SUMMARY
While survival in paediatric acute lymphoblastic leukaemia (ALL) is excellent, survival following relapse is poor. Previous studies suggest proteasome inhibition with chemotherapy improves relapse ALL response rates. This phase 2 Children’s Oncology Group study tested the hypothesis that adding the proteasome inhibitor bortezomib to chemotherapy increases complete response rates (CR2). Evaluable patients (n=135, 103 B-ALL, 22 T-ALL, 10 T-lymphoblastic lymphoma) were treated with reinduction chemotherapy plus bortezomib. Overall CR2 rates were 68%±5% for precursor B-ALL patients (<21 years of age), 63%±7% for very early relapse (<18 months from diagnosis) and 72%±6% for early relapse (18–36 months from diagnosis). Relapsed T-ALL patients had an encouraging CR2 rate of 68%±10%. End of induction minimal residual disease (MRD) significantly predicted survival. MRD negative (MRDneg; MRD <0.01%) rates increased from 29% (post-cycle 1) to 64% following cycle 3. Very early relapse, end-of-induction MRDneg precursor B-ALL patients had 70%±14% 3-year event-free (EFS) and overall survival (OS) rates, vs. 3-year EFS/OS of 0–3% (p=0.0001) for MRDpos (MRD ≥0.01) patients. Early relapse patients had similar outcomes (MRDneg 3-year EFS/OS 58–65% vs. MRDpos 10–19%, EFS p=0.0014). These data suggest that adding bortezomib to chemotherapy in certain ALL subgroups, such as T-cell ALL, is worthy of further investigation. This study is registered at http://www.clinical.trials.gov as NCT00873093.
Keywords: proteasome inhibition, paediatric leukaemia, acute lymphoblastic leukaemia, acute lymphocytic leukaemia, minimal residual disease
INTRODUCTION
Paediatric acute lymphoblastic leukaemia (ALL) has been one of the success stories of modern medicine with overall survival (OS) of over 90% for those with newly diagnosed disease (Hunger & Mullighan, 2015;Pui et al, 2011). However, outcome for those with relapsed or refractory disease is guarded, despite treatment with intensive chemotherapy and stem cell transplant (Freyer et al, 2011;Oskarsson et al, 2016), and new therapeutic strategies are needed for these patients. Patients with relapsed T-ALL have a particularly poor event free survival (EFS) of <15% (Teachey & Hunger, 2013). The treatment of relapsed disease is made more difficult by the development of drug-resistant subclones following initial therapy (Gaynon & Sun, 2016). Patients with relapsed ALL are often highly resistant to corticosteroids, one of the principal components of ALL therapy (Bhadri et al, 2012).
Proteasome inhibitors (PIs), including bortezomib, have been shown to act in synergy with corticosteroids and doxorubicin, both in vitro (Horton et al, 2006;Minderman et al, 2007;Colado et al, 2008) and in vivo (Richardson et al, 2004). PIs have also shown activity against paediatric ALL in the Preclinical Pediatric Testing Program (PPTP) using patient-derived xenografts (Houghton et al, 2008). Agents that improve the efficacy of steroids and anthracyclines could enhance the effectiveness of current therapy regimens for relapsed ALL.
Although much clinical testing of PIs has occurred in multiple myeloma, previous clinical studies have suggested that bortezomib can be safely combined with chemotherapy in paediatric leukaemia and lymphoma (Messinger et al, 2010;Horton et al, 2014;Bertaina et al, 2017). Early phase testing by the Children’s Oncology Group (COG) and the Therapeutic Advances for Childhood Leukemia (TACL) consortia have shown that bortezomib can be safely combined with dexamethasone/prednisone, cytarabine, daunorubicin, etoposide, ifosfamide, vinorelbine, mitoxantrone and idarubicin (Horton et al, 2007;Horton et al, 2014;Horton et al, 2010;Messinger et al, 2010). Toxicities in previous clinical trials have been limited to a low incidence of both peripheral neuropathy and acute respiratory distress syndrome (ARDS) (Horton et al, 2014;Messinger et al, 2012;Bertaina et al, 2017).
Two pilot studies in paediatric patients have suggested that bortezomib can enhance complete remission (CR) rates and OS in patients with relapsed precursor B-ALL (Messinger et al, 2012; Bertaina et al, 2017). Messinger et al (2012) reported 22 ALL patients in second or greater relapse treated with bortezomib and reinduction chemotherapy (vincristine, prednisone, pegylated asparaginase and doxorubicin: VPLD). This study showed that 73% (95% confidence interval [CI] 46–78%) attained a second CR (CR2). Similarly, Bertaina et al (2017) showed a CR2 rate of 73% (95% CI 60–83%) in 37 patients with relapsed precursor B-ALL patients treated with bortezomib-VPLD. In both studies, OS was improved by the achievement of either CR2 (Messinger et al, 2012) or end-of-induction minimal residual disease (MRD) negativity (MRD level <0.1%) (Bertaina et al, 2017).
The COG AALL07P1 Phase 2 clinical trial was conducted to determine if the addition of bortezomib to VPLD could improve CR2 rates for children, adolescents and young adults with first relapse of precursor B-ALL occurring within 3 years of diagnosis, as compared to historical control patients treated with VPLD without bortezomib (AALL01P2) (Raetz et al, 2008a). Response rates included measurement of MRD and showed the strong correlation of end-of-induction MRD status with clinical outcome. Unlike previous bortezomib studies, AALL07P1 stratified precursor B-ALL patients into two risk groups; those <18 months from diagnosis (very early relapse) and those 18–36 months from diagnosis (early relapse) due to the lower response rate in those with very early relapse. AALL07P1 also included a stratum for first-relapse T-ALL and T-lymphoblastic lymphoma (LL) patients.
METHODS
Study Population:
Patients between 1 and 31 years of age with first bone marrow (BM) relapse or combined BM and central nervous system (CNS) relapse, with or without extramedullary disease, and occurring within 36 months of initial diagnosis, were eligible to enrol. Patients were required to have an M3 marrow (>25% blasts) to be eligible. LL patients were required to have a T-cell immunophenotype and measurable disease. All patients were required to have an Eastern Cooperative Oncology Group performance status of 0, 1 or 2, using Lansky if ≤16 years of age, and Karnofsky if >16 years of age. Patients were required to have adequate organ function for enrolment (see supplemental methods). Although patients were required to have recovered from the acute toxic effects of prior therapy, there was no waiting period to start therapy if relapse occurred while receiving standard ALL maintenance therapy. The study was approved by the relevant institutional review boards or ethics committees and all participants or legal guardians gave written informed consent.
