ABSTRACT
Pancreatic ductal adenocarcinoma (PDAC) remains a leading cause of cancer-related death due to the failure of traditional therapies. In the present study, we attempted to construct a lncRNA-miRNA-mRNA network which may modulate PDAC cell proliferation and Gemcitabine-induced cell apoptosis starting from CDK14, a new member of the CDK family and an oncogene in many cancers. Based on TCGA data, a significant positive correlation was observed between lncRNA MSC-AS1 and CDK14. Moreover, MSC-AS1 expression was upregulated in PDAC tissues. Higher MSC-AS1 expression was correlated with poorer prognosis in patients with PDAC. MSC-AS1 knockdown in Panc-1 and BxPC-3 cells significantly inhibited the cell proliferation. Moreover, miR-29b-3p, which has been reported to act as a tumor suppressor, was predicted to bind to both MSC-AS1 and CDK14. Contrary to MSC-AS1, higher miR-29b-3p expression was correlated to better prognosis in patients with PDAC. In both PDAC cell lines, miR-29b-3p negatively regulated MSC-AS1 and CDK14. As confirmed using luciferase reporter gene and RIP assays, MSC-AS1 served as a ceRNA for miR-29b-3p to counteract miR-29b-mediated CDK14 repression. MSC-AS1 knockdown inhibited CDK14 protein levels and PDAC proliferation and enhanced gemcitabine-induced cell death and apoptosis while miR-29b-3p inhibition exerted an opposing effect; the effect of MSC-AS1 knockdown was partially attenuated by miR-29b-3p inhibition. Taken together, we demonstrated that MSC-AS1/miR-29b-3p axis modulates the cell proliferation and GEM-induced cell apoptosis in PDAC cell lines through CDK14. We provided a novel experimental basis for PDAC treatment from the perspective of lncRNA-miRNA-mRNA network.
Keywords: pancreatic ductal adenocarcinoma (PDAC), CDK14, lncRNA MSC-AS1, miR-29b-3p, proliferation, apoptosis
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is among the most frequent cancers with a high mortality and morbidity worldwide 1. Over the past decades, pancreatic cancer deaths have been increasing steadily. In contrary to other digestive tract cancer entities, to a large extent there has been no improvement with the survival in PADC patients by means of chemotherapy, radiotherapy and targeted therapies; in consequence, gene therapy may provide a new approach for patients with PDAC 2.
Cyclin-dependent kinases (CDKs) are considered as key regulatory factors of eukaryotic cell cycle, while their activities are controlled by related cell cycle proteins 3. A new member of the CDK family, namely PFTK1 gene, encodes CDK14 protein. It has been recently reported that CDK14 interacts with cyclin D3 (CCND3) and cyclin Y (CCNY) in order to modulate cell cycle progression and cell proliferation 4,5, it could also serve as an oncogene to modulate a few pathways and cellular mechanisms 6,7. For example, Pang et al. demonstrated that CDK14 upregulates invasiveness and cell motility in hepatocellular carcinoma (HCC) 6. More importantly, we revealed that CDK14 was promoted in tissues in patients with pancreatic cancer. Besides, pancreatic cancer cell proliferation, migration and invasion and the progress of epithelial-to-mesenchymal transition (EMT) were downregulated by CDK14 silence 8. A deeper understanding of the mechanism by which CDK14 could be regulated may help develop novel strategies for PDAC treatment.
Non-coding RNAs (ncRNAs) are the RNA molecules untranslated into proteins 9, such as microRNA (miRNA) and long non-coding RNA (lncRNA). The pivotal roles of non-coding RNAs in PDAC have been demonstrated 10,11. Recently, it has been reported by a study based on next generation sequencing, that in the carcinogenesis of pancreas at least 319 lncRNAs may be dysregulated 12. Moreover, deregulation of lncRNA plays a vital role in the metastatic process 13. Regarding possible mechanisms, via acting as competing endogenous RNAs (ceRNAs) for miRNAs, lncRNAs may counteract miRNA-mediated repression of downstream targets 14. LincRNA-ROR may exert its biological effects via serving as a ceRNA sponging miR-145 and thus promoting Mucin1 (MUC1) expression. As a result, it could promote tumor cell invasion and metastasis in triple negative breast carcinoma 15. LncRNA ZEB2-AS1 increases the growth and invasion of pancreatic cancer cell by upregulating HMGB1 as a ceRNA for miR-204 16. Searching for lncRNA-miRNA network related to CDK14 may provide novel understanding of the mechanism of its function in PDAC.
In the present study, lncRNA MSC-AS1 has been identified to be positively correlated with CDK14 and was examined for its relation with clinical parameters in patients with PDAC and effect on PDAC cell proliferation. Further, miRNAs related to CDK14 and MSC-AS1 were screened for. The effect of selected miRNA on MSC-AS1 and CDK14 and predicted binding between lncRNA, miRNA and mRNA was validated. Finally, the dynamic effect of lncRNA-miRNA axis on CDK14 and cell cycle-related factors and PDAC cell proliferation and apoptosis was evaluated. In summary, we provided a new mechanism for regulating the proliferation of PDAC on the potential therapeutic benefits of lncRNA-miRNA-mRNA regulation.
Materials and methods
Tissue samples
A total of 45 paired PDAC and non-cancerous pancreatic tissue specimens were collected from patients who underwent a surgery at the First Affiliated Hospital of Wenzhou Medical University (Wenzhou, China) under the approval of the Ethic Committee of the First Affiliated Hospital of Wenzhou Medical University, and later stored in liquid nitrogen at −80°C. All patients’ informed consents were obtained. These specimens were evaluated and identified by experienced pathologist; cancer cellularity was enriched by cryostat sectioning and dissection of most cellular area.
Cell line, cell culture and cell transfection
Human pancreatic cancer cell lines PANC-1 and BxPC-3 and were purchased from the cell bank of the Chinese Academy of Sciences (Shanghai, China) and cultured in RPMI-1640 medium (Invitrogen, CA, USA) adding 10% FBS (Gibco, CA, USA) at 37°C in a humidified atmosphere with 5% CO2.
LncRNA MSC-AS1 expression was achieved by transfection of MSC-AS1 shRNA1 or shRNA2 (GeneCopoecia, Guangzhou, China). The expression of miR-29b-3p was achieved by transfection of miR-29b-3p mimics or miR-29b-3p inhibitor (GenePharma, Shanghai, China). All transfections were performed with the help of Lipo2000 (Invitrogen, Waltham, MA, USA).
Real-time PCR
Total RNA was extracted using Trizol reagent (Invitrogen) following the protocol. The SYBR green PCR Master Mix (Qiagen) was used for mRNA expression detection following the protocol. The GAPDH expression was used as an endogenous control. The expression of miRNA was examined by a Hairpin-it TM miRNAs qPCR kit (Genepharma, Shanghai, China) using RNU6B as an endogenous control. 2−ΔΔCT method was used to analyze the relative fold changes.
