Abstract
Introduction
Transbronchial lung cryobiopsy (TBLC) is a novel, minimally invasive technique for obtaining lung tissue for histopathological assessment in interstitial lung disease (ILD). Despite its increasing popularity, the diagnostic accuracy of TBLC is not yet known. The COLDICE Study (Cryobiopsy versus Open Lung biopsy in the Diagnosis of Interstitial lung disease allianCE) aims to evaluate the agreement between TBLC and surgical lung biopsy sampled concurrently from the same patients, for both histopathological and multidisciplinary discussion (MDD) diagnoses.
Methods and analysis
This comparative, multicentre, prospective trial is enrolling patients with ILD requiring surgical lung biopsy to aid with their diagnosis. Participants are consented for both video-assisted thoracoscopic surgical (VATS) biopsy and TBLC within the same anaesthetic episode. Specimens will be blindly assessed by three expert pathologists both individually and by consensus. Each tissue sample will then be considered in conjunction with clinical and radiological data, within a centralised MDD. Each patient will be presented twice in random order, once with TBLC data and once with VATS data. Meeting participants will be blinded to the method of tissue sampling. The accuracy of TBLC will be assessed by agreement with VATS at (1) histopathological analysis and (2) MDD diagnosis. Data will be collected on interobserver agreement between pathologists, interobserver agreement between MDD participants, and detailed clinical and procedural characteristics.
Ethics and dissemination
The study is being conducted in accordance with the International Conference on Harmonisation Guideline for Good Clinical Practice and Australian legislation for the ethical conduct of research.
Trial registration number
Keywords: transbronchial lung cryobiopsy, interstitial lung disease, surgical lung biopsy, diagnostic accuracy
Introduction
Obtaining lung tissue for histopathological assessment remains an important part of the diagnostic algorithm in interstitial lung disease (ILD), required in up to 30% of patients presenting with these diseases. Transbronchial lung cryobiopsy (TBLC) is a recently developed, minimally invasive technique for sampling lung tissue to diagnose ILD.1 TBLC has the potential for a greater diagnostic yield than conventional forceps transbronchial biopsies, due to the larger, architecturally well-preserved specimens which are obtained.2 Furthermore, without the requirement for chest wall incision, the morbidity and health resource utilisation of TBLC appear favourable compared with video-assisted thoracoscopic surgical (VATS) lung biopsy. TBLC is performed by an interventional pulmonologist, usually under general anaesthesia with rigid or flexible endotracheal intubation. Patients are often suitable for discharge from hospital on the same day, with minimal subsequent recovery time. Overall, a sample suitable for histopathological evaluation for ILD diagnosis is obtained in 73%–81% of TBLC procedures compared with up to 95% yield for VATS biopsies.3–5 Of note, these numbers reflect diagnostic yield, rather than diagnostic accuracy, in the absence of direct comparison of the new technique against the conventional standard.
Despite the promise of the new procedure, concerns have been raised around practice standards, safety and histopathological accuracy.4 6 7 An expert consensus paper published by Hetzel et al8 sought to address some of these concerns, particularly in the wake of increasing popularity of TBLC across many centres. Techniques to optimise safety and maximal tissue yield were outlined within the statement in a move towards standardisation, as the body of evidence for TBLC continues to expand. In addition to a lower reported yield, the authors acknowledge reduced diagnostic confidence at pathology assessment for TBLC compared with VATS specimens.8 9 This may be particularly relevant for disease patterns where larger tissue specimens are required, such as usual interstitial pneumonia (UIP) where there is geographical heterogeneity of disease. To date, very few studies have evaluated the TBLC against the current histopathological reference standard VATS biopsy.10 Ravaglia et al11 reported on a retrospective comparison of diagnostic yield for each method in an ILD cohort at a single expert centre, including 150 VATS and 297 TBLC samples from separate patients. A histopathological diagnosis was obtained in approximately 99% of patients with VATS biopsy and 83% of patients who underwent TBLC. Aside from the limitations of retrospective analysis, the study did not include multidisciplinary discussion (MDD) for a definitive clinical-radiological-pathological diagnosis of each case. Tomassetti and colleagues9 compared a historical cohort of 58 patients who had undergone TBLC against 59 separate patients with VATS biopsy at an MDD that comprised expert clinicians. Their findings suggested that a similar degree of diagnostic confidence could be achieved by the addition of either form of biopsy to the clinical and radiological data for MDD diagnosis. This study did not, however, address the issue of the accuracy of the TBLC, with no direct comparison against a larger tissue sample obtained from the same patient. In an attempt to address the question of accuracy, a recent study by Romagnoli et al12 included 21 subjects with ILD with sequential TBLC and VATS tissue sampling. The study suggested only fair agreement between the two, with a kappa concordance coefficient of 0.22 at histopathological assessment and 0.31 at MDD diagnosis. This study, however, was not powered to a specific endpoint and only used a single blinded pathologist after the MDD process. Due to the limitations of each of these studies, it remains uncertain if the diagnostic accuracy of TBLC is sufficient to recommend it as a first-line investigation in ILD. In essence, the TBLC must be carefully assessed for both its histopathological accuracy and its performance within the MDD, recognised internationally as the gold standard for comprehensive ILD diagnosis. This manuscript describes the methodology of the COLDICE Study (Cryobiopsy versus Open Lung biopsy in the Diagnosis of Interstitial lung disease allianCE), a comparative, multicentre trial aiming to definitively evaluate the diagnostic agreement of TBLC with VATS lung biopsy, both at histopathological review and at MDD.