Drug Administration and Trial Design:
Bortezomib was supplied by Millennium Pharmaceuticals (Cambridge MA, now Takeda Pharmaceuticals) through the National Institutes of Health (NIH)-National Cancer Institute (NCI) Cancer Therapy Evaluation Program (CTEP). Bortezomib, 1.3 mg/m2/dose, was given as a 3–5 second intravenous (IV) push for 7 doses on days 1, 4, 8 and 11 in Block 1 and on days 1, 4, and 8 in Block 2. Chemotherapy consisted of three reinduction blocks (Table I) lasting 28–36 days each. Block 1 consisted of a standard VPLD 4-drug induction with bortezomib. Block 2 was a combination of cyclophosphamide and etoposide with bortezomib followed by high-dose (HD) methotrexate. Block 3 was high dose cytarabine on days 1, 2, 8 and 9 with PEG-asparaginase on days 3 and 10.
Table I:
Treatment Plan
| Study Phase | Dosing |
|---|---|
| Block 1 (VPLD) 28 days; response at Day 28–36 | |
| CNS 1 or 2: IT- AraC/ IT- MTX* | Day 1/ Day 15 and 29 |
| CNS3: IT-AraC/IT-MTX + HC + AraC (ITT)* | Day 1/Days 8, 15, 22 and 29 |
| Vincristine 1.5 mg/m2 (max 2 mg) IV | Days 1, 8, 15, and 22 |
| Doxorubicin (60 mg/m2) IV | Day 1 |
| Prednisone (40 mg/m2/day PO divided BID)** | Days 1–28 |
| Bortezomib (1.3 mg/m2) IV | Days 1, 4, 8, and 11 |
| PEG-asparaginase (2500 units/m2) IM | Days 2, 8, 15, and 22 |
| Block 2: 28 days; response at Day 28–36 | |
| CNS1: IT MTX/ CNS3 ITT | Days 1 and 22 |
| Etoposide (100 mg/m2) IV | Days 1–5 |
| Cyclophosphamide (440 mg/m2) IV | Days 1–5 |
| Bortezomib (1.3 mg/m2 ) IV | Days 1, 4, and 8 |
| Methotrexate (5 000 mg/m2) IV | Day 22 |
| Block 3: 28 days; response assessed at Day 28 | |
| Cytarabine (3 000 mg/m2/dose) IV BID | Days 1, 2, 8 and 9 |
| E-coli asparaginase (6 000 units/m2) IM *** | Days 2 and 9 |
IT therapy followed age-based dosing. Day 1: Cytarabine 30 mg (age 1–2 years), 50 mg (age 2–3 years) or 70 mg (>3 years). Subsequent days for CNS-negative: IT MTX: 8 mg (ages 1–2 years), 10 mg (2–3 years), 12 mg (3–9 years) or 15 mg (>9 years). Subsequent days for CNS-positive: MTX: 8 mg, HC: 8 mg, AraC: 16 mg (1–2 y), MTX: 10 mg, HC: 10 mg, AraC: 20 mg (2–3 years); MTX: 12 mg, HC: 12 mg, AraC: 24 mg (3–9 years) or MTX: 15 mg, HC: 15 mg, AraC: 30 mg (>9 years).
Methylprednisolone IV could be substituted for prednisone PO.
Alternative asparaginase regimen: PEG-asparaginase 2500 units/m2 IM on Day 2.
AraC: cytarabine; BID: twice a day; CNS: central nervous system; HC: hydrocortisone; IM: intramuscularly; IT: intrathecal; ITT: intention-to-treat; IV: intravenously; PO: orally; VPLD vincristine, prednisone, pegylated asparaginase and doxorubicin
Assessment of Toxicity
Adverse events were graded according to the NCI Common Toxicity Criteria for Adverse Events, version 4.0 (https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010–06-14_QuickReference_5×7.pdf). A severe toxicity was defined as any Grade 4 non-haematological toxicity related or possibly related to bortezomib with the exception of anorexia, vomiting, dehydration, mucositis, hypofibrinogenaemia and metabolic abnormalities resolving to ≤ Grade 2 within 7 days or the start of the next cycle. Dose-limiting toxicities also included any possibly, probably, or definitely related Grade 3 or 4 non-haematological toxicity that delayed the beginning of the subsequent course of therapy by ≥ 7 days. Local treating investigators determined bortezomib toxicity grade and attribution.
Assessment of Response
Second complete remission (CR2) was defined as achieving an M1 bone marrow (<5% blasts) with no evidence of circulating blasts or extramedullary disease, and with an absolute neutrophil count > 0.75 × 109/l and platelet count > 75 ×109/l. Partial remission (PR) was defined as complete disappearance of circulating blasts and achievement of M2 marrow status (≥ 5% to < 25% blast cells). Progressive disease (PD) was defined an increase of at least 25% or an absolute increase of at least 5 × 109 cells/l (whichever was greater) in circulating leukaemia cells, extramedullary disease, or other laboratory or clinical evidence of PD. Patients not fulfilling criteria for CR, PR or PD were considered to have stable disease (SD). MRD was performed as described previously at a single central reference laboratory (Borowitz et al, 2008). Detectable MRD at a level of 0.01% or greater was designated positive. Designation as MRD negative implied a sensitivity of 1/10 000 cells (<0.01%). Criteria for the assessment of response for patients with lymphoma are provided in the supplemental methods.
Statistical analysis
Clinical trial design:
All patients were analysed on an intention-to-treat basis. Further details for the statistical design of the clinical trial are presented in the supplemental materials. EFS was defined as the time from study enrolment to first event (induction failure, induction death, relapse, second malignant neoplasm or remission death), or date of last contact for those who were event-free. OS was defined as time from study enrolment to death or date of last contact for those who were still alive. The Kaplan- Meier method was used to obtain estimates of EFS and OS (Kaplan & Meier, 2017), and standard error (SE) of estimates were obtained using the method of Peto & Peto (1972). Response results are presented as percentage +/− standard deviation. Chi-square test or Fisher’s exact test were used to compare response rates, and the log-rank test was used to compare survivor curves. P-values <0.05 were considered significant. Statistical analysis was performed using the SAS software, version 9.1 (SAS Institute, Cary, NC) and R (R Development Core Team, Vienna, Austria; http://www.R-project.org).
RESULTS
Demographics and Clinical Trial
One hundred forty-eight patients were enrolled from 2009–2014 (Figure 1); data current as of 30 September 2016 are included in this report. Two enrolled patients were ineligible. Table II provides demographics for all eligible patients. Eleven patients were deemed unevaluable for response: 8 with M1 marrows who began cycle 2 of therapy without attaining a platelet count of 75 ×109/l, 2 patients went off-therapy early due to infections unrelated to bortezomib, and 1 due to treating physician’s choice. The remaining 135 eligible, evaluable patients included 46 precursor B-ALL patients <21 years of age with a very early relapse (<18 months from diagnosis), 54 with an early relapse (18–36 months from diagnosis), 3 precursor-B-ALL patients over 21 years of age, 22 T-ALL patients and 10 T-LL patients. Most patients (71%) were Caucasian and the median age at enrolment was 8 years. All had relapsed following chemotherapy, 8 had relapsed following prior stem cell transplant and 11 had received prior radiotherapy in addition to chemotherapy.