Immunoblotting
Target cells in lysed using RIPA buffer with 1% PMSF; the proteins were extracted and analyzed for protein concentration using the bicinchoninic acid (BCA) protein assay kit (Beyotime Institute of Biotechnology, China). Protein separation was conducted by SDS-PAGE minigel and the blots were then transferred onto PVDF membranes. Afterwards, the membrane was probed with the antibodies listed below: anti-CDK14 (ab104150, Abcam, Cambridge, MA, USA), anti-c-Myc (ab32072, Abcam), anti-CCND1 (ab134175, Abcam), anti-CCNY (ab237677, abcam), anti-CCND3 (ab28283, abcam) and anti-GAPDH (ab8245, Abcam) at 4°C overnight. Thereafter, the blots were incubated with Horseradish peroxidase-conjugated secondary antibody IgG (cat. P0448, Dako, Milano, Italy). Signals visualization was conducted by ECL Substrates (Millipore, MA, USA) using Tubulin as an endogenous protein for normalization. The gray intensity analysis was performed using ImageJ software (NIH).
MTT assay
MTT assay was performed to evaluate cell proliferation. 24 h after seeding into 96-well plates (5 × 103 cells/well), cells were transfected and/or treated as described. 48 h after transfection, 20 μl MTT (at a concentration of 5 mg/ml; Sigma-Aldrich) was added, and the cells were incubated for an additional 4 h in a humidified incubator. 200 μl DMSO was added after the supernatant discarded to dissolve the formazan. OD490 nm value was measured. The proliferation of the non-treated cells (control) was defined as 100%, and the proliferation rate of cells from all other groups was calculated separately from that of the control group.
Colony formation assay
Cells were seeded and transfected as described. Cells were suspended in RPMI-1640 containing 0.35% low-melting agarose and plated onto 0.6% agarose in six-well culture plates at a density of 1 × 105 cells per dish. The plates were incubated for two weeks at 37°C in a 5% CO2 incubator, and the number of colonies was counted after staining with 0.1% crystal violet solution. Colonies with more than 50 cells were manually counted.
Typan blue staining
After transfected and gemcitabine treatment, cells were harvested and resuspended in culture medium. The cell suspensions were mixed with trypan blue solution (0.4% in PBS). Colored (non‑viable) and dye-excluding (viable) cells were counted on a Malassez hemocytometer.
Luciferase reporter assay
For CDK14 3ʹ-UTR or lncRNA MSC-AS1 binding to miR-29b-3p, the fragment of CDK14 3ʹ-UTR or lncRNA MSC-AS1 was amplified by PCR and cloned to the downstream of the Renilla psiCHECK2 vector (Promega, Madison, WI, USA), named wt-CDK14 3ʹ-UTR or wt-MSC-AS1. To generate the CDK14 3ʹ-UTR or lncRNA MSC-AS1 mutant reporter, the seed region of the CDK14 3ʹ-UTR or lncRNA MSC-AS1 was mutated to remove the complementarity to miR-29b-3p, named mut-CDK14 3ʹ-UTR or mut-MSC-AS1. HEK293 cells (ATCC, USA) were seeded into a 24-well plate. After cultured overnight, HEK293 cells were co-transfected with the indicated vectors and miR-29b-3p mimics or miR-29b-3p inhibitor, respectively. Luciferase assays were performed 48 h after transfection using the Dual Luciferase Reporter Assay System (Promega). Renilla luciferase activity was normalized to Firefly luciferase activity for each transfected well.
RNA immunoprecipitation (RIP)
RNA immunoprecipitation was performed using Magna RIP RNA-Binding Protein Immunoprecipitation Kit (17–700, Millipore) following the protocols. RNA for in vitro experiments was transcribed using T7 High YieldRNA Synthesis Kit (E2040S, NEB) following the protocols. CDK14, MSC-AS1 and miR-29b levels in the immunoprecipitates were measured by qRT-PCR.
Flow cytometer assay
For apoptosis analysis, quantification of apoptotic cells was performed with Annexin V-FITC apoptosis detection kit (Keygen, China). Briefly, the cell samples were harvested with 0.25% trypsin without EDTA after 48 hours of infection and then washed twice with ice-cold PBS and re-suspended in 500 μl binding buffer. Then cells were incubated with 5 μl Annexin V-FITC specific antibodies and 5 μl propidium iodide (PI) then incubated for 15–20 minutes in dark and detected by BD Accuri C6 flow cytometer (BD, USA) with the excitation wavelength of Ex = 488 nm and emission wavelength of Em = 530 nm. Each experiment was repeated three times in triplicate.
Statistical analysis
Data are processed using SPSS17.0 statistical software and presented as the mean ±S.D. of results from at least three independent experiments. A Student t test was used for statistical comparison between means where applicable. Differences among more than two groups in the above assays were estimated using one-way ANOVA. Survival curves were constructed and differences among groups were calculated using the Kaplan-Meier method. Significant variables in univariate analyses were used in multivariate analyses according to the Cox regression analyses. *P < 0.05; **P < 0.01.
Results
Selection of lncrna MSC-AS1 and its correlation with the clinical parameters
Due to upregulation of CDK14 (PFTK1) expression and its key role in promoting the proliferation, invasion and EMT of pancreatic cancer tumor cell8, herein, we analyzed the data from TCGA database and found that lncRNA MSC-AS1 was positively correlated with CDK14 (Figure S1(a)). Similar to CDK14, MSC-AS1 expression was also significantly upregulated in pancreatic adenocarcinoma (PAAD) according to TCGA (Figure S1(b)). More importantly, a low MSC-AS1 expression was correlated to a higher percentage of overall survival in PAAD patients (Figure S1(c)). These findings suggest that lncRNA MSC-AS1 may affect the proliferation of PDAC cell.
In order to investigate MSC-AS1 function as well as its correlation with CDK14 in PDAC, we made use of real-time PCR for first examining CDK14 expression as well as MSC-AS1 in 45 paired PDAC and non-cancerous tissue specimens. As revealed by Figure 1(a,b), CDK14 and MSC-AS1 expression were both remarkably upregulated in 45 cases of PDAC tissue specimens, compared to those in non-cancerous tissue specimens. In tissue samples, CDK14 and MSC-AS1 was positively correlated (Figure 1(c)).
Figure 1.
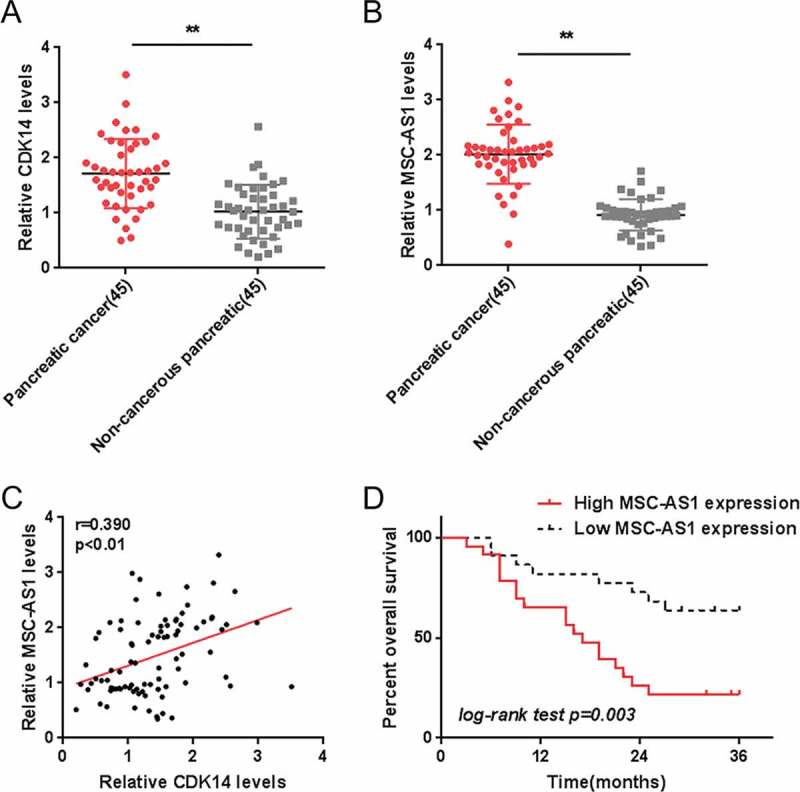
Selection of lncRNA MSC-AS1 and its correlation with the clinical parameters (a-b) Expression of CDK14 and MSC-AS1 in 45 paired PDAC and non-cancerous tissue specimens were examined using real-time PCR assays. (c) The correlation between CDK14 and MSC-AS1 was analyzed using Pearson’s analysis. (d) The overall survival of 45 cases patients with PDAC grouped by MSC-AS1 expression was analyzed using Kaplan-Meier method. The data are presented as mean ±SD of three independent experiments. *P < 0.05, **P < 0.01.