Methods and analysis
Trial design
The COLDICE Study (U1111-1171-6880) is a comparative, multicentre, prospective trial designed to evaluate diagnostic agreement between TBLC and VATS lung biopsy in patients with ILD. Nine expert tertiary hospitals across Australia are participating in the study, with plans to enrol a total of 65 patients. The study protocol (V.5, 2017) follows the STARD guidelines (Standards for Reporting of Diagnostic Accuracy Studies) for evaluating the accuracy of diagnostic tests.13 14 The trial is currently recruiting, with the first subject enrolled on 17 March 2016.
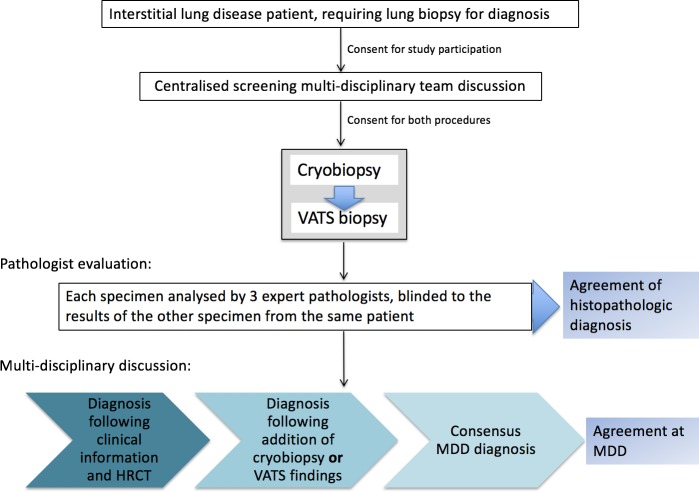
Within the study, VATS and TBLC specimens are obtained serially (during the same anaesthetic) from patients with ILD. Samples will be assessed by blinded centralised expert pathologists and will subsequently be evaluated within the framework of MDD for ability to inform consensus diagnosis and subsequent management (figure 1). The two primary outcomes will be assessed as follows:
Figure 1.
Flow diagram of screening and procedural protocol: COLDICE Study protocol. COLDICE, Cryobiopsy versus Open Lung biopsy in the Diagnosis of Interstitial lung disease allianCE; HRCT, high-resolution CT scan; MDD, multidisciplinary discussion; VATS, video-assisted thoracoscopic surgical.
Diagnostic agreement between TBLC and VATS biopsy histopathology, based on classification schema in current international guidelines.6 15 16
Agreement between consensus clinical-radiological-pathological diagnoses at ILD MDD using either TBLC or VATS pathology specimens.
Study subjects
Sixty-five patients requiring histopathological sampling for ILD diagnosis will be consented and recruited for concurrent TBLC and VATS biopsies. Subjects are being prospectively recruited from the respiratory outpatient clinics at participating sites, following a detailed panel of baseline investigations (table 1). After consideration of inclusion and exclusion criteria (see below), eligible patients are screened through a centralised MDD, which comprised expert ILD physicians, radiologists, rheumatologists and pathologists. Clinical details are presented using a standardised proforma, by the referring physician. High-resolution CT (HRCT) scans performed within 3 months of MDD, following a standard protocol (table 1), are reviewed. Radiological patterns will be interpreted by the central MDD expert radiologists according to current diagnostic guidelines.6 17 If the consensus diagnosis is ‘unclassifiable’ or of low diagnostic confidence, and fit for surgical lung biopsy, then the patient is screened as suitable to proceed. Targeted sites for biopsy identified by the radiologist are relayed to proceduralists.
Table 1.
Baseline assessments for all potential participants
| Domain | Assessments |
| History | Symptoms, date of onset, family history, comorbid diseases, medications, smoking history, exposures (environmental/occupational). |
| Examination | Vital signs, lung auscultation, signs of pulmonary hypertension or right ventricular failure, clubbing, Raynaud’s phenomenon, inflammatory arthritis, sclerodactyly, skin changes, muscle weakness, other connective tissue disease features (eg, Gottron’s papules). |
| Serology | Full blood count, biochemistry, coagulation studies, ANA, ENA, RF, anti-CCP, ds-DNA, ANCA (MPO/PR3), extended panel of myositis antibodies, farmers/pigeon/budgerigar immunoglobulins, ACE, creatine kinase, NT-pro-BNP. |
| Lung function | Spirometry, lung volumes, DLCO. |
| 6MWT | Distance, start SpO2, nadir SpO2. |
| HRCT scan | Images must be obtained volumetrically on a multidetector CT with <1.25 mm slice collimation of axial images using a high-resolution reconstruction algorithm, or non-contiguously with 1–1.5 mm slices obtained at 10 mm intervals. Prone, supine, inspiratory and expiratory views will be acquired. |
anti-CCP, anticyclic citrullinated peptide; ANA, antinuclear antibodies; ANCA, antineutrophil cytoplasmic antibody; ds-DNA, double-stranded DNA.DLCO, diffusing lung capacity for carbon monoxide; ENA, extractable nuclear antigen; HRCT, high-resolution CT; MPO, myeloperoxidase; 6MWT, 6 min walk test; NT-pro-BNP, N-terminal pro-brain natriuretic peptide; PR3, proteinase 3; RF, rheumatoid factor; SpO2, oxygen saturation on pulse oximetry;
Inclusion criteria
Age ≥18 years and ≤80 years.