Figure 1 Legend:
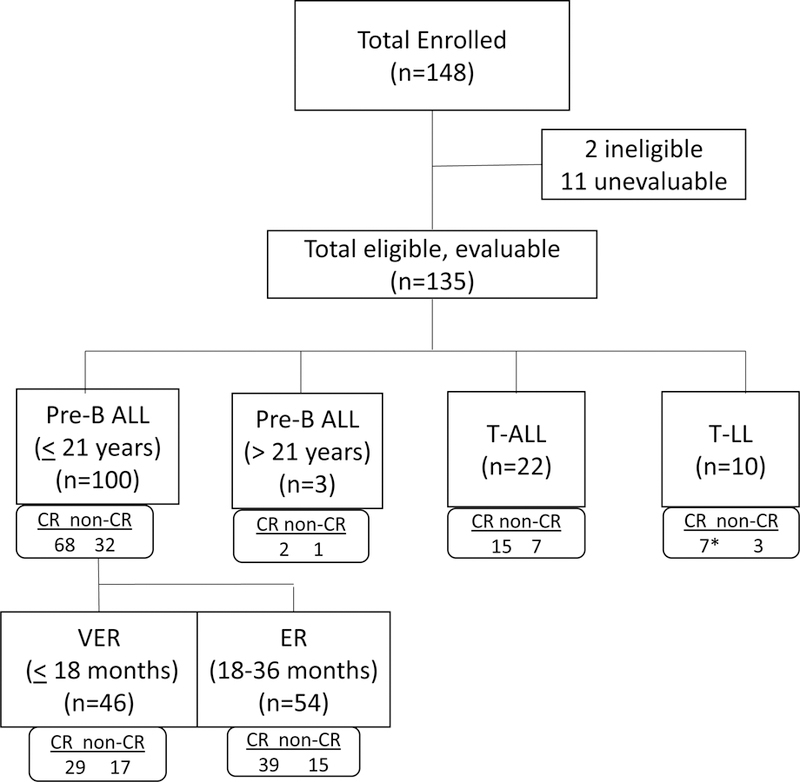
Consort diagram for the Children’s Oncology Group (COG) AALL07P1 clinical trial. ALL: acute lymphoblastic leukaemia; CR: complete remission; ER: early relapse; LL: lymphoblastic lymphoma; VER: very early relapse.
Table II:
Demographics n= 146 eligible patients
| Characteristics | Number (%) |
|---|---|
| Male | 84 (58%) |
| Female | 62 (42%) |
| Age, years (median, range)* | 8 (0–25) |
| Presenting WBC, average (x 109/l) | 40.4 ± 14 |
| Race@ | |
| Caucasian | 103 (70%) |
| African-American | 21 (14%) |
| Asian/Hawaiian/Pacific Islander | 7 (5%) |
| Native American | 2 (1%) |
| Mixed Race or Other | 12 (8%) |
| Ethnicity | |
| Hispanic or Latina | 38 (26%) |
| Non-Hispanic or Latina | 108 (74%) |
| Site/timing of relapse for pre-B (n=110) | |
| BM, very early (<18 months) | 46 (42%) |
| BM, early (18–36 months) | 50 (45%) |
| Combined, very early | 3 (2.7%) |
| Combined, early | 11 (10%) |
| Lineage/strata (evaluable) | |
| Stratum I: very early, pre-B** | 46 |
| Stratum II: early, pre-B*** | 53 |
| Stratum III: pre-B >21 years old | 4 |
| Stratum IV: T-cell ALL | 22 |
| Stratum V: T-cell lymphoma# | 10 |
Age from diagnosis to relapse at study enrolment
very early relapse = <18 months from initial diagnosis.
early relapse = 18–36 months from diagnosis. # with or without central nervous system or extramedullary. @Race percentages total <100% due to rounding.
ALL: acute lymphoblastic leukaemia; BM: bone marrow; WBC: white blood cell count.
Patients received three blocks of therapy: 135 were evaluable after block 1, 99 were evaluable after block 2 and 52 were evaluable after block 3. Thirty-nine percent completed all cycles of chemotherapy. The most common primary reason for discontinuation of therapy was due to physician’s determination that it was in the patient’s best interest (n=27). These patients either went to stem cell transplant (n=13), or were removed from therapy following complications due to adverse events (usually infections) (n=7) or to pursue alternative therapies (n=7). Other reasons for discontinuation of therapy included PD (n=22) and relapse (n=17). Four patients were lost to follow-up. Ninety patients (67%) continued onto additional chemotherapy and 69 (51%) underwent stem cell transplant.
Toxicity
All eligible patients were included in the safety cohort and received a total of 308 cycles of chemotherapy. Overall, bortezomib was well tolerated. Haematological toxicities were common, with >55% of patients having Grade 3 or 4 neutropenia or thrombocytopenia. Common non-haematological adverse events regardless of attribution included metabolic abnormalities, infectious complications and gastrointestinal symptoms (Table S1). There were three toxic deaths on study, all due to infections (Clostridium tertium (n=2) and E. coli (n=1)). Other severe toxicities (regardless of attribution) included 8 episodes of grade 3–4 typhlitis (4 attributed to bortezomib), 5 neuropathy, 4 pancreatitis, 4 enterocolitis (3 attributed to bortezomib) and 1 Grade 3 ARDS (Table III). Bortezomib-related neuropathy included 2 grade 3 peripheral motor neuropathy and 3 grade 3 sensory neuropathy, for an incidence rate of 2.8%. There were no grade 4 neuropathies and all neuropathies were reversible with bortezomib discontinuation.
Table III:
Grade 3–5 Non-Haematological, Non-Infectious Bortezomib-Related Toxicities1 (More than one occurrence in all eligible patients) (n=146)
| Toxicity Grade | Related Grade 3 | Related Grade 4 | Any Grade 5 | Total |
|---|---|---|---|---|
| Neurological | ||||
| Neuropathy-motor | 2 | 0 | 0 | 2 |
| Neuropathy-sensory | 3 | 0 | 0 | 3 |
| Non-haematological, Non-neurological | ||||
| Cardiac | 12 | 0 | 0 | 1 |
| Constitutional | 23 | 0 | 0 | 2 |
| Dermatological | 0 | 0 | 0 | 0 |
| Endocrine | 0 | 0 | 0 | 0 |
| Gastrointestinal | 254 | 34 | 0 | 28 |
| Pancreas/bilirubin | 0 | 0 | 0 | 0 |
| Metabolic | 605 | 105 | 0 | 70 |
| Musculoskeletal | 8 | 0 | 0 | 8 |
| Respiratory | 176 | 26 | 2 | 19 |
| Ocular/visual | 0 | 0 | 0 | 0 |
| Renal/GU | 0 | 1 | 0 | 1 |
| Vascular | 77 | 6 | 0 | 13 |
AE at least possibly attributed to bortezomib.
