Abstract
Background
Upper gastrointestinal bleeding is typically a mild, self‐limiting condition that can affect both preterm and term neonates, although it can be severe particularly when associated with co‐morbidities. Pharmacological interventions with a proton pump inhibitor (PPI), H2 receptor antagonist (H2RA), antacid, bismuth and sucralfate may have effects on both the prevention and treatment of upper gastrointestinal bleeding in infants.
Objectives
To assess how different pharmacological interventions (PPIs, H2RAs, antacids, sucralfate or bismuth salts) administered to preterm and term neonates for the prevention or treatment of upper gastrointestinal bleeding to reduce morbidity and mortality compare with placebo or no treatment, supportive care, or each other.
Search methods
We used the standard search strategy of Cochrane Neonatal to search the Cochrane Central Register of Controlled Trials (CENTRAL 2018, Issue 6), MEDLINE via PubMed (1966 to 12 July 2018), Embase (1980 to 12 July 2018), and CINAHL (1982 to 12 July 2018). We also searched clinical trial databases, conference proceedings, the reference lists of retrieved articles for randomised controlled trials and quasi‐randomised trials, and online for Chinese literature articles.
Selection criteria
We selected randomised, quasi‐randomised and cluster‐randomised trials involving preterm and term neonates. Trials were included if they used a proton pump inhibitor, H2 receptor antagonist, antacid, sucralfate or bismuth either for the prevention or treatment of upper gastrointestinal bleeding.
Data collection and analysis
Two review authors independently assessed the eligibility of studies for inclusion, extracted data and assessed methodological quality. We conducted meta‐analysis using a fixed‐effect model. We used the GRADE approach to assess quality of evidence.
Main results
Eleven studies with 818 infants met the criteria for inclusion in this review.
Four trials with 329 infants assessed the use of an H2 receptor antagonist for prevention of upper gastrointestinal bleeding in high‐risk newborn infants. Meta‐analysis of these four trials identified a reduction in any upper gastrointestinal bleeding when using an H2 receptor antagonist (typical risk ratio (RR) 0.36, 95% confidence interval (CI) 0.22 to 0.58; typical risk difference (RD) −0.20, 95% CI −0.28 to −0.11; number needed to treat for an additional beneficial outcome (NNTB) 5, 95% CI 4 to 9). The quality of evidence was moderate. A single trial with 53 infants assessing prevention of upper gastrointestinal bleeding reported no difference in mortality in infants assigned H2 receptor antagonist versus no treatment; however the quality of evidence was very low.
Seven trials with 489 infants assessed an inhibitor of gastric acid (H2 receptor antagonist or proton pump inhibitor) for treatment of gastrointestinal bleeding in newborn infants. Meta‐analysis of two trials (131 infants) showed no difference in mortality from use of a H2 receptor antagonist compared to no treatment. The quality of evidence was low. Meta‐analysis of two trials (104 infants) showed a reduction in duration of upper gastrointestinal bleeding from use of an inhibitor of gastric acid compared to no treatment (mean difference −1.06 days, 95% CI −1.28 to −0.84). The quality of evidence was very low. Meta‐analysis of six trials (451 infants) showed a reduction in continued upper gastrointestinal bleeding from use of any inhibitor of gastric acid compared to no treatment (typical RR 0.36, 95% CI 0.26 to 0.49; typical RD −0.26, 95% CI −0.33, −0.19; NNTB 4, 95% CI 3 to 5). The quality of evidence was low. There were no significant subgroup differences in duration of upper gastrointestinal bleeding or of continued upper gastrointestinal bleeding according to type of inhibitor of gastric acid. A single trial (38 infants) reported no difference in anaemia requiring blood transfusion from use of a H2 receptor antagonist compared to no treatment.
Although no serious adverse events were reported from the use of a H2 receptor antagonist or proton pump inhibitor, some neonatal morbidities — including necrotising enterocolitis, ventilator‐associated pneumonia, duration of ventilation and respiratory support, and duration of hospital stay — were not reported. Long‐term outcome was not reported.
Authors' conclusions
There is moderate‐quality evidence that use of an H2 receptor antagonist reduces the risk of gastrointestinal bleeding in newborn infants at high risk of gastrointestinal bleeding. There is low‐quality evidence that use of an inhibitor of gastric acid (H2 receptor antagonist or proton pump inhibitor) reduces the duration of upper gastrointestinal bleeding and the incidence of continued gastric bleeding in newborn infants with gastrointestinal bleeding. However, there is no evidence that use of an inhibitor of gastric acid in newborn infants affects mortality or the need for blood transfusion. As no study reported the incidence of necrotising enterocolitis, ventilator‐ or hospital‐associated pneumonia, sepsis, or long‐term outcome, the safety of inhibitors of gastric acid secretion is unclear.
Plain language summary
Pharmacological interventions to prevent and treat upper gastrointestinal bleeding in neonates
Review question To assess how different medications for reducing stomach acidity (proton pump inhibitors, histamine 2 receptor antagonists, antacids) or for protecting the stomach lining (sucralfate or bismuth salts) given to preterm and term infants help to prevent or treat upper gastrointestinal bleeding, reduce suffering of other illnesses and deaths.
Background Upper gastrointestinal bleeding is common in sick newborns admitted to neonatal intensive care. It may be associated with reflux of milk (gastroesophageal reflux) or allergy to milk proteins. Common symptoms include vomiting of material which can be either bloodstained or like coffee grounds in appearance; and black, tarry stools. When occurring in otherwise well newborns it is typically a mild, self‐limiting condition. However, upper gastrointestinal bleeding can be severe particularly when associated with other underlying conditions.
Study characteristics We included randomised controlled trials (RCTs). The search is up to date as of 12 July 2018.
Key results We found 11 trials with 818 infants. We considered no trial to be at low risk of bias.
Four trials included 329 infants in neonatal intensive care units and used a histamine 2 receptor antagonist for prevention of upper gastrointestinal bleeding. These four trials demonstrated a reduction in the incidence of upper gastrointestinal bleeding with a histamine 2 receptor antagonist, but no change in mortality. Outcomes such as serious gastrointestinal problems (e.g. necrotising enterocolitis) and infections were not reported.
Seven trials with 489 infants enrolled sick newborn infants with upper gastrointestinal bleeding and used either a histamine 2 receptor antagonist or a proton pump inhibitor for treatment. Use of a histamine 2 receptor antagonist or proton pump inhibitor in a treatment context was associated with a reduction of both duration of upper gastrointestinal bleeding and continued upper gastrointestinal bleeding; however it did not affect mortality or requirement for blood transfusion. No long‐term follow‐up was reported.
Although there is moderate‐quality evidence that use of an inhibitor of gastric acid reduces the incidence and duration of upper gastrointestinal bleeding in newborn infants, there is insufficient safety data in this population. The implication of this is that caution should be applied when deciding whether to use an inhibitor of gastric acid in sick newborn infants until additional studies are performed.
Quality of the evidence We graded the quality of evidence for prevention of upper gastrointestinal bleeding as low and moderate. We graded the quality of evidence for the treatment of upper gastrointestinal bleeding as low and very low.
Summary of findings
Background
Description of the condition
Upper gastrointestinal bleeding (arising proximal to the ligament of Treitz in the distal duodenum) is typically a mild, self‐limiting condition that can affect both preterm and term neonates. Common signs of upper gastrointestinal bleeding include vomiting of bloodstained material or material with the appearance of coffee grounds (haematemesis); and black, tarry stools (melena) (Green 2003). Upper gastrointestinal bleeding can be diagnosed clinically by the presence of blood‐stained aspirates through indwelling nasogastric or orogastric tubes; haematemesis; or endoscopically by examining the gastric mucosa for bleeding lesions (Green 2003). It is important to differentiate newborn infants who have swallowed maternal blood from those with gastric bleeding (Apt 1955). Severe gastrointestinal bleeding occurs in newborn infants but is more common in older infant patient populations (Romano 2017). A bleeding associated mortality rate of 2.07% has been reported in children admitted with gastrointestinal bleeding in the USA (Attard 2017). Mortalities were significantly more likely to have multiple complex chronic conditions compared to children with a principle diagnosis of gastrointestinal bleeding with a mortality of 0.37%. However, the outcomes of newborns with upper gastrointestinal bleeding have not been similarly reported.
Reported causes of gastrointestinal bleeding in newborns include coagulation disorders, such as vitamin K deficiency, cow’s milk protein allergy, stress‐related gastritis, sepsis, and trauma from placement of nasogastric tubes (Boyle 2008; Chawla 2007; Romano 2017). However population data on the incidence and causes in newborns are limited. Lazzaroni 2002 reported 64 of 5180 newborn babies (1.23%) suffered from upper gastrointestinal bleeding. Endoscopy revealed oesophageal damage in 24/53 infants, gastric and duodenal lesions in 43/52 and 1/52 infants, respectively. No maternal or newborn infant clinical risk factors were identified between cases and controls. A retrospective study undertaken by Kuusela 2000 reported approximately 20% of infants treated in a neonatal intensive care unit had signs of gastrointestinal bleeding, with mechanical ventilation the major risk factor. In mechanically ventilated infants, 53% had gastric mucosal lesions. Risk factors for mucosal lesions included interventional delivery, delayed delivery and hypotension after birth.
Description of the intervention
Acid suppression agents are used in the management of upper gastrointestinal bleeding in the adult population (Mejia 2009). However, evidence‐based recommendations supporting similar benefits for newborn infants are not readily available. The main principle underlying pharmacological intervention is to protect the gastric mucosa from damage and promote healing. In adults with peptic ulcers, gastric acidity has been associated with ongoing mucosal tissue damage and reduced clot formation (Kolkman 1996). Individuals with gastric ulcers and a gastric acidity of pH less than 6 are predisposed to increased fibrinolysis of overlying clots (Green 1978). Although the role of acid in the aetiology of gastric lesions in adults has been described, its role is unclear in neonates (Maki 1993).
Drugs commonly used to treat conditions associated with upper gastrointestinal bleeding include the following.
Proton pump inhibitors (PPIs) ‒ acid suppressors (e.g. omeprazole).
H2 receptor antagonists (H2RAs) ‒ acid suppressors (e.g. ranitidine, cimetidine and famotidine).
Antacids ‒ acid neutralisers (e.g. sodium/calcium carbonate, magnesium/aluminium hydroxide and almagate).
Mucosal protective agents (e.g. sucralfate and bismuth salts).
PPIs, such as omeprazole, are prodrugs that require protonation to exert their inhibitory effect on H+‐K+‐ATPase. This results in decreased gastric acid production (Sachs 2007). PPIs further protect the mucosal lining of the stomach by reducing pepsin secretion (Brunner 1995). H2RAs, such as ranitidine, also act to suppress gastric acid production. They achieve this through binding to H2 receptors on the basolateral side of parietal cells, which results in inhibition of the effects of histamine. H2RAs have a faster onset of action but shorter duration of action compared to PPIs (Australian Medicines Handbook 2013).
Significant adverse effects of PPIs and H2RAs are infrequent or rare and include hypotension, rashes, thrombocytopaenia and insomnia (Australian Medicines Handbook 2013). H2RAs have been associated with an increased risk of necrotizing enterocolitis (NEC) in very low birth weight (VLBW) infants (More 2013; Terrin 2012). In mechanically ventilated patients, PPIs were associated with an increased risk of gastrointestinal haemorrhage, pneumonia and Clostridium difficile infection compared to patients administered H2RAs (MacLaren 2014).
Unlike PPIs and H2RAs, antacids exert their effects within the gut lumen. They both neutralise gastric acid and inhibit pepsin activity (Maton 1999). Sodium bicarbonate, calcium bicarbonate, magnesium hydroxide, aluminium hydroxide and almagate are examples of antacids (Mejia 2009). Possible adverse effects of antacids include hypophosphataemia, hypermagnesaemia, intestinal obstruction and osteomalacia (Australian Medicines Handbook 2013).
Whilst PPIs, H2RAs and antacids predominantly influence acid secretion, sucralfate and bismuth treatment aims to protect the gastric mucosa (Australian Medicines Handbook 2013). Both of these drugs are excreted in faeces (Mejia 2009). Sucralfate may cause adverse constipation and nausea, whilst bismuth more commonly causes blackening of faeces and darkening of the teeth and tongue (Australian Medicines Handbook 2013).
How the intervention might work
In adult patients, PPIs are indicated for both the prevention and treatment of upper gastrointestinal bleeding (Australian Medicines Handbook 2013). Treatment with PPIs has been shown to decrease the rate of re‐bleeding compared to H2RAs or placebo (Leontiadis 2009). This effect has been demonstrated to occur independently of dose, route of administration or geographic location. In adults with upper gastrointestinal bleeding, high‐dose omeprazole infusion has been shown to reduce signs of upper gastrointestinal bleeding and the need for endoscopy therapy (Lau 2007).
Since gastric acid production is not solely regulated by histamine, H2RAs are not as effective as PPIs at reducing acid production. Consequently, H2RA treatment is no longer recommended for treatment in adult patients with acute ulcer bleeding. However, H2RAs may prove to be useful in treating upper gastrointestinal bleeding in neonates (Barkun 2010). Although PPIs have superior acid suppression ability, the shorter onset of action of H2RAs may provide faster relief of upper gastrointestinal bleeding symptoms (Mejia 2009).
In adults, antacid use has largely been replaced by PPIs and H2RAs. Despite this, acid neutralisation may have a greater role in the prophylaxis or treatment of upper gastrointestinal bleeding in neonates.
In response to the acidic environment of the gut, sucralfate promotes mucosal healing via angiogenesis, growth factor delivery and granulation tissue formation at ulcer sites (Tarnawski 1995). Healing mechanisms of bismuth include stimulating prostaglandin and bicarbonate production in addition to inhibiting the growth of Helicobacter pylori in the gut mucosa (Mejia 2009). These mechanisms of mucosal protection may have greater importance in the prophylaxis of upper gastrointestinal bleeding in neonates.
The adverse effects associated with acid suppression agents may occur due to several underlying mechanisms. The importance of gastric acid in clearance of ingested pathogens, for example, has been postulated as an underlying pathway in the pathogenesis of NEC in preterm infants (Martinsen 2005; More 2013; Terrin 2012); and to predispose to ventilator‐associated pneumonia (MacLaren 2014). Inhibiting gastric acid has also been associated with reduced calcium and vitamin B12 absorption, although the significance of this in the neonatal population is unclear (Martinsen 2005). As such, acid suppression agents may represent potentially harmful interventions for preterm and term infants.
Why it is important to do this review
Current recommendations for the prevention and treatment of upper gastrointestinal bleeding in neonates are based on those used for adults, largely due to a lack of studies in neonates. International consensus guidelines recommend intravenous and oral PPIs, but not H2RAs, for the prevention and treatment of acute upper gastrointestinal bleeding in adults (Barkun 2010). However, a recent study by Sreedharan 2010 demonstrated the usefulness of treatment with PPIs in reducing stigmata of recent haemorrhage but not in reducing mortality and re‐bleeding rates. A previous Cochrane Review described benefits of PPI treatment for acute upper gastrointestinal bleeding in adults, but not in neonates (Leontiadis 2006). A Cochrane Review reported moderate evidence was found to support the use of PPIs, along with some evidence to support the use of H2RAs in children with gastroesophageal reflux disease (GORD), based on improvement in symptom scores, pH indices and endoscopic/histological appearances; however, no robust RCT evidence was found regarding treatment of preterm babies with GORD (Tighe 2014). Current guidelines of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) do not make specific recommendations for treatment of newborn or preterm infants with GORD (Vandenplas 2009). The Italian Society of Pediatric Gastroenterology identified three classes of drugs for use in paediatric gastrointestinal bleeding (acid suppression drugs, vasoactive drugs and non‐selective β‐blockers) but notes the literature supporting use of these agents is limited to RCTs in adults (Romano 2017). No guidelines were found that specifically address upper gastrointestinal bleeding in newborn infants. Given that sick newborn infants at risk of, and newborn infants with, upper gastrointestinal bleeding are frequently treated with inhibitors of gastric acid secretion, that bleeding can be severe, and that the safety of treatment has not been reviewed in newborn infants, a systematic review of prevention and treatment was needed. The lack of guidelines/consensus on pharmacological prophylaxis and treatment of upper gastrointestinal bleeding in neonates formed the basis for this Cochrane Review.
Objectives
To assess how different pharmacological interventions (PPIs, H2RAs, antacids, sucralfate or bismuth salts) administered to preterm and term neonates for the prevention or treatment of upper gastrointestinal bleeding to reduce morbidity and mortality compare with placebo or no treatment, supportive care, or each other.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs), quasi‐RCTs and cluster‐RCTs.
Types of participants
We considered all preterm and term infants with, or at risk of, upper gastrointestinal bleeding as eligible for inclusion.
We defined infants at risk of upper gastrointestinal bleeding as those with known upper gastrointestinal bleeding risk factors including born at preterm gestation, birth asphyxia, mechanical ventilation, hypotension, neonatal cyanosis and neonatal seizures (Kuusela 2000; Ombeva 2013).
We defined upper gastrointestinal bleeding as: the macroscopic presence of bloodstained material or material like coffee grounds in gastric aspirates or haematemesis; the microscopic presence of blood in gastric aspirates or haematemesis; or endoscopic examination of the gastric mucosa for bleeding lesions (Green 2003). We excluded infants who had swallowed maternal blood.