Patients with unclear underlying ILD diagnosis after detailed clinical, serological and radiological evaluation at a centralised MDD meeting.
Patients deemed suitable for lung biopsy, without any contraindications listed in box 1.
Patients able to give informed consent.
Box 1. Contraindications for surgical biopsy and study participation.
Resting hypoxaemia (SpO2 <90% on room air).
DLCO <40%.
Severe lung restriction on lung function testing (total lung capacity <50%).
Excessive, uncorrectable bleeding risk (platelets <100 x 109/L, INR >1.5, antiplatelet agents that cannot be safely withheld).
History of adverse reaction to general anaesthesia.
Pulmonary hypertension (estimated right ventricular systolic pressure >40 mm Hg on echocardiogram and/or right ventricular dysfunction).
Advanced comorbidities (including ischaemic heart disease with unstable angina, morbid obesity (BMI >40 kg/m2), current infective illness, uncontrolled severe hypertension, poorly controlled heart failure, myocarditis, severe aortic stenosis, acute pulmonary embolus/thrombus/venous thromboembolic disorders, mental impairment).
BMI, body mass index; DLCO, diffusing lung capacity for carbon monoxide; INR, international normalised ratio; SpO2, oxygen saturation on pulse oximetry.
Exclusion criteria
Patients not able to give informed consent.
Patients in whom confident MDD diagnosis can be obtained without histopathology: that is, HRCT scan demonstrates a definite pattern (including UIP) and/or serological and clinical findings sufficient to make a confident ILD diagnosis following international diagnostic guidelines.6 15 16
Patients considered unsuitable for VATS biopsy (box 1).
Preprocedural training and standardisation
Prior to commencement of cases, participating centres took part in a training workshop using intubated animal cadavers to ensure standardisation of the cryobiopsy technique, including freezing procedures, prophylactic balloon placement and drilling in the management of procedure-related complications.
Procedural protocol
At the time of the dual procedures, patients are anaesthetised by a cardiothoracic anaesthetist. A flexible endotracheal tube or rigid bronchoscope is inserted for airway control. Subjects first undergo TBLC, performed by interventional pulmonologists, and then VATS, performed by thoracic surgeons. Both TBLC and VATS biopsies are obtained from two separate, but corresponding lobes from the same lung, as predetermined at MDD.
TBLC procedure
Prior to TBLC, a deflated endobronchial balloon is placed in the proximal airways for control of bleeding. Using the bronchoscope and an image intensifier, the flexible cryoprobe, either 1.9 mm or 2.4 mm in diameter (Erbe, Germany), is advanced into a peripheral airway until the pleural surface is reached. The probe is withdrawn ~1 cm proximally, and freezing is applied via an external foot pedal, for 4–7 s. The probe with attached tissue specimen is removed, en bloc with the bronchoscope. A second proceduralist inflates the bronchial balloon prophylactically with an air, saline or contrast-filled syringe following withdrawal of the probe and bronchoscope, taking care to keep the bronchial balloon at the predetermined position. The specimen is placed into a saline-filled pot, for later transfer into formalin fixative. The bronchoscope is returned to the biopsy site. The bronchial balloon is deflated to observe for ongoing bleeding. If continued bleeding is seen, the balloon is reinflated for 2–3 min before attempting to redeflate. Additionally, ice-cold saline, epinephrine or tranexamic acid can be instilled at the biopsy site if necessary. This process is repeated to obtain a total of four to six specimens from the two distinct lobes. At completion, the image intensifier or ultrasound machine is used to identify any pneumothorax.
VATS procedure
VATS lung biopsies are performed on the same side as TBLC by the thoracic surgeon, using a standardised surgical technique. Thoracoscopies are done under general anaesthesia using a double-lumen airway, with patients in the lateral decubitus position. Two to three ports are positioned at the discretion of the surgeon. An endoscopic stapling device is used to obtain lung tissue under videoscopic guidance. Two biopsies (one from each of two separate anatomical sites, also sampled at TBLC) are taken. All patients have a chest drain inserted at the completion of the VATS, connected to 20 cm H2O suction. The patient is transferred to the anaesthetic recovery bay for standard postoperative observation.
Decision to abandon procedure
In the setting of anaesthetic or surgical complications, including severe bleeding or haemodynamic instability, a decision to abandon further TBLC or VATS may be made by the interventionalist, surgeon and/or anaesthetist. In such situations, patients would be reviewed for possible transfer to an intensive care or high dependency unit.