Left ventricular systolic dysfunction.
Consitutional AE: pain possibly related to bortezomib.
GI adverse events: mucositis (8), typhlitis (8 total, 4 related to bortezomib), pancreatitis (4), abdominal pain (3), diarrhoea (3), ileus (2), enterocolitis (4 total, 3 related to bortezomib), colitis (2). Two typhlitis and one ileus were Grade 4.
Metabolic AE (Grade 4): Hypokalaemia (4), Hypertriglyceridaemia (3), Hyperglycaemia (2), acidosis (2).
Respiratory AE (Grade 4/5): Dyspnoea (2) including 1 Grade 3 ARDS possibly related to bortezomib, respiratory failure/infection (2 Grade 5).
Vascular AE: hypotension (11, 6 grade 4), hypertension (2)
AE: adverse event; ARDS: acute respiratory distress syndrome; GI: gastrointestinal; GU; genito-urinary
Although infections and their complications were common in this clinical trial (Table S2), they were not significantly different than the rates of infections reported on previous studies in paediatric patients with relapsed ALL (Messinger et al, 2012;Raetz et al, 2008a). Infections involving gram-positive cocci were the most common, with 19 documented Streptococcus or Staphylococcus infections, followed by 9 gram-negative rod infections (E.coli, C. difficile, Moraxalla sp. and Clostridium sp.) and 7 fungal infections. With these infections, there were 30 occurrences of hypotension (11 at least possibly related to bortezomib) and 17 incidences of hypoxia, all but one related to a documented bacterial, fungal, or viral infection. Unlike previous reports with bortezomib in adults, these episodes of hypotension did not appear to be orthostatic in nature, but were related to concomitant infections.
Precursor B-ALL Response
This study used a single-arm stratified two stage design (London & Chang, 2005), which compares response rate for precursor B-ALL patients ≤21 years of age to a fixed response rate estimated from the historical control study AALL01P2, which used identical chemotherapy without bortezomib (Raetz et al, 2008a). Demographic characteristics in AALL07P1 included a median age of 9 years, 45% male and 75% Caucasian. As seen in prior studies of relapsed ALL, younger paediatric patients (<10 years of age) were more likely to achieve a CR than older patients (ages 10–21 years) (CR rate 75% vs. 54%, p=0.04, Fisher’s exact test).
Because CR2 rates varied significantly on AALL01P2 based upon time to relapse (Supplemental methods), we performed pre-specified analyses to compare response rates for very early and early relapse groups between AALL07P1 and AALL01P2. Patients with a very early relapse had a CR2 rate of 64% ± 9% (18/28), which met the pre-defined study endpoint for efficacy. In contrast, the CR2 rate of early relapse B-ALL patients on AALL07P1 was 73 ± 8% (24/33), which was similar to that observed on AALL01P2.
Thirty-nine additional precursor B-ALL patients were enrolled to obtain a more precise estimate of response rates and more detailed bortezomib pharmacokinetic data (Hanley et al, 2017). The overall CR2 rate for the 100 eligible, evaluable precursor B-ALL patients under 21 years of age was 68% ± 5% (68/100 patients), which was not significantly different from the CR2 rate on AALL01P2, 74% ± 6% (p=0.42). The very early risk groups included different high-risk features, as the historical control (AALL01P2) included patients that were Philadelphia chromosome-positive (Ph+) who were treated with a tyrosine kinase inhibitor in addition to chemotherapy. If making a direct comparison, patients with a very early relapse had a CR2 rate of 63% ± 7% (29/46) compared to 56% ± 12% (9/16) on AALL01P2 (p=0.63). The CR2 rate in patients with early relapse was 72% ± 6% (39/54 patients) compared to 81% ± 6% (34/24) on AALL01P2 (p=0.32) (Table S3).
Response rates in the first block of therapy were affected by persistent thrombocytopenia. There were 19 patients that achieved an M1 remission, but had thrombocytopenia at the end of reinduction. Eight of these patients recovered but were deemed unevaluable for response due to starting cycle 2 prior to platelet recovery. Of these 8 patients, 1 was over 21 years of age. If the remaining 7 patients were considered to have complete response without platelet recovery (CRp), the CR+CRp for patients with precursor B-ALL under 21 years of age rate was 75% (75/100), similar to previous reports (Messinger et al, 2012;Bertaina et al, 2017). Although prolonged thrombocytopenias may have been aggravated by bortezomib, the CR+CRp rates were no different than those seen in the historical control group.
Precursor B-ALL Clinical outcome
Outcome was determined at two time points, both at the completion of protocol therapy (4-month EFS) and after 3 years. For the early time point, the overall 4-month EFS was 54% ± 5% for all eligible, evaluable patients, with a 67% ± 6% EFS for those with early relapse and 39% ± 7% for those with very early relapse (Figure S1). Overall survival at 4 months was 83% ± 4% with an OS of 87% ± 5% in the early relapse and 78% ± 6% in the very early relapse patients. These numbers were not statistically different compared to the historical control trial AALL01P2. As seen in earlier COG studies, those with very early relapse had worse outcomes compared to those with early relapse at both early and later time points (Table S3). In the AALL07P1 study, 54 pre-B patients with early relapse had a 3-year OS of 24%. Those with very early relapse (n=46) the 3-year OS was 17% (p=0.043).
Precursor B-ALL minimal residual disease and outcome
Multiple studies have shown that MRD in relapsed ALL predicts long term outcome following treatment, either with chemotherapy or stem cell transplant (Eckert et al, 2015; Eckert et al, 2013; Paganin et al, 2008). This study measured MRD by flow cytometry, at both cut-offs of <0.1% and <0.01%, after each cycle of chemotherapy. As with similar studies (Raetz et al, 2008a) it was noted that the percentage of patients with MRD below the 0.01% cut-off (MRDneg) increased with each treatment cycle (Figure 2). Only 29% of precursor B-ALL patients less than 21 years of age in morphological remission were MRDneg (<0.01%) at the end of reinduction. Following the second cycle, the MRDneg rate increased to 49% (58% using 0.1% cut-off) and then 64% (75% using 0.1% cut-off) after three blocks of therapy. End-of-induction MRDneg (<0.01%) patients included 10/29 (34%) with very early relapse and 17/39 (43%) with early relapse at the end of the third cycle (p=.45).
