Abstract
Many studies have demonstrated that smoking can influence ovarian cancer risk and survival; however, the number of studies investigating this relationship according to histological subtypes is limited. We conducted a review of epidemiologic research that assessed the role of smoking on ovarian cancer risk and survival after diagnosis, specifically capturing studies that discerned between various histological subtypes of this disease. In the majority of studies, current smoking was associated with increased risk of mucinous cancer. There was also evidence of a decreased risk of clear cell and endometrioid histotypes. No significant association was observed between cigarette smoking and serous cancer. In the studies investigating the relationship between smoking and survival, all the studies reported an increased risk of mortality associated with smoking. Smoking appeared to be a risk factor for both ovarian cancer risk and mortality. Future studies need to investigate further a potential link between smoking and ovarian cancer by having a better assessment of exposure to smoking and having a larger number of participants with the ability to detect associations within rare histotypes.
Keywords: smoking cigarettes, ovarian cancer risk, survival, prognosis, review
Introduction
In the United States, ovarian cancer is the fifth leading cause of cancer deaths among females (1). As the deadliest gynecological cancer, ovarian cancer has an overall five-year survival rate of 47% (2). At the time of diagnosis, approximately 60% of women present with distant stage of the disease that is characterized by five-year survival rate of only 29% (2).
The majority of ovarian tumors tend to originate from the ovarian epithelium (3). Epithelial ovarian tumors include four main histotypes: serous (70%), endometrioid (10%), clear cell (13%), and mucinous (3%)(4). These subtypes could be further subdivided into benign, borderline and malignant (3, 5). Benign tumors are characterized by a lack of intense proliferation and invasiveness while borderline tend to exhibit atypical proliferation with the absence of invasiveness, and malignant being characterized by invasiveness (3). Because epithelial ovarian cancer accounts for nearly 90% of all malignant ovarian tumors (3), epidemiologic research focuses mainly on this subtype of ovarian cancer.
According to a recent theory, histological subtypes of ovarian cancer differ by the origin of disease and may originate outside the ovary (6). It has been proposed that high-grade serous cancer can originate in the fallopian tube epithelium; and endometrioid and clear cell cancers may develop from endometrial epithelium implanted on ovarian surface through a retrograde menstrual flow (7, 8). At the same time, the origin of mucinous carcinoma is not clear and some argue that this subtype may arise at the tuboperitoneal junction (8). Such etiological heterogeneity could explain differences in the strength of associations with various risk and survival factors across different histological subtypes. For instance, BRCA mutation is associated with an increased risk of high-grade serous ovarian cancer, while endometriosis is associated with risk of ovarian cancer of endometrioid or clear cell histotypes (9, 10).
Cigarette smoking represents an exposure which has been demonstrated to be linked to ovarian cancer with associations varying across the histotypes. In fact, while epidemiological studies reported either inverse, positive, or no association between smoking and overall ovarian cancer risk, the same and some additional studies have demonstrated that smoking is associated with increased risk of mucinous ovarian cancer (11–37). At the same time, epidemiologic evidence on the association between smoking and risk of ovarian cancer across other histotypes has been inconclusive. Although some studies suggested an inverse association between smoking and risk of endometrioid, serous, and clear cell ovarian cancer, the results were, in general, not statistically significant (11–36, 38). Meanwhile, the role of smoking on ovarian cancer survival and, specifically, in relation to histotype-specific associations has been unclear because very few studies have been conducted on the topic (39–41).
Currently, there is a need for a better understanding of the relationship between smoking and histotype-specific ovarian cancer in the context of a high mortality of ovarian cancer and smoking being one of the leading causes of death in the United States (42, 43) and its ability to impact survival of cancer patients (44, 45). A thorough understanding of the link between smoking and ovarian cancer could allow for the development of more targeted and, therefore, more effective preventive and therapeutic measures.
The results of the most recent pooled analysis of epidemiologic data on smoking and histotype-specific ovarian cancer risk were published in 2016 (35). However, there still is a need to summarize evidence on this topic accumulated by today and to compare and contrast the results of various studies. Therefore, to understand the impact of smoking on ovarian cancer risk and survival after diagnosis and to emphasize epidemiologic research that recognized heterogeneous nature of ovarian cancer by examining histotype-specific associations, we conducted a systematic review that summarized the results of the studies published in the past 20 years.
Materials and Methods
We conducted a literature search through PubMed on smoking and ovarian cancer, using a combination of key words such as “ovary or ovarian”, “cancer or neoplasm or carcinoma” and “tobacco or smoke or smoking”. Studies included were published from 1997 to 2018 and written in English language. Eligible studies included in this review met all of the following criteria: 1) studies with human subjects; 2) observational studies; 3) studies investigating the association between cigarette smoking and epithelial ovarian cancer risk and survival; 4) studies presenting data on odds ratios, relative risk or hazard ratios. Further, we decided to focus on the studies published from 1997 since 1996 was the year when it was first hypothesized that histological subtypes could be etiologically different, specifically, that mucinous ovarian cancer was distinct from the other histotypes (46). Therefore, in this review, we included only those studies that, in addition to reporting overall estimates, presented histotype-specific associations or only focused on histotype-specific associations.
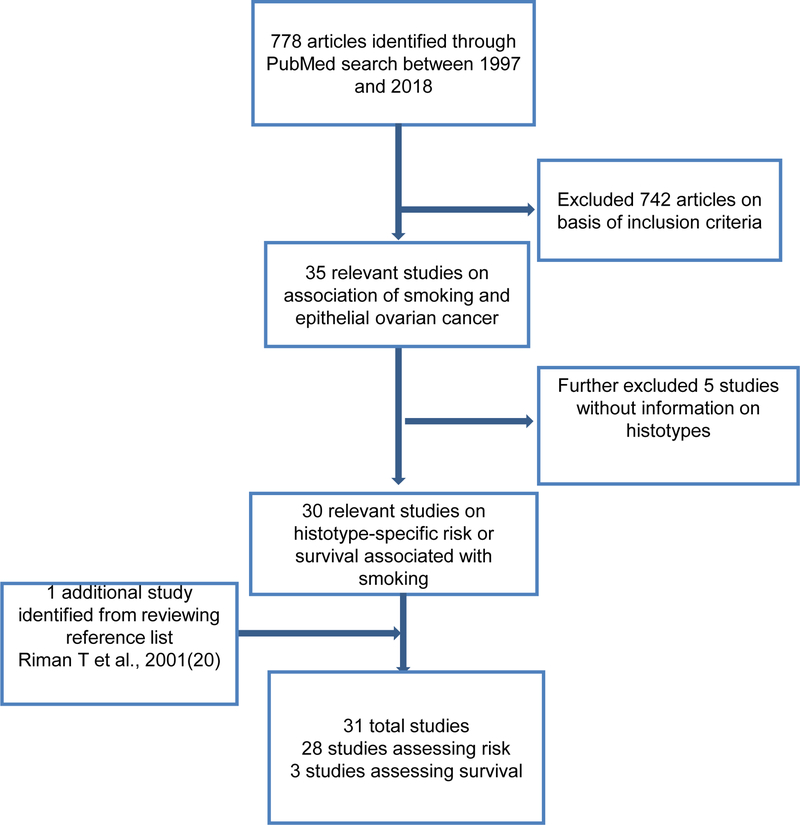
We identified 778 records through our initial search of the PubMed database, and among them 30 studies met the criteria mentioned above (Figure 1). One additional study was identified through a reference list and added to our review. Overall, out of 31 studies that were eligible to be included in the review, 28 were risk and three were survival studies.
Figure 1.
Flowchart of criteria for inclusion of literature on association between smoking and epithelial ovarian cancer risk and survival
Results
Characteristics of 16 case-control, seven cohort, five pooled/meta-analyses, and three survival studies included in this review are listed in Tables 1, 2, 3, and 4, respectively. The tables included information on country where the studies were conducted, sample size, and the main findings. We also presented results of the studies in Figures 2–5 according to the following sequence: Figure 2-if these studies reported an overall association for ovarian cancer risk associated with cigarette smoking; Figures 3, 4, 5- if these studies presented estimates for serous, mucinous, and/or endometrioid histotypes, respectively. We presented results for invasive tumors when studies distinguished between borderline and invasive tumor types.
Table 1.
