ABSTRACT
Objective: To compare the clinicopathological features and chemotherapy response of ovarian clear cell carcinoma (CCC) and endometrioid carcinoma (EC) associated with endometriosis or not.
Methods: This was a retrospective study of 128 patients diagnosed with CCC and EC from 2002 to 2017. Clinicopathological features and chemotherapy response were analyzed.
Results:There were 34 women with endometriosis-associated ovarian cancer (EAOC) and 94 with non-endometriosis associated ovarian cancer (non-EAOC) according to Sampson’s and Scott’s criteria. The mean diagnosis age in the EAOC group was 48.65 vs. 54.39 years in non-EAOC (p = 0.002). Compared with non-EAOC, the EAOC patients were more likely to have an earlier menarche age (13 vs. 14 years, p = 0.001), a higher incidence of infertility (26.47% vs. 10.64%, p = 0.026), and an earlier stage tumor (91.18% vs. 73.40% in stages I–II, p = 0.032). At a median follow-up time of 32.9 months, overall survival among patients with EAOC was significantly longer (109.8 vs. 47.4 months, p = 0.007). Association with endometriosis (p = 0.033) was the significant favorable prognostic factors associated with survival. However, stratifying by stage, the overall survival advantage of EAOC was not significant. Although EAOC had a better prognosis, no difference was observed in chemotherapy response between the two groups (p = 0.535).
Conclusions: The EAOC patients were often diagnosed at a younger age, an earlier stage, and related to nulliparity and infertility. Patients with EAOC had a better prognosis than non-EAOC, early stage rather than association with endometriosis may be the driver of survival.
KEYWORDS: Ovarian cancer, endometriosis, clinicopathological features, prognosis, chemotherapy response
Introduction
Ovarian cancer, the gynecological malignancies with the highest mortality rate, is a serious threat to women’s health and life.1 The case numbers are increasing and overall five-year relative survival is below 45%.2 There are around 22,240 new cases of ovarian cancer and 14,070 ovarian cancer deaths expected in the United States in 2018.3 The causes of ovarian cancer remain unclear and many researches have shown that endometriosis is closely associated with the occurrence of ovarian cancer.
Endometriosis is a common gynecologic disorder, with a prevalence of about 5–15% in women of reproductive age. Endometriosis is considered a benign disease, while it has similar characteristics to invasive neoplasms such as uncontrolled growth, neo-angiogenesis, local invasion and distant spread.1,4 Previous studies have demonstrated an increased epithelial ovarian cancer incidence in women with endometriosis.5,6 The longer the duration of endometriosis, the larger the size of endometrioma, the greater is the risk for developing ovarian cancer.7 The risk of endometriosis-associated ovarian cancer (EAOC) varies by histological sub-types and the greatest risk is associated with ovarian endometrioid (EC) and clear cell carcinoma (CCC).8 Although endometriosis has not been considered to be a precancerous lesion, several studies have provided unequivocal evidence that atypical endometriosis is in fact an intermediate lesion between endometriosis and ovarian cancer.9 However, definite mechanisms involved in the malignant transformation remain not entirely understood. In recent years, novel evidences have emerged, improving our understanding of this entity. Many gene mutations including ARID1A, PIK3CA, MET, and HNF1β may contribute to the malignant transformation of endometriosis.10-13 Oxidative stress, inflammation and steroid hormones also participate in the process. Nowadays, a highly controversial issue is whether EAOC is a different disease or whether it is similar to non-endometriosis associated ovarian cancer (non-EAOC). Some studies have showed that EAOC seems to represent a distinct disease entity with different histological subtypes, early presentation and a relatively favorable outcome, whereas others have failed to find such a difference.7,14-16
In this study, 128 patients diagnosed with CCC and EC in our hospital were divided into EAOC group and non-EAOC group according to Sampson’s and Scott’s criteria.17,18 This study aims at analyzing the clinicopathological features and chemotherapy response of EAOC compared with non-EAOC.
Results
According to Sampson’s and Scott’s criteria,17,18 34 were EAOC and 94 patients were non-EAOC. Table 1 summarizes clinicopathological features for both groups. The mean age of patients in EAOC group was 48.65 ± 8.98 years, whereas it was 54.39 ± 9.05 years in non-EAOC group (p = 0.002). The EAOC patients were more likely to have an earlier menarche age (p = 0.001), a higher incidence of infertility (p = 0.026) and a lower incidence of ascites (p = 0.046) than the non-EAOC patients. We also observed significant differences in dysmenorrhea (p = 0.022) and menstrual disorder (p = 0.040) between the two groups, which may explain the reason for earlier diagnosis in EAOC group. There was no obvious difference in pelvic pain (p = 0.408), abdominal distension (p = 0.391), and vaginal bleeding (p = 0.352). The majority of patients (91.18%) in EAOC group presented at earlier stage (p = 0.032) compared with those in non-EAOC group. As the pathological grade of CCC is generally regarded as high grade in the pathological results, we only compared the EC grade in two groups. Low grade tumor (G1) was more common in EAOC group (68.75%) than in non-EAOC group (51.79%), although the difference was not significant (p = 0.228).
