Abstract
Thyroid disorders have emerged as one of the most common immune-related adverse events (irAEs), yet optimum management and biomarkers to predict vulnerable individuals remain to be explored. High-dose glucocorticoid (HDG) therapy is routinely recommended for irAEs. However, systematic analysis of the impact of glucocorticoid therapy on the outcome of immune checkpoint inhibitor (ICI)–induced thyroid disorders is lacking. We analyzed 151 patients with or without ICI-related thyroid disorders. We divided the patients with -ICI-related thyroid disorders into two subgroups: those with and without HDG treatment. Our results showed no significant differences between HDG and no-HDG groups in terms of the median duration of thyrotoxicosis: 28 (range: 7–85) and 42 (range: 14–273) days, the median time to conversion from thyrotoxicosis to hypothyroidism: 39 days (range: 14–169) and 42 days (range: 14–315) days, the median time to onset of hypothyroidism: 63 (range: 21–190) and 63 (range: 14–489) days, and the median maintenance dose of levothyroxine: 1.5 (range: 0.4–2.3) μg/kg/day, and 1.3 (range: 0.3–2.5) μg/kg/day. The median pretreatment TSH was 2.3 (range: 0.3–5.2) mIU/L and 1.7 (range: 0.5–4.5) mIU/L in patients with and without ICI-related thyroid disorders, respectively. Baseline TSH was significantly higher in patients who developed ICI-related thyroid disorders (P = 0.05). Subgroup analysis revealed significantly higher baseline TSH in male but not female patients with ICI-induced thyroid dysfunction. Our results show that HDG treatment did not improve the outcome of ICI-related thyroid disorders.
Keywords: immune checkpoint inhibitors, immune-related adverse events, thyroid disorders, high dose glucocorticoids, biomarker
Introduction
Unleashing the immune response through the use of immune checkpoint inhibitors has demonstrated significant improvement in overall survival in a broad spectrum of advanced solid as well as liquid malignancies (1–6), but this treatment increases autoimmunity and causes immune-related adverse events (irAEs) (7,8). Thyroid disorders have emerged to be very common irAEs (9). Early identification and proper management of immune checkpoint inhibitor (ICI)–induced thyroid disorders will help to prevent severe consequences such as thyroid storm or myxedema coma, and improve quality of life. High dose glucocorticoid therapy (HDG) is routinely recommended for moderate-severe irAEs (10,11). However, systematic analysis of the impact of HDG on the outcome of ICI-induced thyroid disorders is lacking. In the context of management of hypothyroidism, initiation with 1.6 μg/kg/day was recommended by the American Society of Clinical Oncology (ASCO) clinical practice guideline (11), but no study has evaluated the replacement dose of levothyroxine in ICI-induced hypothyroidism. Additionally, there is no biomarker reported to identify individuals susceptible to ICI-related thyroid disorders. The purpose of this study was to evaluate optimum management of ICI-induced thyroid disorders and to explore the role of baseline TSH as a predictive biomarker of ICI-induced thyroid disorders.
Methods
Study design and patient cohorts
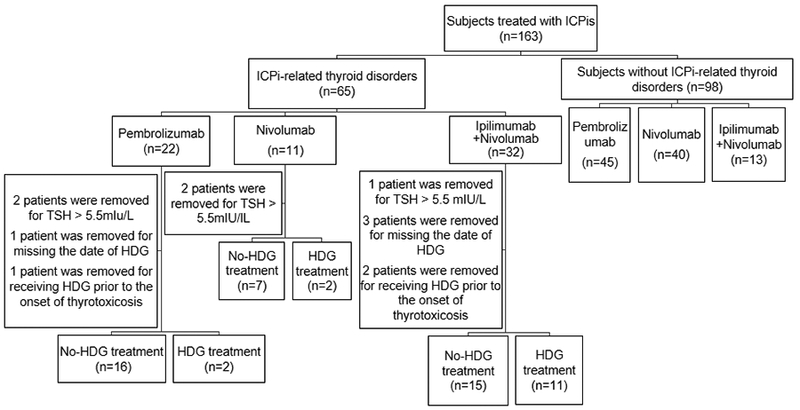
This cohort analysis was performed retrospectively by collecting data from chart reviews with removal of individual identifiers. The patients were identified through a Research Patient Data Registry (RPDR), a program developed at our institution to search patients in the electronic database by diagnosis, laboratory values, medication, and/or keyword. Some of the patients with ICI-related thyroid disorders were evaluated clinically in the outpatient endocrinology clinic of Brigham and Women’s Hospital and received continued longitudinal clinical care. The period for this study was from 12/4/2009 to 8/14/2017. Institutional Review Board approval was obtained for the study. We identified 65 patients with ICI-induced thyroid disorders and 98 individuals who were exposed to ICIs but did not develop thyroid disorders as a control group (Fig. 1). Patients were excluded if they did not have pre-treatment TSH measurements, if pretreatment TSH was greater than up limit of normal range (5.5 mIU/L) by assays in our institutions, if dates of HDG treatment were missing, or if they received HDG prior to the onset of thyrotoxicosis. After exclusions, 53 and 98 patients with and without ICI-induced thyroid disorders, respectively, were analyzed.
Figure 1. Flow chart of study design and subjects. HDG: High dose glucocorticoids.
