Abstract
Evolutionary studies are often limited by missing data that are critical to understanding the history of selection. Selection experiments, which reproduce rapid evolution under controlled conditions, are excellent tools to study how genomes evolve under selection. Here we present a genomic dissection of the Longshanks selection experiment, in which mice were selectively bred over 20 generations for longer tibiae relative to body mass, resulting in 13% longer tibiae in two replicates. We synthesized evolutionary theory, genome sequences and molecular genetics to understand the selection response and found that it involved both polygenic adaptation and discrete loci of major effect, with the strongest loci tending to be selected in parallel between replicates. We show that selection may favor de-repression of bone growth through inactivating two limb enhancers of an inhibitor, Nkx3-2. Our integrative genomic analyses thus show that it is possible to connect individual base-pair changes to the overall selection response.
Research organism: Mouse
eLife digest
Humans have been making use of artificial selection for thousands of years. Much of what we eat, for example, from beef to poultry to cereals, comes from a collection of organisms with genomes that have been completely reshaped by the actions of generations of farmers and breeders. Yet, despite decades of research in evolutionary biology, it remains difficult to predict what will happen to an organism’s genes when selective pressure is applied.
Traits that at first seem simple often arise from layers upon layers of complexity. It can take hundreds if not thousands of tiny changes to many genes, plus just the right alterations to a few key ones, to have a desired effect on a single trait. Also, if you consider that often the genomes of the starting population are unknown and that many traits are under simultaneous selection in wild populations, it becomes clear why many questions remain unanswered.
Castro, Yancoskie, et al. have analyzed an on-going laboratory experiment dubbed “the Longshanks experiment” to explore how an animal’s genome changes under strong selection. Over five years, two independent populations of mice were selectively bred to have longer legs. In each generation, the mice were measured and those with the longest tibia – a bone in the shin – relative to their body mass were allowed to breed. Genetic data were also recorded. Now, Castro, Yancoskie, et al. have analyzed the genetic data up to the first 17 generations in the Longshanks experiment to find out what kind of genes may be relevant to the 13% increase in leg length seen in the mice so far.
This analysis uncovered many genes, possibly thousands, all acting in concert to increase tibia length. But the gene with the largest effect by far was a key developmental gene called Nkx3-2. Mutations in this gene cause a disease called spondylo-megaepiphyseal-metaphyseal dysplasia in people, which can lead to long limbs and a short trunk. Although inactivating this gene completely in mice is lethal, among the founding group of mice in the Longshanks experiment was a rare copy of Nkx3-2. This copy of the gene worked perfectly in all tissues with the exception of the legs, where a genetic switch that controls it was left in the “off” state. Mice inheriting this short stretch of DNA ended up having longer tibia. In effect, these mice held the winning ticket in the genetic lottery that was the Longshanks experiment.
Even in highly controlled experiments that record a great deal of information about the organisms involved, predicting how the genome will change and which genes will be involved is not a straightforward question. Finding out how the genome may change in response to selection is important because scientists can build better models to help with breeding farm animals or crops, or with predicting the consequences of climate change. As a result, experiments such as these may have broad applications in conservation, genomic medicine and agriculture.
Introduction
Understanding how populations adapt to a changing environment is an urgent challenge of global significance. The problem is especially acute for mammal populations, which are often small and fragmented due to widespread habitat loss. Such populations often show increased inbreeding, leading to the loss of genetic diversity (Hoffmann and Sgrò, 2011). Because beneficial alleles in mammals typically come from standing genetic variation rather than new mutations like in microbes, this loss of diversity would ultimately impose a limit on the ability of small populations to adapt. Nonetheless, mammals respond readily to selection in many traits, both in nature and in the laboratory (Darwin, 1859; Gingerich, 2001; Garland and Rose, 2009; Keightley et al., 2001). In quantitative genetics, such traits are interpreted as the overall effect from a large set of loci, each with an infinitesimally small (and undetectable) effect (‘infinitesimal model’). Broadly speaking, the infinitesimal model has performed remarkably well across a wide range of selection experiments, and the model is the basis for commercial breeding (Walsh and Lynch, 2018). However, it remains unclear what type of genomic change is associated with rapid response to selection, especially in small populations where allele frequency changes can be dominated by random genetic drift.
While a large body of theory exists to describe the birth, rise and eventual fixation of adaptive variants under diverse selection scenarios (Maynard Smith and Haigh, 1974; Barton, 1995; Otto and Barton, 2001; Weissman and Barton, 2012; Crow and Kimura, 1965; Hill and Robertson, 1966), few empirical datasets capture sufficient detail on the founding conditions and selection regime to allow full reconstruction of the selection response. This is particularly problematic in nature, where historical samples, environmental measurements and replicates are often missing. Selection experiments, which reproduce rapid evolution under controlled conditions, are therefore excellent tools to understand response to selection—and by extension—adaptive evolution in nature (Garland and Rose, 2009).
Here we describe an integrative, multi-faceted investigation into an artificial selection experiment, called Longshanks, in which mice were selected for increased tibia length relative to body mass (Marchini et al., 2014). The mammalian limb is an ideal model to study the dynamics of complex traits under selection: it is both morphologically complex and functionally diverse, reflecting its adaptive value; and limb development has been studied extensively in mammals, birds and fishes as a genetic and evolutionary paradigm (Petit et al., 2017). The Longshanks selection experiment thus offers the opportunity to study selection response not only from a quantitative and population genetics perspective, but also from a developmental (Marchini and Rolian, 2018) and genomic perspective.
By design, the Longshanks experiment preserves a nearly complete archive of the phenotype (trait measurements) and genotype (via tissue samples) in the pedigree. Previously, Marchini et al. investigated how selection was able to overcome correlation between tibia length and body mass and produced independent changes in tibia length during the first 14 generations of the Longshanks experiment (Marchini et al., 2014). Importantly, that study focused on the phenotypes and inferred genetic correlations indirectly using the pedigree. The current genomic analysis was initiated when the on-going experiment reached generation 17 and extends the previous study by integrating both phenotypic and genetic aspects of the Longshanks experiment. By sequencing the initial and final genomes, the current analysis benefits from direct and highly resolved genetic information. Here, with essentially complete information, we wish to answer a number of important questions regarding the factors that determine and constrain rapid adaptation: Are the observed changes in gene frequency due to selection or random drift? Does rapid selection response of a complex trait proceed through innumerable loci of infinitesimally small effect, or through a few loci of large effect? What type of signature of selection may be associated with this process? Finally, when the same trait changes occur independently, do these depend on changes in the same gene(s) or the same pathways (parallelism)?
Results
Longshanks selection for longer tibiae
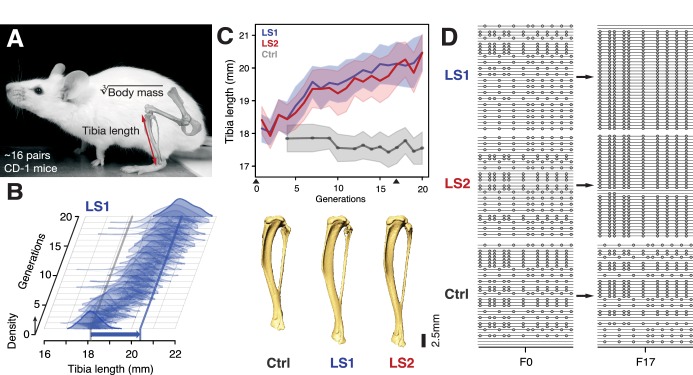
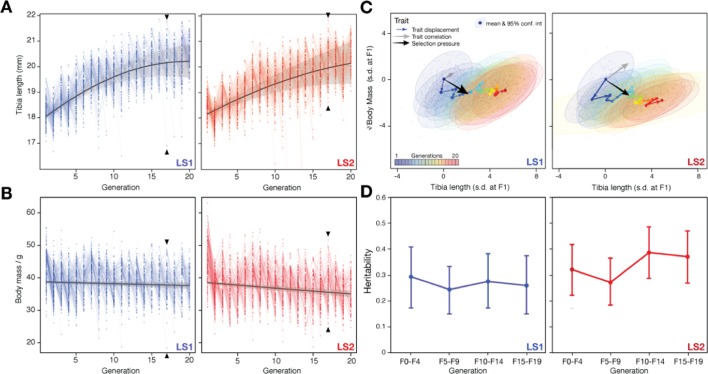
At the start of the Longshanks experiment, we established three base populations with 14 pairs each by sampling from a genetically diverse, commercial mouse stock (Hsd:ICR, also known as CD-1; derived from mixed breeding of classical laboratory mice [Yalcin et al., 2010]). In two replicate ‘Longshanks’ lines (LS1 and LS2), we bred mice by pairing 16 males and females (and excluding sibling pairs) with the longest tibia relative to the cube root of body mass for each sex. This corresponds to 15–20% of all offspring (only details essential to understanding our analysis are summarized here. See Marchini et al., 2014 for a detailed description of the breeding scheme). We kept a third Control line (Ctrl) using an identical breeding scheme, except that breeders were selected at random. In LS1 and LS2, we observed a strong and significant response to selection in tibia length (0.29 and 0.26 Haldane or standard deviations (s.d.) per generation, from a selection differential of 0.73 s.d. in LS1 and 0.62 s.d. in LS2). Over 20 generations, selection for longer relative tibia length produced increases of 5.27 and 4.81 s.d. in LS1 and LS2, respectively (or 12.7% and 13.1% in tibia length), with a modest decrease in body mass (−1.5% in LS1 and −3.7% in LS2; Student’s t-test, p<2 × 10−4 and p<1 × 10−8, respectively; Figure 1B and C; Figure 1—figure supplement 1; n.b. this relationship was in part biased by the F1 generation, which were fed a different diet and phenotyped three weeks later than later generations, see Marchini et al., 2014 for details). By contrast, Ctrl showed no directional change in tibia length or body mass (Figure 1C; Student’s t-test, p>0.05). This approximately 5 s.d. change in 20 generations is rapid compared to typical rates observed in nature (Hendry and Kinnison, 1999, but see Grant and Grant, 2002) but is in line with responses seen in selection experiments (Gingerich, 2001; Keightley et al., 2001; Falconer and Mackay, 1996; Pitchers et al., 2014).
Figure 1. Selection for Longshanks mice produced rapid increase in tibia length.
(A and B) Tibia length varies as a quantitative trait among outbred mice derived from the Hsd:ICR (also known as CD-1) commercial stock. Selective breeding for mice with the longest tibiae relative to body mass within families has produced a strong selection response in tibia length over 20 generations in Longshanks mice (13%, blue arrow, LS1). (C) Both LS1 and LS2 produced replicated rapid increase in tibia length (blue and red; line and shading show mean ±s.d.) compared to random-bred Controls (gray). Arrowheads along the x-axis mark sequenced generations F0 and F17. See Figure 1—figure supplement 1 for body mass data. Lower panel: Representative tibiae from the Ctrl, LS1 and LS2 after 20 generations of selection. (D) Analysis of sequence diversity data (linked variants or haplotypes: lines; variants: dots) may detect signatures of selection, such as selective sweeps (F17 in LS1 and LS2) that result from selection favoring a particular variant (dots), compared to neutral or background patterns (Ctrl). Alternatively, selection may elicit a polygenic response, which may involve minor shifts in allele frequency at many loci and therefore may leave a very different selection signature from the one shown here.
Figure 1—figure supplement 1. Artificial selection allowed detailed reconstruction of selection parameters.
Figure 1—figure supplement 2. Simulating selection on pedigrees.
Simulating selection response: infinitesimal model with linkage
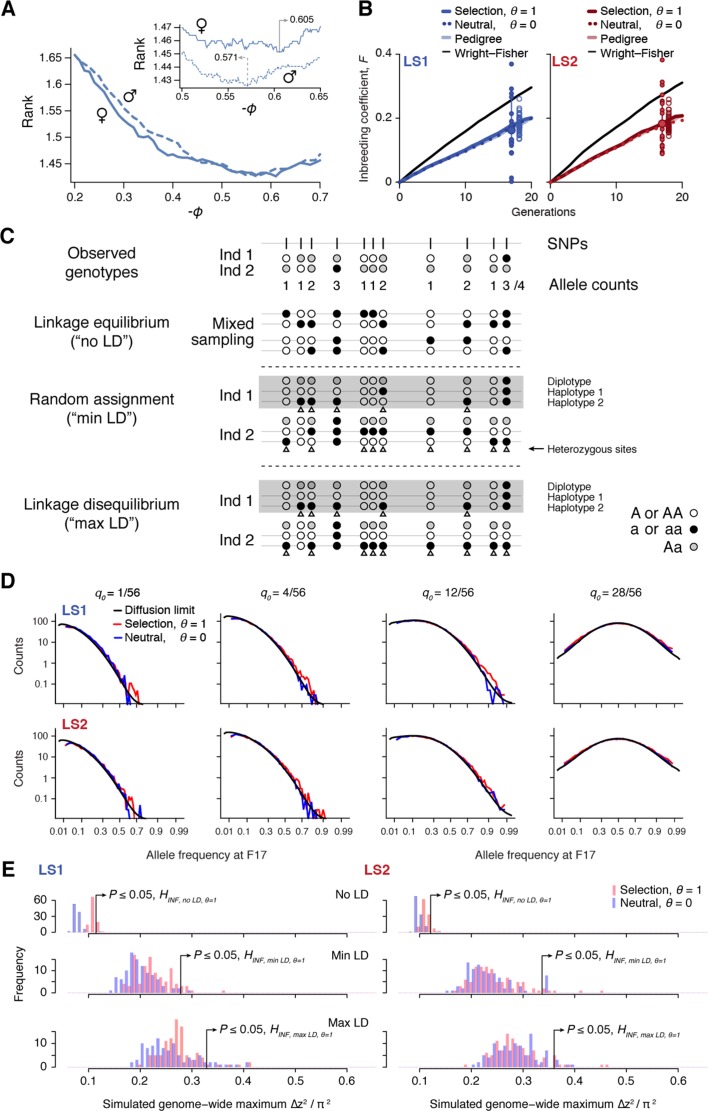
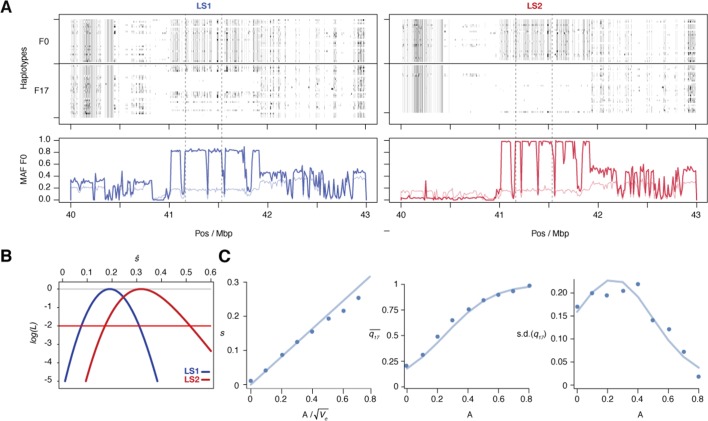
The rapid but generally smooth increase in tibia length in Longshanks is typically interpreted as evidence for a highly dispersed genetic architecture with no individually important loci contributing to the selection response. This is classically described under quantitative genetics as the infinitesimal model. Crucially, the appropriate null hypothesis for the genomic response here should capture “polygenic adaptation” rather than a neutral model. We therefore developed a simulation that faithfully recapitulates the artificial selection experiment by integrating the trait measurements, selection regime, pedigree and genetic diversity of the Longshanks selection experiment, in order to generate an accurate expectation for the genomic response. Using the actual pedigree and trait measurements, we mapped fitness onto tibia length and cube-root body mass as a single composite trait . We estimated from actual data as −0.57, such that the ranking of breeders closely matched the actual composite ranking used to select breeders in the selection experiment, based on and separately (Marchini et al., 2014) (Figure 1—figure supplement 2A). We assumed a maximally polygenic genetic architecture using an “infinitesimal model with linkage” (abbreviated here as HINF), under which the trait is controlled by very many loci, each of infinitesimally small effect (see Appendix for details). Results from simulations seeded with actual genotypes or haplotypes showed that overall, the predicted increase in inbreeding closely matched the observed data (Figure 1—figure supplement 2B). We tested models with varying selection intensity and initial linkage disequilibrium (LD), and for each, ran 100 simulated replicates to determine the significance of changes in allele frequency (Figure 1—figure supplement 2C-E). This flexible quantitative genetics framework allowed us to explore possible changes in genetic diversity over 17 generations of breeding under strong selection.
In simulations, we followed blocks of genomes as they were passed down the pedigree. In order to compare with observations, we seeded the initial genomes with single nucleotide polymorphisms (SNPs) in the same number and initial frequencies as the data. We observed much more variation between chromosomes in overall inbreeding (Figure 1—figure supplement 2B) and in the distribution of allele frequencies (Figure 2—figure supplement 1B) than expected from simulations in which the ancestral SNPs were initially in linkage equilibrium. This can be explained by linkage disequilibrium (LD) between the ancestral SNPs, which greatly increases random variation. Therefore, we based our significance threshold tests on simulations that were seeded with SNPs drawn with LD consistent with the initial haplotypes (Figure 1—figure supplement 2C and E; see Appendix).
Because our simulations assume infinitesimal effects of loci, allele frequency shifts exceeding this stringent threshold would suggest that discrete loci contribute significantly to the selection response. An excess of such loci in either a single LS replicate or in parallel would thus imply a mixed genetic architecture of a few large-effect loci amid an infinitesimal background.
Sequencing the Longshanks mice reveals genomic signatures of selection
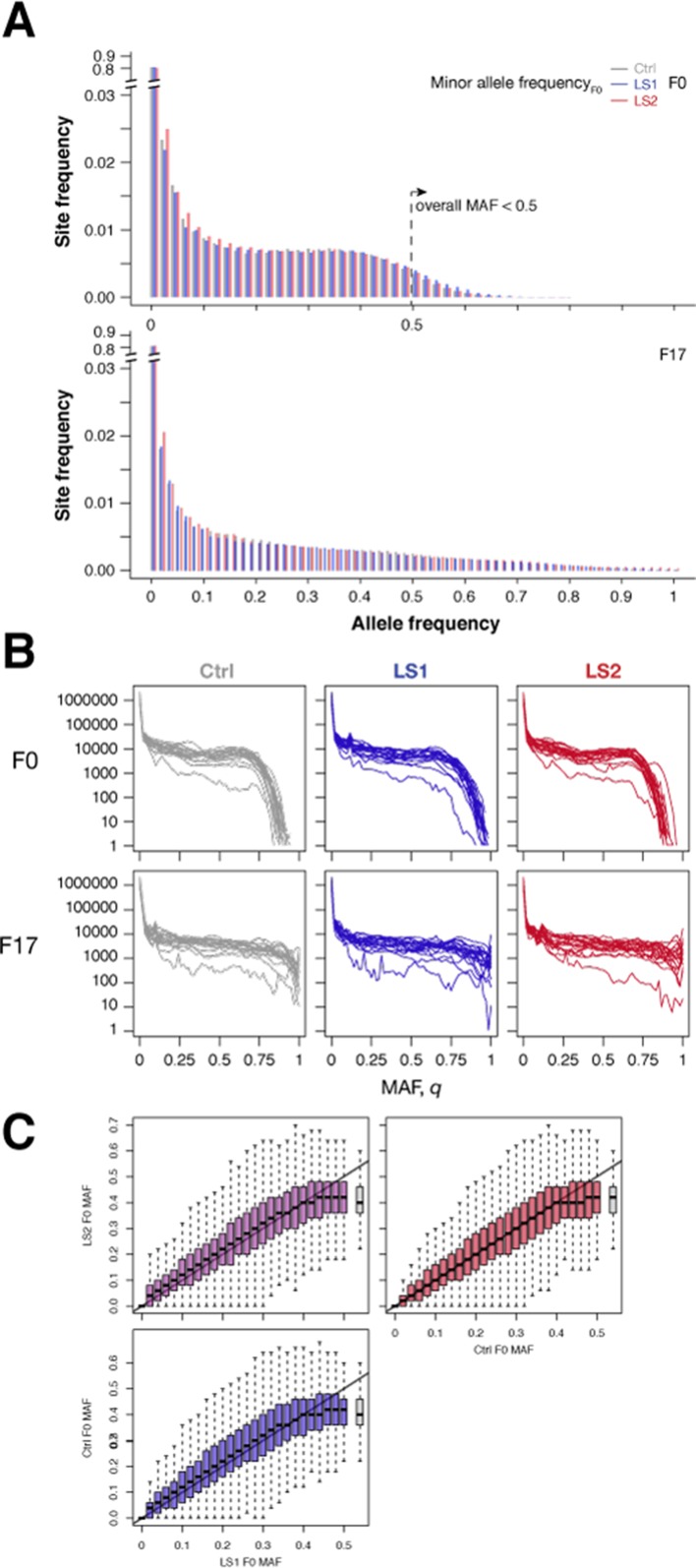
To detect the genomic changes in the actual Longshanks experiment, we sequenced all individuals of the founder (F0) and 17th generation (F17) to an average of 2.91-fold coverage (range: 0.73–20.6×; n = 169 with <10% missing F0 individuals; Supplementary file 1). Across the three lines, we found similar levels of diversity, with an average of 6.7 million (M) segregating SNPs (approximately 0.025%, or 1 SNP per four kbp; Supplementary file 2; Figure 2—figure supplement 1A and Figure 2—figure supplement 2). We checked the founder populations to confirm negligible divergence between the three founder populations (across-line FST on the order of 1 × 10−4), which increased to 0.18 at F17 (Supplementary file 2). This is consistent with random sampling from an outbred breeding stock. By F17, the number of segregating SNPs dropped to around 5.8 M (Supplementary file 2). This 13% drop in diversity (0.9M SNPs genome-wide) is predicted by drift. Our simulations confirmed this and moreover, showed that selection contributed negligibly to the drop in diversity (Appendix, Figure 1—figure supplement 2B, D).