Next, according to the mid-value of MSC-AS1 expression we grouped 45 cases of PDAC specimens into a high MSC-AS1 group with expression of MSC-AS1 greater than the mid-value as well as a low MSC-AS1 group with MSC-AS1 expression less than the mid-value. Then we examined the relation between the expression of MSC-AS1 and clinical indicators. A high MSC-AS1 expression was related to distant metastasis (P = 0.004) as well as advanced TNM stages (P = 0.005) (Table 1). According to the Cox regression model analysis, MSC-AS1 expression, distant metastasis and TNM stage resulted in remarkable differences in overall survival, and MSC-AS1 expression represented a high-risk factor to overall survival of PDAC patients (Table 2). According to Kaplan-Meier analysis we revealed that the overall survival of patients in the high MSC-AS1 group was poorer (Figure 1(d)). The above findings suggest that MSC-AS1 may contribute to PDAC progression.
Table 1.
Correlation of the expression of NR2F1-AS1 & PFTK1 with clinicopathologic features.
| NR2F1-AS1 expression |
PFTK1 expression |
||||||
|---|---|---|---|---|---|---|---|
| Clinic-pathological parameters | High | Low | p | High | Low | p | |
| Distant metastasis | M0 | 4 | 19 | <0.001 | 8 | 15 | 0.025 |
| M1 | 19 | 3 | 15 | 7 | |||
| Gender | female | 11 | 11 | 0.884 | 9 | 13 | 0.181 |
| male | 12 | 11 | 14 | 9 | |||
| Age (years) | <50 | 10 | 9 | 0.862 | 11 | 8 | 0.436 |
| ≥50 | 13 | 13 | 12 | 14 | |||
| TNM stage | I+II | 3 | 18 | <0.001 | 7 | 14 | 0.026 |
| III+IV | 20 | 4 | 16 | 8 | |||
| Pancreas tumor location | Elsewhere | 8 | 11 | 0.302 | 8 | 11 | 0.302 |
| Head | 15 | 11 | 15 | 11 | |||
| Largest tumor dimension(cm) | <3 | 10 | 12 | 0.458 | 10 | 12 | 0.458 |
| ≥3 | 13 | 10 | 13 | 10 | |||
Table 2.
Univariate and multivariate analysis for factors related to overall survival using the COX proportional hazard model.
| Univariate analysis |
Multivariate analysis |
||||||
|---|---|---|---|---|---|---|---|
| Variable | P-value | HR | 95.0% CI | P-value | HR | 95.0% CI | |
| NR2F1-AS1 | High vs Low | <0.001 | 4.580 | 1.956–10.720 | 0.040 | 2.895 | 1.048–8.001 |
| PFTK1 | High vs Low | 0.024 | 2.544 | 1.131–5.721 | 0.036 | 2.473 | 1.059–5.771 |
| Distant metastases | Negative vs Positive | <0.001 | 0.182 | 0.075–0.440 | 0.679 | 0.640 | 0.077–5.295 |
| Gender | Female vs male | 0.622 | 1.214 | 0.562–2.622 | |||
| Age (years) | <50 vs ≥50 | 0.838 | 1.085 | 0.498–2.365 | |||
| TNM stage | I+II vs III+IV | 0.000 | 0.178 | 0.071–0.449 | 0.543 | 0.516 | 0.061–4.342 |
| Pancreas tumor location | Elsewhere vs Head | 0.458 | 0.741 | 0.336–1.635 | |||
| Largest tumor dimension(cm) | <3 vs ≥3 | 0.178 | 0.580 | 0.263–1.282 | |||
MSC-AS1 knockdown inhibited PDAC cell proliferation
Since higher MSC-AS1 expression is related to lower survival in PDAC patients, the specific function of MSC-AS1 on PDAC cells was then detected. As confirmed by real-time PCR, MSC-AS1 was knocked down in two PDAC cell lines, Panc-1 and BxPC-3, by transfecting MSC-AS1 shRNA1 or shRNA2 (Figure 2(a)); As revealed by MTT assays, both MSC-AS1 shRNA1 and shRNA2 dramatically downregulated the proliferation of these two PDAC cell lines (Figure 2(b-c)). The MSC-AS1 shRNA2 has a better suppression effect on cell proliferation. Therefore, MSC-AS shRNA2 was chose for MSC-AS1 knockdown in the further experiments. MSC-AS1 knockdown repressed the colony formation capacity of both PDAC cell lines (Figure 2(d-e)). Based on these data, we reveal that MSC-AS1 knockdown can suppress PDAC cell proliferation.
Figure 2.
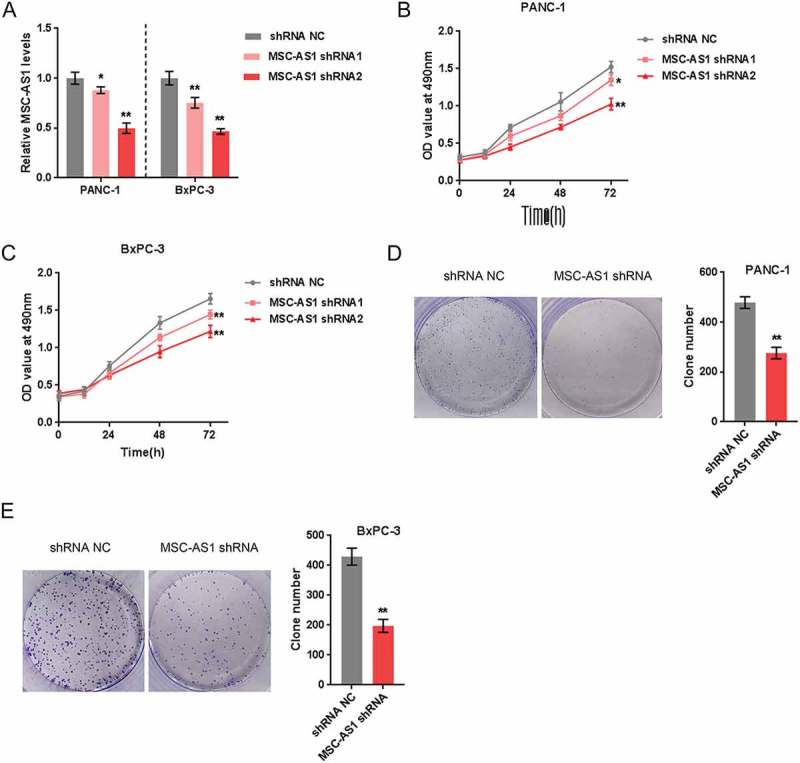
MSC-AS1 knockdown inhibited PDAC cell proliferation (a) MSC-AS1 knockdown in Panc-1 and BxPC-3 cells was achieved by transfection of MSC-AS1 shRNA1 or shRNA2, as confirmed by real-time PCR. (b-c) Panc-1 and BxPC-3 cells were transfected with MSC-AS1 shRNA1 or shRNA2 and examined for cell proliferation using MTT assays. (d-e) Panc-1 and BxPC-3 cells were transfected with MSC-AS1 shRNA2 and examined the colony formation capacity. The data are presented as mean±SD of three independent experiments. *P < 0.05, **P < 0.01.
