Abstract
A rare case of acquired amegakaryocytic thrombocytopenia (AATP) in a 35-year-old woman who presented with anaemia and thrombocytopenia at 22 weeks gestation. The first diagnostic impression was of an evolving aplastic anaemia; however, the patient was simultaneously diagnosed with severe vitamin B12 deficiency in the setting of vegetarianism. Once the cyanocobalamin deficiency was corrected, a repeat bone marrow biopsy revealed an isolated depletion of megakaryocytes, which suggested the diagnosis of AATP. Supportive care was provided for her anaemia and thrombocytopenia and she delivered a healthy baby girl with a normal platelet count. The patient was subsequently started on romiplostim with steady improvement in her platelet counts. This rare AATP case presentation highlights the importance of a well-structured diagnostic approach to thrombocytopenia during pregnancy and supports the successful use of thrombopoietin agonists for the management of AATP.
Keywords: haematology (incl blood transfusion), obstetrics and gynaecology, pregnancy
Background
Acquired amegakaryocytic thrombocytopenia (AATP) is a rare cause of thrombocytopenia. A well-structured diagnostic workup of thrombocytopenia during pregnancy is essential for management that ensures both maternal and fetal well-being. Our case highlights the importance of the systematic approach that enabled our team to diagnose the bone marrow (BM) suppression secondary to vitamin B12 deficiency with an underlying AATP. We also present the successful use of romiplostim, a thrombopoietin (TPO) agonist, for the management of AATP.
Case presentation
A 35-year-old woman, G1P0, presented at 22 weeks gestation and was found to have new onset anaemia and severe thrombocytopenia. She presented with bruising on her lower extremities, gingival bleeding, a white blood cell (WBC) count of 7.9×103/µL, haemoglobin (Hb) of 6.6 g/dL (mean corpuscular volume (MCV) 115) and a platelet count of 12×103/µL. She responded appropriately to two units of packed red blood cells and one unit of platelets. She denied any significant past medical history, smoking, alcohol or illicit drug use, and was not taking any home medications or herbal supplements. Her family history was notable for maternal Hashimoto’s disease, arthritis and lymphocytic hypophysitis and a paternal grandmother with Raynaud’s phenomenon. She had been following a vegetarian diet for 13 years, and her brother, who also followed a vegetarian diet, had been diagnosed with vitamin B12 deficiency. She had recently travelled to China and Japan.
Investigations
The review of her peripheral blood smear showed macroovalocytes, a manual platelet count of 20×103/µL (machine count of 11×103/µL) with large platelets but no platelet clumping or classical hypersegmented neutrophils. She had a normal ferritin, iron, folate, haptoglobin and coagulation screen. Her vitamin B12 level was low at 184 pg/mL (normal range 211–911 pg/mL), and anti-parietal cell or intrinsic factor antibodies were not detected. A comprehensive infectious workup (including parvovirus) was considered and was unremarkable. A haemolysis panel and workup for thrombotic thrombocytopenic purpura (TTP) were also unrevealing. A BM biopsy at 26 weeks gestation showed a hypocellular marrow concerning for aplastic anaemia, with normal cytogenetics and telomere length studies. She was found to have a minor paroxysmal nocturnal haemoglobinuria (PNH) clone (up to 1.64%), deemed clinically insignificant. The patient’s anaemia significantly improved with vitamin B12 supplementation. Her reticulocyte count increased to 6.5% from 2.2% and ultimately normalised along with her MCV.
Differential diagnosis
By far, the most common aetiology of thrombocytopenia during pregnancy is gestational thrombocytopenia (GT) (60%–80%), characterised by a platelet count typically >80×103/µL.1 2 Because of her severe thrombocytopenia and normal absolute neutrophil count, a diagnosis of immune thrombocytopenia (ITP) was first entertained. The absence of schistocytes, normal ADAMST13 level and absence of typical symptoms made TTP, atypical haemolytic uremic syndrome and thrombotic microangiopathies of pregnancy very unlikely.
