Abstract
Processed products from agricultural produce generate a large number of agricultural by-products that contain a number of functional substances. These are often discarded owing to the lack of suitable processing methods. The present study investigated the anti-photoaging properties of fermented rice bran (FRB), soybean cake (FSB) and sesame seed cake (FSC) on ultraviolet B (UVB)-irradiated hairless mouse skin. Results indicated that the oral administration of FRB, FSB and FSC effectively inhibited the UVB irradiation-induced expression of matrix metalloproteinase (MMP)-2, MMP-9, MMP-3 and MMP-13. Reverse transcription-quantitative polymerase chain reaction results also demonstrated that FRB, FSB and FSC significantly inhibited the UVB-induced expression of the genes encoding tumor necrosis factor-α, inducible nitric oxide synthase, interleukin (IL)-6 and IL-1β when compared with the UVB-vehicle group (P<0.05). Additionally, collagen degradation and mast cell infiltration were reduced in hairless mouse skin. Furthermore, UVB-induced wrinkle formation was also significantly reduced in mouse skin compared with the UVB-vehicle group (P<0.05). These results reveal that fermented agricultural by-products may serve as potential functional materials with anti-photoaging activities.
Keywords: bioresource, agricultural by-product, anti-photoaging, matrix metalloproteinase, HR-1 hairless mouse
Introduction
Green growth is a process that aims to maintain sustainable development while maintaining and improving the global environment; thus, it is of great social interest and importance (1). An essential part of achieving green growth involves the recycling of agricultural waste generated during the production of agricultural products (2). The by-products from agricultural activities are abandoned during the processing steps. These abandoned agricultural by-products are rich sources of various functional materials and are now actively evaluated for their efficacy (3). Previous studies have focused on high-value agricultural by-products and bioenergetics (4) and eco-friendly materials (5), where functional improvement using bioconversion is a representative example. Therefore, the present study aimed to conduct a bioconversion process (fermentation) to increase the use of bioresources in the production of functional food materials.
Bioconversion is a technique that is used to produce a desired product from specific precursors with the help of microorganisms. It includes bioprocessing, biosynthesis and biocatalysis (6). The difference between bioconversion and the conventional fermentation process is that the latter starts from a simple raw material, whereas the former produces a product from precursors using the selectivity of a microbial or enzyme substrate (7). In this regard, the bioconversion process is an energy-saving advanced technology (8). The bioconver-sion process may particularly contribute to improved usability and effectiveness in the development of pharmaceuticals and cosmetics (9). In a previous study, it was confirmed that bioconversion increased the production of active ingredients including β-glucan and γ-oryzanol compounds in rice bran, and lignan compounds in sesame seed cake (10). Additionally, it was confirmed that soybean cake glycosides (daidzin, glycitin and genistin) were converted to aglycones (daidzein, glycitein and genistein) (11). Furthermore, bioconversion increased the antioxidant activity of rice bran, soybean cake and sesame seed cake, and altered the anti-allergic activities of sesame seed cake (12).
The skin is the largest organ of the body that controls metabolite excretion and the body temperature. It is constantly exposed to the outside environment and protects the body from external stimuli (13). Ultraviolet (UV) rays, a type of typical external stimulus, have beneficial effects on vitamin D synthesis and sterilization (14,15). Although UV radiation, through sufficient sun exposure, is necessary for humans, a high frequency of external activities and long-term exposure to UV rays may result in skin aging (16).
Based on its wavelength, UV light may be classified as UVA (320-400 nm), UVB (290-320 nm) or UVC (200-290 nm) (17). The ozone layer in the atmosphere absorbs UVC rays completely; however, UVA and UVB rays penetrate through to the Earth's surface (18). Excessive UVB exposure, in particular, induces changes in the epidermis (19). Photodamaged skin is characterized by the development of erythema, edema, hyperplasia, hyperpigmentation, sunburns, drying, reduced elasticity and the formation of wrinkles (20). Furthermore, skin aging may occur due to increased reactive oxygen species (ROS) production in the skin caused by UVB. Although the skin has developed a defense system against ROS, the continuous production of ROS disrupts the enzymatic and non-enzymatic antioxidant defense system in the skin (21). Furthermore, UVB aggravates the inflammatory response by activating and promoting the inflammatory cell infiltration that causes skin damage (22). ROS generated by UVB may affect mitogen-activated protein kinase (MAPK) signaling and activate nuclear factor-κB and activation protein 1 to release inflammatory cytokines including tumor necrosis factor-α (TNF-α), interleukin (IL)-1β and IL-6 (23). In addition, the increase in matrix metalloproteinase (MMP) activity in response to ROS production results in the destruction of the structure and function of the extracellular matrix (ECM) via collagen degradation (24). UV exposure also stimulates the production of cyclooxygenase-2 (COX-2), which aggravates skin inflammation by catalyzing the synthesis of prostaglandin E2 and inducing the expression of inducible nitric oxide synthase (iNOS), which may result in skin inflammation (25).
However, the modern lifestyle choices of people from different age groups place a great emphasis on skin health and beauty (26). A number of cosmetics and foods have been developed to delay and prevent the aging process and to maintain healthy skin (27,28). A study aiming for the alleviation and improvement of skin wrinkles is currently being performed (29). Therefore, it is a worthwhile endeavor to improve the value of agricultural products while meeting the needs of modern people. The present study was undertaken to investigate the anti-photoaging effect of fermented agricultural by-products including fermented rice bran (FRB), soybean cake (FSB) and sesame seed cake (FSC) on UVB-irradiated hairless mouse skin.
Materials and methods
Care of animals and UV irradiation
Seven-week-old female HR-1 hairless mice (weight, 27.9-33.8 g) were provided by the Central Lab. Animal Inc. (Seoul, Korea). The trial was ethically approved by the Institutional Animal Care and Use Committee of Kangwon National University (approval no. KW-170417-1; 15 May, 2017). These mice (n=30) were randomly divided into six groups of five animals per group (Table I). They were housed in a climate-controlled room (22°C at 50% humidity) in 12/12 h light/dark cycles. All the mice had ad libitum access to water and food. The oral administration of FRB, FSB and FSC, which were dissolved in 500 µl vehicle (saline solution) and administered once a day for a total of 8 weeks, was calculated and performed according to the body weight of the mice. In order to equalize the conditions with the sample treatment groups, the UVB vehicle group was orally administered the vehicle alone. The body weight of each mouse was measured every week. The food efficiency rate was calculated and expressed as a percentage using the following formula: Food efficiency ratio (%)=[total weight gain (g)/total food intake (g) ×100]. UV light equipped with a 100 µW/cm2 UVB lamp that had a maximum emission wavelength of 312 nm (Jiatian Trading Co., Ltd.) was used. To determine the 1 minimal erythemal dose (MED), the dorsal skin of the mice was exposed to different doses of UV light and erythema formation was detected after 24 h. Skin aging was performed via 1 MED irradiation three times a week for 8 weeks. HR-1 hairless mice were irradiated with 100 mJ/cm2 of UVB radiation (1 MED=100 mJ/cm2) daily for the first week, followed by UVB irradiation thrice a week at 200 mJ/cm2 from week 2 to 8. Following 8 weeks once the radiation treatment was over, vehicle, topically applied 0.01% retinoic acid (cat. no. R2625; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), FRB, FSB and FSC treatments commenced, and the animals were sacrificed immediately using CO2 gas to acquire skin tissue samples.