Selection of mir-29b-3p and its correlation with the clinical parameters
In general, LncRNAs serve as miRNA “sponges” by sharing common miRNAs responses elements (MRE), inhibiting normal miRNA targeting activity on mRNA 14. To investigate the mechanism of MSC-AS1 modulating PDAC cell proliferation, miRNAs associated with CDK14 and MSC-AS1 were screened for. According to data from TCGA, 26 miRNAs were significantly correlated with CDK14 in 154 cases of patients with PDAC, of them 24 miRNAs were negatively correlated with CDK14. Further, 7 of the 24 miRNAs were predicted to target CDK14, while 5 of them were putative downstream targets of MSC-AS1 (Figure 3(a)). In Panc-1 cells, once MSC-AS1 knocked down, the expression of these 5 miRNAs were all significantly upregulated, miR-29b-3p more upregulated (Figure 3(b)). Consistently, the significant increase in miR-29b-3p expression was correlated with MSC-AS1 knockdown (Figure 3(c)). More importantly, according to previous study, miR-29b-3p was lowly-expressed in the tissues of PDAC 17. Based on these data, miR-29b-3p was selected for further experiments. Contrary to MSC-AS1 and CDK14, miR-29b-3p expression was remarkably inhibited in PDAC tissue specimens (Figure 3(d)). Data from TCGA revealed that a poorer overall survival was more commonly seen in PDAC patients with a lower miR-29b-3p expression (Figure 3(e)). The 45 cases PDAC patients recruited in this study were grouped by the mid-value of miR-29b-3p expression: a high miR-29b-3p group with the expression of miR-29b-3p greater than the mid-value as well as a low miR-29b-3p group with the expression of miR-29b-3p less than the mid-value. Consistent with analysis based on TGCA, lower miR-29b-3p expression resulted in poorer overall survival (Figure 3(f)). In tissue specimens, a negative correlation between miR-29b-3p expression and CDK14 and MSC-AS1 expression was observed (Figure 3(g-h)). According to these figures we reveal that miR-29b-3p may have the opposite impact on proliferation of PDAC cells as MSC-AS1.
Figure 3.
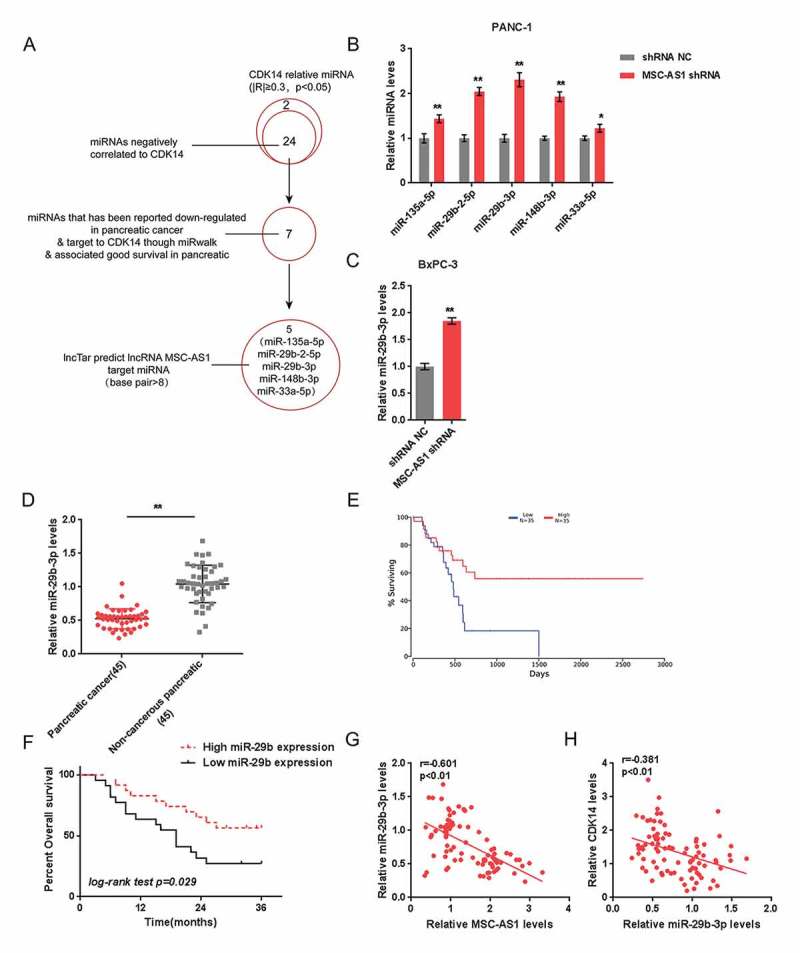
Selection of miR-29b-3p and its correlation with the clinical parameters (a) A schematic diagram showing the selection of miRNAs related to CDK14 and MSC-AS1. (b) Expression of five selected miRNAs, miR-135a-5p, miR-29b-2-5p, miR-29b-3p, miR-148b-3p and miR-33a-5p in response to MSC-AS1 knockdown was examined in Panc-1 cells. (c) miR-29b expression in response to MSC-AS1 knockdown was examined in BxPC-3 cells. (d) Expression of miR-29b-3p in 45 paired PDAC and non-cancerous tissue specimens was examined. The data are presented as mean ± D of three independent experiments. *P < 0.05, **P < 0.01. (e) The overall survival of patients with PDAC recorded in TCGA database was analyzed using Kaplan-Meier method. (f) The overall survival of 45 cases patients with PDAC grouped by miR-29b-3p expression was analyzed using Kaplan-Meier method. (g-h) Correlation between miR-29b-3p and MSC-AS1, between miR-29b-3p and CDK14 was analyzed using Pearson’s analysis.
We counteract mir-29b-mediated repression of CDK14 by using MSC-AS1 as a cerna for mir-29b-3p
As online tools predicted, miR-29b-3p can target both MSC-AS1 and CDK14; next, as confirmed by real-time PCR, once miR-29b-3p mimics or inhibitor transfected, miR-29b-3p was expressed in Panc-1 cell and BxPC-3 cell (Figure 4(a)), taken that we could verify the predicted interaction and regulation. In Panc-1 cell and BxPC-3 cell, MSC-AS1 expression (Figure 4(b)) and CDK14 protein levels (Figure 4(c)) could be downregulated by miR-29b.
Figure 4.