Types of interventions
Pharmacological intervention (H2RAs, PPIs, antacids, sucralfate, bismuth salts, or any combination) administered enterally or parenterally at any dose or frequency with or without co‐administration with other modalities (e.g. ceasing oral feeds; ceasing NSAIDs) with the intention of preventing or treating upper gastrointestinal bleeding, compared with each other, placebo, no intervention or supportive therapy.
We excluded treatment trials for upper gastrointestinal bleeding aimed at correcting underlying bleeding disorders, such as vitamin K administration for vitamin K deficiency or fresh frozen plasma transfusion, as these have already been described in previous Cochrane Reviews (Ardell 2010; Puckett 2000).
We planned to analyse the following comparisons.
a) Comparisons between drug classes
PPIs vs. H2RAs;
PPIs vs. antacids;
PPIs vs. bismuth;
PPIs vs. sucralfate;
PPIs vs. placebo, no treatment or supportive care;
H2RAs vs. antacids;
H2RAs vs. bismuth;
H2RAs vs. sucralfate;
H2RAs vs. placebo, no treatment or supportive care;
Antacids vs. bismuth;
Antacids vs. sucralfate;
Antacids vs. placebo, no treatment or supportive care;
Bismuth vs. sucralfate;
Bismuth vs. placebo, no treatment or supportive care;
Sucralfate vs. placebo, no treatment or supportive care.
b) Comparisons between specific drugs of different classes
Specific H2RAs vs. specific PPIs;
Specific antacids vs. specific PPIs;
Bismuth vs. specific PPIs;
Sucralfate vs. specific PPIs;
Specific antacid vs. specific H2RAs;
Bismuth vs. specific H2RAs;
Sucralfate vs. specific H2RAs;
Specific antacids vs. bismuth;
Specific antacids vs. sucralfate;
Bismuth vs. sucralfate.
c) Comparison between specific drugs within a drug class
Specific H2RAs vs. specific H2RAs;
Specific PPIs vs. specific PPIs;
Specific antacids vs. specific antacids.
d) Comparisons of combinations of specific drugs
H2RAs plus one or more of the following: antacids, sucralfate or bismuth salts vs. PPIs;
H2RAs plus one or more of the following: antacids, sucralfate or bismuth salts vs. placebo, no treatment or supportive care;
PPIs plus one or more of the following; antacids, sucralfate or bismuth salts. vs. H2RAs;
PPIs plus one or more of the following; antacids, sucralfate or bismuth salts. vs. placebo, no treatment or supportive care.
Types of outcome measures
Primary outcomes
All‐cause mortality to near‐term age or discharge.
Any upper gastrointestinal bleeding in infants at risk of upper gastrointestinal bleeding.
Duration of upper gastrointestinal bleeding in infants with upper gastrointestinal bleeding (days).
Secondary outcomes
Gastric lesions detected by endoscopy and macroscopically.
All‐cause infant mortality (i.e. < 1 year of age).
Anaemia requiring blood transfusion.
Volume of blood transfused for treatment of upper gastrointestinal bleeding (mL/kg).
Number of blood transfusions for treatment of upper gastrointestinal bleeding.
Necrotising enterocolitis (NEC) as defined by Bell stages 1 to 4 (confirmed = Bell stage 2 or greater) (Bell 1978).
Time to full feeds (days).
Duration of total parenteral nutrition (days).
Proven infection (culture positive from a normally sterile site).
Neonatal cholestasis ('serum conjugated bilirubin concentration > 17.1 µM and total serum bilirubin < 85.5 µM' or 'serum conjugated bilirubin concentration > 20% of total serum bilirubin if total serum bilirubin is > 85.5 µM').
Ventilator‐associated pneumonia (new or progressive infiltrate with positive respiratory specimens after 48 hours of mechanical ventilation).
Neonatal chronic lung disease (also known as bronchopulmonary dysplasia) (defined according to the 2001 National Institute of Child Health and Development criteria (Jobe 2001): treatment with oxygen > 21% for at least 28 days).
Duration of ventilation (days).
Duration of respiratory support (days).
Duration of hospital stay (days).
Neurodevelopmental disability (defined as neurological abnormality including cerebral palsy on clinical examination or global developmental delay (two or more standard deviations (SDs) below population mean on Bayley Scales of Infant Development or Griffiths Mental Development Scales at any time after term corrected at 1 year, 18 months, 2 years and 5 years postnatal age).
Haemorrhagic shock (at least 15% blood loss).
Thrombocytopenia (platelet count < 150,000/µL).
Retinopathy of prematurity or other severe adverse events.
Serious adverse reactions (potentially related to pharmacological intervention) (post hoc addition to protocol).
Search methods for identification of studies
We used the criteria and standard methods of Cochrane and Cochrane Neonatal (see the Cochrane Neonatal search strategy for specialized register). We searched for errata or retractions from included studies published in full text on PubMed (www.ncbi.nlm.nih.gov/pubmed) on 12 July 2018. We did not limit the search to any particular geographical region, language or timing of publication. Unpublished studies were eligible for review.
Electronic searches
We conducted a comprehensive search — including Cochrane Central Register of Controlled Trials (CENTRAL 2018, Issue 6) in the Cochrane Library, MEDLINE via PubMed (1966 to 12 July 2018), Embase (1980 to 12 July 2018), and CINAHL (1982 to 12 July 2018) — using topic‐specific search terms, plus database‐specific limiters for RCTs and neonates (see Appendix 1 for the full search strategies for each database). We did not apply language restrictions.
Searching other resources
We conducted additional searches of the following resources.
1. Ongoing trials in the following trial registries.
ClinicalTrials.gov (U.S. National Institutes of Health);
World Health Organization International Clinical Trials Registry Platform (ICTRP);
ISRCTN registry;
Australian New Zealand Clinical Trials Registry.
2. Conference abstracts from the following.
Proceedings of the Pediatric Academic Societies (American Pediatric Society, Society for Pediatric Research and European Society for Pediatric Research) from 1990 to current from the journal Pediatric Research and Abstracts Online.
Proceedings of the European Academy of Paediatric Societies (EAPS) (The European Society for Paediatric Research (ESPR), the European Academy of Paediatrics (EAP) and the European Society of Paediatric and Neonatal Intensive Care (ESPNIC) from 2003 to current from Abstracts Online).
Proceedings of the Perinatal Society of Australia and New Zealand (PSANZ) from 1996 to current (handsearch).
3. Reference lists: after reading the identified individual studies, we screened the reference lists of these papers to identify further relevant studies about upper gastrointestinal bleeding.
4. Personal communications.
Where unpublished trials were identified, we contacted the corresponding investigator for information on any unpublished trials potentially eligible for inclusion. Unpublished studies will be eligible for review.
We contacted the corresponding authors of identified RCTs for additional information where necessary.
5. Pharmaceutical companies: we contacted the respective pharmaceutical companies responsible for developments of H2RA, PPI, antacid, sucralfate or bismuth salts products used in the prevention or treatment of upper gastrointestinal bleeding for unpublished studies.
4. Chinese language articles from China/Asia On Demand (CAOD).
Data collection and analysis
We used the standard methods of the Cochrane Neonatal Review Group and Cochrane, as documented in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Selection of studies
Two review authors independently assessed studies identified for inclusion. We resolved any differences through discussion and consensus.
Data extraction and management
Two review authors (DG, MA) independently extracted data using specifically designed data extraction forms. We resolved disagreements through discussion and consensus with a third author (DO). We used the retrieved information to determine trial eligibility, to extract methods and data from eligible trials and for requesting additional unpublished information from authors of original reports. We used Review Manager 2014 to enter and cross‐check data.
Assessment of risk of bias in included studies
Two review authors (DG and MA) independently assessed the risk of bias (low, high, or unclear) of all included trials in the following domains, using the Cochrane ‘Risk of bias’ tool (Higgins 2017).
Sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Any other bias.
When necessary, we requested additional information and clarification of published data from trial authors. We resolved any disagreements by discussion or by inviting a third assessor to arbitrate. See Appendix 2 for a more detailed description of risk of bias for each domain.
Measures of treatment effect
We carried out statistical analyses using Review Manager 2014.
Dichotomous Data
We reported dichotomous data using risk ratios (RRs) with 95% confidence intervals (CIs). We calculated risk difference (RD) and number needed to treat for an additional beneficial outcome (NNTB) or for an additional harmful outcome (NNTH) with 95% CIs where there was a statistically significant difference in RR.
Continuous Data
We reported continuous data using mean difference (MD) with 95% CI.
Unit of analysis issues
When assessing individually randomised controlled trials, the unit of analysis was the participating infant. However, in cluster‐RCTs we planned to use the neonatal unit or the cluster that is randomised as the unit of analysis. We planned to include cluster‐RCTs in the analyses using an estimate of the intra‐cluster correlation co‐efficient (ICC) derived from the trial (if possible), or from another source, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If ICCs from other sources were used, we planned to report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identified both cluster‐RCTs and individual RCTs, we planned to synthesise the relevant information. We considered it reasonable to combine the results from both if there was little heterogeneity between study designs, and the interaction between the effect of intervention and the choice of randomisation unit was considered to be unlikely. As it turned out, we included no cluster‐RCTs in this Cochrane Review.
Dealing with missing data
We obtained missing data from the trial authors when possible. If this was not possible, we conducted analyses on available data (i.e. ignoring the missing data). In addition, we conducted another analysis by using the imputation method (both best‐ and worst‐case scenarios) and the last observation carried forward to the final assessment (LOCF) method for dichotomous and continuous outcome data respectively. For dichotomous outcomes, we conducted both best‐ and worst‐case scenarios and intention‐to‐treat (ITT) analyses with imputation. We planned to compare results obtained from two analysis options to have a better understanding of the robustness of results relative to the different analytic approaches. We considered an imputation approach of best‐case scenarios (i.e. all missing participants in the intervention group did not experience poor outcomes (e.g. death) and all missing participants in the control group experienced poor outcomes); and worst‐case scenarios (i.e. all missing participants in the intervention group experienced the event and all missing participants in the control condition did not). We planned to conduct sensitivity analysis to compare results based on different imputation assumptions (i.e. best‐case vs. worst‐case scenarios). We analysed missing continuous data on an endpoint basis, including only participants with a final assessment, or using LOCF if trial authors reported these data.
Assessment of heterogeneity
We used Review Manager 2014 to assess the heterogeneity of treatment effects between trials. We undertook this assessment using the following two formal statistical models.
The Chi² test, to assess whether observed variability in effect sizes between studies is greater than would be expected by chance. Since this test has low power when the number of studies included in the meta‐analysis is small, we set the probability at the 10% level of significance.
The I² statistic, to ensure that pooling of data is valid. We graded the degree of heterogeneity as either none (< 25%), low (25% to 49%), moderate (50% to 74%) or high (75% to 100%). Where there was evidence of apparent or statistical heterogeneity, we assessed the source of the heterogeneity using sensitivity and subgroup analyses, looking for evidence of bias or methodological differences between trials.
Assessment of reporting biases
We were unable to assess reporting and publication bias by examining degree of asymmetry of funnel plots in Review Manager 5, as all analyses reported on fewer than 10 studies (Review Manager 2014).
Data synthesis
We performed statistical analyses according to the recommendations of the Cochrane Neonatal Review Group (neonatal.cochrane.org). We analysed all infants randomised on an ITT basis and treatment effects in the individual trials. We used a fixed‐effect model to combine the data. For any meta‐analyses analysing categorical outcomes, we calculated typical estimates of RR and RD, each with 95% CIs. For any meta‐analyses analysing continuous outcomes, we calculated the weighted mean difference (WMD) with 95% CIs if outcomes were measured in the same way between trials, and standardized mean difference (SMD) with 95% CIs to combine trials that measure the same outcome using different scales. If high heterogeneity had been apparent, we planned not to report a typical effect. When meta‐analysis was judged to be inappropriate, we analysed and interpreted individual trials separately.
Quality of evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of evidence for the following (clinically relevant) outcomes.
All‐cause mortality to near‐term age or discharge.
Any upper gastrointestinal bleeding in infants at risk of upper gastrointestinal bleeding.
Duration of any upper gastrointestinal bleeding in infants with upper gastrointestinal bleeding (days).
Anaemia requiring blood transfusion.
NEC as defined by Bell stages 1 to 4.
Ventilator‐associated pneumonia (new or progressive infiltrate with positive respiratory specimens after 48 hours of mechanical ventilation).
Duration of hospital stay (days).
Two authors (DG, MA) independently assessed the quality of the evidence for each of the outcomes above. We considered evidence from randomised controlled trials as high quality but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates and presence of publication bias. We used the GRADEpro GDT Guideline Development Tool to create a ‘Summary of findings’ table to report the quality of the evidence.
The GRADE approach results in an assessment of the quality of a body of evidence in one of four grades.
High: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Subgroup analysis and investigation of heterogeneity
Where sufficient data were available, we explored potential sources of clinical heterogeneity through the following a priori subgroup analyses.
Gestational age (< 32' weeks, 32' to 36' weeks, or > 36 weeks' gestation).
Weight for age z‐score.
Risk factor present for the development of upper gastrointestinal bleeding (e.g. stress‐induced gastritis or ulcers; trauma or mechanical ventilation; NSAID use; preterm birth; birth‐asphyxiation; neonatal cyanosis; neonatal seizures).
Higher vs. lower dose of pharmacological intervention (median dosing recommendation as the threshold).
Sensitivity analysis
Where sufficient data were available, we explored methodological heterogeneity through sensitivity analyses. We performed these by including only those trials with adequate allocation concealment, randomisation or blinding of treatment and less than 10% loss to follow‐up.
Results
Description of studies
Results of the search
We screened a total of 388 records (see Figure 1). We identified 296 records through database searching of PubMed, Embase, CINAHL, and the Cochrane Central Register of Controlled Trials, as detailed in Search methods for identification of studies above. Two hundred and fifty records remained after de‐duplication. We identified an additional 53 records through searches of the Chinese literature via China National Knowledge Infrastructure (CNKI) and CAOD (China/Asia on Demand) services; and 85 records were found through searches of ClinicalTrials.gov, ISRCTN registry, World Health Organization International Clinical Trials Registry Platform (ICTRP), Australian New Zealand Clinical Trials Registry and conference abstracts/proceedings.
1.
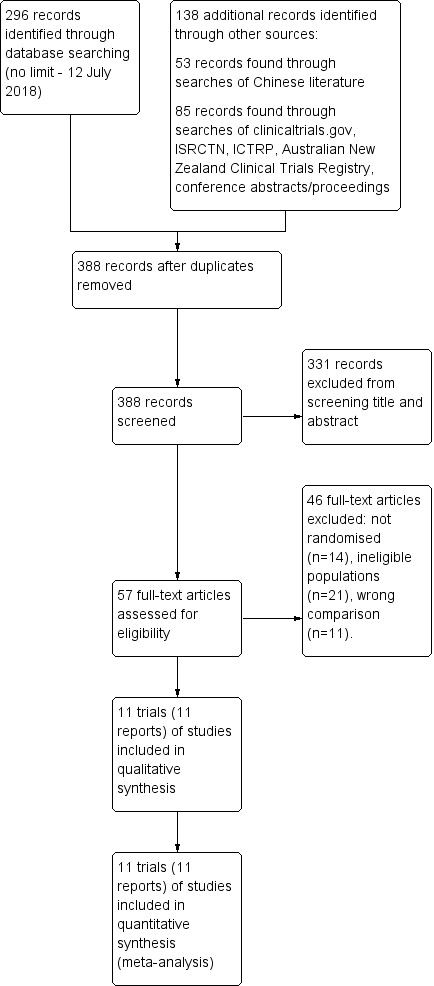
Study flow diagram.
Included studies
We assessed 11 trials as eligible for inclusion (see Characteristics of included studies): seven were trials of treatment in infants with active gastrointestinal bleeding and four were trials of prevention of gastrointestinal bleeding in high‐risk infants.
Participants
Prevention of upper gastrointestinal bleeding
Four trials enrolled infants at high risk of gastrointestinal bleeding with the goal of preventing gastrointestinal haemorrhage (Han 2001; Kuusela 1997; Lu 1995; Pourarian 2005). The criteria for enrolment included term and preterm newborns with severe illness (Han 2001; Lu 1995); newborns commencing mechanical ventilation in the first two days (Kuusela 1997); and term and preterm newborns admitted to a neonatal intensive care unit (NICU) (Pourarian 2005).
Treatment of upper gastrointestinal bleeding
Seven trials enrolled infants with active gastrointestinal bleeding (Deng 2005; Huo 2001; Lin 2011; Liu 2005; Sarna 1991; Yang 2004; Yang 2015). Five studies reported enrolling term and preterm newborns with severe illness (Huo 2001; Lin 2011; Liu 2005; Yang 2004; Yang 2015); and two studies did not report illness severity (Deng 2005; Sarna 1991).
Interventions
Prevention of upper gastrointestinal bleeding
All four studies compared an H2RA versus no treatment. Han 2001 compared cimetidine 5 mg/kg/day for five days versus no treatment; Kuusela 1997 compared ranitidine 5 mg/kg/day intravenously divided into three doses for four days versus no treatment; Lu 1995 compared cimetidine 5 mg/kg/dose intravenously twice daily for three to five days versus no treatment; and Pourarian 2005 compared ranitidine 5 mg/kg/day intravenously, three divided doses for four days irrespective of gastric pH, versus no treatment.