Follow-up assessment
Standard postoperative assessment includes both inpatient and outpatient review, according to clinical need. Assessment for trial purposes includes vital status and postoperative issues at 6 weeks and 6 months postprocedure and at study completion. Lung function test measurements are obtained at 6 months.
Histopathological evaluation for the COLDICE Study
At completion of recruitment, all processed TBLC and VATS specimens will be transferred to the central site. TBLC and VATS specimens will be assigned randomly generated, unique de-identifying code numbers, separate from the study identification number and unrelated to the other specimen of the same patient, allowing for blinded evaluation of each. Three expert pathologists will independently interpret biopsy specimens without information on the nature of biopsy or specific clinical details. Following independent evaluation, they will confer on their findings, recording consensus findings of ‘definite UIP’, ‘probable UIP’, ‘indeterminate for UIP’ or ‘alternative diagnosis’, as defined in the current idiopathic pulmonary fibrosis (IPF) diagnostic guidelines.6 In addition, pathologists will record their consensus-specific histopathological patterns for each case (eg, hypersensitivity pneumonitis (HP), non-specific interstitial pneumonia (NSIP), UIP-IPF and so on), along with any differential diagnoses and degree of diagnostic confidence. A fourth independent pathologist will be consulted if a consensus pattern cannot be agreed on by the three pathologists. Specific histopathological features will also be documented, including the number and size of specimens, presence of any artefact, anatomical structures (eg, pleura, alveoli, airways, blood vessels), and presence and quantification of pathological features (eg, inflammatory cells, granulomata, fibroblastic foci, vasculitis, eosinophils).
MDD of all cases
Following histopathology assessment, each case will be discussed in the setting of a single centralised diagnostic MDD, including the same expert radiologists, pathologists and physicians involved with screening. For this exercise, de-identified patient information will be presented as follows (figure 2):
Figure 2.
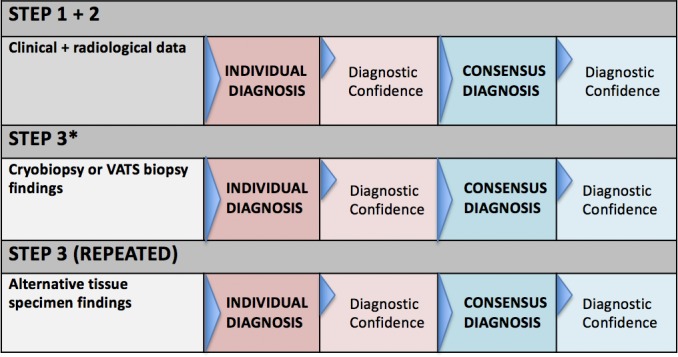
Multidisciplinary team discussion protocol. Stepwise process of multidisciplinary team discussion of each case. *Every patient’s details (steps 1 and 2) to be presented twice, each with addition of histopathology, either cryobiopsy or VATS biopsy, in random order. VATS, video-assisted thoracoscopic surgery.
Clinical history with examination findings and relevant investigations.
HRCT images and findings (consensus between two expert radiologists).
Histopathology pattern of either TBLC or VATS pathology specimen (consensus between three expert pathologists).
To minimise potential for bias of the panel, each biopsy will be considered in the context of the clinical case, without the influence of the other specimen. As such, every patient will be presented twice over the course of the extended MDD, so that 130 cases in total will be discussed (once with a VATS biopsy and once with a cryobiopsy to complement the clinical and radiological data). Cases will be presented in the same random order ascribed to specimens during the histopathological evaluation process so that a single patient’s two presentations cannot be correlated. If by random allocation the paired samples fall within 10 cases of one another, the second sample will be moved to later in the proceedings, so as to minimise the risk of familiarisation with the details of the case. Clinicians within the MDD will not be made aware of the dimensions of the specimen so that the nature of how it was obtained remains unknown.
At the end of each of steps 2 and 3, the ILD diagnosis (following guideline criteria) and degree of diagnostic confidence (detailed below) will be recorded individually by participants on standardised forms. Following individual recordings, a consensus discussion will take place and an agreed-on diagnosis will be recorded at steps 2 and 3. Consensus certainty levels of either ‘Definite’ (90%–100% confident), ‘High’ (70%–89% confident), ‘Low’ (51%–69% confident) or ‘Unclassifiable’ (<50% confident) will also be recorded.18 Data will also be collected on recommended management and predicted disease behaviour.15
Statistical methodology
Primary endpoint analyses
The two coprimary endpoints for the study are (1) agreement between TBLC and VATS histopathological patterns, and (2) agreement between TBLC and VATS consensus MDD diagnoses. For the histopathological primary endpoint assessment, diagnostic groups will be categorised into three histopathology categories: ‘definite or probable UIP’, ‘indeterminate for UIP’ or ‘alternative diagnosis’. The cross-tabulation of the diagnostic groups for the two methods will be presented, with the raw agreement reported together with the combined Cohen’s kappa statistic (stratified by biopsy type) and 95% CI.19 The χ2 test of heterogeneity will assess equality of Cohen’s kappa between diagnostic methods. For the MDD primary endpoint, the same statistical tests will be used to compare the agreement between consensus diagnoses using the histopathological information from the two procedures during the MDD process, following the stepwise presentation of (1) clinical information, (2) HRCT, and (3) either TBLC or VATS histopathology. Study endpoints are shown in table 2.