Definition of thyrotoxicosis and hypothyroidism
Serum TSH, thyroxine (T4), free T4 (FT4), and/or free triiodothyronine (FT3) were measured prior to initiation of ICI treatment, and then every 2–4 weeks as mandated by clinical trial protocols or clinical practice. Thyrotoxicosis was defined as a decreased TSH with an elevated T4, FT4, or FT3. Hypothyroidism was defined as an elevated TSH with a decreased T4, FT4 or FT3. Subclinical hypothyroidism and hyperthyroidism were defined as normal T4, FT4, and FT3 with elevated or decreased TSH, respectively. Reference ranges of normal laboratory values in our institutions were: TSH, 0.5–5.5 mIU/L; T4, 5.0–11.0 μg/dL; FT4, 0.9–1.7 ng/dL; FT3, 2.8–4.4 pg/ml; thyroperoxidase antibodies (TPOAb), < 35 IU/ml; thyroid-stimulating immunoglobulin index (TSII), ≤ 1.3.
Definitions of time to onset of thyroid disorders
Time to onset of thyrotoxicosis was defined as the number of days between the administration of the first dose of ICI and the date of the first documented biochemical evidence of thyrotoxicosis. Duration of thyrotoxicosis was defined as the number of the days between the initial biochemical evidence of thyrotoxicosis and the first subsequent documented biochemical evidence of euthyroidism or hypothyroidism. Time to conversion from thyrotoxicosis to hypothyroidism was defined as the number of days between the initial biochemical evidence of thyrotoxicosis and the first documented biochemical evidence of hypothyroidism. Time to onset of hypothyroidism was defined as the number of days between the administration of the first dose of ICI and the date of the first biochemical documentation of hypothyroidism.
Patient regimens
The patients were divided into subgroups based on the regimens of ICIs received. We analyzed time to onset of thyrotoxicosis and hypothyroidism by different treatments. We evaluated the association of baseline TSH with the development of ICI-induced thyroid disorders. We further divided the patients into two groups: those with and without HDG treatment. Systemic HDG treatment was defined as the administration of glucocorticoids at a dose of more than 7.5 mg prednisone or equivalent daily for more than one week. HDG treatment was given to 15 patients: before the onset of thyrotoxicosis (n=1), after the onset of thyrotoxicosis (n=7), before the onset of hypothyroidism (n=1), or after the onset of hypothyroidism (n=6). Among these 15 patients, 3 patients received HDG treatment for thyrotoxicosis, 10 for other immune-related adverse events (IrAEs), 1 for brain metastasis, and 1 for headache. The patients in the HDG group received prednisone (n=4, at 30–60 mg daily, for 2–6 weeks), dexamethasone (n=2, at 2–6 mg daily for 9 days to 55 weeks), methylprednisolone followed by prednisone (n=5, methylprednisolone at 40–260 mg daily for 1–3 days; then prednisone at 60–120 mg daily for 2–15 weeks), methylprednisolone followed by dexamethasone and prednisone (n=1, methylprednisolone at 180 mg daily for 8 days, then dexamethasone at 36 mg tapered over 4 weeks, then prednisone at 200 mg tapered over 9 weeks), methylprednisolone followed by hydrocortisone (n=1, methylprednisolone at 50 mg daily for 1 day, then hydrocortisone at 50–150 mg daily for 2 weeks), hydrocortisone followed by prednisone (n=1, hydrocortisone at 50 mg daily for 1 day, then prednisone at 80 mg tapered over 2 weeks), or dexamethasone followed by prednisone (n=1, dexamethasone at 8 mg daily for 24 days, prednisone at 80 mg tapered for 46 weeks). We analyzed the impact of HDG on the duration of thyrotoxicosis and the time to conversion from thyrotoxicosis to hypothyroidism, including the patients (n = 8). We also analyzed the time to onset of hypothyroidism (patients treated with HDG before or after the onset of thyrotoxicosis and before the onset of hypothyroidism were included, n=9). We did not analyze the impact of HDG on the time to onset of thyrotoxicosis, because no patients received HDG prior to initiating ICI treatment and only 1 patient received HDG before the onset of thyrotoxicosis. We analyzed the impact of HDG on the duration of thyrotoxicosis, the time to conversion from thyrotoxicosis to hypothyroidism, and the time to onset of hypothyroidism. We evaluated the impact of HDG on the maintenance dose of levothyroxine.
Statistical analysis
Summaries of patient characteristics are primarily descriptive. Comparisons between groups of characteristics measured on a continuous scale are based on Wilcoxon rank-sum or Kruskal-Wallis tests. Comparisons of characteristics measured categorically are based on Fisher’s exact tests. The distributions of time to thyrotoxicosis or hypothyroidism are described using the method of Kaplan-Meier; median times are presented with 95% confidence intervals. A Cox proportional hazards regression model was used to identify predictors of time to develop thyrotoxicosis. Statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA). Statistical significance is defined as p ≤ 0.05; there were no corrections for multiple comparisons.
Results
Study population
Among 53 patients who developed thyroid disorders, 18 (34%) patients received pembrolizumab (pembro), 9 (17%) patients received nivolumab (nivo), and 26 (49%) patients received ipilimumab (ipi) + nivo therapy (Fig. 1). The demographic data are summarized in Table 1.
Table 1.