Treatment of upper gastrointestinal bleeding
Four studies compared an H2RA versus no treatment. Huo 2001 compared cimetidine 3 mg/kg to 5 mg/kg intravenous six to eight hourly until bleeding stopped and then continued three to five days more versus no treatment; Liu 2005 compared famotidine 0.3 mg/kg 12 hourly for 48 to 72 hours until bleeding ceased for 24 hours versus usual therapy (not reported); Sarna 1991 compared ranitidine 0.6 mg/kg loading dose followed by 0.15 mg/kg/hour intravenous infusion until bleeding had ceased for 24 hours versus no treatment; and Yang 2004 compared ranitidine 3 mg/kg/day to 5 mg/kg/day intravenously for three to five days, and Smecta 1/3 packet then 1/4 packet three times a day versus usual therapy (not reported). Three studies compared a proton pump inhibitor versus no treatment. Deng 2005 compared omeprazole 1 mg/kg twice daily for five days plus usual therapy versus usual therapy (not reported); Lin 2011 compared omeprazole 0.6 mg/kg intragastric once daily for five days plus usual therapy versus usual therapy (nil by mouth, stomach washed with bicarbonate); and Yang 2015 compared omeprazole 0.7 mg/kg intragastric daily for five days versus usual therapy (not reported).
Outcomes
Primary outcomes
Mortality was only reported in three of the 11 trials, reporting outcomes of 184 infants (Huo 2001; Kuusela 1997; Sarna 1991). Any upper gastrointestinal bleeding in infants at risk of upper gastrointestinal bleeding was reported by all four studies of prevention of gastrointestinal bleeding (Han 2001; Kuusela 1997; Lu 1995; Pourarian 2005). Duration of upper gastrointestinal bleeding in infants with upper gastrointestinal bleeding was reported by two of seven trials of treatment of gastrointestinal bleeding (Deng 2005; Sarna 1991). Continued upper gastrointestinal bleeding in infants with upper gastrointestinal bleeding was also reported by six of seven trials of treatment of gastrointestinal bleeding (Deng 2005; Huo 2001; Lin 2011; Liu 2005; Yang 2004; Yang 2015).
Secondary outcomes
Data were only reported or extractable for a minority of prespecified outcomes including gastric lesions detected by endoscopy and macroscopically (Kuusela 1997); anaemia requiring blood transfusion, reported by one of 11 trials (Sarna 1991); and an absence of serious adverse reactions, reported by eight of the 11 trials (Han 2001; Huo 2001; Kuusela 1997; Liu 2005; Lu 1995; Pourarian 2005; Sarna 1991; Yang 2004). Yang 2004, however, excluded infants with adverse reactions to medications after randomisation although numbers were unclear. No other neonatal morbidity or long‐term outcome was reported.
Excluded studies
Forty‐six full‐text articles were excluded: 14 were not randomised controlled trials; 21 did not report eligible populations of infants; and 11 reported ineligible comparisons (see Characteristics of excluded studies and Figure 1).
Risk of bias in included studies
See 'Risk of bias' graph and 'Risk of bias' summary (Figure 2; Figure 3).
2.
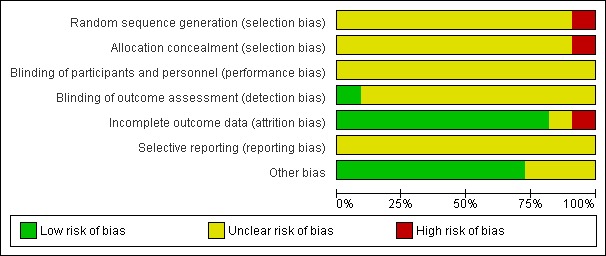
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
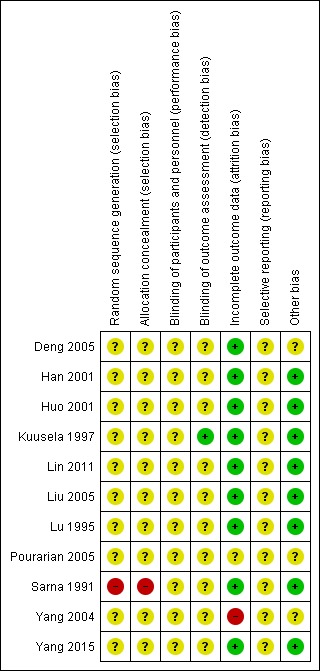
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Allocation methods were not adequately reported for 10 trials so these trials were at unclear risk of selection bias (Deng 2005; Han 2001; Huo 2001; Kuusela 1997; Lin 2011; Liu 2005; Lu 1995; Pourarian 2005; Yang 2004; Yang 2015); and one trial reported alternately allocating infants to intervention so was at high risk of selection bias (Sarna 1991).
Blinding
No trial reported blinding of intervention or measurement, so all trials were at unclear risk of performance bias and detection bias.
Incomplete outcome data
Nine studies were at low risk of attrition bias (Deng 2005; Han 2001; Huo 2001; Kuusela 1997; Lin 2011; Liu 2005; Lu 1995; Sarna 1991; Yang 2015). Yang 2004 reported post‐randomisation losses due to haemorrhagic diseases and adverse effects of medication.
Selective reporting
The protocols were unavailable for all studies so risk of reporting bias was unclear for all studies.
Other potential sources of bias
No other potential sources of bias were found for eight trials (Han 2001; Huo 2001; Kuusela 1997; Lin 2011; Liu 2005; Lu 1995; Sarna 1991; Yang 2015). For three trials this was unclear. Deng 2005 did not report baseline characteristics; Pourarian 2005 reported some baseline differences between groups; and Yang 2004 had an imbalance in numbers between groups.
Effects of interventions
Summary of findings for the main comparison. Prevention of upper gastrointestinal bleeding in newborn infants.
| Prevention of upper gastrointestinal bleeding in newborn infants | ||||||
| Patient or population: newborn infants at risk of gastrointestinal bleeding Settings: neonatal intensive care Intervention: H2RA versus no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Prevention of upper gastrointestinal bleeding | |||||
| All‐cause mortality | 111 per 1000 | 269 per 1000 (78 to 931) | RR 2.42 (0.7 to 8.38) | 53 (1 study) | ⊕⊕⊝⊝ very low1,2 | Single small study comparing ranitidine versus no treatment. Quality of evidence downgraded due to risk of bias and very serious imprecision. |
| Any upper gastrointestinal bleeding in infants at risk of upper gastrointestinal bleeding | 305 per 1000 | 110 per 1000 (67 to 177) | RR 0.36 (0.22 to 0.58) | 329 (4 studies) | ⊕⊕⊕⊝ moderate1 | All studies compared H2RA versus no treatment. Quality of evidence downgraded due to risk of bias. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1 No study at low risk of bias. 2 Single small study. Very wide confidence intervals.
Summary of findings 2. Treatment of upper gastrointestinal bleeding in newborn infants.
| Treatment of upper gastrointestinal bleeding in newborn infants | ||||||
| Patient or population: infants with upper gastrointestinal bleeding Settings: neonatal intensive care Intervention: inhibitor of gastric acid (H2RA and PPIs) versus no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Treatment of upper gastrointestinal bleeding | |||||
| All‐cause mortality | 169 per 1000 | 107 per 1000 (44 to 256) | RR 0.63 (0.26 to 1.51) | 131 (2 studies) | ⊕⊕⊝⊝ low1,2 | Both studies compared H2RA versus no treatment. Quality of evidence downgraded due to risk of bias and imprecision. |
| Duration of upper gastrointestinal bleeding (days) | 1.06 lower (1.28 to 0.84 lower) | 104 (2 studies) | ⊕⊝⊝⊝ very low1,2,3 | 1 study compared PPI versus no treatment. 1 study compared H2RA versus no treatment. No significant subgroup difference. Quality of evidence downgraded due to risk of bias, inconsistency and imprecision. |
||
| Continued upper gastrointestinal bleeding | 400 per 1000 | 144 per 1000 (104 to 196) | RR 0.36 (0.26 to 0.49) | 451 (6 studies) | ⊕⊕⊝⊝ low1,3 | 3 studies compared PPI versus no treatment. 3 studies compared H2RA versus no treatment. No significant subgroup difference. Quality of evidence downgraded due to risk of bias and imprecision. |
| Anaemia requiring blood transfusion | 167 per 1000 | 100 per 1000 (18 to 532) | RR 0.6 (0.11 to 3.19) | 38 (1 study) | ⊕⊕⊝⊝ low1,3 | Single small study comparing ranitidine versus no treatment. Quality of evidence downgraded due to risk of bias and imprecision. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1 No study at low risk of bias. 2 Wide confidence intervals. 3 Moderate heterogeneity between studies.
Comparison 1: Prevention of upper gastrointestinal bleeding
Primary outcomes
Mortality (Analysis 1.1): Kuusela 1997 reported no difference in mortality (RR 2.42, 95% CI 0.70 to 8.38; participants = 53). We downgraded the quality of evidence to very low due to risk of bias and very serious imprecision (single small study comparing ranitidine versus no treatment).
1.1. Analysis.
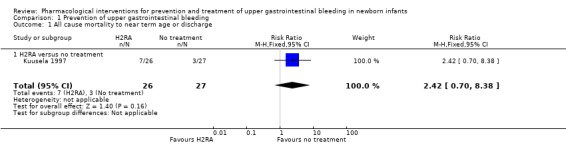
Comparison 1 Prevention of upper gastrointestinal bleeding, Outcome 1 All cause mortality to near term age or discharge.
Any upper gastrointestinal bleeding in infants at risk of upper gastrointestinal bleeding (Analysis 1.2): our meta‐analysis showed a reduction in any upper gastrointestinal bleeding from use of H2RA compared to no treatment (RR 0.36, 95% CI 0.22 to 0.58; participants = 329; studies = 4; I² = 0%). We downgraded the quality of evidence to moderate due to risk of bias.
1.2. Analysis.
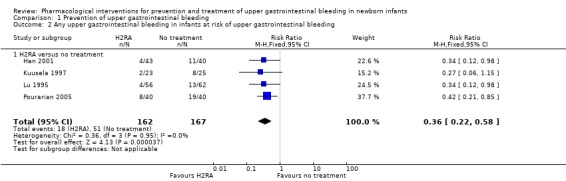
Comparison 1 Prevention of upper gastrointestinal bleeding, Outcome 2 Any upper gastrointestinal bleeding in infants at risk of upper gastrointestinal bleeding.
Secondary outcomes
Gastric lesions detected by endoscopy and macroscopically (Analysis 1.3): Kuusela 1997 reported a reduction in gastric lesions detected by endoscopy from use of an H2RA compared to no treatment (RR 0.49, 95% CI 0.28 to 0.84; participants = 48).
1.3. Analysis.
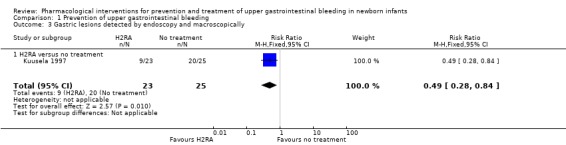
Comparison 1 Prevention of upper gastrointestinal bleeding, Outcome 3 Gastric lesions detected by endoscopy and macroscopically.
Serious adverse reactions (Analysis 1.4): no serious adverse reactions were reported from use of an H2RA (participants = 329; studies = 4).
1.4. Analysis.
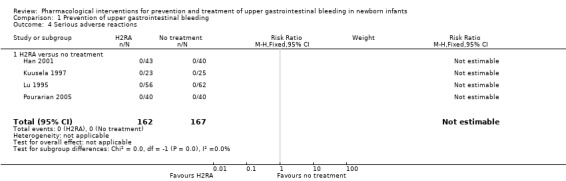
Comparison 1 Prevention of upper gastrointestinal bleeding, Outcome 4 Serious adverse reactions.
No data were available from included trials that assessed other secondary outcomes.
Comparison 2: Treatment of upper gastrointestinal bleeding
Primary outcomes
Mortality (Analysis 2.1): our meta‐analysis showed no significant difference in mortality from use of an H2RA compared to no treatment (typical RR 0.63, 95% CI 0.26 to 1.51; participants = 131; studies = 2; I² = 0%), although the quality of evidence was low. The clinical significance of this result was unclear.
2.1. Analysis.
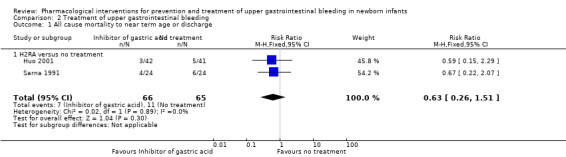
Comparison 2 Treatment of upper gastrointestinal bleeding, Outcome 1 All cause mortality to near term age or discharge.
Duration of upper gastrointestinal bleeding in infants with upper gastrointestinal bleeding (days) (Analysis 2.2): our meta‐analysis showed a reduction in duration of upper gastrointestinal bleeding from use of an inhibitor of gastric acid (PPI or H2RA) compared to no treatment (MD −1.06, 95% CI −1.28 to −0.84; participants = 104; studies = 2; I² = 63%). We downgraded the quality of evidence to very low due to risk of bias, inconsistency and imprecision. Deng 2005 reported a reduction in duration of upper gastrointestinal bleeding from use of a PPI compared to no treatment (MD −1.06, 95% CI −1.28 to −0.84; participants = 66). Sarna 1991 reported no significant difference in duration of upper gastrointestinal bleeding from use of an H2RA compared to no treatment (MD −9.50, 95% CI −19.55 to 0.55; participants = 38). The test for subgroup differences was not significant (P = 0.10; I² = 63.0%).
2.2. Analysis.
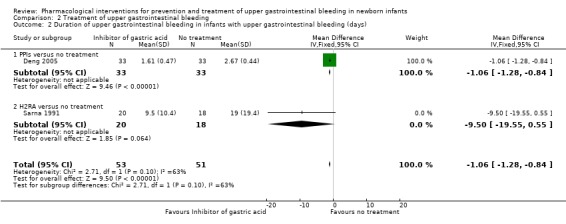
Comparison 2 Treatment of upper gastrointestinal bleeding, Outcome 2 Duration of upper gastrointestinal bleeding in infants with upper gastrointestinal bleeding (days).
Continued upper gastrointestinal bleeding in infants with upper gastrointestinal bleeding at latest time reported (Analysis 2.3): our meta‐analysis showed a reduction in continued upper gastrointestinal bleeding from use of any inhibitor of gastric acid compared to no treatment (typical RR 0.36, 95% CI 0.26 to 0.49; participants = 451; studies = 6; I² = 63%). Heterogeneity was moderate. The quality of evidence was downgraded to low quality due to risk of bias and imprecision. Our meta‐analysis showed a reduction in continued upper gastrointestinal bleeding from use of a PPI compared to no treatment (typical RR 0.39, 95% CI 0.27 to 0.57; participants = 263; studies = 3; I² = 80%). Heterogeneity was high. Our meta‐analysis showed a reduction in continued upper gastrointestinal bleeding from use of an H2RA compared to no treatment (typical RR 0.30, 95% CI 0.17 to 0.54; participants = 188; studies = 3; I² = 0%). The test for subgroup differences was not significant (P = 0.45; I² = 0%).
2.3. Analysis.
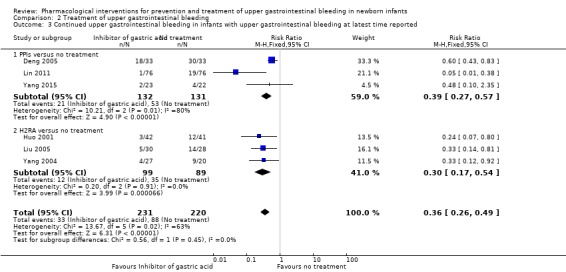
Comparison 2 Treatment of upper gastrointestinal bleeding, Outcome 3 Continued upper gastrointestinal bleeding in infants with upper gastrointestinal bleeding at latest time reported.
Secondary outcomes
Anaemia requiring blood transfusion (Analysis 2.4): Sarna 1991 reported no difference in anaemia requiring blood transfusion from use of an H2RA compared to no treatment (RR 0.60, 95% CI 0.11 to 3.19; participants = 38). We downgraded the quality of evidence to low due to risk of bias and imprecision.
2.4. Analysis.
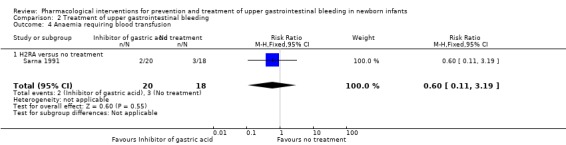
Comparison 2 Treatment of upper gastrointestinal bleeding, Outcome 4 Anaemia requiring blood transfusion.
Serious adverse reactions (Analysis 2.5): no studies of a PPI reported serious adverse reactions. No serious adverse reactions were reported from use of an H2RA (participants = 226; studies = 3).
2.5. Analysis.
Comparison 2 Treatment of upper gastrointestinal bleeding, Outcome 5 Serious adverse reactions.
No data were available from included trials that assessed other secondary outcomes.