Treatment
She was started on prednisone for presumed ITP, and cyanocobalamin for her vitamin B12 deficiency. The platelet count did not improve with these treatments or with a trial of intravenous immunoglobulin (IVIg). She remained severely thrombocytopenic (<10×103/μL) and platelet transfusion dependent to which she consistently responded appropriately, an unusual observation in ITP. After correction of her vitamin B12 deficiency, a repeat BM biopsy at 30 weeks gestation revealed a normocellular marrow with megakaryocytic hypoplasia, consistent with a diagnosis of AATP (figure 1).
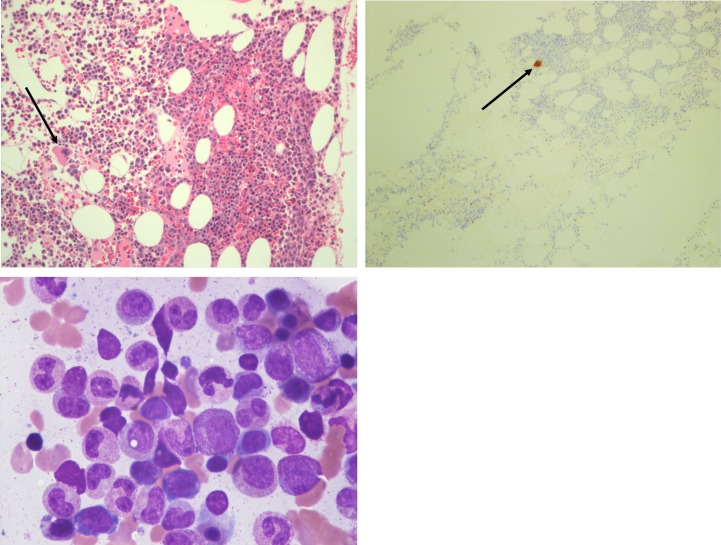
Figure 1.
Bone marrow biopsy pathology. Upper left: normocellular marrow with a solitary megakaryocyte. Upper right: Immunohistochemistry for CD61, a marker for megakaryocytes, showing the marked megakaryocytic hypoplasia with only one megakaryocyte highlighted with the stain. Lower left: bone marrow aspirate smear shows virtually no megakaryocytes with normal myeloid and erythroid lineages maturation.
An interdisciplinary decision was made to provide supportive care until safe delivery at 36 weeks. However, the patient required admission at 35 weeks due to symptoms of pre-eclampsia with severe features. Her liver function tests remained normal and there was no evidence of haemolysis to suggest a diagnosis of HELLP (haemolysis, elevated liver enzymes, low platelets). She was managed with labetalol, magnesium, platelet transfusions and underwent an uncomplicated caesarean delivery of a healthy baby girl with a normal platelet count. She was discharged on postoperative day 5 with a platelet count of 60×103/µL. The patient briefly breast fed but discontinued after a few weeks.
The patient’s anaemia continued to improve but she became progressively more thrombocytopenic with a nadir of 5×103/µL at 4 weeks post partum. We started treatment with romiplostim, and after 3 months of weekly romiplostim at 10 mcg/kg, her platelet count reached 52×103/µL and with no clinical adverse effects. She has completed 1 year of romiplostim therapy, and her platelet count has stabilised at 80–90×103/µL. The frequency of administration is being steadily decreased.
Outcome and follow-up
The patient’s WBC and Hb remained within normal limits, and her platelet count stabilised at 80–90×103/µL on romiplostim. Her liver function tests have remained normal throughout treatment. She remains under close observation with monthly follow-up to determine if there is any change in her disease status.