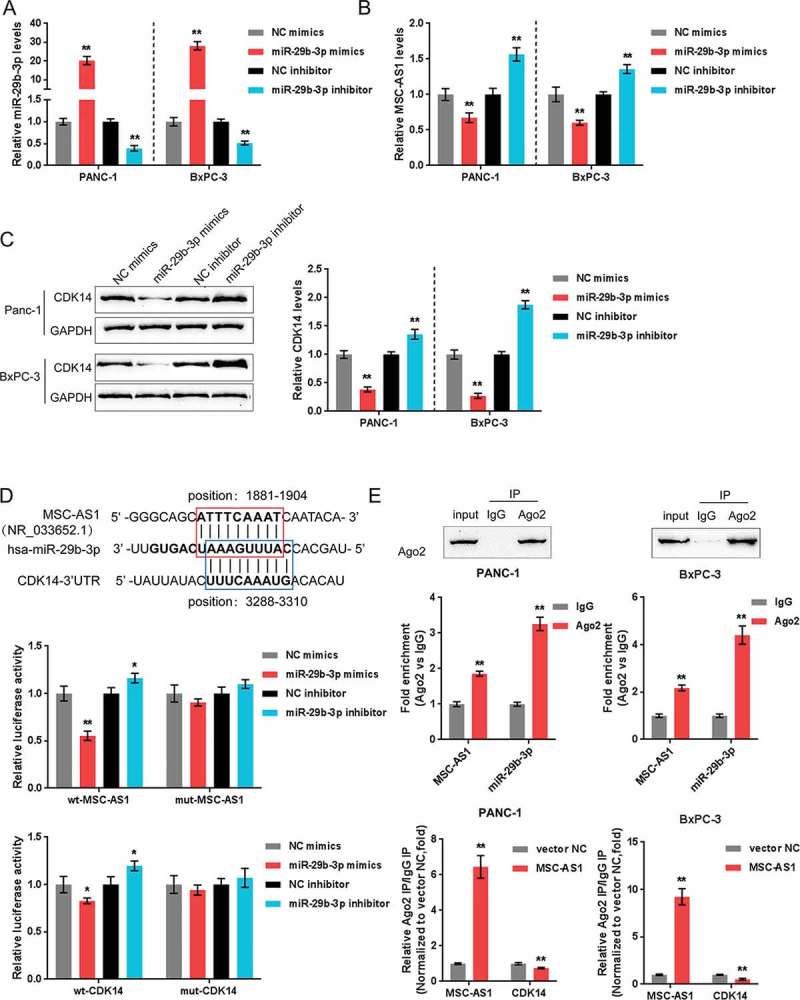
MSC-AS1 serves as a ceRNA for miR-29b-3p to counteract miR-29b-mediated repression of CDK14 (a) miR-29b-3p expression was achieved by transfection of miR-29b-3p mimics or miR-29b-3p inhibitor into Panc-1 and BxPC-3 cells, as confirmed using real-time PCR. (b) Expression of MSC-AS1 in response to miR-29b-3p overexpression or miR-29b-3p inhibition was examined in Panc-1 and BxPC-3 cells. (c) Protein levels of CDK14 in response to miR-29b-3p overexpression or miR-29b-3p inhibition was examined in Panc-1 and BxPC-3 cells. (d) The interaction between miR-29b-3p and CDK14, between miR-29b-3p and MSC-AS1 was validated by luciferase reporter gene assays. (e) Association of miR-29b-3p and MSC-AS1 with AGO2 in Panc-1 and BxPC-3 cells. Detection of AGO2 using Immunoblotting assays. (f) RIP assay in Panc-1 and BxPC-3 cells transfected with vector-NC (negative control) or MSC-AS1 overexpressing vector followed by real-time PCR to detect MSC-AS1 and CDK14 associated with AGO2. The data are presented as mean ±SD of three independent experiments. *P < 0.05, **P < 0.01.
Then we employed luciferase reporter gene assays to verify the respective interactions between miR-29b-3p and MSC-AS1 and CDK14. Two different types of reporter vectors, wild-type vectors and mutant-type vectors were constructed and entitled wt-CDK14 3ʹ-UTR and wt-MSC-AS1, as well as mut-CDK14 3ʹ-UTR and mut-MSC-AS1, containing wild and mutant miR-29b-3p binding site, respectively (Figure 4(d)). HEK293 cells were then co-transfected with these vectors and miR-29b-3p mimics or inhibitor; the luciferase activity of wild-type vector was inhibited bymiR-29b-3p overexpression while amplified by miR-29b-3p inhibition; once the putative miR-29b-3p target site mutated, the elimination of luciferase activity alteration was observed (Figure 4(d)). Next, for further confirming the target we employed the RIP assays. According to the results, we revealed that miR-29b-3p and MSC-AS1 were associated with AGO2 in cells of Panc-1 and BxPC-3 (Figure 4(e)). We extracted RNA from the protein deposits, and the levels of MSC-AS1 and miR-29b-3p were more than 2 times higher than IgG group (Figure 4(e)). Next, RIP assays were performed on these two PDAC cell lines transfected with MSC-AS1 overexpressing vector and then examined for the levels of MSC-AS1 and CDK14. Upon MSC-AS1 overexpression, the levels of MSC-AS1 in the protein precipitate was dramatically upregulated while the levels of CDK14 were obviously downregulated, compared to control vector group (Figure 4(f)). According to the above findings, we reveal that MSC-AS1 directly targets miR-29b-3p as a ceRNA for miR-29b-3p, thereby neutralizing miR-29b-3p-mediated CDK14 inhibition.
Msc-as1/mir-29b-3p axis modulates PDAC cell proliferation through CDK14
We examined the impact of MSC-AS1/miR-29b-3p axis on CDK14 as well as other cell cycle-related proteins, c-Myc, cyclin D1, cyclin D3 and cyclin Y, and then verified the dynamic effect of this axis on PDAC cell proliferation. As shown in Figure 5(a-f), MSC-AS1 knockdown significantly downregulated while miR-29b-3p inhibition upregulated the protein levels of CDK14, c-Myc, cyclin D1 as well as cyclin D3. The cyclin Y expression showed an opposite trend; miR-29b-3p inhibition partially restored the effect of MSC-AS1 knockdown. Next, the cell proliferation and colony formation of PDAC cell lines were examined. Consistently, MSC-AS1 knockdown significantly suppressed the cell activity and colony formation of both PDAC cell lines, whereas the cellular effect of miR-29b-3p inhibition was opposite; miR-29b-3p inhibition partially restored the effect of MSC-AS1 knockdown (Figure 6 (a-c)). These data indicate that MSC-AS1/miR-29b-3p axis modulates PDAC cell proliferation through CDK14 and cell cycle-related factors.
Figure 5.