Table 1.
Clinicopathological features in patients with CCC and EC associated or not with endometriosis.
| EAOC |
Non-EAOC |
||
|---|---|---|---|
| (n = 34) | (n = 94) | ||
| Clinicopathological features | N(%)/x(_±s/M | N(%)/x(_±s/M | p |
| Age | |||
| Mean ± SD | 48.65 ± 8.98 (32–63) |
54.39 ± 9.05 (31–74) |
0.002* |
| Median age of menarche | 13 | 14 | 0.001* |
| Menopausal status | 0.211 | ||
| Premenopausal | 11 (32.36%) | 42(44.68%) | |
| Postmenopausal | 23 (67.64%) | 52 (55.32%) | |
| Personal history | |||
| Infertility | 9 (26.47%) | 10 (10.64%) | 0.026* |
| Symptoms | |||
| Dysmenorrhea | 15 (44.12%) | 22 (23.40%) | 0.022* |
| Pelvic pain | 18 (52.94%) | 42 (44.68%) | 0.408 |
| Abdominal distension | 14 (41.18%) | 31 (32.40%) | 0.391 |
| Menstrual disorder | 12 (35.29%) | 17 (18.09%) | 0.040* |
| Vaginal bleeding | 6 (17.65%) | 24 (25.53%) | 0.352 |
| Tumor diameter (cm) | 12.15 | 10.00 | 0.100 |
| Histology | 0.207 | ||
| EC | 16 (47.06%) | 56(59.57%) | |
| CCC | 18 (52.94%) | 38(40.43%) | |
| FIGO stage | 0.032* | ||
| I–II | 31 (91.18%) | 69 (73.40%) | |
| III–IV | 3 (8.82%) | 25 (26.60%) | |
| EC grade | 0.228 | ||
| Low grade | 11(68.75%) | 29 (51.79%) | |
| High grade | 5(31.25%) | 27 (48.21%) | |
| Ascites | 15 (44.12%) | 60 (63.83%) | 0.046* |
| Tumor markers | |||
| CA125 | 0.105 | ||
| <35 U/mL | 11(32.35%) | 24 (25.53%) | |
| 35–600 U/mL | 18 (52.94%) | 38 (40.43%) | |
| >600 U/mL | 5(14.71%) | 32 (34.04%) | |
| CA199 (U/mL) | 131.21 | 49.86 | 0.520 |
| HE4 (pmol/L) | 152.00 | 152.00 | 0.369 |
*p < 0.05. EAOC, endometriosis-associated ovarian cancer; non-EAOC, non-endometriosis-associated ovarian cancer; M, median.
Tumor markers in ovarian cancer mainly included serum CA125, CA199, and HE4. Serum CA125 were divided into low (<35 U/mL), middle (35–600 U/mL) and high (>600 U/mL) level. Among 128 patients, the proportion of low and middle level CA125 was higher in EAOC group than in non-EAOC group, whereas it did not show a significant difference (p = 0.105). There was also no difference in CA199 (p = 0.520) and HE4 (p = 0.369) level between the two groups.
After a median follow-up of 32.9 months (range, 0.2–164.7 months), 10 patients (29.41%) in EAOC group versus 31 patients (32.98%) in non-EAOC group died from tumor recurrence or distant metastasis (p = 0.702). Patients with EAOC had longer overall survival time, 109.8 months (95% CI 77.085–142.515) versus 47.4 months (95% CI 39.540–55.260) in non-EAOC patients (p = 0.011). Furthermore, the 5 year Kaplan-Meier estimate of survival rate was 67.8% in 34 patients of EAOC group and was 34.3% in 94 patients of non-EAOC group (Figure 1A, p = 0.009).
Figure 1.