Clinical characteristics of patients with ICI-related thyroid disorders.
| Characteristic | Pembro | Nivo | Ipi+ Nivo | Total |
|---|---|---|---|---|
| Number | 18 | 9 | 26 | 53 |
| Age, years | ||||
| Median (range) | 62 (32–82) | 60 (42–81) | 58 (34–78) | 60 (32–82) |
| Gender, n(%) | ||||
| Female | 10(56) | 9(100) | 15(58) | 34(64) |
| Male | 8(44) | 0 (0) | 11(42) | 19(36) |
| Malignancy, n(%) | ||||
| Melanoma | 12(67) | 3(33) | 21(81) | 36(68) |
| Leiomyosarcoma | 1(6) | 0(0) | 0(0) | 1(2) |
| Breast cancer | 3(17) | 0(0) | 0(0) | 3(6) |
| Lung cancer | 1(6) | 0(0) | 0(0) | 1(2) |
| Glioblastoma | 1(6) | 2(22) | 0(0) | 3(6) |
| Renal cell carcinoma | 0(0) | 0(0) | 1(4) | 1(2) |
| Meningioma | 0(0) | 3(33) | 0(0) | 3(6) |
| Bladder cancer | 0(0) | 1(11) | 1(0) | 2(4) |
| Ovarian cancer | 0(0) | 0(0) | 2(0) | 2(4) |
| Malignant neoplasm of esophagus | 0(0) | 0(0) | 1(4) | 1(2) |
| Preexisting thyroid disorder, n(%) | ||||
| Yes | 4(22) | 3(33) | 1(4) | 8(15) |
| No | 14(78) | 6(67) | 25(96) | 45(85) |
Time to onset of thyrotoxicosis and hypothyroidism
ICI-related thyroid disorders presented as hypothyroidism only (21%), thyrotoxicosis only (4%), or thyrotoxicosis followed by hypothyroidism (75%) (Supplementary Table S1). Onset of thyrotoxicosis and hypothyroidism was analyzed by the method of Kaplan-Meier survival analysis. The follow-up of patients who did not develop thyrotoxicosis is censored at the time of hypothyroidism development. Median time to onset of thyrotoxicosis after initiation of ICIs was 44 days (range: 19–447), 56 days (range: 13–126), and 21 days (range: 7–64), in the pembro, nivo, and nivo+ipi groups, respectively (Fig. 2A, p = 0.018). The p values were 0.593, 0.004, and 0.158 between pembro and nivo, pembro and ipi+nivo, and nivo and ipi+nivo, respectively. Median time to onset of hypothyroidism after initiation of ICIs was 84 days (range: 43–544), 84 days (range: 14–154), and 62 days (range: 21–141) in the pembro, nivo, and nivo+ipi groups, respectively (Fig. 2B, p = 0.003). The p values were 0.333, 0.002, and 0.057 between pembro and nivo, pembro and ipi+nivo, and nivo and ipi+nivo, respectively. Patients in the combination nivo+ipi group had significantly shorter times to develop both thyrotoxicosis and hypothyroidism than those in pembro and nivo monotherapy groups (Fig. 2A and B). Forty of 42 patients (95%) who had thyrotoxicosis converted to hypothyroidism (Supplementary Table S1). There was no difference in the time to onset of hypothyroidism in patients with or without preceding thyrotoxicosis (Supplementary Fig. S1).
Figure 2. Time to onset of thyrotoxicosis and hypothyroidism by different treatment regimens.
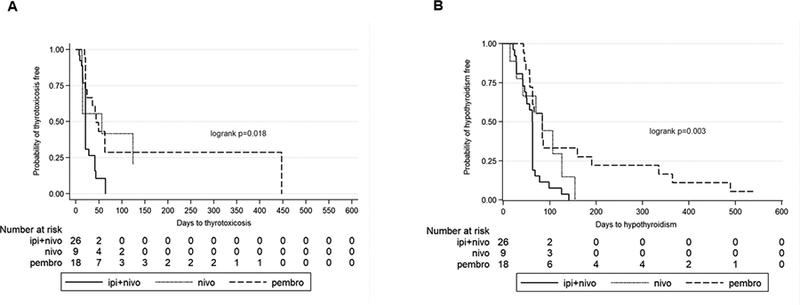
Onset of thyrotoxicosis and hypothyroidism was analyzed by the method of Kaplan-Meier. A, Onset of thyrotoxicosis after first treatment with ICI. The follow-up of patients who did not develop thyrotoxicosis is censored at the time of development of hypothyroidism. Median Onset Time of thyrotoxicosis (Median with range, days): pembro: 44, (range: 19–447); nivo: 56 (range: 13–126); ipi + nivo: 21 (range: 7–64). B, Onset of hypothyroidism after first treatment with ICI. Median onset time of hypothyroidism (Median with range, Days): pembro: 84 (range: 43–544); nivo: 84 (range: 14 – 154); ipi + nivo: 62 (range: 21–141). Significant difference is defined as P < 0.05.