Table 2.
Primary and secondary study endpoints
| Primary |
|
| Secondary |
|
MDD, multidisciplinary discussion; TBLC, transbronchial lung cryobiopsy; VATS, video-assisted thoracoscopic surgery.
Secondary endpoint analysis
Raw agreement, along with the kappa value and 95% CI, will be measured to demonstrate degree of concordance between the specific histopathological patterns for TBLC and VATS (eg, HP, NSIP, UIP-IPF and so on). Kappa statistics will be calculated by biopsy type (as described for the primary outcome) to measure interobserver agreement between (1) histopathology assessment by pathologists (separately for each procedure type); (2) diagnosis by individual MDD participants after step 2; and (3) diagnosis by individual MDD participants after step 3 (separately for each procedure type). McNemar’s test will be used to assess if the proportion of diagnosed cases changes after the addition of histopathological assessment at MDD (with separate tests performed for the two procedure types). As an exploratory analysis, logistic regression models will be used to investigate clinical, procedural and histopathological characteristics that are associated with agreement between diagnostic methods. For all statistical tests, a p value <0.05 will be considered statistically significant. A kappa value equal to or less than 0.20 indicates poor agreement, 0.21–0.40 fair agreement, 0.41–0.60 moderate agreement, 0.61–0.80 good agreement and 0.81–1.00 excellent agreement.
Sample size
Assuming that 55%, 15% and 30% of cases in this population would be classified as definite/probable for UIP pattern, indeterminate for UIP pattern and an alternative diagnosis, and a true kappa of 0.8, a sample of 62 patients will allow estimation of a kappa statistic with a 95% CI, with a lower bound of at least 0.6 (indicating good agreement). Allowing for 5% missing data (due to patient dropout), we plan to recruit 65 patients to this study. Demographic and clinical characteristics of the sample will be summarised using counts and percentages for categorical variables, and means and SDs (or medians and IQRs) for continuous variables.
Trial oversight and ethical conduct
The COLDICE Study Steering Committee will oversee the study, meeting regularly to review processes and recruitment. An Independent Data Safety and Monitoring Board has been convened to adjudicate on serious adverse events and other safety issues. The study will be conducted following the International Conference on Harmonisation Guideline for Good Clinical Practice. All subjects will undergo informed consent before proceeding with any trial-related activities. They will also undergo specific procedural consent with the surgeon and interventional pulmonologist, in accordance with Australian health legislation.
Patient and public involvement
In Australia, we have already identified timely and accurate diagnosis as an important patient priority.20 However, the need to obtain lung tissue in many patients who are not fit enough to undergo surgical biopsy remains an important clinical paradox that hampers ILD diagnosis. If a safer method for sampling lung parenchyma (ie, TBLC) can provide similar results to the more invasive VATS technique, then the landscape for ILD diagnosis may be changed for the better. Although patients were not directly involved in designing the study protocol, this specific health priority formed the basis of the COLDICE research question. Indeed, the COLDICE trial was the inaugural study of Pulmonary fibrosis Australasian Clinical Trials Network, a portal for bringing patients, researchers and clinicians together for patient-centred clinical trials (https://pact.lungfoundation.com.au/wp-content/uploads/sites/2/2019/02/PACT-Clinician-COLDICE.pdf).
At study completion, patients will be engaged through the Pulmonary Fibrosis Consumer Advisory Group (an initiative of the Lung Foundation Australia). A summary statement in lay terminology will be created with the assistance of patient representatives for sharing results with the study participants and to the wider public.
Discussion
Through comprehensive evaluation, including expert pathology assessment and MDD, the COLDICE Study seeks to address unanswered questions regarding TBLC. Due to potential limitations of transbronchial tissue sampling, there is a strong need to assess this novel diagnostic technique rigorously, before its incorporation into guidelines.
Rationale for study design
The study will assess diagnostic accuracy of lung tissue obtained from the TBLC compared with the current standard, the VATS biopsy. The diagnostic accuracy of a smaller tissue sample can only reliably be evaluated by comparing it against the larger tissue sample obtained from the same patient. Although a randomised controlled trial may be a better study design to compare the safety profile of each of the two biopsy methods, it would not allow for the assessment of diagnostic agreement. Thus, TBLC must be validated against the current histopathological reference standard VATS biopsy for the diagnosis of ILD. Furthermore, the histopathological findings are only one component of the comprehensive MDD diagnosis that also encompasses the important metrics of exposure, demography, serology and radiology. The COLDICE Study necessarily includes two coprimary endpoints, that is, the histopathological agreement and the MDD agreement, which will delineate the role of the TBLC in ILD diagnosis.