Discussion
Thrombocytopenia is common during pregnancy, with up to 10% of women having a platelet count of <150×103/µL at the time of delivery, and 1% of women with platelet count <100×103/µL during the course of pregnancy and delivery.1 The majority of cases are attributed to GT, a benign and self-limited condition that resolves on delivery.1–4
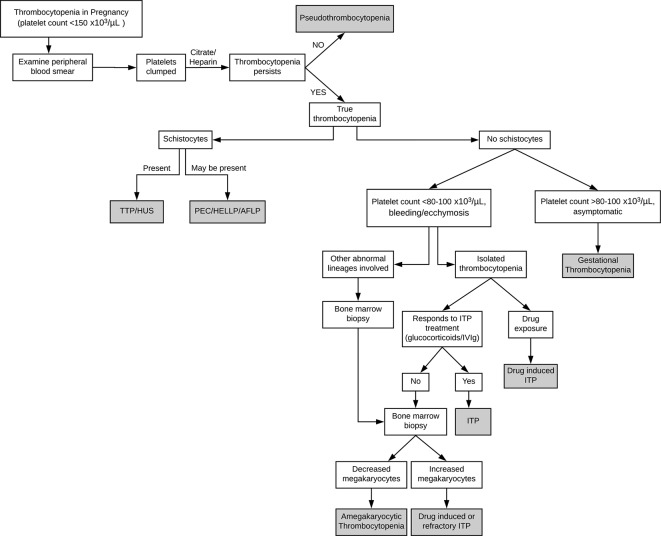
Primary care physicians, obstetricians and haematologists may encounter patients with thrombocytopenia during pregnancy. The initial evaluation should consist of a thorough history and physical examination, confirmation of gestational age and assessment of the severity of thrombocytopenia (figure 2). Additional history should be aimed at identifying nutritional deficiencies, travel history, medications and herbal supplements, toxic exposures to heavy metals, family history of bleeding disorders and personal pregnancy history.3–5 A mild asymptomatic thrombocytopenia during the third trimester suggests the diagnosis of GT, and further workup is not required. If a symptomatic and/or significant (<80×103/µL) thrombocytopenia is noted or if associated with schistocytes, neutropenia or anaemia, further evaluation should be pursued. Laboratory testing should include liver function testing, evaluation for disseminated intravascular coagulopathy, reticulocyte count to assess BM function, direct antiglobulin test to assess for concurrent autoimmune haemolytic process, a full anaemia workup if indicated and virologic testing for HIV, hepatitis and cytomegalovirus infections for reactive/secondary thrombocytopenia.4 In clinical situations suggesting autoimmunity, testing for anti-phospholipid and anti-nuclear antibodies is appropriate. If ITP is suspected and the patient does not respond to standard therapy, a BM biopsy is then warranted.
Figure 2.
Diagnostic algorithm for thrombocytopenia in pregnancy. AFLP, acute fatty liver of pregnancy; HELLP, haemolysis, elevated liver enzymes, low platelets; HUS, haemolytic uremic syndrome; ITP, immune thrombocytopenia; IVIg, intravenous immunoglobulin; PEC, pre-eclampsia; TTP, thrombotic thrombocytopenic purpura.
AATP is an extremely rare haematologic disorder with <50 cases published in the literature.6 It is characterised by severe thrombocytopenia with complete or near-complete absence of megakaryocytes in the BM, and with otherwise normal haematopoiesis. The underlying mechanism for AATP is not fully elucidated but is thought to be related to an anti-TPO autoantibody, directed against the TPO receptor, possibly a defect in the early progenitor cells of the megakaryocyte lineage or a cell-mediated suppression of megakaryocytopoiesis.6 7 AATP may be associated with dysplastic changes and be an early precursor of myelodysplastic syndrome or aplastic anaemia.8–11
To our knowledge, only one other case of AATP during pregnancy has been reported.12 The patient had a known history of AATP with a partial response to cyclosporine, which she remained on throughout her pregnancy.