We conclude that despite the strong selection on the LS lines, there was little perturbation to genome-wide diversity. Indeed, the changes in diversity in 17 generations were remarkably similar in all three lines, despite Ctrl not having experienced selection on relative tibia length (Figure 2—figure supplement 1A). Hence, and consistent with our simulation results (Figure 1—figure supplement 2B,D), changes in global genome diversity had little power to distinguish selection from neutral drift despite the strong phenotypic selection response.
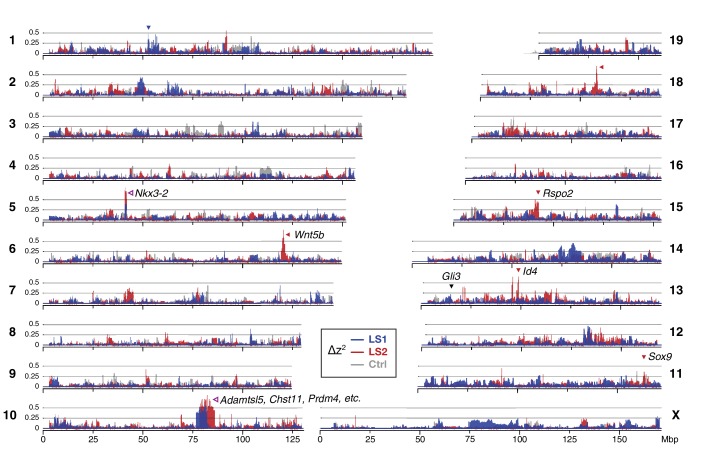
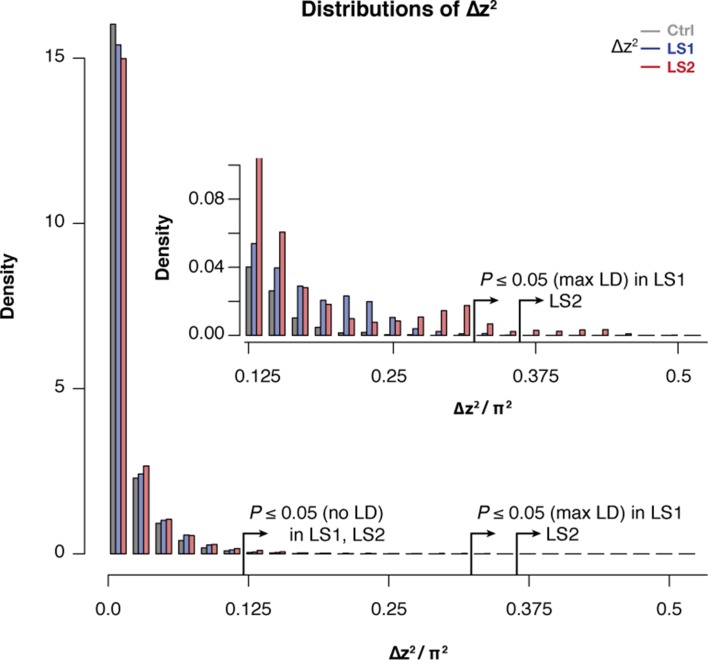
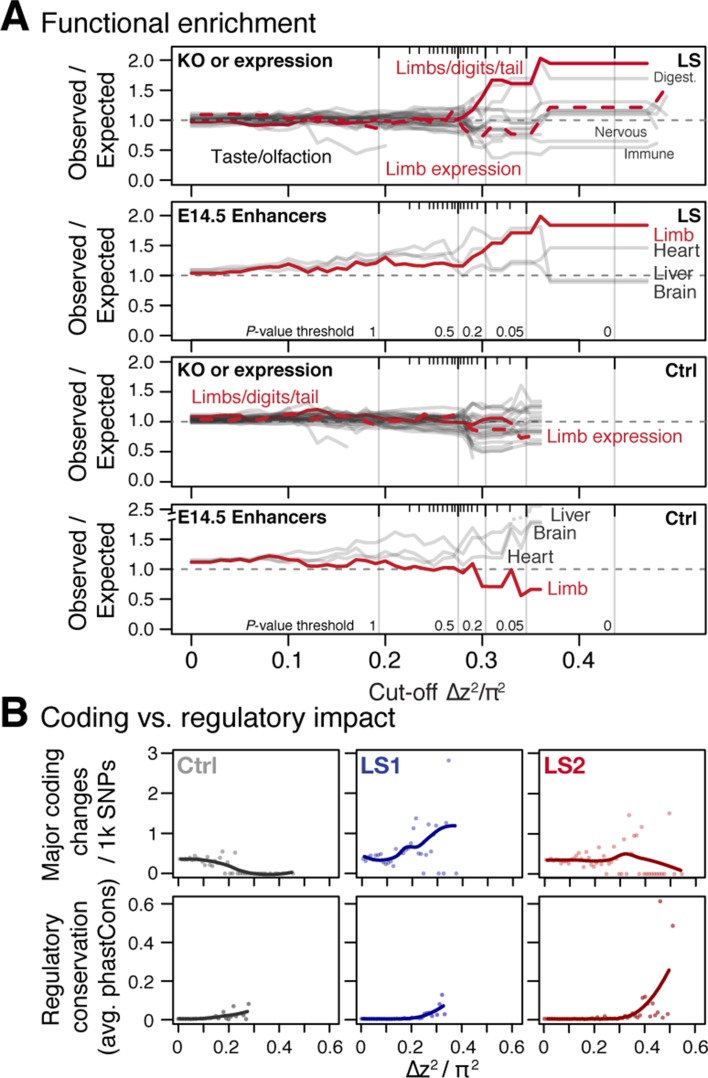
We next asked whether specific loci reveal more definitive differences between the LS replicates and Ctrl (and from infinitesimal predictions). We calculated ∆z2, the square of an arcsine transformed allele frequency difference between F0 and F17; this has an expected variance of 1/2Ne per generation, independent of starting frequency, and ranges from 0 to π2. We averaged ∆z2 within 10 kbp windows (see Methods for details), and found 169 windows belonging to eight clusters (i.e., loci) that had significant shifts in allele frequency in LS1 and/or LS2 (corresponding to 9 and 164 clustered windows respectively at p≤0.05 under HINF, max LD; ∆z2 ≥0.33 π2; genome-wide ∆z2 = 0.02 ± 0.03 π2; Figure 2; Figure 1—figure supplement 2D, Figure 2—figure supplement 2, Figure 2—figure supplement 3; see Methods for details) and 8 windows in three clusters in Ctrl (genome-wide ∆z2 = 0.01 ± 0.02 π2). The eight loci in Longshanks each overlapped between 2 to 179 genes and together contained 11 candidate genes with known roles in bone, cartilage and/or limb development (e.g., Nkx3-2 and Sox9; Table 1; Figure 2—figure supplement 3, Figure 2—figure supplement 4). Four out of the eight loci contain genes with a ‘short tibia’ or ‘short limb’ knockout phenotype (Table 1; p≤0.032 from 1000 permutations, see Methods for details). Of the broader set of genes at these loci with any limb knockout phenotypes, only fibrillin 2 (Fbn2) is polymorphic for SNPs coding for different amino acids, suggesting that for the majority of loci with large shifts in allele frequency, gene regulation was likely important in the selection response (Figure 2—figure supplement 4; Supplementary file 3; see Appendix for further analyses on enrichment in gene functions, protein-coding vs. cis-acting changes and clustering with loci affecting human height).
Figure 2. Widespread genomic response to selection for increased tibia length.
Allele frequency shifts between generations F0 and F17 in LS1, LS2 and Ctrl lines are shown as ∆z2 profiles across the genome (plotted here as fraction of its range from 0 to π2). The Ctrl ∆z2 profile (gray) confirmed our expectation from theory and simulation that drift, inbreeding and genetic linkage could combine to generate large ∆z2 shifts even without selection. Nonetheless the LS1 (blue) and LS2 (red) profiles show a greater number of strong and parallel shifts than Ctrl. These selective sweeps provide support for the contribution of discrete loci to selection response (arrowheads, blue: LS1; red: LS2; purple: parallel; see also Figure 1—figure supplement 2E, Figure 2—figure supplement 2, Figure 2—figure supplement 3) beyond a polygenic background, which may explain a majority of the selection response and yet leave little discernible selection signature. Candidate genes are highlighted (Table 1). An additional a priori candidate limb regulator Gli3 is indicated with a black arrowhead.
Figure 2—figure supplement 1. Broad similarity in molecular diversity in the founder populations for the Longshanks lines and the Control line.
Figure 2—figure supplement 2. Selected lines showed more extreme values of ∆z2 than the Control line.
Figure 2—figure supplement 3. Detailed ∆z2 profiles at the 8 Longshanks significant loci.
Figure 2—figure supplement 4. Loci associated with selection response in Longshanks lines show enrichment for limb function likely associated with cis-acting mechanisms.
Table 1. Major loci likely contributing to the selection response.
These eight loci show significant allele frequency shifts in ∆z2 and are ordered according to their estimated selection coefficients according to Haldane (1932). Shown for each locus are the full hitchhiking spans, peak location and their size covering the core windows, the overlapping TAD and the number of genes found in it. The two top-ranked loci show shifts in parallel in both LS1 and LS2, with the remaining six showing line-specific response (LS1: 1; LS2: 5). Candidate genes found within the TAD with limb, cartilage, or bone developmental knockout phenotype functions are shown, with asterisks (*) marking those with a ‘short tibia’ knockout phenotype (see also Figure 2—figure supplement 3 and Supplementary file 3 for full table).
| Rk | Chr | Span (Mbp) | Peak | Core (kbp) | TAD (kbp) |
Genes | ∆q | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LS1 | LS2 | Ctrl | Type | Candidate genes | |||||||
| 1 | 5 | 38.95–45.13 | 41.77 | 900 | 720 | 3 | 0.69 | 0.86 | −0.14 | Parallel | Nkx3-2 |
| 2 | 10 | 77.47–87.69 | 81.07 | 5360 | 6520 | 175 | 0.79 | 0.88 | −0.04 | Parallel | Sbno5, Aes, Adamtsl5*, Chst11*, Cry1, Prdm4* |
| 3 | 18 | 53.63–63.50 | 58.18 | 220 | 520 | 4 | 0.05 | 0.78 | −0.06 | LS2-specific | - |
| 4 | 13 | 35.59–55.21 | 48.65 | 70 | 2600 | 22 | 0.24 | 0.80 | −0.03 | LS2-specific | Id4 |
| 5 | 1 | 53.16–57.13 | 55.27 | 10 | 720 | 4 | 0.65 | 0.01 | −0.23 | LS1-specific | - |
| 6 | 15 | 31.92–44.43 | 41.54 | 10 | 680 | 3 | −0.23 | 0.66 | 0.02 | LS2-specific | Rspo2* |
| 7 | 6 | 118.65–125.25 | 120.30 | 130 | 1360 | 12 | −0.03 | 0.79 | −0.15 | LS2-specific | Wnt5b* |
| 8 | 11 | 111.10–115.06 | 113.42 | 10 | 2120 | 2 | −0.14 | 0.66 | −0.15 | LS2-specific | Sox9* |
Rk, Rank.
Chr, Chromosome.
Core, Span of 10 kbp windows above HINF, max LD p≤0.05 significance threshold.
TAD, Merged span of topologically associating domains (TAD) overlapping the core span. TADs mark segments along a chromosome that share a common regulatory mechanism. Data from Dixon et al. (2012).
Candidate genes, Genes within the TAD span showing ‘short tibia’, ‘short limbs’, ‘abnormal osteoblast morphology’ or ‘abnormal cartilage morphology’ knockout phenotypes are listed, with * marking those with ‘short tibia’.
Taken together, two major observations stand out from our genomic survey. One, a polygenic, infinitesimal selection model with strong LD among marker SNPs performed better than moderate LD or no LD (Figure 1—figure supplement 2E); and two, we nevertheless find more discrete loci in LS1 and LS2 than in Ctrl, beyond the significance threshold set by the infinitesimal model (Figure 2; Figure 2—figure supplement 2). Thus, we conclude that although the genetic basis of the selection response in the Longshanks experiment may be largely polygenic, evidence strongly suggests discrete loci with major effect, even when each line is considered separately.
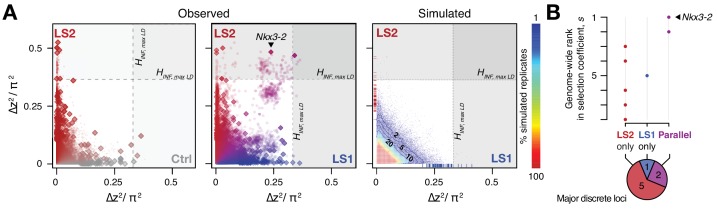
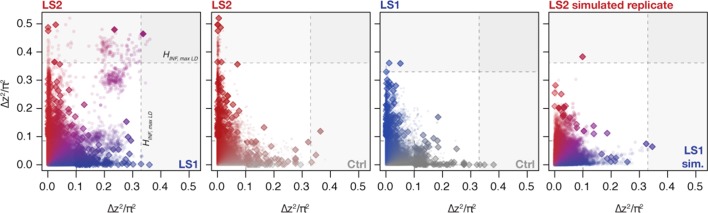
We next tested the repeatability of the selection response at the gene/locus level using the two LS replicates. If the founding populations shared the same selectively favored variants, we may observe parallelism or co-incident selective sweeps, as long as selection could overcome random drift. Indeed, the ∆z2 profiles of LS1 and LS2 were more similar to each other than to Ctrl (Figure 2 and 3A; Figure 3—figure supplement 1; Pearson’s correlation in ∆z2 from 10 kbp windows: LS1–LS2: 0.21 vs. LS1–Ctrl: 0.06 and LS2–Ctrl: 0.05). Whereas previous genomic studies with multiple natural or artificial selection replicates focused mainly on detecting parallel loci (Burke et al., 2010; Jones et al., 2012; Chan et al., 2012; Kelly and Hughes, 2018), here we have the possibility to quantify parallelism and determine the selection value of a given locus. Six out of eight significant loci at the HINF, max LD threshold were line-specific, even though all eight selected alleles were present in the F0 generation in both lines. This prevalence of line-specific loci was consistent under different significance thresholds. However, the two remaining loci that ranked first and second by selection coefficient were parallel, both with s > 0.3 (Figure 3B; note that as outliers, the selection coefficient may be substantially overestimated, but their rank order should remain the same), supporting the idea that the probability of parallelism can be high among those loci with the greatest selection advantage (Orr, 2005).
Figure 3. Selection response in the Longshanks lines was largely line-specific, but the strongest signals occurred in parallel.
(A) Allele frequencies showed greater shifts in LS2 (red) than in Ctrl (gray; left panel; diamonds: peak windows; dots: other 10 kbp windows; see Figure 3—figure supplement 1 for Ctrl vs. LS1 and Appendix for details). Changes in the two lines were not correlated with each other. In contrast, there were many more parallel changes in a comparison between LS1 (blue) vs. LS2 (red; middle panel; adjacent windows appear as clusters due to hitchhiking). The overall distribution closely matches simulated results under the infinitesimal model with maximal linkage disequilibrium (HINF, max LD; right heatmap summarizes the percentage seen in 100 simulated replicates), with most of the windows showing little to no shift (red hues near 0; see also Figure 3—figure supplement 1 for an example replicate). Tick marks along the axes show genome-wide maximum ∆z2 shifts in each of 100 replicate simulations in LS1 (x-axis, blue) and LS2 (y-axis, red), from which we derived line-specific thresholds at the p≤0.05 significance level. While the frequency shifts from simulations matched the bulk of the observed data well, no simulation recovered the strong parallel shifts observed between LS1 and LS2 (compare middle to right panel, points along the diagonal). (B) Genome-wide ranking based on estimated selection coefficients s among the candidate discrete loci at p≤0.05 under HINF, max LD. While six out of eight total loci showed significant shifts in only LS1 or LS2, the two loci with the highest selection coefficients were likely selected in parallel in both LS1 and LS2 (also see middle panel in A).
Figure 3—figure supplement 1. Changes in ∆z2 across lines.
Finding just two parallel loci out of 8 discrete loci may appear to be low, given the genetic similarity in the founding generation and the identical selection applied to both Longshanks replicates. However, one should bear in mind the very many genetic paths to increasing tibia length under an infinitesimal model, and that the effect of drift is expected to be very strong in these small populations. In larger populations, the shift in the balance from drift to selection should result in selection being able to favor increasingly subtle variants and thus produce a greater proportion of parallel loci. However, we expect the trend of parallelism being enriched among the top loci to hold.
In contrast to the subtle differences within each line in changes in global diversity over 17 generations (Figure 2 and Figure 2—figure supplement 2), we found the signature of parallelism to be significantly enriched in the comparison between the selected replicates (χ2 test, LS1–LS2: p≤1 × 10−10), as opposed to comparisons between each selected line and Ctrl (LS1–Ctrl: p>0.01 and LS2–Ctrl: p>0.2, both non-significant after correcting for multiple testing), or between simulated replicates (Figure 3—figure supplement 1; see Appendix for details). Because the parallel selected loci between LS1 and LS2 have the highest selection coefficients and parallelism is not generally expected in our populations, these loci provide the strongest evidence for the role of discrete major loci. As such, the top-ranked parallel locus is the prime candidate for molecular dissection (see Figure 4—figure supplement 1 and Appendix ‘Molecular dissection of Gli3’ for an additional a priori candidate locus with known limb function).
