ABSTRACT
Most of gastric carcinoma (GC) is attributed to infection by Helicobacter pylori (H. pylori) but there is increasing evidence that the positive H. pylori status correlates with better prognosis in GC. The H. pylori-induced cellular immune response may suppress cancer and in this work, recombinant pcDNA3 plasmids encoding various fragments of H. pylori virulence genes of cagA, vacA and babA are constructed and combined into groups to immunize BALB/c mice. The activated splenic CD3+ T cells are purified and the anticancer effects are investigated in vitro and in vivo. The H. pylori DNA vaccines induce a shift in the response from Th1 to Th2 that mimicks the immune status in patients of GC with chronic H. pylori infection. The stimulated CD3+ T cells inhibit the growth of human GC cells in vitro and adoptive transfusions of the CD3+ T cells suppress the growth of GC xenograft in vivo. The effects may be caused by the larger ratios of infiltrated CD8+/CD4+ T cells, reduced infiltration of regulatory FOXP3+ T cells, and enhanced apoptosis induced by upregulation of Caspase-9/Caspase-3 and downregulation of Survivin. Our results reveal the potential immunotherapeutic value of H. pylori vaccine-activated CD3+ T cells in those with advanced GC.
KEYWORDS: Gastric carcinoma, Helicobacter pylori, prognosis, immune response, DNA vaccine, adoptive transfusion, immunotherapy
Introduction
Gastric carcinoma (GC) leads to high world-wide morbidity and mortality rates and over 80% of the global GC burden is attributed to infection of Helicobacter pylori (H. pylori) that has been classified as a type 1 carcinogen by the International Agency for Research on Cancer.1-3 The cytotoxin-associated gene A (CagA) and vacuolating toxin A (VacA) are key virulence factors of H. pylori and determine the infection type I (CagA+, VacA+) or II (CagA−, VacA−).4 Another virulence factor, the blood-group antigen-binding adhesin (BabA) as an outer membrane protein, can mediate the adherence and localization of H. pylori subsequently inducing mucosal inflammation and preneoplastic lesions in the stomach.5,6 The H. pylori strains with specific genotype of triple-positive babA2, vacAs1, and cagA exhibit significant correlation with intestinal metaplasia and prevalence of GC.7,8
The majority of data suggest that H. pylori causes inflammation, ulcers, atrophy, and even carcinoma of the stomach.9,10 However, there is new evidence of a new link between H. pylori infection and better outcome in GC patients and increasing interest to uncover the other role of H. pylori being a friend rather than a foe.11-16 Besides participating in pathogenesis, many virulence factors including CagA, VacA, and BabA are still strong immunogens and stimulate persistent nonsterile immunity in the host even throughout a lifetime.17,18 The H. pylori-activated immune responses have been suggested to improve prognosis in patients with GC and activated T cells may play more critical roles than antibodies induced by activated B-cells in the battle against cancer.18-21
Herein, we mimick the immune status of chronic H. pylori infection to study the H. pylori vaccine-induced immunotherapeutic effects in GC. Plasmid vaccines encoding various fragments of cagA, vacA and babA genes are constructed and combined into groups to immunize BALB/c mice. The activated CD3+ pan-T cells are purified from the spleen of immunized mice and their anticancer effects are assessed in vitro and in vivo. Our results show that the H. pylori vaccine-activated CD3+ T cells inhibit the growth of cancer cells and have large potential in immunotherapy of GC.
Results
Construction of H. pylori DNA vaccines
Since the cagA, vacA, and babA genes are toxic to animals, we amplified the truncated fragments containing cagA 1–3 (c-1, c-2 and c-3), vacA 1–2 (v-1 and v-2), and babA 1–2 (b-1 and b-2) by the polymerase chain reaction (PCR) that was used to construct the DNA vaccines (Figure 1(a)). All the gene fragments are identifed by 1% agarose gel electrophoresis using molecular weight marker standards (Figure 1(b)). After inserting into the pMD18-T plasmid by the T-A cloning technique, the gene fragments with the correct sequences confirmed by DNA sequencing are sub-cloned into the pcDNA3 vector. The various recombinant pcDNA3 plasmids are transiently transfected into HEK 293-T cells and the expression of target proteins encoded by corresponding inserted genes is verified by Western blotting analysis (Figure 1(c)). Therefore, the pcDNA3 plasmid vaccines expressing the mentioned 7 fragments of H. pylori cagA, vacA and babA genes are successfully constructed.
Figure 1.
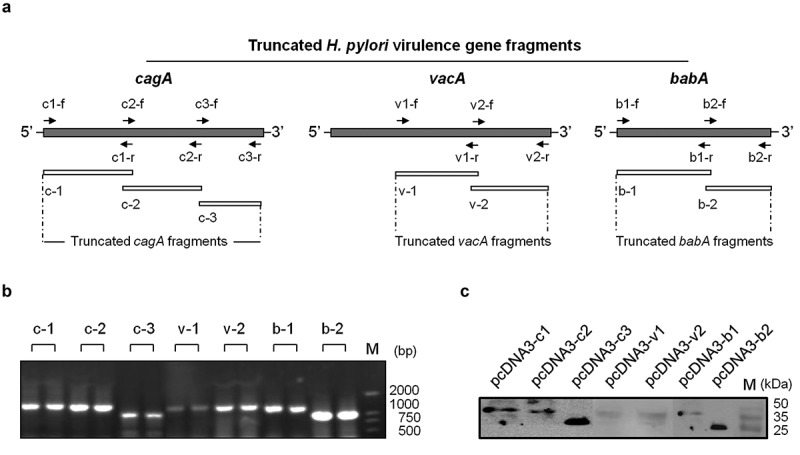
Construction of H. pylori DNA vaccines. (a). Schematic diagram of H. pylori virulence genes of cagA, vacA and babA and truncated fragments with the loci of corresponding primer pairs. (b). Electrophoretic analysis of the PCR products of the 7 target fragments by separation of a 1% agarose gel. (c). Western blot analysis of the expression of recombinant plasmid vaccines including pcDNA3-c1, pcDNA3-c2, pcDNA3-c3, pcDNA3-v1, pcDNA3-v2, pcDNA3-b1, and pcDNA3-b2, in transiently transfected HEK 293-T cells.
Immune response induced by H. pylori DNA vaccines
All the recombinant H. pylori DNA vaccines are inoculated into the BALB/c mice and the strength and subtypes of the corresponding immune responses are evaluated. The enzyme-linked immunosorbent assay (ELISA) was adopted to detect both the IgG subclasses and the levels of interleukin (IL)-2, IL-4, IL-6, IL-10, IL-12, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α in the serum of immunized mice. After the last immunization, the IgG1 and IgG2a levels are elevated notably by 2.1 to 3.1 times and 4.2 to 5.4 times in the vaccinated groups, respectively. The IgG1/IgG2a ratio decreases by about 50% following immunization of the H. pylori DNA vaccines indicating a stronger IgG2a response induced by the DNA vaccines (Figure 2(a)). The cytokine response to the vaccines is also evaluated. The serum IL-2 and IFN-γ concentrations are reduced by nearly 50% and 95%, respectively, in all vaccinated groups. After vaccination, the TNF-α levels increase by 3.4 to 6.8 times compared to the baseline. The IL-4 and IL-10 levels of the 7 immunized groups also increase by 1.4 to 2.1 and 3.4 to 13.3 times, respectively. The IL-12 levels increase only in C1, C3 and V2 groups by 2.6 to 4.1 times. However, serum levels of IL-6 in all groups and IL-12 in C2, V1, B1 and B2 groups show no significant changes (Figure 2(a)). Based on the IgG1 and IgG2a responses and dynamic changes of IL-2, IL-10 and IL-4 concentrations, the major immune response subtype is likely a mixed pattern of T helper (Th) 1 and 2 cells but with polarization to the Th2 pattern following vaccination (Figure 2(b)).