We described a case of AATP with concomitant vitamin B12 deficiency during pregnancy that was successfully treated with romiplostim after delivery. Our patient has a significant family history of autoimmunity that could allude to an autoimmune process. Our initial differential diagnosis included more common conditions aplastic anaemia and ITP. The persistently normal WBC and the lack of response to standard therapy for ITP prompted a repeat BM biopsy after correction of her vitamin B12 deficiency and revealed the diagnosis of AATP.
Recent case reports have shown the utility of TPO receptor agonists for the treatment of AATP.13 Romiplostim is a peptide-based TPO receptor agonist approved for the treatment of ITP and is administered weekly as a subcutaneous injection.14–16 Once the platelet count improves to >50×103/µL, the medication is tapered to its lowest effective dose. Romiplostim and eltrombopag, a non-peptide TPO receptor agonist, have been used off-label for the successful treatment of AATP.13 In our case, romiplostim was selected given its favourable side effect profile in a non-breastfeeding postpartum patient, its ease of administration and lack of immunosuppressive effects.16
Patient’s perspective.
At first, my obstetrician called me with a chuckle. ‘Something has gone seriously wrong with your blood sample—can you come back in for a retest?’ I was 22 weeks pregnant and had just passed all of the other tests successfully—surely something must have gone wrong in the lab. But, the next day the second round of blood tests confirmed earlier results, my platelet levels had fallen to 7000. My obstetrician called my office this time, getting my secretary to interrupt my freshman public speaking class. She instructed me to stop teaching class, get into a taxi and immediately go to an oncologist who was on the line. Within hours I had my first bone marrow biopsy and blood transfusions. This was the start of 3 months of on-and-off hospitalisations and every-other-day blood transfusions leading up to the premature birth of my daughter. She was born happy and healthy, though just under 5 lbs. Thankfully, due to the exceptional preparation by my doctors, both the baby and I were able to avoid entering the ICU and were able to recover in the maternity ward, leaving 5 days after her birth.
Today, my daughter is 14 months old and thriving. I have been receiving romiplostim injections since the summer, and it has been months since I have had to have a blood transfusion. This experience has taught me how lucky I was, to have my daughter in a large city, with multiple teams of doctors who could quickly respond to the many symptoms that I was experiencing. They were consistently straightforward about the problems that I was experiencing, the rareness of those problems and clarified what they did and did not know about my condition. My ears perked up every time I heard that someone new had been consulted. I quickly googled doctor’s names, NHS studies, and any term that a nurse, doctor or administrator mentioned, and thankfully the hospital staff took the time to explain to me what I was reading. As an academic, I had always been interested in the ways that scientific rhetoric is translated for the public, but I never expected to gain such a first-hand experience. I still worry about the diagnosis—with something so rare it is unclear when, how and even if, I will fully recover. Yet, I am fully satisfied with the treatment plan, and I look forward to my visits with the haematologists. I am grateful for the care that my daughter and I received, and the help we have had after her birth to ensure that I am steadily improving.
Learning points.
Thrombocytopenia attributed to ITP but which does not respond to standard therapy with glucocorticoids and IVIg requires a BM biopsy evaluation.
Thrombocytopenia accompanied by additional cell lineage abnormalities requires a workup for nutritional deficiencies or BM processes and mandates a BM biopsy evaluation.
The diagnosis of AATP is one of exclusion based on the isolated decrease/absence of megakaryocytes on a BM biopsy.
New effective therapies for the treatment of AATP include TPO agonists such as eltrombopag and romiplostim.