Molecular dissection of the Nkx3-2 locus highlights cis-acting changes
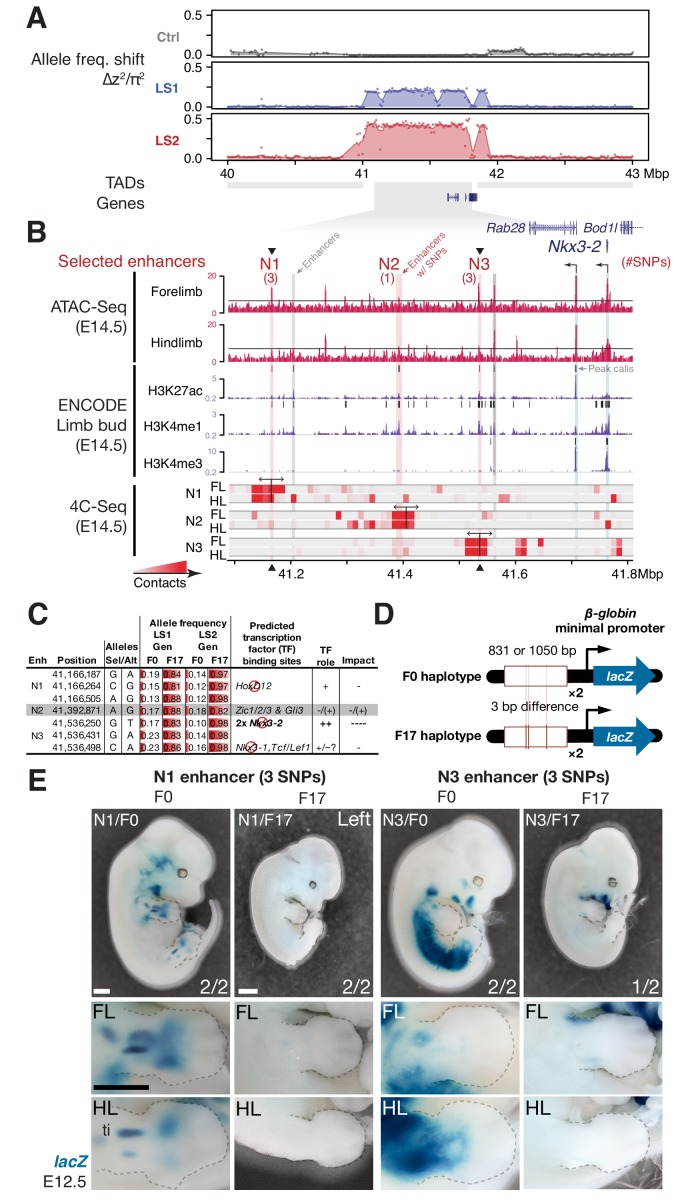
Between the two major parallel loci, we chose the locus on chromosome 5 (Chr5) at 41–42 Mbp for functional validation because it showed the strongest estimated selection coefficient, its signature of selection was clear, and crucially for functional characterization, it contains only three genes, including Nkx3-2 (also known as Bapx1), a known regulator of bone maturation (Figure 2 and 4A) (Provot et al., 2006). At this locus, the pattern of variation resembles a selective sweep spanning 1 Mbp (Figure 4A). Comparison between F0 and F17 individuals revealed no recombinant in this entire region (Figure 5—figure supplement 1A, top panel), precluding fine-mapping using recombinants. We then analyzed the genes in this region to identify the likely target(s) of selection. First, we determined that no coding changes existed for either Rab28 or Nkx3-2, the two genes located within the topologically associating domain (TAD, which mark chromosome segments with shared gene regulatory logic) (Dixon et al., 2012). We then performed in situ hybridization and detected robust expression of Nkx3-2 and Rab28 in the developing fore- and hind limb buds of Ctrl, LS1 and LS2 E12.5, in a domain broadly overlapping the presumptive zeugopod, the region including the tibia (Figure 4—figure supplement 2B). A third gene, Bod1l, straddled the TAD boundary with its promoter located in the neighboring TAD, making its regulation by sequences in the selected locus unlikely. Consistent with this, Bod1l showed only weak or undetectable expression in the developing limb bud (Figure 4—figure supplement 2A). We next combined ENCODE chromatin profiles and our own ATAC-Seq data to identify limb enhancers in the focal TAD. Here we found three novel enhancer candidates (N1, N2 and N3) carrying three, one and three SNPs respectively, all of which showed significant allele frequency shifts in LS1 and LS2 (Figure 4B and C; Figure 5—figure supplement 1A). Chromosome conformation capture assays showed that the N1 and N3 sequences formed long-range looping contacts with the Nkx3-2 promoter—a hallmark of enhancers—despite as much as 600 kbp of intervening sequence (Figure 4B). We next used transgenic reporter assays to determine whether these sequences could drive expression in the limbs. Here, we were not only interested in whether the sequence encoded enhancer activity, but specifically whether the SNPs would affect the activity (Figure 4C and D). An examination of the predicted transcription factor binding sites showed that both the N1 and N3 enhancers contain multiple SNPs with consistent directional impact on the putative enhancer activity (Figure 4C). In contrast, the N2 enhancer contains only a single SNP and is predicted to have inconsistent effect on its activity. We therefore excluded the N2 enhancer from further testing. We found that the F0 alleles of the N1 and N3 enhancers (three SNPs each in about one kbp) drove robust and consistent lacZ expression in the developing limb buds (N1 and N3) as well as in expanded trunk domains (N3) at E12.5 (Figure 4E). In contrast, transgenic reporters carrying the selected F17 alleles of the N1 and N3 enhancers drove consistently weak, nearly undetectable lacZ expression (Figure 4E). Thus, switching from the F0 to the F17 enhancer alleles led to a nearly complete loss in activity (‘loss-of-function’) at developmental stage E12.5. This is consistent with the role of Nkx3-2 as a repressor in long bone maturation (Provot et al., 2006). It should be noted that even though our selective regime favored an increase in the target phenotype (tibia length), at the molecular level we expect advantageous loss- and gain-of-function variants to be equally likely favored by selection. In fact, in an additional functional validation example at the Gli3 locus, we found a gain-of-function enhancer variant that may have been favored at that locus (see Figure 4—figure supplement 1 and Appendix ‘Molecular dissection of Gli3’).
Figure 4. Strong parallel selection response at the bone maturation repressor Nkx3-2 locus was associated with decreased activity of two enhancers.
(A) ∆z2 in this region of chromosome five showed strong parallel differentiation spanning 1 Mbp in both Longshanks but not in the Control line. This 1 Mbp region contains three genes: Nkx3-2, Rab28 and Bod1l (whose promoter lies outside the TAD boundary, shown as gray boxes). Although an originally rare allele in all lines, this region swept almost to fixation by generation 17 in LS2 (Figure 5—figure supplement 1A). (B) Chromatin profiles [ATAC-Seq, red, (Buenrostro et al., 2013); ENCODE histone modifications, purple] from E14.5 developing limb buds revealed five putative limb enhancers (gray and red shading) in the TAD, three of which contained SNPs showing significant frequency shifts. Chromosome conformation capture assays (4C-Seq) from E14.5 limb buds from the N1, N2 and N3 enhancer viewpoints (bi-directional arrows) showed significant long-range looping between the enhancers and sequences around the Nkx3-2 promoter (heat-map from gray to red showing increasing contacts; Promoters are shown with black arrows and blue vertical shading). (C) Selected alleles at 7 SNPs found within the N1, N2, and N3 enhancers increased ~0.75 in frequency in both LS1 and LS2. Selected alleles at three of these sites are predicted to lead to loss (red inhibition circles) of transcription factor binding sites in the Nkx3-2 pathway (including a SNP in N3 causing loss of two adjoining Nkx3-2 binding sites) and thus reduce enhancer activity in N1 and N3. (D, E) Transient transgenic reporter assays of the N1 and N3 enhancers showed that the F0 alleles drove robust and consistent expression at centers of future cartilage condensation (N1) and broader domains of Nkx3-2 expression (N3) in E12.5 fore- and hind limb buds (FL, HL; ti: tibia). Fractions indicate the number of embryos showing similar lacZ staining out of all transgenic embryos. Substituting the F17 enhancer allele (i.e., replacing three positions each in N1 and N3) led to little observable limb bud expression in both the N1/F17 and N3/F17 embryos, suggesting that selection response for longer tibia involved de-repression of bone maturation through a loss-of-function regulatory allele of Nkx3-2 at this locus. Scale bar: 1 mm for both magnifications.
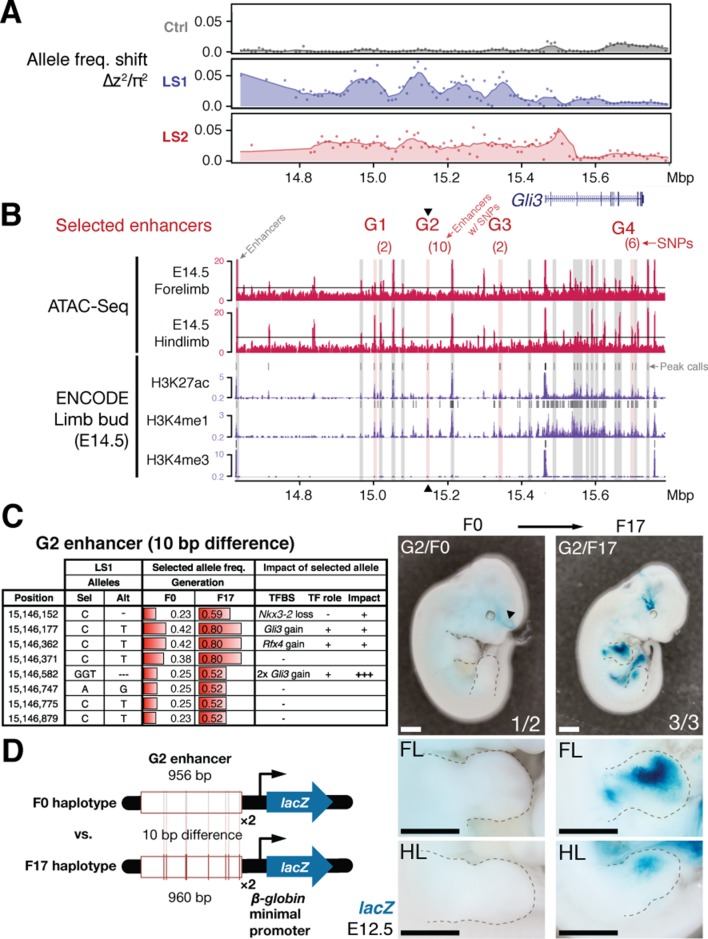
Figure 4—figure supplement 1. An enhancer in chromosome 13 boosts Gli3 expression during limb bud development.
Figure 4—figure supplement 2. Gene expression patterns at the Gli3 and Nkx3-2 candidate intervals.
At the Nkx3-2 locus, we hypothesize that the F17 allele causes de-repression of bone and/or cartilage formation by reducing enhancer activity and Nkx3-2 expression. Crucially, the F0 N1 enhancer showed activity that presages future long bone cartilage condensation in the limb (Figure 4E). That is, the observed expression pattern recalls previous results that suggest that undetected early expression of Nkx3-2 may mark the boundaries and size of limb bone precursors, including the tibia (Sivakamasundari et al., 2012). Conversely, over-expression of Nkx3-2 has been shown to cause shortened tibia (even loss) in mice (Bren-Mattison et al., 2011; Tribioli and Lufkin, 2006). In humans, homozygous frameshift mutations in NKX3-2 cause the rare disorder spondylo-megaepiphyseal-metaphyseal dysplasia (SMMD; OMIM: 613330), which is characterized by short-trunk, long-limbed dwarfism and bow-leggedness (Hellemans et al., 2009). The affected bones in SMMD patients broadly correspond to the expression domains of the two novel N1 (limbs) and N3 (limbs and trunk) enhancers. Instead of wholesale loss of Nkx3-2 expression, which would have been lethal in mice (Akazawa et al., 2000) or likely cause major defects similar to SMMD patients (Hellemans et al., 2009), our in situ hybridization data did not reveal qualitative differences in Nkx3-2 expression domains between Ctrl or LS embryos (Figure 4—figure supplement 2B). Taken together, our results recapitulate the key features of a cis-acting mode of adaptation: Nkx3-2 is a broadly expressed pleiotropic transcription factor that is lethal when knocked out (Akazawa et al., 2000). We found no amino acid changes between the F0 and F17 alleles that could impact protein function. Rather, selection favored changes in tissue-specific expression by modular enhancers. By combining population genetics, functional genomics and developmental genetic techniques, we were able to dissect a megabase-long locus and present data supporting the identification of up to six candidate quantitative trait nucleotides (QTNs). In mice, this represents a rare example of genetic dissection of a trait to the base-pair level.
Linking molecular mechanisms to evolutionary consequence
We next aimed to determine the evolutionary relevance of the Nkx3-2 enhancer variants at the molecular and the population levels. At the strongly expressed N3/F0 ‘trunk and limb’ enhancer, we note that the SNPs in the F17 selected allele lead to disrupted Nkx3-1 and Nkx3-2 binding sites (Figure 4C and 5A; UNIPROBE database [Berger et al., 2008]). This suggests that the selected SNPs may disrupt an auto-feedback loop to decrease Nkx3-2 activity in the limb bud and trunk domains (Figure 5A). Using a GFP transgenic reporter assay in stickleback fish embryos, we found that the mouse N1/F0 enhancer allele was capable of driving expression in the distal cells but not in the fin rays of the developing fins (Figure 5A). This pattern recapitulates fin expression of nkx3.2 in fish, which gives rise to endochondral radials (homologous to ulna/tibia in mice) (Crotwell and Mabee, 2007). Our results suggest that strong selection may have favored the weaker N1/F17 and N3/F17 enhancer alleles in the context of the Longshanks selection regime despite the deep functional conservation of the F0 variants.
Figure 5. Linking base-pair changes to rapid morphological evolution.
(A) At the Nkx3-2 locus, we identified two long-range enhancers, N1 and N3 (circles), located 600 and 230 kbp away, respectively. During development, they drive partially overlapping expression domains in limbs (N1 and N3) and trunk (N3), which are body regions that may correlate positively (tibia length) and possibly negatively (trunk with body mass) with the Longshanks selection regime. For both enhancers, the selected F17 alleles carry loss-of-function variants (gray crosses). Two out of three SNPs in the N3 F17 enhancer are predicted to disrupt an auto-feedback loop, likely reducing Nkx3-2 expression in the trunk and limb regions. Conversely, the enhancer function of the strong N1 F0 allele is evolutionarily conserved in fishes, demonstrated by its ability to drive consistent GFP expression (green) in the pectoral fins (pf, outlined) and branchial arches (white arrowhead, left) in transgenic stickleback embryos at 11 days post-fertilization. The N1 enhancer can recapitulate nkx3.2 expression in distal cells specifically in the endochondral radial domain in developing fins (black arrowheads, right). Scale bar: 250 µm for both magnifications. (B) Allele frequency of the selected allele (minor allele at F0, q) at N3 over 20 generations (blue: LS1; red: LS2; gray broken line: Ctrl; results from N1 were nearly identical due to tight linkage). Observed frequencies from genotyped generations in the Ctrl line are marked with filled circles. Dashed lines indicate missing Ctrl generations. Open circles at generations 0 and 17 indicate allele frequencies from whole genome sequencing. The allele frequency fluctuated in Ctrl due to random drift but followed a generally linear increase in the selected lines from around 0.17 to 0.85 (LS1) and 0.98 (LS2) by generation 17. Shaded contours mark expected allelic trajectories under varying selection coefficients starting from 0.17 (red horizontal line; the average starting allele frequency between LS1 and LS2 founders). The gray shaded region marks the 95% confidence interval under random drift.
Figure 5—figure supplement 1. Selection at the Nkx3-2 locus.
Using theory and simulations, we went beyond qualitative molecular dissection to quantitatively estimate the selection coefficient at the Nkx3-2 locus and its contribution to the total selection response in the Longshanks mice. We retraced the selective sweep of the Nkx3-2 N1 and N3 alleles through targeted genotyping in 1569 mice across all 20 generations. The selected allele steadily increased from around 0.17 to 0.85 in LS1 and 0.98 in LS2 but fluctuated around 0.25 in Ctrl (Figure 5B). We estimated that such a change of around 0.7 in allele frequency would correspond to a selection coefficient s of ~0.24 ± 0.12 at this locus (Figure 5—figure supplement 1B; see Appendix section on ‘Estimating selection coefficient of the top-ranking locus, Nkx3-2, from changes in allele frequency’). By extending our simulation framework to allow for a major locus against an infinitesimal background, we find that the Nkx3-2 locus would contribute 9.4% of the total selection response (limits 3.6–15.5%; see Appendix section ‘Estimating selection coefficient’ for details) in order to produce a shift of 0.7 in allele frequency over 17 generations. To avoid inflation stemming from estimating from outliers, we also independently estimated the contribution of the Nkx3-2 locus using a linear mixed animal model based on the full genotyped series mentioned above (see Appendix section ‘Estimating selection coefficient, animal model’ for details). Using this alternative approach, we estimated that each selected allele increases tibia length by 0.36% (N = 1569, 95% conf. int.: 0.07–0.64%, p=0.0171). Multiplying the effect with the increase in the allele frequency suggests that the Nkx3-2 locus alone would account for approximately 4% of the overall 12.9% increase in tibia length. This lower estimate of around 4% is nonetheless within the bounds of the estimate from simulations. Together, both approaches indicate that the Nkx3-2 locus contributes substantially to the selection response.
Discussion
A defining task of our time is to understand the factors that determine and constrain how small populations respond to sudden environmental changes. Here, we analyze the replicated and controlled Longshanks experiment to characterize the genomic changes that occur as small experimental populations respond to selection.
An important conclusion from the Longshanks experiment is that selection response can be steady and robust even in extremely bottlenecked populations. That is, we found that tibia length increased readily and repeatedly in response to selection even with as few as 14–16 breeding pairs per generation. The sustained response was possible because the lines were founded with enough standing variation, and generation 17 was still only a fraction of the way to the expected limit for the selection response at ~2Ne generations (Robertson, 1960), estimated here to be around 90 (see legend for Figure 1—figure supplement 2B; Appendix on ‘Estimating the selection coefficient’). Although other selective breeding studies using a similar base population of mice encountered selection limits at around generation 20–25 (possibly due to countervailing selection rather than loss of genetic variance) for high voluntary wheel running behavior (Careau et al., 2013) and for nest-building behavior (Bult and Lynch, 2000), here all evidence suggests that the Longshanks mice should continue to show increases in tibia length for many more generations.
The estimated Ne of 46 in the Longshanks experiment, while small, is comparable to those in natural populations like the Soay sheep (McRae et al., 2005), Darwin’s finches (Grant and Grant, 1992) or Tasmanian devils (Epstein et al., 2016) (this last study documents a rapid and parallel evolutionary response to transmissible tumors). These populations span a wide range of time in sustained bottlenecking, from the most recent in Tasmanian devils, to likely many millions of years in Darwin’s finches. Accordingly, we also expect very different dynamics during short- vs. long-term selection response: for a short bout of selection, such as the 20 generations analyzed in this study, selection response depends overwhelmingly on standing genetic variation, with little to no contribution from de novo mutations (Hill, 1982; Weber and Diggins, 1990). Over the long term, however, de novo mutations would contribute increasingly to selection response. In the Longshanks experiment, we observe a robust early response to selection (Figure 1B and Figure 1—figure supplement 1), and a gradual decrease in sequence diversity, consistent with the effect of drift (Figure 1—figure supplement 2B and Figure 2—figure supplement 1A, Supplementary file 2). There has long been broad empirical support for adaptation from standing genetic variation in nature (Jones et al., 2012; Epstein et al., 2016; Hancock et al., 2011) and breeding (Sheng et al., 2015). At least in the short-run, our result demonstrating robust selection response in the Longshanks experiment provides grounds for some optimism regarding the ability of populations to respond rapidly to changes in their environment.