Figure 2.
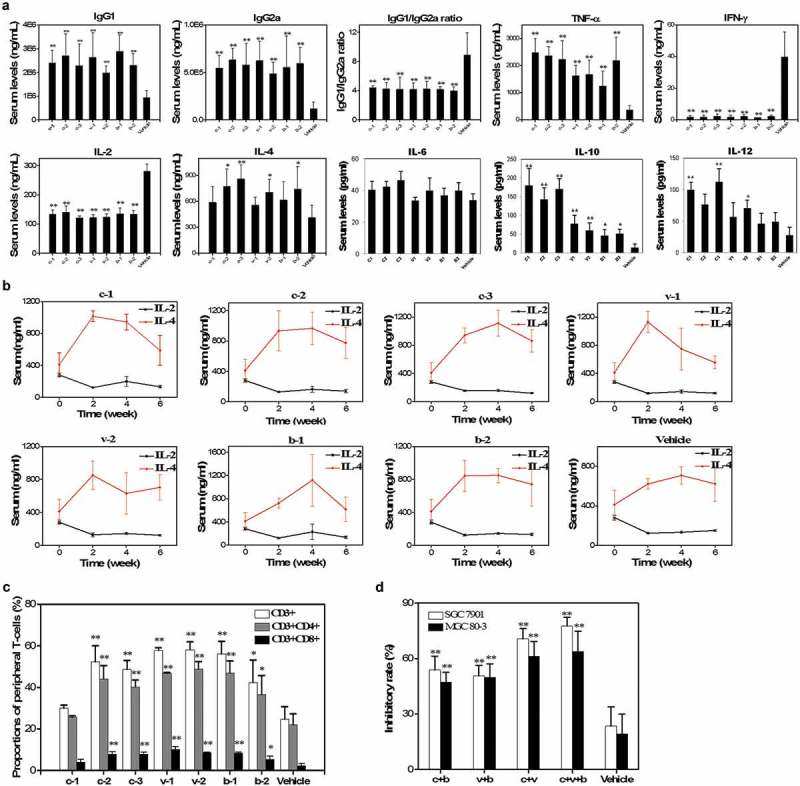
Evaluation of the immune responses and inhibitory effects of splenic T-cells elicited by H. pylori DNA vaccines. (a). Serum IgG1 and IgG2a levels and the relative ratios and concentrations of TNF-α, IFN-γ, IL-2, IL-4, IL-6, IL-10 and IL-12 assessed by ELISA in various groups of immunized mice. (b). Dynamic changing curves of serum IL-2 and IL-4 from 0 to 6 weeks after vaccination determined by ELISA. (c). Subpopulations of peripheral CD3+, CD4+ and CD8+ T-cells in immunized mice evaluated by flow cytometry. (d). Inhibitory rates of H. pylori-activated splenic T-cells on SGC 7901 and MGC 80–3 cell lines cultured in vitro. **, P < 0.01 and *, P < 0.05.
To identify the peripheral T-cell subpopulations, blood samples taken from animals after the last vaccination were subjected to flow cytometry. Compared to the group of vehicles, the proportions of CD3+, CD4+, and CD8+ T-cell subgroups in the immunized mice are elevated by the DNA vaccines except the c-1 group (Figure 2(c)).
Therefore, H. pylori DNA vaccination activates the Th cell response and converts the Th1 pro-inflammatory to Th2 anti-inflammatory response. This basically mimics the immune status of hosts with chronic H. pylori infection and also promotes TNF-α secretion and T-cell proliferation.
In vitro anticancer effect of H. pylori vaccine-activated CD3+ T cells
To verify the in vitro anticancer effect, H. pylori vaccine-activated splenic CD3+ T cells were purified from the immunized mice by immunomagnetic beads and used as the effector cells and co-cultured with two kinds of GC cells, SGC 7901 and MGC 80–3 cell lines, respectively. Considering the close relationship between GC and H. pylori infection of type 1 (CagA+, VacA+), various CD3+ T cells were combined at equal ratios into 4 experimental groups: c + b (c − 1 + c −2 + c − 3 + b-1 + b − 2), v + b (v − 1 + v − 2 + b − 1 + b − 2), c + v (c − 1 + c − 2 + c − 3 + v − 1 + v − 2) and c + v + b (c − 1 + c − 2 + c − 3 + v − 1 + v − 2 + b − 1 + b − 2). The SGC 7901 and MGC 80–3 cell lines are co-cultured in vitro with differently combined CD3+ T cells from groups of c + b, v + b, c + v and c + v + b, respectively.
All the immunotherapy groups exhibit enhanced inhibitory effects compared to the pcDNA3 vehicle control (23.5 ± 10.3%; 19.2 ± 10.8%) in both GC cell lines (P < 0.01) (Figure 2(d)). The combined CD3+ T cells from the c + v + b group show the highest inhibitory effects in both SGC 7901 (77.6 ± 4.7%) and MGC 80–3 (63.9 ± 11.0%) cells. Moreover, the CD3+ T cells from the c + v (70.7 ± 5.5%; 61.2 ± 8.1%), c + b (53.9 ± 7.4%; 47.2 ± 5.4%), and v + b (50.7 ± 5.6%; 49.8 ± 7.4%) groups exhibit significant anticancer effects in the two GC cell lines.
In vivo anticancer effect of H. pylori vaccine-activated CD3+ T cells
The nude BALB/c mice are burdened with SGC 7901 cells and undergo adoptive CD3+ T-cell transfusion. Tumor growth in the immunotherapy groups is suppressed with more tumor necrosis as well as smaller tumor volume and weight than the vehicle control (Figure 3(a-c)). In all the groups, the mouse body weight does not change significantly (Figure 3(d)). The anti-GC effects of the H. pylori vaccine-activated T-cells are discernible as manifested by the change in the tumor volume early at day 8 after CD3+ T-cell transfer and continue to be enhanced for at least one more week (Figure 3(b)). The decrease in the tumor weight in the groups of c + v and c + v + b is significant compared to the vehicle control (Figure 3(c)).
Figure 3.