Table I.
Design and treatment of HR-1 hairless mice groups.
| Groups | Treatment | Number |
|---|---|---|
| HR-1 normal | HR-1 without any treatment (control) | 5 |
| UVB vehicle | UVB irradiation vehicle | 5 |
| UVB RA | UVB + 0.01% retinoic acid (topical ointment) | 5 |
| UVB FRB | UVB + FRB 40 mg/kg (oral administration) | 5 |
| UVB FSB | UVB + FSB 40 mg/kg (oral administration) | 5 |
| UVB FSC | UVB + FSC 40 mg/kg (oral administration) | 5 |
FRB, fermented rice bran; FSB, fermented soybean cake; FSC, fermented sesame seed cake; UVB, ultraviolet B; RA, retinoic acid.
Preparation of FRB, FSB and FSC
The samples used in the present study were provided by STR Biotech Co., Ltd. (Chuncheon, Korea). Rice, soybean and sesame seeds were harvested in Geochang, Korea, between August 2014 and October 2014. The samples were identified by Dr Sea Kwan Oh at the National Institute of Crop Science (Milyang, Korea). These were fermented with Lentinula edodes (Shiitake mushrooms) using a fermentation system. Fermentation was performed as previously described (12). In brief, L. edodes mycelia were isolated from the mushroom fruit body and cultured in potato dextrose agar (PDA) medium (Difco; BD Biosciences, Franklin Lakes, NJ, USA). The genetic identity of the fungus was confirmed by the Korean Center of Microorganisms (Seoul, Korea). The mycelia cultured on PDA media were inoculated in 50 ml of a liquid medium containing 2% glucose, 0.5% yeast extract, 0.5% soy peptone, 0.2% mono-potassium phosphate (KH2PO4), 0.05% magnesium sulfate (MgSO4) and 0.002% ferrous sulfate (FeSO4). The experiments were conducted in 250 ml Erlenmeyer flasks at 28°C for 5 days in a rotary shaker, and the resulting broth was used to seed the main liquid culture. Liquid culture media containing agricultural by-products (rice bran, soybean cake and sesame seed cake) were treated with amylase and cellulase at 60°C for 60 min to enzymatically digest the articulate materials containing carbohydrates. The culture mass was subsequently adjusted by bringing the pH to 6.0 using hydrochloric acid (HCl), followed by sterilization in an autoclave. Further experiments were performed in a 5 l fermenter (working volume of 3 l) at 28°C and 1,050 × g by inoculating the media with the cultured mycelia (10%). Subsequent to 7 days, the culture was treated with an enzyme mixture containing cellulase, hemicellulase, pectinase, glucanase, mannase and arabinase (Sigma-Aldrich; Merck KGaA) at 50°C for 60 min to lyse the cell walls. The enzyme-treated culture mass was extracted at 90°C following 1 h and freeze-dried to make a powder.
Measurement of wrinkle formation
The degree of UVB-induced skin aging was measured by investigating wrinkle formation. HR-1 hairless mice were anesthetized with intraperitoneal injections of 0.1 ml 8% chloral hydrate (320 mg/kg) for up to 8 weeks at 2-week intervals, following which the UVB-irradiated dorsal area was photographed at a magnification of ×400 using a USB digital Optical microscope. Skin wrinkles were investigated using the DETAX System 2 and Double-Stick Dis (3M Deutschland GmbH, Walheim, Germany). The wrinkles were also analyzed using a Dermo Bella wrinkle analyzer (Chowis Co. Ltd., Seongnam, Korea). Wrinkle formation was assessed according to the scoring system established by Bissett et al (30): Grade 0, no coarse wrinkles; grade 1, few shallow coarse wrinkles; grade 2, some coarse wrinkles; and grade 3, several deep coarse wrinkles.
Measurement of transepidermal water loss (TEWL)
TEWL was measured following a previously reported method (30). Mice were maintained in a climate-controlled room (22°C and 50% humidity) for 30 min. The dorsal skin of each mouse was examined using a Corneometer® CM825 probe (Courage + Khazaka electronic GmbH, Koln, Germany), which was placed in close contact with the surface of the dorsal skin and lightly pressed to record the skin moisture content.
Measurement of β-glucosidase activity
To measure β-glucosidase activity, the separated epidermis was pulverized using phosphate-buffered saline (1x, pH 7.2) supplemented with 100 µM phenylmethanesulfonyl fluoride (PMSF) and centrifuged at 10,000 × g for 5 min at 4°C. The separated supernatant was reacted with citrate-phosphate buffer (pH 5.6, 5 mM sodium taurocholate) containing 4-methylumbellifery-β-D- glucopyranoside (4-MUG) at 37°C for 60 min. The reaction was terminated by adding 200 mM carbonate-bicarbonate buffer (pH 10.5) and the fluorescence intensity of 4-methylumbelliferone (4-MU) converted from 4-MUG was measured using a spectrofluorometer (Hitachi, Ltd., Tokyo, Japan) at excitation and emission wavelengths of 360 and 450 nm, respectively. The 4-MU concentrations ranging from 0 to 300 nM were used as the standard for fluorescence measurements.
Analysis of histological staining
Histological analysis was performed to determine epidermal thickness, collagen fiber structure and mast cells. Dorsal skin tissues from each experimental group were fixed in 10% formalin at 21°C for 48 h. These were washed, dehydrated, permeated and embedded using the Paraffin Embedding Station (Leica Microsystems GmbH, Wetzlar, Germany). Hematoxylin & eosin (H&E) staining (each 25°C for 3 and 1 min, respectively) was performed to measure epidermal thickness. Masson's trichrome (M-T) and toluidine blue staining (each 25°C for 10 and 30 min, respectively) were also used to analyze the collagen fiber structure and mast cell infiltration, respectively.