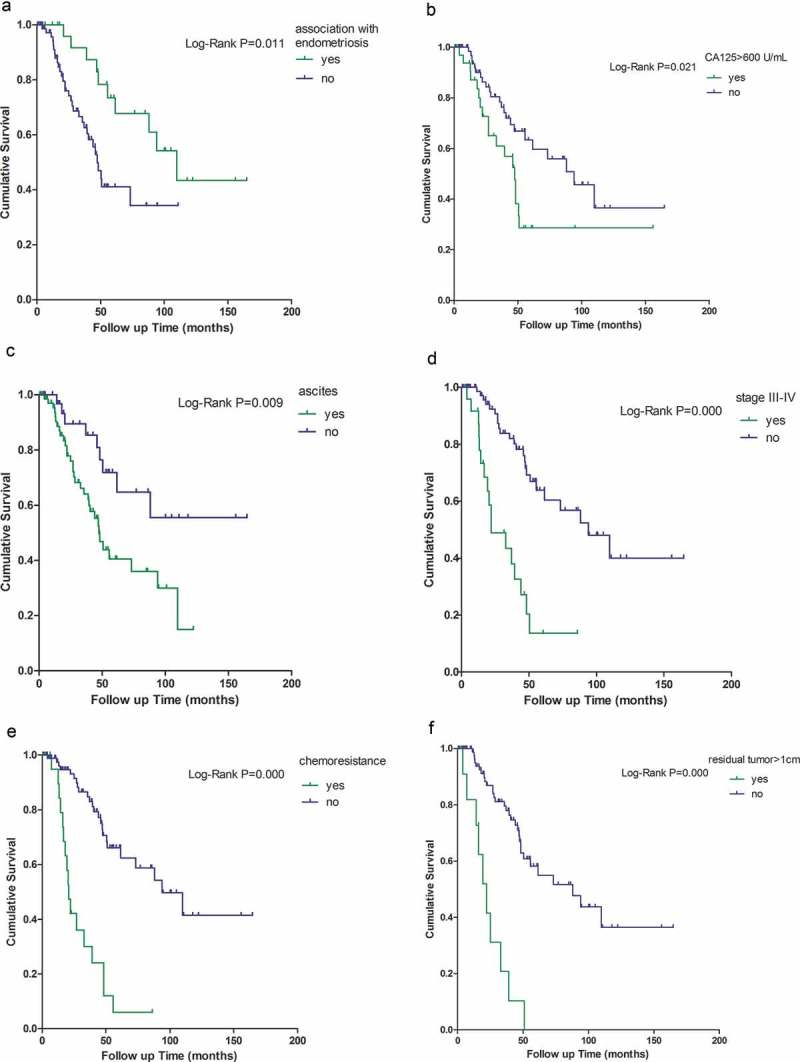
Multi-variable analysis of overall survival.
Kaplan-Meier survival functions in ovarian cancer associated with (A) endometriosis, (B) CA125 > 600 U/mL, (C) ascites, (D) at stage III–IV, (E) chemo-resistance, and (F) residual tumor>1cm. Log-rank p < 0.05 makes a significant difference at five-year survival. The censored cases precludes unequivocal conclusion.
The Kaplan-Meier survival analysis showed that CA125 > 600 U/mL, presence of ascites, advanced stage (III–IV), residual tumor>1cm, and chemo-resistance were risk factors for survival, whereas association with endometriosis was a protective factor for survival (Figure 1). Cox regression analysis further confirmed that ascites (p = 0.045, HR = 2.274, 95%CI 1.020–5.069), stages III–IV (p = 0.001, HR = 3.743, 95%CI 1.683–8.327), residual tumor≥1cm (p = 0.004, HR = 3.423, 95%CI 1.470–7.970), and chemo-resistance (p = 0.000, HR = 4.300, 95% CI 1.932–9.570) could increase the death hazard ratio, but association with endometriosis (p = 0.033, HR = 0.423, 95%CI 0.192–0.933) could decrease the death hazard ratio (Table 2). The potential bias should not be ignored because early stage tumor (stages I–II) was more common in EAOC group. We further performed multi-variable Cox regression analysis after stratifying by stage, and then found association with endometriosis had no statistically significant impact on the survival (Table 3). Association with endometriosis was not an independent predictor of better survival in ovarian cancer. Chemo-resistance is related to poor prognosis of ovarian cancer patients. Next, multi-variable analysis was performed to seek factors affecting chemo-resistance. Histological type of CCC (p = 0.016, OR = 4.364, 95%CI 1.312–14.523), CA125 > 600 U/mL (p = 0.038, OR = 3.782, 95%CI 1.078–13.274), and residual tumor > 1cm (p = 0.008, OR = 8.262, 95%CI 1.743–39.155) might be high risk factors for chemo-resistance. Association with endometriosis (p = 0.535) and advanced stage (p = 0.211) seemed not relevant with chemo-resistance (Table 4).
Table 2.