Summary of Fifteen Case-Control Studies on Association between Smoking and Epithelial Ovarian Cancer risk
| Reference Author / Date | Country | Study Design | Sample Size Case/control | Exposure of Interest | Main Findings* OR, 95% CI | Adjustment and Stratification Variables |
|---|---|---|---|---|---|---|
| Kelemen et al., 2017 (11) | US | Population based case-control study | 613/752 | Smoking status, daily smoking, pack-years, duration of smoking | Increased risk of serous ovarian cancer among ever smokers (1.46, 1.11–1.92). Increased risk of serous ovarian cancer among former smokers who quit within 0–2 years of diagnosis (5.48, 3.04–9.86). |
Age, geographic location, BMI, parity, infertility, menopausal status, education, alcohol and coffee |
| Rossing et al., 2008(12) | US | Population based case-control | 812/1,313 | Smoking status, years since last smoked, years smoked | Increased risk of borderline mucinous tumor among ever smoker (1.8, 1.2–2.9); Increased risk of invasive serous ovarian cancer among women who smoked in previous 15 years (1.4, 1.1–1.9). |
Stratified by invasiveness; adjusted for age, county of residence, year of diagnosis/reference date, number of full term births, duration of hormonal contraception and education |
| Soegaard et al., 2007(13) | Denmark | Population based case-control | 554/1,564 | Smoking status, smoking duration, daily smoking | Increased risk of mucinous ovarian cancer among current smokers (1.78, 1.01–3.15). | Adjusted for age, pregnancy, additional pregnancies (linear), duration of oral contraceptive use (linear) |
| Jordan et al., 2007(29) | Australia | Population based case control | 323/1487 | Smoking status and pack-years | Increased risk of benign (2.7, 1.6–4.4), borderline (2.7, 1.7–4.4) and invasive mucinous tumors (2.1, 0.9–5.0) among smokers with 20 or more pack-years. | Adjusted for age, education, parity, use of hormonal contraceptive, hysterectomy, tubal sterilization |
| Baker et al., 2006(14) | US | Hospital based case-control | 434/868 | Smoking status, daily smoking, smoking duration, pack-years | Decreased risk of ovarian cancer among current smokers (0.53, 0.32–0.88). | Adjusted for age, residence, income, BMI, history of a vaginal infection, year of participation, duration of breastfeeding, daily exposure to second hand smoke (ETS), and multiplicative interaction between income and ETS exposure |
| Huusom et al., 2006(15) | Denmark | Population based case-control | 202/1,564 | Smoking status, duration of smoking | Increased risk of borderline mucinous tumor among current smokers (2.10, 1.22–3.60). | Adjusted for age, childbirth, number of additional births, age at first birth, breastfeeding, duration of oral contraceptives, intake of milk |
| Pan et al., 2004(16) | Canada | Population based case-control study | 442/2,135 | Smoking status, pack-years, daily smoking, starting and quitting age, years since quitting | Increased risk of mucinous ovarian cancer among smokers (OR=1.77, 1.06–2.96); Dose response relationship existed with smoking years, daily smoking and smoking pack-years. |
Adjusted for 10-year age group, province of residence, parity, menopause status, years of menstruation, education, BMI, physical activity, total energy intake, alcohol consumption |
| Zhang et al., 2004(30) | US | Hospital based case-control study | 709/951 | Smoking status, daily smoking, pack-years | Increased risk of mucinous ovarian cancer among former smokers (2.5, 1.1–5.4), and among women who smoked more than one pack per day (2.9, 1.2–7.5). | Stratified by histologic subtypes; adjusted for race, education, BMI, age at menarche, menopausal status, parity, oral contraceptive use, and postmenopausal hormone use |
| Riman et al., 2004(17) | Sweden | Population based case-control study | 655/3899 | Smoking status | Decreased risk of ovarian cancer among current smokers whose daily consumption was 1–10 cigarettes (0.7, 0.52–0.94). | Adjusted for age, parity, BMI, age at menopause as categorized variables, duration of oral contraceptive use, and ever use of hormone replacement therapy |
| Goodman et al., 2003(18) | US | Population based case control study | 558/607 | Smoking status | No association between active smoking and invasive ovarian cancer by histotypes. Increased risk of borderline serous tumors among smokers (1.91, 1.09–3.34). |
Stratified by histologic subtypes. Adjusted for age, ethnicity, education, study site, use of oral contraceptives, parity, and tubal ligation |
| Modugno et al., 2002(31) | US | Population based case-control study | 767/ 1,367 | Smoking status, years since initiation, age at initiation, pack-years | Increased risk of mucinous ovarian cancer among smokers (1.9, 1.3–2.9). Dose response association with pack-years existed. |
Adjusted for use of oral contraceptive use, number of live birth, age, tubal ligation, family history of ovarian cancer |
| Green et al., 2001(19) | Australia | Population based case-control study | 794/855 | Smoking status, maximum daily smoking, initiation age, years since stopping | Increased risk of ovarian cancer among smokers (1.5, 1.2–1.9); Increased risk of borderline mucinous tumor among former smokers (OR=2.6, 1.2–5.2) and current smokers (OR=4.0, 1.9–8.3). |
Stratified by smoking status, maximum daily smoking, invasiveness; adjusted for age, age-squared, education, BMI, duration of oral contraceptive use, tubal ligation, alcohol consumption, caffeine consumption |
| Riman et al., 2001(20) | Sweden | Population based case-control study | 193/3899 | Smoking status | Increased risk of borderline mucinous tumors among current smokers. | Adjusted for age, parity, BMI, age at menopause, ever use of oral contraceptives |
| Kuper et al., 2000 (37) | US | Population-based case-control | 549/516 | Years of smoking, daily smoking, pack-years | No association was observed between smoking and ovarian cancer risk | Adjusted for age, center, marital status, parity, BMI, use of oral contraceptives, family history of breast, ovarian or prostate cancer, tubal ligation, education, alcohol use and pack-years |
| Marchbanks et al., 2000(36) | US | Population based case-control | 447/ 3868 | Smoking status, years since stopping, years since initiation | Increased risk of mucinous ovarian cancer among current smokers (OR=2.9, 1.7–4.9) | Adjusted for study site, age, parity, oral contraceptive use |
| Nagle et al. 2008(38) | Australia | Population based case-control study | 232/1508 | Smoking status, Pack-years |
No association between smoking and endometrioid ovarian cancer. Decreased risk of clear cell ovarian cancer among former smokers (0.4, 0.2–0.7) and dose-response association with pack-years (p=0.03). |
Adjusted for age, education, parity, hormone contraceptive use. |
Never smokers or nonsmokers as reference group.
Table 2.
Summary of Seven Cohort Studies on Association between Smoking and Epithelial Ovarian Cancer Risk
| Reference Author / Date | Country | Sample Size/ Follow-up years | Exposure of Interest | Main Findings* HR or RR, 95% CI | Adjustment and Stratification Variables |
|---|---|---|---|---|---|
| Licaj et al., 2017(21) | Norway | 300,398 women, 2336 cases/ 19 years | Smoking duration in years, pack-years, daily smoking | Increased risk of borderline (2.26, 1.71–2.97) and invasive mucinous ovarian cancer (1.78, 1.20–2.64). | Number of children, age at first childbirth, BMI, physical activity; invasiveness; birth cohort |
| Licaj et al., 2016(22) | Norway | 154,234 women aged 34–70 years, 915 cases/13.2 years | Smoking duration in years (0–19, 20+), pack-years, initiation age | Increased risk of borderline mucinous tumors among current smokers (2.17, 1.06–4.45). | Age at menarche, number of full-term pregnancies, age at first full-term birth, age at last birth, infertility, menopausal status, age at menopause, education, physical activity, alcohol, BMI, oral contraceptive use, duration of oral contraceptive use, HRT, age at start using HRT, history of breast cancer in mother; stratified by invasiveness |
| Gram et al., 2012(23) | Europe | 326, 831 women, 836 cases/13 years | Smoking status, starting age, smoking duration, pack-years, time since quitting, daily consumption | Increased risk of mucinous ovarian cancer among current smokers (1.85, 1.08–3.16). | Stratified by center, age; adjusted for number of full-term pregnancy, duration of oral contraceptive use |
| Gates et al. 2010(24) | US | 221,866 women, 721 cases | Smoking status | Decreased risk of endometrioid ovarian cancer among former smokers (0.59, 0.39–0.90). | Adjusted for age, parity, breastfeeding, oral contraceptive use, tubal ligation, hysterectomy, estrogen use, age at natural menopause, BMI, physical activity, talc use, family history |
| Gram et al., 2008(25) | Norway, Sweden | 103,081 women aged 30–50 years, 343 cases/14 years | Smoking status, age of initiation, smoking duration, pack-years, daily consumption | Increased risk of borderline tumor among former smokers (2.2, 1.0–4.7) and current smokers (2.7, 1.2–5.7). Dose response relationship existed with pack-years. | Adjusted for age, nulliparous, menopausal status, duration of hormonal contraceptive use |
| Tworoger et al., 2008(32) | US | 110,454 women, 737 cases/28 years | Smoking status, smoking duration, pack-years | Increased risk of mucinous cancer among former smokers (2.02, 1.15–3.55) and current smokers (2.22, 1.16–4.24). | Adjusted for age, parity, oral contraceptive use, postmenopausal hormone use, tubal ligation, BMI |
| Terry et al., 2003(26) | Canada | 89,835 women aged 40–59, 454 cases/16.5 years | Daily smoking, smoking years, pack-years, starting age, years since stopping | Increased risk of ovarian cancer among smokers who smoked for 40 years or more (2.50, 1.37–4.56). | Adjusted for age, treatment allocation, study centre, quetelet’s index, education, physical activity, oral contraceptive use, HRT, parity, age of menarche, menopausal status |
Never smokers or nonsmokers as reference group.