Measurement of MMP-2 using an enzyme-linked immunosorbent assay (ELISA)
To measure the MMP-2 protein expression levels in the dorsal skin tissue extracted from HR-1 hairless mice, an MMP-2 ELISA kit (QC126; R&D Systems, Inc., Minneapolis, MN, USA) was used according to the manufacturer's protocol. Skin tissue samples were homogenized for 2 min on ice using Tissue-Tearor (BioSpec Products, Inc., Bartlesville, OK, USA) and resuspended in 100 mg/ml Tris-buffered saline (1x, pH 7.6) containing 1 mM PMSF, 1 mM EDTA and protease inhibitors (Sigma-Aldrich; Merck KGaA). The homogenates were centrifuged at 10,000 × g for 30 min at 4°C. To determine the protein concentration, a Bradford protein assay kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used according to the manufacturer's protocol. The MMP-2 ELISA kit was used to measure protein expression and a monoclonal antibody specific for total MMP-2 was pre-coated onto 96-well microplate. The 200 µl assay diluent was added to the wells and incubated at room temperature for 1 h. Subsequent to diluting the standard solution and supernatants 20 times, the microplate was washed, followed by the addition of 100 µl standard and supernatant to each well and incubation for 2 h at room temperature. The plate was further incubated at room temperature for 1 h with a 200 µl Biotin-labeled antibody (detection antibody), followed by treatment with a 200 µl tetramethylbenzidine substrate solution for 1 h at room temperature in the dark. Stop solution was added to each well and the absorbance was measured at 450 nm using a microplate spectrophotometer. All of the antibodies and solutions used to measure MMP-2 protein levels were included in the MMP-2 ELISA kit.
Measurement of MMPs and cytokines using reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
RT-qPCR was performed by modifying a previously published protocol (25) to determine the effect of FRB, FSB and FSC (40 mg/kg) on MMPs (MMP-2,9,3 and 13) and inflammation-associated cytokines including TNF-α, COX-2, iNOS, IL-6 and IL-1β. Total cellular RNA was extracted from skin tissue using the phenol-chloroform method using RNAzol (Tel-Test, Inc., Friendswood, TX, USA). In brief, 3 µg total RNA was used to synthesize cDNA using the ReverTra Ace® qPCR RT kit (Toyobo Life Science, Osaka, Japan). The 7500 Fast Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA) was used for RT-qPCR (according to the manufacturer's protocol) along with the primer sequences mentioned in Table II. TaqMan probes containing carboxyfluorescein dye were used to determine mRNA expression. A mouse GAPDH probe set (cat no. 4352339E, VIC/MGB Probe, Primer Limited; Applied Biosystems; Thermo Fisher Scientific, Inc.) was used as an internal standard. The final concentration of the primer was 200 nM. The standard PCR conditions were 50°C for 2 min and 94°C for 10 min, followed by 40 cycles of 94°C for 1 min and 60°C for 1 min. The number of cycles at which the emission intensity of the sample rose above the baseline value represented the relative quantity (RQ), which was proportional to the target concentration. The RQ value of the target group was used as the internal control and fold changes in the relative abundance of transcripts were calculated using the 2-∆∆cq method (31).
Table II.
Primer sequences for reverse transcription-quantitative polymerase chain reaction analysis.
| Target gene | Sequences (5′-3′) |
|---|---|
| MMP-2 | F: CAGGGAATGAGTACTGGGTCTATT |
| R ACTCCAGTTAAAGGCAGCATCTAC | |
| MMP-3 | F: TGGACCTGGAAATGTTTTGG |
| R: ATCAAAGTGGGCATCTCCAT | |
| MMP-9 | F: AATCTCTTCTAGAGACTGGGAAGGAG |
| R: AGCTGATTGACTAAAGTAGCTGGA | |
| MMP-13 | F: CCTCTTCTTCTCCGGAAACC |
| R: GGTAGTCTTGGTCCATGGTATGA | |
| TNF-α | F: TTCTGTCTACTGAACTTCGGGGTGATCGGTCC |
| R: GTATGAGATAGCAAATCGGCTGACGGTGTGGG | |
| COX-2 | F: AGTGATCGAAGACTACGTGCAA |
| R: GGGATTTCCCATAAGTCCTTTC | |
| iNOS | F: CGAAACGCTTCACTTCCA |
| R: TGAGCCTATATTGCTGTGGCT | |
| IL-6 | F: TCCAGTTGCCTTCTTGGGAC |
| R: GTGTAATTAAGCCTCCGACTTG | |
| IL-1β | F: CAACCAACAAGTGATATTCTCCATG |
| R: AGATCCACACTCTCAGCTGCA | |
| GAPDH | F: GTGAGGCCGGTGCTGAGTAT |
| R: CATCCTGCACCACCAACTGCTTAGCC |
F, forward; R, reverse; MMP, matrix metalloproteinase; TNF-α, tumor necrosis factor-α; COX-2, cyclooxygenase-2; iNOS, inducible nitric oxide synthase; IL, interleukin.
Statistical analysis
Statistical analyses were performed using the statistical package SPSS version 24.0 (IBM Corp., Armonk, NY, USA). For continuous variables normality was checked. The appropriate nonparametric test was selected for the variables not normally distributed. One-way analysis of variance and Kruskal-Wallis tests were used for comparing multiple groups with Scheffe's and Bonferroni's post-hoc comparisons, respectively. Results were expressed as the mean ± standard deviation. P<0.05 was considered to indicate a statistically significant difference.
Results
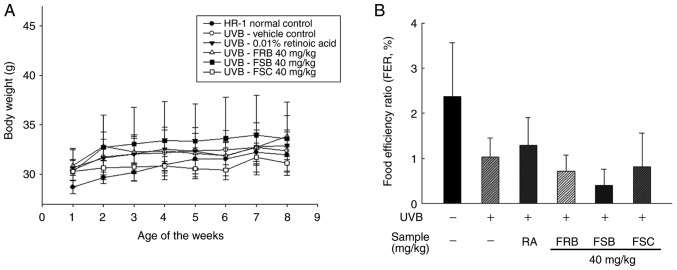
Effects of FRB, FSB and FSC on body weight and food efficiency rate
Body weights were measured weekly from week 1 to 8 (Fig. 1A). No significant difference in the mean body weight of mice was observed between the six groups following 3 weeks. The initial mean body weight in the normal control group was 28.7±0.66 g, which was lower compared with that of the other groups. However, the UVB vehicle group exhibited no significant difference in body weight when compared with the other groups subsequent to 3 weeks. Evaluation of the food efficiency ratio [total weight gain (g)/total food intake (g) ×100] revealed no significant differences between the normal control group and UVB-irradiated groups during the 8 experimental weeks (Fig. 1B). All UVB-irradiated groups did not significantly differ compared with that of the normal control group; however, the mean values decreased to 1.03±0.42% following UVB treatment from 2.37±1.19 (control group). Additionally, the intake of FRB, FSB and FSC (40 mg/kg) groups were decreased to 0.72±0.36, 0.40±0.36 and 0.82±0.74%, respectively.
Figure 1.
Effect of FRB, FSB and FSC on the body weights of hairless mice. (A) Body weights and (B) food efficiency rates of the mice from the six groups. The body weight of each mouse was measured once a week and the food efficiency rate was calculated using the equation: Total weight gain/total food intake ×100. All values are presented as the mean ± standard deviation. The Kruskal-Wallis nonparametric test and Bonferroni's post-hoc test were used to control for multiple comparisons. FRB, fermented rice bran; FSB, fermented soybean cake; FSC, fermented sesame seed cake; UVB, ultraviolet B; RA, retinoic acid.