Variable analysis of overall survival.
| Kaplan–Meier |
Cox-regression | |||
|---|---|---|---|---|
| test |
Analysis |
|||
| variable | Log-rank p p value |
p value |
HR | 95%CI |
| Association with endometriosis | 0.011* | 0.033* | 0.423 | 0.192–0.933 |
| Serum CA125 > 600 U/mL | 0.021* | 0.346 | 0.702 | 0.337–1.464 |
| Ascites | 0.009* | 0.045* | 2.274 | 1.020–5.069 |
| Stages III–IV | 0.000* | 0.001* | 3.743 | 1.683–8.327 |
| Chemo-resistance | 0.000* | 0.000* | 4.300 | 1.932–9.570 |
| Residual tumor > 1 cm | 0.000* | 0.004* | 3.423 | 1.470–7.970 |
| Histological type of CCC | 0.610 | – | – | – |
*p < 0.05. HR, hazard ratio; CI, confidence interval.
Table 3.
Variable analysis of overall survival after stratifying for stage.
| Variable | Cox-regression analysis |
||
|---|---|---|---|
| p value | HR | 95%CI | |
| Stages I–II | |||
| Association with endometriosis | 0.162 | 0.504 | 0.193–1.318 |
| Serum CA125 > 600 U/mL | 0.725 | 0.834 | 0.304–2.291 |
| Ascites | 0.063 | 2.731 | 0.945–7.889 |
| Chemo-resistance | 0.003* | 4.300 | 1.633–11.323 |
| Residual tumor > 1 cm | 0.040* | 3.479 | 1.062–11.400 |
| Stages III–IV | |||
| Association with endometriosis | 0.037* | 0.068 | 0.005–0.848 |
| Serum CA125 > 600 U/mL | 0.527 | 0.656 | 0.178–2.417 |
| Ascites | 0.481 | 1.779 | 0.359–8.822 |
| Chemo-resistance | 0.007* | 10.120 | 1.882–54.415 |
| Residual tumor > 1 cm | 0.027* | 3.653 | 1.156–11.539 |
*p < 0.05. HR, hazard ratio; CI, confidence interval.
Table 4.
Variable analysis of chemo-resistance.
| variable | Logistic regression analysis |
||
|---|---|---|---|
| p | OR | 95%CI | |
| Association with endometriosis | 0.535 | 1.494 | 0.421–5.299 |
| Histological type of CCC | 0.016* | 4.364 | 1.312–14.523 |
| Serum CA125 > 600 U/mL | 0.038* | 3.782 | 1.078–13.274 |
| Residual tumors>1 cm | 0.008* | 8.262 | 1.743–39.155 |
| Stages III–IV | 0.211 | 2.277 | 0.628–8.260 |
*p < 0.05. OR, odds ratio; CI, confidence interval.
Discussion
CCC and EC were considered as “EAOC”, and thus our study mainly focused on the two histologic sub-types. In our series, the proportion of EAOC was 26.6% which is lower than that reported (33.3%) in the literature. Due to limited sampling for pathologic evaluation, it is not extensive enough to detect all foci of endometriosis adjacent to tumors. Furthermore, some ovarian tumors overgrow rapidly and replaced the original ectopic endometrium, which may lead to the disappearance of histological transition region from endometriosis into ovarian cancer.
Many studies have been performed in order to understand whether EAOC is a clinically distinct entity from non-EAOC. According to the currently accepted dualistic model of Kurman and Shih for the pathogenesis of epithelial ovarian cancer,19 type-I tumors comprise low-grade serous, low-grade endometrioid, clear cell, mucinous carcinomas and malignant Brenner tumors, which are usually developed in a stepwise fashion from well-established precursor lesions such as cystadenofibroma, borderline tumors, and endometriosis. Type-I tumors typically present as large masses confined to one ovary, with low stage, and have a good prognosis. Tumor cells are characterized by mutations of KRAS, BRAF, PTEN, PIK3A, ARID1A, CTNNB1 and microsatellite instability, but rare p53 activation and low chromosomal instability.20,21 In contrast, type-II tumors comprise high-grade serous, high-grade endometrioid, malignant mixed mesodermal tumors (carcinosarcomas), and undifferentiated carcinomas. They are not associated with the morphologically detectable precursor lesions but highly aggressive present in advanced stage.
The EAOC patients were more likely to exhibit the favorable features of type I tumors, often being diagnosed at a younger age, an earlier stage, a better prognosis, and are related to nulliparity and infertility.22,23 Although there was no significant difference in EC grade between the two groups due to small sample size, the trend could be observed from lower grade tumors in EAOC that type-I tumors were more frequent among those with EAOC; on the contrary, type-II tumors were more frequent high-grade in the non-EAOC group. The interpretation of these potential observations on clinicopathological features was that EAOC might represent a single entity distinct from ovarian cancer without endometriosis.