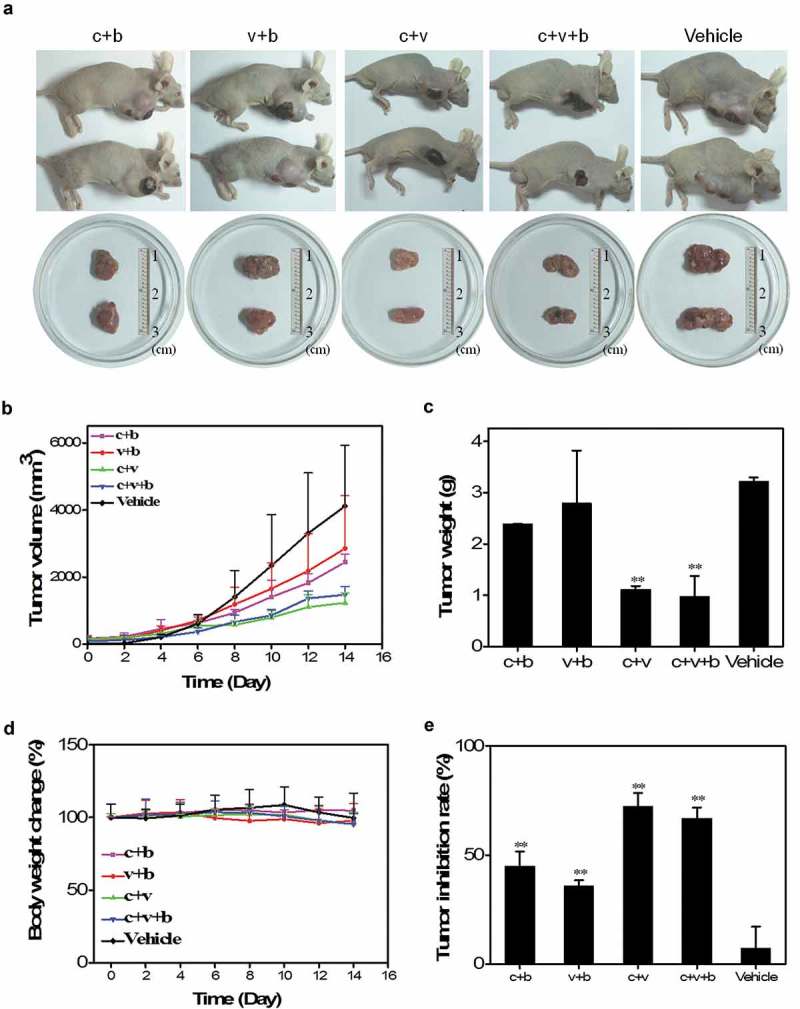
In vivo anticancer effects of splenic T-cells activated by H. pylori vaccines. (a). Representative tumor nodules excised from GC-bearing mice after adoptive T-cell transfusions. (b). Volume curves of tumor nodules in mice from 0 to 14 days after T-cell transfusions. (c). Average weights of tumor nodules removed from the mice. (d). Curves of body weight change of mice from 0 to 14 days after adoptive transfusions. (e). Tumor inhibition rates in the different experimental groups. **, P < 0.01.
The mean tumor inhibition rates of the c + v, c + v + b, c + b, and v + b groups based on the changes in the tumor volume at day 14 reach 72.3%, 66.8%, 45.0%, and 35.8%, respectively, which are significantly higher than that of the vehicle control group (Figure 3(e)). The results suggest that adoptive transfusions of CD3+ T cells activated by the H. pylori DNA vaccines effectively inhibit the growth of GC in vivo.
Subpopulation of tumor-infiltrated immune cells after CD3+ T-cell transfusion
The subgroups of infiltrated CD3+, CD4+ and CD8+ T cells in the subcutaneous xenograft of GC are examined by immunohistochemistry (IHC) and the positivity index and the tumor-infiltrated CD4+ T cells in all immunotherapy groups show decreased levels (P < 0.01) (Figure 4(a)). However, infiltration of the CD8+ T cells in the cancerous tissues appears to be almost unaltered by adoptive transfusions of the H. pylori DNA vaccine-activated CD3+ T cells. Correspondingly, the ratios of the infiltrated CD8+/CD4+ T cells of all the immunotherapy groups increase significantly (Figure 4(b)). In addition, staining of the regulatory FOXP3+ T (Treg) cells in the experimental groups decreases compared to the vehicle control (Figure 4(c,d)). Notably, neither the infiltrations of CD56+ natural killer (NK) cells (Figure 4(e,f)), CD68+ macrophages, and CD86+ M1 and CD163+ M2 macrophages nor the ratios of infiltrated CD163+/CD86+ macrophages show significant changes (Figure 4(g,h)).
Figure 4.
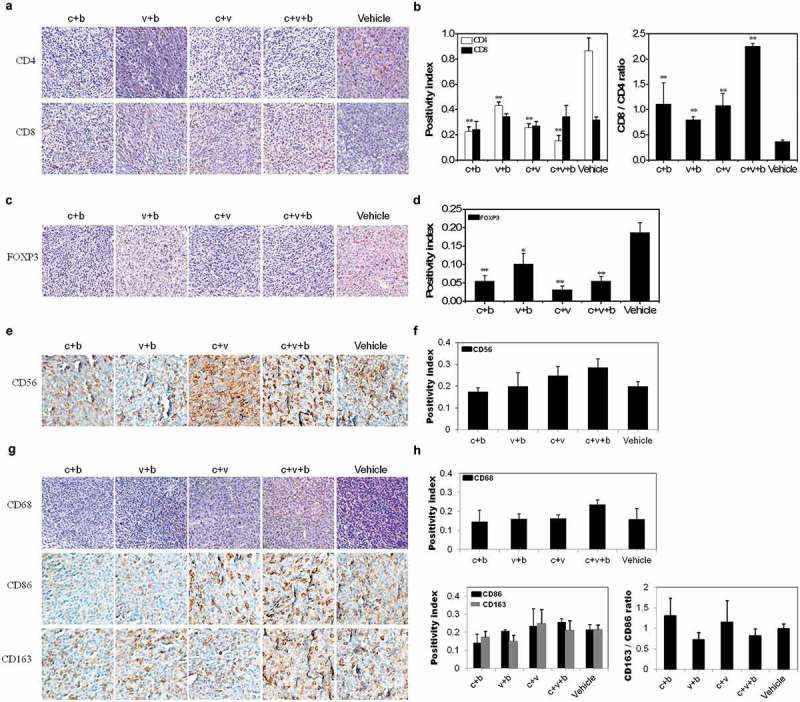
Assessment of infiltrated immune cell subpopulations in the xenograft tumor after adoptive T-cell transfusions. (a). Representative stains of the infiltration of CD4+ and CD8+ T cells. Magnification, 20 × . (b). Positivity index and CD8+/CD4+ ratios of the infiltration of T cells in various groups. (c). Representative stains of the infiltration of FOXP3+ T cells. Magnification, 20 × . (d). Positivity index of the infiltration of FOXP3+ T cells. (e). Representative stains of the infiltrated CD56+ NK cells. Magnification, 40 × . (f). Positivity index of the infiltration of CD56+ NK cells. (g). Representative stains of the infiltrated CD68+ macrophages (Magnification, 20 ×), and CD86+ M1 and CD163+ M2 macrophages (Magnification, 40 ×). (h). Positivity index of the infiltrations of CD68+, CD86+ and CD163+ macrophages and CD163+/CD86+ ratios of the infiltration of macrophages in various groups. **, P < 0.01 and *, P < 0.05.
Apoptosis and antiapoptosis pathways induced by H. pylori vaccine-activated CD3+ T cells
To further identify the molecular mediators involved in the anticancer effects of adoptive immunotherapy using H. pylori vaccine-activated CD3+ T cells, several major signaling molecules known to be closely associated with the apoptosis and antiapoptosis pathways in GC are detected by Western blotting.22-24 Caspase-9 and −3 in the c + v + b and c + v groups are significantly up-regulated, while Survivin in all the immunotherapy groups is significantly down-regulated compared to the vehicle control. The expression of Caspase-8 is not influenced significantly by infusion of the H. pylori DNA vaccine-activated CD3+ T cells (Figure 5), suggesting that cellular apoptosis in the xenograft of GC may be enhanced by upregulation of Caspase-9/Caspase-3 and downregulation of Survivin after adoptive transfusions of the H. pylori vaccine-activated CD3+ T cells.
Figure 5.
