ABSTRACT
Background: Among all breast cancer subtypes, triple-negative breast cancer (TNBC) has aggressive clinical manifestations including more frequent relapses and metastases. The roles of PD-L1 expression and tumor-infiltrating lymphocytes (TILs) in TNBC clinicopathological behaviors and patients’ survival outcomes remain unclear.
Methods: TNBC (108 cases) patients with at least 5-year follow-up were analyzed for PD-L1 expression and TILs by immunohistochemistry. We also analyzed the relationships between PD-L1 expression, TILs and clinicopathological characteristics. Furthermore, we explored the effect of PD-L1 expression and TILs on prognosis as illustrated by disease-free survival (DFS).
Results: The expression of PD-L1 was related to more aggressive clinicopathological behaviors in TNBC patients including a larger tumor size, higher incidence of PL-1-ALN, more frequent distant metastasis, and a reduced disease-free survival. In contrast, patients with high-level TILs showed less aggressive disease progression hence a better prognosis compared to patients with low-level TILs. Among patients with high-level TILs, PD-L1 expression was correlated with adverse prognosis.
Conclusions: Expression of PD-L1 and low-level TILs in TNBC patients were associated with adverse clinical outcomes. However, the positive impact of high-level TILs was attenuated by PD-L1 expression. Our results suggest potential biomarkers for a selection of indicated cases in the TNBC patients for anti-PD-L1/anti-PD1 immunotherapy.
KEYWORDS: PD-L1, TILs, TNBC, immunotherapy, prognosis
Introduction
Triple-negative breast cancer (TNBC) is a group of clinically and molecularly heterogeneous diseases defined as breast carcinomas negative for estrogen receptor (ER) and progesterone receptor (PR) protein expression, and negative for human epidermal growth factor receptor type 2 (HER2) protein overexpression or gene amplification.1 There are various transcriptional subtypes and histologic patterns of TNBC, with the majority of the cases being invasive ductal carcinomas with high grade, in particular, the “basal-like” type by molecular classification. TNBC accounts for 15–20% of all kinds of breast cancers approximately and demonstrates an aggressive clinical manifestation.2 Compared to other breast cancer subtypes, organ metastasis of TNBC is more likely involving the lung and brain.3 At present, in the adjuvant, neoadjuvant, and metastatic settings, applying chemotherapy is a standard treatment strategy for early-stage or metastatic TNBC. Although TNBC are in general more sensitive to chemotherapy than other subtypes, it has higher rates of relapse and distant metastasis.4–6 To further improve the survival outcome of TNBC patients, many researchers drive large-scale clinical investigational efforts to evaluate these novel therapeutic strategies.
Breast cancer is not traditionally considered strongly antigenic. Recent studies, however, found that the existence of tumor-infiltrating lymphocytes (TILs) predicted a good reaction to neoadjuvant chemotherapy,7–11 showing that TILs may mediate host immune defense against the tumor. Several studies have found a higher level of TILs in TNBCs compared with hormone receptor-positive breast cancer.7 Recently, some results with immune checkpoint inhibitors such as programmed death-1 ligand 1 (PD-L1) in melanoma and even in non-small cell lung cancer have found new interest in TILs and their association with tumor immunity.12,13 PD-L1 is a kind of transmembrane protein of B7 family which plays an important role in limiting the cytotoxic immune reaction by binding with programmed death-1 (PD-1) receptor expressed on immune effector cells to avoid autoimmune diseases.14 Its expression in the tumor cell and existence in the tumor microenvironment have been related to the existence of TILs. The bind between PD-1 expressed by TILs and PD-L1 expressed by tumor cells may facilitate the tumor to escape from the antitumor immunity in the breast cancer microenvironment.
The purpose of the research was to investigate the association of PD-L1 with TILs among TNBC patients. The expression of PD-L1 on the tumor cell of surgical specimens from a cohort of TNBC patients was assessed by immunohistochemical study. The predictive and prognostic values of PD-L1 and TILs were investigated separately, and the correlated influence of PD-L1 expression in the breast cancer tumor cells and TILs in the tumor microenvironment on the disease-free survival (DFS) was analyzed.
Methods
Patients and breast cancer tissue specimens
The patient cohort of this study consisted of 108 patients with histologically confirmed triple-negative breast cancer in China Medical University affiliated hospital from January 2008 to October 2011. Cases meeting the following criteria were selected for the study: (a) surgical resection was performed; (b) diagnosis of triple-negative breast cancer on the surgical specimen was confirmed by pathologists; (c) at least 10 axillary lymph nodes were dissected and evaluated by pathologists. The clinical information was obtained through a telephone conversation or in-person visit in outpatient settings. The DFS was defined as the period from operation date to the date when local recurrence or distant metastasis was diagnosed. This protocol (2018PS336K) was approved by the Institutional Review Board (IRB) of China Medical University.