In this study, EAOC was significantly more frequently diagnosed at an early stage, consistent with previous findings. As one typical symptom of patients with endometriosis, the incidence of dysmenorrhea in the group of EAOC was higher than non-EAOC. It is possible that women with associated endometriosis seek medical help because of their symptoms and were incidentally found to have ovarian cancer; on the other hand, patients without associated endometriosis may have significant symptoms only in later stages. That could explain why most EAOC patients were diagnosed at an earlier stage. We also found menarche age in females of EAOC group was earlier than that of non-EAOC group. A meta-analysis suggested that early age at menarche was associated with a very modest increase in endometriosis risk.24 Earlier menarche age may increase opportunities for retrograde menstruation, resulting in the increased incidence of endometriosis and thus EAOC. Infertility was another symptom of endometriosis and infertility rate in ovarian cancer with endometriosis was significantly higher than the group without endometriosis.25 Brinton found that among infertile women, a significantly higher prevalence of ovarian cancer in these patients with endometriosis than the general population without endometriosis.26 Stewart further reported that the risk of ovarian cancer was decreased if women diagnosed with endometriosis had children.27
Serum CA125 is the most commonly measured tumor marker for epithelial ovarian cancers. It is also elevated in conditions of endometriosis, uterine adenomyosis, pelvic inflammatory disease. We recorded no statistically significant difference in the preoperative serum CA125 between the EAOC and non-EAOC groups. This is consistent with previous studies.28,29 While Wang and Lim found that patients with EAOCs had significantly lower levels of CA125,30,31 a high level (over 200 U/mL) and/or rapid rise of serum CA125 levels should make one highly suspicious of the eventual development of EAOC in a woman with endometriosis.32 In addition to CA125, human epididymis protein 4 (HE4) is also used in ovarian cancer screening. HE4 is highly elevated in specific subtypes of ovarian cancers.33 In our study, no significant differences of HE4 level were observed in the two groups. One possible reason is that serum HE4 is elevated in malignant ovarian diseases, whereas it remains normal in all benign lesions, including endometriosis.33
In patients with EAOCs, a significantly longer overall survival was recorded, both in our and in other studies.1,22 Meanwhile, we observed a higher 5-year survival rate for EAOC compared with the non-EAOC group. In multi-variable analysis of overall survival, association with endometriosis and advanced stages (III–IV) had a statistically significant impact on the survival. To understood further whether the impact of association with endometriosis on the survival was unexpected due to stage variance, multi-variable Cox regression analysis of survival after stratifying for stage was chosen. We had not found survival difference at both early and advanced stage. The best explanation for this is that association with endometriosis leads to a higher prevalence of early-stage and low-grade tumors, and thus a much better survival rate than non-EAOC. These survival analysis findings showed that stage at diagnosis seems to be more important to prognosis than association with endometriosis alone, which is consistent with findings of previous studies.34
Poor prognosis of epithelial ovarian cancers is closely related to chemo-resistance. In our study, we found that chemo-resistance is a risk factor for overall survival. For patients with epithelial ovarian cancer, recommended postoperative treatment is still the standard intravenous taxane-carboplatin regimens (with paclitaxel or docetaxel).35 Most CCC patients are resistant to taxane, or platinum-based chemotherapy, which may be associated with decreased drug accumulation, increased drug detoxification, an increased DNA repair activity, and low cell proliferation.36 As endometriosis develops to EAOC, many resistance-related protein expressions such as mismatch repair protein, hMLH1 protein, and XRCC5 may change, and thus decrease drug resistance.37-39 However, no significant difference in chemo-resistance was observed between EAOC and non-EAOC groups as to parity via logistic regression analysis in our study (Table 4). One possible reason is the small sample size. The other possible reason is that we may actually have missed some cases of EAOC because of lacking malignant transformation point. The relationship of association with endometriosis in ovarian cancers and chemo-resistance needs to be further explored to look for potential molecular targets for decreasing clinical drug resistance.
In conclusion, EAOC has different clinicopathological features compared to non-EAOC. EAOC appears to confer a better overall survival which may be related to the earlier stage. Despite the fact that no differences in chemo-resistance are observed, detection of the resistance-related proteins may be a basis for a targeted therapy, and the clinical and therapeutic significance of these findings need be supported by further research.