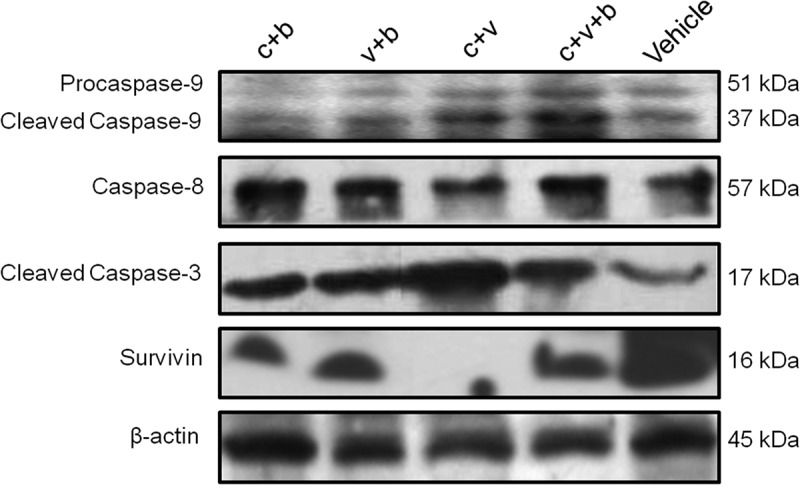
Western blot analysis of apoptosis/antiapoptosis signalings including Caspase-9, Caspase-8, Cleaved Caspase-3 and Survivin in the xenograft tumor after adoptive T-cell transfusions.
Discussion
Clinical data show that GC patients with positive H. pylori infection (Hp-GC) tend to have better outcome than those with negative H. pylori infection (nHp-GC), regardless of whether they receive surgery or not.11-16,25-27 The chronic infection status and immune response induced by H. pylori may favorably affect progression and prognosis of GC after the bacteria have initially led to carcinogenesis.20,21,28 The objective of this study is to verify this possibility and explore the associated molecular mechanisms.
The association between seronegative H. pylori status and poor outcome in resected GC patients by univariate analysis was reported in 1995.29 In 2006 and 2009, two large prospective trials performed in Germany and Italy confirmed the beneficial prognostic role of the positive H. pylori status for longer relapse-free survival (RFS) and overall survival (OS) in curatively resected GC. According to these studies, the median RFS and OS in Hp-GC patients are 56.7 and 61.9 months, respectively, compared to 19.2 and 19.2 months in nHp-GC patients. The 5-year survival rates are 57.0% versus 24.0% for Hp-GC and nHp-GC patients, respectively.14,15 Some retrospective studies confirm the role of the positive H. pylori status as a beneficial predicative factor, but several reports do not.11-13,16,25,30-35 As shown by the meta-analysis of 12 studies including 2454 patients, the pooled hazard ratio is 0.71 (95%CI: 0.57–0.87; P = 0.001) for OS in patients with Hp-GC compared to those with nHp-GC, thereby suggesting a protective role for H. pylori infection in the prognosis of GC.27 In addition, patients with Hp-GC in the advanced stages show better response to chemotherapy besides longer median OS compared to those with nHp-GC.26
However, the reasons for the different outcome of Hp-GC and nHp-GC patients are still unclear. Some investigators have suggested that the increased microsatellite instability caused by H. pylori infection may improve the prognosis of GC but others have presented conflicting findings.36-39 Furthermore, H. pylori infection has been reported to suppress constitutive secretion of the macrophage migration inhibitory factor (MIF), a potential negative prognostic factor associated with the advanced tumor stages and poor patient survival in GC.40,41 Because CagA, VacA, and BabA mimic and bind to specific receptors or surface molecules on gastric epithelial cells and platelets, anti-CagA, anti-VacA, and anti-BabA antibodies may mediate killing and even suppress metastasis of cancer cells in an autoimmune reaction.28 In addition, H. pylori infection activates monocytes, macrophages, and dendritic cells correlating with increased capacity to stimulate antigen processing and presentation as well as T-cell activation and proliferation to raise the antitumor response besides just inflammation in the host.18-20,40 Therefore, it has been postulated that the anticancer immune response may be promoted in Hp-GC.
Herein, to verify the anticancer role of immune responses in Hp-GC, DNA vaccines targeting the major virulence genes (cagA, vacA and babA) of H. pylori are constructed and used to immunize mice to mimic the immune responses caused by chronic H. pylori infection. Several strategies are employed to ensure successful mimicry. Firstly, to avoid the toxicity of the products, the cagA, vacA, and babA genes are truncated into 7 fragments containing c-1, c-2, c-3, v-1, v-2, b-1, and b-2 as the independent antigenic targets of DNA vaccines. Secondly, both the IgG subtypes and Th1 and Th2 responses are dynamically determined after vaccination to evaluate the category and strength of the immune response induced by the H. pylori vaccines. Finally, many virulence factors of H. pylori including CagA, Vac,A and BabA can evoke the production of associated antibodies. However, the T-cell response usually plays a more critical anticancer role than the humoral immune response induced by B cells.20,21 Hence, the splenic CD3+ T cells activated by the DNA vaccines are classified into 4 groups (c + b, c + v, v + b, and c + v + b) and used for adoptive transfusions to mimic the cellular immune status after type Ι (CagA+, VacA+) H. pylori infection, which is most closely associated with development of GC.4
Generally, a Th1-type response is promoted during acute H. pylori infection and leads to serious inflammation and cellular damage in the stomach mainly due to increased secretion of IFN-γ.18,19,42 The Th2 response inhibits gastritis caused by Th1 cytokines and controls H. pylori infection indicating the positive role of the Th2 pathway in preventing H. pylori-related diseases in spite of some conflicting data on the supposedly protective role of the Th1 response.18-20,42,43 The Th1-biased immune response is known to be induced early by H. pylori infection but polarization from the Th1 to Th2 responses plays a more important role during chronic infection and carcinogenesis and progression of Hp-GC.21,44 Here, the increasing trend of IL-4 and IL-10 expression and decreasing tendency of IgG1/IgG2a ratio and IL-2 and IFN-γ expressions are supported by previous detection in Hp-GC patients.21 Thus, DNA vaccination induces a shift from the Th1 to Th2 responses that fundamentally mimic the changing immune status in the host of GC with chronic H. pylori infection.21,45 The elevated TNF-α level is identified after vaccination to mimic the changes in the TNF-α expression induced by H. pylori infection in patients with chronic gastritis, intestinal metaplasia, dysplasia and GC.46 In addition, the H. pylori DNA vaccines stimulate significant proliferation of both peripheral CD3+CD4+ and CD3+CD8+ T cells in mice and the enhanced proliferation and effector roles of activated CD3+ T cells are thought to likely inhibit tumor growth and improve prognosis of Hp-GC. The splenic CD3+ T cells activated by the vaccines are isolated and subjected to co-culturing with the GC cell lines and adoptive transfusions in a tumor-bearing mouse model. Significant suppression of cancer is observed and the mean tumor inhibition rates are 35.8% – 72.3% in vivo and 77.6 ± 4.7% in vitro, indicating the anticancer effects of the H. pylori vaccine-induced CD3+ T cells.