Antibodies
Polyclonal rabbit antihuman PD-L1 antibody was purchased from CST Company (PD-L1 (E1L3N) XP Rabbit mAb). The dilution of the antibody and antibody applications were performed according to the manufacturer instructions. The horseradish peroxidase-conjugated secondary antibody was purchased from Gene Tech (Shanghai) Company Ltd.
Immunohistochemistry
The surgical specimens were fixed in 4% formaldehyde, embedded in paraffin, and sectioned at 5μm. After deparaffinization with xylene and rehydration with an ethanol series, the sections were incubated with the primary anti-PD-L1 antibody (1:300) at 4°C overnight. Subsequently, the sections were incubated with the secondary antibody at 37°C for 30 min, followed by development using a DAB kit purchased from Gene Tech (Shanghai) Company Limited.
To examine the lymphocytic infiltration level, the specimens were fixed in a 4% formaldehyde, processed for paraffin embedding and sectioned at 5μm. The sections were subsequently placed on slides coated with (3-aminopropyl) triethoxysilane and stained with hematoxylin and eosin.
PD-L1 expression analysis
The PD-L1 expression was semi-quantitated using the following scoring method: score 0 if <1% cancer cells expressed PD-L1 in the cytoplasm and/or on the cell membrane, score 1 if ≥1 and <5% cancer cells expressed PD-L1, score 2 if ≥5% but <10% cancer cells expressed PD-L1, and score 3 if ≥10% cancer cells expressed PD-L1. Specimens with score 1, 2 and 3 were considered positive for PD-L1 expression.15
Lymphocyte infiltration analysis
Lymphocytic infiltration levels in the tumor beds and adjacent tissues were evaluated. The level was scored 0 if lymphocytes were absent. Score 1, 2 and 3 was given if the ratio of lymphocyte vs. tumor cells was <30%, 30%-60%, and >60%, respectively. The overall lymphocyte infiltration grades were calculated by adding the scores of the cancer bed lymphocyte infiltration level and adjacent lymphocyte infiltration level. High-level infiltration was defined as the overall grade equal to or higher than 3. Grade below 3 was considered as low level infiltration.16,17
Statistical analysis
Data were analyzed using SPSS 17.0 software. Differences of clinicopathological characteristics between groups with and without PD-L1 expression, groups with high and low-level lymphocytic infiltration were tested using Chi-square test and Fisher’s extract test. Kaplan–Meier test was used to analyze DFS data. The log-rank test was utilized to compare survival outcome between groups. p-V alue below 0.05 was defined as statistically significant.
Results
Patients characteristics
A total of 108 female patients diagnosed with TNBC were analyzed. These patients’ age ranged from 26 to 74 with an average of 52. Fourteen patients received neoadjuvant therapy. Twenty-nine patients were found to have lymph node metastases. Fifteen patients developed local recurrence or distant organ metastases during the follow-up period.
PD-L1 expression was associated with larger tumor size and increased PL-1-ALN metastasis
Twenty-four out of 108 patients (22%) were found to be positive for PD-L1 expression by immunohistochemistry stain. PD-L1 in the tumor was expressed in a focal or patchy pattern. Interestingly, PD-L1 positive tumor cells were located adjacent to tumor infiltrating lymphocytes (Figure 1). Both membrane and cytoplasm PD-L1 expressions were observed (Figure 1A and 1B). We subsequently compared the clinical and histological characteristics between PD-L1 negative and PD-L1 positive groups. No differences were identified in age, menopausal status, neoadjuvant therapy, histological grade or ki-67 status. However, the average tumor size was significantly larger in the PD-L1 positive group compared to PD-L1 negative group (p = 0.035). In addition, patients in the PD-L1 positive group had more positive level-1-axillary lymph nodes (PL-1-ALN) (p = 0.041). (Table 1).
Figure 1.
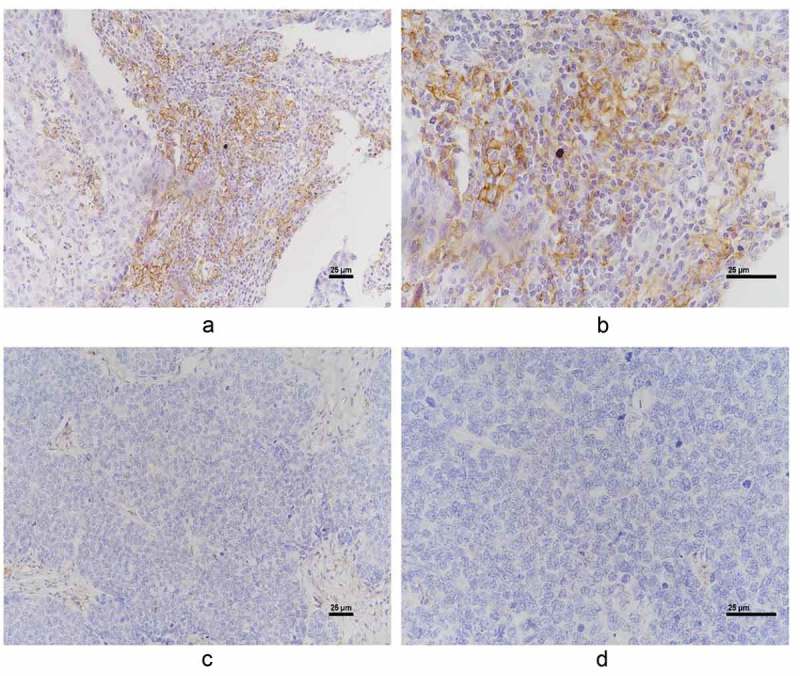
Representative positive PD-L1 (A and B. A, x 200 magnification. B, x 400 magnification) and negative PD-L1 immunohistochemical staining (C and D. C, x 200 magnification. D, x 400 magnification).