Figure 2 Legend:
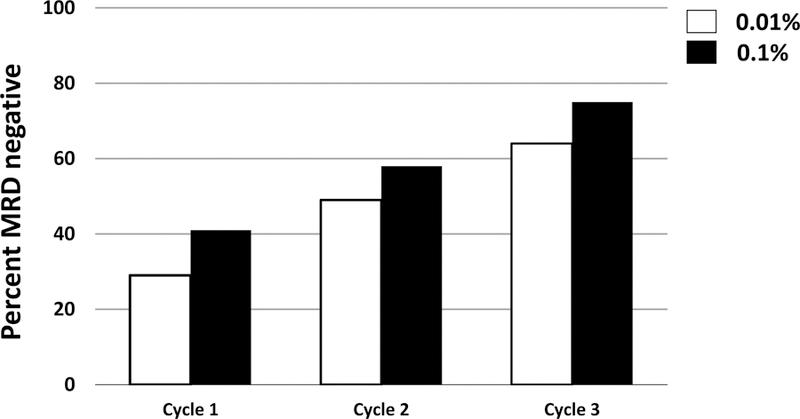
Percentage of patients in complete remission with an MRD from bone marrow below either 0.01% (white bars) or 0.1% (black bars) at the end of reinduction (cycle 1), cycle 2 and cycle 3 of chemo-therapy. Patient number for MRD assessment varied by therapy block (n=88 cycle 1, n-49 cycle 2, n=31 cycle 3). MRD: minimal residual disease
End-of-induction MRD was strongly predictive of EFS for both very early relapse (n=40) and early relapse (n=48) (Figure 3). Three-year EFS was 70% ± 14% in the very early relapse MRDneg patients (n=10), vs. 3% ± 3% in MRDpos patients (n=30) at the end of induction (MRD cut-off 0.01%; p<0.0001) (Figure 3A). For early relapse patients (Figure 3B), the 3-year EFS rates were 58% ± 19% for MRDneg patients (n=17), and 10% ± 9% for MRDpos patients (n=31) (p<0.0001). Similar results were seen in OS (Figures 3C, D). Very early relapse patients attaining MRDneg status had a 3-year OS of 70% ± 19% vs. 0% for MRDpos patients (p=0.0003) and the early relapse patients attaining MRDneg status had an OS of 65% ± 17% vs. 19% ± 17% if MRDpos after induction (p=0.0014).
Figure 3 Legend:



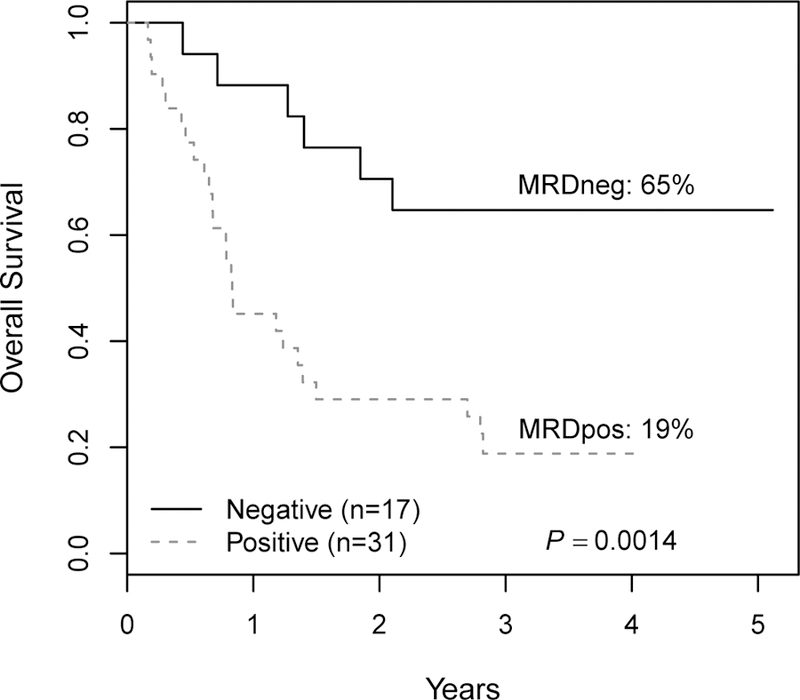
Survival as a function of MRD in pre-B ALL patients less than 21 years of age in early relapse. A: Three-year EFS for patients relapsing <18 months from diagnosis (very early relapse) stratified by MRD status at the end of the first cycle of therapy (reinduction). B: 3-year EFS for patients relapsing 18–36 months from diagnosis (early relapse) stratified by MRD status. C: Three-year overall survival for very early relapse patients stratified by MRD status. D: 3-year EFS for early relapse patients stratified by MRD status. EFS: event-free survival; MRD: minimal residual disease; MRDneg: minimal residual disease negative; MRDpos: minimal residual disease positive.
T-ALL Response, MRD and outcome
The CR2 rate for T-ALL patients in first relapse was 68% ± 10% (15/22 patients). Among the 10 lymphoblastic lymphoma patients on this study, 7 patients had an overall response including CR (n=1), CRu (n=2) and PR (n=4) with this regimen. Two patients who did not achieve a CR after one cycle subsequently attained a CR after 2 treatment cycles.
Our data suggests that MRD can be predictive of survival in patients with T-ALL. Using an MRD cut-off of 0.01%, 3/4 MRDneg patients were alive after 3 years (Figure 4). In contrast, only 7/16 (44%) patients that remained MRDpos at the end of the first cycle of therapy were alive after 3 years. Interestingly, these data suggest that MRD may be less predictive of subsequent treatment failure for T-ALL than for precursor B-ALL treated with bortezomib-containing regimens, because many of the T-ALL patients treated with bortezomib with MRD positivity at the end of induction survived up to 5 years following treatment. They also suggest that bortezomib may have been more effective in T-ALL than in precursor B-ALL.
Figure 4 Legend:


Outcome by MRD status (cut-off 0.01%) in patients with T-ALL. A: Event free survival in MRD negative patients (EFS 75%) and in MRD positive patients (43% until censored). B: Overall survival in MRD negative patients (EFS 67%) vs. MRD positive patients (EFS 43%). ALL: acute lymphoblastic leukaemia; EFS: event-free survival; MRD: minimal residual disease; MRDneg: minimal residual disease negative; MRDpos: minimal residual disease positive.
DISCUSSION
This study determined that the addition of 7 doses of bortezomib to VPLD chemotherapy is safe, and achieved its primary response endpoint within the first 61 patients, achieving an overall CR2 rate of 68% ± 6% in precursor B-ALL patients under 21 years of age. There was a suggestion of increased response rate in very early relapse patients (CR2=64%); however the small sample size in the historical control group (n=16) and a heterogeneous comparator populations between studies are potential explanations for the lack of a statistically significance differences with the addition of bortezomib to reinduction therapy.
Despite encouraging CR2 response rates, outcomes for relapsed leukaemia remains poor, with 3-year EFS of 17%−24%. This study confirmed the predictive power of MRD in relapsed B-ALL, as MRDneg patients had superior survival (58%−70% EFS) compared to MRDpos patients (3%−10% EFS). Finally, for relapsed T-ALL patients, the addition of bortezomib to VPLD resulted in a favourable CR2 rate (68%) compared to very limited data in the historical control trial (5/7 T-ALL patients failed to attain CR2 at the end of block 1 in AALL01P2).