The anti-GC roles of CD3+ T cells activated by the H. pylori vaccines are attributed to several mechanisms. The changes in the infiltrated T-cell subpopulations and corresponding cellular immune response have been investigated.20 In this study, infiltration of CD4+ T cells decreases significantly but those of CD8+ T cells, CD56+ NK cells, CD68+ macrophages, and CD86+ M1 and CD163+ M2 macrophages are almost unchanged after adoptive T-cell transfusions. The ratios of the infiltrated CD8+/CD4+ T cells consequently increase suggesting that CD8+ T-cell-dominated polarization is induced, meaning that the specific anti-tumor effects contribute to better prognosis.20,47,48 In addition, specific proliferation and anticancer effects of T cells are primarily based on the recognition and binding between antigens and T cell receptors (TCRs). Thus, a cross-reaction between GC and H. pylori antigens reported previously can be inferred.28,49-52 It may also be an autoimmune mechanism for enhanced killing of cancer cells by the homed activated T cells but further verification is needed. The FOXP3+ Treg cells that can inhibit a variety of immune responses are important mediators of the tumor immune tolerance.53-55 Since the proportions of FOXP3+ T cells decrease after adoptive immunotherapy in this study, transfusion of CD3+ T cells activated by the H. pylori vaccines probably suppresses proliferation or infiltration of Treg cells and subsequently the Treg-induced immune tolerance as well. Furthermore, apoptosis is promoted after CD3+ T-cell transfusion possibly due to upregulation of Caspase-9/Caspase-3 and downregulation of Survivin, which correlate closely to the growth, invasion, and metastasis of GC.22-24
To address the limitations, the DNA vaccines described here are specifically constructed to mimic the immune status induced by chronic H. pylori infection (particularly of type І) and it differs from most of the other reported vaccines used to protect against infection. Our vaccines and strategy of combined immunization, which may not evoke the strongest immune response, are designed specifically for the purpose. Transfusions of activated CD3+ T cells into the GC-bearing mouse model are used to observe the influence of the H. pylori-induced T cell responses on progression of GC. It is not equivalent to the more complicated situation of natural Hp-GC. Therefore, further studies are needed to explore both the aspects of H. pylori immunogens and the host. Firstly, the TCR gene spectrum of tumor-infiltrated lymphocytes (particularly CD3+ T cells) needs to be identified and compared with the epitope sequences of H. pylori antigens to verify the molecular basis of the specific anticancer effects of activated T cells. Secondly, a standard mouse model with primary Hp-GC should be established albeit with difficulties, in which natural influences of the H. pylori-related immune responses on the prognosis of GC may be reconstructed and clarified.
Materials and methods
Vectors, cell lines and animals
The pMD18-T plasmid was a product of TaKaRa Dalian (Dalian, Liaoning Province, China) and the pcDNA3 vector was obtained from Invitrogen (Carlsbad, CA, USA). The HEK 293-T cells and human GC cell lines SGC 7901 and MGC 80–3 were purchased from Shanghai Cell Bank of Chinese Academy of Sciences (Shanghai, China) and incubated in Dulbecco’s modified Eagle’s medium (DMEM) (Hyclone, Logan, UT, USA) with 10% fetal bovine serum (Hyclone), 100 U/ml penicillin, and 100 mg/ml streptomycin (Sigma-Aldrich, St. Louis, MO, USA). The six-week-old normal and nude (nu/nu) female BALB/c mice were purchased from Chinese Academy of Medical Sciences (Beijing, China) and maintained under specific pathogen-free (SPF) conditions in the Comparative Medical Center of Jinling Hospital (Nanjing, Jiangsu Province, China). The animal experiments were performed in accordance with the recommendations for the proper use and care of laboratory animals by the Ethics Committee of Jinling Hospital.
Construction of H. pylori DNA vaccines
The total DNA of H. pylori J99 strain was kindly provided by Professor Fei-Fei She (Fujian Medical University, Fuzhou, Fujian Province, China). All the truncated fragments (c-1, c-2, c-3, v-1, v-2, b-1 and b-2) of cagA, vacA, and babA genes were amplified by PCR (Table 1) and used to construct DNA vaccines as follows. The target 7 PCR products were inserted into the pMD18-T vector using the T-A cloning technique and the correct sequences were confirmed by DNA sequencing. Afterwards, all the gene fragments were sub-cloned into pcDNA3 and the resulting plasmids were prepared using the PureYield™ Plasmid Maxiprep System (Promega, Madison, WI, USA) according to the manufacturer’s protocol. To verify the eukaryotic expression, the constructed plasmids were transiently transfected into HEK 293-T cells using LipofectamineTM 2000 (Invitrogen). The cells were harvested and lysed 48 h after transfection and the total cellular proteins were subjected to Western blot analysis using the rabbit anti-H. pylori polyclonal antibody (pAb) (LifeSpan BioSciences, Seattle, WA, USA) according to the manufacturer’s instructions.
Table 1.
PCR primers for truncated fragments of cagA, vacA and babA genes of H. pylori.
| Gene fragment | Primer pair | Sequence | Gene loci (nt) | Target length |
|---|---|---|---|---|
| c-1 | c1-f | 5ʹ-GGTACCATGACTAACGAAGCCAT-3’ | 1–17 | 1367 bp |
| c1-r | 5ʹ-CCTAGGGAAACAAAAGCAATGTGAT-3’ | 1337–1355 | ||
| c-2 | c2-f | 5ʹ-GGTACCATGATCACATTGCTTTTGTTTC-3’ | 1337–1355 | 1253 bp |
| c2-r | 5ʹ-CCTAGGAGATAACCCATTACCGACTA-3’ | 2555–2574 | ||
| c-3 | c3-f | 5ʹ-GGTACCATGGTTATCTAAAGCAGAAGCC-3’ | 2568–2586 | 950 bp |
| c3-r | 5ʹ-CCTAGGAAGATTTTTGGAAACCACCTTT-3’ | 3481–3502 | ||
| v-1 | v1-f | 5ʹ-CCTAGGATGGCGGTGTCAATCTGTCCAA −3’ | 1274–1291 | 1283bp |
| v1-r | 5ʹ-CTCGAGATTAGTGGTGTTTGTGGGTAAGT-3’ | 2519–2541 | ||
| v-2 | v2-f | 5ʹ-CCTAGGATGACCAACTTACCCACAAACAC-3’ | 2515–2534 | 1331bp |
| v2-r | 5ʹ-CTCGAGTGGCTTGCGTTGGAAATCAA-3’ | 3811–3830 | ||
| b-1 | b1-f | 5ʹ-GGTACCATGAAAAAACACATCCTT- 3’ | 1–18 | 1325bp |
| b1-r | 5ʹ-CCTAGGGTAACTGTGCCTGGAGC- 3’ | 1297–1313 | ||
| b-2 | b2-f | 5ʹ-GGTACCATGGCTCCAGGCACAGTTAC-3’ | 1297–1313 | 953bp |
| b2-r | 5ʹ- CCTAGGTAATAAGCGAACACGTA- 3’ | 2218–2234 |
Note: The underlined oligo nucleotides are Kpn I (GGTACC), BamH I (CCTAGG), Xho I (CTCGAG) enzyme sites, respectively. Abbreviations: f, forward primer; r, reverse primer; nt, nucleotide; bp, base pair.
Immunization of mice by DNA vaccines
DNA vaccination (100 μg of plasmid combined with 3.4% PVP40/0.01 M PBS as the adjuvant) was performed on random groups of BALB/c mice. The immunogen mixture was injected into the double hind thigh (0.3 ml per mouse). All the animals (5 per group) received immunization every two weeks for three rounds and 0.3 ml of the orbit blood were sampled for each inoculation. The orbit blood samples were also taken two weeks after the last vaccination and the empty pcDNA3 was used as the vehicle control. The animals were sacrificed by cervical dislocation and the spleens were taken out to prepare the single-cell suspensions. The splenic cells and blood samples were then used to determine the associated immune response before separation and transfusion of the splenic T-cells.
ELISA
IgG1 and IgG2a levels in the serum of immunized mice were assessed by the corresponding Mouse ELISA Quantitation Kit (Bethyl Laboratories, Montgomery, TX, USA). The concentrations of IL-2, IL-4, IL-6, IL-10, IL-12, IFN-γ and TNF-α were determined by the double-antibody sandwich ELISA method using mouse Quick EIATM kits (Dakewe Biotech, Shenzhen, China). All tests were performed in duplicate according to the recommended procedures of the manufacturers.