Effect of HDG on the outcome of thyroid disorders
There were 11, 2, and 40 patients in this analysis who manifested with hypothyroidism only, thyrotoxicosis only, and thyrotoxicosis followed by hypothyroidism respectively (Supplementary Table S1). Of the 42 patients with thyrotoxicosis (thyrotoxicosis only + thyrotoxicosis followed by hypothyroidism), 8 received HDG (19%) before or after the onset of thyrotoxicosis. The median number of days from initiation of immunotherapy to initiation of HDG treatment was 26 days. Among 40 patients who developed hypothyroidism following thyrotoxicosis, 8 patients received HDG before the onset of hypothyroidism. Among 51 patients who developed hypothyroidism (hypothyroidism only + thyrotoxicosis followed by hypothyroidism), 9 patients received HDG prior to the development of hypothyroidism. Most of the patients in this study did not receive HDG for their thyroid disorders because thyroid-related symptoms were usually mild, even if thyroid function tests showed markedly abnormal TSH, free T4, and free T3. HDG was generally used to treat other irAEs, except in 3 patients who were treated for severe symptomatic thyrotoxicosis.
Figure 3 illustrates the impact of HDG on the outcome of thyroid disorders. The median duration of thyrotoxicosis in the HDG and no HDG groups was 28 days (range: 7–85) and 42 days (range: 14–273), respectively. There was no significant difference in the median duration of thyrotoxicosis between the two groups (Fig. 3A, Wilcoxon rank-sum P = 0.26). The median time to conversion from thyrotoxicosis to hypothyroidism was 39 days (range: 14–169) and 42 days (range: 14–315) in the HDG and no HDG groups, respectively, which were not significantly different (Fig. 3B, Wilcoxon rank-sum P = 0.92). The median time to onset of hypothyroidism was 63 days (range: 21–190) and 63 days (range: 14–489) in the HDG and no HDG groups, with no significant difference (Fig. 3C, Wilcoxon rank-sum P = 0.50). None of the patients had an identified remission of their hypothyroidism during the study period.
Figure 3. The effect of systemic high-dose glucocorticoid (HDG) treatment on ICI-related thyroid disorders.
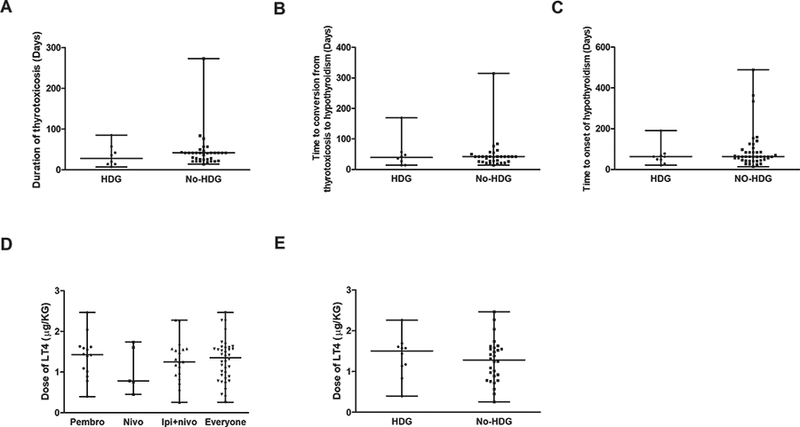
A, Duration of thyrotoxicosis. Lines represent median with range; grey dots represent individual values. The difference between the two groups was not statistically significant (Wilcoxon rank-sum P = 0.26). B, Time to conversion from thyrotoxicosis to hypothyroidism in patients with or without HDG treatment. The difference between two groups was not statistically significant (Wilcoxon rank-sum P = 0.92). C, The time to onset of hypothyroidism after the initial treatment with ICI in patients with or without treatment with HDG. The difference between the two groups was not statistically significant (Wilcoxon rank-sum P = 0.50). D, Dose of levothyroxine (LT4) needed to achieve euthyroidism (μg/kg body weight/day). There was no significant difference among the groups (pembro, nivo, ipi+nivo) (Kruskal-Wallis Test P = 0.64.). E, Impact of HDG on the dose of LT4 needed to achieve euthyroidism. There was no significant difference between the patients with or without HDG treatment (Wilcoxon rank-sum P = 0.46). For each panel, lines represent median with range; grey dots represent individual values.
Effect of HDG on the maintenance dose of levothyroxine
We defined the maintenance dose of levothyroxine (LT4) as the dose needed to reach biochemical euthyroidism, i.e., normal TSH with normal T4 or FT4. We calculated the dose of LT4 (μg) relative to body weight (kg). We typically started LT4 at a dose of 0.8–1.0 μg/kg/day if patients were younger than 60 years and had no known cardiac disorders. In patients older than 60 years or those with known cardiac disease, we started with a lower dose of LT4 (25–50 μg daily) and increased the dose more slowly, usually taking 2–3 months to reach euthyroidism. In this analysis of levothyroxine maintenance dose, we excluded patients whose thyroid function tests did not reach euthyroidism during the study period. Among 38 patients who had data for levothyroxine dose and reached euthyroidism, the median dose of LT4 was 1.4 (range: 0.3–2.5) μg/kg/day. There was no significant difference in levothyroxine maintenance dose among nivo-, pembro-, or combination nivo + ipi-induced hypothyroidism (Fig. 3D, Kruskal-Wallis Test, P = 0.64). We evaluated the impact of HDG on the dose of LT4 needed to achieve euthyroidism. The doses of LT4 were 1.5 (range: 0.4–2.3) μg/kg/day and 1.3 (range: 0.3–2.5) μg/kg/day in patients with and without HDG treatment, which were not significantly different (Fig. 3E, Wilcoxon rank-sum P = 0.46).