Table 3.
Summary of Five Pooled and Meta-analysis Studies on Association between Smoking and Epithelial Ovarian Cancer Risk
| Reference Author / Date | Country | Study Design | Sample Size | Exposure of Interest | Main Findings* RR or OR, 95% CI | Adjustment and Stratification Variables |
|---|---|---|---|---|---|---|
| Wentzensen et al., 2016(35) | North America, Europe, Asia | Pooled analysis of 21 cohort studies | 1.3million women, 5584 invasive cases | Smoking status (ever, never), pack-years | Increased risk of mucinous cancer among smokers (1.26, 1.08–1.46); Decreased risk of clear cell cancer among smokers (0.72, 0.55–0.94). |
Adjusted by age at study entry, parity, duration of oral contraceptive use; stratified by birth year and cohort |
| Faber et al., 2013(27) | Australia, Europe, North America | Pooled analysis of 21 case-control studies (19 OCAC, 2 non-OCAC) | 11,972 invasive and 2,752 borderline cases, 19,066 controls | Smoking status, daily consumption, smoking duration, age at initiation, time from cessation to diagnosis | Increased risk of borderline (1.83, 1.39–2.41) and invasive mucinous tumors (1.31,1.03–1.65) among current smokers. Decreased risk of clear cell ovarian cancer among former smokers (0.77, 0.66–0.91) and current smokers (0.74, 0.56–0.98). |
Adjusted for parity, breastfeeding, oral contraceptive use, family history of breast/ovarian cancer, education; stratified by invasiveness |
| Beral et al., 2012(28) | Worldwide | Meta-analysis of 51 cohort and case-control studies | 28,114 cases, 94,942 without ovarian cancer | Smoking status | Higher increased risk of borderline than fully malignant mucinous cancer among current smokers (2.25, 1.91–2.65 vs 1.49, 1.28–1.73). Decreased risk of endometrioid (0.81, 0.72–0.92) and clear cell ovarian cancer (0.80, 0.65–0.97) among current smokers. |
Stratified by study, age at diagnosis, menopausal status or hysterectomy, BMI, menopausal hormone therapy; adjusted by parity, duration of oral contraceptive use |
| Jordan et al., 2006(33) | North America, Australia, Sweden | Meta-analysis of case-control, pooled and cohort studies | 6474 cases and 16,863 controls | Smoking status, pack-years, years since stopping smoking | Increased risk of mucinous cancer among current smokers (2.1, 1.7–2.7), and dose response relationship existed. Decreased risk of clear cell cancer among current smokers (0.6, 0.3–0.9). |
All studies adjusted for age. Studies also adjusted for different factors including parity, oral contraceptive use, family history, tubal ligation, education |
| Kurian AW et al., 2005(34) | US | Pooled analysis of 10 case-control studies | 1834 invasive cases and 7484 controls | Smoking status | Increased risk of mucinous ovarian cancer among current smokers (2.4, 1.5–3.8) | Adjusted for parity and oral contraceptive use |
Never smokers or nonsmokers as reference group.
Table 4.
Summary of Three Studies on Association between Smoking and Epithelial Ovarian Cancer Survival
| Reference Author/ Date | Country | Sample Size | Exposure of Interest | Main Findings* HR and 95% CI | Adjustment and Stratification Variables |
|---|---|---|---|---|---|
| Kim SJ et al., 2017 (41) | Canada | 1421 invasive cases | smoking status (never, ever) | Increased risk of death with smoking (1.25,1.01–1.54); Increased risk of death with smoking for mucinous cancer (2.52, 1.01–6.33). |
adjusted for age at diagnosis, histology, stage, and residual disease |
| Praestegaard et al., 2017 (39) | Worldwide | 9114 cases | smoking status, daily consumption, smoking duration, time from cessation to diagnosis | Worse survival for mucinous (current smoker: 1.91, 1.01–3.65) and serous ovarian cancer (current smoker: 1.11, 1.0–1.23; former smoker: 1.12, 1.04–1.20). | age, race/ethnicity, tumor stage |
| Kelemen et al., 2016 (40) | Canada | 427 cases with chemotherapy | smoking status, neoadjuvant chemotherapy status | Shorter overall (8.56, 1.50–48.7) and progression-free survival (5.74, 1.05–31.4) for mucinous ovarian cancer among current smokers receiving adjuvant chemotherapy | adjusted for age at diagnosis, ethnicity, stage and residual disease |
Never smokers or nonsmokers as reference group.
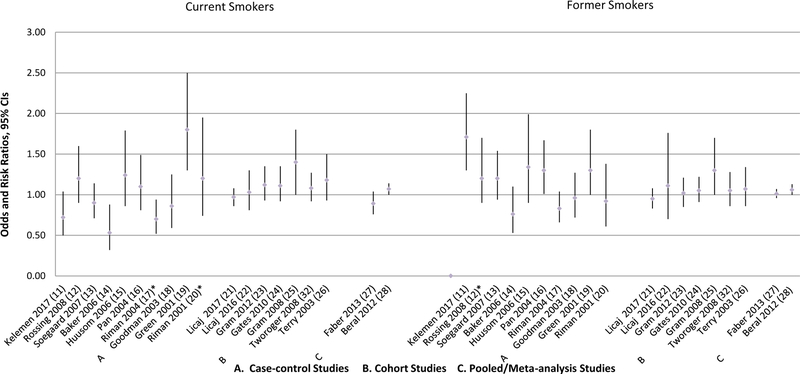
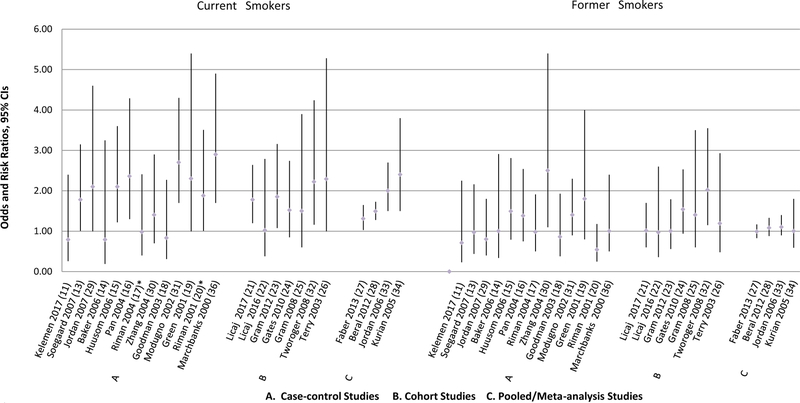
Figure 2.
Overall Epithelial Ovarian Cancer Risk Associated with Cigarette Smoking
*More categories were presented in the paper.
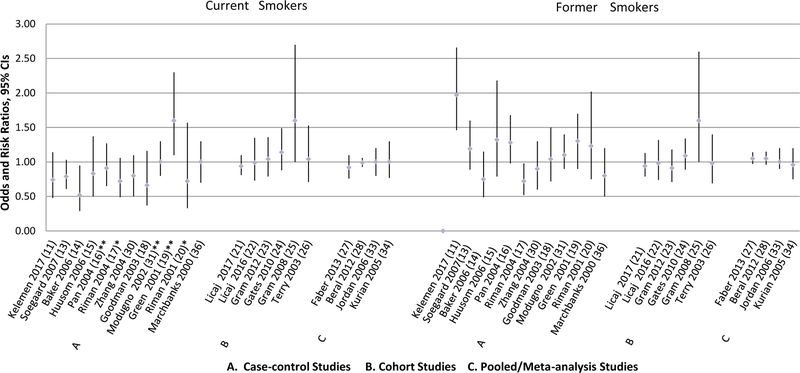
Figure 5.
Endometrioid Epithelial Ovarian Cancer Risk Associated with Cigarette Smoking
* More categories were presented in the paper.
Figure 3.
Serous Epithelial Ovarian Cancer Risk Associated with Cigarette Smoking
*More categories were presented in the paper. **Results were reported for non-mucinous tumors.