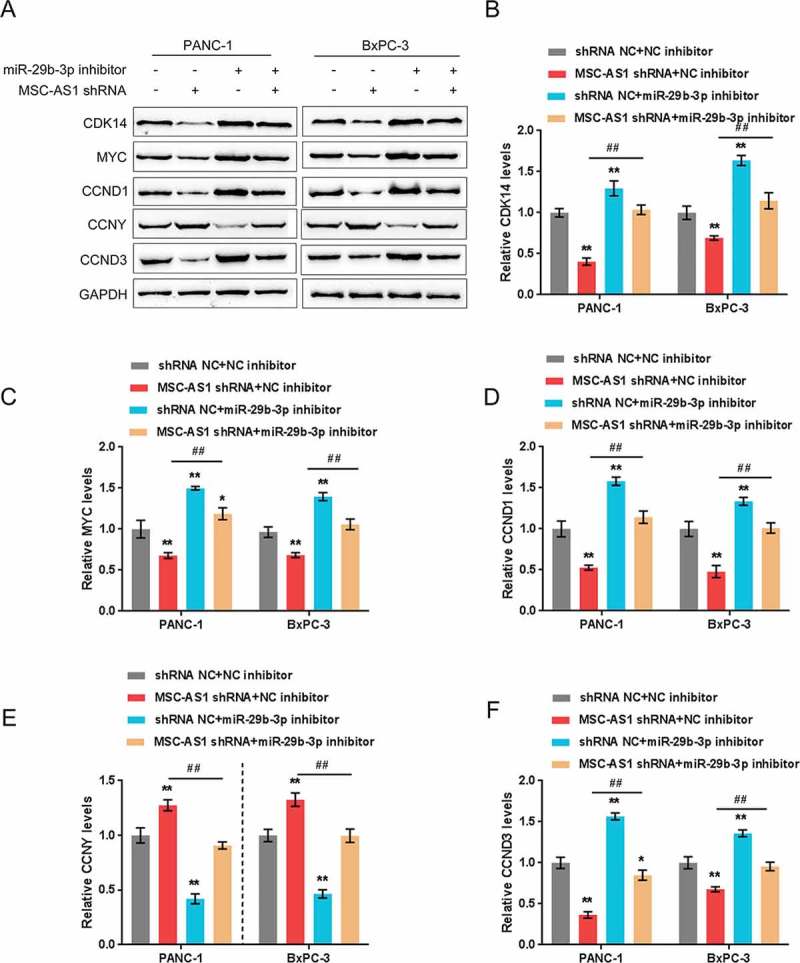
MSC-AS1/miR-29b-3p axis modulates CDK14 and cell cycle-related proteins expression in PDAC cell. (a)Panc-1 and BxPC-3 cells were co-transfected with miR-29b-3p inhibitor and MSC-AS1 shRNA and then examined for protein levels of CDK14, c-Myc, cyclin D1, cyclin Y and cyclin D3 using westernblot, the protein density analysis were shown in (b-f). The data are presented as mean ±SD of three independent experiments. *P < 0.05, **P < 0.01, compared to control group; ##P < 0.01, compared to MSC-AS1 shRNA group.
Figure 6.
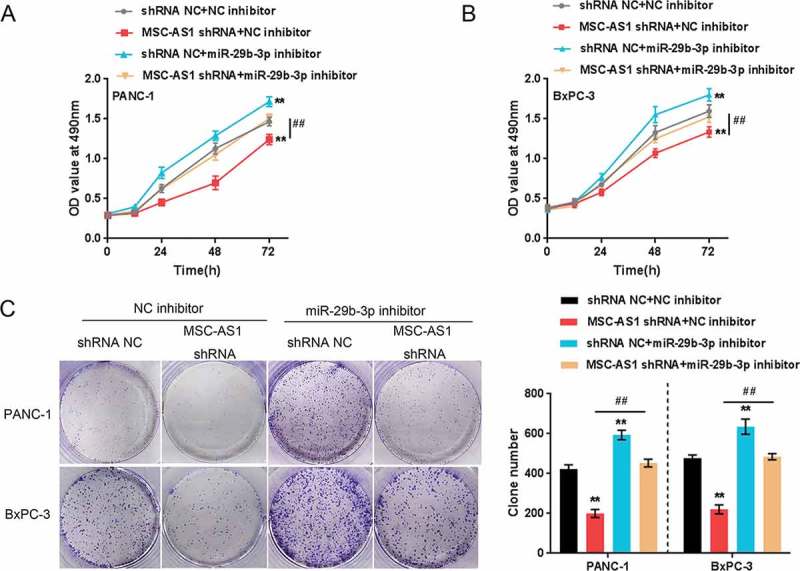
MSC-AS1/miR-29b-3p axis modulates PDAC cell proliferation through CDK14. Panc-1 and BxPC-3 cells were co-transfected with miR-29b-3p inhibitor and MSC-AS1 shRNA and then examined for cell proliferation (a-b) and colony formation capacity (c). The data are presented as mean ± SD of three independent experiments. *P < 0.05, **P < 0.01, compared to control group; ##P < 0.01, compared to MSC-AS1 shRNA group.
MSC-As1/miR-29b-3p axis modulates gemcitabine-mediated PDAC cell apoptosis
Gemcitabine (GEM) is used as the standard chemotherapy for unresectable pancreatic cancer due to its ability to prolong the overall survival and improve life quality 18. Here, the effect of MSC-AS1/miR-29b-3p axis on GEM-mediated PDAC cell death and apoptosis was evaluated. We co-transfected Panc-1 cell and BxPC-3 cell with miR-29b-3p inhibitor and MSC-AS1 shRNA in the presence or absence of 20 nM GEM and examined for cell death and apoptosis using typan blue staining and Flow Cytometer assays. As shown in Figure 6, GEM treatment significantly induced cytotoxicity and apoptosis in PDAC cells; GEM-induced cell death and apoptosis was positively regulated by MSC-AS1 silence while negatively regulated by miR-29b-3p inhibition; miR-29b-3p inhibition partly reduced the effect of MSC-AS1 silence on cell death and apoptosis (Figure 7). The above findings demonstrate that MSC-AS1/miR-29b-3p axis can also have an effect on the sensitivity of PDAC cells to GEM treatment.
7.
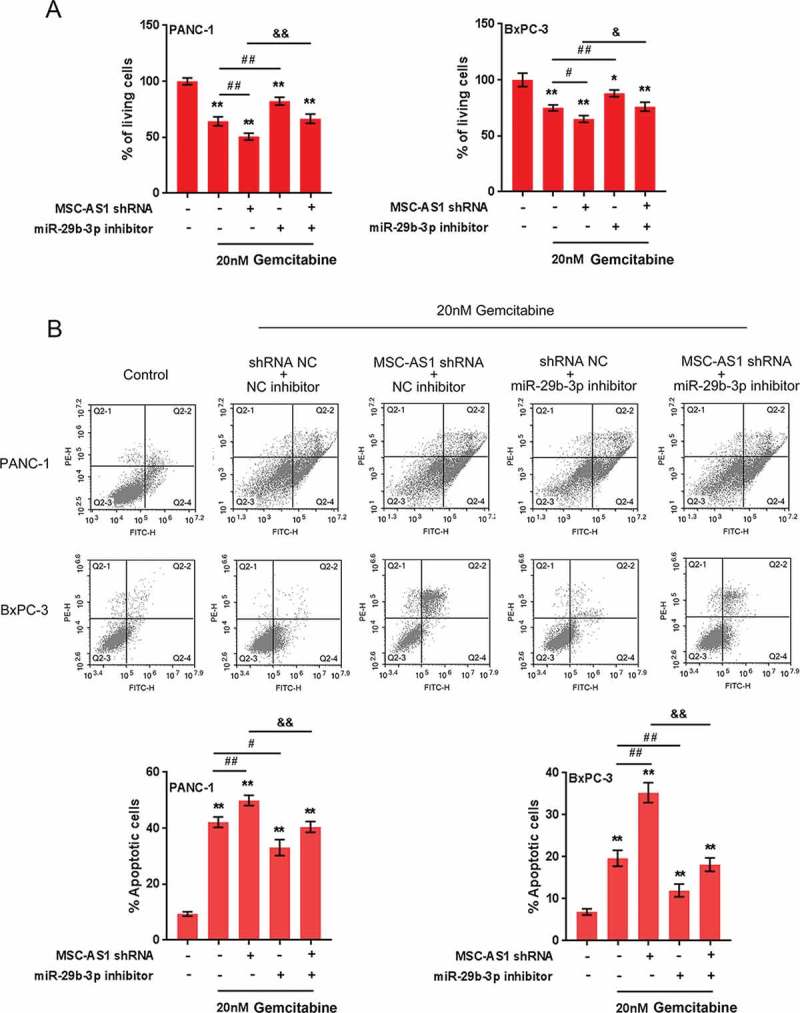
MSC-AS1/miR-29b-3p axis modulates Gemcitabine-mediated PDAC cell death and apoptosis Panc-1 and BxPC-3 cells were co-transfected with miR-29b-3p inhibitor and MSC-AS1 shRNA in the presence or absence of 20 nM GEM treatment and examined for cell death (a) and apoptosis (b). The data are presented as mean ±SD of three independent experiments. *P < 0.05, **P < 0.01, compared to control group; ##P < 0.01, compared to GEM treatment group; &&P < 0.01, compared to MSC-AS1 shRNA + GEM group.