Subgroup analyses
We could not report most prespecified subgroup analyses. Four trials enrolled infants at high risk of gastrointestinal bleeding with the goal of preventing gastrointestinal haemorrhage (Han 2001; Kuusela 1997; Lu 1995; Pourarian 2005). The criteria for enrolment included term and preterm newborns with severe illness (Han 2001; Lu 1995); newborns commencing mechanical ventilation in the first two days (Kuusela 1997); and term and preterm newborns admitted to NICU (Pourarian 2005).
Seven trials enrolled infants with active gastrointestinal bleeding (Deng 2005; Huo 2001; Lin 2011; Liu 2005; Sarna 1991; Yang 2004; Yang 2015). Five studies reported enrolling term and preterm newborns with severe illness (Huo 2001; Lin 2011; Liu 2005; Yang 2004; Yang 2015); whilst two studies did not report illness severity (Deng 2005; Sarna 1991).
We could not extract data separately for the following subgroup analyses: preterm and term gestation infants; according to weight for age z‐score; and risk factor present for the development of upper gastrointestinal bleeding. However, the majority of studies included sick term and preterm infants.
Higher versus lower dose of pharmacological intervention (median dosing recommendation as the threshold)
Primary outcomes
Mortality (Analysis 3.1): Huo 2001 reported no significant difference in mortality from use of higher dose H2RA (cimetidine) compared to no treatment (RR 0.59, 95% CI 0.15 to 2.29; participants = 83). Sarna 1991 reported no significant difference in mortality from use of lower dose H2RA (raniditine) compared to no treatment (RR 0.67, 95% CI 0.22 to 2.07; participants = 48). Test for subgroup differences was not significant (P = 0.89; I² = 0%).
3.1. Analysis.
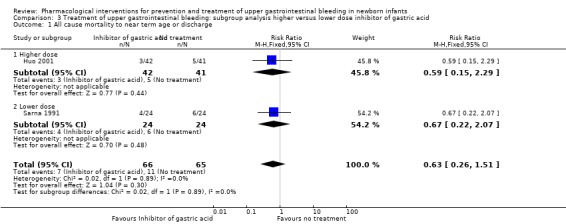
Comparison 3 Treatment of upper gastrointestinal bleeding: subgroup analysis higher versus lower dose inhibitor of gastric acid, Outcome 1 All cause mortality to near term age or discharge.
Duration of upper gastrointestinal bleeding in infants with upper gastrointestinal bleeding (days) (Analysis 3.2): Deng 2005 reported a reduction in duration of upper gastrointestinal bleeding from use of higher dose PPI (omeprazole) compared to no treatment (MD −1.06, 95% CI −1.28 to −0.84; participants = 66). Sarna 1991 reported no significant difference in duration of upper gastrointestinal bleeding from use of lower dose H2RA (ranitidine) compared to no treatment (MD −9.50, 95% CI −19.55 to 0.55; participants = 38). Test for subgroup differences was not significant (P = 0.10; I² = 63.0%).
3.2. Analysis.
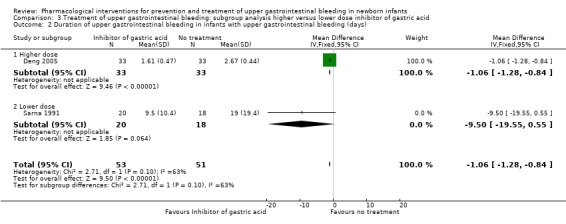
Comparison 3 Treatment of upper gastrointestinal bleeding: subgroup analysis higher versus lower dose inhibitor of gastric acid, Outcome 2 Duration of upper gastrointestinal bleeding in infants with upper gastrointestinal bleeding (days).
Continued upper gastrointestinal bleeding in infants with upper gastrointestinal bleeding at latest time reported (Analysis 3.3): our meta‐analysis showed a reduction in continued upper gastrointestinal bleeding from use of higher dose inhibitor of gastric acid compared to no treatment (RR 0.46, 95% CI 0.33 to 0.64; participants = 207; studies = 3; I² = 53%). Heterogeneity was moderate. Our meta‐analysis showed a reduction in continued upper gastrointestinal bleeding from use of lower dose inhibitor of gastric acid compared to no treatment (RR 0.19, 95% CI 0.09 to 0.42; participants = 244; studies = 3; I² = 50%). Heterogeneity was moderate. The test for subgroup differences was significant (P = 0.05; I² = 74.9%).
3.3. Analysis.
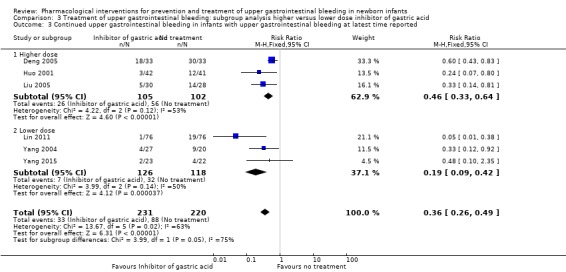
Comparison 3 Treatment of upper gastrointestinal bleeding: subgroup analysis higher versus lower dose inhibitor of gastric acid, Outcome 3 Continued upper gastrointestinal bleeding in infants with upper gastrointestinal bleeding at latest time reported.
Sensitivity analyses
We assessed no studies as being at low risk of bias with regard to adequate allocation concealment, randomisation, blinding of treatment and less than 10% loss to follow‐up. However, for the majority of studies the criteria for bias were unclear due to a failure to report details.
Discussion
Summary of main results
Prevention of upper gastrointestinal bleeding
A single trial with 53 infants reported no difference in mortality from use of an H2RA compared to no treatment. We graded the quality of evidence as very low with downgrading due to risk of bias and very serious imprecision. Meta‐analysis of four trials with 329 infants found a reduction in any upper gastrointestinal bleeding from use of H2RA compared to no treatment (typical risk difference (RD) −0.20, 95% CI −0.28 to −0.11; NNTB 5, 95% CI 4 to 9). We graded the quality of evidence as moderate with downgrading due to risk of bias. For secondary outcomes, our review found a single study reporting a reduction in gastric lesions detected by endoscopy from use of an H2RA compared to no treatment. Four trials with 329 infants reported no serious adverse reactions from use of an H2RA compared to no treatment. No study reported the incidence of necrotising enterocolitis, ventilator‐ or hospital‐associated pneumonia, sepsis, or long‐term outcome.
Treatment of upper gastrointestinal bleeding
Meta‐analysis of two trials including 131 infants found no significant difference in mortality from use of an H2RA compared to no treatment for treatment of gastrointestinal bleeding. We graded the quality of evidence as low, with downgrading due to risk of bias and imprecision. Meta‐analysis of two trials including 104 infants found a reduction in duration of upper gastrointestinal bleeding from use of any inhibitor of gastric acid compared to no treatment (MD −1.06 days, 95% CI −1.28 to −0.84). Meta‐analysis of six trials including 451 infants showed a reduction in continued upper gastrointestinal bleeding from use of any inhibitor of gastric acid compared to no treatment (typical RD −0.26, 95% CI −0.33, −0.19; NNTB 4, 95% CI 3 to 5). No study reported the incidence of necrotising enterocolitis, ventilator‐ or hospital‐associated pneumonia, sepsis, or long‐term outcome. There were no significant subgroup differences in duration of upper gastrointestinal bleeding or of continued upper gastrointestinal bleeding according to type of inhibitor of gastric acid (PPI versus H2RA). Data regarding adverse effects are limited. A single trial with 38 infants reported no difference in anaemia requiring blood transfusion from use of an H2RA compared to no treatment. No studies of a PPI reported serious adverse reactions. Three trials including 226 infants reported no serious adverse reactions from use of an H2RA.
A subgroup analysis of higher versus lower doses of inhibitor of gastric acid did not identify any significant subgroup differences for mortality or duration of upper gastrointestinal bleeding in infants with upper gastrointestinal bleeding. Although an analysis of continued upper gastrointestinal bleeding in infants with upper gastrointestinal bleeding found a significant subgroup difference between higher versus lower dose trials, the greatest effect was in the lower dose group. This review found no evidence that use of higher doses of inhibitor of gastric acid results in a further reduction in gastrointestinal bleeding in newborn infants.
Overall completeness and applicability of evidence
The trials included in this review enrolled term and preterm infants at high risk of gastrointestinal bleeding on the basis of illness severity or need for NICU and mechanical ventilation, or infants with gastrointestinal bleeding, most commonly due to concomitant illness. The evidence is most likely to be applicable to sick term and near‐term infants at high risk of gastrointestinal bleeding or with active bleeding. Several trials excluded infants with abnormal bleeding times or clotting (Han 2001; Liu 2005; Pourarian 2005; Sarna 1991; Yang 2004). It is unclear if treatment with an inhibitor of gastric acid is effective in infants with these disorders.
The trials addressed the effects of a single PPI, omeprazole, for treatment of upper gastrointestinal bleeding, and H2RAs including cimetidine, famotidine and ranitidine for the prevention and treatment of upper gastrointestinal bleeding. Studies of H2RA gave cimetidine, famotidine or ranitidine intravenously until bleeding ceased. Studies of PPI gave omeprazole via the intragastric tube. One trial of ranitidine also used Smecta (a natural silicate of aluminium and magnesium) in the treatment group (Yang 2004); and another trial of omeprazole orally also reported usual treatment in both groups consisting of nil by mouth and bicarbonate (HCO₃) stomach wash (Lin 2011). There was no trial in newborn infants that reported the use of sucralfate.
Few studies reported mortality so the analysis is substantially underpowered. Studies consistently reported H2RA prevented gastrointestinal bleeding in high‐risk infants, and both H2RAs and PPIs reduced duration of gastrointestinal bleeding and continued gastrointestinal bleeding in infants with gastrointestinal bleeding. A single study reported no effect on anaemia requiring blood transfusion.
Other important neonatal morbidities including necrotising enterocolitis, ventilator‐associated pneumonia, duration of ventilation and respiratory support, and duration of hospital stay were not reported. Long‐term outcomes have not been reported. We could not perform subgroup analyses according to gestation age, weight for age z‐score and risk factor for development of upper gastrointestinal bleeding. However, the trials in this review predominately enrolled term or near‐term infants who were sick, required mechanical ventilation, or had upper gastrointestinal bleeding.
Quality of the evidence
For prevention of upper gastrointestinal bleeding in newborn infants, we assessed the quality of evidence for mortality as very low, with downgrading due to risk of bias and very serious imprecision. The quality of evidence for any upper gastrointestinal bleeding was assessed as moderate, with downgrading due to risk of bias. The analyses had narrow confidence intervals and we found no heterogeneity between studies. All studies compared H2RA versus no treatment. See Table 1.
For treatment of upper gastrointestinal bleeding in newborn infants, we assessed the quality of evidence for mortality as low with downgrading due to risk of bias and imprecision. Both studies compared H2RA versus no treatment. We assessed the quality of evidence for duration of upper gastrointestinal bleeding as very low with downgrading due to risk of bias, inconsistency and imprecision. One study compared PPI versus no treatment; and one study compared H2RA versus no treatment. We found no significant subgroup difference. We assessed the quality of evidence for continued upper gastrointestinal bleeding as low, with downgrading due to risk of bias and imprecision. Three studies compared PPI versus no treatment and three studies compared H2RA versus no treatment. No significant subgroup difference was found. We assessed the quality of evidence for anaemia requiring blood transfusion as low, with downgrading due to risk of bias and imprecision. A single small study compared ranitidine versus no treatment.
Ten of the 11 trials included in this review did not report methods of sequence generation, allocation concealment, use of a placebo or attempts to blind participants, personnel or outcome assessment. The majority of these are published in Chinese language journals. It is unclear if this reflects a concern about potential for bias in the included studies or simply relates to reporting standards in Chinese language journals.
Potential biases in the review process
We undertook an extensive literature search for published and unpublished studies including non‐English publications. We found a substantial number of Chinese language articles and they were translated by one of the authors (KL). It is unclear whether the search tools used identified all Chinese language studies.
The review used standard Cochrane methodology with a pre‐published protocol. We prespecified comparisons and outcomes. We added a single outcome post hoc: serious adverse reactions. Two independent reviewers cross‐checked eligibility, risk of bias and data extraction. We used Google Translate to cross‐check Chinese language articles.
As assessments of risk of bias in this review are substantially affected by the under‐reporting of methodology (including methods of sequence generation, allocation concealment, use of a placebo or attempts to blind participants, personnel or outcome assessment), this review has the potential to under‐ or over‐report the quality of evidence.
Agreements and disagreements with other studies or reviews
Stress ulcer prophylaxis has been a standard of care in intensive care patients (Eastwood 2014; Krag 2015; Lam 1999; Shears 2016). Stress ulcer prophylaxis is also commonly used in paediatric intensive care patients (Araujo 2010; Costarino 2015). However, recent systematic reviews and meta‐analyses have failed to find conclusive evidence that use of H2RAs or PPIs in intensive care patients reduced gastrointestinal bleeding without increasing the incidence of ventilator‐ or hospital‐associated pneumonia (Alhazzani 2017; Krag 2015; Marik 2010; Shan 2013). Marik 2010 reported that in those patients who were fed enterally, stress ulcer prophylaxis did not alter the risk of gastrointestinal bleeding; overall H2RAs did not increase the risk of hospital‐acquired pneumonia; however, this complication was increased in the subgroup of patients who were fed enterally. Shan 2013 reported 'inhibitor of gastric acid' medication significantly increased the incidence of hospital‐acquired pneumonia when compared with sucralfate in 11 trials; 'inhibitor of gastric acid' therapy significantly reduced the incidence of clinically significant bleeding compared with sucralfate; however, it did not lower the incidence of overt bleeding; and there was no significant difference between inhibitor of gastric acid group and sucralfate group on either ICU mortality or hospitalisation mortality in 11 studies. Krag 2015 performed a systematic review of 20 trials with 1971 intensive care patients randomised to stress ulcer prophylaxis. All were judged as having a high risk of bias. There was no statistically significant difference in mortality or hospital‐acquired pneumonia, but significant gastrointestinal bleeding. Alhazzani 2017 reported a meta‐analysis of five trials including 604 patients of PPIs versus placebo in intensive care patients and found no statistically significant difference in the risk of upper gastrointestinal bleeding, infections, or mortality.
We found no other published systematic review of inhibitor of gastric acid prophylaxis or treatment in newborn infants. We found no guidelines around the use of inhibitor of gastric acid prophylaxis or treatment in newborn infants. A literature review raised concern regarding the association between inhibitors of gastric acid used for upper gastrointestinal bleeding or gastroesophageal reflux in preterm infants and an increased incidence of necrotising enterocolitis (More 2013). One case‐control and one prospective cohort study including 11,346 preterm infants were included. The prospective cohort study also reported a higher incidence of infection (sepsis, pneumonia, urinary tract infection) with use of inhibitors of gastric acid. Another review of the literature of treatment of children with gastro‐oesophageal reflux reported adverse effects in at least 23% of children treated with H2RAs and 34% of those treated with PPIs, mostly including headaches, diarrhoea, nausea (H2RAs and PPIs) and constipation (PPIs) (Cohen 2015).
Authors' conclusions
Implications for practice.
There is moderate‐quality evidence that the use of a histamine 2 receptor antagonist (H2RA) reduces the risk of gastrointestinal bleeding in newborn infants at high risk of gastrointestinal bleeding. There is low‐quality evidence that use of an inhibitor of gastric acid (H2RA or proton pump inhibitor) reduces the duration of upper gastrointestinal bleeding and reduces the incidence of continued gastric bleeding in newborn infants with gastrointestinal bleeding. However, there is no evidence that use of an inhibitor of gastric acid in newborn infants affects mortality or the need for blood transfusion. As no study reported the incidence of necrotising enterocolitis, ventilator‐ or hospital‐associated pneumonia, sepsis, or long‐term outcome, the safety of inhibitors of gastric acid secretion remains unclear. There is no evidence that there is additional benefit from use of a higher dose of an H2RA or a proton pump inhibitor for prevention or treatment of gastrointestinal bleeding in newborn infants.
Implications for research.
Further trials of the use of an inhibitor of gastric acid (H2RA or PPI) in newborn infants are required to determine if the clinical effectiveness in reducing gastrointestinal bleeding and duration outweighs the potential harms. Trials should report and be powered to detect clinically important effects of the rate of necrotising enterocolitis in preterm infants, ventilator‐ and hospital‐acquired infection, and sepsis. Trials comparing the use of H2RA versus a PPI, and use of intravenous versus intragastric administration of medication are needed.
Acknowledgements
We would like to thank Jesús López‐Herce, Dincer Yildizdas, Jacques Lacroix and Bahareh Imani for providing further information about their studies on request.
The Methods section of this review is based on a standard template used by Cochrane Neonatal.