Rationale for comparing TBLC with VATS lung biopsy in ILD
The addition of surgically obtained lung tissue can make a significant impact on confident ILD diagnosis at MDD. Indeed, the chief reason behind the majority of ‘unclassifiable’ ILD is the absence of adequate lung tissue to accompany other clinical data.21 International IPF registry data reveal VATS biopsy rates of 13%–24%.22 23 In clinical trial populations, where accurate classification is essential and disease is generally milder, these rates are even higher, at 30%–55%.24–26 However, VATS biopsy is associated with potential complications for the patient with ILD, with risk of acute exacerbation of ILD, persistent air leak, post-thoracotomy pain syndrome and death. Many patients are considered unsuitable for VATS, and thus remain unclassifiable and often without specific treatment options. Less invasive strategies, including bronchoalveolar lavage, transbronchial forceps biopsy and blood biomarkers, are generally of insufficient sensitivity to inform accurate diagnosis.6 17
As an emerging modality, the TBLC holds promise for a relatively safer and cost-effective alternative to surgery. Indeed, in many centres the enthusiasm for TBLC has led to a dramatic increase in tissue sampling in new patients with ILD.23 There are, however, valid concerns that a tissue specimen many-fold smaller in magnitude than the current standard may be more vulnerable to sampling error and incorrect histopathological interpretation.7 Although cross-sectional studies consistently show reasonable diagnostic ‘yield’ with TBLC, the diagnostic accuracy of TBLC against VATS biopsy has not yet been demonstrated. The COLDICE Study will be the largest prospective multicentre study to address this important question.
Potential limitations of the study
We acknowledge selection bias in the study population, through necessary enrolment of only those patients robust enough to withstand VATS lung biopsy. This will mean that any findings may not be generalisable to sicker patients with more advanced disease. Furthermore, in performing the dual procedures concurrently, the true adverse event rate for each technique will not be measurable. We also specifically chose to exclude comparison with conventional forceps biopsies given the limited diagnostic utility of this sampling technique, particularly in IPF.6 17
The safety profile of TBLC
There are many potential advantages of TBLC over VATS, including faster recovery time and lower risk of adverse events. The impact of prolonged chest wall pain following VATS is under-recognised and can be largely avoided with the TBLC. Although no direct comparison has been made, the risk of death with TBLC appears favourable over VATS, with respective reported mortality rates of 0.3% and 1.7% for elective procedures.3 27 It follows that the better safety profile could potentially translate into lower healthcare utilisation and cost savings.
The risk for TBLC, however, is not negligible, with a number of meta-analyses showing bleeding rates of 14%–39% and pneumothoraces in 10%–12%.3 4 As more centres have started to use TBLC, it is not surprising that diagnostic yields are lower and adverse events are more frequent than initially reported.8 Poor patient selection and operator inexperience are likely to be important contributing factors, highlighting the need for further evidence and standardised practice before general implementation of TBLC.
The future role of histopathology in ILD
In an era of increasingly personalised medicine, accurate diagnosis is the key to targeted therapy. As a specific example, antifibrotic agents nintedanib and pirfenidone are currently approved by funding bodies for the diagnosis of IPF only. While it is possible that the indications for these two antifibrotic drugs may broaden to include other forms of fibrotic lung disease, emerging agents remain tested only in very homogeneous, well-defined IPF populations. As with lung cancer, combination therapies may one day be standard for ILD sufferers, with specific treatment choices driven by molecular and immunological characteristics of the diseased tissue. It is already evident from the work of Oldham et al28 that different genotypes within IPF respond differentially to therapy, hinting at an imperative for meticulous evaluation. It is also apparent that many disease subtypes behave diversely, with variable natural histories and long-term outlook.29 While surgical lung biopsy may be falling out of favour for some, it is clear that lung tissue will continue to be an important source of information for ILD management.
If the TBLC is demonstrated to have satisfactory safety and accuracy attributes, it may reasonably supplant the VATS biopsy in the majority of ILD cases that require tissue diagnosis. If, however, the potential benefits come at the expense of diagnostic exactitude, then the role of TBLC must be carefully reviewed before further widespread use.
Acknowledgments
The authors wish to acknowledge the contributions of Venerino Poletti, Juergen Hetzel and Martin Hetzel for their advice on study design and procedural competency standards.
Footnotes
Collaborators: David Arnold, Christopher Cao, Amy Cashmore, Shannon Cleary, Bruce French, Monika Geis, Laura Glenn, Benjamin Harris, Michael Hibbert, Alvin Ing, Allen James, Helen E. Jo, Qi Tina Lin, Graham Meredith, Christopher Merry, Benjamin Ng, Martin Phillips, Anand Pudipeddi, Jessica Rhodes, Tajalli Saghaie, Rajesh Thomas, Claire Thomson, Scott Twaddell, Susanne Webster, Jeremy Wrobel, Peter Wu and Christopher Oldmeadow.
Contributors: LKT, EMTL, TC, CG, JPW, PJT, WC, MPV, MS and GR are members of the COLDICE Steering Committee and have all contributed to the design of the study. JM, AMM, SL, GD and EM have also had input into the study protocol. All authors met the criteria for authorship, with oversight of all stages of manuscript writing. Each takes full responsibility for the content of the manuscript.