By combining pedigree records with sequencing of founder individuals, our data had sufficient detail to allow precise modeling of trait response, with predicted shifts in allele frequency distribution that closely matched our results (e.g. Figure 1—figure supplement 2D). Furthermore, we functionally validated loci that showed allele frequency shifts outside the model’s predictions and found key enhancers of major effect. Connecting trait changes to allele frequency changes at specific loci has been a longstanding objective in selection experiments, with a number of notable early attempts (e.g., Keightley et al., 1996). To date, we know of only a few studies that attempt to explicitly link traits with changes in allele frequencies (Kessner and Novembre, 2015; Rice and Townsend, 2012; Chen et al., 2019; Nuzhdin et al., 1999) and none have systematically tested the underlying architecture against an infinitesimal background. Here, our results imply a mixed genetic architecture with a few discrete loci of large effect amid an infinitesimal background. It remains to be seen whether other evolve-and-resequence (E&R) studies, with different selection pressures and population parameters, may reveal similar results.
To put our finding of a mixed genetic architecture into perspective, it is worth noting that the infinitesimal model is still the most predictive model by far in practical quantitative genetics, for diverse domesticated species from cattle to crops, despite its intrinsically unrealistic assumptions (Hill et al., 2008; Lynch and Walsh, 1998; Hill and Zhang, 2012). In general, current genomic data for many traits is consistent with a very large number of loci, each with a small effect. From a practical point of view, however, the use of an infinitesimal model does not preclude the presence or indeed the importance of a few major effect loci. Rather, it simply assumes that they are rare enough to allow reasonable model fit (Walsh and Lynch, 2018, page 878). Here, we note that it is actually not clear how one might parameterize a generally applicable predictive oligogenic model with more than a single major effect locus. In this study, while we consider the most likely genetic architecture underlying selection response for tibia length to be a small number of major effect loci together with a polygenic background, we cannot reject other alternative models that could also account for the observed response, such as an effectively infinitesimal model with linkage, as well as models with a few major trait loci.
Among other classical examples of complex traits, such as height or body weight, that may have been subjected to selection, we observe a range of genetic architectures in ways often tightly connected to their population size and/or selection history. Height in humans is often cited as the classical complex trait under possible selection of unknown (and much debated) intensity (see Turchin et al., 2012; Berg and Coop, 2014; Barton et al., 2019). It shows high heritability and a highly dispersed genetic architecture (with the top-ranked locus accounting for only 0.8% of the variation explained in cosmopolitan European populations) (Weedon et al., 2007; Wood et al., 2014). In contrast, as few as 4 to 6 loci account for 83% and 50% of the variation in height in horses and dogs, respectively (Makvandi-Nejad et al., 2012; Rimbault et al., 2013). In both horses and dogs, selection has been strong and sustained, and breed-specific populations tend to be small. Interestingly, and in line with our experiment, the major allele at the IGF1 locus stems from a standing genetic variant, despite many factors that may theoretically favor large-effect de novo mutations (Sutter et al., 2007). In chickens, modern breeding practice and selection from large populations yielded a highly polygenic genetic architecture for body weight, with some of the best empirical evidence for epistasis (Carlborg et al., 2006; Wahlberg et al., 2009; Rubin et al., 2010; Pettersson et al., 2013). Similarly, results from many selection experiments in Drosophila suggest that the genetic architecture underlying selection response may involve many genes (Jha et al., 2015; Reeves and Tautz, 2017; Orozco-terWengel et al., 2012; Turner et al., 2011). By contrast, the extreme tail of the effect size distribution (as inferred from ∆z2) from the Longshanks experiment appears to account for a substantial part of the selection response, presumably due to the combined effects of relatively low diversity in commercial mouse stocks and the small founding populations. But unlike these previous QTL studies or selection experiments, in which either the genetic architecture of a trait or the selection value were estimated separately, sometimes from only few parental individuals or lines, E&R studies sample a much broader pool of alleles and continually compete them against each other. Thus, our approach allowed simultaneous inference of genetic architecture and distribution of effect sizes, is more likely to be representative of the population at large, and is more akin to genome-wide association studies (GWAS), except that here we can also directly connect a trait to its selective value and capture the trajectory of any given allele.
Parallel evolution is often seen as a hallmark for detecting selection (Chan et al., 2012; Schluter et al., 2004; Chan et al., 2010; Martin and Orgogozo, 2013). We investigated the factors that contribute to parallelism in allele frequency shifts over 17 generations by contrasting the two Longshanks replicates against the Control line. However, we observed little parallelism between selected lines and Ctrl, or between simulated replicates under selection, even though the simulated haplotypes were sampled directly from actual founders. This underscores that parallelism depends on both shared selection pressure (absent in Ctrl) and the availability of large-effect loci that confer a substantial selection advantage (absent under the infinitesimal model; Figure 3; Figure 3—figure supplement 1). With increasing population size, selection would be better able to detect variants with more subtle effects. This would in turn lower the threshold beyond which the selective advantage of an allele would become deterministic, that is, exhibit parallelism.
Through in-depth dissection of the Nkx3-2 locus, our data show in fine detail how the selective value of standing variants depends strongly on the selection regime: the originally common F0 variant of the N1 enhancer shows deep functional conservation and can evidently recapitulate fin nkx3.2 expression in fishes (Figure 5A). Yet, in the Longshanks experiment selection strongly favored the weaker allele (Figure 5B). In fact, our molecular dissection of two loci show that both gain-of-function (Gli3) and loss-of-function (Nkx3-2) variants could be favored by selection (Figure 4E and 5A; Figure 4—figure supplement 1D). Through synthesis of multiple lines of evidence, our work uncovered the key role of Nkx3-2, which was not an obvious candidate gene like Gli3 due to the lack of abnormal limb phenotype in Nkx3-2 knockout mice. To our surprise, the same loss of NKX3-2 function in human SMMD patients manifests in opposite ways in different bone types as short trunk and long limbs (Hellemans et al., 2009). This matches the expression domains of our N1 (limb) and N3 (limb and trunk) enhancers (Figure 5A). Evidently, in the absence of lethal coding mutations, the F17 haplotype was doubly beneficial at both enhancers for the limb and potentially also trunk target tissues under the novel selection regime in the Longshanks selection experiment. We estimate that these enhancer variants, along with any other tightly linked beneficial SNPs, segregate as a single locus, which in turn contributes ~10% of the overall selection response.
Despite our efforts to uncover the mechanism underlying the selective advantage of the Nkx3-2 locus, much remains unknown. For example, it remains unclear how such a major allele could segregate in the general mouse stock (and as the reference C57BL/6J allele, no less). It could be that this allele has the same effect in the general mouse population but is conditionally neutral under non-selective breeding and simply escaped notice. However, our preliminary exploration in a panel of C57BL/6-by-DBA/2 (‘BXD’) mice suggested otherwise: mapping of tibia length or mineral density did not reveal this locus as a major QTL determining tibia length (unpublished data kindly provided by Weikuan Gu), suggesting that this allele’s effect on tibia length may depend on the genetic background. Alternatively, the broader C57BL/6 allele could be linked to a compensatory mutation that became uncoupled among the founders of the Longshanks lines. Finally, although we do observe the specific N1 and N3 SNP positions as variable across the rodent and indeed the broader mammalian lineages, further work is needed to determine their effect, if any, on limb development.
Conclusion
Using the Longshanks selection experiment and synthesizing theory, empirical data and molecular genetics, we show that it is possible to identify some of the individual SNPs that have contributed to the response to selection on morphology. In particular, discrete, large-effect loci are revealed by their parallel response. Further work should focus on dissecting the mechanisms behind the dynamics of selective sweeps and/or polygenic adaptation by re-sequencing the entire selection pedigree, testing how the selection response depends on the genetic architecture, and the extent to which linkage places a fundamental limit on our inference of selection. Improved understanding in these areas may have broad implications for conservation, rapid adaptation to climate change and quantitative genetics in medicine, agriculture and in nature.
Materials and methods
Animal care and use
All experimental procedures described in this study have been approved by the applicable University institutional ethics committee for animal welfare at the University of Calgary (HSACC Protocols M08146 and AC13-0077); or local competent authority: Landesdirektion Sachsen, Germany, permit number 24–9168.11-9/2012-5.
Reference genome assembly
All co-ordinates in the mouse genome refer to Mus musculus reference mm10, which is derived from GRCm38.
Code and data availability
Sequence data have been deposited in the SRA database under accession number SRP165718 and GEO under GSE121564, GSE121565 and GSE121566. Non-sequence data have been deposited at Dryad, doi:10.5061/dryad.0q2h6tk. Analytical code and additional notes have been deposited in the following repository: https://github.com/evolgenomics/Longshanks (Evolgenomics, 2019; copy archived at https://github.com/elifesciences-publications/Longshanks). Additional raw data and code are hosted via our institute's FTP servers at http://ftp.tuebingen.mpg.de/fml/ag-chan/Longshanks/.
Pedigree data
Tibia length and body weight phenotypes were measured as previously described (Marchini et al., 2014). A total of 1332 Control, 3054 LS1, and 3101 LS2 individuals were recorded. Five outlier individuals with a skeletal dysplasia of unknown etiology were removed from LS2 and excluded from further analysis. Missing data in LS2 were filled in with random individuals that best matched the pedigree. Trait data were analyzed to determine response to selection based on the measured traits and their rank orders based on the selection index.
Simulations
Simulations were based on the actual pedigree and selection scheme, following one chromosome at a time. Each chromosome was represented by a set of junctions, which recorded the boundaries between genomes originating from different founder genomes; at the end, the SNP genotype was reconstructed by seeding each block of genome with the appropriate ancestral haplotype. This procedure is much more efficient than following each of the very large number of SNP markers. Crossovers were uniformly distributed, at a rate equal to the map length (Cox et al., 2009). Trait value was determined by a component due to an infinitesimal background (Vg); a component determined by the sum of effects of 104 evenly spaced discrete loci (Vs); and a Gaussian non-genetic component (Ve). The two genetic components had variance proportional to the corresponding map length, and the heritability was estimated from the observed trait values (see Appendix section ‘Major considerations’). In each generation, the actual number of male and female offspring were generated from each breeding pair, and the male and female with the largest trait value were chosen to breed.
SNP genotypes were assigned to the founder genomes with their observed frequencies. However, to reproduce the correct variability requires that we assign founder haplotypes. This is not straightforward, because low-coverage individual genotypes cannot be phased reliably, and heterozygotes are frequently mis-called as homozygotes. We compared three procedures, which were applied within intervals that share the same ancestry: assigning haplotypes in linkage equilibrium (LE, or ‘no LD’); assigning the two alleles at heterozygous sites in each individual to its two haplotypes at random, which minimizes linkage disequilibrium but is consistent with observed diploid genotypes (‘min LD’); and assigning alleles at heterozygous sites in each individual to the ‘reference’ and ‘alternate’ haplotype consistently within an interval, which maximizes linkage disequilibrium (‘max LD’) (Figure 1—figure supplement 2C). For details, see legend in Figure 1—figure supplement 2.
Significance thresholds
To obtain significance thresholds, we summarized the genome-wide maximum ∆z2 shift for each replicate of the simulated LS1 and LS2 lines, averaged within 10 kb windows, and grouped by the selection intensity and extent of linkage disequilibrium (LD). From this distribution of genome-wide maximum ∆z2 we obtained the critical value for the corresponding significance threshold (typically the 95th quantile or p=0.05) under each selection and LD model (Figure 3A; Figure 1—figure supplement 2E). This procedure controls for the effect of linkage and hitchhiking, line-specific pedigree structure, and selection strength.
Sequencing, genotyping and phasing pipeline
Sequencing libraries for high-throughput sequencing were generated using TruSeq or Nextera DNA Library Prep Kits (Illumina, Inc, San Diego, USA) according to manufacturer’s recommendations or using equivalent Tn5 transposase expressed in-house as previously described (Picelli et al., 2014). Briefly, genomic DNA was extracted from ear clips by standard Protease K digestion (New England Biolabs GmbH, Frankfurt am Main, Germany) followed by AmpureXP bead (Beckman Coulter GmbH, Krefeld, Germany) purification. Extracted high-molecular weight DNA was sheared with a Covaris S2 (Woburn, MA, USA) or ‘tagmented’ by commercial or purified Tn5-transposase according to manufacturer’s recommendations. Each sample was individually barcoded (single-indexed as N501 with N7XX variable barcodes; all oligonucleotides used in this study were synthesized by Integrated DNA Technologies, Coralville, Iowa, USA) and pooled for high-throughput sequencing by a HiSeq 3000 (Illumina) at the Genome Core Facility at the MPI Tübingen Campus. Sequenced data were pre-processed using a pipeline consisting of data clean-up, mapping, base-calling and analysis from software fastQC v0.10.1 (Andrews, 2016); trimmomatic v0.33 (Bolger et al., 2014); bwa v0.7.10-r789 (Li and Durbin, 2010); GATK v3.4–0-gf196186 modules BQSR, MarkDuplicates, IndelRealignment (McKenna et al., 2010; DePristo et al., 2011). Genotype calls were performed using the GATK HaplotypeCaller under the GENOTYPE_GIVEN_ALLELES mode using a set of high-quality SNP calls made available by the Wellcome Trust Sanger Centre (Mouse Genomes Project version three dbSNP v137 release [Keane et al., 2011]), after filtering for sites segregating among inbred lines that may have contributed to the original seven female and two male CD-1 founders, namely 129S1/SvImJ, AKR/J, BALB/cJ, BTBR T+Itpr3 tf/J, C3H/HeJ, C57BL/6NJ, CAST/EiJ, DBA/2J, FVB/NJ, KK/HiJ, MOLF/EiJ, NOD/ShiLtJ, NZO/HlLtJ, NZW/LacJ, PWK/PhJ and WSB/EiJ based on (Yalcin et al., 2010). We consider a combined ~100x coverage sufficient to recover any of the 18 CD-1 founding haplotypes still segregating at a given locus. The raw genotypes were phased with Beagle v4.1 (Browning and Browning, 2016) based on genotype posterior likelihoods using a genetic map interpolated from the mouse reference map (Cox et al., 2009) and imputed from the same putative CD-1 source lines as the reference panel. The site frequency spectra (SFS) were evaluated to ensure genotype quality (Figure 2—figure supplement 1A).
Population genetics summary statistics
Summary statistics of the F0 and F17 samples were calculated genome-wide (Weir–Cockerham FST, π, heterozygosity, allele frequencies p and q) in adjacent 10 kbp windows or on a per site basis using VCFtools v0.1.14 (Danecek et al., 2011). The summary statistic ∆z2 was the squared within-line difference in arcsine square-root transformed MAF q; it ranges from 0 to π2. The resulting data were further processed by custom bash, Perl and R v3.2.0 (R Development Core Team, 2015) scripts.
Peak loci and filtering for hitchhiking windows
Peak loci were defined by a descending rank ordering of all 10 kbp windows, and from each peak signal the windows were extended by 100 SNPs to each side, until no single SNP rising above a ∆z2 shift of 0.2 π2 was detected. A total of 810 peaks were found with a ∆z2 shift ≥0.2 for LS1 and LS2. Following the same procedure, we found 766 peaks in Ctrl.
Candidate genes
To determine whether genes with related developmental roles were associated with the selected variants, the topologically associating domains (TADs) derived from mouse embryonic stem cells as defined elsewhere (Dixon et al., 2012) were re-mapped onto mm10 co-ordinates. Genes within the TAD overlapping within 500 kbp of the peak window (‘core span’) were then cross-referenced against annotated knockout phenotypes (Mouse Genome Informatics, http://www.informatics.jax.org). This broader overlap was chosen to include genes whose regulatory sequences (e.g., enhancers), but not necessarily their gene bodies, fall close to the peak window. We highlight candidate genes showing limb- and bone-related phenotypes, e.g., with altered limb bone lengths or epiphyseal growth plate morphology, as observed in Longshanks mice (Marchini and Rolian, 2018), of the following categories (along with their Mammalian Phenotype Ontology term and the number of genes): ‘abnormal tibia morphology/MP:0000558’ (212 genes), ‘short limbs/MP:0000547’ and ‘short tibia/MP:0002764’ (223 genes), ‘abnormal cartilage morphology/MP:0000163’ (321 genes), ‘abnormal osteoblast morphology/MP:0004986’ (122 genes). Note that we excluded compound mutants or those conditional mutant phenotypes involving transgenes. To determine if the overlap with these genes wassignificant, we performed 1000 permutations of the core span using bedtools v2.22.1 shuffle with the -noOverlapping option (Quinlan and Hall, 2010) and excluding ChrY, ChrM and unassembled scaffolds. We then followed the exact procedure as above to determine the number of genes in the overlapping TAD belonging to each category. We reported the quantile rank as the P-value, ignoring ties. To determine other genes in the region, we list all genes falling within the entire hitchhiking window (Supplementary file 3).
Identification of putative limb enhancers
We downloaded publicly available chromatin profiles, derived from E14.5 limbs, for the histone H3 lysine-4 (K4) or lysine-27 (K27) mono-/tri-methylation or acetylation marks (H3K4me1, H3K4me3 and H3K27ac) generated by the ENCODE Consortium (Shen et al., 2012). We intersected the peak calls for the enhancer-associated marks H3K4me1 and H3K27ac and filtered out peaks overlapping promoters (H3K4me3 and promoter annotation according to the FANTOM5 Consortium [Forrest et al., 2014]).
Enrichment analysis
To calculate enrichment through the whole range of ∆z2, a similar procedure was taken as in Candidate genes above. For knockout gene functions, genes contained in TADs within 500 kbp of peak windows were included in the analysis. We used the complete database of annotated knockout phenotypes for genes or spontaneous mutations, after removing phenotypes reported under conditional or polygenic mutants. For gene expression data, we retained all genes which have been reported as being expressed in any of the limb structures, by tracing each anatomy ontological term through its parent terms, up to the top-level groupings, e.g., ‘limb’, in the Mouse Genomic Informatics Gene Expression Database (Finger et al., 2017). For E14.5 enhancers, we used a raw 500 kbp overlap with the peak windows because enhancers, unlike genes, may not have intermediaries and may instead represent direct selection targets.
For coding mutations, we first annotated all SNPs for their putative effects using snpEff v4.0e (Cingolani et al., 2012). To accurately capture the per-site impact of coding mutations, we used per-site ∆z2 instead of the averaged 10 kbp window. For each population, we divided all segregating SNPs into up to 0.02 bands based on per-site ∆z2. We then tracked the impact of coding mutations in genes known to be expressed in limbs, as above. We reported the sum of all missense (‘moderate’ impact), frame-shift, stop codon gain or loss sites (‘high impact’). A linear regression was used to evaluate the relationship between ∆z2 and the average impact of coding SNPs (SNPs with high or moderate impact to all coding SNPs).
For regulatory mutations, we used the same bins spanning the range of ∆z2, but focused on the subset of SNPs falling within the ENCODE E14.5 limb enhancers. We then obtained a weighted average conservation score based on an averaged phastCons (Pollard et al., 2010) or phyloP (Siepel et al., 2005) score in ±250 bp flanking the SNP, calculated from a 60-way alignment between placental mammal genomes (downloaded from the UCSC Genome Browser [Kent et al., 2002]). We reported the average conservation score of all SNPs within the bin and fitted a linear regression on log-scale. In particular, phastCons scores range from 0 (un-conserved) to 1 (fully conserved), whereas phyloP is the of the P-value of the phylogenetic tree, expressed as a positive score for conservation and a negative score for lineage-specific accelerated change. We favored using phastCons for its simpler interpretation.