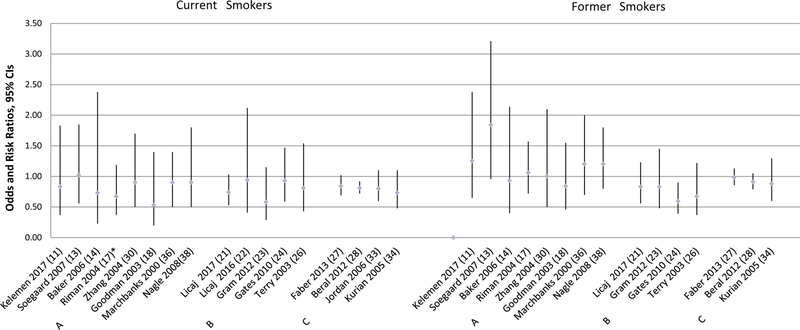
Figure 4.
Mucinous Epithelial Ovarian Cancer Risk Associated with Cigarette Smoking
*More categories were presented in the paper.
To increase comparability among the study results and to avoid a problem of a limited power, we presented the results for the smoking status variable that had only three categories: never smokers/non-smokers; current smokers, and former smokers. Two studies, one case-control by Kuper et al.(37) and one pooled analysis by Wentzensen et al.(35) did not parameterize smoking by employing this variable. Therefore, the results of these analyses were discussed in a separate section and included in the tables but were not presented in the figures.
Smoking and Overall Epithelial Ovarian Cancer Risk
Current Smokers
As shown in Figure 2, 10 case-control studies reported on the association between current smoking and epithelial ovarian cancer (11–20). One study by Green et al. observed a significantly increased risk of overall ovarian cancer (OR=1.8, 95% CI 1.3–2.5) when borderline and invasive cases were combined in the analyses (19). When these subtypes were examined separately, current smoking was associated with elevated risk of ovarian cancer in both cases, OR=1.7; 95% CI 1.2–2.4 and OR=2.4; 95% CI 1.4–4.1 for invasive and borderline tumors, respectively. In the study by Riman et al., smoking more than 10 cigarettes per day among current smoking was associated with increased risk of ovarian cancer, OR=1.66; 95% CI 1.04–2.63, while smoking less than that was not associated with ovarian cancer, OR=1.20; 95% CI 0.74–1.95 (20). Two case-control studies by Baker et al.(14) and Riman et al. (17) found a decreased risk of ovarian cancer associated with current smoking. The other six case-control studies found no significant associations for current smoking (11–13, 15, 16, 18). Four case-control studies had no data on overall ovarian cancer risk (29–31, 36), therefore, these results were not shown in Figure 2.
Seven cohort studies presented results on overall ovarian cancer risk (21–26, 32). Among them only one reported an increased risk associated with current smoking (HR=1.4, 95% CI 1.0–1.8), when borderline and invasive cases were combined (25). The association was more pronounced for borderline, HR=2.7; 95% 1.2–5.7, and more attenuated for invasive tumors, HR=1.1; 95% CI 0.8–1.7, when these two types were analyzed separately.
One pooled study that used data from the Ovarian Cancer Association Consortium (OCAC) by Faber et al. (27) and one meta-analysis by Beral et al. (28) presented results on the overall ovarian cancer risk associated with current smoking status. While Faber et al. did not observe significant association between current smoking and overall invasive ovarian cancer risk, they found an increased risk of borderline ovarian tumors among current smokers (OR=1.36, 95% CI 1.13–1.64)(27). Beral et al. found increased risk of overall ovarian cancer among current smokers (RR=1.07, 95% CI 1.00–1.14), with borderline and invasive cases combined together (28). Three other studies either did not present the estimates for the overall ovarian cancer (33, 34) or did not use the current/former/never parameterization of smoking variable (35).
Former Smokers
As shown in Figure 2, two case-control studies by Green et al. (19) and Pan et al.(16) found approximately 30% increase in overall ovarian cancer risk among former smokers. Rossing et al. reported increased risk of ovarian cancer among those who discontinued smoking 6–15 years prior to reference date, OR=1.3; 95% CI 1.0–1.8 and OR=1.8; 95% CI=1.1–2.9 for all and borderline tumors, respectively (12). No association was observed If smoking was discontinued within 2–5 years or more than 15 years prior to reference date. In the study by Kelemen et al. (11) a significant increase of ovarian cancer risk was observed among former smokers (OR=1.71, 95% CI 1.30–2.25) and, in particular, among ex-smokers who quit within two years prior to reference date (OR=4.24, 95% CI 2.44–7.36), as no significant results were observed among former smokers who quit more than two years prior to reference date (11). Further, among former smokers, greater number of pack-years (not more than 20 pack-years) and smoking more than 20 years was also associated with increased risk of ovarian cancer, OR= 1.93; 95% CI 1.29–2.89, and OR=1.87; 95% CI 1.28–2.75, respectively. No significant association for former smoking was observed in the other case-control analyses (13–15, 18, 20) (Figure 2).
Only the cohort study by Gram et al. (25) and meta-analysis by Beral et al.(28) found an increased risk of overall ovarian cancer among former smokers (HR=1.3, 95% CI 1.0–1.7; and RR=1.06, 95% CI 1.00–1.13, respectively), while other cohort or pooled/meta-analyses studies observed no association (21–24, 26, 27, 32).
Smoking and Serous Ovarian Cancer Risk
Current Smokers
As shown in Figure 3, twelve case-control studies estimated the association between smoking and serous ovarian cancer risk and the majority of studies found a decreased but nonsignificant risk of serous ovarian cancer associated with current smoking (11, 13, 15, 18, 20, 30) and one study reported null association (36). Two case-control studies observed a significantly decreased risk of serous ovarian cancer associated with current smoking, OR=0.52, 95% CI 0.29–0.95 for Baker et al.(14) and OR=0.53; 95% CI 0.33–0.88 for Riman et al.(17) if women were currently smoking 11 or more cigarettes per day.
Three studies examined the association within non-mucinous histotype (16, 19, 31). However, because a large proportion of non-mucinous ovarian cancer is being presented by serous cancer (33), these results were included among the results for the studies that investigated the associations for serous histotype (Figure 3). In the study by Green et al.(19), an increased risk of nonmucinous cancer was observed among current smokers (OR=1.6, 95% CI 1.1–2.3). On the contrary, Modugno et al (31) and Pan et al. (16) observed no association between current smoking and non-mucinous ovarian cancer.
Six cohort studies reported results on association between serous ovarian cancer risk and smoking (21–26), but only one cohort study by Gram et al. found an increased risk when borderline and invasive cases were combined together (HR=1.6, 95% CI 1.0–2.7) (25). However, the association became non-significant when referent group included never and passive smokers instead of just never smokers, HR=1.3; 95% CI 0.9–1.9. No pooled or meta-analysis studies found any significant association between serous ovarian cancer risk and current smoking (27, 28, 33, 34).
Former Smokers
As shown in Figure 3, among 12 case-control studies only in a study by Kelemen et al. it was found that former smoking is significantly associated with an increased risk of serous ovarian cancer (OR=1.97, 95% CI 1.46–2.66), driven largely by those who quit within two years of diagnosis (OR=5.48, 95% CI 3.04–9.86) (11). In addition, in the study by Riman et al. former smoking was inversely associated with risk of serous cancer, OR=0.72; 95% CI 0.52–0.98 (17). One cohort study found an increased risk among former smokers (HR=1.6, 95% CI 1.0–2.6)(25). Similarly to current smokers, in this study, confidence interval included a null value when the referent category was changed from including just never users to including both never and passive smokers. No other case-control, cohort or pooled studies observed significant results among former smokers.
Smoking and Mucinous Ovarian Cancer Risk
Current Smokers
As shown in Figure 4, 13 case-control studies presented estimates of the association between current smoking and mucinous ovarian cancer risk. Three of the eight studies combined borderline and invasive mucinous cases together (13, 31, 36) and two presented estimates for borderline tumors only (15, 20). Seven studies reported a significantly increased risk of mucinous ovarian cancer associated with current smoking with estimates ranging from 2.1 to 2.9 (15, 16, 19, 20, 29, 31, 36). In the study by Soegaard et al, increased risk for current smoking was observed when compared to never and former smokers combined, OR=1.78; 95% CI 1.01–3.15; however, the association became slightly attenuated and non-significant when current smoking was compared to never smokers (13). Five studies observed no association for mucinous ovarian cancer (11, 14, 17, 18, 30).