Flow cytometry
Flow cytometry was adopted to detect the peripheral T-cell subpopulations in blood samples taken from vaccinated animals of each group. Briefly, about 100 μl of the fresh whole blood under anticoagulation were subjected to red blood cell (RBC) lysis in 1× RBC Lysis Buffer (eBioscience, San Diego, CA, USA). The cell surface was stained with a cocktail of antibodies against CD3e PE-Cy5, CD4 PE and CD8a FITC (eBioscience) according to the manufacturer’s protocols. The cells were washed and analyzed on a LSRII flow cytometer (BD Pharmingen, San Diego, CA, USA) and FlowJo 7.6.5 software (Tree Star, Ashland, OR, USA).
Purification of splenic CD3+ pan-T cells
The single splenic cell suspensions were prepared after the spleens were extracted from the immunized mice. The Pan T Cell Isolation Kit II (Miltenyi Biotec, Auburn, CA, USA) and MiniMACS™ Separator were used according to the recommended procedures. The pure CD3+ pan-T cells were prepared by depletion of non-T cells including the B cells, NK cells, dendritic cells, macrophages, granulocytes, endothelial and erythroid cells. The isolated CD3+ T cells were subjected to flow cytometry using a cocktail of antibodies against CD3e PE-Cy5, CD4 PE, and CD8a FITC (eBioscience, San Diego, CA, USA) on a LSRII flow cytometer (BD Pharmingen, San Diego, CA, USA) and analyzed by FlowJo 7.6.5 software (Tree Star, Ashland, OR, USA).
Cell viability assay
The in vitro anticancer effect of H. pylori vaccine-activated CD3+ T cells was determined by MTT assay (Sangon Biotech, Shanghai, China) according to the manufacturer’s protocols. Both the target cells of SGC 7901 and MGC 80–3 were co-cultured with effector cells in 96-well plates at a ratio of 1: 25 for 24 h. The splenic CD3+ T-cells obtained from the mice immunized with the vehicle served as the negative control. The inhibitory efficacy of effector cells was calculated according to the following formula: Inhibitory rate (%) = [(1 – optimum A of experimental group)/optimum A of control] × 100%. The experiments were performed in triplicate.
In vivo anticancer efficacy evaluation
A nude BALB/c mouse model with GC xenograft was established and subjected to adoptive transfusions of H. pylori-activated CD3+ T cells. Briefly, female mice 6 weeks old were inoculated with 1 × 107 SGC 7901 cells in the right forelimb armpit. When the subcutaneous tumor nodules grew to about 100 mm3, 2 × 107 CD3+ T cells were transfused through the caudal vein every three days for three rounds. The tumor volume (V) was determined with calipers every two days and calculated by the equation: V = a × b2/2, where a and b indicate the major and minor axes of the tumor, respectively. The mice were sacrificed by cervical dislocation two weeks after the last T-cell transfusion and the tumor nodules were removed and used in subsequent investigation.
IHC
The tumor tissue sections were subjected to IHC detection of the subpopulation of infiltrated immune cells by the EnVision method. Briefly, the sections were washed with xylene, rehydrated with alcohol, and washed again with PBS. The samples were incubated overnight at 4 °C with pAbs of anti-CD4 (Santa Cruz Biotechnology, Santa Cruz, CA, USA; 1:50), anti-CD8 (Santa Cruz Biotechnology, 1:50), anti-FOXP3 (Abgent, San Diego, CA, USA; 1:50), anti-CD56 (Dako, Carpinteria, CA, USA; 1:50), anti-CD68 (Dako, 1:100), anti-CD86 (Dako, 1:100) and anti-CD163 (Dako, 1:500), respectively. The primary antibodies were removed and components of the Envision-plus detection system (Dako) were applied. The slides were counterstained with hematoxylin and coverslipped with an aqueous mounting medium. The slides were examined by optical microscopy by two researchers and the positivity index was calculated according to the ratio of the stained cells.
Western blotting
Western blot analysis was performed to determine apoptosis and antiapoptosis pathways in the GC xenograft after adoptive transfusions of CD3+ T cells. Equal amounts of proteins from the tumor tissues were separated by 12% SDS-PAGE and electrophoretically transferred to PVDF membranes. After blocking with 5% non-fat milk, the membranes were probed with primary antibodies including rabbit anti-Survivin pAb (Santa Cruz Biotechnology, 1:50), mouse monoclonal antibodies (mAb) of anti-Caspase 9 (Cell Signaling Technology, Beverly, MA, USA; 1:1000), anti-Caspase 8 (Cell Signaling Technology, 1:1000), and anti-Cleaved Caspase 3 (Cell Signaling Technology, 1:1000). The membranes were washed and incubated with HRP-conjugated goat anti-rabbit/mouse IgG (Cell Signaling Technology, 1:20000) and the immunoreactive bands were visualized by enhanced chemiluminescence (ECL) on the SuperSignal substrate system (Pierce, Rockford, IL, USA). The level of β-actin was detected by a rabbit mAb (Cell Signaling Technology, 1:5000).
Statistical analysis
Each value represents mean ± standard deviation (SD) of at least two different experiments and the software package SPSS 13.0 was used in the statistical analysis. Comparisons between individual data points were made using the Student’s t-test, with statistical significance defined as P value < 0.05 (*, P < 0.05; **, P < 0.01).
Funding Statement
This work was jointly supported by grants from the National Natural Science Foundation of China [Nos. 30901733, 81771977] (L.X., H.C.); the Open Research Fund of State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics [No. 2017KF08] (L.X., H.C.); and grants from the Research Fund of Jinling Hospital [Nos. 2011M020, 2010M039] (X.M., X.L.).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.de Martel C, Ferlay J, S F, Vignat J, Bray F, Forman D, Plummer M.. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13:607–615. PMID:22575588. doi: 10.1016/S1470-2045(12)70137-7. [DOI] [PubMed] [Google Scholar]
- 2.Helicobacter and Cancer Collaborative Group Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut. 2001;49:347–353. PMID:11511555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.IARC Working Group Schistosomes, liver flukes and Helicobacter pylori. IARC working group on the evaluation of carcinogenic risks to humans. Lyon, 7–14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1–241. PMID:7715068. [PMC free article] [PubMed] [Google Scholar]
- 4.Petersen AM, Blom J, Andersen LP, Krogfelt KA.. Role of strain type, AGS cells and fetal calf serum in Helicobacter pylori adhesion and invasion assays. FEMS Immunol Med Microbiol. 2000;29:59–67. PMID:10967262. doi: 10.1111/j.1574-695X.2000.tb01506.x. [DOI] [PubMed] [Google Scholar]