Table 1.
Correlations between the PD-L1 expression and clinicopathological characteristics.
| Variables | PD-L1 expression (%) | No PD-L1 expression (%) | p-Value |
|---|---|---|---|
| No. of patients | 24(22) | 84(78) | |
| Age (years) | 0.393 | ||
| ≤45 | 4 (16.7) | 21 (25.0) | |
| >45 | 20 (83.3) | 63 (75.0) | |
| Menopausal status | 0.071 | ||
| Premenopausal | 7 (29.2) | 42 (50.0) | |
| Postmenopausal | 17 (70.8) | 42 (50.0) | |
| Neoadjuvant therapy | 0.937 | ||
| Yes | 3 (12.5) | 10 (11.9) | |
| No | 21 (87.5) | 74 (88.1) | |
| Tumor size (cm) | 0.035 | ||
| Median (range) | 2.09 (1.0–3.5) | 2.49 (1.0–6.0) | |
| No. of PL-1-ALN | 0.041 | ||
| Median (range) | 1.79 (0–13) | 0.69 (0–11) | |
| Histological grade | 0.295 | ||
| II | 12 (50.0) | 52 (61.9) | |
| III | 12 (50.0) | 32 (38.1) | |
| Ki67 status | 0.285 | ||
| ≤20 | 4 (16.7) | 23 (27.4) | |
| >20 | 20 (83.3) | 61 (72.6) | |
| Distant metastasis | 0.002 | ||
| Yes | 8 (33.3) | 7 (8.3) | |
| No | 16 (66.7) | 77 (91.7) | |
| DFS | |||
| Median (range) | 72.4 (8–114) | 95.4 (9–120) | <0.001 |
High-level lymphocyte infiltration was related to smaller tumor size and a lower metastatic rate
Lymphocyte infiltration was evaluated on HE-stained specimens. We were able to detect lymphocytes both in the tumor and in the surrounding tissue (Figure 2). In fact, 84.3% of the specimens showed high-level lymphocyte infiltration. We subsequently compared the clinical and histological characteristics between the high level and low-level lymphocyte infiltration groups. No differences were found in terms of age, menopausal status, neoadjuvant therapy, numbers of PL-1-ALN, histological grade or ki-67 status (Table 2). However, patients with high-level lymphocyte infiltration had significantly smaller tumor size and a lower distant metastasis rate compared to the low lymphocyte infiltration group (p = 0.024 and 0.044, respectively; Table 2).
Figure 2.
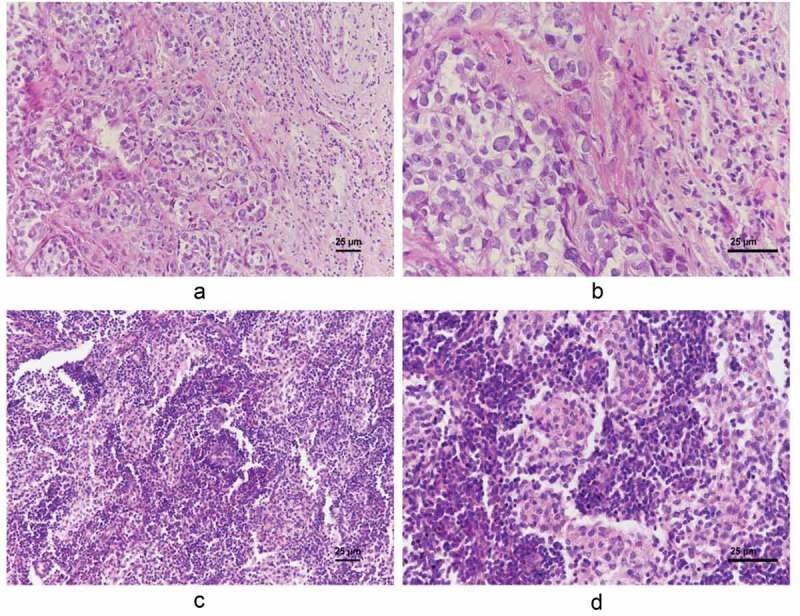
Representative low-level (A and B. A, x 200 magnification. B, x 400 magnification) and high-level lymphocyte infiltration (C and D. C, x 200 magnification. D, x 400 magnification).