Discussion
Here, lncRNA MSC-AS1 was positively associated with CDK14 and was upregulated in PAAD and PDAC tissues. A high MSC-AS1 expression was associated with a poorer prognosis in PDAC patients. MSC-AS1 knockdown inhibited the proliferation of PDAC cells. According to online tools analyses, miR-29b-3p might bind to CDK14 and MSC-AS1. MiR-29b-3p expression was dramatically reduced in the tissues of PDAC, while a low miR-29b-3p expression was associated with a poorer prognosis in PDAC patients. MSC-AS1 exerted its biological effect as a ceRNA for miR-29b-3p to counteract miR-29b-meidated suppression of CDK14, therefore modulating PDAC cell proliferation and apoptosis.
The oncogenic roles of CDK14 have been widely recognized. The overexpression of CDK14 is considered as a promoting factor for breast cancer cell proliferation, migration and invasion by promoting CDK14-DVL1-β-catenin signaling 19. In ovarian cancer, CDK14 knockdown inhibited the cell proliferation, migration and invasion in vitro and reduced the tumor volume in vivo by inhibiting Wnt signaling 20. In pancreatic cancer, CDK14 knockdown downregulated the pancreatic cancer cell proliferation, migration and invasion as well as the epithelial-to-mesenchymal transition (EMT) progress 8. In the present study, lncRNA MSC-AS1 was found to be positively correlated with CDK14 and upregulated in PDAC tissue samples. More importantly, a higher MSC-AS1 expression was more commonly observed in PDAC patients with a poorer prognosis, suggesting that MSC-AS1 could affect PDAC, potentially via a CDK14-related way.
LncRNA can modulate multi behaviors of PDAC cells, including proliferation, invasion, migration and apoptosis. Ye et al. 21 reported that lncRNA FEZF1-AS1 may endogenously compete with ZNF312B for miR-107 binding, leading to ZNF312B derepression; FEZF1-AS1/miR-107/ZNF312B axis can modulate the proliferation, colony formation, migration, and invasion of PDAC cells in vitro. Another lncRNA, MALAT1, sponges miR-217 to modulate KRAS expression as a competing endogenous RNA, therefore modulating pancreatic tumor cell growth, as well as cell cycle progress and migration and invasion of tumor cell 22. In the present study, MSC-AS1 silence significantly downregulated the cell proliferation and the capacity of colony formation. To investigate the underlying mechanism, we searched for miRNAs related to both CDK14 and MSC-AS1 to construct a lncRNA-miRNA-mRNA network that could affect the cell proliferation of PDAC.
As predicted by online tools, miR-29b-3p may bind to both CDK14 and MSC-AS1. miR-29b-3p exerts its function of tumor suppressor in breast 23 and colorectal cancer 24, both through interacting with lncRNA H19 but targeting different downstream targets, namely PGRN and DNMT3B. In pancreatic cancer, according to previous study, a low miR-29b-3p expression in tumor tissues was observed17. Consistently, significant reducedmiR-29b-3p expression was observed in 45 cases tissues of PDAC. Better prognosis in PDAC patients was related to a higher miR-29b-3p expression. Above all, we revealed miR-29b-3p may also serve as a tumor suppressor in PDAC. Thus, we further validated the putative binding of miR-29b-3p to CDK14 and MSC-AS1. Consistent with online tools prediction, miR-29b-3p was a direct downstream target of MSC-AS1, while miR-29b could directly target the 3ʹ-UTR of CDK14. In RIP experiment with AGO2 antibody, the levels of CDK14 in precipitated protein were obviously lower upon MSC-AS1 overexpression than those in vector-NC group, indicating that MSC-AS1 may counteract miR-29b-meidated CDK14 repression as a ceRNA for miR-29b-3p.
As we have mentioned, CDK14 as a new member of CDK family commonly acts as an oncogene. CDK14 can interact with cyclin D3 and cyclin Y which is considered as its regulatory subunit to modulate cell cycle progression and cell proliferation, and upregulate Wnt pathway through mediating its downstream proteins c-Myc, cyclin D1 and cell cycle-dependent low-density-lipoprotein receptor-related protein 6 (LRP6) 25. Here, MSC-AS1 silence remarkably downregulated, while miR-29b inhibition upregulated the protein levels of CDK14, as well as c-Myc, cyclin Y cyclin D1 and cyclin D3. Consistently, the cell proliferation of PDAC cell lines was negatively regulated by MSC-AS1 knockdown while positively regulated by miR-29b-3p inhibition; miR-29b-3p inhibition partially reversed the effect of MSC-AS1 knockdown, indicating that MSC-AS1/miR-29b-3p axis modulates PDAC cell proliferation through CDK14. More importantly, MSC-AS1 knockdown further enhanced, while miR-29b-3p inhibition attenuated GEM-induced PDAC cell death and apoptosis, suggesting that MSC-AS1/miR-29b-3p may also affect the resistance of PDAC to GEM treatment, which needs further in vivo validation in future research.
Above all, we verified that MSC-AS1/miR-29b-3p axis modulates the cell proliferation and GEM-induced cell death and apoptosis in PDAC cell lines through CDK14. Our findings provide a rationale for further studies of PDAC treatment on the potential therapeutic benefits of lncRNA-miRNA-mRNA network.
Funding Statement
This work was supported by the Key Laboratory of Diagnosis and Treatment of Severe Hepato-Pancreatic Diseases of Zhejiang Province;Wenzhou Science and Technology Bureau Project [Y20150059]
Acknowledgments
This study was supported by Wenzhou Science and Technology Bureau Project(Y20150059) and Key Laboratory of Diagnosis and Treatment of Severe Hepato-Pancreatic Diseases of Zhejiang Province.