- 5.Ishijima N, Suzuki M, Ashida H, Ichikawa Y, Kanegae Y, Saito I, Borén T, Haas R, Sasakawa C, Mimuro H. BabA-mediated adherence is a potentiator of the Helicobacter pylori type IV secretion system activity. J Biol Chem. 2011;286:25256–25264. PMID:21596743. doi: 10.1074/jbc.M111.233601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hage N, Howard T, Phillips C, Brassington C, Overman R, Debreczeni J, Gellert P, Stolnik S, Winkler GS, Falcone FH. Structural basis of Lewis(b) antigen binding by the Helicobacter pylori adhesin BabA. Sci Adv. 2015;1:e1500315 PMID:26601230. doi: 10.1126/sciadv.1500315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerhard M, Lehn N, Neumayer N, Borén T, Rad R, Schepp W, Miehlke S, Classen M, Prinz C. Clinical relevance of the Helicobacter pylori gene for blood-group antigen-binding adhesin. Proc Natl Acad Sci USA. 1999;96:12778–12783. PMID:10535999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zambon CF, Navaglia F, Basso D, Rugge M, Plebani M. Helicobacter pylori babA2, cagA, and s1 vacA genes work synergistically in causing intestinal metaplasia. J Clin Pathol. 2003;56:287–291. PMID:12663641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mégraud F, Bessède E, Varon C. Helicobacter pylori infection and gastric carcinoma. Clin Microbiol Infect. 2015;21:984–990. PMID:26086571. doi: 10.1016/j.cmi.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Bornschein J, Kandulski A, Selgrad M, Malfertheiner P. From gastric inflammation to gastric cancer. Dig Dis. 2010;28:609–614. PMID:21088411. doi: 10.1159/000320061. [DOI] [PubMed] [Google Scholar]
- 11.Jung DH, Lee YC, Kim JH, Chung H, Park JC, Shin SK, Lee SK, Kim HI, Hyung WJ, Noh SH. Postoperative Helicobacter pylori infection as a prognostic factor for gastric cancer patients after curative resection. Gut Liver. 2017;11:635–641. PMID:28395509. doi: 10.5009/gnl16397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsai KF, Liou JM, Chen MJ, Chen CC, Kuo SH, Lai IR, Yeh KH, Lin MT, Wang HP, Cheng AL, et al. Distinct clinicopathological features and prognosis of Helicobacter pylori negative gastric cancer. PLoS One. 2017;12:e0170942 PMID:28152027. doi: 10.1371/journal.pone.0170942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Postlewait LM, Squires MH 3rd, Kooby DA, Poultsides GA, Weber SM, Bloomston M, Fields RC, Pawlik TM, Votanopoulos KI, Schmidt CR, et al. Preoperative Helicobacter pylori infection is associated with increased survival after resection of gastric adenocarcinoma. Ann Surg Oncol. 2016;23:1225–1233. PMID:26553442. doi: 10.1245/s10434-015-4953-x. [DOI] [PubMed] [Google Scholar]
- 14.Meimarakis G, Winter H, Assmann I, Kopp R, Lehn N, Kist M, Stolte M, Jauch KW, Hatz RA. Helicobacter pylori as a prognostic indicator after curative resection of gastric carcinoma: a prospective study. Lancet Oncol. 2006;7:211–222. PMID:16510330. doi: 10.1016/S1470-2045(06)70586-1. [DOI] [PubMed] [Google Scholar]
- 15.Marrelli D, Pedrazzani C, Berardi A, Corso G, Neri A, Garosi L, Vindigni C, Santucci A, Figura N, Roviello F. Negative Helicobacter pylori status is associated with poor prognosis in patients with gastric. Cancer. 2009;115:2071–2080. PMID:19280589. doi: 10.1002/cncr.24253. [DOI] [PubMed] [Google Scholar]
- 16.Kang SY, Han JH, Ahn MS, Lee HW, Jeong SH, Park JS, Cho YK, Han SU, Kim YB, Kim JH, et al. Helicobacter pylori infection as an independent prognostic factor for locally advanced gastric cancer patients treated with adjuvant chemotherapy after curative resection. Int J Cancer. 2012;130:948–958. PMID:21425257. doi: 10.1002/ijc.26081. [DOI] [PubMed] [Google Scholar]
- 17.Velin D, Michetti P. Immunology of Helicobacter pylori infection. Digestion. 2006;73:116–123. PMID:16788292. doi: 10.1159/000094043. [DOI] [PubMed] [Google Scholar]
- 18.Robinson K, Argent RH, Atherton JC. The inflammatory and immune response to Helicobacter pylori infection. Best Pract Res Clin Gastroenterol. 2007;21:237–259. PMID:17382275. doi: 10.1016/j.bpg.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Larussa T, Leone I, Suraci E, Imeneo M, Luzza F. Helicobacter pylori and T helper cells: mechanisms of immune escape and tolerance. J Immunol Res. 2015;2015:981328 PMID:26525279. doi: 10.1155/2015/981328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Den Engel NK, Winter H, Rüttinger D, Shau I, Schiller M, Mayer B, Moudgil T, Meimarakis G, Stolte M, Jauch KW, et al. Characterization of immune responses in gastric cancer patients: a possible impact of H. pylori to polarize a tumor-specific type 1 response? Clin Immunol. 2006;120:285–296. PMID:16765089. doi: 10.1016/j.clim.2006.04.566. [DOI] [PubMed] [Google Scholar]
- 21.Wang SK, Zhu HF, He BS, Zhang ZY, Chen ZT, Wang ZZ, Wu GL. CagA+ H pylori infection is associated with polarization of T helper cell immune responses in gastric carcinogenesis. World J Gastroenterol. 2007;13:2923–2931. PMID:17589941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frejlich E, Rudno-Rudzińska J, Janiszewski K, Salomon L, Kotulski K, Pelzer O, Grzebieniak Z, Tarnawa R, Kielan W. Caspases and their role in gastric cancer. Adv Clin Exp Med. 2013;22:593–602. PMID:23986221. [PubMed] [Google Scholar]
- 23.Zhang J, Zhu Z, Sun Z, Sun X, Wang Z, Xu H. Survivin gene expression increases gastric cancer cell lymphatic metastasis by upregulating vascular endothelial growth factor-C expression levels. Mol Med Rep. 2014;9:600–606. PMID:24337012. doi: 10.3892/mmr.2013.1858. [DOI] [PubMed] [Google Scholar]
- 24.Gu Y, Jin S, Wang F, Hua Y, Yang L, Shu Y, Zhang Z, Guo R. Clinicopathological significance of PI3K, Akt and survivin expression in gastric cancer. Biomed Pharmacother. 2014;68:471–475. PMID:24726064. doi: 10.1016/j.biopha.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 25.Wang F, Sun GP, Zou YF, Zhong F, Ma T, Li XQ, Wu D. Helicobacter pylori infection predicts favorable outcome in patients with gastric cancer. Curr Oncol. 2013;20:e388–95. PMID:24155636. doi: 10.3747/co.20.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi IK, Sung HJ, Lee JH, Kim JS, Seo JH. The relationship between Helicobacter pylori infection and the effects of chemotherapy in patients with advanced or metastatic gastric cancer. Cancer Chemother Pharmacol. 2012;70:555–558. PMID:22871922. doi: 10.1007/s00280-012-1944-5. [DOI] [PubMed] [Google Scholar]
- 27.Wang F, Sun G, Zou Y, Zhong F, Ma T, Li X. Protective role of Helicobacter pylori infection in prognosis of gastric cancer: evidence from 2,454 patients with gastric cancer. PLoS One. 2013;8:e62440 PMID:23667477. doi: 10.1371/journal.pone.0062440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xue LJ, Su QS, Yang JH, Lin Y. Autoimmune responses induced by Helicobacter pylori improve the prognosis of gastric carcinoma. Med Hypotheses. 2008;70:273–276. PMID:17681432. doi: 10.1016/j.mehy.2007.05.045. [DOI] [PubMed] [Google Scholar]
- 29.Lee WJ, Lin JT, Shun CT, Lee WC, Yu SC, Lee PH, Chang KJ, Wei TC, Chen KM. Comparison between resectable gastric adenocarcinomas seropositive and seronegative for Helicobacter pylori. Br J Surg. 1995;82:802–805. PMID:7627516. [DOI] [PubMed] [Google Scholar]
- 30.Yoon H, Kim N, Lee HS, Shin CM, Park YS, Lee DH, Jung HC, Song IS. Helicobacter pylori-negative gastric cancer in South Korea: incidence and clinicopathologic characteristics. Helicobacter. 2011;16:382–388. PMID:21923684. doi: 10.1111/j.1523-5378.2011.00859.x. [DOI] [PubMed] [Google Scholar]