Funding: The COLDICE study is an investigator-initiated study that has received inkind and/or financial support from the following commercial entities: Erbe Elektromedizin, Medtronic, Cook Medical, Rymed, Karl Storz and Olympus. Institutional research funding has also been received from The University of Sydney and Hunter Medical Research Institute. The study has the endorsement of both the Australian and New Zealand Interventional Pulmonology (ANZIP) Group and the Pulmonary Fibrosis Australasian Clinical Trials (PACT) Network. Administration of trial funding has been undertaken by the Baird Institute. Funding sources will have no involvement in the study design, study conduct, data analysis or manuscript preparation.
Competing interests: LKT received study-related unrestricted educational grants or inkind support from the aforementioned commercial entities, on behalf of the COLDICE Investigator Team. MPV sits on the advisory boards of Medtronic and Edwards Lifesciences, and consults for Edwards Lifesciences and Abbott. GR provides consultation for Bellerophon, Biogen, BMS, FibroGen, Gilead, Nitto, Revistan, Promedior, Sanofi, Veracyte, Roche-Genentech, Avalyn and Boehringer Ingelheim. JM is an unpaid collaborator in the Veracyte BRAVE Trial.
Patient consent for publication: Not required.
Ethics approval: The study will be conducted following the International Conference on Harmonisation Guideline for Good Clinical Practice. Full ethics approval has been granted by both the lead site Sydney Local Health District (Royal Prince Alfred Hospital) Human Research Ethics Committee (X15-0180 and HREC/15/RPAH/240), and Sir Charles Gairdner Group Human Research Ethics Committee (trial no 2015-143) for Western Australian sites. Site-specific approvals have been granted at each participating site.
Provenance and peer review: Not commissioned; externally peer reviewed.
Contributor Information
COLDICE Investigator Team:
David Arnold, Christopher Cao, Amy Cashmore, Shannon Cleary, Bruce French, Monika Geis, Laura Glenn, Benjamin Harris, Michael Hibbert, Alvin Ing, Allen James, Helen E. Jo, Qi Tina Lin, Graham Meredith, Christopher Merry, Benjamin Ng, Martin Phillips, Anand Pudipeddi, Jessica Rhodes, Tajalli Saghaie, Rajesh Thomas, Claire Thomson, Scott Twaddell, Susanne Webster, Jeremy Wrobel, Peter Wu, and Christopher Oldmeadow
References
- 1.Babiak A, Hetzel J, Krishna G, et al. . Transbronchial cryobiopsy: a new tool for lung biopsies. Respiration 2009;78:203–8. 10.1159/000203987 [DOI] [PubMed] [Google Scholar]
- 2.Pajares V, Puzo C, Castillo D, et al. . Diagnostic yield of transbronchial cryobiopsy in interstitial lung disease: a randomized trial. Respirology 2014;19:900–6. 10.1111/resp.12322 [DOI] [PubMed] [Google Scholar]
- 3.Sethi J, Ali MS, Mohananey D, et al. . Are transbronchial Cryobiopsies ready for prime time?: a systematic review and meta-analysis. J Bronchology Interv Pulmonol 2019;26:22–32. 10.1097/LBR.0000000000000519 [DOI] [PubMed] [Google Scholar]
- 4.Johannson KA, Marcoux VS, Ronksley PE, et al. . Diagnostic yield and complications of transbronchial lung Cryobiopsy for interstitial lung disease. A systematic review and Metaanalysis. Ann Am Thorac Soc 2016;13:1828–38. 10.1513/AnnalsATS.201606-461SR [DOI] [PubMed] [Google Scholar]
- 5.Han Q, Luo Q, Xie J-X, et al. . Diagnostic yield and postoperative mortality associated with surgical lung biopsy for evaluation of interstitial lung diseases: a systematic review and meta-analysis. J Thorac Cardiovasc Surg 2015;149:1394–401. 10.1016/j.jtcvs.2014.12.057 [DOI] [PubMed] [Google Scholar]
- 6.Raghu G, Remy-Jardin M, Myers JL, et al. . Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 2018;198:e44–68. 10.1164/rccm.201807-1255ST [DOI] [PubMed] [Google Scholar]
- 7.Patel NM, Borczuk AC, Lederer DJ. Cryobiopsy in the diagnosis of interstitial lung disease. A step forward or back? Am J Respir Crit Care Med 2016;193:707–9. 10.1164/rccm.201511-2313ED [DOI] [PubMed] [Google Scholar]
- 8.Hetzel J, Maldonado F, Ravaglia C, et al. . Transbronchial Cryobiopsies for the diagnosis of diffuse parenchymal lung diseases: expert statement from the Cryobiopsy Working Group on safety and utility and a call for standardization of the procedure. Respiration 2018;95:188–200. 10.1159/000484055 [DOI] [PubMed] [Google Scholar]
- 9.Tomassetti S, Wells AU, Costabel U, et al. . Bronchoscopic lung Cryobiopsy increases diagnostic confidence in the multidisciplinary diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2016;193:745–52. 10.1164/rccm.201504-0711OC [DOI] [PubMed] [Google Scholar]