Patients and methods
The study includes patients with CCC and EC diagnosed in the department of obstetrics and gynecology of Tianjin Medical University General Hospital between 2002 and 2017. All patients underwent hysterectomy, bilateral salpingo-oophorectomy, omentectomy, and lymph node resection at our center according to national comprehensive cancer network (NCCN) guideline. Most patients received platinum-based chemotherapy after surgery. Patients were considered to be chemotherapy-resistant if the duration from completion of the last regimen to tumor recurrence was less than 6 months or tumor progressed during chemotherapy. Patients receiving neoadjuvant chemotherapy were excluded. The study was approved by the Institute Research Ethics Committee of Tianjin Medical University General Hospital.
A histologic review of all cases was performed by experienced gynecologic pathologists at our institution. The criteria defined cases associated with endometriosis which included that: (1) tumor is adjacent to unequivocal foci of endometriosis, (2) absence of any other primary tumor, (3) the presence of tissue resembling endometrial stroma surrounding epithelial glands.17 Scott added a transition from endometriosis to neoplastic epithelium (or stromal component) to the criteria.18 According to Sampson’s and Scott’s criteria, cases were divided into two groups. Thirty-four of the patients were found to be associated with endometriosis, the remaining ninety-four patients were considered as ovarian cancer not associated with endometriosis. All patients were followed up to December 2017.
The following data were collected in a tabulated manner: age, personal history, symptoms, presence or absence of ascites, diameter of tumor, pathological type, grade, and stage of the tumor, and preoperative level of serum CA125 and HE4.
Statistical analysis was performed for determining the significance values using SPSS 21.0 (IBM Corp.,Armonk,NY,USA). A descriptive analysis was performed to describe the two groups. Differences in means for age were evaluated for significance using two independent sample t tests. The non-normal distribution data such as menarche, tumor diameter, CA199, and HE4 level, was evaluated for significance using the Mann-Whitney test. The difference in proportions for categorical variables, including menopausal status, infertility, dysmenorrhea, pelvic pain, abdominal distension, menstrual disorder, vaginal bleeding, histology, FIGO stage, EC grade, and ascites were evaluated for significance using χ2 test or Fisher exact test. Differences for ranked data like CA125 level were evaluated for significance using the rank-sum test. Kaplan–Meier test and Cox regression analysis were used to determine survival prognostic factors. Multiple factors of chemotherapy response were analyzed using logistic regression model. A p value <0.05 was accepted as statistically significant.
Funding Statement
This work was supported by the National Natural Science Foundation of China [grant number 81472761 to GL] and the Natural Science Foundation of Tianjin City [grant number 14JCYBJC25300 to GL].
Acknowledgments
The authors thank Wenjing Song and Jing Yang in the department of pathology of Tianjin Medical University General Hospital. They are the experienced gynecologic pathologists and responsible for the pathological reports of the patients.
Conflict of interest
The authors are financially independent and have no potential conflict of interest.
References
- 1.Kumar S, Munkarah A, Arabi H, Bandyopadhyay S, Semaan A, Hayek K, Garg G, Morris R, Ali-Fehmi R.. Prognostic analysis of ovarian cancer associated with endometriosis. Am J Obstet Gynecol. 2011;204:63.e1. doi: 10.1016/j.ajog.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 2.Webb PM, Jordan SJ. Epidemiology of epithelial ovarian cancer. Best Pract Res Cl Ob. 2017;41:3. doi: 10.1016/j.bpobgyn.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 4.Gorp TV, Amant F, Neven P, Vergote I, Moerman P. Endometriosis and the development of malignant tumours of the pelvis. A Review of Literature. Best Pract Res Cl Ob. 2004;18:349–371. doi: 10.1016/j.bpobgyn.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Vercellini P, Viganò P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 2014;10:261. doi: 10.1038/nrendo.2013.255. [DOI] [PubMed] [Google Scholar]