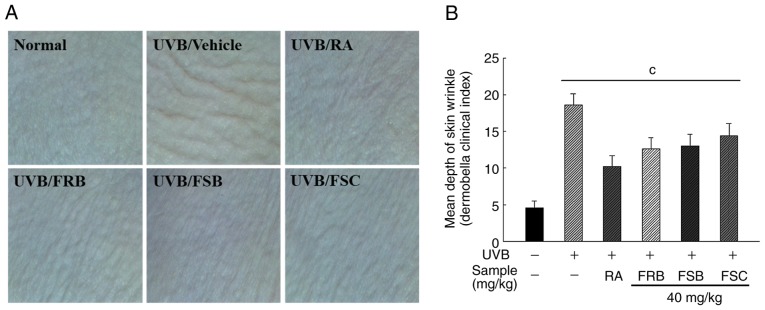
Inhibition of UVB-induced wrinkle formation by FRB, FSB and FSC
To measure the effect of FRB, FSB and FSC (40 mg/kg each) on wrinkle formation, HR-1 hairless mice were irradiated with UVB in the dorsal region for 8 weeks to induce photoaging. Skin wrinkle depth was measured using a Dermo Bella 3D analyzer (Fig. 2). Wrinkle formation and depth due to photoaging was observed to significantly increase (P<0.001) in the UVB vehicle group (18.60±1.52) when compared with the normal group (4.60±0.89). However, the group treated with retinoic acid exhibited a significant decrease (P<0.001) in the Dermo Bella clinical index value (10.20±1.48) when compared with the UVB vehicle group. The oral administration of FRB, FSB and FSC (40 mg/kg) also reduced the Dermo Bella clinical index to 12.6±1.52, 13.00±1.58 and 14.40±1.67, respectively.
Figure 2.
Effects of FRB, FSB and FSC on UVB-induced wrinkle formation in hairless mice. (A) Features of the dorsal skin and (B) mean skin wrinkle depth of hairless mice exposed to UVB for 8 weeks. Wrinkle formation was measured via replica grading in the final week and investigated using a scoring system. All values are presented as the mean ± standard deviation. One-way analysis of variance and Scheffe's post-hoc test were used to control for multiple comparisons. cP<0.001 vs. normal control mice. FRB, fermented rice bran; FSB, fermented soybean cake; FSC, fermented sesame seed cake; UVB, ultraviolet B; RA, retinoic acid.
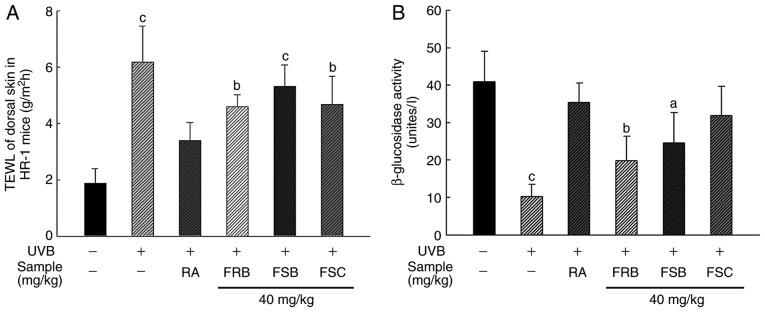
Effects of FRB, FSB and FSC on TEWL and β-Glucosidase activity
The stratum corneum of the skin is the outermost layer that prevents the excessive loss of moisture from the body. It is comprised of 10-20% moisture, which prevents the skin surface from drying and reduces skin barrier anomalies (32,33). Ceramide is a lipid component that constitutes the stratum corneum and is known to have a skin moisturizing effect, while β-glucosidase is an enzyme that regenerates ceramide from glycosylceramide and acyl glycosylceramide (34,35). Therefore, the moisture content of the stratum corneum is an index used to monitor skin damage. To evaluate the protective effect of FRB, FSB and FSC (40 mg/kg) against water loss, the UVB-irradiated dorsal skin of mice was analyzed. As presented in Fig. 3A, UVB irradiation caused a 3.3-fold increase in the TEWL in the skin compared with the normal group. However, retinoic acid treatment decreased the TEWL value by 1.3-, 1.2- and 1.3-fold in mice orally administered with FRB, FSB and FSC, respectively compared with the UVB vehicle group. β-glucosidase was used as an index to evaluate skin aging in the present study. As presented in Fig. 3B, β-glucosidase activity reduced 4.0-fold following UVB irradiation when compared with the normal group, which was recovered following retinoic acid treatment. β-glucosidase activity was also restored following the oral administration of FRB, FSB and FSC (40 mg/kg). FSC treatment particularly restored activity by ~1.3-fold. Therefore, FRB, FSB and FSC (40 mg/kg) may prevent photoaging, as observed from the TEWL and β-glucosidase assay results.
Figure 3.
Moisturizing effect of FRB, FSB and FSC on UVB-induced hairless mice. (A) TEWL in dorsal skin and (B) β-glucosidase activity. To estimate the TEWL, the dorsal skin of each mouse was examined using an appropriate probe. β-glucosidase activity was measured via an enzyme assay that monitored the conversion of 4-methylumbellifery-β-D-glucopyranoside to 4-methylumbelliferone. All values are presented as the mean ± standard deviation. One-way analysis of variance and Scheffe's post-hoc test were used to control for multiple comparisons. aP<0.05, bP<0.01 and cP<0.001 vs. normal control mice. TEWL, transepidermal water loss; FRB, fermented rice bran; FSB, fermented soybean cake; FSC, fermented sesame seed cake; UVB, ultraviolet B; RA, retinoic acid.
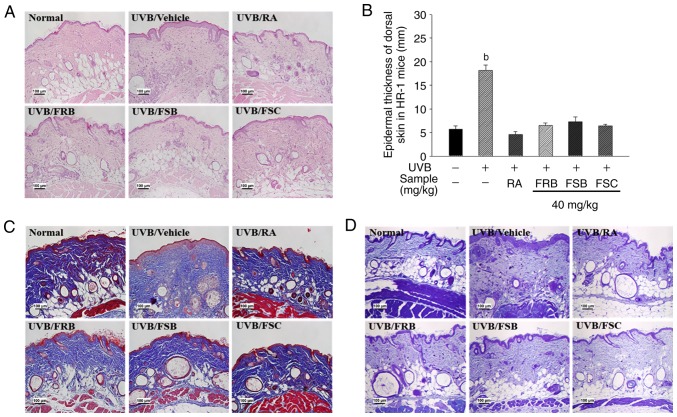
Effects of FRB, FSB and FSC on histological change
To evaluate the positive effect of FRB, FSB and FSC (40 mg/kg) on skin wrinkles, the epidermal thickness of the dorsal skin of UVB-irradiated HR-1 mice was measured via H&E staining (Fig. 4A and B). The epidermal thickness was observed to increase in the UVB vehicle group when compared with the normal control group. However, the mice orally administrated with FRB, FSB and FSC exhibited a significant decrease (P<0.01) in epidermal thickness compared with the UVB vehicle group. As presented in Fig. 4C, additional changes were observed in the collagen fiber structures in dorsal skin tissue via M-T staining. The intensity of M-T staining decreased in the UVB vehicle group when compared with the normal control group. This result indicates an acceleration in the wrinkle formation process owing to collagen degradation. However, the collagen fiber numbers increased in mice orally administered FRB, FSB and FSC when compared with those in the UVB vehicle group. Mast cell distribution and the degree of degranulation in the dermis and subcutaneous layer are presented in Fig. 4D. Mast cell infiltration around the dermis substantially increased in the UVB vehicle group when compared with the normal control group, whereas this effect was reduced in the tissues of mice treated with FRB, FSB and FSC (40 mg/kg). These results demonstrate that FRB, FSB and FSC inhibit UVB-induced changes in epidermal thickness, collagen degradation and mast cell infiltration.