Unique to this study was CR2 assessment stratified by time to relapse in B-ALL patients. Those relapsing very early (<18 months from diagnosis) had a worse CR2 rate and OS than those with an early time to relapse (18–36 months from diagnosis) (Raetz et al 2008a). In this study, the overall CR2 rate for all precursor B-ALL patients under the age of 21 years (n=100) was 68% ± 5% (68/100 patients), with a CR rate of 63% ± 7% in patients with very early relapse (29/46 patients; AALL01P2 CR2 rate 45% without Ph+ patients) and 72% ± 6% in those patients with early relapse (39/54 patients).
Although it is unclear why very early relapse patients might respond better to bortezomib, it is possible that proteasome inhibition interferes with drug resistance mechanisms. Nuclear factor (NF)-κB and immunoproteasomes were examined in this trial (Niewerth et al, 2016). Although the addition of bortezomib was associated with substantial decreases in NF-κB activity and increases in immunoproteasome activity, neither of these factors statistically correlated with outcome. More research is needed to determine the mechanisms of bortezomib action in paediatric ALL.
The CR2 rates in this study are comparable to two prior pilot studies combining bortezomib with similar reinduction regimens. Messinger et al (2012) enrolled 22 patients with pre-B ALL and 14/22 patients achieved a CR2 (64% ± 10%). Similarly, Bertaina et al (2017) enrolled 37 patients, 23 of whom achieved CR2. Although these studies had large confidence intervals due to small sample size, both suggested that bortezomib increased CR2 when combined with standard reinduction. This study expands on their work to suggest that response rate differs by length from diagnosis to relapse, with those at highest risk (<18 months from diagnosis) possibly benefitting most from the addition of proteasome inhibition.
This study had a lower induction mortality than either of the previous studies. In both prior studies (Messinger et al 2012; Bertaina et al 2017) the induction treatment regimens included dexamethasone instead of prednisone, which probably accounted for the differences in treatment-related mortality. Both studies had a higher rate of Grade 4 and 5 infections, including toxic deaths.
One key prognostic factor for sustained CR is the early achievement of MRD negativity. Prior research has shown that patients with evidence of MRD at the end of remission induction are prone to early relapse (Goto, 2015;Coustan-Smith et al, 2003;Eckert et al, 2015;Borowitz et al, 2008). This association is well known in newly diagnosed ALL, where several studies have shown a direct correlation between end-of-induction MRD and EFS (Campana, 2010;Pui et al, 2017;Borowitz et al, 2008;Nyvold et al, 2002). The correlation between MRD and survival in relapsed patients, however, is less clear (Eckert et al, 2013;Paganin et al, 2008). In this study we show that patients with relapsed precursor B-ALL who attain early MRD negativity had significantly improved EFS of 58–70%, with survival rates of <10% in those who remain MRDpos after reinduction. The study reported by Bertaina et al (2017) had similar results for 27 patients, with those attaining MRDneg status having a 68% OS (n=14) vs. 0% OS for patients with MRD+ status after induction (n=13) (MRD cut-off 0.1%). In addition to paediatric ALL patients receiving chemotherapy (Campana, 2010;Pui et al, 2017;Borowitz et al, 2008;Nyvold et al, 2002), it has also been demonstrated that MRD correlates with improved long-term survival in adult ALL patients receiving standard chemotherapy (Jabbour, 2017), inotuzumab (Jabbour, 2017) and blinatumomab (Zugmaier, 2015). This study suggests that MRD response following the first block of therapy is most important in determining overall outcome in first relapse of paediatric ALL, and that extremely few precursor B-ALL patients that were MRDpos at the end of reinduction were long-term survivors regardless of therapy. This is very different to the situation in newly diagnosed ALL, where patients who are MRDpos at the end of induction who become MRDneg with 4–8 weeks of additional therapy have a relatively favourable outcome (Conter et al, 2010;Borowitz et al, 2015). This suggests that relapsed patients not responding to bortezomib within the first cycle will need alternate therapies, such as blinatumomab, inotuzumab or chimeric antigen receptor T-cell therapy (Maloney & Gore, 2018).
Despite the encouraging survival rates in patients with negative MRD following reinduction, the overall outcome for patients with precursor B-ALL in early first relapse remains poor. In this study, overall 3-year EFS was 21% and 3-year OS was 25%, similar to outcomes in previous COG studies (Table IV). In the historical control COG study AALL01P2, patients with very early relapse had a very high risk of relapse and had a subsequently poor outcome, with a 3-year event-free survival (EFS) of only 13% (Raetz et al, 2008a). Patients with early relapse on AALL01P2 had similar outcomes to this study, with a 3-year EFS of 23%. EFS and OS on this study were also similar to those seen in ADVL04P2, a study which added epratuzumab to the standard VPLD reinduction chemotherapy established in AALL01P2; however, there was an increased EFS for those with an early relapse on the epratuzumab study, who had a 3-year OS of 45% (Raetz et al, 2008b). Small sample size is one potential reason why the differences between these studies and their historical control populations did not reach statistical significance. Allogeneic stem cell transplantation as curative therapy following reinduction for those that are MRDneg is also supported by our results; however, two long-term survivors with precursor B-ALL did not undergo stem cell transplant following bortezomib-containing reinduction.
Table IV:
Outcome for very early and early relapse pre-B ALL (<36 months from diagnosis)
| Group | Study period | Very Early Relapse | Early Relapse | ||||
|---|---|---|---|---|---|---|---|
| Patients (n) | 3-year EFS (%) | 3-year OS (%) | Patients (n) | 3-year EFS (%) | 3-year OS (%) | ||
| Nguyen et al (2008) | 1988–2002 | 412 | ND | 12 | 324 | ND | 24 |
| AALL01P2 | 2003–2005 | 15 | 11 | 16 | 44 | 24 | 29 |
| AALL04P2 | 2005–2011 | 42 | 10 | 8 | 72 | 39 | 45 |
| AALL07P1 | 2009–2013 | 49 | 16 | 18 | 61 | 23 | 29 |
Patients with T-ALL responded well to this bortezomib-containing regimen. Our study enrolled 32 patients with T-cell disease (T-ALL and T-LL) and demonstrated a CR2 rate of 68% ± 10% for T-ALL patients. Outcome for patients with T-ALL achieving MRD negative status following reinduction was also favourable (75% ± 37% 3-year EFS and 67% ± 8% 3-year OS). This CR2 rate is an important finding, as most studies have shown a particularly poor outcome for patients with relapsed T-ALL. In the historical control trial (AALL01P2) there were only 2 T-ALL responders in the small number of treated patients with relapsed T-ALL (n=7)( Raetz et al 2008a). The most promising previous T-ALL trials have been in studies including nelarabine, which demonstrated a CR2 rate of 48% in relapsed paediatric patients (n=33) (Berg et al, 2005) and a CR2 rate of 36% in adults as a single agent (Gokbuget et al, 2011). Other agents have had limited response in relapsed T-ALL (Raetz et al, 2008a). Although numbers were very limited, patients with T-cell lymphoblastic lymphoma also showed a response to bortezomib-containing chemotherapy, with 6 of 10 achieving an overall response.