Table 2.
Correlations between the Lymphocytic infiltration and clinicopathologic characteristics.
| Variables | Low-level infiltration (%) | High-level infiltration (%) | p-Value |
|---|---|---|---|
| No. of patients | 17 (15.7) | 91 (84.3) | |
| Age (years) | 0.558 | ||
| ≤45 | 3 (17.6) | 22 (24.2) | |
| >45 | 14 (82.4) | 69 (75.8) | |
| Menopausal status | 0.150 | ||
| Premenopausal | 5 (29.4) | 44 (48.4) | |
| Postmenopausal | 12 (70.6) | 47 (51.6) | |
| Neoadjuvant therapy | 0.113 | ||
| Yes | 13 (76.5) | 82 (90.1) | |
| No | 4 (23.5) | 9 (9.9) | |
| Tumor size (cm) | 0.024 | ||
| Median (range) | 2.8 (2.0–5.0) | 2.3 (1.0–6.0) | |
| No. of PL-1-ALN | 0.087 | ||
| Median (range) | 1.8 (0–11) | 0.7 (0–13) | |
| Histological grade | 0.968 | ||
| II | 10 (58.8) | 54 (59.3) | |
| III | 7 (41.2) | 37 (40.7) | |
| Ki67 status | 0.647 | ||
| ≤20 | 5 (29.4) | 22 (24.2) | |
| >20 | 12 (70.6) | 69 (75.8) | |
| Distant metastasis | 0.044 | ||
| Yes | 5 (29.4) | 10 (11.0) | |
| No | 12 (70.6) | 81 (89.0) | |
| DFS | |||
| Median (range) | 76.8 (8–114) | 92.8 (9–120) | <0.001 |
PD-L1 expression and the low-level lymphocyte infiltration were related to shorter disease-free survival (DFS) and poorer prognosis
Although TNBC patients generally show aggressive clinical behavior, we questioned if PD-L1 expression made any differences in the long-term prognosis in TNBC patients. In fact, patients with PD-L1 expression exhibited reduced DFS (average 72.4 months) during at least 5-year follow-up compared with patients without PD-L1 expression (average 95.4 months) (p = 0.001) (Figure 3A and Table 3). In addition, the ratio of local recurrence/distant metastasis was significantly higher in PD-L1 positive group (33.3%) than in the PD-L1 negative group (8.3%) (p = 0.002).
Figure 3.
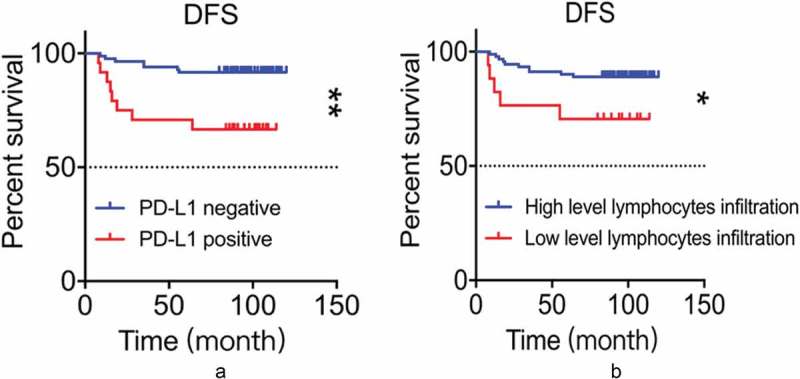
A: PD-L1 expression was associated with reduced DFS (p = 0.001, log-rank test). B: High level of lymphocytic infiltration was associated with a longer DFS (p = 0.026, log-rank test).
Table 3.
PD-L1 expression and Prognosis.
| Variables | PD-L1 positive patient No. (%) | PD-L1 negative patient No. (%) | p-Value |
|---|---|---|---|
| No. of patients | 24 (22) | 84 (78) | |
| Distant metastasis | 0.002 | ||
| Yes | 8 (33.3) | 7 (8.3) | |
| No | 16 (66.7) | 77 (91.7) | |
| DFS | |||
| Median (range) | 72.4 (8–114) | 95.4 (9–120) | <0.001 |
Lymphocyte infiltration is another factor we proposed to play an important role in the prognosis of TNBC patients. Patients with high-level lymphocyte infiltration showed a longer DFS (average 92.8 months) compared to those with low-level lymphocyte infiltration (average 76.8 months) during follow-up (p = 0.026 Figure 3B). Also, the local recurrence and distant metastasis rate was 29.4% in the group with high-level lymphocyte infiltration, significantly higher than 11.0% in low-level lymphocyte infiltration group (p = 0.044).