Figure 4.
Inhibition of UVB-induced histological alteration by FRB, FSB and FSC in hairless mice. Histological alterations of epidermal thickness, collagen degradation and mast cell infiltration were analyzed using (A) haemotoxylin and eosin staining, (B) epidermal thickness of dorsal skin, (C) Masson's trichrome staining and (D) toluidine blue staining, respectively. All values are presented as the mean ± standard deviation. A Kruskal-Wallis nonparametric test and Bonferroni's post-hoc tests were used to control for multiple comparisons. bP<0.01 vs. normal control mice. FRB, fermented rice bran; FSB, fermented soybean cake; FSC, fermented sesame seed cake; UVB, ultraviolet B; RA, retinoic acid.
Effects of FRB, FSB and FSC oral intake on MMP expression
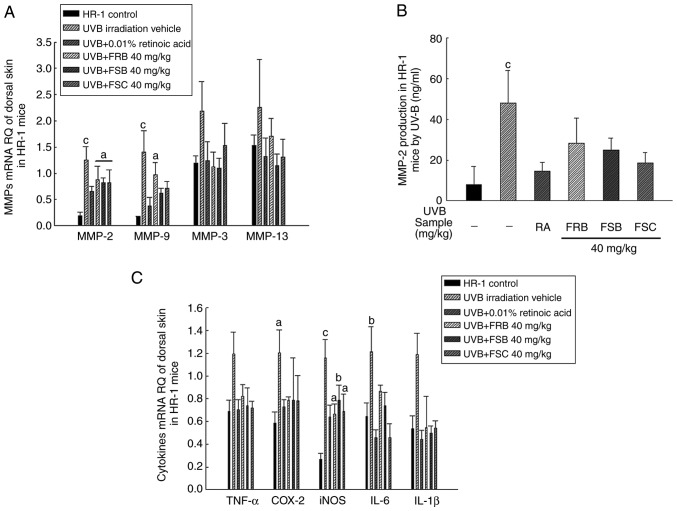
MMPs are zinc-dependent endopeptidases that are formed via the UVB irradiation-induced activation of MAPK signaling pathway transcription factors. Functionally, MMPs are classified as stromelysins (including MMP-3, -10 and -11), gelatinases (MMP-2 and -9) and collagenases (MMP-1, -8 and -13) on the basis of their substrate specificity (36). Stromelysins may cleave type IV collagen, in addition to proteoglycans, laminin and fibronectin (37). Collagenases specifically degrade collagen type I, II and III into characteristic 3/4 and 1/4 fragments (38). Subsequent to the initial cleavage, the collagen triple helix becomes denatured, and the dissociated polypeptide chains (gelatin molecules) may then be degraded by gelatinases (39). Gelatinase enzymes are able to break down type IV, V and VII collagen and exert activity against denatured collagen molecules (gelatin) (40). To determine the effect of FRB, FSB and FSC (40 mg/kg) on MMP expression, HR-1 hairless mice were irradiated with UVB to induce wrinkles, and the expression of wrinkle-associated genes, including collagenases (MMP-13), gelatinases (MMP-2 and -9), and stromelysins (MMP-3), were analyzed. As presented in Fig. 5A, MMP-2 and MMP-9 mRNA expression levels increased in the UVB vehicle group when compared with the normal control group; however, FSB and FSC (in MMP-9 only; 40 mg/kg) treatment significantly decreased (P<0.05) the expression levels of these mRNAs when compared with the UVB vehicle group. No significant difference in MMP-3 and MMP-13 mRNA expression levels were observed between the different groups; however, the mean values decreased in mice orally treated with FRB, FSB and FSC when compared with those in the UVB vehicle group. Similar to the MMP-2 mRNA gene expression results, MMP-2 protein expression levels were significantly inhibited (P<0.05) in mice treated with FSB and FSC compared with the UVB vehicle group (Fig. 5B). These results indicate that FRB, FSB and FSC (40 mg/kg) blocked collagen and gelatin degradation by inhibiting MMP activity. These results were sufficient to explain the inhibition of collagen and gelatin degradation, but the measurement of MMP-1 expression may provide more definite results. The skin is composed of collagen type II in addition to type I and III (41). MMP-13 cleaves collagen type II in preference to collagens type I and III (42). To complement these results, the determination of MMP-1 expression and immunohistochemistry for MMPs-induced wrinkle formation will be performed in future studies.
Figure 5.
Effects of FRB, FSB and FSC on MMP and cytokine expression in UVB-induced hairless mice. (A) MMP-2, MMP-9, MMP-3 and MMP-13 expression levels determined using RT-qPCR. Total cellular RNA was extracted using RNAzol from skin tissues. Next, 3 µg total RNA was used for cDNA synthesis via RT-qPCR with the appropriate primer sequences. (B) Enzyme-linked immunosorbent assay kit-mediated determination of the MMP-2 protein expression. (C) Measurement of inflammation-associated cytokines using RT-qPCR. All values are presented as the mean ± standard deviation. A Kruskal-Wallis nonparametric test and Bonferroni's post-hoc tests were used for MMP-3, MMP-13, TNF-α and IL-1β groups and one-way analysis of variance and Scheffe's post-hoc test were used to control for multiple comparisons. aP<0.05, bP<0.01 and cP<0.001 vs. normal control mice. RQ, MMP, matrix metalloproteinase; TNF-α, tumor necrosis factor-α; COX-2, cyclooxygenase-2; iNOS, inducible nitric oxide synthase; IL, interleukin; FRB, fermented rice bran; FSB, fermented soybean cake; FSC, fermented sesame seed cake; UVB, ultraviolet B; RA, retinoic acid; RT-qPCR, reverse transcription-quantitative polymerase chain reaction.