Appendices
Appendix 1. Cochrane Neonatal searches
Pubmed:
((("Gastrointestinal Hemorrhage"[Mesh] OR (gastrointestinal AND (bleed* OR hemorrhag* OR haemorrhag*)) OR Hematochezia* OR "Hematemesis"[Mesh] OR hematemesis OR haematemesis OR "Gastritis"[Mesh] OR "Stomach Ulcer"[Mesh] OR (gastric AND mucos* AND lesion*))) AND (((((("Histamine H2 Antagonists"[Mesh] OR "Histamine H2 Antagonists" [Pharmacological Action] OR (histamine AND h2 AND antagonist) OR Burimamide OR Cimetidine OR ebrotidine OR etintidine OR Famotidine OR lafutidine OR loxtidine OR Metiamide OR mifentidine OR Nizatidine OR oxmetidine OR Ranitidine OR "roxatidine acetate" OR tiotidine OR zolantidine)) OR ((Proton Pump Inhibitors[Mesh] OR “proton pump inhibitors”[tiab] OR “proton pump inhibitor”[tiab] OR PPI*[tiab] OR Omeprazole[MeSH] OR omeprazole[tiab] or losec[tiab] or nexium[tiab] or prilosec[tiab] or rapinex[tiab] or zegerid[tiab] or ocid[tiab] or Omepral[tiab] or Omez[tiab] OR Esomeprazole[MeSH] OR Esomeprazole[tiab] OR Nexium[tiab] OR Alenia[tiab] OR Nexiam[tiab] OR Lansoprazole[MeSH] OR lansoprazole[tiab] OR lanzoprazole[tiab] OR agopton[tiab] OR Levant[tiab] OR lanzor[tiab] OR prevacid[tiab] OR takepron[tiab] OR zoton[tiab] OR pantoprazole[tiab] OR protium[tiab] OR protonix[tiab] OR Pantozol[tiab] OR Pantor[tiab] OR Pantoloc[tiab] OR Controloc[tiab] OR ilaprazole[tiab] OR Rabeprazole[MeSH] OR rabeprazole[tiab] OR aciphex[tiab] OR dexrabeprazole[tiab] OR pariet[tiab] OR Dexlansoprazole[MeSH] OR Dexlansoprazole[tiab] OR Kapidex[tiab] OR Dexilant[tiab] OR tenatoprazole[tiab] OR benatoprazole[tiab]))) OR ("Antacids"[Mesh] OR "Antacids"[Pharmacological Action] OR antacid* OR bismuth OR ((alkalinizing OR antigastralgic) AND agent*) OR aluminum or aldrox or algeldrate or alhydrogel or alugel or amphojel or basalgel or brasivil or dialume or nephrox or pepsamer or rocgel OR "calcium carbonate" or aragonite or calcite or "calcium milk" or Chalk or limestone or marble or vaterite OR magnesium or brucite or magnesia OR "aceglutamide aluminum" or "alexitol sodium" or algicon or Almagate or almagel or alubifar or alugastrin or andursil or attapulgite or bicarbonate or carbex or "dihydroxyaluminum sodium carbonate" or gaviscon or hydrotalcite or magaldrate or Mylanta or novaluzid or rennie or solugastril or titralac or vangatalcite)) OR ("Sucralfate"[Mesh] OR sucralfate or sulfate or antepsin or carafate or ulcerban or ulcogant or ulsanic)) OR ((Gastro*[tiab] AND mucosa*[tiab] AND (protect*[tiab] AND (agent*[tiab] OR drug[tiab] OR medicine[tiab] OR medication[tiab]))) OR (Gastric[tiab] mucosa*[tiab] AND (protect*[tiab] AND (agent*[tiab] OR drug[tiab] OR medicine[tiab] OR medication[tiab]))) OR (stomach[tiab] mucosa*[tiab] AND (protect*[tiab] AND (agent*[tiab] OR drug[tiab] OR medicine[tiab] OR medication[tiab])))))) AND (((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh])))
Embase:
| 1 | exp gastrointestinal hemorrhage/ |
| 2 | (gastrointestinal adj2 (bleed* or hemorrhag* or haemorrhag*)).mp. |
| 3 | (Hematochezia* or hematemesis or haematemesis).mp. |
| 4 | exp hematemesis/ |
| 5 | exp gastritis/ |
| 6 | exp stomach ulcer/ |
| 7 | (gastric and mucos* and lesion*).mp. |
| 8 | 1 or 2 or 3 or 4 or 5 or 6 or 7 |
| 9 | exp histamine H2 receptor antagonist/ |
| 10 | (histamine and h2 and antagonist).mp. |
| 11 | (Burimamide or Cimetidine or ebrotidine or etintidine or Famotidine or lafutidine or loxtidine or Metiamide or mifentidine or Nizatidine or oxmetidine or Ranitidine or "roxatidine acetate" or tiotidine or zolantidine).mp. |
| 12 | 9 or 10 or 11 |
| 13 | exp proton pump inhibitor/ |
| 14 | (proton adj pump adj inhibitor*).ti,ab,kw. |
| 15 | exp esomeprazole/ |
| 16 | (Esomeprazole or Nexium or Esotrex or Alenia or Escz or Esofag or Nexiam).ti,ab,kw. |
| 17 | exp omeprazole/ |
| 18 | (omeprazole or losec or nexium or prilosec or rapinex or zegerid or ocid or Lomac or Omepral or Omez).ti,ab,kw. |
| 19 | (pantoprazole or protium or protonix or Pantotab or Pantopan or Pantozol or Pantor or Pantoloc or Astropan or Controloc or Pantecta or Inipomp or Somac or Pantodac or Zurcal or Zentro).ti,ab,kw. |
| 20 | exp rabeprazole/ |
| 21 | (rabeprazole or aciphex or dexrabeprazole or pariet or Zechin or Rabecid or Nzole‐D or Rabeloc).ti,ab,kw. |
| 22 | exp lansoprazole/ |
| 23 | (lansoprazole or lanzoprazole or agopton or bamalite or Inhibitol or Levant or Lupizole or lanzor or monolitum or ogast or ogastro or opiren or prevacid or prezal or promeco or takepron or ulpax or zoton).ti,ab,kw. |
| 24 | exp dexlansoprazole/ |
| 25 | (Dexlansoprazole or Kapidex or Dexilant).ti,ab,kw. |
| 26 | (tenatoprazole or benatoprazole).ti,ab,kw. |
| 27 | 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 |
| 28 | exp sucralfate/ |
| 29 | (sucralfate or sulfate or antepsin or carafate or ulcerban or ulcogant or ulsanic).mp. |
| 30 | 28 or 29 |
| 31 | (protect* and (agent* or drug or medicine or medication)).mp. |
| 32 | (gastro* adj2 mucosa*).mp. |
| 33 | gastric mucosa*.mp. |
| 34 | stomach mucosa*.mp. |
| 35 | 32 or 33 or 34 |
| 36 | 31 and 35 |
| 37 | 12 or 27 or 30 or 36 |
| 38 | (infan* or newborn or neonat* or premature or very low birth weight or low birth weight or VLBW or LBW).mp. |
| 39 | exp infant/ |
| 40 | 38 or 39 |
| 41 | (human not animal).mp. |
| 42 | (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial).mp. |
| 43 | 40 and 41 and 42 |
| 44 | 8 and 37 and 43 |
| 45 | from 44 keep 1‐123 |
CINAHL:
| S34 | S9 AND S32 AND S33 |
| S33 | (infan* OR newborn OR neonat* OR premature OR low birth weight OR VLBW OR LBW) AND (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial) |
| S32 | S13 OR S16 OR S21 OR S24 OR S27 OR S29 OR S31 |
| S31 | S26 AND S30 |
| S30 | stomach mucosa* |
| S29 | S26 AND S28 |
| S28 | Gastric mucosa* |
| S27 | S25 AND S26 |
| S26 | protect* AND ( agent* OR drug OR medicine OR medication ) |
| S25 | Gastro* AND mucosa* |
| S24 | S22 OR S23 |
| S23 | sucralfate or sulfate or antepsin or carafate or ulcerban or ulcogant or ulsanic |
| S22 | (MH "Sucralfate") |
| S21 | S17 OR S18 OR S19 OR S20 |
| S20 | aluminum or aldrox or algeldrate or alhydrogel or alugel or amphojel or basalgel or brasivil or dialume or nephrox or pepsamer or rocgel OR "calcium carbonate" or aragonite or calcite or "calcium milk" or Chalk or limestone or marble or vaterite OR magnesium or brucite or magnesia OR "aceglutamide aluminum" or "alexitol sodium" or algicon or Almagate or almagel or alubifar or alugastrin or andursil or attapulgite or bicarbonate or carbex or "dihydroxyaluminum sodium carbonate" or gaviscon or hydrotalcite or magaldrate or Mylanta or novaluzid or rennie or solugastril or titralac or vangatalcite |
| S19 | ( alkalinizing OR antigastralgic ) AND agent* |
| S18 | antacid* OR bismuth |
| S17 | (MH "Antacids+") |
| S16 | S14 OR S15 |
| S15 | “proton pump inhibitors” OR “proton pump inhibitor” OR PPI* OR omeprazole or losec or nexium or prilosec or rapinex or zegerid or ocid or Omepral or Omez OR Esomeprazole OR Nexium OR Alenia OR Nexiam OR lansoprazole OR lanzoprazole OR agopton OR Levant OR lanzor OR prevacid OR takepron OR zoton OR pantoprazole OR protium OR protonix OR Pantozol OR Pantor OR Pantoloc OR Controloc OR ilaprazole OR rabeprazole OR aciphex OR dexrabeprazole OR pariet OR Dexlansoprazole OR Kapidex OR Dexilant OR tenatoprazole OR benatoprazole |
| S14 | (MH "Proton Pump Inhibitors+") |
| S13 | S10 OR S11 OR S12 |
| S12 | Burimamide OR Cimetidine OR ebrotidine OR etintidine OR Famotidine OR lafutidine OR loxtidine OR Metiamide OR mifentidine OR Nizatidine OR oxmetidine OR Ranitidine OR "roxatidine acetate" OR tiotidine OR zolantidine |
| S11 | histamine AND h2 AND antagonist |
| S10 | (MH "Histamine H2 Antagonists+") |
| S9 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 |
| S8 | gastric AND mucos* AND lesion* |
| S7 | (MH "Peptic Ulcer+") |
| S6 | (MH "Gastritis+") |
| S5 | hematemesis OR haematemesis |
| S4 | (MH "Hematemesis") |
| S3 | Hematochezia* |
| S2 | gastrointestinal AND ( bleed* OR hemorrhag* OR haemorrhag* ) |
| S1 | (MH "Gastrointestinal Hemorrhage+") |
Cochrane Central Register of Controlled Trials:
| 1 | MESH DESCRIPTOR Gastrointestinal Hemorrhage EXPLODE ALL AND CENTRAL:TARGET |
| 2 | MESH DESCRIPTOR Hematemesis EXPLODE ALL AND CENTRAL:TARGET |
| 3 | MESH DESCRIPTOR Gastritis EXPLODE ALL AND CENTRAL:TARGET |
| 4 | MESH DESCRIPTOR Stomach Ulcer EXPLODE ALL AND CENTRAL:TARGET |
| 5 | gastrointestinal AND (bleed* OR hemorrhag* OR haemorrhag*) AND CENTRAL:TARGET |
| 6 | Hematochezia* AND CENTRAL:TARGET |
| 7 | hematemesis OR haematemesis AND CENTRAL:TARGET |
| 8 | gastric AND mucos* AND lesion* AND CENTRAL:TARGET |
| 9 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 |
| 10 | MESH DESCRIPTOR Histamine H2 Antagonists EXPLODE ALL AND CENTRAL:TARGET |
| 11 | histamine AND h2 AND antagonist AND CENTRAL:TARGET |
| 12 | Burimamide OR Cimetidine OR ebrotidine OR etintidine OR Famotidine OR lafutidine OR loxtidine OR Metiamide OR mifentidine OR Nizatidine OR oxmetidine OR Ranitidine OR "roxatidine acetate" OR tiotidine OR zolantidine AND CENTRAL:TARGET |
| 13 | #10 OR #11 OR #12 |
| 14 | MESH DESCRIPTOR Proton Pump Inhibitors EXPLODE ALL AND CENTRAL:TARGET |
| 15 | (Proton NEXT Pump NEXT inhibitor*):TI,AB,KW AND CENTRAL:TARGET |
| 16 | MESH DESCRIPTOR Esomeprazole EXPLODE ALL AND CENTRAL:TARGET |
| 17 | (Esomeprazole or Nexium or Esotrex or Alenia or Escz or Esofag or Nexiam):TI,AB,KW AND CENTRAL:TARGET |
| 18 | MESH DESCRIPTOR Omeprazole EXPLODE ALL AND CENTRAL:TARGET |
| 19 | (omeprazole or losec or nexium or prilosec or rapinex or zegerid or ocid or Lomac or Omepral or Omez):TI,AB,KW AND CENTRAL:TARGET |
| 20 | (pantoprazole or protium or protonix or Pantotab or Pantopan or Pantozol or Pantor or Pantoloc or Astropan or Controloc or Pantecta or Inipomp or Somac or Pantodac or Zurcal or Zentro):TI,AB,KW AND CENTRAL:TARGET |
| 21 | MESH DESCRIPTOR Rabeprazole EXPLODE ALL AND CENTRAL:TARGET |
| 22 | (rabeprazole or aciphex or dexrabeprazole or pariet or Zechin or Rabecid or Nzole‐D or Rabeloc):TI,AB,KW AND CENTRAL:TARGET |
| 23 | MESH DESCRIPTOR Lansoprazole EXPLODE ALL AND CENTRAL:TARGET |
| 24 | (lansoprazole or lanzoprazole or agopton or bamalite or Inhibitol or Levant or Lupizole or lanzor or monolitum or ogast or ogastro or opiren or prevacid or prezal or promeco or takepron or ulpax or zoton):TI,AB,KW AND CENTRAL:TARGET |
| 25 | MESH DESCRIPTOR Dexlansoprazole EXPLODE ALL AND CENTRAL:TARGET |
| 26 | (Dexlansoprazole or Kapidex or Dexilant):TI,AB,KW AND CENTRAL:TARGET |
| 27 | (tenatoprazole or benatoprazole):TI,AB,KW AND CENTRAL:TARGET |
| 28 | #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 |
| 29 | MESH DESCRIPTOR Antacids EXPLODE ALL AND CENTRAL:TARGET |
| 30 | antacid* OR bismuth AND CENTRAL:TARGET |
| 31 | (alkalinizing OR antigastralgic) AND agent* AND CENTRAL:TARGET |
| 32 | aluminum or aldrox or algeldrate or alhydrogel or alugel or amphojel or basalgel or brasivil or dialume or nephrox or pepsamer or rocgel OR "calcium carbonate" or aragonite or calcite or "calcium milk" or Chalk or limestone or marble or vaterite OR magnesium or brucite or magnesia OR "aceglutamide aluminum" or "alexitol sodium" or algicon or Almagate or almagel or alubifar or alugastrin or andursil or attapulgite or bicarbonate or carbex or "dihydroxyaluminum sodium carbonate" or gaviscon or hydrotalcite or magaldrate or Mylanta or novaluzid or rennie or solugastril or titralac or vangatalcite AND CENTRAL:TARGET |
| 33 | MESH DESCRIPTOR Sucralfate EXPLODE ALL AND CENTRAL:TARGET |
| 34 | sucralfate or sulfate or antepsin or carafate or ulcerban or ulcogant or ulsanic AND CENTRAL:TARGET |
| 35 | MESH DESCRIPTOR Gastric Mucosa EXPLODE ALL AND CENTRAL:TARGET |
| 36 | gastro* NEAR2 mucosa* AND CENTRAL:TARGET |
| 37 | gastric NEAR2 mucosa* AND CENTRAL:TARGET |
| 38 | stomach NEAR2 mucosa* AND CENTRAL:TARGET |
| 39 | protect* and (agent* or drug or medicine or medication) AND CENTRAL:TARGET |
| 40 | #35 OR #36 OR #37 OR #38 |
| 41 | #39 AND #40 |
| 42 | (infan* or newborn or neonat* or premature or preterm or very low birth weight or low birth weight or VLBW or LBW) AND CENTRAL:TARGET |
| 43 | #41 OR #13 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 |
| 44 | #9 AND #42 AND #43 |
Appendix 2. Risk of bias tool
We used the standard methods of Cochrane and Cochrane Neonatal to assess the methodological quality of the trials. For each trial, we sought information regarding the method of randomisation, blinding and reporting of all outcomes of all the infants enrolled in the trial. We assessed each criterion as being at a low, high, or unclear risk of bias. Two review authors () independently assessed each study. Disagreements were resolved by discussion. We added this information to the table Characteristics of included studies. We evaluated the following issues and enter the findings into the risk of bias table:
1. Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorized the method used to generate the allocation sequence as:
low risk (any truly random process e.g. random number table; computer random number generator);
high risk (any non‐random process e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk.
2. Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorized the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk.
3. Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we categorized the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or class of outcomes. We categorized the methods as:
low risk, high risk or unclear risk for participants; and
low risk, high risk or unclear risk for personnel.
4. Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we categorized the methods used to blind outcome assessment. Blinding was assessed separately for different outcomes or class of outcomes. We categorized the methods as:
low risk for outcome assessors;
high risk for outcome assessors; or
unclear risk for outcome assessors.
5. Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorized the methods as:
low risk (< 20% missing data);
high risk (≥ 20% missing data); or
unclear risk.
6. Selective reporting bias. Are reports of the study free of suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. For studies in which study protocols were published in advance, we compared prespecified outcomes versus outcomes eventually reported in the published results. If the study protocol was not published in advance, we contacted study authors to gain access to the study protocol. We assessed the methods as:
low risk (where it is clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk (where not all the study's prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified outcomes of interest and are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported); or
unclear risk.