In conclusion, PD-L1 expression and low-level lymphocyte infiltration are adverse factors for patients with TNBC with regard to local recurrence, distant metastasis, and DFS.
PD-L1 expression attenuates the positive impacts of high-level TILs on DFS
To explore the clinical significance of PD-L1 expression on patients’ prognosis in the background of high-level lymphocytic infiltration, we subdivide the group with high-level lymphocytic infiltration into two sub-groups based on PD-L1 expression. Average DFS in PD-L1 positive sub-group (75.95 months) was shorter than that in PD-L1 negative sub-group (97.56 months) (p = 0.001. Figure 4A). In addition, the local recurrence/distant metastasis rate was 30.0% in PD-L1 positive patients, significantly higher than 5.6% in PD-L1 negative patients (p = 0.002). In conclusion, PD-L1 expression had an overall significant unfavorable impact on the prognosis of patients with high-level lymphocyte infiltration, indicating that PD-L1 expression attenuated the positive impacts of tumor-infiltrating lymphocytes on overall prognosis in TNBC patients.
Figure 4.
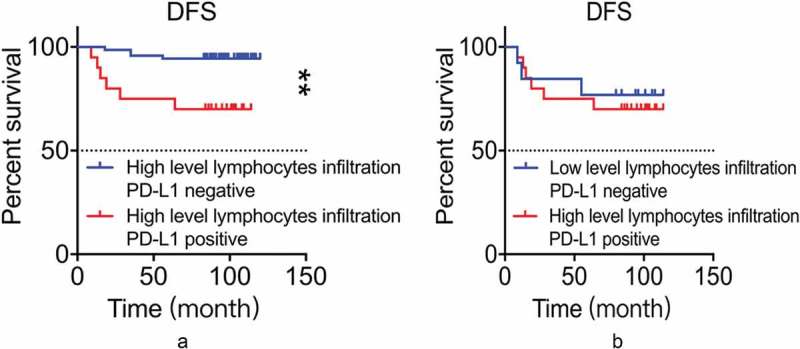
A: PD-L1 was associated with worse DFS in the presence of high-level lymphocytic infiltration (p = 0.001, log-rank test). B: Result of comparison on DFS between the group of PD-L1-/low level lymphocytic infiltration and the group of PDL1+/high level lymphocytic infiltration (p = 0.709, log-rank test).
These results raised the question of which factor, PD-L1 expression or lymphocytic infiltration levels, has a more significant prognostic effect. To answer the question, we compared the prognosis of the group with negative PD-L1 and low-level lymphocytic infiltration and the group with positive PD-L1 and high-level lymphocytic infiltration. The local recurrence rate/distant metastasis rate (23.1%) in PD-L1 negative and low-level lymphocytic infiltration group was slightly lower than that in PD-L1 positive and high-level lymphocytic infiltration group (30.0%). However, the difference is not statistically significant (p = 0.562). Similarly, patients in PD-L1 negative and low-level lymphocyte infiltration group had DFS of 83.62 months compared to slightly but not statistically significant shorter DFS of 75.95 months in PD-L1 positive and high-level lymphocyte infiltration group (p = 0.709. Figure 4B).
Discussion and conclusions
Deficiency in the antitumor immune surveillance is the hallmark for the development and progression of cancer.18 Expression of PD-L1 on the tumor cells has been revealed to be one of the multiple mechanisms used by the tumor to avoid recognizing by the host immune system. TNBC is a group of clinically and molecularly heterogeneous breast cancer accounting for approximately 15–20% of all breast cancer, which demonstrates a poorer prognosis compared to other hormonal receptor-positive breast cancers. The finding of the presence of TILs in breast cancer, especially the presence of a higher level of TILs in TNBC has arisen extensive basic and clinical investigational interests in the correlation between the roles of TILs and PD-L1 in the development of breast cancer and its prognostic and predictive values.
In the research, we identified the ratio of PD-L1 expression is 22% in TNBC, at the lower range compared to the previous studies, which demonstrated 20–34% positive expression rates of PD-L1 in TNBC.19–21 We used large tissue sections from surgical specimen instead of core biopsy specimens in the study to minimize sampling error. Similar to most reports, the expression of PD-L1 by these tumor cells was in a focal or patchy pattern other than an evenly distributed pattern, and the PD-L1 expressed tumor cells were generally seen proximal to infiltrative lymphocytes. The expression was positively related to tumor size and the number of positive level-1-axillary lymph node but not related to the age, menopausal status, histological grade or ki-67 status. Wimberly H. et al. found that PD-L1 expression had a positive predictive value to neoadjuvant chemotherapy.21 In our study, the predictive response in this regard was not observed, probably due to the low number of the patients underwent neoadjuvant chemotherapy (total 13 patients, 3 positive for PD-L1 and 10 negative for PD-L1). Survival analysis demonstrated that PD-L1 expression was negatively related to DFS. The ratio of local recurrence/distant metastasis was 33.3% in PD-L1 positive group versus 8.3% in the PD-L1 negative group, and the mean DFS was 72.4 months in the PD-L1 positive group versus 95.4 months in the PD-L1 negative group, and with statistical significances. Our observation showed that PD-L1 expression can become a kind of negative prognostic biomarker for the outcome of TNBC.