Effect of FRB, FSB and FSC oral intake on inflammatory cytokine expression
UVB-induced skin inflammation is a major cause of skin aging (43). Exposure of the skin to UVB radiation is known to enhance the levels of proinflammatory cytokines including TNF-α, IL-1β and IL-6 (44). Interestingly, a number of natural compounds have been demonstrated to exert anti-photoaging effects by inhibiting inflammatory cytokines (45). In the present study, a similar increase was observed in inflammatory cytokine levels following UVB irradiation, which were reduced following FRB, FSB and FSC treatments (40 mg/kg). To determine the effect of FRB, FSB and FSC (40 mg/kg) on inflammatory cytokine levels, HR-1 hairless mice were irradiated with UVB to induce skin inflammation, following which the inflammation-associated cytokines including TNF-α, COX-2, iNOS, IL-6 and IL-1β, were measured. The mRNA expression level of these inflammatory cytokines were observed to increase following UVB irradiation when compared with the normal group. However, the inhibition of UVB-induced cytokine expression was also confirmed subsequent to FRB (TNF-α, COX-2, iNOS, IL-1β, IL-6 all P<0.05), FSB (TNF-α and IL-6, P<0.05; IL-1β, P<0.001) and FSC (TNF-α and iNOS, P<0.05; IL-6 and IL-1β, P<0.001) treatments when compared with the UVB vehicle group (Fig. 5C). These observations suggest that FRB, FSB and FSC effectively inhibit the inflammatory response.
Discussion
Agricultural waste biomass is currently one of the most important challenges facing environmental protection, which has gained attention in the past several decades (46). The recycling of abandoned agricultural waste is meaningful not only for environmental protection but also for its potential value as functional food ingredients. The current study therefore assessed the possibility of FRB, FSB and FSC as ingredients for anti-photoaging. Skin is naturally composed of antioxidant defence systems against UV-induced ROS generation. However, these antioxidant defences are insufficient when exposed to solar radiation (47). ROS generated by UVB may affect MAPK signaling and inflammatory cytokines (23). Activated MAPK signaling increases the activity of various MMPs, leading to the structural and functional disruption of the ECM (24).
Wrinkle formation is caused by the decreased elasticity of the skin and is characteristic of aging (48). UVB is a typical extrinsic aging factor that reduces skin elasticity via wrinkle formation and is known to cause acute and chronic reactions in human skin (49). Exposure to UV light causes skin symptoms of skin inflammation, including erythema, edema, epidermis thickening, keratin thickening and increased skin pigmentation (50). Long-term exposure to UVB may also cause severe skin lesions, resulting in cell death and malignancy (51). The dermis is composed of collagen, elastin and hyaluronic acid (52). Photodamaged skin exhibits decreased elasticity due to decreased fibroblast function (53). Furthermore, photo-damage causes elastin fibers to reduce in number and diameter, and exhibit morphological changes (54). UV radiation also causes the degranulation of mast cells and the infiltration of chronic inflammatory cells (55,56). MMP expression also results in wrinkle formation via the decomposition of collagen and elastin, which are the main components of ECM (57).
The present study demonstrated that fermented agricultural by-products (FRB, FSB and FSC) may attenuate UVB-induced inflammatory cytokine production, collagen breakdown and mast cell infiltration in hairless mice. These products were also demonstrated to substantially reduce UVB-induced TEWL and wrinkle formation in mouse skin and upregulate β-glucosidase expression. Furthermore, the mRNA expression of a certain MMPs were revealed to be notably regulated by the fermented agricultural by-products. In conclusion, the results of the present study indicated that discarded agricultural by-products may be recycled via bioconversion and used as functional materials in the prevention of UVB-induced skin damage. However, the present study did not distinguish the efficacy of Lentinula edodes (shiitake) used for fermentation. Thus, future studies should determine the effect of fermentation with Lentinula edodes (shiitake) and assess the changes in bioconversion mechanisms that are involved with antioxidants and anti-photoaging.
Acknowledgments
Not applicable.
Abbreviations
- ECM
extracellular matrix
- ELISA
enzyme-linked immunosorbent assay
- H&E
hematoxylin and eosin
- MMP
matrix metalloproteinase
- UV
ultraviolet
- TNF-α
tumor necrosis factor-α
- COX-2
cyclooxygenase-2
- iNOS
inducible nitric oxide synthase
- TEWL
transepidermal water loss
- M-T
Masson's trichrome
Funding
The present study was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries, the High Value-added Food Technology Development Program (grant no. 314076-3) and a research grant from Kangwon National University in 2017. Additionally, the present study was supported by the National Research Foundation of Korea grant funded by the Korean Government (grant no. NRF-2018 H1A2A1062634; Fostering Core Leaders of the Future Basic Science Program/Global Ph.D. Fellowship Program).
Availability of data and materials
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
Authors' contributions
OHL, YCK and SJL designed the current study. SIC, TDJ, BYC, SHC, WSS and XH performed the experiments. SIC and TDJ wrote the manuscript. All authors performed data analysis, and drafted/critically revised the manuscript. All authors approval the version final version of the manuscript and agree to be accountable for all aspects of the study.
Ethics approval and consent to participate
The use of animals in the current study was approved by the Institutional Animal Care and Use Committee of Kangwon National University (approval no. KW-170417-1; 15 May 2017).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests, financial or otherwise, associated with this publication.