Impact of coding variants
Using the same SNP effect annotations described in the section above, we checked whether any specific SNP with significant site-wise ∆z2 in either LS1 or LS2 cause amino acid changes or protein disruptions and are known to cause limb defects when knocked out. For each position we examined outgroup sequences using the 60-way placental mammal alignment to determine the ancestral amino acid state and whether the selected variant was consistent with purifying vs. diversifying selection. The resulting 12 genes that matched these criteria are listed in Supplementary file 4.
Association with human height loci
We downloaded the set of 697 SNPs associated with loci for human height (Wood et al., 2014) to test if these loci cluster with the selected loci in the Longshanks lines. In order to facilitate mapping to mouse co-ordinates, each human SNP was expanded to 100 kbp centering on the SNP and converted to mm10 positions using the liftOver tool with the multiple mapping option disabled (Kent et al., 2002). We were able to assign positions in 655 out of the 697 total SNPs. Then for each of the 810 loci above the HINF, no LD threshold in the selected Longshanks lines, the minimal distance to any of the mapped human loci was determined using bedtools closest with the -d option (Quinlan and Hall, 2010). When a region actually overlapped, a distance of 0 bp was assigned. To generate a permuted set, the 810 loci were randomly shuffled across the mouse autosomes using the bedtools shuffle program with the -noOverlapping option. Then the exact same procedure as the actual data was followed to determine the closest interval. The resulting permuted intervals followed an approximately normal distribution, with observed results falling completely below the range of permuted results, that is, closer to height-associated human SNPs.
In situ hybridization
Detection of specific gene transcripts were performed as previously described in Brown et al., 2005. Probes against Nkx3-2, Rab28, Bod1l and Gli3 were amplified from cDNA from wildtype C57BL/6NJ mouse embryos (Supplementary file 5). Amplified fragments were cloned into pJET1.2/blunt plasmid backbones in both sense and anti-sense orientations using the CloneJET PCR Kit (Thermo Fisher Scientific, Schwerte, Germany) and confirmed by Sanger sequencing using the included forward and reverse primers. Probe plasmids have also been deposited with Addgene. In vitro transcription from the T7 promoter was performed using the MAXIscript T7 in vitro Transcription Kit (Thermo Fisher Scientific) supplemented with Digoxigenin-11-UTP (Sigma-Aldrich) (MPI Tübingen), or with T7 RNA polymerase (Promega) in the presence of DIG RNA labeling mix (Roche) (University of Calgary). Following TURBO DNase (Thermo Fisher Scientific) digestion, probes were cleaned using SigmaSpin Sequencing Reaction Clean-Up columns (Sigma-Aldrich) (MPI Tübingen), or using Illustra MicroSpin G-50 columns (GE Healthcare) (University of Calgary). During testing of probe designs, sense controls were used in parallel reactions to establish background non-specific binding.
ATAC-seq library preparation and sequencing pipeline
ATAC-seq was performed on dissected C57BL/6NJ E14.5 forelimb and hindlimb. Nuclei preparation and tagmentation were performed as previously described in Buenrostro et al. (2013), with the following modifications. To minimize endogenous protease activity, cells were strictly limited to 5 + 5 min of collagenase A treatment at 37°C, with frequent pipetting to aid dissociation into single-cell suspensions. Following wash steps and cell lysis, 50,000 nuclei were tagmented with expressed Tn5 transposase. Each tagmented sample was then purified by MinElute columns (Qiagen) and amplified with Q5 High-Fidelity DNA Polymerase (New England Biolabs) using a uniquely barcoded i7-index primer (N701-N7XX) and the N501 i5-index primer. PCR thermocycler programs were 72°C for 4 min, 98°C for 30 s, 6 cycles of 98°C for 10 s, 65°C for 30 s, 72°C for 1 min, and final extension at 72°C for 4 min. PCR-enriched samples were taken through a double size selection with PEG-based SPRI beads (Beckman Coulter) first with 0.5X ratio of PEG/beads to remove DNA fragments longer than 600 bp, followed by 1.8X PEG/beads ratio in order to select for Fraction A as described in Milani et al. (2016). Pooled libraries were run on the HiSeq 3000 (Illumina) at the Genome Core Facility at the MPI Tübingen Campus to obtain 150 bp paired end reads, which were aligned to mouse mm10 genome using bowtie2 v.2.1.0 (Langmead and Salzberg, 2012). Peaks were called using MACS14 v.2.1 (Zhang et al., 2008).
Multiplexed chromosome conformation capture (4C-Seq)
Chromosome conformation capture (3C) template was prepared from pooled E14.5 liver, forelimb and hindlimb buds (n = 5–6 C57BL/6NJ embryos per replicate), with improvements to the primer extension and library amplification steps following (Sexton et al., 2012). The template was amplified with Q5 High-Fidelity Polymerase (New England Biolabs GmbH, Frankfurt am Main, Germany) using a 4C adapter-specific primer and a pool of 6 Nkx3-2 enhancer viewpoint primers (and, in a separate experiment, a pool of 8 Gli3 enhancer-specific viewpoint primers; Supplementary file 6). Amplified fragments were prepared for Illumina sequencing by ligation of TruSeq adapters, followed by PCR enrichment. Pooled libraries were sequenced by a HiSeq 3000 (Illumina) at the Genome Core Facility at the MPI Tübingen Campus with single-end, 150 bp reads. Sequence data were processed using a pipeline consisting of data clean-up, mapping, and analysis based upon cutadapt v1.10 (Martin, 2011); bwa v0.7.10-r789 (Li and Durbin, 2010); samtools v1.2 (Li et al., 2009); bedtools (Quinlan and Hall, 2010) and R v3.2.0 (R Development Core Team, 2015). Alignments were filtered for ENCODE blacklisted regions (ENCODE Project Consortium, 2012) and those with MAPQ scores below 30 were excluded from analysis. Filtered alignments were binned into genome-wide BglII fragments, normalized to Reads Per Kilobase of transcript per Million mapped reads (RPKM), and plotted and visualized in R.
Plasmid construction
Putative limb enhancers corresponding to the F0 and F17 alleles of the Gli3 G2 and Nkx3-2 N1 and N3 enhancers were amplified from genomic DNA of Longshanks mice from the LS1 F0 (nine mice) and F17 (10 mice) generations and sub-cloned into pJET1.2/blunt plasmid backbone using the CloneJET PCR Kit (Thermo Fisher Scientific) and alleles were confirmed by Sanger sequencing using the included forward and reverse primers (Supplementary file 7). Each allele of each enhancer was then cloned as tandem duplicates with junction SalI and XhoI sites upstream of a β-globin minimal promoter in our reporter vector (see below). Constructs were screened for the enhancer variant using Sanger sequencing. All SNPs were further confirmed against the rest of the population through direct amplicon sequencing.
The base reporter construct pBeta-lacZ-attBx2 consists of a β-globin minimal promoter followed by a lacZ reporter gene derived from pRS16, with the entire reporter cassette flanked by double attB sites. The pBeta-lacZ-attBx2 plasmid and its full sequence have been deposited and is available at Addgene.
Pronuclear injection of F0 and F17 enhancer-reporter constructs in mice
The reporter constructs containing the appropriate allele of each of the three enhancers were linearized with ScaI (or BsaI in the case of the N3 F0 allele due to the gain of a ScaI site) and purified. Microinjection into mouse zygotes was performed essentially as described (DiLeone et al., 2000).
At 12 d after the embryo transfer, the gestation was terminated and embryos were individually dissected, fixed in 4% paraformaldehyde for 45 min and stored in PBS. All manipulations were performed by R.N. or under R.N.’s supervision at the Transgenic Core Facility at the Max Planck Institute of Molecular Cell Biology and Genetics, Dresden, Germany. Yolk sacs from embryos were separately collected for genotyping and all embryos were stained for lacZ expression as previously described (Mortlock et al., 2003). Embryos were scored for lacZ staining, with positive expression assigned if the pattern was consistently observed in at least two embryos.
Genotyping of time series at the Nkx3-2 N3 locus
Allele-specific primers terminating on SNPs that discriminate between the F0 from the F17 N3 enhancer alleles were designed (rs33219710 and rs33600994; Supplementary file 8). The amplicons were optimized as a qPCR reaction to give allele-specific, present/absent amplifications (typically no amplification for the absent allele, otherwise average ∆Ct >10). Genotyping on the entire breeding pedigree of LS1 (n = 602), LS2 (n = 579) and Ctrl (n = 389) was performed in duplicates for each allele on a Bio-Rad CFX384 Touch instrument (Bio-Rad Laboratories GmbH, Munich, Germany) with SYBR Select Master Mix for CFX (Thermo Fisher Scientific) and the following qPCR program: 50°C for 2 min, 95°C for 2 min, 40 cycles of 95°C for 15 s, 58°C for 10 s, 72°C for 10 s. In each qPCR run we included individuals of each genotype (LS F17 selected homozygotes, heterozygotes and F0 major allele homozygotes). For the few samples with discordant results between replicates, DNA was re-extracted and re-genotyped or otherwise excluded.
Transgenic reporter assays in stickleback fish
In sticklebacks, transgenic reporter assays were carried out using the reporter construct pBHR (Chan et al., 2010). The reporter consists of a zebrafish heat shock protein 70 (Hsp70) promoter followed by an eGFP reporter gene, with the entire reporter cassette flanked by tol2 transposon sequences for transposase-directed genomic integration. The Nkx3-2 N1/F0 enhancer allele was cloned as tandem duplicates using the NheI and EcoRV restriction sites upstream of the Hsp70 promoter. Enhancer orientation and sequence was confirmed by Sanger sequencing. Transient transgenic stickleback embryos were generated by co-microinjecting the plasmid (final concentration: 10 ng/µl) and tol2 transposase mRNA (40 ng/µl) into freshly fertilized eggs at the one-cell stage as described in Chan et al. (2010).
Acknowledgements
We thank Felicity Jones for input into experimental design, helpful discussion and improving the manuscript. We thank the Rolian, Barton, Chan and Jones Labs members for support, insightful scientific discussion and improving the manuscript. We thank the Rolian lab members, the Animal Resource Centre staff at the University of Calgary, and MPI Dresden Animal Facility staff for animal husbandry. We thank Derek Lundberg for help with library preparation automation. We thank Christa Lanz, Rebecca Schwab and Ilja Bezrukov for assistance with high-throughput sequencing and associated data processing; Andre Noll for high-performance computing support; the MPI Tübingen IT team for computational support. We thank Felicity Jones and the Jones Lab for help with stickleback microinjections. pRS16 was a gift from François Spitz. We thank Mirna Marinič for creating an earlier version of the transgenic reporter plasmid. We are indebted to Gemma Puixeu Sala, William G Hill, Peter Keightley for input and discussion on data analysis and simulation. We are also indebted to Stefan Mundlos, Przemko Tylzanowki, Weikuan Gu for suggested experiments and sharing unpublished data. We thank Sean B Carroll, Andrew Clark, John Kelly (reviewer), David Kingsley, Jonathan Pritchard, Matthew Rockman (reviewer), Gregory Wray, and Magnus Nordborg (reviewer) for thoughtful input that has greatly improved our manuscript. JPLC is supported by the International Max Planck Research School ‘From Molecules to Organisms’. SB and NB are supported by IST Austria. CR is supported by Discovery Grant #4181932 from the Natural Sciences and Engineering Research Council of Canada and by the Faculty of Veterinary Medicine at the University of Calgary. YFC is supported by the Max Planck Society.
Appendix 1
Major considerations in constructing the simulations
In the Longshanks experiment, the highest-ranking male and the highest-ranking female from each family were chosen to breed with the highest-ranking mice from other families within a line (i.e., disallowing sibling matings). Thus, if we disregard non-Mendelian segregation, and the fraction of failed litters (15%), selection acts solely within families, on the measured traits. Such selection does not distort the pedigree and allows us to follow the evolution of each chromosome separately.
Our simulations track the inheritance of continuous genomes by following the junctions between regions with different ancestry. In principle, we should simulate selection under the infinitesimal model by following the contributions to the trait of continuous blocks of chromosomes across the whole genome. However, this is computationally challenging, since the contributions of all the blocks defined by every recombination event have to be tracked. Instead, we follow a large number of discrete biallelic loci checking that the number is sufficiently large to approach the infinitesimal limit (Figure 1—figure supplement 2D). We made a further slight approximation by only explicitly modeling discrete loci on one chromosome at a time. We divided the breeding value of an individual into two components. The first, Vg, is a contribution from a large number of unlinked loci, due to genes on all but the focal chromosome, as represented by the infinitesimal model. The values of this component amongst offspring are normally distributed around the mean of the parents, with its variance being:
where: is the initial genetic variance, and
, are the probabilities of identity between distinct genes in each parent, ,; , are calculated from the pedigree;
is the fraction of genome on the focal chromosome.
The second component, Vs, is the sum of contributions from a large number, , of discrete loci, evenly spaced along the focal chromosome (here we used 10,000), and contributing a fraction of the initial additive variance. We choose these to have equal effects and random signs, ±, such that initial allele frequencies , and equal effects , such that . The initial population consists of 28 diploid individuals, matching the experiment, and loci have initial frequencies of 1, 4, 12 and 28 out of the diploid total of 56 alleles, in equal proportions. Inheritance is assumed to be autosomal, with no sex-linkage. This choice of equal effects approaches most closely to the infinitesimal model, for a given number of loci.
The decrease in genetic variance due to random drift is measured by the inbreeding coefficient, defined as the probability of identity by descent, relative to the initial population. We distinguish the identity between two distinct genes within a diploid individual, , from the probability of identity between two genes in different individuals, . The overall mean identity between two genes chosen independently and at random from all genes is
.
The proportion of heterozygotes in the population decreases by a factor of , the variance in allele frequency increases with , and the genetic diversity,
, decreases as .
Figure 1—figure supplement 2B shows that in the absence of selection, the identity increases slower than expected under the Wright–Fisher model with the actual population sizes (compare light shaded lines with black lines). These differences are a consequence of the circular mating scheme, which was designed to slow the loss of variation. The dotted line shows the average , estimated from the loss of heterozygosity in 50 replicate neutral simulations, each with 104 loci on a chromosome of length R=1 Morgan. These are close to the prediction from the pedigree (light shaded lines), validating the simulations.
The thick colored line in Figure 1—figure supplement 2B shows , estimated in the same way from simulations that include truncation selection on a trait with within-family variance = 0.584 (a value we abbreviate as ), which matches the observed selection response and parent-offspring regression. The rate of drift, as measured by the gradient in over time, is significantly faster in simulations with selection, by 6.7% in LS1 and 9.8% in LS2 (Student’s t-test P ≤ 0.008 in LS1 and P ≤ 0.0005 in LS2). However, this effect of selection would not be detectable from any one replicate, since the standard deviation of the rate of drift, relative to the mean rate, is ~13% between replicates. On average, the observed loss of heterozygosity fits closely to that expected from the pedigree (large dot with error bars), though there is wide variation among chromosomes (filled dots), which is substantially higher than seen in simulations seeded with SNP at linkage equilibrium (compare filled and open dots).
We then performed 100 simulations, seeding each founding generation with actual genotypes and using actual pedigrees, selection pressure or heritability parameters (within-family heritability of the fitness dimension: 0.51). A main conclusion from our modelling is that the overall allele frequencies were hardly perturbed by varying selection from random drift to even doubling the selection intensity. Upon closer examination, it became clear that under the standard “infinitesimal” model, selection could generate a weak but detectable excess of allele frequency sweeps compared to strict neutrality with no selection (Figure 1—figure supplement 2D, SNP classes 1/56 and 4/56). However, it would take many replicates (assuming no parallelism) for this excess to become statistically significant. Taken at face value, this result echoes many “evolve-and-resequence” (E&R) experiments based on diverse base populations that show only weak evidence of selective sweeps at specific loci (Burke et al., 2010; Orozco-terWengel et al., 2012).
Broader patterns and analyses of parallelism
On a broader scale, we also observed greater extent of parallelism globally than in the simulated results or with the empirical Ctrl line. For example, out of the 2405 and 2991 loci found above the HINF, no LD cut-off in LS1 and LS2, 398 were found in both lines (13%; χ2 test, N ~ 150,000 windows; χ2 = 2901.4, d.f. = 1, p≤1 × 10−10); whereas we found only 10 or seven overlaps in Ctrl–LS1 or Ctrl–LS2 comparisons, respectively. This difference between the LS1–LS2 in contrast to Ctrl–LS1 or Ctrl–LS2 is statistically significant (940 significant Ctrl loci at the HINF, no LD threshold; N ~ 150,000 windows; Ctrl–LS1: χ2 = 0.7; Ctrl–LS2: χ2 = 6.0; both p=n.s.; see also Figure 3—figure supplement 1). In fact, there was not a single window out of a total of 8.4 million windows from the 100 HINF, max LD replicates where both simulated LS1 and LS2 replicates simultaneously cleared the less stringent HINF, no LD threshold. In contrast to our earlier analysis in single LS replicates, the parallel selected loci in both LS replicates loci may provide the strongest evidence yet to reject the infinitesimal model.
Heritability estimate by an animal model
We estimated heritability using linear mixed effect “animal models” with maximum likelihood (Figure 1—figure supplement 1D) in the R package MCMCglmm v2.5 (Hadfield, 2010; following guide by de Villemereuil, 2019). Because the animal model makes inference of the parameter estimates to the base population, to compare heritability as it changed over time we estimated heritability in blocks of 5 generations F0–4, 5–9, 10–14, and 15–19, separately for each selected line. In testing each block, we used the full pedigree to build the relationship matrix but only phenotypes from the individuals in those generations. As an alternative, we tested each block with a truncated pedigree, in which the first generation of each block is treated as unrelated (i.e., the base population). The two methods produced similar results. In all analyses, we standardized the composite trait (= tibia length in mm; = cube-root body mass in ; see Simulating selection response: infinitesimal model with linkage in main text) within each generation and line to account for fuctuations in mean and variance (Careau et al., 2013). The phenotypic variance was partitioned as VP = fixed effects + VA + VR, where fixed effects were sex, age, and litter size, VA was additive genetic variance, and VR was residual variance. Heritability was estimated as h2 = VA /(VA + VR).
Enrichment for genes with functional impact on limb development
To determine what types of molecular changes may have mediated the selection response, we performed a gene set enrichment analysis. We asked if the outlier loci found in the Longshanks lines were enriched for genes affecting limb development (as indicated by their knockout phenotypes) and found increasingly significant enrichment as the allele frequency shift ∆z2 cut-off became increasingly stringent (Figure 2—figure supplement 4A). The ‘limb/digital/tail’ category of affected anatomical systems in the Mouse Genomic Informatics Gene Expression Database (Finger et al., 2017) showed the greatest excess of observed-to-expected ratio out of all 28 phenotype categories (the excluded ‘normal’ category also showed no enrichment). In contrast, genes showing knock-out phenotypes in most other categories did not show similar enrichment as ∆z2 became more stringent (Figure 2—figure supplement 4A). For genes expressed in limb tissue, there was a similar, but weaker increase, with the enrichment only appearing at higher ∆z2 cut-off. We did not observe similar enrichment using data and thresholds derived from Ctrl (Figure 2—figure supplement 4A, lower panels). To investigate the impact on regulatory sequences, we obtained 21,211 limb enhancers predicted by ENCODE chromatin profile at a stage immediately preceding bone formation (Theiler Stage 23, at approximately embryonic day E14.5) (Shen et al., 2012). We found likewise an enrichment throughout the range of significance cut-offs (Figure 2—figure supplement 4A). Again, there was no similar enrichment in Ctrl.
Clustering with loci associated with human height
Since tibia lengths directly affect human height, we tested if an association exists between loci controlling human height (Wood et al., 2014) and a set of 810 loci at the p≤0.05 significance level under HINF, no LD described here. After remapping the human loci to their orthologous mouse positions (n = 655 out of 697 total height loci; data from the GIANT Consortium), we detected significant clustering with the 810 peak loci (mean pairwise distance to remapped height loci: 1.41 Mbp vs. mean 1.69 Mbp from 1000 permutations of shuffled peak loci, range: 1.45–1.93 Mbp; n = 655 height loci and 810 peak loci; p<0.001, permutations). We interpret this clustering to suggest that a shared and conserved genetic program exist between human height and tibia length and/or body mass.