In agreement with the previous studies,7 high-level of lymphocytic infiltration was observed in the majority of the patient cohort (84.3%). High-level lymphocytic infiltration was associated with a smaller average primary tumor size and less distant metastasis, but not with age, menopausal status, neoadjuvant therapy, numbers of positive level-1-axillary lymph node, histological grade or ki-67 status. The mean DFS was 92.8 months in high-level lymphocytic infiltration group and 76.8 months in low-level lymphocytic infiltration group. Presence of high-level TILs suggested an immune response to tumor-associated antigens, albeit the response was insufficient given the fact that the tumor had already developed. The observation that high-level TILs was associated with longer DFS indicated that TILs had positive prognostic significance in TNBC.
While PD-L1 expression and TILs proved to be independently prognostic significant for TNBCs in our study, it would be interesting to explore the correlated influence of the two markers on the long-term prognosis. In overall, the presence of high-level TILs demonstrated certain degrees of antitumor immunity as presented by a prolonged EFS, the patients with positive PD-L1 expression within this group, however, showed a significant worse prognosis, compared with those with negative PD-L1 expression and low-level lymphocytic infiltration, even though the difference was not statistically significant (Figure 4B). Although this combined consequence might be multifactorial in nature, the attenuating or blocking effect of PD-L1 on the antitumor immunity of the TILs in the tumor microenvironment should be the first priority in reasoning, given the up-to-date knowledge on the interaction between PD-L1 and PD-1 on the surfaces of lymphocytes. This interpretation is of particular importance in that the combination of PD-L1 expression and presence of high-level TIL may not only can serve as a prognostic marker but also can be a predictive marker for PD-L1/PD-1 axis immunotherapy.
It has been known that there is no expression of PD-L1 on normal breast cancer epithelial tissues, but it is expressed on many kinds of cancers including renal cell carcinoma,22,23 ovarian cancer, pancreatic cancer,24,25 gastric cancer,26 hepatocellular carcinoma, and esophageal cancer as well as breast cancer as discussed above.27,28 Early phase I trials with PD-L1 inhibitors in TNBC reported an overall response rate of up to 19%.29 A deep understanding on the interaction between TILs and PD-L1 would lead to a better selection of these TNBC patients suitable for this therapeutic option. In a comprehensive review article, Chen, L. and his colleague proposed a “adaptive resistance hypothesis” in 2015.13 The hypothesis suggested that the “adaptive resistance” was initiated by the recognization of antigens on the surface of tumor cells by TILs. By specific bind via T cell receptor, TILs, as well as macrophages and/or even dendritic cells in the tumor microenvironment would release IFN-γ and further induce the expression of PD-L1 on the tumor cells. While IFN-γ can easily promote the functions of TIL effector via stimulating their differentiation and promoting the processing and presentation of antigen,30,31 induced PD-L1 down-regulates the TILs, serving as a negative feedback loop to inhibit tumor immunity. These cells expressing PD-L1 use many mechanisms to inhibit tumor immunity, including the induction of IL-10 production, T cell apoptosis, energy, and even functional exhaustion. PD-L1 can also act as a kind of molecular shield and protect cancer cells from lysis by the function of cytotoxic T lymphocytes.14,32–35 Our observations of focal or patchy expression of PD-L1 by TNBC tumor cells proximal to TILs and an attenuated antitumor immunity of TILs in the existence of PD-L1 are in agreement with this hypothesis. While high-level TILs were present in most tumors in our study, their absence was seen in some cases. The reasons may include weak tumor antigenicity, defected systemic antitumor immune response or induced lymphocyte apoptosis in the tumor microenvironment by immune checkpoint inhibitors like PD-L1.
In short, our results have shown that 22% of cases of TNBC expressed PD-L1 and 84% had high-level TILs approximately. Both PD-L1 expression by the tumor cells and the presence of high-level TILs demonstrated prognostic significance. The positive impact of TILs on the long-term prognosis was attenuated in the presence of PD-L1 expression. Our results also suggest potential biomarkers for selection of suitable cases for anti-PD-L1/anti-PD1 immunotherapy in the TNBC patient population, and inclusion of the evaluations on the two biomarkers in the pathology report may have important clinical values.
In conclusion, our results show that the expression of PD-L1 and low-level lymphocyte infiltration can predict a worse DFS in TNBC patients, and PD-L1 expression in TNBC attenuates the positive impacts of lymphocytes infiltration on DFS. Therefore, our results imply two predictors for TNBC patients’ survival outcome and potential biomarkers for a selection of indicated cases for anti-PD-L1/anti-PD1 immunotherapy in TNBC patients. Especially, inclusion of the evaluations on the two biomarkers in pathology reports may have important clinical values. The definition of PD-L1 expression and lymphocytes infiltration may affect the decision of immunotherapy strategy, and in the end, it can affect TNBC patients’ prognosis.