Footnotes
Contributors: BSZ and BM are haematology/oncology fellows, SMEJ is a clinical pathologist and ASR is a haematology/oncology attending and they all contributed to caring for this patient as well as the conception, preparation, writing and reviewing of the case report. SMEJ was responsible for reviewing the patient’s bone marrow biopsy pathology specimen. All authors approved the final manuscript and contributed substantially to its preparation.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Reese JA, Peck JD, Deschamps DR, et al. Platelet Counts during Pregnancy. N Engl J Med 2018;379:32–43. 10.1056/NEJMoa1802897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Townsley DM. Hematologic complications of pregnancy. Semin Hematol 2013;50:222–31. 10.1053/j.seminhematol.2013.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gernsheimer T, James AH, Stasi R. How I treat thrombocytopenia in pregnancy. Blood 2013;121:38–47. 10.1182/blood-2012-08-448944 [DOI] [PubMed] [Google Scholar]
- 4. Bergmann F, Rath W. The Differential Diagnosis of Thrombocytopenia in Pregnancy. Dtsch Ärzteblatt Int 2015;112:795–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. American College of Obstetricians and Gynecologists Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol 2013;122:1122–31. 10.1097/01.AOG.0000437382.03963.88 [DOI] [PubMed] [Google Scholar]
- 6. Agarwal N, Spahr JE, Werner TL, et al. Acquired amegakaryocytic thrombocytopenic purpura. Am J Hematol 2006;81:132–5. 10.1002/ajh.20510 [DOI] [PubMed] [Google Scholar]
- 7. Hoffman R, Briddell RA, van Besien K, et al. Acquired cyclic amegakaryocytic thrombocytopenia associated with an immunoglobulin blocking the action of granulocyte-macrophage colony-stimulating factor. N Engl J Med 1989;321:97–102. 10.1056/NEJM198907133210207 [DOI] [PubMed] [Google Scholar]
- 8. Novotný JP, Köhler B, Max R, et al. Acquired Amegakaryocytic Thrombocytopenic Purpura Progressing into Aplastic Anemia. Prague Med Rep 2017;118:147–55. 10.14712/23362936.2017.16 [DOI] [PubMed] [Google Scholar]
- 9. King JA, Elkhalifa MY, Latour LF. Rapid progression of acquired amegakaryocytic thrombocytopenia to aplastic anemia. South Med J 1997;90:91–4. 10.1097/00007611-199701000-00024 [DOI] [PubMed] [Google Scholar]
- 10. Eddou H, Zinebi A, Khalloufi A, et al. [Acquired amegacaryocytic thrombocytopenic purpura hiding acute myeloid leukemia]. Pan Afr Med J 2017;26:26–32. 10.11604/pamj.2017.26.32.9215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Erkurt MA, Kaya E, Baran M, et al. Rapid progression of acquired amegakaryocytic thrombocytopenia to myelodysplastic syndrome: case report. Turk J Haematol 2005;22:205–8. [PubMed] [Google Scholar]
- 12. Usala E, Simula MP, Massidda M, et al. Pregnancy outcome and disease course in a woman with acquired amegacariocytic thrombocytopenia. Haematologica 2011;96:97. [Google Scholar]
- 13. Cela I, Miller IJ, Katz RS, et al. Successful treatment of amegakaryocytic thrombocytopenia with eltrombopag in a patient with systemic lupus erythematosus (SLE). Clin Adv Hematol Oncol 2010;8:806–9. [PubMed] [Google Scholar]
- 14. Kuter DJ, Bussel JB, Newland A, et al. Long-term treatment with romiplostim in patients with chronic immune thrombocytopenia: safety and efficacy. Br J Haematol 2013;161:411–23. 10.1111/bjh.12260 [DOI] [PubMed] [Google Scholar]
- 15. Bussel JB, Buchanan GR, Nugent DJ, et al. A randomized, double-blind study of romiplostim to determine its safety and efficacy in children with immune thrombocytopenia. Blood 2011;118:28–36. 10.1182/blood-2010-10-313908 [DOI] [PubMed] [Google Scholar]
- 16. Cines DB, Gernsheimer T, Wasser J, et al. Integrated analysis of long-term safety in patients with chronic immune thrombocytopaenia (ITP) treated with the thrombopoietin (TPO) receptor agonist romiplostim. Int J Hematol 2015;102:259–70. 10.1007/s12185-015-1837-6 [DOI] [PubMed] [Google Scholar]