7. Other sources of bias. Was the study apparently free of other problems that could put it at a high risk of bias?
For each included study, we described any important concerns we had about other possible sources of bias (for example, whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
low risk;
high risk; or
unclear risk.
If needed, we explored the impact of the level of bias through undertaking sensitivity analyses.
Data and analyses
Comparison 1. Prevention of upper gastrointestinal bleeding.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All cause mortality to near term age or discharge | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.42 [0.70, 8.38] |
| 1.1 H2RA versus no treatment | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.42 [0.70, 8.38] |
| 2 Any upper gastrointestinal bleeding in infants at risk of upper gastrointestinal bleeding | 4 | 329 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.22, 0.58] |
| 2.1 H2RA versus no treatment | 4 | 329 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.22, 0.58] |
| 3 Gastric lesions detected by endoscopy and macroscopically | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.28, 0.84] |
| 3.1 H2RA versus no treatment | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.28, 0.84] |
| 4 Serious adverse reactions | 4 | 329 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 H2RA versus no treatment | 4 | 329 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Treatment of upper gastrointestinal bleeding.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All cause mortality to near term age or discharge | 2 | 131 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.26, 1.51] |
| 1.1 H2RA versus no treatment | 2 | 131 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.26, 1.51] |
| 2 Duration of upper gastrointestinal bleeding in infants with upper gastrointestinal bleeding (days) | 2 | 104 | Mean Difference (IV, Fixed, 95% CI) | ‐1.06 [‐1.28, ‐0.84] |
| 2.1 PPIs versus no treatment | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐1.06 [‐1.28, ‐0.84] |
| 2.2 H2RA versus no treatment | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐9.5 [‐19.55, 0.55] |
| 3 Continued upper gastrointestinal bleeding in infants with upper gastrointestinal bleeding at latest time reported | 6 | 451 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.26, 0.49] |
| 3.1 PPIs versus no treatment | 3 | 263 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.27, 0.57] |
| 3.2 H2RA versus no treatment | 3 | 188 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.17, 0.54] |
| 4 Anaemia requiring blood transfusion | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.11, 3.19] |
| 4.1 H2RA versus no treatment | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.11, 3.19] |
| 5 Serious adverse reactions | 4 | 226 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 H2RA versus no treatment | 4 | 226 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Treatment of upper gastrointestinal bleeding: subgroup analysis higher versus lower dose inhibitor of gastric acid.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All cause mortality to near term age or discharge | 2 | 131 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.26, 1.51] |
| 1.1 Higher dose | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.15, 2.29] |
| 1.2 Lower dose | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.22, 2.07] |
| 2 Duration of upper gastrointestinal bleeding in infants with upper gastrointestinal bleeding (days) | 2 | 104 | Mean Difference (IV, Fixed, 95% CI) | ‐1.06 [‐1.28, ‐0.84] |
| 2.1 Higher dose | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐1.06 [‐1.28, ‐0.84] |
| 2.2 Lower dose | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐9.5 [‐19.55, 0.55] |
| 3 Continued upper gastrointestinal bleeding in infants with upper gastrointestinal bleeding at latest time reported | 6 | 451 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.26, 0.49] |
| 3.1 Higher dose | 3 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.33, 0.64] |
| 3.2 Lower dose | 3 | 244 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.09, 0.42] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Deng 2005.
| Methods | Randomised controlled trial in China. | |
| Participants |
Inclusion criteria: newborns with upper gastrointestinal haemorrhage day 3 to 28 (n = 66). Exclusion criteria: none reported. Average age 7 to 8 days. |
|
| Interventions |
Intervention (n = 33): omeprazole 1 mg/kg twice daily for 5 days and normal therapy. Control (n = 33): normal therapy. Both groups given vitamin K and nil by mouth. |
|
| Outcomes | Curative rate: no haematemesis or melena at 24 hours. | |
| Notes | It is unclear if there were any adverse reactions attributable to the intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported: "randomly divided". |
| Allocation concealment (selection bias) | Unclear risk | Method not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses reported. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Unclear risk | No baseline characteristics given although age at presentation and duration of bleeding similar between groups. |
Han 2001.
| Methods | Clinical trial in China. | |
| Participants |
Inclusion criteria: newborns age 1 to 12 days. Most infants with severe illness. Term and preterm. Exclusion criteria: if swallowed maternal blood, bleeding outside gastrointestinal tract, abnormal clotting, constipation, necrotising enterocolitis. |
|
| Interventions |
Intervention (n = 43): cimetidine 5 mg/kg per day for 5 days. Control (n = 40): no cimetidine. |
|
| Outcomes | Acute gastric mucosal lesion (diagnosed by vomiting of bright red or coffee‐like liquid or faecal occult blood positive or tarry stool). | |
| Notes | Authors did not believe there were any adverse reactions attributable to the intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported 'random'. |
| Allocation concealment (selection bias) | Unclear risk | Method not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None reported. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | Similar baseline characteristics between groups. |
Huo 2001.
| Methods | Randomised controlled trial in China 1997 to 2000. | |
| Participants |
Inclusion criteria: critical neonates (term and preterm) age 3 hours to 14 days complicated with upper alimentary canal haemorrhage. All with normal clotting times. Exclusion criteria: none reported. |
|
| Interventions |
Intervention (n = 42): cimetidine 3 to 5 mg/kg intravenous drip every 6 to 8 hours until bleeding stopped and then continued 3 to 5 days more. Control (n = 41): no cimetidine. 2 groups had stomach wash‐out with 1% dicarbonate, thrombin 400 unit poured into stomach through stomach tube and metachysis in case of massive haemorrhage. 7 control infants received cimetidine after 48 hours. |
|
| Outcomes |
|
|
| Notes | Authors did not believe there were any adverse reactions attributable to the intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported: "randomly divided". |
| Allocation concealment (selection bias) | Unclear risk | Method not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None reported. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | Similar baseline characteristics between groups. |
Kuusela 1997.
| Methods | Randomised controlled trial in Finland. | |
| Participants |
Inclusion criteria: infants starting mechanical ventilation during the first 2 hours of life in the neonatal ICU. Exclusion criteria: infants who did not undergo mechanical ventilation during the first 2 hours of life in the neonatal ICU. Further information regarding participants: 53 mechanically ventilated newborn infants with data for 48 reported: mean gestational age 32 weeks (range 24 to 41 weeks). Ranitidine group mean and 95% CI (31 (29 to 33)); control group mean and 95% CI (32 (29 to 32)). Birth weight in grams (mean (95% CI)): ranitidine group 1678 (1241 to 2114); control group 1972 (1534 to 2412). Study period: 10 months. |
|
| Interventions |
Intervention (n = 23): ranitidine: 5 mg/kg/day intravenous, divided into 3 doses throughout 4 days Control (n = 25): no prophylaxis (no placebo was available). |
|
| Outcomes |
|
|
| Notes | Conflicts of interest: none reported. Supported, in part, by a grant from the Finnish Foundation of Pediatric Research. Authors did not believe there were any adverse reactions attributable to the intervention. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported: "mechanically ventilated newborns at the neonatal ICU were randomised into either the treatment group (prophylactic intravenous ranitidine [5 mg/kg body weight/day] divided into three doses throughout 4 days) or the control group (no prophylaxis)." "The first randomisation block included 20 envelopes in random order for both groups, followed by two randomisation blocks of ten envelopes for the more preterm babies. Babies closer to term were randomised in one block with 20 envelopes." Method of random sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | The methods of allocation concealment were not specified. It is not reported if envelopes were sealed and opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Reported: "Only the sequence number of the patient was written in the patient records by the nurse responsible for medication. Thus, the attending physicians, the endoscopist, and the pathologist remained blinded as to the treatment group." "The clinical data on the patients up to the age of 4 weeks were collected from patients’ records after the children had been discharged but before the opening of the randomisation code." There was no placebo ‒ no treatment was used in the control group so adequacy of clinical blinding is unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Reported: "the attending physicians, the endoscopist, and the pathologist remained blinded as to the treatment group." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 neonates (9% ‒ 3 treatment group; 2 control group) dropped out of the study after the randomisation and before gastroscopic evaluation. These patients were not included in the study because of early death (2 patients at gestational age of < 33 weeks and 1 patient at gestational age of ≥ 33 weeks) and because of oesophageal atresia (1 patient in both groups). |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘low risk’ or ‘high risk’ ‒ protocol not available. |
| Other bias | Low risk | No other bias noted. Infant characteristics were similar between intervention and control groups. |
Lin 2011.
| Methods | Randomised controlled trial in China. | |
| Participants |
Inclusion criteria: sick term and preterm newborns with stress ulcer day 1 to 8 age (n = 152). Exclusion criteria: none reported. |
|
| Interventions |
Intervention (n = 76): conventional therapy combined with omeprazole 0.6 mg/kg intragastric once daily for 5 days. Control (n = 76): conventional therapy. Both groups nil by mouth, stomach washed with bicarbonate. |
|
| Outcomes | Efficacy ‒ gastric aspirate bleed and melena to 48 hours. Bleeding after 48 hours no benefit. | |
| Notes | It is unclear if there were any adverse reactions attributable to the intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported: "randomly divided". |
| Allocation concealment (selection bias) | Unclear risk | Method not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses reported. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | Similar baseline characteristics between groups. |
Liu 2005.
| Methods | Randomised controlled trial in China 2002 to 2004. | |
| Participants |
Inclusion criteria: haemorrhage of digestive tract in sick term and preterm newborn infants 16 hours and 14 days (n = 58). Exclusion criteria: vitamin K deficiency, platelet or clotting abnormality, swallowed blood and congenital gastrointestinal abnormality. |
|
| Interventions |
Intervention (n = 30): famotidine 0.3 mg/kg 12 hourly continued for 48 to 72 hours until bleeding ceased for 24 hours. Control (n = 28): classical therapy. Both groups received vitamin K. |
|
| Outcomes | Efficacy ‒ stopped bleeding by 48 hours. | |
| Notes | Authors did not believe there were any adverse reactions attributable to the intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported: "divided randomly". |
| Allocation concealment (selection bias) | Unclear risk | Method not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses reported. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. |
| Other bias | Low risk | Similar baseline characteristics between groups. |
Lu 1995.
| Methods | Randomised controlled trial in China January 1990 to October 1992. | |
| Participants |
Inclusion criteria: sick term and preterm newborns at high risk of gastric bleeding. Exclusion criteria: none reported. |
|
| Interventions |
Intervention (n = 56): cimetidine 5 mg/kg/dose intravenously in 10% glucose twice daily for 3 to 5 days. Control (n = 62): no treatment. All infants given vitamin K 5 mg/day [route not reported] for 3 days. |
|
| Outcomes |
|
|
| Notes | Authors did not believe there were any adverse reactions attributable to the intervention. It was unclear how bleeding time was determined, given that these infants did not have bleeding initially. As a result, it could not be included in the 'duration of upper gastrointestinal bleeding in infants with upper gastrointestinal bleeding (days)' outcome. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported: "randomly assigned". |
| Allocation concealment (selection bias) | Unclear risk | Method not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None reported. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Unclear risk | Similar baseline characteristics between groups. |
Pourarian 2005.
| Methods | Randomised controlled trial in Iran. | |
| Participants |
Inclusion criteria: neonates admitted to the neonatal intensive care unit (NICU) ward irrespective of gestational age. Exclusion criteria: renal failure (serum creatinine > 1.5 mg/dl), nasal or pharyngeal bleeding or bleeding tendency, abnormal PT, PTT, platelet count, abnormal liver function test or received oral feeding. |
|
| Interventions |
Intervention (n = 40): prophylactic intravenous ranitidine 5 mg/kg/day (3 divided doses) for 4 days irrespective of gastric pH. Control group (n = 40): received no prophylactic treatment. |
|
| Outcomes |
|
|
| Notes | Gastric bleeding rates calculated from percentages reported in publication. Authors did not believe there were any adverse reactions attributable to the intervention. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information given. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. No placebo reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Percentages reported so denominator unclear. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Unclear risk | Some baseline differences in clinical conditions between groups. |
Sarna 1991.
| Methods | Quasi‐randomised controlled trial January 1990 to July 1990. | |
| Participants |
Inclusion criteria: neonates with gastric bleeding Exclusion criteria: all neonates who were septicaemic, preterm, had prolonged prothrombin time or developed disseminated intravascular coagulation. Further information regarding participants: 48 term neonates mean gestational age: ranitidine group 38.3 ± 2.2 weeks versus control group 38.6 ± 2.2 weeks (P > 0.05). Mean birth weight: ranitidine group 2.4 ± 0.6 versus control group 2.6 ± 0.8 kg. |
|
| Interventions | Intervention (n = 20): ranitidine: 0.6 mg/kg loading dose followed by 0.15 mg/kg/hour intravenous infusion until bleeding had ceased for 24 hours. Control (n = 18): supportive therapy. | |
| Outcomes |
|
|
| Notes | Conflicts of interest: not reported. Authors did not believe there were any adverse reactions attributable to the intervention. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Reported: "The neonates with gastric bleeding were alternatively, divided into two groups, on the basis of odd and even admission numbers." Quasi‐random allocation. |
| Allocation concealment (selection bias) | High risk | The methods of allocation concealment were not specified and the study was quasi‐random. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of assessors not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10 (4 treatment group, 6 control) of 48 infants (21%) not reported for other clinical outcomes who died. 3 infants died after intracranial haemorrhage; 3 from hypoxic‐ischaemic encephalopathy; and 4 from respiratory distress syndrome. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. |
| Other bias | Low risk | Infant characteristics were similar between intervention and control groups. |
Yang 2004.
| Methods | Randomised controlled trial in China. | |
| Participants |
Inclusion criteria: sick term and preterm neonates with upper gastrointestinal haemorrhage. Exclusion criteria: gastrointestinal tract abnormality; haemorrhagic diseases; adverse effect of medication. |
|
| Interventions |
Intervention (n = 27): ranitidine 3 to 5 mg/kg/day intravenous infusion for 3 to 5 days, and Smecta* 1/3 packet then 1/4 packet 3 times a day (* diosmectite, a natural silicate of aluminium and magnesium used as an intestinal adsorbent) and normal therapy. Control (n = 20): same therapy was performed in control group, except ranitidine and Smecta. Normal therapy: all infants nil by mouth, given vitamin K and 'hepatic enzymes' to stop bleeding. |
|
| Outcomes |
|
|
| Notes | Reported duration of bleeding intervention 1.7 days versus control 3.6 days (no SD). Authors did not believe there were any adverse reactions attributable to the intervention. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported: 'randomly divided'. Method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Probable post‐allocation losses due to haemorrhagic diseases; adverse effect of medication. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Unclear risk | Infant characteristics were similar between intervention and control groups. Group size imbalance. |
Yang 2015.
| Methods | Randomised controlled trial in China October 2013 to October 2014. | |
| Participants |
Inclusion criteria: sick neonatal patients with stress ulcer (blood in gastric aspirate and/or stool] (mean gestation 38.5 versus 38.6 weeks). Exclusion criteria: congenital anomalies; medication side effect. |
|
| Interventions |
Intervention (n = 23): omeprazole 0.7 mg/kg in saline intragastric daily for 5 days. Control (n = 22): conventional medicine treatment. All infants nil by mouth; given bicarbonate gastric washout and vitamin K. |
|
| Outcomes | Efficacy ‒ gastric aspirates and stool bleeding. Failure = bleeding at 24 and 48 hours. | |
| Notes | It is unclear if there were any adverse reactions attributable to the intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported: "separated them into control group and study group at random". Note this was not reported in Chinese language version. |
| Allocation concealment (selection bias) | Unclear risk | Method not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None reported. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | Infant characteristics were similar between intervention and control groups. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aanpreung 1998 | Study did not concern infants treated within the neonatal period. |
| Agarwal 1990 | Not a randomised trial (controlled clinical trial). |
| Bush 1987 | Not a randomised trial. |
| Cannon 1987 | Study did not concern infants treated within the neonatal period. |
| Chen 2010a | Study did not concern infants treated within the neonatal period. Trial of omeprazole versus omeprazole plus thrombin. |
| Chen 2010b | Study did not concern infants treated within the neonatal period. |
| Chen 2015 | Trial of different doses of omeprazole in newborns with stress ulceration. There was no control group without omeprazole. |
| Duan 2002 | Control group thrombin. |
| Fan 2010 | Study did not concern infants treated within the neonatal period. |
| Fontana 1993a | Not a randomised trial (prospective cohort study). |
| Fontana 1993b | Not a randomised trial (no control group). |
| Guillet 2006 | Not a randomised trial (case‐control study). |
| Han 2014 | Study did not concern infants treated within the neonatal period. |
| He 2004 | Retrospective study. |
| Hencz 1984 | Randomised study but only 1 of the participants was an infant. |
| Ito 1986 | Not a randomised trial. |
| Ke 2016 | Compared different doses of the same PPI (omeprazole 0.8 mg/kg/day versus infusion of 2 mg/kg/day), but did not compare to a control group without omeprazole. |
| Kelly 1993 | Not a randomised trial (case series of 10 consecutive infants). |
| Kentrup 1996 | Not a randomised trial. |
| Kuusela 1998 | Trial of different dose of ranitidine in newborn infants with gastrointestinal bleeding. No control group. |
| Lacroix 1986 | Newborns were not stratified in randomisation. Mean age of study participants was 1.85 ± 3.25 years (range 10 days to 14.5 years). |
| Lai 2014 | Omeprazole and haemocoagulase combined with norepinephrine for haemorrhagic disease of newborn versus conventional treatment of regular fasting, blood transfusion, indwelling stomach tube. Study included haemostatic treatments. |
| Li 2006 | Study did not concern infants treated within the neonatal period. |
| Liu 2002 | Study did not concern infants treated within the neonatal period. |
| Liu 2013 | Study comparing omeprazole and thrombin versus omeprazole alone. |
| Liu 2014 | Omeprazole versus no omeprazole but Vitamin K given in control group only. |
| Lopez‐Herce 1992 | Study did not concern infants treated within the neonatal period. |
| Metz 1993 | Study did not concern infants treated within the neonatal period. |
| Osteyee 1994 | Study did not concern infants treated within the neonatal period. |
| Ouyang 2012 | Retrospective study. |
| Simeone 1997 | Study did not concern infants treated within the neonatal period. |
| Solana 2013 | Study did not concern infants treated within the neonatal period. |
| Solana 2014 | Study did not concern infants treated within the neonatal period. |
| Song 2009 | Study did not concern infants treated within the neonatal period. |
| Song 2011 | 3‐arm RCT ‒ 2 arms ineligible as included thrombostatic agent (reptilase). |
| Sung 2014 | Study did not concern infants treated within the neonatal period. |
| Terrin 2012 | Not a randomised trial (prospective cohort study). |
| Vandenplas 1987 | Not a randomised trial (controlled clinical trial). |
| Wang 2005 | No control group. |
| Wang 2011 | Study did not concern infants treated within the neonatal period. |
| Wei 2004 | Trial of thrombin and cimetidine versus no treatment. |
| Wei 2013 | Trial of different doses of omeprazole for upper gastrointestinal bleeding in newborn infants. |
| Wu 2008 | No control group. |
| Xiaohua 2012 | Comparison of omeprazole versus norepinephrine. |
| Yildizdas 2002 | Study did not concern infants treated within the neonatal period. |
| Zhang 2003 | Study did not concern infants treated within the neonatal period. |
Differences between protocol and review
We have added an outcome to assess for adverse reactions to pharmacological interventions which was not included in the original protocol.