These results, along with other recent studies, suggest that alternative therapies continue to be needed for patients with relapsed or refractory ALL. Plans for moving bortezomib forward in the COG consortium will be prioritized based on the early results of other promising drugs in development for pre-B ALL, including immunotherapies such as blinatumomab, inotuzumab and chimeric-antigen receptor (CAR)-T cell therapy, which have demonstrated particularly promising results in relapsed/refractory B-ALL (Maloney & Gore, 2018;Maude et al, 2014;Maude et al, 2015), and targeted agents (dasatinib and ruxolitinib) which hold promise for certain subgroups of Philadelphia-like ALL. Based on these results, the Therapeutic Advances in Childhood Leukemia and Lymphoma (TACL) consortium is testing the oral proteasome inhibitor ixazomib for relapsed ALL/lymphoma in an upcoming clinical trial (NCT03817320). Based on the promising results of bortezomib in T-ALL, a NCI-sponsored Children’s Oncology Group Phase 3 study, AALL1231 (NCT02112916), is currently randomizing patients with newly-diagnosed T-ALL to standard induction therapy vs. standard therapy with bortezomib.
Supplementary Material
ACKNOWLEDGEMENTS
Gaye Jenkins provided technical research support. TMH was funded in part by NIH-K12-CA90433, NIH K23-CA113775, NIH-R01-CA193776, Texas Children’s Cancer Center, and Hyundai Hope on Wheels Research Award. JAW is the Women’s Auxiliary Millennium Chair in Haematology/ Oncology, The Hospital for Sick Children; EAR is a KiDS of NYU Foundation Professor at NYU Langone Health. SPH is the Jeffrey E. Perelman Distinguished Chair in the Department of Pediatrics, Children’s Hospital of Philadelphia and the St. Baldrick’s Foundation. Research reported in this publication was supported by grants U10 CA98543, U10 CA98413, U10 CA180886, 1U24-CA196173 and U10 CA180899 from the National Institutes of Health, and by St. Baldrick’s Foundation. Bortezomib was kindly supplied by Millennium Pharmaceuticals (now Takeda) through a CRADA with the NIH NCI Cancer Therapy Evaluation Program (CTEP). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Trial Registry: clinicaltrials.gov Identification Number: NCT00873093
Competing Interests: SPH has received honoraria from Jazz Pharmaceuticals and consulting fees from Novartis. JAW has received honoraria from Jazz Pharmaceuticals, Janssen Pharmaceuticals and Shire. TMH has received research funding from Takeda/Millennium Pharmaceuticals.
References
- Berg SL, Blaney SM, Devidas M, Lampkin TA, Murgo A, Bernstein M, Billett A, Kurtzberg J, Reaman G, Gaynon P, Whitlock J, Krailo M, & Harris MB (2005) Phase II study of nelarabine (compound 506U78) in children and young adults with refractory T-cell malignancies: a report from the Children’s Oncology Group. J.Clin.Oncol, 23, 3376–3382. [DOI] [PubMed] [Google Scholar]
- Bertaina A, Vinti L, Strocchio L, Gaspari S, Caruso R, Algeri M, Coletti V, Gurnari C, Romano M, Cefalo MG, Girardi K, Trevisan V, Bertaina V, Merli P, & Locatelli F (2017) The combination of bortezomib with chemotherapy to treat relapsed/refractory acute lymphoblastic leukaemia of childhood. Br.J.Haematol, 176, 629–636. [DOI] [PubMed] [Google Scholar]
- Bhadri VA, Trahair TN, & Lock RB (2012) Glucocorticoid resistance in paediatric acute lymphoblastic leukaemia. J.Paediatr.Child.Health, 48, 634–640. [DOI] [PubMed] [Google Scholar]
- Borowitz MJ, Devidas M, Hunger SP, Bowman WP, Carroll AJ, Carroll WL, Linda S, Martin PL, Pullen DJ, Viswanatha D, Willman CL, Winick N, & Camitta BM (2008) Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: a Children’s Oncology Group study. Blood, 111, 5477–5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowitz MJ, Wood BL, Devidas M, Loh ML, Raetz EA, Salzer WL, Nachman JB, Carroll AJ, Heerema NA, Gastier-Foster JM, Willman CL, Dai Y, Winick NJ, Hunger SP, Carroll WL, & Larsen E (2015) Prognostic significance of minimal residual disease in high risk B-ALL: a report from Children’s Oncology Group study AALL0232. Blood, 126, 964–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campana D (2010) Progress of minimal residual disease studies in childhood acute leukemia. Curr.Hematol.Malig.Rep, 5, 169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colado E, Alvarez-Fernandez S, Maiso P, Martin-Sanchez J, Vidriales MB, Garayoa M, Ocio EM, Montero JC, Pandiella A, & San Miguel JF (2008) The effect of the proteasome inhibitor bortezomib on acute myeloid leukemia cells and drug resistance associated with the CD34+ immature phenotype. Haematologica, 93, 57–66. [DOI] [PubMed] [Google Scholar]
- Conter V, Bartram CR, Valsecchi MG, Schrauder A, Panzer-Grumayer R, Moricke A, Arico M, Zimmermann M, Mann G, De RG, Stanulla M, Locatelli F, Basso G, Niggli F, Barisone E, Henze G, Ludwig WD, Haas OA, Cazzaniga G, Koehler R, Silvestri D, Bradtke J, Parasole R, Beier R, van Dongen JJ, Biondi A, & Schrappe M (2010) Molecular response to treatment redefines all prognostic factors in children and adolescents with B-cell precursor acute lymphoblastic leukemia: results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood, 115, 3206–3214. [DOI] [PubMed] [Google Scholar]
- Coustan-Smith E, Ribeiro RC, Rubnitz JE, Razzouk BI, Pui CH, Pounds S, Andreansky M, Behm FG, Raimondi SC, Shurtleff SA, Downing JR, & Campana D (2003) Clinical significance of residual disease during treatment in childhood acute myeloid leukaemia. Br.J.Haematol, 123, 243–252. [DOI] [PubMed] [Google Scholar]
- Eckert C, Henze G, Seeger K, Hagedorn N, Mann G, Panzer-Grumayer R, Peters C, Klingebiel T, Borkhardt A, Schrappe M, Schrauder A, Escherich G, Sramkova L, Niggli F, Hitzler J, & von Stackelberg A (2013) Use of allogeneic hematopoietic stem-cell transplantation based on minimal residual disease response improves outcomes for children with relapsed acute lymphoblastic leukemia in the intermediate-risk group. J.Clin.Oncol, 31, 2736–2742. [DOI] [PubMed] [Google Scholar]