Genome-wide analysis of the role of coding vs. cis-acting changes in response to selection
We examined the potential functional impact of coding or regulatory changes as a function of ∆z2 in all three lines. For coding changes, we tracked the functional consequences of coding SNPs of moderate to high impact (missense mutations, gain or loss of stop codons, or frame-shifts). Whereas we found only mixed evidence of increased coding changes as ∆z2 increased in the LS lines, there was a depletion of coding changes in Ctrl line as ∆z2 increased, possibly due to purifying or background selection (Figure 2—figure supplement 4B; linear regression, LS1: p≤0.015, slope >0; LS2: p=0.62, n.s., slope ≈ 0; Ctrl: p≤5.72 × 10−9, slope <0).
For regulatory changes, we used sequence conservation in limb enhancers overlapping a SNP as a proxy for functional impact. In contrast to the situation for coding changes, where the correlations differed between LS1 and LS2, the potential impact of regulatory changes increased significantly as a function of ∆z2 in both LS lines (Figure 2—figure supplement 4B): within limb enhancers, SNP-flanking sequences became increasingly conserved at highly differentiated SNPs (phastCons conservation score, ranging from 0 to 1 for unconserved to completely conserved positions; linear regression, log-scale, p<1.05 × 10−9 for both, slopes > 0). This relationship also exists for the Ctrl line, albeit principally from lower ∆z2 and conservation values (p<0.8 × 10−3, slope >0; Figure 2—figure supplement 4B). Taken together, our enrichment analysis suggests that while both coding and regulatory changes were selected in the Longshanks experiment, the overall selection response may depend more consistently on cis-regulatory changes, especially for developmental regulators involved in limb, bone and/or cartilage development (Table 1; Supplementary file 3; c.f. Supplementary file 4 for coding changes). This is a key prediction of the ‘cis-regulatory hypothesis’, especially in its original scope on morphological traits (Carroll, 2008).
Genes with amino acid changes of potentially major impact
We have further identified 12 candidate genes with likely functional impact on limb development due to specific amino acid changes showing large frequency shifts (albeit only one, Fbn2, cleared the stringent p≤0.05 HINF, max LD threshold; six in LS1, nine in LS2, of which three were shared; Supplementary file 4). Consistent with strong selection for tibia development, all 12 genes show limb or tail phenotypes when knocked out, e.g., ‘short limbs’ for the collagen gene Col27a1 knockout. Most of these genes encode for structural cellular components, e.g., myosin, fibrillin and collagen (Myo10; Fbn2; and Col27a1 respectively), with Fuz (fuzzy planar cell polarity protein) being the only classical developmental regulator gene. All but one of these genes have also been shown to have widespread pleiotropic effects with broad expression domains, and their knockouts were often lethal (eight out of 12) and/or exhibit defects in additional organ systems (11 out of 12). Based on this observation, we anticipate that the phenotypic impact of these selected coding missense SNPs (n.b. not knockout) would not be restricted to tibia or bone development.
Molecular dissection of Gli3, a candidate limb regulator, reveal gain-of-function cis-acting changes
We anticipated that genes related to major limb patterning, like Gli3, may contribute to the selection response ( Mo et al., 1997; Nakamura et al., 2015). We thus performed an in-depth molecular dissection of Gli3, an important early limb developmental regulator on chromosome 13 (Chr13; Figure 4—figure supplement 1A). This locus showed a substantial shift in minor allele frequency of up to 0.42 in LS1 (∆q, 98th quantile genome-wide, but below the HINF, max LD threshold to qualify as a discrete major locus). We performed functional validation of Gli3, given its limb function (Büscher et al., 1997) and considering that Gli3 could be among the many minor loci in the polygenic background contributing to the selection response in LS1.
At the Gli3 locus we could only find conservative amino acid changes (D1090E and I1326V) that are unlikely to impact protein function. Because the signal in LS1 was stronger in the 5’ flanking intergenic region, we examined the Gli3 cis-regulatory topologically associating domain (TADs, which mark chromosome segments with shared gene regulatory logic) (Dixon et al., 2012) and identified putative enhancers using chromatin modification marks from the ENCODE project and our own ATAC-Seq data (Figure 4—figure supplement 1B) (Buenrostro et al., 2013; Shen et al., 2012). Four putative enhancers carried SNPs with large allele frequency changes. Among them, an upstream putative enhancer G2 (956 bp) carried 6 SNPs along with two 1- and 3 bp insertion/deletion (‘indel’) with putative functional impact due to predicted gain or loss in transcription factor binding sites (Figure 4—figure supplement 1C). We tested the G2 putative enhancer in a transgenic reporter assay by placing its sequence as a tandem duplicate upstream of a lacZ reporter gene (see Methods for details). We found that only the F17 LS1 allele was able to drive consistent lacZ expression in the developing limb buds (Figure 4—figure supplement 1D). Importantly, this enhancer was active not only in the shaft of the limb bud but also in the anterior hand/foot plate, a major domain of Gli3 expression and function (Figure 4—figure supplement 1A). Furthermore, substitution of the enhancer sequence with the F0 allele (10 differences out of 956 or 960 bp) abolished lacZ expression (Figure 4—figure supplement 1D). This showed that 10 or fewer changes within this novel enhancer sequence were sufficient to convert the inactive F0 allele into an active limb enhancer corresponding to the selected F17 allele (‘gain-of-function’), suggesting that a standing genetic variant of the F17 allele may have been selectively favored because it drove stronger expression of Gli3, a gene essential for tibia development (Akiyama et al., 2015, but see Koziel et al., 2005).
Estimating the selection coefficient of the top-ranking locus, Nkx3-2, from changes in allele frequency
The significant locus on Chr5 containing Nkx3-2 shows strong changes in SNP frequency in both LS1 and LS2. Here, we estimate the strength of selection on this locus, and the corresponding effect on the selected trait. We approximate by assuming two alternative alleles, and find the selection coefficient implied by the observed parallel changes in allele frequency; we then set bounds on this estimate that take account of random drift. Finally, we use simulations that condition on the known pedigree to estimate the effect on the trait required to cause the observed strong frequency changes; these show that linked selection has little effect on the single-locus estimates.
We see strong and parallel changes in allele frequency at multiple steps. There are 14 non-overlapping 10kb windows that have a mean square change in arc-sin transformed allele frequency of in both LS1 and LS2, spanning a 260 kbp region and including 807 SNP. SNP frequencies are tightly clustered, corresponding to two alternative haplotypes (Figure 5 and Figure 5—figure supplement 1A). The initial (untransformed) allele frequencies average q0 = 0.18, 0.17 in LS1, LS2, respectively, and the final frequencies average q17 = 0.84, 0.98, respectively (also see Figure 5—figure supplement 1A, lower panel). These frequencies depend on the arbitrary threshold for which windows to include. However, this makes little difference, relative to the wide bounds on our estimates.
Under constant selection, changes linearly with time, at a rate equal to the selection coefficient, . Therefore, a naive estimate of selection is given by (Haldane, 1932) thus, = 0.19, 0.32 for q in LS1, LS2, and averages 0.26. Here, males and females with longest tibia are chosen to breed; the strength of selection on an additive allele depends on the fraction selected and the within-family trait variance. The former is kept constant, and there is little loss of variance due to drift (~0.17). Thus, assuming constant selection is reasonable (Figure 5B), unless there is strong dominance.
To set bounds on this estimate, we must account for random drift. The predicted loss of diversity over 17 generations, based on the pedigree, is =0.173, 0.175 for LS1, LS2, which corresponds to an effective size , respectively (note that due to differences in estimation methodology, this differs slightly from that mentioned in Figure 1—figure supplement 2 but is largely consistent). Therefore, we calculate the matrix of transition probabilities for a Wright–Fisher population with rounded to 90, 89 copies for LS1, LS2, over a range of selection,. This yields the probability that the number of copies would change from the rounded values of 16/90 to 75/90 in LS1, and from 14/89 to 87/89 in LS2—that is, the likelihood of , given the observed changes in allele frequency, and the known . There is no significant loss of likelihood by assuming the same selection in both lines; overall, = 0.24 (limits 0.13–0.36; Figure 5—figure supplement 1B).
Estimating the selection coefficient, accounting for linked loci
The estimates above using the simple approach do not account for selection on linked loci, and do not give the effect on the composite trait. We therefore simulated conditional on the pedigree and on the actual selection regime, as described above, but including an additive allele with effect A at the candidate locus on Chr5. The genetic variance associated with the unlinked infinitesimal background, and across Chr5, were reduced in proportion, to keep the overall heritability the same as before 0.539. The selection coefficient inferred from the simulated changes in allele frequency was approximately proportional to the effect on the trait, with best fit (Figure 5—figure supplement 1C, left). Assuming this relationship, we can compare the mean and standard deviation of allele frequency from simulations with linked selection, with that predicted by the single locus Wright–Fisher model (points vs. line in Figure 5—figure supplement 1C, middle and right). These agree well, showing that linked selection does not appreciably change the distribution of allele frequencies at a single locus. This is consistent with Figure 1—figure supplement 2D, which shows that linked selection only inflates the tail of the allele frequency distribution, an effect that would not be detectable at a single locus.
Combining our estimates of the selection coefficient with the relation , we estimate that the locus on Chr5 has effect , with 2-unit support limits to . This single locus is responsible for ~9.4% of the total selection response (limits 3.6–15.5%).
This analysis does not allow for the inflation of effect that might arise from multiple testing. This is hard to estimate, because it depends on the distribution of effects across the genome, and also on the excess variation in estimates due to LD in the founder population. However, we note that if the effect of this locus is large enough that it would certainly be detected in this study, then there is no estimation bias from this source.
We also assume that there are two haplotypes, each with a definite effect. There might in fact be heterogeneity in the effects of each haplotype, for two reasons. First, this region might have had heterogeneous effects in the founder population, with multiple alleles at multiple causal loci. Second, as recombination breaks up the founder genomes, blocks of genome would become associated with different backgrounds. To the extent that genetic variation is spread evenly over an infinitesimal background, this latter effect is accounted for by our simulations, and has little consequence. However, we have not tested whether the data might be explained by more than two alleles, possibly at more than one discrete locus. Testing such complex models would be challenging, and we do not believe that such test would have much power. However, the estimates of selection made here should be regarded as effective values that may reflect a more complex reality.
Estimating the contribution of the Nkx3-2 locus using an animal model
We used a linear mixed ‘animal model’ to estimate the effect of the enhancer N3 (of the major locus in Nkx3-2) on the composite selected trait , see Section 'Simulating selection response: infinitesimal model with linkage' and Figure 1—figure supplement 2A. The model was:
where: fixed effects = sex, generation, litter size (i.e., number of siblings in family), genotype at N3 (0, 1, or 2 copies of F17 allele), and replicate line
VA = additive genetic variance
VR = residual variance
We found a small but significant effect of the genotype at enhancer N3 on the composite trait (mean effect = 0.36%; 95% CI: 0.069–0.64%; p=0.017). Given the same body mass , the mean effect corresponds to 0.36% increase in tibia length per copy of the F17 allele, or ~1% of the variance in tibia length at generation F01. The observed increase of this allele from ~0.18 to 0.91, averaged over the two lines, implies that it accounts for ~4% of the total selection response. This is within the confidence limits in the main text, based on the change in SNP frequency (3.6–15.5%) and note that the latter may be biased upwards by ascertainment. However, the exact effect of the allele is difficult to pinpoint in any given generation or population due the nature of the composite trait and change in variance in the composite trait over generations.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Contributor Information
Campbell Rolian, Email: cprolian@ucalgary.ca.
Yingguang Frank Chan, Email: frank.chan@tue.mpg.de.
Magnus Nordborg, Austrian Academy of Sciences, Austria.
Patricia J Wittkopp, University of Michigan, United States.
Funding Information
This paper was supported by the following grant:
Natural Sciences and Engineering Research Council of Canada 4181932 to Campbell Rolian.
Additional information
Competing interests
No competing interests declared.
Author contributions
Data curation, Formal analysis, Validation, Investigation, Visualization, Methodology, Writing—original draft, Writing—review and editing.
Data curation, Formal analysis, Validation, Investigation, Methodology, Writing—original draft, Writing—review and editing.
Resources, Data curation, Methodology, Writing—review and editing.
Software, Formal analysis, Methodology, Writing—review and editing.
Formal analysis, Validation, Visualization, Writing—review and editing.
Resources, Data curation, Formal analysis, Validation, Investigation, Methodology, Writing—review and editing.
Resources, Data curation, Validation, Writing—review and editing.
Resources, Investigation, Methodology, Writing—review and editing.
Formal analysis, Investigation, Visualization, Methodology, Writing—review and editing.
Supervision, Investigation, Methodology, Writing—review and editing.
Conceptualization, Data curation, Software, Formal analysis, Supervision, Validation, Investigation, Visualization, Methodology, Writing—original draft, Writing—review and editing.
Conceptualization, Resources, Data curation, Formal analysis, Supervision, Funding acquisition, Validation, Investigation, Visualization, Methodology, Writing—original draft, Project administration, Writing—review and editing.
Conceptualization, Resources, Data curation, Software, Formal analysis, Supervision, Funding acquisition, Validation, Investigation, Visualization, Methodology, Writing—original draft, Project administration, Writing—review and editing.
Ethics
Animal experimentation: All experimental procedures described in this study have been approved by the applicable University institutional ethics committee for animal welfare at the University of Calgary (HSACC Protocols M08146 and AC13-0077); or local competent authority: Landesdirektion Sachsen, Germany, permit number 24-9168.11-9/2012-5.
Additional files
For each line and generation, we individually barcoded all available individuals and pooled for sequencing. We aimed for a sequencing depth of around 100x coverage for 50–64 haplotypes per sample. Since the CD-1 mice were founded by an original import of 7 inbred females and two inbred males, we expect a maximum of 18 segregating haplotypes at any given locus. This sequencing design should give sufficient coverage to recover allele frequencies and possibly genotypes genome-wide. Our successful genome-wide imputation results validated this strategy.
As expected, there is a general trend of decrease in diversity after 17 generations of breeding. Globally, there was a 13% decrease in diversity, but F17 populations still retained on average ~5.8M segregating SNPs (diagonal). There was very little population differentiation, as indicated by low FST among the three founder populations, however FST increases by at least 100-fold among lines by generation F17 (above diagonal, orange boxes). Within-line FST is intermediate in this respect, reaching about half of the differentiation observed between lines.
Listed here are the eight loci shown in Table 1, with additional details on the core span and the TAD span used to identify candidate genes, and a full list of genes within the full span.
Listed are genes carrying large frequency changing SNPs affecting amino acid residues. Highlighted cells indicate the line with greater frequency changes ≥ 0.34 (red text with shading). Other suggestive changes are also shown with red numbers in unshaded cells. The changed amino acids are marked using standard notations, with the directionality indicated as ‘purifying’ or ‘diversifying’ with respect to a 60-way protein sequence alignment with other placental mammals. The conservation score based on phastCons was calculated at the SNP position itself, ranging from 0 (no conservation) to 1 (complete conservation) among the 60 placental mammals. For each gene, reported knockout phenotypes of the ‘limbs/digits/tail’ category is reported, along with whether lethality was reported in any of the alleles, excluding compound genotypes. A summary of the mutant phenotype as reported by the Mouse Genome Informatics database is also included to highlight any systemic defects beyond the ‘limbs/digits/tail’ category (lethal phenotypes reported in bold).
The 4C-seq adapter and adapter-specific primer sequences are given in Sexton et al. (2012). N2-DS denotes its location as 18 kbp downstream of the actual N2 enhancer. All viewpoints are pointed towards Nkx3-2 gene body ('+' strand).
Each of the amplicons are tagged with SalI (forward) or XhoI (reverse) sites (underlined) for concatenation and flanked by EcoRV sites (underlined and bold) for insertion into the pBeta-lacZ-attBx2 reporter vector upstream of the β-globin minimal promoter.
The primers were designed to target two SNPs (bold) in the N3 enhancer, rs33219710 and rs33600994.
Data availability
Sequencing data have been deposited in SRA (accession number SRP165718), GEO (accession numbers GSE121564, GSE121565 and GSE121566). Non-sequence data have been deposited at Dryad (doi:10.5061/dryad.0q2h6tk). Analytical code and additional notes have been deposited in the following repository: https://github.com/evolgenomics/Longshanks (copy archived at https://github.com/elifesciences-publications/Longshanks). Additional raw data and code are hosted via our institute's FTP servers at http://ftp.tuebingen.mpg.de/fml/ag-chan/Longshanks/.
The following datasets were generated:
Castro JPL, Yancoskie MN, Marchini M, Belohlavy S, Hiramatsu L, Kučka M, Beluch WH, Naumann R, Skuplik IO, Cobb J, Barton NH, Rolian CP, Chan YF. 2019. An integrative genomic analysis of the Longshanks selection experiment for longer limbs in mice. NCBI Sequence Read Archive. SRP165718
Castro JPL, Yancoskie MN, Marchini M, Belohlavy S, Hiramatsu L, Kučka M, Beluch WH, Naumann R, Skuplik IO, Cobb J, Barton NH, Rolian CP, Chan YF. 2019. An integrative genomic analysis of the Longshanks selection experiment for longer limbs in mice. NCBI Gene Expression Omnibus. GSE121564
Castro JPL, Yancoskie MN, Marchini M, Belohlavy S, Hiramatsu L, Kučka M, Beluch WH, Naumann R, Skuplik IO, Cobb J, Barton NH, Rolian CP, Chan YF. 2019. An integrative genomic analysis of the Longshanks selection experiment for longer limbs in mice. NCBI Gene Expression Omnibus. GSE121565
Castro JPL, Yancoskie MN, Marchini M, Belohlavy S, Hiramatsu L, Kučka M, Beluch WH, Naumann R, Skuplik IO, Cobb J, Barton NH, Rolian CP, Chan YF. 2019. An integrative genomic analysis of the Longshanks selection experiment for longer limbs in mice. NCBI Gene Expression Omnibus. GSE121566
Castro JPL, Yancoskie MN, Marchini M, Belohlavy S, Hiramatsu L, Kučka M, Beluch WH, Naumann R, Skuplik IO, Cobb J, Barton NH, Rolian CP, Chan YF. 2019. Data from: An integrative genomic analysis of the Longshanks selection experiment for longer limbs in mice. Dryad Digital Repository.