Baseline TSH concentrationin patients with ICI-related thyroid disorders
The median pretreatment TSH for patients who developed ICI-induced thyroid disorders was 2.3 (range: 0.3–5.2 mIU/L) compared with 1.7 (range: 0.5–4.5 mIU/L) for those who did not. Patients who developed ICI-related thyroid disorders had significantly higher baseline TSH than patients who did not (p = 0.05) (Table 2). Male patients with ICI-induced thyroid disorders had significantly higher pre-treatment TSH than the remaining three subgroups of patients (p = 0.02) (Table 2). When we performed further analyses in subgroups of patients whose initial presentation was with hypothyroidism or thyrotoxicosis, significantly higher pretreatment TSH was observed in only the group whose initial presentation was with hypothyroidism but not in those who presented with thyrotoxicosis (Supplementary Fig. S2). Among males, baseline TSH values were significantly higher in those with initial manifestations of either hypothyroidism or thyrotoxicosis. In contrast, in females, there was no significant difference in baseline TSH among any of the groups (Table 2 and Supplementary Fig. S2). In patients with ICI-induced thyroid disorders, there was no difference in pretreatment TSH according to ICI regimen or HDG treatment (Supplementary Table S2). To validate the findings, we analyzed baseline TSH from 44 patients (all female) enrolled in a prospective randomized phase 2 open label study of eribulin mesylate + pembrolizumab for patients with metastatic hormone receptor (HR) positive breast cancer. Seven patients were excluded from the analysis, because 6 had pretreatment abnormal TSH and 1 did not have a pretreatment TSH performed. Nine patients developed thyroid disorders. The median pretreatment TSH was 1.64 (range: 0.58–3.40 mIU/L) and 1.64 (range: 0.63–4.60 mIU/L) in patients with or without pembrolizumab-induced thyroid disorders (Supplementary Table S3). There was no significant difference in pretreatment TSH values between the patients with or without pembrolizumab-induced thyroid toxicity. There were no thyroid disorders observed in the arm with eribulin mesylate monotherapy.
Table 2.
Baseline (pre-treatment) TSH concentrations (mIU/L) in patients with or without ICI-induced thyroid disorders.
| Thyroid disorders | N | Min | Median | Max | |
|---|---|---|---|---|---|
| TSH (mIU/L) | |||||
| Yes# | 53 | 0.28 | 2.30 | 5.21 | |
| No | 98 | 0.50 | 1.70 | 4.45 | |
| Overall | 151 | 0.28 | 1.76 | 5.21 | |
| Sex | |||||
| Yes | F | 34 | 0.28 | 1.66 | 5.21 |
| M## | 19 | 0.51 | 2.98 | 4.83 | |
| No | F | 32 | 0.78 | 1.49 | 4.45 |
| M | 66 | 0.50 | 1.74 | 3.90 | |
All: All patients. Yes: Patients developed ICI-induced thyroid disorders. No: Patient did not develop ICI-induced thyroiditis. F: Female. M: Male.
Patients who developed ICI-related disorders had significantly higher baseline TSH compared with patients who did not (Wilcoxon rank-sum P = 0.05).
Males with ICI disorders had significantly higher pre-treatment TSH than the remaining three subgroups of patients (Kruskal-Wallis P = 0.02).
Thyroid antibodies and FT3/FT4 ratio
Nineteen patients in this study were tested for TPOAb, of which eight patients (42%) were positive. Among those tested, 60% (3/5), 50% (1/2), and 33% (4/12) had positive TPOAb in the pembro, nivo, and ipi+nivo groups, respectively. Seventeen patients were tested for TSII during thyrotoxicosis. Only 1 (6%) had detectable TSII, and this patient subsequently developed hypothyroidism. The calculated FT3/FT4 ratio was 1.8 (range: 0.6–2.7) in 21 patients during the thyrotoxicosis phase.
Discussion
Thyroid disorders have emerged as one of the most common adverse events associated with anti–PD-1 monotherapy and with combination anti–PD-1 and anti–CTLA-4 therapy (2,12–14). To explore the proper management of ICI-related thyroid disorders and to identify predictive biomarkers, we investigated the nature of ICI-induced thyroid disorders, the role of HDG on the outcome of thyroid disorder, the maintenance dose of levothyroxine, and the pretreatment TSH in patients with or without ICI-induced thyroid disorders.
Thyroid disorders associated with ICI therapy have been described as hyperthyroidism, hypothyroidism, and thyroiditis in previous reports (2,3,15). Consistent with previous work (16), the findings in the current study demonstrated that most immune checkpoint blockade–related thyroid disorders are the result of thyroiditis. This conclusion is supported by the findings that 96% of the patients with ICI-related thyroid disorders developed hypothyroidism at the end of this study. The evolution of ICI-induced thyroid disorders follows the typical time course of thyroiditis (17). The onset time of thyrotoxicosis and hypothyroidism between pembro and nivo was not statistically significant. The median FT3/FT4 ratio during thyrotoxicosis was 1.8 (range: 0.6–2.7), consistent with thyroiditis (18). Although 1 patient in this study had positive TSII, this patient eventually developed hypothyroidism, indicating she developed thyroiditis as well.