Seven cohort studies presented results on mucinous ovarian cancer risk associated with current smoking, and four of them found significant positive association between mucinous ovarian cancer risk and current smoking (21, 23, 26, 32) (Figure 4). Two cohort studies by Gram et al. and Gates et al. found positive but nonsignificant associations (24, 25). Among the aforementioned six studies three combined borderline and invasive mucinous cases in their analyses (23, 24, 32). The cohort study by Licaj et al. reported an increased risk of borderline mucinous tumors among current smokers (HR=2.17, 95% CI 1.06–4.45) but did not find a significant association for invasive mucinous ovarian cancer (22). Four pooled/meta-analysis studies reported results on mucinous ovarian cancer risk associated with current smoking and all four found a significantly increased risk, and the estimates ranged between 1.31 to 2.4 (27, 28, 33, 34) (Figure 4).
Former Smokers
As shown in Figure 4, only one case-control study by Zhang et al. found a significantly increased risk of mucinous ovarian cancer among former smokers (OR=2.5, 95% CI 1.1–5.4) (30) and no association was observed in other studies (11, 13–18, 20, 29, 31, 36). In the case-control study by Green et al. (19), the authors did not find any significant association between former smoking and invasive mucinous cancer risk but they observed a significantly increased risk of borderline mucinous ovarian cancer (OR= 2.6, 95% CI 1.2–5.2). Only one cohort study by Tworoger et al. reported an increased risk of mucinous ovarian cancer among former smokers (RR=2.02, 95% CI 1.15–3.55) (32). No other cohort study, pooled or meta-analysis observed the association between former smoking and risk of mucinous ovarian cancer (Figure 4).
Smoking and Endometrioid Ovarian Cancer Risk
Current Smokers
Eight case-control studies presented results on endometrioid ovarian cancer risk and none of them observed any statistically significant association for current smoking, although, almost all the studies reported inverse associations (Figure 5) (11, 13, 14, 17, 18, 30, 36, 38). Similarly, among the five cohort studies that conducted the analyses on investigating the relationship between current smoking and risk of endometrioid ovarian cancer, all the studies observed nonsignificant inverse association (Figure 5)(21–24, 26). All of the four pooled/meta-analysis studies observed an inverse association for current smoking, and estimates ranged between 0.73–0.84 (27, 28, 33, 34)(Figure 5). However, only results from the meta-analysis by Beral et al. reached statistical significance (RR=0.81, 95% CI 0.72–0.92) (28).
Former smokers
As shown in Figure 5, for the association between former smoking and endometrioid cancer risk, results from the case-control studies were mixed in terms of direction and none of the estimates were statistically significant (11, 13, 14, 17, 18, 30, 36, 38). Only four cohort studies presented results associated with former smoking and all of them observed an inverse association (21, 23, 24, 26), but only the results from study by Gates et al. (24) were significant (RR=0.59, 95% CI 0.39–0.90). Finally, among the three pooled/meta-analysis studies that presented results associated with former smoking, the associations reported were inverse but all the confidence intervals included the null value (Figure 5) (27, 28, 34).
Smoking and Clear Cell Ovarian Cancer Risk
Current and former smoking
Four case-control studies presented results on clear cell ovarian cancer risk and all reported nonsignificant inverse associations current smoking (11, 14, 17, 18). Similarly, among these studies, for former smoking, the associations were inverse except for the study by Kelemen et al. (11) where the authors reported the OR=1.95, 95% CI 0.52–7.29. None of the cohort studies investigated the relationship between smoking and ovarian cancer risk according to this histological subtype.
Four pooled/ meta-analysis studies assessed the association between smoking and clear cell ovarian cancer risk; and all of them observed a significantly decreased risk among current smokers, pOR= 0.74, 95% CI 0.56–0.98 for Faber et al. (27); RR=0.80, 95% CI 0.65–0.97 for Beral et al. (28); and RR=0.6, 95% CI0.3–0.9 for Jordan et al. (33). In the study by Kurian et al. (34), the estimate was, although inverse, non-significant. For former smoking, Faber et al. (27) observed an inverse association between former smoking and risk of clear cell ovarian cancer. In the studies by Beral et al. (28)and Kurian et al.(34) the associations were inverse and non-significant.
Adittional studies
There were three studies, two case-control (12, 37) and one pooled analysis (35) that did not present the results by the common variable for smoking status with current/former/never categories. The study by Kuper et al.(37) reported, however, an increased risk of ovarian cancer associated with a prolonged exposure to smoking. In fact, for smoking for 20–40 years, OR was equal to 1.45; 95% CI 1.04–2.01 in the overall group. They also reported an increased risk of invasive serous cancer associated with years of tobacco use and elevated risk of mucinous ovarian cancer for women who smoked more than 40 cigarettes per day. In another case-control study, although the results for current/former tobacco use for the overall study population were included, the results for mucinous and serous tumors were not presented by this smoking variable (12). Instead, a variable “years since last smoked” with the categories ≤15 and >15 years was used, with the estimated OR being equal to 2.6; 95% CI 1.6–4.2 for borderline mucinous, OR=2.7; 95% CI=1.1–6.5 for invasive mucinous, and OR=1.4; 95% CI=1.1–1.9 for invasive serous for smoking within 15 years before the diagnosis or reference date (12).
Finally, in one the largest prospective studies of smoking and ovarian cancer to date, based on data from the Ovarian Cancer Cohort Consortium, smoking was associated with an increased risk of mucinous ovarian cancer, RR=1.27; 95% CI=1.10–1.59 for ever smoking and RR=1.26; 95% CI 1.08–1.46 per 20 pack-years (35). At the same time, the authors reported a decreased risk of clear cell cancer, RR=0.72; 95% CI 0.55–0.94. No association was observed in the overall sample and among other histotypes.
Smoking and Epithelial Ovarian Cancer Survival
Only three studies investigated the impact of cigarette smoking on ovarian cancer survival overall and according to histological subtypes (39–41) with one study being based on consortium data (39). Kelemen et al. found significantly increased risk of mortality among both current and former smokers receiving adjuvant chemotherapy; however, this association was limited to cases diagnosed with mucinous ovarian cancer (40). Similarly, in the study by Kim et al. (41), it was observed that increased risk of death associated with smoking was limited to mucinous cancer (HR=2.52, 95% CI 1.01–6.33). In the pooled analysis, Praestegaard et al. (39) also reported significantly increased risk of mortality among current smokers diagnosed with mucinous cancer, pHR=1.91; 95% CI 1.01–3.65. Also, for serous ovarian cancer, both current and former smoking was associated with increased mortality, pHR= 1.11; 95% CI 1.00–1.23, and pHR=1.12; 95% CI 1.04–1.20, respectively. In the overall study sample, the associations were of a similar magnitude among both current and former smokers, pHR=1.17; 95% CI 1.08–1.28, and pHR=1.10; 95% CI 1.02–1.18, respectively.
Discussion
Epidemiological evidence summarized in current review suggests a positive association between current cigarette smoking and mucinous ovarian cancer risk. Certain biological mechanisms could explain the observed findings for mucinous histotype. One of them refers to carcinogenic nature of smoking. In fact, according to Surgeon General’s Report of 2014, there are more than 7000 chemicals and 69 known carcinogens in tobacco and tobacco smoke (47). The metabolizing intermediates of these foreign chemicals and carcinogens can bind to DNA and form DNA adducts which can lead to miscoding of DNA replication and permanent mutations (47). Permanent mutations in oncogenes like KRAS or tumor suppressor genes like TP53 can trigger further mutations and lead to carcinogenic processes (47). Significantly elevated levels of DNA adducts have been detected in various tissues among smokers (48).
Moreover, the mechanism underlying the association between smoking and mucinous ovarian cancer relates to the fact that most primary mucinous cancer cells resemble intestinal epithelial cells (33), and that, in many cases, ovarian mucinous carcinomas has a histochemical profile similar to gastrointestinal cancers (4). As smoking has been consistently shown to be associated with mucinous gastrointestinal cancers and adenomatous colorectal polyps, a precursor of colorectal cancer (33), it could also be implicated in the development of mucinous ovarian cancer the same way it may cause mucinous cancers of gastrointestinal system.
It is also important to note that many studies included in current review observed associations of a similar magnitude between smoking and borderline mucinous tumors (12, 15, 19–22, 28). Compared to invasive mucinous tumors, the association between current smoking and borderline mucinous tumors was, generally, more pronounced. The explanation to this observation could be that latent period from precursor lesions to cancer development might take decades and that cigarette smoking might cause carcinogenesis in the early phase (49).
The results of the studies included in this review also suggest that smoking could be associated with a reduced risk of clear cell and endometrioid ovarian cancer. Clear cell cancer shares similar genetic profile with endometrial epithelium, and case studies have found that a high percentage of women with ovarian cancer of this histotype also tend to have endometriosis (3, 8). Therefore, it was postulated that, similar to endometriosis, most clear cell cancer might develop from endometrium through retrograde menstrual flow (8). Smoking is also related to the decreased risk of endometriosis and decreased risk of endometrial cancer especially among postmenopausal women (50, 51). Because endometriosis and endometrial cancer are both estrogen-dependent, anti-estrogenic effect of smoking has been suspected to be the underlying biological mechanism for decreased risk of both conditions among smokers (50, 52).