The following previously published datasets were used:
Keane TM, Goodstadt L, Danecek P, White MA, Wong K. 2011. Mouse Genomes Project version 3 dbSNP v137 release. Wellcome Sanger Institute. dbSNP v137 release
Shen Y, Yue F, McCleary DF, Ye Z, Edsall L, Kuan S, Wagner U, Dixon J, Lee L, Lobanenkov VV, Ren B. 2012. A map of the cis-regulatory sequences in the mouse genome. ENCODE Experiment Matrix. Mouse E14.5 Limb
Smith CL, Blake JA, Kadin JA, Richardson JE, Bult CJ, the Mouse Genome Database Group. 2018. Mouse knockout phenotypes. Mouse Genome Informatics. MGI_PhenotypicAllele
Wood AR, Esko T, Yang J, Vedantam S. 2014. Defining the role of common variation in the genomic and biological architecture of adult human height. GIANT consortium. GWAS Anthropometric 2014 Height
References
- Akazawa H, Komuro I, Sugitani Y, Yazaki Y, Nagai R, Noda T. Targeted disruption of the homeobox transcription factor Bapx1 results in lethal skeletal dysplasia with asplenia and gastroduodenal malformation. Genes to Cells. 2000;5:499–513. doi: 10.1046/j.1365-2443.2000.00339.x. [DOI] [PubMed] [Google Scholar]
- Akiyama R, Kawakami H, Wong J, Oishi I, Nishinakamura R, Kawakami Y. Sall4-Gli3 system in early limb progenitors is essential for the development of limb skeletal elements. PNAS. 2015;112:5075–5080. doi: 10.1073/pnas.1421949112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews S. FastQC A quality control tool for high throughput sequence data. [August 5, 2016];2016 http://www.bioinformatics.babraham.ac.uk/projects/fastqc/
- Barton NH. Linkage and the limits to natural selection. Genetics. 1995;140:821–841. doi: 10.1093/genetics/140.2.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton NH, Hermisson J, Nordborg M. Why structure matters. eLife. 2019;8:e45380. doi: 10.7554/eLife.45380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg JJ, Coop G. A population genetic signal of polygenic adaptation. PLOS Genetics. 2014;10:e1004412. doi: 10.1371/journal.pgen.1004412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger MF, Badis G, Gehrke AR, Talukder S, Philippakis AA, Peña-Castillo L, Alleyne TM, Mnaimneh S, Botvinnik OB, Chan ET, Khalid F, Zhang W, Newburger D, Jaeger SA, Morris QD, Bulyk ML, Hughes TR. Variation in homeodomain DNA binding revealed by high-resolution analysis of sequence preferences. Cell. 2008;133:1266–1276. doi: 10.1016/j.cell.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bren-Mattison Y, Hausburg M, Olwin BB. Growth of limb muscle is dependent on skeletal-derived Indian hedgehog. Developmental Biology. 2011;356:486–495. doi: 10.1016/j.ydbio.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SD, Chambon P, de Angelis MH, Eumorphia Consortium EMPReSS: standardized phenotype screens for functional annotation of the mouse genome. Nature Genetics. 2005;37:1155. doi: 10.1038/ng1105-1155. [DOI] [PubMed] [Google Scholar]
- Browning BL, Browning SR. Genotype imputation with millions of reference samples. The American Journal of Human Genetics. 2016;98:116–126. doi: 10.1016/j.ajhg.2015.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buenrostro JD, Giresi PG, Zaba LC, Chang HY, Greenleaf WJ. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nature Methods. 2013;10:1213–1218. doi: 10.1038/nmeth.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bult A, Lynch CB. Breaking through artificial selection limits of an adaptive behavior in mice and the consequences for correlated responses. Behavior Genetics. 2000;30:193–206. doi: 10.1023/a:1001962124005. [DOI] [PubMed] [Google Scholar]
- Burke MK, Dunham JP, Shahrestani P, Thornton KR, Rose MR, Long AD. Genome-wide analysis of a long-term evolution experiment with Drosophila. Nature. 2010;467:587–590. doi: 10.1038/nature09352. [DOI] [PubMed] [Google Scholar]
- Büscher D, Bosse B, Heymer J, Rüther U. Evidence for genetic control of Sonic hedgehog by Gli3 in mouse limb development. Mechanisms of Development. 1997;62:175–182. doi: 10.1016/S0925-4773(97)00656-4. [DOI] [PubMed] [Google Scholar]
- Careau V, Wolak ME, Carter PA, Garland T. Limits to behavioral evolution: the quantitative genetics of a complex trait under directional selection. Evolution. 2013;67:3102–3119. doi: 10.1111/evo.12200. [DOI] [PubMed] [Google Scholar]
- Carlborg O, Jacobsson L, Ahgren P, Siegel P, Andersson L. Epistasis and the release of genetic variation during long-term selection. Nature Genetics. 2006;38:418–420. doi: 10.1038/ng1761. [DOI] [PubMed] [Google Scholar]
- Carroll SB. Evo-devo and an expanding evolutionary synthesis: a genetic theory of morphological evolution. Cell. 2008;134:25–36. doi: 10.1016/j.cell.2008.06.030. [DOI] [PubMed] [Google Scholar]
- Chan YF, Marks ME, Jones FC, Villarreal G, Shapiro MD, Brady SD, Southwick AM, Absher DM, Grimwood J, Schmutz J, Myers RM, Petrov D, Jónsson B, Schluter D, Bell MA, Kingsley DM. Adaptive evolution of pelvic reduction in sticklebacks by recurrent deletion of a Pitx1 enhancer. Science. 2010;327:302–305. doi: 10.1126/science.1182213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan YF, Jones FC, McConnell E, Bryk J, Bünger L, Tautz D. Parallel selection mapping using artificially selected mice reveals body weight control loci. Current Biology. 2012;22:794–800. doi: 10.1016/j.cub.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Chen N, Juric I, Cosgrove EJ, Bowman R, Fitzpatrick JW, Schoech SJ, Clark AG, Coop G. Allele frequency dynamics in a pedigreed natural population. PNAS. 2019;116:2158–2164. doi: 10.1073/pnas.1813852116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cingolani P, Platts A, Wang leL, Coon M, Nguyen T, Wang L, Land SJ, Lu X, Ruden DM. A program for annotating and predicting the effects of single Nucleotide Polymorphisms, SnpEff: snps in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly. 2012;6:80–92. doi: 10.4161/fly.19695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox A, Ackert-Bicknell CL, Dumont BL, Ding Y, Bell JT, Brockmann GA, Wergedal JE, Bult C, Paigen B, Flint J, Tsaih SW, Churchill GA, Broman KW. A new standard genetic map for the laboratory mouse. Genetics. 2009;182:1335–1344. doi: 10.1534/genetics.109.105486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotwell PL, Mabee PM. Gene expression patterns underlying proximal-distal skeletal segmentation in late-stage zebrafish, danio rerio. Developmental Dynamics. 2007;236:3111–3128. doi: 10.1002/dvdy.21352. [DOI] [PubMed] [Google Scholar]
- Crow JF, Kimura M. Evolution in sexual and asexual populations. The American Naturalist. 1965;99:439–450. doi: 10.1086/282389. [DOI] [Google Scholar]
- Danecek P, Auton A, Abecasis G, Albers CA, Banks E, DePristo MA, Handsaker RE, Lunter G, Marth GT, Sherry ST, McVean G, Durbin R, 1000 Genomes Project Analysis Group The variant call format and VCFtools. Bioinformatics. 2011;27:2156–2158. doi: 10.1093/bioinformatics/btr330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin C. On the Origin of Species by Means of Natural Selection. London: John Murray; 1859. [Google Scholar]
- de Villemereuil P. Estimation of a biological trait heritability using the animal model: how to use the R package MCMCglmm. [March 21, 2019];2019 http://devillemereuil.legtux.org/wp-content/uploads/2012/12/tuto_en.pdf
- DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, Hartl C, Philippakis AA, del Angel G, Rivas MA, Hanna M, McKenna A, Fennell TJ, Kernytsky AM, Sivachenko AY, Cibulskis K, Gabriel SB, Altshuler D, Daly MJ. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nature Genetics. 2011;43:491–498. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiLeone RJ, Marcus GA, Johnson MD, Kingsley DM. Efficient studies of long-distance Bmp5 gene regulation using bacterial artificial chromosomes. PNAS. 2000;97:1612–1617. doi: 10.1073/pnas.97.4.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon JR, Selvaraj S, Yue F, Kim A, Li Y, Shen Y, Hu M, Liu JS, Ren B. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature. 2012;485:376–380. doi: 10.1038/nature11082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENCODE Project Consortium An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein B, Jones M, Hamede R, Hendricks S, McCallum H, Murchison EP, Schönfeld B, Wiench C, Hohenlohe P, Storfer A. Rapid evolutionary response to a transmissible cancer in Tasmanian devils. Nature Communications. 2016;7:12684. doi: 10.1038/ncomms12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evolgenomics . Github; 2019. https://github.com/evolgenomics/Longshanks [Google Scholar]
- Falconer DS, Mackay TF. Introduction to Quantitative Genetics. Fourth Edition. London: Pearson; 1996. [Google Scholar]
- Finger JH, Smith CM, Hayamizu TF, McCright IJ, Xu J, Law M, Shaw DR, Baldarelli RM, Beal JS, Blodgett O, Campbell JW, Corbani LE, Lewis JR, Forthofer KL, Frost PJ, Giannatto SC, Hutchins LN, Miers DB, Motenko H, Stone KR, Eppig JT, Kadin JA, Richardson JE, Ringwald M. The mouse gene expression database (GXD): 2017 update. Nucleic Acids Research. 2017;45:D730–D736. doi: 10.1093/nar/gkw1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest AR, Kawaji H, Rehli M, Baillie JK, de Hoon MJ, Haberle V, Lassmann T, Kulakovskiy IV, Lizio M, Itoh M, Andersson R, Mungall CJ, Meehan TF, Schmeier S, Bertin N, Jørgensen M, Dimont E, Arner E, Schmidl C, Schaefer U, Medvedeva YA, Plessy C, Vitezic M, Severin J, Semple C, Ishizu Y, Young RS, Francescatto M, Alam I, Albanese D, Altschuler GM, Arakawa T, Archer JA, Arner P, Babina M, Rennie S, Balwierz PJ, Beckhouse AG, Pradhan-Bhatt S, Blake JA, Blumenthal A, Bodega B, Bonetti A, Briggs J, Brombacher F, Burroughs AM, Califano A, Cannistraci CV, Carbajo D, Chen Y, Chierici M, Ciani Y, Clevers HC, Dalla E, Davis CA, Detmar M, Diehl AD, Dohi T, Drabløs F, Edge AS, Edinger M, Ekwall K, Endoh M, Enomoto H, Fagiolini M, Fairbairn L, Fang H, Farach-Carson MC, Faulkner GJ, Favorov AV, Fisher ME, Frith MC, Fujita R, Fukuda S, Furlanello C, Furino M, Furusawa J, Geijtenbeek TB, Gibson AP, Gingeras T, Goldowitz D, Gough J, Guhl S, Guler R, Gustincich S, Ha TJ, Hamaguchi M, Hara M, Harbers M, Harshbarger J, Hasegawa A, Hasegawa Y, Hashimoto T, Herlyn M, Hitchens KJ, Ho Sui SJ, Hofmann OM, Hoof I, Hori F, Huminiecki L, Iida K, Ikawa T, Jankovic BR, Jia H, Joshi A, Jurman G, Kaczkowski B, Kai C, Kaida K, Kaiho A, Kajiyama K, Kanamori-Katayama M, Kasianov AS, Kasukawa T, Katayama S, Kato S, Kawaguchi S, Kawamoto H, Kawamura YI, Kawashima T, Kempfle JS, Kenna TJ, Kere J, Khachigian LM, Kitamura T, Klinken SP, Knox AJ, Kojima M, Kojima S, Kondo N, Koseki H, Koyasu S, Krampitz S, Kubosaki A, Kwon AT, Laros JF, Lee W, Lennartsson A, Li K, Lilje B, Lipovich L, Mackay-Sim A, Manabe R, Mar JC, Marchand B, Mathelier A, Mejhert N, Meynert A, Mizuno Y, de Lima Morais DA, Morikawa H, Morimoto M, Moro K, Motakis E, Motohashi H, Mummery CL, Murata M, Nagao-Sato S, Nakachi Y, Nakahara F, Nakamura T, Nakamura Y, Nakazato K, van Nimwegen E, Ninomiya N, Nishiyori H, Noma S, Noma S, Noazaki T, Ogishima S, Ohkura N, Ohimiya H, Ohno H, Ohshima M, Okada-Hatakeyama M, Okazaki Y, Orlando V, Ovchinnikov DA, Pain A, Passier R, Patrikakis M, Persson H, Piazza S, Prendergast JG, Rackham OJ, Ramilowski JA, Rashid M, Ravasi T, Rizzu P, Roncador M, Roy S, Rye MB, Saijyo E, Sajantila A, Saka A, Sakaguchi S, Sakai M, Sato H, Savvi S, Saxena A, Schneider C, Schultes EA, Schulze-Tanzil GG, Schwegmann A, Sengstag T, Sheng G, Shimoji H, Shimoni Y, Shin JW, Simon C, Sugiyama D, Sugiyama T, Suzuki M, Suzuki N, Swoboda RK, 't Hoen PA, Tagami M, Takahashi N, Takai J, Tanaka H, Tatsukawa H, Tatum Z, Thompson M, Toyodo H, Toyoda T, Valen E, van de Wetering M, van den Berg LM, Verado R, Vijayan D, Vorontsov IE, Wasserman WW, Watanabe S, Wells CA, Winteringham LN, Wolvetang E, Wood EJ, Yamaguchi Y, Yamamoto M, Yoneda M, Yonekura Y, Yoshida S, Zabierowski SE, Zhang PG, Zhao X, Zucchelli S, Summers KM, Suzuki H, Daub CO, Kawai J, Heutink P, Hide W, Freeman TC, Lenhard B, Bajic VB, Taylor MS, Makeev VJ, Sandelin A, Hume DA, Carninci P, Hayashizaki Y, FANTOM Consortium and the RIKEN PMI and CLST (DGT) A promoter-level mammalian expression atlas. Nature. 2014;507:462–470. doi: 10.1038/nature13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland T, Rose MR. Experimental Evolution: Concepts, Methods, and Applications of Selection Experiments. Berkeley: University of California Press; 2009. [Google Scholar]
- Gingerich PD. Rates of evolution on the time scale of the evolutionary process. Genetica. 2001;112-113:127–144. [PubMed] [Google Scholar]
- Grant PR, Grant BR. Demography and the genetically effective sizes of two populations of Darwin's Finches. Ecology. 1992;73:766–784. doi: 10.2307/1940156. [DOI] [Google Scholar]
- Grant PR, Grant BR. Unpredictable evolution in a 30-year study of Darwin's finches. Science. 2002;296:707–711. doi: 10.1126/science.1070315. [DOI] [PubMed] [Google Scholar]
- Hadfield J. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. Journal of Statistical Software. 2010;33 doi: 10.18637/jss.v033.i02. [DOI] [Google Scholar]
- Haldane JBS. The Causes of Evolution. London: Green and Co; 1932. [Google Scholar]
- Hancock AM, Witonsky DB, Alkorta-Aranburu G, Beall CM, Gebremedhin A, Sukernik R, Utermann G, Pritchard JK, Coop G, Di Rienzo A. Adaptations to climate-mediated selective pressures in humans. PLOS Genetics. 2011;7:e1001375. doi: 10.1371/journal.pgen.1001375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellemans J, Simon M, Dheedene A, Alanay Y, Mihci E, Rifai L, Sefiani A, van Bever Y, Meradji M, Superti-Furga A, Mortier G. Homozygous inactivating mutations in the NKX3-2 gene result in spondylo-megaepiphyseal-metaphyseal dysplasia. The American Journal of Human Genetics. 2009;85:916–922. doi: 10.1016/j.ajhg.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry AP, Kinnison MT. Perspective: the pace of modern life: measuring rates of contemporary microevolution. Evolution. 1999;53:1637–1653. doi: 10.1111/j.1558-5646.1999.tb04550.x. [DOI] [PubMed] [Google Scholar]
- Hill WG. Predictions of response to artificial selection from new mutations. Genetical Research. 1982;40:255–278. doi: 10.1017/S0016672300019145. [DOI] [PubMed] [Google Scholar]
- Hill WG, Goddard ME, Visscher PM. Data and theory point to mainly additive genetic variance for complex traits. PLOS Genetics. 2008;4:e1000008. doi: 10.1371/journal.pgen.1000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill WG, Robertson A. The effect of linkage on limits to artificial selection. Genetical Research. 1966;8:269–294. doi: 10.1017/S0016672300010156. [DOI] [PubMed] [Google Scholar]
- Hill WG, Zhang XS. On the pleiotropic structure of the genotype-phenotype map and the evolvability of complex organisms. Genetics. 2012;190:1131–1137. doi: 10.1534/genetics.111.135681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann AA, Sgrò CM. Climate change and evolutionary adaptation. Nature. 2011;470:479–485. doi: 10.1038/nature09670. [DOI] [PubMed] [Google Scholar]
- Jha AR, Miles CM, Lippert NR, Brown CD, White KP, Kreitman M. Whole-genome resequencing of experimental populations reveals polygenic basis of egg-size variation in Drosophila melanogaster. Molecular Biology and Evolution. 2015;32:2616–2632. doi: 10.1093/molbev/msv136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones FC, Grabherr MG, Chan YF, Russell P, Mauceli E, Johnson J, Swofford R, Pirun M, Zody MC, White S, Birney E, Searle S, Schmutz J, Grimwood J, Dickson MC, Myers RM, Miller CT, Summers BR, Knecht AK, Brady SD, Zhang H, Pollen AA, Howes T, Amemiya C, Baldwin J, Bloom T, Jaffe DB, Nicol R, Wilkinson J, Lander ES, Di Palma F, Lindblad-Toh K, Kingsley DM, Broad Institute Genome Sequencing Platform & Whole Genome Assembly Team The genomic basis of adaptive evolution in threespine sticklebacks. Nature. 2012;484:55–61. doi: 10.1038/nature10944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane TM, Goodstadt L, Danecek P, White MA, Wong K, Yalcin B, Heger A, Agam A, Slater G, Goodson M, Furlotte NA, Eskin E, Nellåker C, Whitley H, Cleak J, Janowitz D, Hernandez-Pliego P, Edwards A, Belgard TG, Oliver PL, McIntyre RE, Bhomra A, Nicod J, Gan X, Yuan W, van der Weyden L, Steward CA, Bala S, Stalker J, Mott R, Durbin R, Jackson IJ, Czechanski A, Guerra-Assunção JA, Donahue LR, Reinholdt LG, Payseur BA, Ponting CP, Birney E, Flint J, Adams DJ. Mouse genomic variation and its effect on phenotypes and gene regulation. Nature. 2011;477:289–294. doi: 10.1038/nature10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keightley PD, Hardge T, May L, Bulfield G. A genetic map of quantitative trait loci for body weight in the mouse. Genetics. 1996;142:227–235. doi: 10.1093/genetics/142.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keightley PD, Bünger L, Renne U, Buis RC. In: Encyclopedia of Genetics. Reeve E C R, editor. Chicago: Fitzroy Dearborn Publishers; 2001. [Google Scholar]
- Kelly JK, Hughes K. An examination of the evolve-and-resequence method using Drosophila simulans. bioRxiv. 2018 doi: 10.1101/337188. [DOI]
- Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, Haussler D. The human genome browser at UCSC. Genome Research. 2002;12:996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessner D, Novembre J. Power analysis of artificial selection experiments using efficient whole genome simulation of quantitative traits. Genetics. 2015;199:991–1005. doi: 10.1534/genetics.115.175075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koziel L, Wuelling M, Schneider S, Vortkamp A. Gli3 acts as a repressor downstream of Ihh in regulating two distinct steps of chondrocyte differentiation. Development. 2005;132:5249–5260. doi: 10.1242/dev.02097. [DOI] [PubMed] [Google Scholar]