The role of HDG in the management of ICI-related thyroid disorders remains unclear. High dose steroid treatment is very effective in shortening the period of thyrotoxicosis in naturally occurring autoimmune thyroiditis (19). HDG is recommended by American Thyroid Association guidelines for management of thyrotoxicosis induced by destructive thyroiditis (20). Although ICI-induced thyroiditis typically manifests with less severe symptoms, the thyroid function test results can be extremely abnormal. It is therefore relevant to investigate the role of HDG in the management of ICI-induced thyroiditis. We evaluated the impact of HDG treatment on the outcome of ICI-related thyroid disorders. We found that HDG did not significantly alter the duration of thyrotoxicosis or the time to conversion from thyrotoxicosis to hypothyroidism, suggesting that HDG did not alter the evolution of ICI-induced thyroid disorders. There was no significant difference in pre-ICI–treatment TSH between the HDG and no HDG groups. The severity of thyroid disorders was therefore not a confounding factor in determining the impact of HDG on the outcome of thyroid disorders. Our results suggest that HDG did not improve the outcome of ICI-related thyroid disorders. We therefore do not recommend routine use of HDG in patients who develop ICI-related thyroid disorders such as thyrotoxicosis.
The average maintenance dose of levothyroxine in this study was 1.29 μg/kg/day, which is lower than the maintenance dose needed in the athyroid patients (1.6–1.8 μg/kg/day) (21–25). The current recommendation for the starting dose of levothyroxine is 1.6 μg/kg/day (11); based on our findings, we suggest revising the guidelines to start with a lower dose, 0.8–1.0 μg/kg/day. The maintenance dose of LT4 in HDG and non-HDG groups was not significantly different, suggesting that HDG did not prevent or reduce ICI-induced thyroid damage.
Our findings that the pretreatment median TSH value was significantly higher in patients who developed ICI-related thyroid disorders than in patients without ICI-related thyroid disorders are unexpected. In subgroup analysis, only male patients who developed ICI-related thyroid disorders had significantly higher pretreatment TSH than control male patients, but there was no significant difference between female patients with or without ICI-related thyroid disorders. In our validation cohort of female patients with breast cancer, there was no significant difference in pre-pembrolizumab–treatment TSH between the patients with or without pembrolizumab-induced thyroid toxicity. Pending prospective studies in both male and female patients, the role of baseline TSH as a predictive biomarker for the development of ICI-related thyroid dysfunction remains to be determined.
Thyroid autoantibodies have been suggested as biomarkers to predict development of ICI-related thyroiditis, but some studies do not support this suggestion (26–36). In the current study, we found that 42% of tested patients had positive TPOAb. Our study showed a trend of a higher incidence of TPOAb in anti–PD-1 monotherapy–induced thyroid disorders than in combination therapy, but the difference did not reach statistical significance. The role of thyroid autoantibodies in ICI-induced thyroid disorders remains uncertain. Future prospective, randomized clinical studies may help to clarify the uncertainty.
The symptoms of ICI-related thyrotoxicosis and hypothyroidism at first presentation were usually mild and non-specific. Despite their mild symptoms, the biochemical tests usually showed a very high TSH of 31.5 (range: 2.4–187.8) mIU/L in hypothyroidism, and a very high FT4 of 2.8 (range: 1.0–7.7) ng/dL in thyrotoxicosis. As the thyroid receptor is a nuclear receptor that regulates gene expression by binding to hormone response elements(37), acute changes in thyroid hormone may take time to induce genomic change. Indeed, it takes more than a week for patients with hypothyroidism to feel better after initiating levothyroxine (38). During the acute period of rapid and marked changes in thyroid hormone, patients with ICI-induced thyroid disorders might not become symptomatic for a period of time if left uncorrected. If attention is not given to these patients early in the course of their thyroid disorder because of mild symptomatology, serious consequences such as life-threatening thyroid storm (39) or myxedema coma (40) can occur. Close monitoring and treatment with a beta-blocker in patients with thyrotoxicosis, as well as timely replacement with levothyroxine in patients with hypothyroidism will help to prevent these serious consequences.
In summary, with increasing use of ICI therapy in a broad spectrum of malignancies, it is advantageous for clinicians to learn the diagnosis and management of ICI-induced thyroiditis, one of the most common irAEs. Based on this and previous studies (16,26,28,31,32,34,35), we recommend measuring TSH and FT4 before the initiation of ICI treatment and repeating them before each cycle of ICI, for at least 5 cycles. In our practice, supportive care with or without a beta blocker is sufficient in most patients in thyrotoxicotic phase. We do not routinely use HDG treatment for thyrotoxicosis. We use HDG in patients who present with symptoms suggestive of thyroid storm such as tachycardia, fever, and mental status change, or in patients with cardiac disease. When patients develop hypothyroidism, we initiate levothyroxine replacement, usually starting with 0.8–1 μg/kg/day in young patients without cardiac disease, or with a lower dose of 25–50 μg/day in elderly patients (> 60 years) or patients with cardiac disease, titrating the levothyroxine dose every 3–4 weeks based on the TSH and FT4. Finally, the role of pre-treatment TSH as a useful biomarker to predict ICI-induced thyroiditis remains to be determined.
This study has limitations due to its retrospective design. To confirm our findings of the impact of high dose corticosteroids on the outcome of ICI-induced thyroiditis, the maintenance dose of levothyroxine in the phase of hypothyroidism, as well as the association between pretreatment TSH and development of ICI-induced thyroiditis, future randomized prospective clinical trials will be necessary. Furthermore, the recommendations based on this retrospective study could be strengthened or revised by the future randomized prospective studies.