As it was mentioned before, endometrioid and clear cell ovarian cancer might share similar etiology, and, similar to clear cell cancer, endometrioid cancer has been found to be associated with endometriosis (53–55). Therefore, anti-estrogenic effect of smoking could potentially explain a decreased risk of endometrioid and clear cell ovarian cancer among smokers. It is important to emphasize, however, that, even though the inverse associations were observed, they were mostly non-significant even among pooled or meta-analysis studies. One of the explanation could be that because a lack of statistical power to be able to detect association. It could also be that misclassification of endometrioid ovarian cancer cases could attenuate the estimated measure of association. Many cases of endometrioid subtype, especially of high grade, diagnosed in the past are now considered serous ovarian cancer (4). As a result, the association between smoking and endometrioid ovarian cancer could have been attenuated in the earlier studies due to the lack of association between smoking and serous ovarian cancer.
The results of epidemiologic studies included in current review suggest that cigarette smoking is not associated with risk of serous ovarian cancer. Two counterbalanced mechanisms were proposed to explain the null association between serous cancer and smoking. On one hand, carcinogenic effect of smoking might increase serous cancer risk. On the other hand, its anti-estrogenic effect might decrease serous cancer risk, since non-mucinous cancer has been reported to be more responsive to hormonal factors than mucinous cancer (33, 56). As a result, the ultimate effect might become neutral(33).
What requires further attention is that, although the associations were mostly non-significant, there was some discrepancy of the direction of the estimated associations depending on the study design. In fact, among current smokers, the estimates were mostly inverse for case-control studies and mostly positive among cohort studies. Such difference could have several explanations. One of them is the control selection. For instance, Baker et al. used hospital controls who usually smoke more than general population and this could attenuate or even reverse the actual association between smoking and cancer risk (14). Another reason is a possible survival bias affecting the findings of the case-control studies. In fact, in case-control studies there could have been a higher proportion of less severe and less aggressive cases, and, quite likely, a higher proportion of low-grade serous ovarian cancer tumors. There is evidence of low-grade serous ovarian cancer being linked to endometriosis (55), which, similarly to proposed mechanisms for the association between smoking and clear cell and endometrioid cancers, could explain inverse associations among serous ovarian tumors observed in case-control studies.
In our review, only three studies reported on the histotype-specific associations between smoking and ovarian cancer survival. All three studies observed increased risk of mortality within mucinous subtype (39–41), and one reported on adverse survival among women diagnosed with serous histotype (39). Such findings correspond to the results of the studies reporting adverse prognostic impact of smoking on a variety of cancers including lung, head and neck, and breast cancer (57, 58). However, more studies should be conducted in order to make a more definite conclusion about smoking and survival during the postdiagnostic period among patients diagnosed with certain histotypes of ovarian cancer.
The unfavorable influence of smoking on survival among ovarian cancer patients could be due to both direct and indirect mechanisms. In in vitro model, cigarette smoke extract promoted cancer cell proliferation and increased VEGF expression which led to increased angiogenesis (59). Tobacco carcinogens could induce genetic mutations that could lead to more aggressive cancer (60). Additionally, smoking might increase cancer mortality through promoting inflammation and suppressing immune function (47, 60). Long-term smokers usually have other smoking-associated conditions such as chronic obstructive pulmonary disease and cardiovascular disease. Furthermore, smoking is associated with presence of other adverse lifestyle habits and comorbidities which may contribute to worse survival.
Study Limitations
As mentioned before, studies on epithelial ovarian cancer face a common limitations of a small number of cases and insufficient statistical power that would prevent from conducting analysis stratified by histological subtypes, and, specifically, for the least frequently occurring histotypes. For example, in the EPIC cohort study, which is, to date, one of the largest prospective cohort studies on smoking and epithelial ovarian cancer, there was only 83 mucinous cases (23). Moreover, further stratification of the sample by categories of smoking exposure lowers the number of cases in each category of these smoking variables. Analyzing such associations by additionally stratifying by histological subtypes becomes nearly impossible due to restricted power. Because the main aim of this review was to investigate epidemiologic evidence on the associations between smoking and histotype-specific ovarian cancer, we were able to present only the findings for the current/former/never smoking variables. Moreover, a limited number of cases for each histotype may have resulted in the inability to detect the presence of association if one existed or in widening of confidence intervals resulting in nonsignificant findings. Future studies may need to address this important point by emphasizing other variables that characterize exposure to smoking such as duration, intensity, and frequency of smoking and evaluate presence of a dose-response relationship.
One way to alleviate the problem of insufficient sample size is to combine several histotypes together. For instance, merging together borderline and invasive mucinous cases was a common practice in earlier studies when assessing risk of mucinous ovarian cancer associated with smoking (23, 32, 36). If, as reported by some studies in this review, the association between smoking and mucinous ovarian cancer is stronger among borderline tumors compared to invasive tumors, then combining these two types could lead to overestimation of the true association between smoking and invasive mucinous cancer. Similarly, combining all the non-mucinous histotypes into one “nonmucinous ovarian cancer” category could have obscured a possible association between current smoking and endometrioid histotype.
Another common limitation of ovarian cancer studies, as it was pointed out earlier, is misclassification of cases according to histological subtype. Very few individual studies included in this review reported having systematic or central pathology review. But even if such pathology review were to be implemented, misclassification of cases could have only been mitigated not eliminated. As it was mentioned earlier, there was misclassification of serous subtype as endometrioid ovarian cancer in earlier studies. There were also misclassification of primary and secondary ovarian cancer, and misclassification of borderline and invasive tumors, especially among mucinous tumor (61). Compared to the other histological subtypes of epithelial ovarian cancer, mucinous cancer is relatively uncommon, accounting for about 2–10% of all primary ovarian cancers, but in earlier studies mucinous ovarian cancer was diagnosed much more frequently (29). For example, in two most recent case-control studies invasive mucinous cases accounted for no more than 5% of total invasive cases (11, 12), but represented more than 12% in three earlier case-control studies (16, 30, 36).
Advances in pathological techniques have led to a subsequent drop in the number of mucinous cancers cases, particularly because it was determined that some primary mucinous cancers, especially of advanced stage, were actually cases of secondary cancer metastasized from gastrointestinal or cervical cancer (4). As a result of this misclassification, the association between smoking and mucinous ovarian cancer risk could have been overestimated because smoking is an established risk factor for both colorectal and cervical cancers (50). According to Seidman et al., another reason for more diagnoses of invasive mucinous tumor in the past was that some borderline mucinous tumors were misclassified as invasive (62). Such misclassification could have also explained some variation in the measures of association observed within the studies of a similar design.
One of the possible solution to attenuate a problem of a restricted statistical power and, partially, misclassification could be combining the histotypes according to the origin of disease, into Type I and Type II tumors (6). To our knowledge, none of the studies examined attempted to combine the histotypes according to this proposed model. To do so, future studies may need to focus on a more thorough pathological distinction between the ovarian cancer cases, paying a particular attention to grade of the tumors.
Third, misclassification of smoking exposure may occur due to recall bias which could be unintentional or intentional. According to Kelemen et al., underreporting of smoking behavior among current smokers was common and it could have attenuated the positive association between current smoking and cancer risk and mortality (40). Missing information on smoking could also cause misclassification of smoking exposure. For instance, cohort studies often collected information on smoking at enrollment but no updated information tends to be collected during follow–up period when some patients can change their smoking behavior. Studies on survival also reported possibility of misclassification of smoking exposure due to missing information on smoking after diagnosis (60, 63).
Fourth, selection bias with case-control studies could occur due to underrepresentation of smokers among controls because controls who are willing to participate tend to be healthier than non-responders. This selection bias could lead to overestimation of the positive association between smoking and risk of mucinous ovarian cancer. Selection bias could also stem from underrepresentation of advanced cases. In the case-control study by Kelemen et al., 14% of newly diagnosed cases were non-responders due to rapid death after diagnosis (11). Another case-control study had 11.4% of non-responders due to death (16), while many other studies did not report such statistics. Serous ovarian cancer is usually diagnosed at advanced stage, while endometrioid, clear cell, and mucinous ovarian cancers are usually detected at a lower stage (4), so underrepresentation of advanced cases might disproportionally affect the histotype of serous cancer. If, as reported, smoking is associated with worse survival primarily among cases at advanced stage, underrepresentation of advanced cases could lead to attenuated association between smoking and risk of serous ovarian cancer. Survival bias could explain differences in the measures of associations observed across case-control studies.