- 6.Somigliana E, Vigano’ P, Parazzini F, Stoppelli S, Giambattista E, Vercellini P. Association between endometriosis and cancer: a comprehensive review and a critical analysis of clinical and epidemiological evidence. Gynecol Oncol. 2006;101:331–341. doi: 10.1016/j.ygyno.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 7.Nagle CM, Olsen CM, Webb PM, Jordan SJ, Whiteman DC, Green AC. Endometrioid and clear cell ovarian cancers: a comparative analysis of risk factors. Eur J Cancer. 2008;44:2477. doi: 10.1016/j.ejca.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Pearce CL, Templeman C, Rossing MA, Lee A, Near AM, Webb PM, Nagle CM, Doherty JA, Cushing-Haugen KL, Wicklund KG. Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case-control studies. Lancet Oncol. 2012;13:385–394. doi: 10.1016/S1470-2045(11)70404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moll UM, Chumas JC, Chalas E, Mann WJ. Ovarian carcinoma arising in atypical endometriosis. Obstet Gynecol. 1990;75:537–539. [PubMed] [Google Scholar]
- 10.Siufi NJ, Kho RM, Siufi DF, Baracat EC, Anderson KS, Abrão MS. Cellular, histologic, and molecular changes associated with endometriosis and ovarian cancer. J Minim Invasive Gynecol. 2014;21:55–63. doi: 10.1016/j.jmig.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 11.Pavone ME, Lyttle BM. Endometriosis and ovarian cancer: links, risks, and challenges faced. Int J Womens Health. 2015;7:663–672. doi: 10.2147/IJWH.S66824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krawczyk N, Banys-Paluchowski M, Schmidt D, Ulrich U, Fehm T. Endometriosis-associated Malignancy. Geburtshilfe Frauenheilkd. 2016;76:176. doi: 10.1055/s-0035-1558239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farfaras A, Danielis M, Papavasileiou S, Barbounaki S, Bilirakis E, Skolarikos P. Insight into the mechanisms of endometriosis associated ovarian cancers development. Cancer Res Front. 2016;2:112–125. doi: 10.17980/2015.1. [DOI] [Google Scholar]
- 14.Scarfone G, Bergamini A, Noli S, Villa A, Cipriani S, Taccagni G, Vigano’ P, Candiani M, Parazzini F, Mangili G. Characteristics of clear cell ovarian cancer arising from endometriosis: a two center cohort study. Gynecol Oncol. 2014;133:480. doi: 10.1016/j.ygyno.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 15.Kim HS, Chung HH, Kim JW, Park NH, Song YS. ISO–4–1 Risk and prognosis of ovarian cancer in women with endometriosis: a meta-analysis(group 4 oncology 2,IS Award Candidate,International Session). J Bacteriol. 2001;182:6975–6982. [Google Scholar]
- 16.Depriest PD, Banks ER, Powell DE, Jr JRVN, Gallion HH, Puls LE, Hunter JE, Kryscio RJ, Royalty MB. Endometrioid carcinoma of the ovary and endometriosis: the association in postmenopausal women. Gynecol Oncol. 1992;47:71–75. [DOI] [PubMed] [Google Scholar]
- 17.Sampson JA. Endometrial carcinoma of the ovary arising in endometrial tissue in that organ. Am J Obstet Gynecol. 1925;9:111–114. doi: 10.1016/S0002-9378(25)90949-0. [DOI] [Google Scholar]
- 18.Scott RB. Malignant changes in endometriosis. Obstet Gynecol. 1953;2:283. [PubMed] [Google Scholar]
- 19.Kurman RJ, Ie-Ming S. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer–shifting the paradigm. Hum Pathol. 2011;42:918–931. doi: 10.1016/j.humpath.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones S, Wang TL, Iem S, Mao TL, Nakayama K, Roden R, Glas R, Slamon D, Jr DL, Vogelstein B. Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science. 2010;330:228. doi: 10.1126/science.1196333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wiegand KC, Shah SP, Al-Agha OM, Zhao Y, Tse K, Zeng T, Senz J, McConechy MK, Anglesio MS, Kalloger SE, et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med. 2010;363:1532–1543. doi: 10.1056/NEJMoa1008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim HS, Kim TH, Chung HH, Song YS. Risk and prognosis of ovarian cancer in women with endometriosis: a meta-analysis. Br J Cancer. 2014;110:1878–1890. doi: 10.1038/bjc.2014.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mangili G, Bergamini A, Taccagni G, Gentile C, Panina P, Viganò P, Candiani M. Unraveling the two entities of endometrioid ovarian cancer: A single center clinical experience. Gynecol Oncol. 2012;126:403–407. doi: 10.1016/j.ygyno.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Nnoaham KE, Premila W, Jharna K, Kennedy SH, Zondervan KT. Is early age at menarche a risk factor for endometriosis? A systematic review and meta-analysis of case-control studies. Fertil Steril. 2012;98:702–712. doi: 10.1016/j.fertnstert.2012.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kobayashi H, Sumimoto K, Moniwa N, Imai M, Takakura K, Kuromaki T, Morioka E, Arisawa K, Terao T. Risk of developing ovarian cancer among women with ovarian endometrioma: a cohort study in Shizuoka, Japan. Int J Gynecol Cancer. 2007;17:37–43. doi: 10.1111/j.1525-1438.2006.00754.x. [DOI] [PubMed] [Google Scholar]