The background of the abstract was updated to reflect the association of upper gastrointestinal bleeding with mortality.
The Description of the condition within the Background has been updated to further reflect the associated adverse outcomes of upper gastrointestinal bleeding and the epidemiology of gastric lesions and upper gastrointestinal bleeding.
The Description of the intervention section has been updated to highlight the unclear aetiology of acid in gastric lesions in neonates. The Description of the intervention section has also been updated to ensure NEC is described as associated with, rather than caused by, H2RAs.
A section has been added to the How the intervention might work section to describe adverse effects of acid suppression agents.
The Subgroup analysis and investigation of heterogeneity section has been updated to ensure infants of age 32 weeks are now included in a subgroup.
Contributions of authors
The protocol was developed by Daniel S Green and Mohamed E Abdel‐Latif. All authors provided feedback on the content of the protocol.
The review manuscript was developed in Review Manager 5 (RevMan 5) by Daniel S Green (Review Manager 2014). Daniel S Green and Mohamed E Abdel‐Latif independently performed electronic database searches, assessed the quality of the evidence and synthesized the evidence. All authors provided feedback on the content of the draft and approved the final manuscript.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Vermont Oxford Network, USA.
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families.
-
National Institute for Health Research (NIHR), UK.
This report is independent research funded by a UK NIHR Cochrane Programme Grant (16/114/03). The views expressed in this publication are those of the review authors and are not necessarily those of the National Health Service, the NIHR, or the UK Department of Health.
Declarations of interest
All authors declare no known conflict of interest.
New
References
References to studies included in this review
Deng 2005 {published data only}
- Deng HL, Long JH, Mo K. Investigation of the effects of Omeprazole therapy on neonates with upper gastrointestinal hemorrhage. Hebei Medicine 2005;11(8):723‐5. [Google Scholar]
Han 2001 {published data only}
- Han M, Ye XQ, Yuan JQ. Cimetidine in prevention of acute gastric mucosal lesion of newborns. Journal of Bengbu Medical College 2001;26(4):315‐6. [Google Scholar]
Huo 2001 {published data only}
- Huo K. Cimetidine in treating upper alimentary canal hemorrhage after critical neonates in 42 cases. Journal of Pediatric Pharmacy 2001;7(3):25‐6. [Google Scholar]
Kuusela 1997 {published data only}
- Kuusela AL, Ruuska T, Karikoski R, Laippala P, Ikonen RS, Janas M, et al. A randomized, controlled study of prophylactic ranitidine in preventing stress‐induced gastric mucosal lesions in neonatal intensive care unit patients. Critical Care Medicine 1997;25(2):346‐51. [PUBMED: 9034275] [DOI] [PubMed] [Google Scholar]
Lin 2011 {published data only}
- Lin XY. The efficacy of stomach injection method applied stress ulcer omeprazole treatment of neonatal. China Practical Medicine 2011;6(32):14‐5. [Google Scholar]
Liu 2005 {published data only}
- Liu H. Observations of effect of Famotidine on hemorrhage of digestive tract in newborn. China Journal of Modern Medicine 2005;15(12):1870‐1, 4. [Google Scholar]
Lu 1995 {published data only}
- Lu WQ. The effect of cimetidine in preventing from acute gastric mucosal lesion of neonates. Journal of Neonatology 1995;10(1):23‐4. [Google Scholar]
Pourarian 2005 {published data only}
- Pourarian S, Imani B, Imanieh MH. Prophylactic ranitidine in prevention of GI bleeding in neonatal intensive care units. Iranian Journal of Medical Sciences 2005;30(4):178‐81. [Google Scholar]
Sarna 1991 {published data only}
- Sarna MS, Saili A, Dutta AK, Sharma D. Stress associated gastric bleeding in newborn‐‐role of ranitidine. Indian Pediatrics 1991;28(11):1305‐8. [PUBMED: 1808052] [PubMed] [Google Scholar]
Yang 2004 {published data only}
- Yang J, Zhao QM, Zhang Y. Ranitidine and smecta in treatment of neonates with upper gastrointestinal hemorrhage. Journal of Pediatric Pharmacy 2004;10(2):38‐9. [Google Scholar]
Yang 2015 {published data only}
- Yang D. The efficacy observation of Omeprazole in treatment of neonatal stress ulcer. China Health Standard Management 2015;6(7):221‐2. [Google Scholar]
References to studies excluded from this review
Aanpreung 1998 {published data only}
- Aanpreung P, Vanprapar N, Susiva C, Parkpreaw C, Boonyachart C. A randomized clinical trial comparing the efficacy of ranitidine and famotidine on intragastric acidity in critically ill pediatric patients. Chotmaihet thangphaet [Journal of the Medical Association of Thailand] 1998;81(3):185‐9. [PUBMED: 9623009] [PubMed] [Google Scholar]
Agarwal 1990 {published data only}
- Agarwal AK, Saili A, Pandey KK, Saxena AK, Sarna MS, Dutta AK. Role of cimetidine in prevention and treatment of stress induced gastric bleeding in neonates. Indian Pediatrics 1990;27(5):465‐9. [PUBMED: 2276774] [PubMed] [Google Scholar]
Bush 1987 {published data only}
- Bush A, Busst CM, Knight WB, Shinebourne EA. Cardiovascular effects of tolazoline and ranitidine. Archives of Disease in Childhood 1987;62(3):241‐6. [PUBMED: 3566316] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cannon 1987 {published data only}
- Cannon LA, Heiselman D, Gardner W, Jones J. Prophylaxis of upper gastrointestinal tract bleeding in mechanically ventilated patients. A randomized study comparing the efficacy of sucralfate, cimetidine, and antacids. Archives of Internal Medicine 1987;147(12):2101‐6. [PUBMED: 3500684] [PubMed] [Google Scholar]
Chen 2010a {published data only}
- Chen Y. The effect of 80 cases of gastrointestinal bleeding treated by thrombin and omeprazole. Guide of China Medicine 2010;8(27):26‐7. [Google Scholar]
Chen 2010b {published data only}
- Chen JY, Wang MX, Wang HM. Study on effect of pantoprazole, omeprazole and famotidine on treatment of peptic ulcer. Modern Medicine and Health 2010;26(11):1620‐1. [Google Scholar]
Chen 2015 {published data only}
- Chen JQ. Clinical efficacy and safety of continuous intravenous infusion of low dosage omeprazole for the treatment of neonatal stress ulcer. Chinese Journal of Clinical Pharmacology 2015, (18):1812‐4. [Google Scholar]
Duan 2002 {published data only}
- Duan H, Wang K. Study on the efficacy of ranitidine on upper gastrointestinal hemorrhage in neonates. Chinese Journal of Gastroenterology and Hepatology 2002;11(3):267‐8. [Google Scholar]
Fan 2010 {published data only}
- Fan Y, Mou L, Li W, Yan H. Clinical study of reptilase combined with famotidine treatment for neonatal gastrointestinal hemorrhage. Modern Preventive Medicine 2010;37(23):4588‐9. [Google Scholar]
Fontana 1993a {published data only}
- Fontana M, Tornaghi R, Petrillo M, Lora E, Bianchi Porro G, Principi N. Ranitidine treatment in newborn infants: effects on gastric acidity and serum prolactin levels. Journal of Pediatric Gastroenterology and Nutrition 1993;16(4):406‐11. [PUBMED: 8315550] [PubMed] [Google Scholar]
Fontana 1993b {published data only}
- Fontana M, Massironi E, Rossi A, Vaglia P, Gancia G P, Tagliabue P, et al. Ranitidine pharmacokinetics in newborn infants. Archives of Disease in Childhood 1993;68(5):602‐3. [PUBMED: 8323366] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guillet 2006 {published data only}
- Guillet R, Stoll BJ, Cotten CM, Gantz M, McDonald S, Poole WK, et al. Association of H2‐blocker therapy and higher incidence of necrotizing enterocolitis in very low birth weight infants. Pediatrics 2006;117(2):e137‐42. [DOI: 10.1542/peds.2005-1543; PUBMED: 16390920] [DOI] [PubMed] [Google Scholar]
Han 2014 {published data only}
- Han L. Analysis on the clinical efficacy of omeprazole for treatment of infantile gastrointestinal hemorrhage. China Medicine and Pharmacy 2014;9(4):78‐9, 93. [Google Scholar]
He 2004 {published data only}
- He F, Li Q, Gong X. The curative effect compare of several medications for neonate's upper alimentary tract bleeding. Journal of Pediatric Pharmacy 2004;10(6):19‐20. [Google Scholar]
Hencz 1984 {published data only}
- Hencz P, Baltas B, Tekulics P. Cimetidine therapy of stress ulcer caused by life‐threatening illnesses in children [Gyermekkori heveny eletveszelyallapotokat kisero stressz ulcus kezelese cimetidinnel]. Orvosi Hetilap 1984;125(33):2005‐7. [PUBMED: 6384876] [PubMed] [Google Scholar]
Ito 1986 {published data only}
- Ito S, Mizobuchi T, Shimizu I, Ohura M, Matsumura M, Kishi S, et al. An elevated type of gastric ulcer scar in patients treated with cimetidine. Endoscopy 1986;18(1):7‐9. [DOI: 10.1055/s-2007-1018311; PUBMED: 3948808] [DOI] [PubMed] [Google Scholar]
Ke 2016 {published data only}
- Ke M, Yan K, Rong L. Efficacy and safety of continuous low dose infusion of micro pump with omeprazole in the treatment of neonatal stress ulcer. Journal of Pediatric Pharmacy 2016;22(6):19‐21. [Google Scholar]
Kelly 1993 {published data only}
- Kelly EJ, Chatfield SL, Brownlee KG, Ng PC, Newell SJ, Dear PR, et al. The effect of intravenous ranitidine on the intragastric pH of preterm infants receiving dexamethasone. Archives of Disease in Childhood 1993;69(1 Spec No):37‐9. [PUBMED: 8346951] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kentrup 1996 {published data only}
- Kentrup H, Skopnik H, Wolter L, Engelhart W, Nauert C, Heimann G. Antacids for postoperative prevention of stress ulcer in infants: a dose finding study [Antazida zur postoperativen stressuljusprophylaxe bei suglingen: studie zur dosisfindung]. Klinische Padiatrie 1996;208(1):14‐6. [PUBMED: 8851320] [DOI] [PubMed] [Google Scholar]
Kuusela 1998 {published data only}
- Kuusela AL. Long‐term gastric pH monitoring for determining optimal dose of ranitidine for critically ill preterm and term neonates. Archives of Disease in Childhood 1998;78(2):F151‐3. [PUBMED: 9577289] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lacroix 1986 {published data only}
- Lacroix J, Infante‐Rivard C, Gauthier M, Rousseau E, Doesburg N. Upper gastrointestinal tract bleeding acquired in a pediatric intensive care unit: prophylaxis trial with cimetidine. Journal of Pediatrics 1986;108(6):1015‐8. [PUBMED: 3519913] [DOI] [PubMed] [Google Scholar]
Lai 2014 {published data only}
- Lai YZ. Curative effect of therapeutic alliance of omeprazole and hemocoagulase combined with norepinephrine for hemorrhagic disease of newborn. Shenzhen Journal of Integrated Traditional Chinese and Western Medicine 2014;24(2):17‐9. [Google Scholar]
Li 2006 {published data only}
- Li JJ, Zhou HJ, Sun SZ, Zhang S, Zhang BB. Observations on the therapeutic effects of omeprazole in small‐volume continues infusion in the treatment of stress ulcer. Acta Academiae Medicinae CPAPF 2006;15(6):560‐2. [Google Scholar]
Liu 2002 {published data only}
- Liu R, Wei C, Wang X, Yin Z. The efficacy of losec vs famotidine in the treatment of bleeding duodenal ulcer. Shanghai Medical and Pharmaceutical Journal 2002;23(7):299‐301. [Google Scholar]
Liu 2013 {published data only}
- Liu HM. Omeprazole combined thrombin treatment of neonatal upper gastrointestinal hemorrhage curative effect observation. For all Health 2013, (19). [Google Scholar]
Liu 2014 {published data only}
- Liu YC, Zhang J. Nursing care in neonates with gastrointestinal bleeding administered omeprazole via a gastric tube. World Chinese Journal of Digestology 2014;22(28):4356‐9. [DOI: 10.11569/wcjd.v22.i28.4356] [DOI] [Google Scholar]
Lopez‐Herce 1992 {published data only}
- Lopez‐Herce J, Dorao P, Elola P, Delgado MA, Ruza F, Madero R. Frequency and prophylaxis of upper gastrointestinal hemorrhage in critically ill children: a prospective study comparing the efficacy of almagate, ranitidine, and sucralfate. The Gastrointestinal Hemorrhage Study Group. Critical Care Medicine 1992;20(8):1082‐9. [PUBMED: 1643886] [DOI] [PubMed] [Google Scholar]
Metz 1993 {published data only}
- Metz CA, Livingston DH, Smith JS, Larson GM, Wilson TH. Impact of multiple risk factors and ranitidine prophylaxis on the development of stress‐related upper gastrointestinal bleeding: a prospective, multicenter, double‐blind, randomized trial. The Ranitidine Head Injury Study Group. Critical Care Medicine 1993;21(12):1844‐9. [PUBMED: 8252888] [DOI] [PubMed] [Google Scholar]
Osteyee 1994 {published data only}
- Osteyee JL, Banner W Jr. Effects of two dosing regimens of intravenous ranitidine on gastric pH in critically ill children. American Journal of Critical Care 1994;3(4):267‐72. [PUBMED: 7920954] [PubMed] [Google Scholar]
Ouyang 2012 {published data only}
- Ouyang XT. The analysis of the efficacy for reptilase plus omeprazole in the treatment of neonatal gastrointestinal bleeding. National Medical Frontiers of China 2012;7(16):36‐7. [Google Scholar]
Simeone 1997 {published data only}
- Simeone D, Caria MC, Miele E, Staiano A. Treatment of childhood peptic esophagitis: a double‐blind placebo‐controlled trial of nizatidine. Journal of Pediatric Gastroenterology and Nutrition 1997;25(1):51‐5. [PUBMED: 9226527] [DOI] [PubMed] [Google Scholar]
Solana 2013 {published data only}
- Solana MJ, Lopez‐Herce J, Sanchez A, Sanchez C, Urbano J, Lopez D, et al. 0.5 mg/kg versus 1 mg/kg of intravenous omeprazole for the prophylaxis of gastrointestinal bleeding in critically ill children: a randomized study. The Journal of Pediatrics 2013;162(4):776‐782.e1. [10.1016/j.jpeds.2012.10.010. Epub 2012 Nov 10.; PUBMED: 23149178] [DOI] [PubMed] [Google Scholar]
Solana 2014 {published data only}
- Solana MJ, Colom H, Lopez‐Herce J, Urbano J, Gonzalez R, Lopez J, et al. Population pharmacokinetics of omeprazole in critically ill pediatric patients. Therapeutic Drug Monitoring 2014;36(4):519‐27. [DOI: 10.1097/FTD.0000000000000033; PUBMED: 24365987] [DOI] [PubMed] [Google Scholar]
Song 2009 {published data only}
- Song W, Xiujun W, Yi W. Clinical comparison of Famotidine and Omeprazole in the treatment of children gastrointestinal bleeding. Shaanxi Medical Journal 2009‐05, (5):598‐9. [Google Scholar]
Song 2011 {published data only}
- Song XM. Observation on the curative effects of famotidine or omeprazole combined with reptilase in treatment of neonatal gastrointestinal hemorrhage. Maternal and Child Health Care of China 2011, (27):4276‐7. [Google Scholar]