Funding Statement
This work was supported by the China Medical University Major Construction Project (No. 2017ZDZX05), National Natural Science Foundation of China (81572609 and 81872159), Liaoning Colleges Innovative Talent Support Program (Name: Cancer Stem Cell Origin and Biological Behavior), and Liaoning Province College Student Innovation Training Project (201810159068).
Abbreviations
| TNBC | triple-negative breast cancer |
| TILs | tumor-infiltrating lymphocytes |
| PL-1-ALN | positive level-1-axillary lymph node |
| DFS | disease-free survival |
| ER | estrogen receptor |
| PR | progesterone receptor |
| HER2 | human epidermal growth factor receptor type 2 |
| PD-L1 | programmed death-1 ligand 1 |
| PD-1 | programmed death-1 |
| TBS | Tris-buffered saline |
Acknowledgments
The authors would like to thank Lisha Sun and Hao Zhang for their assistance in data analysis.
References
- 1.Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 2.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P, Narod SA.. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13(15 Pt 1):4429–4434. DOI: 10.1158/1078-0432.ccr-06-3045. [DOI] [PubMed] [Google Scholar]
- 3.Smid M, Wang Y, Zhang Y, Sieuwerts AM, Yu J, Klijn JG, Foekens JA, Martens JW. Subtypes of breast cancer show preferential site of relapse. Cancer Res. 2008;68(9):3108–3114. DOI: 10.1158/0008-5472.can-07-5644. [DOI] [PubMed] [Google Scholar]
- 4.Rouzier R, Perou CM, Symmans WF, Ibrahim N, Cristofanilli M, Anderson K, Hess KR, Stec J, Ayers M, Wagner P, et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res. 2005;11(16):5678–5685. doi: 10.1158/1078-0432.ccr-04-2421. [DOI] [PubMed] [Google Scholar]
- 5.Liedtke C, Mazouni C, Hess KR, André F, Tordai A, Mejia JA, Symmans WF, Gonzalez-Angulo AM, Hennessy B, Green M, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26(8):1275–1281. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 6.Carey LA, Dees EC, Sawyer L, Gatti L, Moore DT, Collichio F, Ollila DW, Sartor CI, Graham ML, Perou CM. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13(8):2329–2334. DOI: 10.1158/1078-0432.ccr-06-1109. [DOI] [PubMed] [Google Scholar]
- 7.Loi S, Michiels S, Salgado R, Sirtaine N, Jose V, Fumagalli D, Kellokumpu-Lehtinen PL, Bono P, Kataja V, Desmedt C, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol. 2014;25(8):1544–1550. doi: 10.1093/annonc/mdu112. [DOI] [PubMed] [Google Scholar]
- 8.Denkert C, Loibl S, Noske A, Roller M, Müller BM, Komor M, Budczies J, Darb-Esfahani S, Kronenwett R, Hanusch C, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28:105–113. DOI: 10.1200/JCO.2009.23.7370. [DOI] [PubMed] [Google Scholar]
- 9.Hornychova H, Melichar B, Tomsova M, Mergancova J, Urminska H, Ryska A. Tumor-infiltrating lymphocytes predict response to neoadjuvant chemotherapy in patients with breast carcinoma. Cancer Invest. 2008;26:1024–1031. DOI: 10.1080/07357900802098165. [DOI] [PubMed] [Google Scholar]
- 10.Yamaguchi R, Tanaka M, Yano A, Tse GM, Yamaguchi M, Koura K, Kanomata N, Kawaguchi A, Akiba J, Naito Y, et al. Tumor-infiltrating lymphocytes are important pathologic predictors for neoadjuvant chemotherapy in patients with breast cancer. Hum Pathol. 2012;43:1688–1694. DOI: 10.1016/j.humpath.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 11.Seo AN, Lee HJ, Kim EJ, Kim HJ, Jang MH, Lee HE, Kim YJ, Kim JH, Park SY. Tumour-infiltrating CD8+ lymphocytes as an independent predictive factor for pathological complete response to primary systemic therapy in breast cancer. Br J Cancer. 2013;109:2705–2713. DOI: 10.1038/bjc.2013.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. DOI: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen L. Han X Anti–PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest. 2015;125(9):3384–3391. DOI: 10.1172/JCI80011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. DOI: 10.1038/nm0902-1039c. [DOI] [PubMed] [Google Scholar]
- 15.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. DOI: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schalper KA, Velcheti V, Carvajal D, Wimberly H, Brown J, Pusztai L, Dl R. In situ tumor PD-L1 mRNA expression is associated with increased TILs and better outcome in breast carcinomas. Clin Cancer Res. 2014;20(10):2773–2782. DOI: 10.1158/1078-0432.CCR-13-2702 15. [DOI] [PubMed] [Google Scholar]