Supplementary Material
Acknowledgements
This work was supported by the National Institutes of Health [K08 HD070957 to L. Min] and National Natural Science Foundation of China [81300698 to C.J. Ma].
Funding: This work was supported by the National Institutes of Health [K08 HD070957 to L. Min] and National Natural Science Foundation of China [81300698 to C.J. Ma].
Disclosures:
Dana-Farber Cancer Institute and BWH receive clinical trial support from Bristol-Myers Squibb (F.S.H., S.M.T., U.B.K., L.M.), Merck (S.M.T), and Genentech (S.M.T).
References
- 1.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363(8):711–23 doi 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med 2015;373(1):23–34 doi 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med 2015;372(26):2521–32 doi 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 4.Lee L, Gupta M, Sahasranaman S. Immune Checkpoint inhibitors: An introduction to the next-generation cancer immunotherapy. J Clin Pharmacol 2016;56(2):157–69 doi 10.1002/jcph.591. [DOI] [PubMed] [Google Scholar]
- 5.Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N Engl J Med 2017;376(25):2415–26 doi 10.1056/NEJMoa1613493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bins S, van Meerten E, Mathijssen RH. Nivolumab for Squamous-Cell Cancer of Head and Neck. N Engl J Med 2017;376(6):595–6 doi 10.1056/NEJMc1615565. [DOI] [PubMed] [Google Scholar]
- 7.Naidoo J, Page DB, Li BT, Connell LC, Schindler K, Lacouture ME, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol 2015;26(12):2375–91 doi 10.1093/annonc/mdv383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weber JS, Yang JC, Atkins MB, Disis ML. Toxicities of Immunotherapy for the Practitioner. J Clin Oncol 2015;33(18):2092–9 doi 10.1200/JCO.2014.60.0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barroso-Sousa R, Barry WT, Garrido-Castro AC, Hodi FS, Min L, Krop IE, et al. Incidence of Endocrine Dysfunction Following the Use of Different Immune Checkpoint Inhibitor Regimens: A Systematic Review and Meta-analysis . JAMA Oncol 2017. doi 10.1001/jamaoncol.2017.30642655010 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Villadolid J, Amin A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl Lung Cancer Res 2015;4(5):560–75 doi 10.3978/j.issn.2218-6751.2015.06.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline . J Clin Oncol 2018:JCO2017776385 doi 10.1200/JCO.2017.77.6385. [DOI] [PubMed] [Google Scholar]
- 12.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366(26):2443–54 doi 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013;369(2):134–44 doi 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larkin J, Lao CD, Urba WJ, McDermott DF, Horak C, Jiang J, et al. Efficacy and Safety of Nivolumab in Patients With BRAF V600 Mutant and BRAF Wild-Type Advanced Melanoma: A Pooled Analysis of 4 Clinical Trials. JAMA Oncol 2015;1(4):433–40 doi 10.1001/jamaoncol.2015.1184. [DOI] [PubMed] [Google Scholar]
- 15.Ryder M, Callahan M, Postow MA, Wolchok J, Fagin JA. Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: a comprehensive retrospective review from a single institution. Endocr Relat Cancer 2014;21(2):371–81 doi 10.1530/ERC-13-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee H, Hodi FS, Giobbie-Hurder A, Ott PA, Buchbinder EI, Haq R, et al. Characterization of Thyroid Disorders in Patients Receiving Immune Checkpoint Inhibition Therapy. Cancer Immunol Res 2017. doi 10.1158/2326-6066.CIR-17-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shrestha RT, Hennessey J. Acute and Subacute, and Riedel’s Thyroiditis. In: De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM, et al. , editors. Endotext; South Dartmouth (MA)2000. [Google Scholar]
- 18.Sriphrapradang C, Bhasipol A. Differentiating Graves’ disease from subacute thyroiditis using ratio of serum free triiodothyronine to free thyroxine. Annals of medicine and surgery 2016;10:69–72 doi 10.1016/j.amsu.2016.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nikolai TF, Coombs GJ, McKenzie AK, Miller RW, Weir GJ Jr., Treatment of lymphocytic thyroiditis with spontaneously resolving hyperthyroidism (silent thyroiditis). Arch Intern Med 1982;142(13):2281–3. [DOI] [PubMed] [Google Scholar]
- 20.Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL, et al. 2016 American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis. Thyroid 2016;26(10):1343–421 doi 10.1089/thy.2016.0229. [DOI] [PubMed] [Google Scholar]
- 21.Mandel SJ, Brent GA, Larsen PR. Levothyroxine therapy in patients with thyroid disease. Annals of internal medicine 1993;119(6):492–502. [DOI] [PubMed] [Google Scholar]