References
- 1.Schmalensee R. From 'Green Growth' to sound policies: An overview. Energy Econ. 2012;34(Suppl 1):S2–S6. doi: 10.1016/j.eneco.2012.08.041. [DOI] [Google Scholar]
- 2.Jänicke M. 'Green growth': From a growing eco-industry to economic sustainability. Energ Policy. 2012;48:13–21. doi: 10.1016/j.enpol.2012.04.045. [DOI] [Google Scholar]
- 3.Peschel W, Dieckmann W, Sonnenschein M, Plescher A. High antioxidant potential of pressing residues from evening primrose in comparison to other oilseed cakes and plant antioxidants. Ind Crop Prod. 2007;25:44–54. doi: 10.1016/j.indcrop.2006.07.002. [DOI] [Google Scholar]
- 4.Asadullah M. Barriers of commercial power generation using biomass gasification gas: A review. Renew Sust Energ Rev. 2013;24:201–215. [Google Scholar]
- 5.Iwata T. Biodegradable and bio-based polymers: Future prospects of eco-friendly plastics. Angew Chem Int Ed Engl. 2015;54:3210–3215. doi: 10.1002/anie.201410770. [DOI] [PubMed] [Google Scholar]
- 6.Perkins C, Siddiqui S, Puri M, Demain AL. Biotechnological applications of microbial bioconversions. Crit Rev Biotechnol. 2016;36:1050–1065. doi: 10.3109/07388551.2015.1083943. [DOI] [PubMed] [Google Scholar]
- 7.Cho YH, Cho JS, Lee GW. Antioxidant activity of wood vinegar by bioconversion. J Korea Acad Industr Coop Soc. 2011;12:4434–4442. doi: 10.5762/KAIS.2011.12.10.4434. [DOI] [Google Scholar]
- 8.Kiran EU, Trzcinski AP, NG WJ, Liu Y. Bioconversion of food waste to energy: A review. Fuel. 2014;134:389–399. doi: 10.1016/j.fuel.2014.05.074. [DOI] [Google Scholar]
- 9.Sanchez S, Demain AL. Enzymes and bioconversions of industrial, pharmaceutical, and biotechnological significance. Org Process Res Dev. 2011;15:224–230. doi: 10.1021/op100302x. [DOI] [Google Scholar]
- 10.Jung TD, Shin GH, Kim JM, Choi SI, Lee JH, Lee SJ, Park SJ, Woo KS, Oh SK, Lee OH. Comparative analysis of γ-oryzanol, β-glucan, total phenolic content and antioxidant activity in fermented rice bran of different varieties. Nutrients. 2017;9:E517. doi: 10.3390/nu9060571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jung TD, Shin GH, Kim JM, Oh JW, Choi SI, Lee JH, Lee SJ, Heo IY, Park SJ, Kim HT, et al. Assessment of validation method for bioactive contents of fermented soybean extracts by bioconversion and their antioxidant activities. J Korean Soc Food Sci Nutr. 2016;45:680–689. doi: 10.3746/jkfn.2016.45.5.680. [DOI] [Google Scholar]
- 12.Jung TD, Choi SI, Choi SH, Cho BY, Sim WS, Han-Xionggao, Lee SJ, Park SJ, Kim DB, Kim YC, et al. Changes in the anti-allergic activities of sesame by bioconversion. Nutrients. 2018;10:E210. doi: 10.3390/nu10020210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kruk J, Duchnik E. Oxidative stress and skin diseases: Possible role of physical activity. Asian Pac J Cancer Prev. 2014;15:561–568. doi: 10.7314/APJCP.2014.15.2.561. [DOI] [PubMed] [Google Scholar]
- 14.Webb AR, Engelsen O. Calculated ultraviolet exposure levels for a healthy vitamin D status. Photochem Photobiol. 2006;82:1697–1703. doi: 10.1111/j.1751-1097.2006.tb09833.x. [DOI] [PubMed] [Google Scholar]
- 15.Taylor GJ, Bannister GC, Leeming JP. Wound disinfection with ultraviolet radiation. J Hosp Infect. 1995;30:85–93. doi: 10.1016/0195-6701(95)90148-5. [DOI] [PubMed] [Google Scholar]
- 16.Imokawa G. Mechanism of UVB-induced wrinkling of the skin: Paracrine cytokine linkage between keratinocytes and fibroblasts leading to the stimulation of elastase. J Investig Dermatol Symp Proc. 2009;14:36–43. doi: 10.1038/jidsymp.2009.11. [DOI] [PubMed] [Google Scholar]
- 17.Matts PJ. Solar ultraviolet radiation: Definitions and terminology. Dermatol Clin. 2006;24:1–8. doi: 10.1016/j.det.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Algaba I, Riva A. In vitro measurement of the ultraviolet protection factor of apparel textiles. Color Technol. 2002;118:52–58. doi: 10.1111/j.1478-4408.2002.tb00137.x. [DOI] [Google Scholar]
- 19.Kim DB, Shin GH, Kim JM, Kim YH, Lee JH, Lee JS, Song HJ, Choe SY, Park IJ, Cho JH, Lee OH. Antioxidant and anti-ageing activities of citrus-based juice mixture. Food Chem. 2016;194:920–927. doi: 10.1016/j.foodchem.2015.08.094. [DOI] [PubMed] [Google Scholar]
- 20.Afaq F, Mukhtar H. Botanical antioxidants in the prevention of photocarcinogenesis and photoaging. Exp Dermatol. 2006;15:678–684. doi: 10.1111/j.1600-0625.2006.00466.x. [DOI] [PubMed] [Google Scholar]
- 21.Jurkiewicz BA, Bissett DL, Buettner GR. Effect of topically applied tocopherol on ultraviolet radiation-mediated free radical damage in skin. J Invest Dermatol. 1995;104:484–488. doi: 10.1111/1523-1747.ep12605921. [DOI] [PubMed] [Google Scholar]
- 22.Weichenthal M, Godorr M, Altenhoff J, Neuber K, Breitbart EW. Effects of whole-body UVB irradiation on cytokine production by peripheral blood mononuclear cells from stage I melanoma patients. Arch Dermatol Res. 2000;292:348–353. doi: 10.1007/s004030000140. [DOI] [PubMed] [Google Scholar]
- 23.Muthusamy V, Piva TJ. The UV response of the skin: A review of the MAPK, NFkappaB and TNFalpha signal transduction pathways. Arch Dermatol Res. 2010;302:5–17. doi: 10.1007/s00403-009-0994-y. [DOI] [PubMed] [Google Scholar]
- 24.Miyachi Y. Photoaging from an oxidative standpoint. J Dermatol Sci. 1995;9:79–86. doi: 10.1016/0923-1811(94)00363-J. [DOI] [PubMed] [Google Scholar]
- 25.Choi SH, Choi SI, Jung TD, Cho BY, Lee JH, Kim SH, Yoon SA, Ham YM, Yoon WJ, Cho JH, Lee OH. Anti-photoaging effect of jeju putgyul (unripe citrus) extracts on human dermal fibroblasts and ultraviolet B-induced hairless mouse skin. Int J Mol Sci. 2017;18:E2052. doi: 10.3390/ijms18102052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar S. Exploratory analysis of global cosmetic industry: Major players, technology and market trends. Technovation. 2005;25:1263–1272. doi: 10.1016/j.technovation.2004.07.003. [DOI] [Google Scholar]
- 27.Landriscina A, Rosen J, Friedman A. Nanotechnology, inflammation and the skin barrier: Innovative approaches for skin health and cosmesis. Cosmetics. 2015;2:177–186. doi: 10.3390/cosmetics2020177. [DOI] [Google Scholar]
- 28.Jeong SC, Park JH, Kim JH. The development trend of skin beauty food with skin protection effects from natural source. Asian J Beauty Cosmetol. 2013;11:203–212. [Google Scholar]
- 29.Yaar M, Gilchrest BA. Aging versus photo aging: Postulated mechanisms and effectors. J Investing Dermatol Symp Proc. 1998;3:47–51. [PubMed] [Google Scholar]