- Langmead B, Salzberg SL. Fast gapped-read alignment with bowtie 2. Nature Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, 1000 Genome Project Data Processing Subgroup The sequence alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Durbin R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics. 2010;26:589–595. doi: 10.1093/bioinformatics/btp698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch M, Walsh B. Genetics and Analysis of Quantitative Traits. Sinauer; 1998. [Google Scholar]
- Makvandi-Nejad S, Hoffman GE, Allen JJ, Chu E, Gu E, Chandler AM, Loredo AI, Bellone RR, Mezey JG, Brooks SA, Sutter NB. Four loci explain 83% of size variation in the horse. PLOS ONE. 2012;7:e39929. doi: 10.1371/journal.pone.0039929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchini M, Sparrow LM, Cosman MN, Dowhanik A, Krueger CB, Hallgrimsson B, Rolian C. Impacts of genetic correlation on the independent evolution of body mass and skeletal size in mammals. BMC Evolutionary Biology. 2014;14:258. doi: 10.1186/s12862-014-0258-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchini M, Rolian C. Artificial selection sheds light on developmental mechanisms of limb elongation. Evolution. 2018;72:825–837. doi: 10.1111/evo.13447. [DOI] [PubMed] [Google Scholar]
- Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. 2011;10 doi: 10.14806/ej.17.1.200. [DOI] [Google Scholar]
- Martin A, Orgogozo V. The loci of repeated evolution: a catalog of genetic hotspots of phenotypic variation. Evolution. 2013;22:1235–1250. doi: 10.1111/evo.12081. [DOI] [PubMed] [Google Scholar]
- Maynard Smith J, Haigh J. The hitch-hiking effect of a favourable gene. Genetical Research. 1974;23:23–35. doi: 10.1017/S0016672300014634. [DOI] [PubMed] [Google Scholar]
- McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, DePristo MA. The genome analysis toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Research. 2010;20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae AF, Pemberton JM, Visscher PM. Modeling linkage disequilibrium in natural populations: the example of the Soay sheep population of St. Kilda, Scotland. Genetics. 2005;171:251–258. doi: 10.1534/genetics.105.040972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milani P, Escalante-Chong R, Shelley BC, Patel-Murray NL, Xin X, Adam M, Mandefro B, Sareen D, Svendsen CN, Fraenkel E. Cell freezing protocol suitable for ATAC-Seq on motor neurons derived from human induced pluripotent stem cells. Scientific Reports. 2016;6:25474. doi: 10.1038/srep25474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo R, Freer AM, Zinyk DL, Crackower MA, Michaud J, Heng HH, Chik KW, Shi XM, Tsui LC, Cheng SH, Joyner AL, Hui C. Specific and redundant functions of Gli2 and Gli3 zinc finger genes in skeletal patterning and development. Development. 1997;124:113–123. doi: 10.1242/dev.124.1.113. [DOI] [PubMed] [Google Scholar]
- Mortlock DP, Guenther C, Kingsley DM. A general approach for identifying distant regulatory elements applied to the Gdf6 gene. Genome Research. 2003;13:2069–2081. doi: 10.1101/gr.1306003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Klomp J, Pieretti J, Schneider I, Gehrke AR, Shubin NH. Molecular mechanisms underlying the exceptional adaptations of batoid fins. PNAS. 2015;112:15940–15945. doi: 10.1073/pnas.1521818112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuzhdin SV, Dilda CL, Mackay TF. The genetic architecture of selection response. Inferences from fine-scale mapping of bristle number quantitative trait loci in Drosophila melanogaster. Genetics. 1999;153:1317–1331. doi: 10.1093/genetics/153.3.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco-terWengel P, Kapun M, Nolte V, Kofler R, Flatt T, Schlötterer C. Adaptation of Drosophila to a novel laboratory environment reveals temporally heterogeneous trajectories of selected alleles. Molecular Ecology. 2012;21:4931–4941. doi: 10.1111/j.1365-294X.2012.05673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr HA. The probability of parallel evolution. Evolution. 2005;59:216–220. doi: 10.1111/j.0014-3820.2005.tb00907.x. [DOI] [PubMed] [Google Scholar]
- Otto SP, Barton NH. Selection for recombination in small populations. Evolution. 2001;55:1921–1931. doi: 10.1111/j.0014-3820.2001.tb01310.x. [DOI] [PubMed] [Google Scholar]
- Petit F, Sears KE, Ahituv N. Limb development: a paradigm of gene regulation. Nature Reviews Genetics. 2017;18:245–258. doi: 10.1038/nrg.2016.167. [DOI] [PubMed] [Google Scholar]
- Pettersson ME, Johansson AM, Siegel PB, Carlborg Örjan. Dynamics of adaptive alleles in divergently selected body weight lines of chickens. G3: Genes|Genomes|Genetics. 2013;3:2305–2312. doi: 10.1534/g3.113.008375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picelli S, Björklund AK, Reinius B, Sagasser S, Winberg G, Sandberg R. Tn5 transposase and tagmentation procedures for massively scaled sequencing projects. Genome Research. 2014;24:2033–2040. doi: 10.1101/gr.177881.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitchers W, Wolf JB, Tregenza T, Hunt J, Dworkin I. Evolutionary rates for multivariate traits: the role of selection and genetic variation. Philosophical Transactions of the Royal Society B: Biological Sciences. 2014;369:20130252. doi: 10.1098/rstb.2013.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard KS, Hubisz MJ, Rosenbloom KR, Siepel A. Detection of nonneutral substitution rates on mammalian phylogenies. Genome Research. 2010;20:110–121. doi: 10.1101/gr.097857.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provot S, Kempf H, Murtaugh LC, Chung UI, Kim DW, Chyung J, Kronenberg HM, Lassar AB. Nkx3.2/Bapx1 acts as a negative regulator of chondrocyte maturation. Development. 2006;133:651–662. doi: 10.1242/dev.02258. [DOI] [PubMed] [Google Scholar]
- Quinlan AR, Hall IM. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26:841–842. doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team . Vienna, Austria: R Foundation for Statasticial Computing; 2015. [Google Scholar]
- Reeves RG, Tautz D. Automated phenotyping indicates pupal size in Drosophila is a highly heritable trait with an apparent polygenic basis. G3: Genes|Genomes|Genetics. 2017;7:1277–1286. doi: 10.1534/g3.117.039883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice DP, Townsend JP. A test for selection employing quantitative trait locus and mutation accumulation data. Genetics. 2012;190:1533–1545. doi: 10.1534/genetics.111.137075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimbault M, Beale HC, Schoenebeck JJ, Hoopes BC, Allen JJ, Kilroy-Glynn P, Wayne RK, Sutter NB, Ostrander EA. Derived variants at six genes explain nearly half of size reduction in dog breeds. Genome Research. 2013;23:1985–1995. doi: 10.1101/gr.157339.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson A. A theory of limits in artificial selection. Proceedings of the Royal Society of London. Series B. Biological Sciences. 1960;153:234–249. doi: 10.1098/rspb.1960.0099. [DOI] [PubMed] [Google Scholar]
- Rubin CJ, Zody MC, Eriksson J, Meadows JR, Sherwood E, Webster MT, Jiang L, Ingman M, Sharpe T, Ka S, Hallböök F, Besnier F, Carlborg O, Bed'hom B, Tixier-Boichard M, Jensen P, Siegel P, Lindblad-Toh K, Andersson L. Whole-genome resequencing reveals loci under selection during chicken domestication. Nature. 2010;464:587–591. doi: 10.1038/nature08832. [DOI] [PubMed] [Google Scholar]
- Schluter D, Clifford EA, Nemethy M, McKinnon JS. Parallel evolution and inheritance of quantitative traits. The American Naturalist. 2004;163:809–822. doi: 10.1086/383621. [DOI] [PubMed] [Google Scholar]
- Sexton T, Kurukuti S, Mitchell JA, Umlauf D, Nagano T, Fraser P. Sensitive detection of chromatin coassociations using enhanced chromosome conformation capture on chip. Nature Protocols. 2012;7:1335–1350. doi: 10.1038/nprot.2012.071. [DOI] [PubMed] [Google Scholar]
- Shen Y, Yue F, McCleary DF, Ye Z, Edsall L, Kuan S, Wagner U, Dixon J, Lee L, Lobanenkov VV, Ren B. A map of the cis-regulatory sequences in the mouse genome. Nature. 2012;488:116–120. doi: 10.1038/nature11243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng Z, Pettersson ME, Honaker CF, Siegel PB, Carlborg Örjan. Standing genetic variation as a major contributor to adaptation in the Virginia chicken lines selection experiment. Genome Biology. 2015;16:219. doi: 10.1186/s13059-015-0785-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siepel A, Bejerano G, Pedersen JS, Hinrichs AS, Hou M, Rosenbloom K, Clawson H, Spieth J, Hillier LW, Richards S, Weinstock GM, Wilson RK, Gibbs RA, Kent WJ, Miller W, Haussler D. Evolutionarily conserved elements in vertebrate, insect, worm, and yeast genomes. Genome Research. 2005;15:1034–1050. doi: 10.1101/gr.3715005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivakamasundari V, Chan HY, Yap SP, Xing X, Kraus P, Lufkin T. New Bapx1(Cre-EGFP) mouse lines for lineage tracing and conditional knockout studies. Genesis. 2012;50:375–383. doi: 10.1002/dvg.20802. [DOI] [PubMed] [Google Scholar]
- Sutter NB, Bustamante CD, Chase K, Gray MM, Zhao K, Zhu L, Padhukasahasram B, Karlins E, Davis S, Jones PG, Quignon P, Johnson GS, Parker HG, Fretwell N, Mosher DS, Lawler DF, Satyaraj E, Nordborg M, Lark KG, Wayne RK, Ostrander EA. A single IGF1 allele is a major determinant of small size in dogs. Science. 2007;316:112–115. doi: 10.1126/science.1137045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribioli C, Lufkin T. Bapx1 homeobox gene gain-of-function mice show preaxial polydactyly and activated shh signaling in the developing limb. Developmental Dynamics. 2006;235:2483–2492. doi: 10.1002/dvdy.20867. [DOI] [PubMed] [Google Scholar]
- Turchin MC, Chiang CW, Palmer CD, Sankararaman S, Reich D, Hirschhorn JN, Genetic Investigation of ANthropometric Traits (GIANT) Consortium Evidence of widespread selection on standing variation in Europe at height-associated SNPs. Nature Genetics. 2012;44:1015–1019. doi: 10.1038/ng.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner TL, Stewart AD, Fields AT, Rice WR, Tarone AM. Population-based resequencing of experimentally evolved populations reveals the genetic basis of body size variation in Drosophila melanogaster. PLOS Genetics. 2011;7:e1001336. doi: 10.1371/journal.pgen.1001336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlberg P, Carlborg O, Foglio M, Tordoir X, Syvänen AC, Lathrop M, Gut IG, Siegel PB, Andersson L. Genetic analysis of an F(2) intercross between two chicken lines divergently selected for body-weight. BMC Genomics. 2009;10:248. doi: 10.1186/1471-2164-10-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh B, Lynch M. Evolution and Selection of Quantitative Traits. Oxford University Press; 2018. The infinitesimal model and its extensions. [Google Scholar]
- Weber KE, Diggins LT. Increased selection response in larger populations. II. selection for ethanol vapor resistance in Drosophila melanogaster at two population sizes. Genetics. 1990;125:585–597. doi: 10.1093/genetics/125.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weedon MN, Lettre G, Freathy RM, Lindgren CM, Voight BF, Perry JR, Elliott KS, Hackett R, Guiducci C, Shields B, Zeggini E, Lango H, Lyssenko V, Timpson NJ, Burtt NP, Rayner NW, Saxena R, Ardlie K, Tobias JH, Ness AR, Ring SM, Palmer CN, Morris AD, Peltonen L, Salomaa V, Davey Smith G, Groop LC, Hattersley AT, McCarthy MI, Hirschhorn JN, Frayling TM, Diabetes Genetics Initiative. Wellcome Trust Case Control Consortium A common variant of HMGA2 is associated with adult and childhood height in the general population. Nature Genetics. 2007;39:1245–1250. doi: 10.1038/ng2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman DB, Barton NH. Limits to the rate of adaptive substitution in sexual populations. PLOS Genetics. 2012;8:e1002740. doi: 10.1371/journal.pgen.1002740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood AR, Esko T, Yang J, Vedantam S, Pers TH, Gustafsson S, Chu AY, Estrada K, Luan J, Kutalik Z, Amin N, Buchkovich ML, Croteau-Chonka DC, Day FR, Duan Y, Fall T, Fehrmann R, Ferreira T, Jackson AU, Karjalainen J, Lo KS, Locke AE, Mägi R, Mihailov E, Porcu E, Randall JC, Scherag A, Vinkhuyzen AA, Westra HJ, Winkler TW, Workalemahu T, Zhao JH, Absher D, Albrecht E, Anderson D, Baron J, Beekman M, Demirkan A, Ehret GB, Feenstra B, Feitosa MF, Fischer K, Fraser RM, Goel A, Gong J, Justice AE, Kanoni S, Kleber ME, Kristiansson K, Lim U, Lotay V, Lui JC, Mangino M, Mateo Leach I, Medina-Gomez C, Nalls MA, Nyholt DR, Palmer CD, Pasko D, Pechlivanis S, Prokopenko I, Ried JS, Ripke S, Shungin D, Stancáková A, Strawbridge RJ, Sung YJ, Tanaka T, Teumer A, Trompet S, van der Laan SW, van Setten J, Van Vliet-Ostaptchouk JV, Wang Z, Yengo L, Zhang W, Afzal U, Arnlöv J, Arscott GM, Bandinelli S, Barrett A, Bellis C, Bennett AJ, Berne C, Blüher M, Bolton JL, Böttcher Y, Boyd HA, Bruinenberg M, Buckley BM, Buyske S, Caspersen IH, Chines PS, Clarke R, Claudi-Boehm S, Cooper M, Daw EW, De Jong PA, Deelen J, Delgado G, Denny JC, Dhonukshe-Rutten R, Dimitriou M, Doney AS, Dörr M, Eklund N, Eury E, Folkersen L, Garcia ME, Geller F, Giedraitis V, Go AS, Grallert H, Grammer TB, Gräßler J, Grönberg H, de Groot LC, Groves CJ, Haessler J, Hall P, Haller T, Hallmans G, Hannemann A, Hartman CA, Hassinen M, Hayward C, Heard-Costa NL, Helmer Q, Hemani G, Henders AK, Hillege HL, Hlatky MA, Hoffmann W, Hoffmann P, Holmen O, Houwing-Duistermaat JJ, Illig T, Isaacs A, James AL, Jeff J, Johansen B, Johansson Å, Jolley J, Juliusdottir T, Junttila J, Kho AN, Kinnunen L, Klopp N, Kocher T, Kratzer W, Lichtner P, Lind L, Lindström J, Lobbens S, Lorentzon M, Lu Y, Lyssenko V, Magnusson PK, Mahajan A, Maillard M, McArdle WL, McKenzie CA, McLachlan S, McLaren PJ, Menni C, Merger S, Milani L, Moayyeri A, Monda KL, Morken MA, Müller G, Müller-Nurasyid M, Musk AW, Narisu N, Nauck M, Nolte IM, Nöthen MM, Oozageer L, Pilz S, Rayner NW, Renstrom F, Robertson NR, Rose LM, Roussel R, Sanna S, Scharnagl H, Scholtens S, Schumacher FR, Schunkert H, Scott RA, Sehmi J, Seufferlein T, Shi J, Silventoinen K, Smit JH, Smith AV, Smolonska J, Stanton AV, Stirrups K, Stott DJ, Stringham HM, Sundström J, Swertz MA, Syvänen AC, Tayo BO, Thorleifsson G, Tyrer JP, van Dijk S, van Schoor NM, van der Velde N, van Heemst D, van Oort FV, Vermeulen SH, Verweij N, Vonk JM, Waite LL, Waldenberger M, Wennauer R, Wilkens LR, Willenborg C, Wilsgaard T, Wojczynski MK, Wong A, Wright AF, Zhang Q, Arveiler D, Bakker SJ, Beilby J, Bergman RN, Bergmann S, Biffar R, Blangero J, Boomsma DI, Bornstein SR, Bovet P, Brambilla P, Brown MJ, Campbell H, Caulfield MJ, Chakravarti A, Collins R, Collins FS, Crawford DC, Cupples LA, Danesh J, de Faire U, den Ruijter HM, Erbel R, Erdmann J, Eriksson JG, Farrall M, Ferrannini E, Ferrières J, Ford I, Forouhi NG, Forrester T, Gansevoort RT, Gejman PV, Gieger C, Golay A, Gottesman O, Gudnason V, Gyllensten U, Haas DW, Hall AS, Harris TB, Hattersley AT, Heath AC, Hengstenberg C, Hicks AA, Hindorff LA, Hingorani AD, Hofman A, Hovingh GK, Humphries SE, Hunt SC, Hypponen E, Jacobs KB, Jarvelin MR, Jousilahti P, Jula AM, Kaprio J, Kastelein JJ, Kayser M, Kee F, Keinanen-Kiukaanniemi SM, Kiemeney LA, Kooner JS, Kooperberg C, Koskinen S, Kovacs P, Kraja AT, Kumari M, Kuusisto J, Lakka TA, Langenberg C, Le Marchand L, Lehtimäki T, Lupoli S, Madden PA, Männistö S, Manunta P, Marette A, Matise TC, McKnight B, Meitinger T, Moll FL, Montgomery GW, Morris AD, Morris AP, Murray JC, Nelis M, Ohlsson C, Oldehinkel AJ, Ong KK, Ouwehand WH, Pasterkamp G, Peters A, Pramstaller PP, Price JF, Qi L, Raitakari OT, Rankinen T, Rao DC, Rice TK, Ritchie M, Rudan I, Salomaa V, Samani NJ, Saramies J, Sarzynski MA, Schwarz PE, Sebert S, Sever P, Shuldiner AR, Sinisalo J, Steinthorsdottir V, Stolk RP, Tardif JC, Tönjes A, Tremblay A, Tremoli E, Virtamo J, Vohl MC, Amouyel P, Asselbergs FW, Assimes TL, Bochud M, Boehm BO, Boerwinkle E, Bottinger EP, Bouchard C, Cauchi S, Chambers JC, Chanock SJ, Cooper RS, de Bakker PI, Dedoussis G, Ferrucci L, Franks PW, Froguel P, Groop LC, Haiman CA, Hamsten A, Hayes MG, Hui J, Hunter DJ, Hveem K, Jukema JW, Kaplan RC, Kivimaki M, Kuh D, Laakso M, Liu Y, Martin NG, März W, Melbye M, Moebus S, Munroe PB, Njølstad I, Oostra BA, Palmer CN, Pedersen NL, Perola M, Pérusse L, Peters U, Powell JE, Power C, Quertermous T, Rauramaa R, Reinmaa E, Ridker PM, Rivadeneira F, Rotter JI, Saaristo TE, Saleheen D, Schlessinger D, Slagboom PE, Snieder H, Spector TD, Strauch K, Stumvoll M, Tuomilehto J, Uusitupa M, van der Harst P, Völzke H, Walker M, Wareham NJ, Watkins H, Wichmann HE, Wilson JF, Zanen P, Deloukas P, Heid IM, Lindgren CM, Mohlke KL, Speliotes EK, Thorsteinsdottir U, Barroso I, Fox CS, North KE, Strachan DP, Beckmann JS, Berndt SI, Boehnke M, Borecki IB, McCarthy MI, Metspalu A, Stefansson K, Uitterlinden AG, van Duijn CM, Franke L, Willer CJ, Price AL, Lettre G, Loos RJ, Weedon MN, Ingelsson E, O'Connell JR, Abecasis GR, Chasman DI, Goddard ME, Visscher PM, Hirschhorn JN, Frayling TM, Electronic Medical Records and Genomics (eMEMERGEGE) Consortium. MIGen Consortium. PAGEGE Consortium. LifeLines Cohort Study Defining the role of common variation in the genomic and biological architecture of adult human height. Nature Genetics. 2014;46:1173–1186. doi: 10.1038/ng.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalcin B, Nicod J, Bhomra A, Davidson S, Cleak J, Farinelli L, Østerås M, Whitley A, Yuan W, Gan X, Goodson M, Klenerman P, Satpathy A, Mathis D, Benoist C, Adams DJ, Mott R, Flint J. Commercially available outbred mice for genome-wide association studies. PLOS Genetics. 2010;6:e1001085. doi: 10.1371/journal.pgen.1001085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, Nusbaum C, Myers RM, Brown M, Li W, Liu XS. Model-based analysis of ChIP-Seq (MACS) Genome Biology. 2008;9:R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]