- 31.Hur H, Lee SR, Xuan Y, Kim YB, Lim YA, Cho YK, Han SU. The effects of Helicobacter pylori on the prognosis of patients with curatively resected gastric cancers in a population with high infection rate. J Korean Surg Soc. 2012;83:203–211. PMID:23091792. doi: 10.4174/jkss.2012.83.4.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolb JM, Ozbek U, Harpaz N, Holcombe RF, Ang C. Effect of Helicobacter pylori infection on outcomes in resected gastric and gastroesophageal junction cancer. J Gastrointest Oncol. 2017;8:583–588. PMID:28736645. doi: 10.21037/jgo.2017.01.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qiu HB, Zhang LY, Keshari RP, Wang GQ, Zhou ZW, Xu DZ, Wang W, Zhan YQ, Li W. Relationship between H. Pylori infection and clinicopathological features and prognosis of gastric cancer. BMC Cancer. 2010;10:374 PMID:20637122. doi: 10.1186/1471-2407-10-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Syrios J, Sougioultzis S, Xynos ID, Kavantzas N, Kosmas C, Agrogiannis G, Griniatsos J, Karavokyros I, Pikoulis E, Patsouris ES, et al. Survival in patients with stage IV noncardia gastric cancer - the influence of DNA ploidy and Helicobacter pylori infection. BMC Cancer. 2012;12:264 PMID:22892134. doi: 10.1186/1471-2407-12-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li G, Wang Z, Wang Z, Xu J, Cui J, Cai S, Zhan W, He Y. Gastric cancer patients with Helicobacter pylori infection have a poor prognosis. J Surg Oncol. 2013;108:421–426. PMID:24037736. doi: 10.1002/jso.23417. [DOI] [PubMed] [Google Scholar]
- 36.Chung WC, Jung SH, Lee KM, Paik CN, Kwak JW, Jung JH, Yoo JY, Lee MK, Chung IS. Genetic instability in gastric epithelial neoplasias categorized by the revised vienna classification. Gut Liver. 2010;4:179–185. PMID:20559519. doi: 10.5009/gnl.2010.4.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corso G, Pedrazzani C, Marrelli D, Pascale V, Pinto E, Roviello F. Correlation of microsatellite instability at multiple loci with long-term survival in advanced gastric carcinoma. Arch Surg. 2009;144:722–727. PMID:19687375. doi: 10.1001/archsurg.2009.42. [DOI] [PubMed] [Google Scholar]
- 38.Kim HJ, Kim N, Yoon H, Choi YJ, Lee JY, Kwon YH, Yoon K, Jo HJ, Shin CM, Park YS, et al. Comparison between resectable Helicobacter pylori-negative and -positive gastric cancers. Gut Liver. 2016;10:212–219. PMID:26087794. doi: 10.5009/gnl14416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kang JU, Koo SH. Assessment of the beneficial loci and prognostic implications of microsatellite instability in gastric carcinoma. Mol Med Rep. 2011;4:1175–1181. PMID:21805035. doi: 10.3892/mmr.2011.541. [DOI] [PubMed] [Google Scholar]
- 40.Fehlings M, Drobbe L, Moos V, Renner Viveros P, Hagen J, Beigier-Bompadre M, Pang E, Belogolova E, Churin Y, Schneider T, et al. Comparative analysis of the interaction of Helicobacter pylori with human dendritic cells, macrophages, and monocytes. Infect Immun. 2012;80:2724–2734. PMID:22615251. doi: 10.1128/IAI.00381-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He LJ, Xie D, Hu PJ, Liao YJ, Deng HX, Kung HF, Zhu SL. Macrophage migration inhibitory factor as a potential prognostic factor in gastric cancer. World J Gastroenterol. 2015;21:9916–9926. PMID:26379396. doi: 10.3748/wjg.v21.i34.9916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mohammadi M, Nedrud J, Redline R, Lycke N, Czinn SJ. Murine CD4 T-cell response to Helicobacter infection: TH1 cells enhance gastritis and TH2 cells reduce bacterial load. Gastroenterology. 1997;113:1848–1857. PMID:9394724. [DOI] [PubMed] [Google Scholar]
- 43.Taylor JM, Ziman ME, Canfield DR, Vajdy M, Solnick JV. Effects of a Th1- versus a Th2-biased immune response in protection against Helicobacter pylori challenge in mice. Microb Pathog. 2008;44:20–27. PMID:17683897. doi: 10.1016/j.micpath.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kayhan B, Arasli M, Eren H, Aydemir S, Kayhan B, Aktas E, Tekin I. Analysis of peripheral blood lymphocyte phenotypes and Th1/Th2 cytokines profile in the systemic immune responses of Helicobacter pylori infected individuals. Microbiol Immunol. 2008;52:531–538. PMID:19090832. doi: 10.1111/j.1348-0421.2008.00066.x. [DOI] [PubMed] [Google Scholar]
- 45.Martínez-Becerra F, Castillo-Rojas G, Ponce de León S, López-Vidal Y. IgG subclasses against Helicobacter pylori isolates: an important tool for disease characterization. Scand J Immunol. 2012;76:26–32. PMID:22686508. doi: 10.1111/j.1365-3083.2012.02699.x. [DOI] [PubMed] [Google Scholar]
- 46.Senthilkumar C, Niranjali S, Jayanthi V, Ramesh T, Devaraj H. Molecular and histological evaluation of tumor necrosis factor-alpha expression in Helicobacter pylori-mediated gastric carcinogenesis. J Cancer Res Clin Oncol. 2011;137:577–583. PMID:20512382. doi: 10.1007/s00432-010-0921-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang W, Liu K, Guo Q, Cheng J, Shen L, Cao Y, Wu J, Shi J, Cao H, Liu B, et al. Tumor-infiltrating immune cells and prognosis in gastric cancer: a systematic review and meta-analysis. Oncotarget. 2017;8:62312–62329. PMID:28977947. doi: 10.18632/oncotarget.17602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. PMID:22419253. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 49.Takahashi T, Yujiri T, Shinohara K, Inoue Y, Sato Y, Fujii Y, Okubo M, Zaitsu Y, Ariyoshi K, Nakamura Y, et al. Molecular mimicry by Helicobacter pylori CagA protein may be involved in the pathogenesis of H. pylori-associated chronic idiopathic thrombocytopenic purpura. Br J Haematol. 2004;124:91–96. PMID:14675413. [DOI] [PubMed] [Google Scholar]
- 50.Baldari CT, Lanzavecchia A, Telford JL. Immune subversion by Helicobacter pylori. Trends Immunol. 2005;26:199–207. PMID:15797510. doi: 10.1016/j.it.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 51.Hennig EE, Mernaugh R, Edl J, Cao P, Cover TL. Heterogeneity among Helicobacter pylori strains in expression of the outer membrane protein BabA. Infect Immun. 2004;72:3429–3435. PMID:15155649. doi: 10.1128/IAI.72.6.3429-3435.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nurgalieva ZZ, Conner ME, Opekun AR, Zheng CQ, Elliott SN, Ernst PB, Osato M, Estes MK, Graham DY. B-cell and T-cell immune responses to experimental Helicobacter pylori infection in humans. Infect Immun. 2005;73:2999–3006. PMID:15845507. doi: 10.1128/IAI.73.5.2999-3006.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shen Z, Zhou S, Wang Y, Li RL, Zhong C, Liang C, Sun Y. Higher intratumoral infiltrated Foxp3+ Treg numbers and Foxp3+/CD8+ ratio are associated with adverse prognosis in resectable gastric cancer. J Cancer Res Clin Oncol. 2010;136:1585–1595. PMID:20221835. doi: 10.1007/s00432-010-0816-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jang TJ. The number of Foxp3-positive regulatory T cells is increased in Helicobacter pylori gastritis and gastric cancer. Pathol Res Pract. 2010;206:34–38. PMID:19819643. doi: 10.1016/j.prp.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 55.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. PMID:18510923. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]