- 10.Maldonado F, Moua T, Skalski J. Parenchymal cryobiopsies for interstitial lung diseases: a step forward in disease management. Respirology 2014;19:773–4. 10.1111/resp.12332 [DOI] [PubMed] [Google Scholar]
- 11.Ravaglia C, Bonifazi M, Wells AU, et al. . Safety and diagnostic yield of transbronchial lung Cryobiopsy in diffuse parenchymal lung diseases: a comparative study versus video-assisted thoracoscopic lung biopsy and a systematic review of the literature. Respiration 2016;91:215–27. 10.1159/000444089 [DOI] [PubMed] [Google Scholar]
- 12.Romagnoli M, Colby TV, Berthet J-P, et al. . Poor concordance between sequential transbronchial lung Cryobiopsy and surgical lung biopsy in the diagnosis of diffuse interstitial lung diseases. Am J Respir Crit Care Med. In Press2019;199:1249–56. 10.1164/rccm.201810-1947OC [DOI] [PubMed] [Google Scholar]
- 13.Knottnerus JA, Tugwell P. The standards for reporting of diagnostic accuracy. J Clin Epidemiol 2003;56 10.1016/j.jclinepi.2003.10.001 [DOI] [PubMed] [Google Scholar]
- 14.Cohen JF, Korevaar DA, Altman DG, et al. . STARD 2015 guidelines for reporting diagnostic accuracy studies: explanation and elaboration. BMJ Open 2016;6:e012799 10.1136/bmjopen-2016-012799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Travis WD, Costabel U, Hansell DM, et al. . An official American thoracic Society/European Respiratory Society statement: update of the International multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188:733–48. 10.1164/rccm.201308-1483ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raghu G. Idiopathic pulmonary fibrosis: guidelines for diagnosis and clinical management have advanced from consensus-based in 2000 to evidence-based in 2011. Eur Respir J 2011;37:743–6. 10.1183/09031936.00017711 [DOI] [PubMed] [Google Scholar]
- 17.Raghu G, Collard HR, Egan JJ, et al. . An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788–824. 10.1164/rccm.2009-040GL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryerson CJ, Corte TJ, Lee JS, et al. . A standardized diagnostic ontology for fibrotic interstitial lung disease. An international Working Group perspective. Am J Respir Crit Care Med 2017;196:1249–54. 10.1164/rccm.201702-0400PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleiss JL, Levin B, Paik MC. Statistical methods for rates and proportions. 3rd New Jersey: Wylie, 2003. [Google Scholar]
- 20.Burnett K, Glaspole I, Holland AE. Understanding the patient's experience of care in idiopathic pulmonary fibrosis. Respirology 2019;24:270–7. 10.1111/resp.13414 [DOI] [PubMed] [Google Scholar]
- 21.Ryerson CJ, Urbania TH, Richeldi L, et al. . Prevalence and prognosis of unclassifiable interstitial lung disease. Eur Respir J 2013;42:750–7. 10.1183/09031936.00131912 [DOI] [PubMed] [Google Scholar]
- 22.Jo HE, Glaspole I, Grainge C, et al. . Baseline characteristics of idiopathic pulmonary fibrosis: analysis from the Australian idiopathic pulmonary fibrosis registry. Eur Respir J 2017;49 10.1183/13993003.01592-2016 [DOI] [PubMed] [Google Scholar]
- 23.Guenther A, Krauss E, Tello S, et al. . The European IPF registry (eurIPFreg): baseline characteristics and survival of patients with idiopathic pulmonary fibrosis. Respir Res 2018;19 10.1186/s12931-018-0845-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noble PW, Albera C, Bradford WZ, et al. . Pirfenidone in patients with idiopathic pulmonary fibrosis (capacity): two randomised trials. Lancet 2011;377:1760–9. 10.1016/S0140-6736(11)60405-4 [DOI] [PubMed] [Google Scholar]
- 25.King TE, Bradford WZ, Castro-Bernardini S, et al. . A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 2014;370:2083–92. 10.1056/NEJMoa1402582 [DOI] [PubMed] [Google Scholar]
- 26.Richeldi L, Costabel U, Selman M, et al. . Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med 2011;365:1079–87. 10.1056/NEJMoa1103690 [DOI] [PubMed] [Google Scholar]
- 27.Hutchinson JP, Fogarty AW, McKeever TM, et al. . In-hospital mortality after surgical lung biopsy for interstitial lung disease in the United States. 2000 to 2011. Am J Respir Crit Care Med 2016;193:1161–7. 10.1164/rccm.201508-1632OC [DOI] [PubMed] [Google Scholar]
- 28.Oldham JM, Ma S-F, Martinez FJ, et al. . Tollip, MUC5B, and the response to N-acetylcysteine among individuals with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2015;192:1475–82. 10.1164/rccm.201505-1010OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park JH, Kim DS, Park I-N, et al. . Prognosis of fibrotic interstitial pneumonia: idiopathic versus collagen vascular disease-related subtypes. Am J Respir Crit Care Med 2007;175:705–11. 10.1164/rccm.200607-912OC [DOI] [PubMed] [Google Scholar]