- 17.Ali HR, Glont SE, Blows FM, Provenzano E, Dawson SJ, Liu B, Hiller L, Dunn J, Poole CJ, Bowden S, et al. PD-L1 protein expression in breast cancer is rare, enriched in basal-like tumours and associated with infiltrating lymphocytes. Ann Oncol. 2015;26(7):1488–1493. DOI: 10.1093/annonc/mdv192. [DOI] [PubMed] [Google Scholar]
- 18.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646:74 DOI: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 19.Ghebeh H, Mohammed S, Al-Omair A, Qattan A, Lehe C, Al-Qudaihi G, Elkum N, Alshabanah M, Bin Amer S, Tulbah A, et al. The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia. 2006;8:190–198. DOI: 10.1593/neo.05733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mittendorf EA, Philips AV, Meric-Bernstam F, Qiao N, Wu Y, Harrington S, Su X, Wang Y, Gonzalez-Angulo AM, Akcakanat A, et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014;2(4):361–370. doi: 10.1158/2326-6066.CIR-13-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wimberly H, Brown JR, Schalper K, Haack H, Silver MR, Nixon C, Bossuyt V, Pusztai L, Lannin DR, Rimm DL. PD-L1 expression correlates with tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy in breast cancer. Cancer Immunol Res. 2014;3(4):326–332. DOI: 10.1158/2326-6066.CIR-14-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thompson RH, Kuntz SM, Leibovich BC, Dong H, Lohse CM, Webster WS, Sengupta S, Frank I, Parker AS, Zincke H, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66:3381–3385. DOI: 10.1158/0008-5472.can-05-4303. [DOI] [PubMed] [Google Scholar]
- 23.Thompson RH, Dong H, Kwon ED. Implications of B7-H1 expression in clear cell carcinoma of the kidney for prognostication and therapy. Clin Cancer Res. 2007;13:709s–15s. DOI: 10.1158/1078-0432.ccr-06-1868. [DOI] [PubMed] [Google Scholar]
- 24.Nomi T, Sho M, Akahori T, Hamada K, Kubo A, Kanehiro H, Nakamura S, Enomoto K, Yagita H, Azuma M, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007;13:2151–2157. DOI: DOI: 10.1158/1078-0432.ccr-06-2746. [DOI] [PubMed] [Google Scholar]
- 25.Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104:3360–3365. DOI: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu C, Zhu Y, Jiang J, Zhao J, Zhang XG, Xu N. Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochem. 2006;108:19–24. DOI: 10.1016/j.acthis.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Ohigashi Y, Sho M, Yamada Y, Tsurui Y, Hamada K, Ikeda N, Mizuno T, Yoriki R, Kashizuka H, Yane K, et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res. 2005;11:2947–2953. DOI: 10.1158/1078-0432.ccr-04-1469. [DOI] [PubMed] [Google Scholar]
- 28.Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, Zhou J, Li BZ, Shi YH, Xiao YS, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15:971–979. DOI: 10.1158/1078-0432.CCR-08-1608. [DOI] [PubMed] [Google Scholar]
- 29.Isha Dua AR. Immunotherapy for triple-negative breast cancer: a focus on immune checkpoint inhibitors. Ajho. 2017;13(4):20–27. DOI: 10.1038/s41388-018-0451-5. [DOI] [Google Scholar]
- 30.Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–795. DOI: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 31.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75(2):163–189. DOI: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 32.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5(12):1365–1369. DOI: 10.1016/s0887-7963(00)80157-1. [DOI] [PubMed] [Google Scholar]
- 33.Selenko-Gebauer N, Majdic O, Szekeres A, Höfler G, Guthann E, Korthäuer U, Zlabinger G, Steinberger P, Pickl WF, Stockinger H, et al. B7-H1 (programmed death-1 ligand) on dendritic cells is involved in the induction and maintenance of T cell anergy. J Immunol. 2003;170(7):3637–3644. doi: 10.4049/jimmunol.170.7.3637. [DOI] [PubMed] [Google Scholar]
- 34.Tsushima F, Yao S, Shin T, Flies A, Flies S, Xu H, Tamada K, Pardoll DM, Chen L. Interaction between B7-H1 and PD-1 determines initiation and reversal of T-cell anergy. Blood. 2007;110(1):180–185. DOI: 10.1182/blood-2006-11-060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldberg MV, Maris CH, Hipkiss EL, Flies AS, Zhen L, Tuder RM, Grosso JF, Harris TJ, Getnet D, Whartenby KA, et al. Role of PD-1 and its ligand, B7-H1, in early fate decisions of CD8 T cells. Blood. 2007;110(1):186–192. doi: 10.1182/blood-2006-12-062422. [DOI] [PMC free article] [PubMed] [Google Scholar]