- 26.Brinton LA, Lamb EJ, Moghissi KS, Scoccia B, Althuis MD, Mabie JE, Westhoff CL. Ovarian cancer risk associated with varying causes of infertility. Fertil Steril. 2004;82:405–414. doi: 10.1016/j.fertnstert.2004.02.109. [DOI] [PubMed] [Google Scholar]
- 27.Stewart LM, Holman CD, Aboagyesarfo P, Finn JC, Preen DB, Hart R. In vitro fertilization, endometriosis, nulliparity and ovarian cancer risk. Gynecol Oncol. 2013;128:260–264. doi: 10.1016/j.ygyno.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 28.Komiyama SI, Aoki D, Tominaga E, Susumu N, Udagawa Y, Nozawa S. Prognosis of Japanese patients with ovarian clear cell carcinoma associated with pelvic endometriosis: clinicopathologic evaluation. Gynecol Oncol. 1999;72:342–346. [DOI] [PubMed] [Google Scholar]
- 29.Davis M, Rauh-Hain JA, Andrade C, Ii DMB, Schorge JO, Horowitz NS, May T, Carmen MGD. Comparison of clinical outcomes of patients with clear cell and endometrioid ovarian cancer associated with endometriosis to papillary serous carcinoma of the ovary. Gynecol Oncol. 2014;132:760–766. [DOI] [PubMed] [Google Scholar]
- 30.Wang S, Qiu L, Lang JH, Shen K, Yang JX, Huang HF, Pan LY, Wu M. Clinical analysis of ovarian epithelial carcinoma with coexisting pelvic endometriosis. Am J Obstet Gynecol. 2013;208:413.e1. doi: 10.1016/j.ajog.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 31.Lim MC, Lee DO, Kang S, Seo SS, Lee BY, Park SY. Clinical manifestations in patients with ovarian clear cell carcinoma with or without co-existing endometriosis. Gynecol Endocrinol. 2009;25:435–440. doi: 10.1080/09513590902770131. [DOI] [PubMed] [Google Scholar]
- 32.Andersen MR, Goff BA, Lowe KA, Scholler N, Bergan L, Drescher CW, Paley P, Urban N. Use of a Symptom Index, CA125, and HE4 to predict ovarian cancer. Gynecol Oncol. 2010;116:378. doi: 10.1016/j.ygyno.2009.10.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drapkin R, Horsten HHV, Lin Y, S MokC, C CrumP, W WelchR, Hecht JL. Human epididymis protein 4 (HE4) is a secreted glycoprotein that is overexpressed by serous and endometrioid ovarian carcinomas. Cancer Res. 2005;65:2162–2169. doi: 10.1158/0008-5472.CAN-04-3924. [DOI] [PubMed] [Google Scholar]
- 34.Ma CLK. Figo stage, histology, histologic grade, age and race as prognostic factors in determining survival for cancers of the female gynecological system: an analysis of 1973-87 SEER cases of cancers of the endometrium, cervix, ovary, vulva, and vagina. Semin Surg Oncol. 2010;10:31–46. [DOI] [PubMed] [Google Scholar]
- 35.Magazzino F, Katsaros D, Ottaiano A, Gadducci A, Pisano C, Sorio R, Rabaiotti E, Scambia G, Cormio G, Scarampi L. Surgical and medical treatment of clear cell ovarian cancer: results from the Multicenter Italian Trials in Ovarian Cancer (MITO) 9 retrospective study. J Hepatol. 2011;56:567. [DOI] [PubMed] [Google Scholar]
- 36.Itamochi H, Kigawa J, Terakawa N. Mechanisms of chemoresistance and poor prognosis in ovarian clear cell carcinoma. Cancer Sci. 2008;99:653. doi: 10.1111/j.1349-7006.2008.00747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fuseya C, Horiuchi A, Hayashi A, Suzuki A, Miyamoto T, Hayashi T, Shiozawa T. Involvement of pelvic inflammation-related mismatch repair abnormalities and microsatellite instability in the malignant transformation of ovarian endometriosis. Hum Pathol. 2012;43:1964–1972. doi: 10.1016/j.humpath.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 38.Ren F, Wang D, Jiang Y, Ren F. Epigenetic inactivation of hMLH1 in the malignant transformation of ovarian endometriosis. Arch Gynecol Obstet. 2012;285:215–221. doi: 10.1007/s00404-011-1922-x. [DOI] [PubMed] [Google Scholar]
- 39.Jr WM, Liu S, Hua Y, Kwok JS, Samuel A, Hou L, Shoni M, Lu S, Sandberg EM, Keryan A. Molecular changes in endometriosis-associated ovarian clear cell carcinoma. Eur J Cancer. 2015;51:1831–1842. doi: 10.1016/j.ejca.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]