Sung 2014 {published data only}
- Sung JJ, Suen BY, Wu JC, Lau JY, Ching JY, Lee VW, et al. Effects of intravenous and oral esomeprazole in the prevention of recurrent bleeding from peptic ulcers after endoscopic therapy. American Journal of Gastroenterology 2014;109(7):1005‐10. [DOI: 10.1038/ajg.2014.105; PUBMED: 24777150] [DOI] [PubMed] [Google Scholar]
Terrin 2012 {published data only}
- Terrin G, Passariello A, Curtis M, Manguso F, Salvia G, Lega L, et al. Ranitidine is associated with infections, necrotizing enterocolitis, and fatal outcome in newborns. Pediatrics 2012;129(1):e40‐5. [DOI: 10.1542/peds.2011-0796; PUBMED: 22157140] [DOI] [PubMed] [Google Scholar]
Vandenplas 1987 {published data only}
- Vandenplas Y, Sacre L. The use of cimetidine in newborns. American Journal of Perinatology 1987;4(2):131‐3. [DOI: 10.1055/s-2007-999755; PUBMED: 3494461] [DOI] [PubMed] [Google Scholar]
Wang 2005 {published data only}
- Wang WZ, Li SL. Study on effects of famotidine in neonates with acute gastric mucous membrance damage. Journal of Pediatric Pharmacy 2005;11(4):33‐4. [Google Scholar]
Wang 2011 {published data only}
- Wang YX, Chen XM, Zhu FS. A multi‐center clinical trial to evaluate the efficacy and safety of intravenous nizatidine in the management of peptic ulcer bleeding. World Chinese Journal of Digestology 2011;19(18):1963‐7. [Google Scholar]
Wei 2004 {published data only}
- Wei X. Clinical observation of thrombine and cimetidine in the treatment of neonatal hematemesis. Journal of Pediatric Pharmacy 2004;10(1):46‐7. [Google Scholar]
Wei 2013 {published data only}
- Wei D, Yanming T, Zhoujie P. Efficacy of Continuous Intravenous Infusion of Low‐Dose Omeprazole in the Treatment of Neonatal Stress Ulcer. Journal of Pediatric Pharmacy 2013;19(1):12‐4. [Google Scholar]
Wu 2008 {published data only}
- Wu YY. Efficacy and safety of famotidine for the treatment of stress ulcers in neonates. Chinese Journal of Contemporary Pediatrics 2008;10(5):593‐5. [PubMed] [Google Scholar]
Xiaohua 2012 {published data only}
- Xiaohua J, Yan L, Xiaosong S. Effect comparison of omeprazole and norepinephrine in treating ulcer hemorrhage in the newborn. Journal of Pediatric Pharmacy 2012;18(3):20‐2. [Google Scholar]
Yildizdas 2002 {published data only}
- Yildizdas D, Yapicioglu H, Yilmaz HL. Occurrence of ventilator‐associated pneumonia in mechanically ventilated pediatric intensive care patients during stress ulcer prophylaxis with sucralfate, ranitidine, and omeprazole. Journal of Critical Care 2002;17(4):240‐5. [DOI: 10.1053/jcrc.2002.36761; PUBMED: 12501151] [DOI] [PubMed] [Google Scholar]
Zhang 2003 {published data only}
- Zhang H. Omeprazole and cimetidine in treatment of stress ulcer hemorrhage: a randomized controlled clinical trial. Chinese Journal of New Drugs and Clinical Remedies 2003;22(7):407‐9. [Google Scholar]
Additional references
Alhazzani 2017
- Alhazzani W, Guyatt G, Alshahrani M, Deane AM, Marshall JC, Hall R, et al. Withholding pantoprazole for stress ulcer prophylaxis in critically ill patients: a pilot randomized clinical trial and meta‐analysis. Critical Care Medicine 2017;45(7):1121‐9. [DOI: 10.1097/CCM.0000000000002461; PUBMED: 28459708] [DOI] [PubMed] [Google Scholar]
Apt 1955
- Apt L, Downey WS Jr. Melena neonatorum: the swallowed blood syndrome; a simple test for the differentiation of adult and fetal hemoglobin in bloody stools. Journal of Pediatrics 1955;47(1):6‐12. [PUBMED: 14392548] [DOI] [PubMed] [Google Scholar]
Araujo 2010
- Araujo TE, Vieira SM, Carvalho PR. Stress ulcer prophylaxis in pediatric intensive care units. Jornal de Pediatria 2010;86(6):525‐30. [DOI: ; PUBMED: 21140039] [DOI] [PubMed] [Google Scholar]
Ardell 2010
- Ardell S, Offringa M, Soll R. Prophylactic vitamin K for the prevention of vitamin K deficiency bleeding in preterm neonates. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD008342; PUBMED: 29401369] [DOI] [PMC free article] [PubMed] [Google Scholar]
Attard 2017
- Attard TM, Miller M, Pant C, Kumar A, Thomson M. Mortality associated with gastrointestinal bleeding in children: A retrospective cohort study. World Journal of Gastroenterology 2017;23:1608‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Australian Medicines Handbook 2013
- Australian Medicines Handbook. Australian Medicines Handbook. Adelaide (AU): Newstyle Printing, 2013. [Google Scholar]
Barkun 2010
- Barkun AN, Bardou M, Kuipers EJ, Sung J, Hunt RH, Martel M, et al. International consensus recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding. Annals of Internal Medicine 2010;152(2):101‐13. [DOI: 10.7326/0003-4819-152-2-201001190-00009; PUBMED: 20083829] [DOI] [PubMed] [Google Scholar]
Bell 1978
- Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Annals of Surgery 1978;187(1):1‐7. [PUBMED: PMC1396409] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boyle 2008
- Boyle JT. Gastrointestinal bleeding in infants and children. Pediatrics in Review 2008;29(2):39‐52. [DOI: 10.1542/pir.29-2-39; PUBMED: 18245300] [DOI] [PubMed] [Google Scholar]
Brunner 1995
- Brunner G, Hell M, Hengels KJ, Hennig U, Fuchs W. Influence of lansoprazole on intragastric 24‐hour pH, meal‐stimulated gastric acid secretion, and concentrations of gastrointestinal hormones and enzymes in serum and gastric juice in healthy volunteers. Digestion 1995;56(2):137‐44. [DOI: 10.1159/000201233; PUBMED: 7750667] [DOI] [PubMed] [Google Scholar]
Chawla 2007
- Chawla S, Seth D, Mahajan P, Kamat D. Upper gastrointestinal bleeding in children. Clinical Pediatrics 2007;46(1):16‐21. [DOI: 10.1177/1084713806297151; PUBMED: 17164504] [DOI] [PubMed] [Google Scholar]
Cohen 2015
- Cohen S, Bueno de Mesquita M, Mimouni FB. Adverse effects reported in the use of gastroesophageal reflux disease treatments in children: a 10 years literature review. British Journal of Clinical Pharmacology 2015;80(2):200‐8. [DOI: 10.1111/bcp.12619; PUBMED: 25752807] [DOI] [PMC free article] [PubMed] [Google Scholar]
Costarino 2015
- Costarino AT, Dai D, Feng R, Feudtner C, Guevara JP. Gastric acid suppressant prophylaxis in pediatric intensive care: current practice as reflected in a large administrative database. Pediatric Critical Care Medicine 2015;16(7):605‐12. [DOI: 10.1097/PCC.0000000000000427; PUBMED: 25901549] [DOI] [PubMed] [Google Scholar]
Eastwood 2014
- Eastwood GM, Litton E, Bellomo R, Bailey MJ, Festa M, Beasley RW, Young PJ. Opinions and practice of stress ulcer prophylaxis in Australian and New Zealand intensive care units. Critical Care and Resuscitation 2014;16(3):170‐4. [PUBMED: 25161018] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 12 July 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Green 1978
- Green FW Jr, Kaplan MM, Curtis LE, Levine PH. Effect of acid and pepsin on blood coagulation and platelet aggregation. A possible contributor prolonged gastroduodenal mucosal hemorrhage. Gastroenterology 1978;74(1):38‐43. [PUBMED: 21830] [PubMed] [Google Scholar]
Green 2003
- Green BT, Rockey DC. Acute gastrointestinal bleeding. Seminars in Gastrointestinal Disease 2003;14(2):44‐65. [PUBMED: 12889580] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2017
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from training.cochrane.org/handbook.
Jobe 2001
- Jobe AH, Bancalari E. Bronchopulmonary dysplasia. American Journal of Respiratory and Critical Care Medicine 2001;163:1723‐9. [DOI: ; PUBMED: 11401896] [DOI] [PubMed] [Google Scholar]
Kolkman 1996
- Kolkman JJ, Meuwissen SG. A review on treatment of bleeding peptic ulcer: a collaborative task of gastroenterologist and surgeon. Scandinavian Journal of Gastroenterology 1996;218:16‐25. [PUBMED: 8865446] [DOI] [PubMed] [Google Scholar]
Krag 2015
- Krag M, Perner A, Wetterslev J, Wise MP, Borthwick M, Bendel S, et al. Stress ulcer prophylaxis in the intensive care unit: an international survey of 97 units in 11 countries. Acta Anaesthesiologica Scandinavica 2015;59:576‐85. [DOI: 10.1111/aas.12508; PUBMED: 25880349 ] [DOI] [PubMed] [Google Scholar]
Kuusela 2000
- Kuusela AL, Mäki M, Ruuska T, Laippala P. Stress‐induced gastric findings in critically ill newborn infants: frequency and risk factors. Intensive Care Medicine 2000;26(10):1501‐6. [PUBMED: 11126263] [DOI] [PubMed] [Google Scholar]
Lam 1999
- Lam NP, Le PD, Crawford SY, Patel S. National survey of stress ulcer prophylaxis. Critical Care Medicine 1999;27(1):98‐103. [PUBMED: 9934901] [DOI] [PubMed] [Google Scholar]
Lau 2007
- Lau JY, Leung WK, Wu JC, Chan FK, Wong VW, Chiu PW, et al. Omeprazole before endoscopy in patients with gastrointestinal bleeding. New England Journal of Medicine 2007;356(16):1631‐40. [DOI: 10.1056/NEJMoa065703; PUBMED: 17442905] [DOI] [PubMed] [Google Scholar]
Lazzaroni 2002
- Lazzaroni M, Petrillo M, Tornaghi R, Massironi E, Sainaghi M, Principi N, et al. Upper GI bleeding in healthy full‐term infants: a case‐control study. American Journal of Gastroenterology 2002;97(1):89‐94. [DOI: 10.1111/j.1572-0241.2002.05443.x; PUBMED: 11808975] [DOI] [PubMed] [Google Scholar]
Leontiadis 2006
- Leontiadis GI, Sharma VK, Howden CW. Proton pump inhibitor treatment for acute peptic ulcer bleeding. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD002094.pub3; PUBMED: 15266462] [DOI] [PubMed] [Google Scholar]
Leontiadis 2009
- Leontiadis GI, Howden CW. The role of proton pump inhibitors in the management of upper gastrointestinal bleeding. Gastroenterology Clinics of North America 2009;38(2):199‐213. [PUBMED: 19446254] [DOI] [PubMed] [Google Scholar]
MacLaren 2014
- MacLaren R, Reynolds PM, Allen RR. Histamine‐2 receptor antagonists vs proton pump inhibitors on gastrointestinal tract hemorrhage and infectious complications in the intensive care unit. JAMA Internal Medicine 2014;174(4):564‐74. [DOI: 10.1001/jamainternmed.2013.14673; PUBMED: 24535015] [DOI] [PubMed] [Google Scholar]
Maki 1993
- Maki M, Ruuska T, Kuusela AL, Karikoski‐Leo R, Ikonen RS. High prevalence of asymptomatic esophageal and gastric lesions in preterm infants in intensive care. Critical care medicine 1993;21(12):1863‐7. [PUBMED: 8252891] [DOI] [PubMed] [Google Scholar]
Marik 2010
- Marik PE, Vasu T, Hirani A, Pachinburavan M. Stress ulcer prophylaxis in the new millennium: a systematic review and meta‐analysis. Critical Care Medicine 2010;38(11):2222‐8. [DOI: 10.1097/CCM.0b013e3181f17adf; PUBMED: 20711074] [DOI] [PubMed] [Google Scholar]
Martinsen 2005
- Martinsen TC, Bergh K, Waldum HL. Gastric juice: a barrier against infectious diseases. Basic & Clinical Pharmacology & Toxicology 2005;96(2):94‐102. [PUBMED: 15679471] [DOI] [PubMed] [Google Scholar]
Maton 1999
- Maton PN, Burton ME. Antacids revisited: a review of their clinical pharmacology and recommended therapeutic use. Drugs 1999;57(6):855‐70. [PUBMED: 10400401] [DOI] [PubMed] [Google Scholar]
Mejia 2009
- Mejia A, Kraft WK. Acid peptic diseases: pharmacological approach to treatment. Expert Review of Clinical Pharmacology 2009;2(3):295‐314. [DOI: 10.1586/ecp.09.8; PUBMED: 21822447] [DOI] [PMC free article] [PubMed] [Google Scholar]
More 2013
- More K, Athalye‐Jape G, Rao S, Patole S. Association of inhibitors of gastric acid secretion and higher incidence of necrotizing enterocolitis in preterm very low‐birth‐weight infants. American Journal of Perinatology 2013;30(10):849‐56. [DOI: 10.1055/s-0033-1333671; PUBMED: 23359235] [DOI] [PubMed] [Google Scholar]
Ombeva 2013
- Ombeva OM, Ndeezi G, Mugalu J. Upper GI bleeding among neonates admitted to Mulago Hospital, Kampala, Uganda: a prospective cohort study. African Health Sciences 2013;13(3):741‐7. [DOI: 10.4314/ahs.v13i3.32; PUBMED: 24250316] [DOI] [PMC free article] [PubMed] [Google Scholar]
Puckett 2000
- Puckett RM, Offringa M. Prophylactic vitamin K for vitamin K deficiency bleeding in neonates. Cochrane Database of Systematic Reviews 2000, Issue 4. [DOI: 10.1002/14651858.CD002776; PUBMED: 11034761] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Romano 2017
- Romano C, Oliva S, Martellossi S, Miele E, Arrigo S, Graziani MG, Cardile S, Gaiani F, de'Angelis GL, Torroni F. Pediatric gastrointestinal bleeding: Perspectives from the Italian Society of Pediatric Gastroenterology. World Journal of Gastroenterology 2017;23:1328‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sachs 2007
- Sachs G, Shin JM, Vagin O, Lambrecht N, Yakubov I, Munson K. The gastric H,K ATPase as a drug target: past, present, and future. Journal of Clinical Gastroenterology 2007;41 Suppl 2:S226‐42. [DOI: 10.1097/MCG.0b013e31803233b7; PUBMED: 17575528] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Shan 2013
- Shan L, Li X, Liu K, Sun MN, Yao ZX, Li LD. Relationship between antacid therapy and hospital acquired pneumonia in critically ill patients: a meta‐analysis. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2013;25(6):360‐4. [DOI: 10.3760/cma.j.issn.2095-4352.2013.06.011; PUBMED: 23739571 ] [DOI] [PubMed] [Google Scholar]
Shears 2016
- Shears M, Alhazzani W, Marshall JC, Muscedere J, Hall R, English SW, et al. Stress ulcer prophylaxis in critical illness: a Canadian survey. Canadian Journal of Anaesthesia 2016;63(6):718‐24. [DOI: 10.1007/s12630-016-0612-3; PUBMED: 26911559] [DOI] [PubMed] [Google Scholar]
Sreedharan 2010
- Sreedharan A, Martin J, Leontiadis GI, Dorward S, Howden CW, Forman D, et al. Proton pump inhibitor treatment initiated prior to endoscopic diagnosis in upper gastrointestinal bleeding. Cochrane Database of Systematic Reviews 2010, Issue 7. [DOI: 10.1002/14651858.CD005415.pub3; PUBMED: 20614440] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tarnawski 1995
- Tarnawski A, Tanoue K, Santos AM, Sarfeh IJ. Cellular and molecular mechanisms of gastric ulcer healing. Is the quality of mucosal scar affected by treatment?. Scandinavian Journal of Gastroenterology 1995;210:9‐14. [PUBMED: 8578218] [DOI] [PubMed] [Google Scholar]
Tighe 2014
- Tighe M, Afzal NA, Bevan A, Hayen A, Munro A, Beattie RM. Pharmacological treatment of children with gastro‐oesophageal reflux. Cochrane Database of Systematic Reviews 2014, Issue 11. [DOI: 10.1002/14651858.CD008550.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vandenplas 2009
- Vandenplas Y, Rudolph CD, Lorenzo C, Hassall E, Liptak G, Mazur L, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN). Journal of Pediatric Gastroenterology and Nutrition 2009;49(4):498‐547. [DOI] [PubMed] [Google Scholar]