- 22.Sex Jonklaas J. and age differences in levothyroxine dosage requirement. Endocr Pract 2010;16(1):71–9 doi 10.4158/EP09257.OR. [DOI] [PubMed] [Google Scholar]
- 23.Olubowale O, Chadwick DR. Optimization of thyroxine replacement therapy after total or near-total thyroidectomy for benign thyroid disease. The British journal of surgery 2006;93(1):57–60 doi 10.1002/bjs.5157. [DOI] [PubMed] [Google Scholar]
- 24.Gordon MB, Gordon MS. Variations in adequate levothyroxine replacement therapy in patients with different causes of hypothyroidism. Endocr Pract 1999;5(5):233–8 doi 10.4158/EP.5.5.233. [DOI] [PubMed] [Google Scholar]
- 25.Roos A, Linn-Rasker SP, van Domburg RT, Tijssen JP, Berghout A. The starting dose of levothyroxine in primary hypothyroidism treatment: a prospective, randomized, double-blind trial. Archives of internal medicine 2005;165(15):1714–20 doi 10.1001/archinte.165.15.1714. [DOI] [PubMed] [Google Scholar]
- 26.Morganstein DL, Lai Z, Spain L, Diem S, Levine D, Mace C, et al. Thyroid abnormalities following the use of cytotoxic T-lymphocyte antigen-4 and programmed death receptor protein-1 inhibitors in the treatment of melanoma. Clin Endocrinol (Oxf) 2017;86(4):614–20 doi 10.1111/cen.13297. [DOI] [PubMed] [Google Scholar]
- 27.Scott E, Long GV, Guminski A, Clifton-Bligh RJ, Menzies AM, Tsang VH. The Spectrum, Incidence, Kinetics, and Management of Endocrinopathies with Immune Checkpoint Inhibitors for Metastatic Melanoma. Eur J Endocrinol 2017. doi EJE-17–0810 [pii] 10.1530/EJE-17-0810. [DOI] [PubMed] [Google Scholar]
- 28.de Filette J, Jansen Y, Schreuer M, Everaert H, Velkeniers B, Neyns B, et al. Incidence of Thyroid-Related Adverse Events in Melanoma Patients Treated With Pembrolizumab. J Clin Endocrinol Metab 2016;101(11):4431–9 doi 10.1210/jc.2016-2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guaraldi F, La Selva R, Sama MT, D’Angelo V, Gori D, Fava P, et al. Characterization and implications of thyroid dysfunction induced by immune checkpoint inhibitors in real-life clinical practice: a long-term prospective study from a referral institution. J Endocrinol Invest 2017. doi 10.1007/s40618-017-0772-1 10.1007/s40618–017-0772–1 [pii]. [DOI] [PubMed] [Google Scholar]
- 30.Yamazaki H, Iwasaki H, Yamashita T, Yoshida T, Suganuma N, Yamanaka T, et al. Potential Risk Factors for Nivolumab-induced Thyroid Dysfunction. In Vivo 2017;31(6):1225–8 doi 31/6/1225 [pii] 10.21873/invivo.11195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delivanis DA, Gustafson MP, Bornschlegl S, Merten MM, Kottschade L, Withers S, et al. Pembrolizumab-Induced Thyroiditis: Comprehensive Clinical Review and Insights Into Underlying Involved Mechanisms. J Clin Endocrinol Metab 2017;102(8):2770–80 doi 10.1210/jc.2017-004483805504 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orlov S, Salari F, Kashat L, Walfish PG. Induction of painless thyroiditis in patients receiving programmed death 1 receptor immunotherapy for metastatic malignancies. J Clin Endocrinol Metab 2015;100(5):1738–41 doi 10.1210/jc.2014-4560. [DOI] [PubMed] [Google Scholar]
- 33.O’Malley G, Lee HJ, Parekh S, Galsky MD, Smith CB, Friedlander P, et al. Rapid Evolution of Thyroid Dysfunction in Patients Treated with Nivolumab. Endocr Pract 2017;23(10):1223–31 doi 10.4158/EP171832.OR. [DOI] [PubMed] [Google Scholar]
- 34.Yamauchi I, Sakane Y, Fukuda Y, Fujii T, Taura D, Hirata M, et al. Clinical Features of Nivolumab-Induced Thyroiditis: A Case Series Study. Thyroid 2017;27(7):894–901 doi 10.1089/thy.2016.0562. [DOI] [PubMed] [Google Scholar]
- 35.Alhusseini M, Samantray J. Hypothyroidism in Cancer Patients on Immune Checkpoint Inhibitors with anti-PD1 Agents: Insights on Underlying Mechanisms. Exp Clin Endocrinol Diabetes 2017;125(4):267–9 doi 10.1055/s-0042-119528. [DOI] [PubMed] [Google Scholar]
- 36.Maekura T, Naito M, Tahara M, Ikegami N, Kimura Y, Sonobe S, et al. Predictive Factors of Nivolumab-induced Hypothyroidism in Patients with Non-small Cell Lung Cancer. In Vivo 2017;31(5):1035–9 doi 31/5/1035 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev 2001;81(3):1097–142 doi 10.1152/physrev.2001.81.3.1097. [DOI] [PubMed] [Google Scholar]
- 38.Vaidya B, Pearce SH. Management of hypothyroidism in adults. BMJ 2008;337:a801 doi 10.1136/bmj.a801. [DOI] [PubMed] [Google Scholar]
- 39.Burch HB, Wartofsky L. Life-threatening thyrotoxicosis. Thyroid storm . Endocrinology and metabolism clinics of North America 1993;22(2):263–77. [PubMed] [Google Scholar]
- 40.Fliers E, Wiersinga WM. Myxedema coma . Reviews in endocrine & metabolic disorders 2003;4(2):137–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