Finally, sources of our review were all from published literature, so the interpretation of the results could have been affected by publication bias. There was also overlapping of results as some individual studies were a part of one or more pooled or meta-analysis included in the current review. Such duplication of the results should be remembered when interpreting the results of these studies.
Conclusions and Implication
In conclusion, no significant association between cigarette smoking and overall ovarian cancer risk was consistently observed among studies included in this review. The only significant association observed across case-control, cohort, and pooled studies was an increased risk of mucinous ovarian cancer associated with current smoking. However, we cannot rule out the fact that this association could be overestimated due to misclassification of mucinous cases and inclusion of borderline tumors. There was also some evidence on a decreased, although non-significant, risk of clear cell and endometrioid ovarian cancer associated with smoking, Overall, smoking appeared to have a negative impact on ovarian cancer survival, but its subtype-specific impact was not clear due to the insufficient amount of epidemiologic evidence.
International epidemiological collaborations formed in the past decade have dramatically increased sample size and statistical power in ovarian cancer research, therefore, allowing further investigation of the links between such important lifestyle factors as smoking with uncommon histological subtypes including endometrioid, clear cell and mucinous (64). However, in order to have a better understanding of the relationship between smoking habits and ovarian cancer, future studies should be conducted with a particular attention being paid to the collection of a detailed information on smoking habits both prior and after the diagnosis and a more thorough classification of ovarian tumors.
Acknowledgements
Funding: A.N. Minlikeeva was supported by National Cancer Institute (NCI) NIH/NCI 4R25CA113951 and NIH/NCI P50CA159981;
K.B. Moysich was supported by NIH/NCI (2R25CA113951, R01CA095023, R01CA126841, P50CA159981) and the Roswell Park Alliance Foundation.
Footnotes
Conflict of interest: No potential conflicts of interest were disclosed.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.SEER. Cancer Stat Facts: Ovarian Cancer 2017. [Available from: https://seer.cancer.gov/statfacts/html/ovary.html.
- 3.Chen VW, Ruiz B, Killeen JL, Coté TR, Wu XC, Correa CN. Pathology and classification of ovarian tumors. Cancer 2003;97(10 Suppl):2631–42. [DOI] [PubMed] [Google Scholar]
- 4.McCluggage WG. Morphological subtypes of ovarian carcinoma: a review with emphasis on new developments and pathogenesis. Pathology 2011;43(5):420–32. [DOI] [PubMed] [Google Scholar]
- 5.Meinhold-Heerlein I, Fotopoulou C, Harter P, Kurzeder C, Mustea A, Wimberger P, et al. The new WHO classification of ovarian, fallopian tube, and primary peritoneal cancer and its clinical implications. Arch Gynecol Obstet 2016;293(4):695–700. [DOI] [PubMed] [Google Scholar]
- 6.Kurman RJ, Shih IM. The Dualistic Model of Ovarian Carcinogenesis: Revisited, Revised, and Expanded. Am J Pathol 2016;186(4):733–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Przybycin CG, Kurman RJ, Ronnett BM, Shih Ie M, Vang R. Are all pelvic (nonuterine) serous carcinomas of tubal origin? Am J Surg Pathol 2010;34(10):1407–16. [DOI] [PubMed] [Google Scholar]
- 8.Kurman RJ, Shih Ie M. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol 2010;34(3):433–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilks CB, Prat J. Ovarian carcinoma pathology and genetics: recent advances. Hum Pathol 2009;40(9):1213–23. [DOI] [PubMed] [Google Scholar]
- 10.Wiegand KC, Shah SP, Al-Agha OM, Zhao Y, Tse K, Zeng T, et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med 2010;363(16):1532–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelemen LE, Abbott S, Qin B, Peres LC, Moorman PG, Wallace K, et al. Cigarette smoking and the association with serous ovarian cancer in African American women: African American Cancer Epidemiology Study (AACES). Cancer Causes Control 2017;28(7):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rossing MA, Cushing-Haugen KL, Wicklund KG, Weiss NS. Cigarette smoking and risk of epithelial ovarian cancer. Cancer Causes Control 2008;19(4):413–20. [DOI] [PubMed] [Google Scholar]
- 13.Soegaard M, Jensen A, Høgdall E, Christensen L, Høgdall C, Blaakaer J, et al. Different risk factor profiles for mucinous and nonmucinous ovarian cancer: results from the Danish MALOVA study. Cancer Epidemiol Biomarkers Prev 2007;16(6):1160–6. [DOI] [PubMed] [Google Scholar]
- 14.Baker JA, Odunuga OO, Rodabaugh KJ, Reid ME, Menezes RJ, Moysich KB. Active and passive smoking and risk of ovarian cancer. Int J Gynecol Cancer 2006;16 Suppl 1:211–8. [DOI] [PubMed] [Google Scholar]
- 15.Huusom LD, Frederiksen K, Høgdall EV, Glud E, Christensen L, Høgdall CK, et al. Association of reproductive factors, oral contraceptive use and selected lifestyle factors with the risk of ovarian borderline tumors: a Danish case-control study. Cancer Causes Control 2006;17(6):821–9. [DOI] [PubMed] [Google Scholar]
- 16.Pan SY, Ugnat AM, Mao Y, Wen SW, Johnson KC, Group CCRER. Association of cigarette smoking with the risk of ovarian cancer. Int J Cancer 2004;111(1):124–30. [DOI] [PubMed] [Google Scholar]
- 17.Riman T, Dickman PW, Nilsson S, Nordlinder H, Magnusson CM, Persson IR. Some life-style factors and the risk of invasive epithelial ovarian cancer in Swedish women. Eur J Epidemiol 2004;19(11):1011–9. [DOI] [PubMed] [Google Scholar]
- 18.Goodman MT, Tung KH. Active and passive tobacco smoking and the risk of borderline and invasive ovarian cancer (United States). Cancer Causes Control 2003;14(6):569–77. [DOI] [PubMed] [Google Scholar]
- 19.Green A, Purdie D, Bain C, Siskind V, Webb PM. Cigarette smoking and risk of epithelial ovarian cancer (Australia). Cancer Causes Control 2001;12(8):713–9. [DOI] [PubMed] [Google Scholar]
- 20.Riman T, Dickman PW, Nilsson S, Correia N, Nordlinder H, Magnusson CM, et al. Risk factors for epithelial borderline ovarian tumors: results of a Swedish case-control study. Gynecol Oncol 2001;83(3):575–85. [DOI] [PubMed] [Google Scholar]
- 21.Licaj I, Jacobsen BK, Selmer RM, Maskarinec G, Weiderpass E, Gram IT. Smoking and risk of ovarian cancer by histological subtypes: an analysis among 300 000 Norwegian women. Br J Cancer 2017;116(2):270–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Licaj I, Lukic M, Jareid M, Lund E, Braaten T, Gram IT. Epithelial ovarian cancer subtypes attributable to smoking in the Norwegian Women and Cancer Study, 2012. Cancer Med 2016;5(4):720–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gram IT, Lukanova A, Brill I, Braaten T, Lund E, Lundin E, et al. Cigarette smoking and risk of histological subtypes of epithelial ovarian cancer in the EPIC cohort study. Int J Cancer 2012;130(9):2204–10. [DOI] [PubMed] [Google Scholar]
- 24.Gates MA, Rosner BA, Hecht JL, Tworoger SS. Risk factors for epithelial ovarian cancer by histologic subtype. Am J Epidemiol 2010;171(1):45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gram IT, Braaten T, Adami HO, Lund E, Weiderpass E. Cigarette smoking and risk of borderline and invasive epithelial ovarian cancer. Int J Cancer 2008;122(3):647–52. [DOI] [PubMed] [Google Scholar]
- 26.Terry PD, Miller AB, Jones JG, Rohan TE. Cigarette smoking and the risk of invasive epithelial ovarian cancer in a prospective cohort study. Eur J Cancer 2003;39(8):1157–64. [DOI] [PubMed] [Google Scholar]
