 10.16288/j.yczz.15-267. In Chinese. [DOI] [PubMed] [Google Scholar]
- 83.Antunes DM, Kalmbach KH, Wang F, Dracxler RC, Seth-Smith ML, Kramer Y, Buldo-Licciardi J, Kohlrausch FB, Keefe DL. A single-cell assay for telomere DNA content shows increasing telomere length heterogeneity, as well as increasing mean telomere length in human spermatozoa with advancing age. J Assist Reprod Genet. 2015;32:1685–1690. doi: 10.1007/s10815-015-0574-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang Q, Zhang N, Zhao F, Zhao W, Dai S, Liu J, Bukhari I, Xin H, Niu W, Sun Y. Processing of semen by density gradient centrifugation selects spermatozoa with longer telomeres for assisted reproduction techniques. Reprod Biomed Online. 2015;31:44–50. doi: 10.1016/j.rbmo.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 85.Yang Q, Luo X, Bai R, Zhao F, Dai S, Li F, Zhu J, Liu J, Niu W, Sun Y. Shorter leukocyte telomere length is associated with risk of nonobstructive azoospermia. Fertil Steril. 2018;110:648–654.e1. doi: 10.1016/j.fertnstert.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 86.Heidary H, Pouresmaeili F, Mirfakhraie R, Omrani MD, Ghaedi H, Fazeli Z, Sayban S, Ghafouri-Fard S, Azargashb E, Shokri F. An association study between longitudinal changes of leukocyte telomere and the risk of azoospermia in a population of Iranian infertile men. Iran Biomed J. 2018;22:231–236. doi: 10.29252/ibj.22.4.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jørgensen PB, Fedder J, Koelvraa S, Graakjaer J. Age-dependence of relative telomere length profiles during spermatogenesis in man. Maturitas. 2013;75:380–385. doi: 10.1016/j.maturitas.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 88.Pech MF, Garbuzov A, Hasegawa K, Sukhwani M, Zhang RJ, Benayoun BA, Brockman SA, Lin S, Brunet A, Orwig KE, et al. High telomerase is a hallmark of undifferentiated spermatogonia and is required for maintenance of male germline stem cells. Genes Dev. 2015;29:2420–2434. doi: 10.1101/gad.271783.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ling X, Zhang G, Chen Q, Yang H, Sun L, Zhou N, Wang Z, Zou P, Wang X, Cui Z, et al. Shorter sperm telomere length in association with exposure to polycyclic aromatic hydrocarbons: Results from the MARHCS cohort study in Chongqing, China and in vivo animal experiments. Environ Int. 2016;95:79–85. doi: 10.1016/j.envint.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 90.Liu M, Hales BF, Robaire B. Effects of four chemotherapeutic agents, bleomycin, etoposide, cisplatin, and cyclophosphamide, on DNA damage and telomeres in a mouse spermatogonial cell line. Biol Reprod. 2014;90:72. doi: 10.1095/biolreprod.114.117754. [DOI] [PubMed] [Google Scholar]
- 91.Dracxler RC, Oh C, Kalmbach K, Wang F, Liu L, Kallas EG, Giret MT, Seth-Smith ML, Antunes D, Keefe DL, et al. Peripheral blood telomere content is greater in patients with endometriosis than in controls. Reprod Sci. 2014;21:1465–1471. doi: 10.1177/1933719114527353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.