- 30.Bissett DL, Hannonand DP, Orr TV. An animal model of solar-aged skin: Histological, physical, and visible changes in UV-irradiated hairless mouse skin. Photochem Photobiol. 1987;46:367–378. doi: 10.1111/j.1751-1097.1987.tb04783.x. [DOI] [PubMed] [Google Scholar]
- 31.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 32.Kwon SB, Lee GT, Choi SJ, Lee NK, Park HW, Lee KS, Lee KK, Ahn KJ, An IS. The effect of glycerin, hyaluronic acid and silicone oil on the hydration, moisturization and transepidermal water loss in human skin. Asian J Beauty Cosmetol. 2013;11:761–768. [Google Scholar]
- 33.Nasir A. Diseases associated with cutaneous barrier dysfunction: Basic science aspects and clinical perspectives. In: Monteiro-Riviere NA, editor. Toxicology of the Skin. Informa Healthcare; New York: 2010. pp. 203–279. [Google Scholar]
- 34.Kitatani K, Sheldon K, Rajagopalan V, Anelli V, Jenkins RW, Sun Y, Grabowski GA, Obeid LM, Hannun YA. Involvement of acid beta-glucosidase 1 in the salvage pathway of ceramide formation. J Biol Chem. 2009;284:12972–12978. doi: 10.1074/jbc.M802790200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sirikudta W, Kulthanan K, Varothai S, Nuchkull P. Moisturizers for patients with atopic dermatitis: An overview. J Allergy Ther. 2013;4:1–6. doi: 10.4172/2155-6121.1000143. [DOI] [Google Scholar]
- 36.Gkouveris I, Nikitakis N, Aseervatham J, Rao N, Ogbureke KUE. Matrix metalloproteinases in head and neck cancer: Current perspectives. Metalloproteinases Med. 2017;4:47–61. doi: 10.2147/MNM.S105770. [DOI] [Google Scholar]
- 37.Chin JR, Murphy G, Werb Z. Stromelysin, a connective tissue-degrading metalloendopeptidase secreted by stimulated rabbit synovial fibroblasts in parallel with collage-nase. Biosynthesis, isolation, characterization, and substrates. J Biol Chem. 1985;260:12367–12376. [PubMed] [Google Scholar]
- 38.Sunami E, Tsuno N, Osada T, Saito S, Kitayama J, Tomozawa S, Tsuruo T, Shibata Y, Muto T, Nagawa H. MMP-1 is a prognostic marker for hematogenous metastasis of colorectal cancer. Oncologist. 2000;5:108–114. doi: 10.1634/theoncologist.5-2-108. [DOI] [PubMed] [Google Scholar]
- 39.Fini ME, Girard MT. The pattern of metalloproteinase expression by corneal fibroblasts is altered by passage in cell culture. J Cell Sci. 1990;97:373–383. doi: 10.1242/jcs.97.2.373. [DOI] [PubMed] [Google Scholar]
- 40.Murphy G, Hembry RM, McGarrity AM, Reynolds JJ, Henderson B. Gelatinase (type IV collagenase) immunolocalization in cells and tissues: Use of an antiserum to rabbit bone gelatinase that identifies high and low Mr forms. J Cell Sci. 92:487–495. doi: 10.1242/jcs.92.3.487. [DOI] [PubMed] [Google Scholar]
- 41.Meigel WN, Gay S, Weber L. Dermal architecture and collagen type distribution. Arch Dermatol Res. 1977;259:1–10. doi: 10.1007/BF00562732. [DOI] [PubMed] [Google Scholar]
- 42.Robichaud TK, Steffensen B, Fields GB. Exosite interactions impact matrix metalloproteinase collagen specificities. J Biol Chem. 2011;286:37535–37542. doi: 10.1074/jbc.M111.273391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pillai S, Oresajo C, Hayward J. Ultraviolet radiation and skin aging: Roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation-a review. Int J Cosmet Sci. 2005;27:17–34. doi: 10.1111/j.1467-2494.2004.00241.x. [DOI] [PubMed] [Google Scholar]
- 44.Nichols JA, Katiyar SK. Skin photoprotection by natural polyphenols: Anti-inflammatory, antioxidant and DNA repair mechanisms. Arch Dermatol Res. 2010;302:71–83. doi: 10.1007/s00403-009-1001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen CC, Chiang AN, Liu HN, Chang YT. EGb-761 prevents ultraviolet B-induced photoaging via inactivation of mitogen-activated protein kinases and proinflammatory cytokine expression. J Dermatol Sci. 2014;75:55–62. doi: 10.1016/j.jdermsci.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 46.Fo KY, Hameed BH. Utilization of rice husk ash as novel adsorbent: A judicious recycling of the colloidal agricultural waste. Adv Colloid Interfac Sci. 2009;152:39–47. doi: 10.1016/j.cis.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 47.Puglia C, Offerta A, Saija A, Trombetta D, Venera C. Protective effect of red orange extract supplementation against UV-induced skin damages: Photoaging and solar lentigines. J Cosmet Dermatol. 2014;13:151–157. doi: 10.1111/jocd.12083. [DOI] [PubMed] [Google Scholar]
- 48.Davies KJ. Protein damage and degradation by oxygen radical. I. general aspects. J Biol Chem. 1987;262:9895–9901. [PubMed] [Google Scholar]
- 49.Bissett DL, Chatterjee R, Hannon DP. Photoprotective effect of superoxide-scavenging antioxidants against ultraviolet radiation-induced chronic skin damage in the hairless mouse. Photodermatol Photoimmunol Photomed. 1990;7:56–62. [PubMed] [Google Scholar]
- 50.Korać RR, Khambholja KM. Potential of herbs in skin protection from ultraviolet radiation. Pharmacog Rev. 2011;5:164–173. doi: 10.4103/0973-7847.91114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Choi WH, Ann HS, Choi TY, Jin SY, Ahn RM. Effects of natural extracts on UVB-induced pigmentation and inflammation in C57BL/6 mouse skin. Korean J Environ Health Sci. 2006;32:492–498. [Google Scholar]
- 52.Nanashima N, Horie K, Maeda H, Tomisawa T, Kitajima M, Nakamura T. Blackcurrant anthocyanins increase the levels of collagen, elastin and hyaluronic acid in human skin fibroblasts and ovariectomized rats. Nutrients. 2018;10:E495. doi: 10.3390/nu10040495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Varani J, Schuger L, Dame MK, Leonard C, Fligiel SE, Kang S, Fisher GJ, Voorhees JJ. Reduced fibroblast interaction with intact collagen as a mechanism for depressed collagen synthesis in photodamaged skin. J Investig Dermatol. 2004;122:1471–1479. doi: 10.1111/j.0022-202X.2004.22614.x. [DOI] [PubMed] [Google Scholar]
- 54.Frances C, Robert L. Elastin and elastic fibers in normal and pathologic skin. Int J Dermatol. 1984;23:166–179. doi: 10.1111/j.1365-4362.1984.tb04506.x. [DOI] [PubMed] [Google Scholar]
- 55.Kligman LH, Kligman AM. The nature of photoaging: Its prevention and repair. Photodermatol. 1986;3:215–227. [PubMed] [Google Scholar]
- 56.Foote CS. Photosensitized oxidation and singlet oxygen; consequences in biological systems. In: Pryor WA, editor. Free Radicals in Biology. Academic Press; New York: 1976. pp. 85–133. [DOI] [Google Scholar]
- 57.Hwang BM, Noh EM, Kim JS, Kim JM, You YO, Hwang JK, Kwon KB, Lee YR. Curcumininhibits UVB-induced matrix metalloproteinase-1/3 expression by suppressing the MAPK-p38/JNK pathways in human dermal fibroblasts. Exp Dermatol. 2013;22:371–374. doi: 10.1111/exd.12137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.