- 27.Faber MT, Kjaer SK, Dehlendorff C, Chang-Claude J, Andersen KK, Hogdall E, et al. Cigarette smoking and risk of ovarian cancer: a pooled analysis of 21 case-control studies. Cancer Causes Control 2013;24(5):989–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beral V, Gaitskell K, Hermon C, Moser K, Reeves G, Peto R. Ovarian cancer and smoking: individual participant meta-analysis including 28,114 women with ovarian cancer from 51 epidemiological studies. Lancet Oncol 2012;13(9):946–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jordan SJ, Green AC, Whiteman DC, Webb PM, Group AOCS. Risk factors for benign, borderline and invasive mucinous ovarian tumors: epidemiological evidence of a neoplastic continuum? Gynecol Oncol 2007;107(2):223–30. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Coogan PF, Palmer JR, Strom BL, Rosenberg L. Cigarette smoking and increased risk of mucinous epithelial ovarian cancer. Am J Epidemiol 2004;159(2):133–9. [DOI] [PubMed] [Google Scholar]
- 31.Modugno F, Ness RB, Cottreau CM. Cigarette smoking and the risk of mucinous and nonmucinous epithelial ovarian cancer. Epidemiology 2002;13(4):467–71. [DOI] [PubMed] [Google Scholar]
- 32.Tworoger SS, Gertig DM, Gates MA, Hecht JL, Hankinson SE. Caffeine, alcohol, smoking, and the risk of incident epithelial ovarian cancer. Cancer 2008;112(5):1169–77. [DOI] [PubMed] [Google Scholar]
- 33.Jordan SJ, Whiteman DC, Purdie DM, Green AC, Webb PM. Does smoking increase risk of ovarian cancer? A systematic review. Gynecol Oncol 2006;103(3):1122–9. [DOI] [PubMed] [Google Scholar]
- 34.Kurian AW, Balise RR, McGuire V, Whittemore AS. Histologic types of epithelial ovarian cancer: have they different risk factors? Gynecol Oncol 2005;96(2):520–30. [DOI] [PubMed] [Google Scholar]
- 35.Wentzensen N, Poole EM, Trabert B, White E, Arslan AA, Patel AV, et al. Ovarian Cancer Risk Factors by Histologic Subtype: An Analysis From the Ovarian Cancer Cohort Consortium. J Clin Oncol 2016;34(24):2888–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marchbanks PA, Wilson H, Bastos E, Cramer DW, Schildkraut JM, Peterson HB. Cigarette smoking and epithelial ovarian cancer by histologic type. Obstet Gynecol 2000;95(2):255–60. [DOI] [PubMed] [Google Scholar]
- 37.Kuper H, Titus-Ernstoff L, Harlow BL, Cramer DW. Population based study of coffee, alcohol and tobacco use and risk of ovarian cancer. Int J Cancer 2000;88(2):313–8. [DOI] [PubMed] [Google Scholar]
- 38.Nagle CM, Olsen CM, Webb PM, Jordan SJ, Whiteman DC, Green AC, et al. Endometrioid and clear cell ovarian cancers: a comparative analysis of risk factors. Eur J Cancer 2008;44(16):2477–84. [DOI] [PubMed] [Google Scholar]
- 39.Praestegaard C, Jensen A, Jensen SM, Nielsen TS, Webb PM, Nagle CM, et al. Cigarette smoking is associated with adverse survival among women with ovarian cancer: Results from a pooled analysis of 19 studies. Int J Cancer 2017;140(11):2422–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kelemen LE, Warren GW, Koziak JM, Köbel M, Steed H. Smoking may modify the association between neoadjuvant chemotherapy and survival from ovarian cancer. Gynecol Oncol 2016;140(1):124–30. [DOI] [PubMed] [Google Scholar]
- 41.Kim SJ, Rosen B, Fan I, Ivanova A, McLaughlin JR, Risch H, et al. Epidemiologic factors that predict long-term survival following a diagnosis of epithelial ovarian cancer. Br J Cancer 2017;116(7):964–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA 2004;291(10):1238–45. [DOI] [PubMed] [Google Scholar]
- 43.Luo J, Horn K, Ockene JK, Simon MS, Stefanick ML, Tong E, et al. Interaction between smoking and obesity and the risk of developing breast cancer among postmenopausal women: the Women’s Health Initiative Observational Study. Am J Epidemiol 2011;174(8):919–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vijayvergia N, Denlinger CS. Lifestyle Factors in Cancer Survivorship: Where We Are and Where We Are Headed. J Pers Med 2015;5(3):243–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poole EM, Konstantinopoulos PA, Terry KL. Prognostic implications of reproductive and lifestyle factors in ovarian cancer. Gynecol Oncol 2016;142(3):574–87. [DOI] [PubMed] [Google Scholar]
- 46.Risch HA, Marrett LD, Jain M, Howe GR. Differences in risk factors for epithelial ovarian cancer by histologic type. Results of a case-control study. Am J Epidemiol 1996;144(4):363–72. [DOI] [PubMed] [Google Scholar]
- 47.U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress. A Report of Surgeon General Washington, DC: U.S. Department of Health and Human Services, Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- 48.Phillips DH. DNA adducts as markers of exposure and risk. Mutat Res 2005;577(1–2):284–92. [DOI] [PubMed] [Google Scholar]
- 49.Giovannucci E, Martínez ME. Tobacco, colorectal cancer, and adenomas: a review of the evidence. J Natl Cancer Inst 1996;88(23):1717–30. [DOI] [PubMed] [Google Scholar]
- 50.IARC. Monographs on the evaluation of carcinogenic risks to humans.personal habits and indoor combustion. A review of human carcinogens Lyon, France: IARC Press; 2009. [Google Scholar]
- 51.Parasar P, Ozcan P, Terry KL. Endometriosis: Epidemiology, Diagnosis and Clinical Management. Curr Obstet Gynecol Rep 2017;6(1):34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McLeod BS, Retzloff MG. Epidemiology of endometriosis: an assessment of risk factors. Clin Obstet Gynecol 2010;53(2):389–96. [DOI] [PubMed] [Google Scholar]
- 53.Ogawa S, Kaku T, Amada S, Kobayashi H, Hirakawa T, Ariyoshi K, et al. Ovarian endometriosis associated with ovarian carcinoma: a clinicopathological and immunohistochemical study. Gynecol Oncol 2000;77(2):298–304. [DOI] [PubMed] [Google Scholar]
- 54.Sainz de la Cuesta R, Eichhorn JH, Rice LW, Fuller AF, Nikrui N, Goff BA. Histologic transformation of benign endometriosis to early epithelial ovarian cancer. Gynecol Oncol 1996;60(2):238–44. [DOI] [PubMed] [Google Scholar]
- 55.Pearce CL, Templeman C, Rossing MA, Lee A, Near AM, Webb PM, et al. Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case-control studies. Lancet Oncol 2012;13(4):385–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Purdie DM, Siskind V, Bain CJ, Webb PM, Green AC. Reproduction-related risk factors for mucinous and nonmucinous epithelial ovarian cancer. Am J Epidemiol 2001;153(9):860–4. [DOI] [PubMed] [Google Scholar]
- 57.Warren GW, Kasza KA, Reid ME, Cummings KM, Marshall JR. Smoking at diagnosis and survival in cancer patients. Int J Cancer 2013;132(2):401–10. [DOI] [PubMed] [Google Scholar]
- 58.Ebbert JO, Williams BA, Sun Z, Aubry MC, Wampfler JA, Garces YI, et al. Duration of smoking abstinence as a predictor for non-small-cell lung cancer survival in women. Lung Cancer 2005;47(2):165–72. [DOI] [PubMed] [Google Scholar]
- 59.Ye YN, Wu WK, Shin VY, Cho CH. A mechanistic study of colon cancer growth promoted by cigarette smoke extract. Eur J Pharmacol 2005;519(1–2):52–7. [DOI] [PubMed] [Google Scholar]
- 60.Kjaerbye-Thygesen A, Frederiksen K, Høgdall EV, Glud E, Christensen L, Høgdall CK, et al. Smoking and overweight: negative prognostic factors in stage III epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev 2006;15(4):798–803. [DOI] [PubMed] [Google Scholar]
- 61.Seidman JD, Kurman RJ, Ronnett BM. Primary and metastatic mucinous adenocarcinomas in the ovaries: incidence in routine practice with a new approach to improve intraoperative diagnosis. Am J Surg Pathol 2003;27(7):985–93. [DOI] [PubMed] [Google Scholar]
- 62.Seidman JD, Horkayne-Szakaly I, Haiba M, Boice CR, Kurman RJ, Ronnett BM. The histologic type and stage distribution of ovarian carcinomas of surface epithelial origin. Int J Gynecol Pathol 2004;23(1):41–4. [DOI] [PubMed] [Google Scholar]
- 63.Nagle CM, Bain CJ, Webb PM. Cigarette smoking and survival after ovarian cancer diagnosis. Cancer Epidemiol Biomarkers Prev 2006;15(12):2557–60. [DOI] [PubMed] [Google Scholar]
- 64.Cannioto RA, Trabert B, Poole EM, Schildkraut JM. Ovarian cancer epidemiology in the era of collaborative team science. Cancer Causes Control 2017;28(5):487–95. [DOI] [PMC free article] [PubMed] [Google Scholar]