Disclosure statement
No potential conflicts of interest were disclosed
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website
References
- 1.Yabar CS, Winter JM.. Pancreatic cancer: a review. Gastroenterol Clin North Am. 2016;45:429–445. doi: 10.1016/j.gtc.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Vassaux G, Angelova A, Baril P, Midoux P, Rommelaere J, Cordelier P.. The promise of gene therapy for pancreatic cancer. Hum Gene Ther. 2016;27:127–133. doi: 10.1089/hum.2015.141. [DOI] [PubMed] [Google Scholar]
- 3.Sherr CJ, Roberts JM. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 2004;18:2699–2711. doi: 10.1101/gad.1256504. [DOI] [PubMed] [Google Scholar]
- 4.Shu F, Lv S, Qin Y, Ma X, Wang X, Peng X, Luo Y, Xu B-E, Sun X, Wu J. Functional characterization of human PFTK1 as a cyclin-dependent kinase. Proc Natl Acad Sci U S A. 2007;104:9248–9253. doi: 10.1073/pnas.0703327104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang M, Gao Y, Yang T, Zhu X, Chen J, Cyclin Y. A novel membrane-associated cyclin, interacts with PFTK1. FEBS Lett. 2009;583:2171–2178. doi: 10.1016/j.febslet.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 6.Pang EY-T, Bai AH-C, To K-F, Sy SM-H, Wong NL-Y, Lai PB-S, Squire JA, Wong N. Identification of PFTAIRE protein kinase 1, a novel cell division cycle-2 related gene, in the motile phenotype of hepatocellular carcinoma cells. Hepatology. 2007;46:436–445. doi: 10.1002/hep.21691. [DOI] [PubMed] [Google Scholar]
- 7.Sun T, Co NN, Wong N. PFTK1 interacts with cyclin Y to activate non-canonical Wnt signaling in hepatocellular carcinoma. Biochem Biophys Res Commun. 2014;449:163–168. doi: 10.1016/j.bbrc.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Zheng L, Zhou Z, He Z. Knockdown of PFTK1 inhibits tumor cell proliferation, invasion and epithelial-to-mesenchymal transition in pancreatic cancer. Int J Clin Exp Pathol. 2015;8:14005–14012. [PMC free article] [PubMed] [Google Scholar]
- 9.Hüttenhofer A, Schattner P, Polacek N. Non-coding RNAs: hope or hype? Trends Genet. 2005;21:289–297. doi: 10.1016/j.tig.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Taucher V, Mangge H, Haybaeck J. Non-coding RNAs in pancreatic cancer: challenges and opportunities for clinical application. Cell Oncol (Dordr). 2016;39:295–318. doi: 10.1007/s13402-016-0275-7. [DOI] [PubMed] [Google Scholar]
- 11.Tang YT, Xu XH, Yang XD, Hao J, Cao H, Zhu W, Zhang S, Cao J. Role of non-coding RNAs in pancreatic cancer: the bane of the microworld. World J Gastroenterol. 2014;20:9405–9417. doi: 10.3748/wjg.v20.i28.9405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Q, Jiang H, Ping C, Shen R, Liu T, Li J, Qian Y, Tang Y, Cheng S, Yao W, et al. Exploring the Wnt pathway-associated LncRNAs and genes involved in pancreatic carcinogenesis driven by Tp53 mutation. Pharm Res. 2015;32:793–805. doi: 10.1007/s11095-013-1269-z. [DOI] [PubMed] [Google Scholar]
- 13.Tahira AC, Kubrusly MS, Faria MF, Dazzani B, Fonseca RS, Maracaja-Coutinho V, Verjovski-Almeida S, Machado MC, Reis EM. Long noncoding intronic RNAs are differentially expressed in primary and metastatic pancreatic cancer. Mol Cancer. 2011;10:141. doi: 10.1186/1476-4598-10-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sen R, Ghosal S, Das S, Balti S, Chakrabarti J. Competing endogenous RNA: the key to posttranscriptional regulation. Sci World J. 2014;2014:896206. doi: 10.1155/2014/896206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma J, Yang Y, Huo D, Wang Z, Zhai X, Chen J, Sun H, An W, Jie J, Yang P. LincRNA-RoR/miR-145 promote invasion and metastasis in triple-negative breast cancer via targeting MUC1. Biochem Biophys Res Commun. 2018;500:614–620. doi: 10.1016/j.bbrc.2018.04.119. [DOI] [PubMed] [Google Scholar]
- 16.Gao H, Gong N, Ma Z, Miao X, Chen J, Cao Y, Zhang G. LncRNA ZEB2-AS1 promotes pancreatic cancer cell growth and invasion through regulating the miR-204/HMGB1 axis. Int J Biol Macromol. 2018;116:545–551. doi: 10.1016/j.ijbiomac.2018.05.044. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Zhao J, Huang J, Tang H, Yu S, Chen Y. The regulatory roles of miRNA and methylation on oncogene and tumor suppressor gene expression in pancreatic cancer cells. Biochem Biophys Res Commun. 2012;425:51–57. doi: 10.1016/j.bbrc.2012.07.047. [DOI] [PubMed] [Google Scholar]
- 18.Kurihara T, Kogo M, Ishii M, Shimada K, Yoneyama K, Kitamura K, Shimizu S, Yoshida H, Kiuchi Y. Chemotherapy-induced neutropenia as a prognostic factor in patients with unresectable pancreatic cancer. Cancer Chemother Pharmacol. 2015;76:1217–1224. doi: 10.1007/s00280-015-2887-4. [DOI] [PubMed] [Google Scholar]
- 19.Gu X, Wang Y, Wang H, Ni Q, Zhang C, Zhu J, Huang W, Xu P, Mao G, Yang S. Upregulated PFTK1 promotes tumor cell proliferation, migration, and invasion in breast cancer. Med Oncol. 2015;32:195. doi: 10.1007/s12032-015-0641-8. [DOI] [PubMed] [Google Scholar]
- 20.Ou-Yang J, Huang L-H, Sun -X-X. Cyclin-dependent kinase 14 promotes cell proliferation, migration and invasion in ovarian cancer by inhibiting Wnt signaling pathway. Gynecol Obstet Invest. 2017;82:230–239. doi: 10.1159/000447632. [DOI] [PubMed] [Google Scholar]
- 21.Ye H, Zhou Q, Zheng S, Li G, Lin Q, Ye L, Wang Y, Wei L, Zhao X, Li W, et al. FEZF1-AS1/miR-107/ZNF312B axis facilitates progression and Warburg effect in pancreatic ductal adenocarcinoma. Cell Death Dis. 2018;9:34. doi: 10.1038/s41419-017-0052-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu P, Yang H, Zhang J, Peng X, Lu Z, Tong W, Chen J. The lncRNA MALAT1 acts as a competing endogenous RNA to regulate KRAS expression by sponging miR-217 in pancreatic ductal adenocarcinoma. Sci Rep. 2017;7:5186. doi: 10.1038/s41598-017-05274-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lv M, Zhong Z, Huang M, Tian Q, Jiang R, Chen J. lncRNA H19 regulates epithelial-mesenchymal transition and metastasis of bladder cancer by miR-29b-3p as competing endogenous RNA. Biochim Biophys Acta. 2017;1864:1887–1899. doi: 10.1016/j.bbamcr.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Ding D, Li C, Zhao T, Li D, Yang L, Zhang B. LncRNA H19/miR-29b-3p/PGRN axis promoted epithelial-mesenchymal transition of colorectal cancer cells by acting on Wnt signaling. Mol Cells. 2018;41:423–435. doi: 10.14348/molcells.2018.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duan C, Liu Y, Lu L, Cai R, Xue H, Mao X, Chen C, Qian R, Zhang D, Shen A. CDK14 Contributes to reactive gliosis via interaction with Cyclin Y in rat model of spinal cord injury. J Mol Neurosci. 2015;57:571–579. doi: 10.1007/s12031-015-0639-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