- Eckert C, Hagedorn N, Sramkova L, Mann G, Panzer-Grumayer R, Peters C, Bourquin JP, Klingebiel T, Borkhardt A, Cario G, Alten J, Escherich G, Astrahantseff K, Seeger K, Henze G, & von Stackelberg A (2015) Monitoring minimal residual disease in children with high-risk relapses of acute lymphoblastic leukemia: prognostic relevance of early and late assessment. Leukemia, 29, 1648–1655. [DOI] [PubMed] [Google Scholar]
- Freyer DR, Devidas M, La M, Carroll WL, Gaynon PS, Hunger SP, & Seibel NL (2011) Postrelapse survival in childhood acute lymphoblastic leukemia is independent of initial treatment intensity: a report from the Children’s Oncology Group. Blood, 117, 3010–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynon PS & Sun W (2016) Oligoclonality and new agent evaluation in acute lymphoblastic leukaemia. Br.J.Haematol, 173, 950–957. [DOI] [PubMed] [Google Scholar]
- Gokbuget N, Basara N, Baurmann H, Beck J, Bruggemann M, Diedrich H, Guldenzoph B, Hartung G, Horst HA, Huttmann A, Kobbe G, Naumann R, Ratei R, Reichle A, Serve H, Stelljes M, Viardot A, Wattad M, & Hoelzer D (2011) High single-drug activity of nelarabine in relapsed T-lymphoblastic leukemia/lymphoma offers curative option with subsequent stem cell transplantation. Blood, 118, 3504–3511. [DOI] [PubMed] [Google Scholar]
- Goto H (2015) Childhood relapsed acute lymphoblastic leukemia: Biology and recent treatment progress. Pediatr.Int, 57, 1059–1066. [DOI] [PubMed] [Google Scholar]
- Hanley MJ, Mould DR, Taylor TJ, Gupta N, Suryanarayan K, Neuwirth R, Esseltine DL, Horton TM, Aplenc R, Alonzo TA, Lu X, Milton A, & Venkatakrishnan K (2017) Population Pharmacokinetic Analysis of Bortezomib in Pediatric Leukemia Patients: Model-Based Support for Body Surface Area-Based Dosing Over the 2- to 16-Year Age Range. J.Clin.Pharmacol, 57, 1183–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton TM, Gannavarapu A, Blaney SM, D’Argenio DZ, Plon SE, & Berg SL (2006) Bortezomib interactions with chemotherapy agents in acute leukemia in vitro. Cancer Chemother.Pharmacol, 58, 13–23. [DOI] [PubMed] [Google Scholar]
- Horton TM, Pati D, Plon SE, Thompson PA, Bomgaars LR, Adamson PC, Ingle AM, Wright JJ, Brockman AH, Paton M, & Blaney S (2007) A Phase 1 Study of the Proteasome Inhibitor Bortezomib in Pediatric Patients with Refractory Leukemia: a Children’s Oncology Group Study. Clin Cancer Res, 13, 1516–1522. [DOI] [PubMed] [Google Scholar]
- Horton TM, Sposto R, Brown P, Reynolds CP, Hunger SP, Winick NJ, Raetz EA, Carroll WL, Arceci RJ, Borowitz MJ, Gaynon PS, Gore L, Jeha S, Maurer BJ, Siegel SE, Biondi A, Kearns PR, Narendran A, Silverman LB, Smith MA, Zwaan CM, & Whitlock JA (2010) Toxicity assessment of molecularly targeted drugs incorporated into multiagent chemotherapy regimens for pediatric acute lymphocytic leukemia (ALL): Review from an international consensus conference. Pediatr.Blood Cancer, 54, 872–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton TM, Perentesis JM, Gamis AS, Alonzo TA, Gerbing RB, Ballard J, Adlard K, Howard DS, Smith FO, & Moscow JA (2014) A phase 2 study of bortezomib combined with reinduction chemotherapy in children and young adults with recurrent, refractory or secondary acute myeloid leukemia: A Children’s Oncology Group (COG) study. Pediatr.Blood Cancer, 61, 1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton PJ, Morton CL, Kolb EA, Lock R, Carol H, Reynolds CP, Keshelava N, Maris JM, Keir ST, Wu J, & Smith MA (2008) Initial testing (stage 1) of the proteasome inhibitor bortezomib by the pediatric preclinical testing program. Pediatr.Blood Cancer, 50, 37–45. [DOI] [PubMed] [Google Scholar]
- Hunger SP & Mullighan CG (2015) Acute Lymphoblastic Leukemia in Children. N.Engl.J.Med, 373, 1541–1552. [DOI] [PubMed] [Google Scholar]
- Jabbour EE (2017) Differential impact of minimal residual disease negativity according to the salvage status in patients with relapsed/refractory B-cell acute lymphoblastic leukemia. Cancer, 123, 294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan EL & Meier P (2017) Nonparametric estimation from incomplete observations. American Statistical Association, 53, 457–481. [Google Scholar]
- London WB & Chang MN (2005) One- and two-stage designs for stratified phase II clinical trials. Stat.Med, 24, 2597–2611. [DOI] [PubMed] [Google Scholar]
- Maloney KW & Gore L (2018) Agents in Development for Childhood Acute Lymphoblastic Leukemia. Paediatr.Drugs, 20, 111–120. [DOI] [PubMed] [Google Scholar]
- Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, Mahnke YD, Melenhorst JJ, Rheingold SR, Shen A, Teachey DT, Levine BL, June CH, Porter DL, & Grupp SA (2014) Chimeric antigen receptor T cells for sustained remissions in leukemia. N.Engl.J.Med, 371, 1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maude SL, Teachey DT, Porter DL, & Grupp SA (2015) CD19-targeted chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Blood, 125, 4017–4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messinger Y, Gaynon P, Raetz E, Hutchinson R, Dubois S, Glade-Bender J, Sposto R, van der Giessen J, Eckroth E, & Bostrom BC (2010) Phase I study of bortezomib combined with chemotherapy in children with relapsed childhood acute lymphoblastic leukemia (ALL): a report from the therapeutic advances in childhood leukemia (TACL) consortium. Pediatr.Blood Cancer, 55, 254–259. [DOI] [PubMed] [Google Scholar]
- Messinger YH, Gaynon PS, Sposto R, van der Giessen J, Eckroth E, Malvar J, & Bostrom BC (2012) Bortezomib with chemotherapy is highly active in advanced B-precursor acute lymphoblastic leukemia: Therapeutic Advances in Childhood Leukemia & Lymphoma (TACL) Study. Blood, 120, 285–290. [DOI] [PubMed] [Google Scholar]
- Minderman H, Zhou Y, O’loughlin KL, & Baer MR (2007) Bortezomib activity and in vitro interactions with anthracyclines and cytarabine in acute myeloid leukemia cells are independent of multidrug resistance mechanisms and p53 status. Cancer Chemother.Pharmacol, 60, 245–255. [DOI] [PubMed] [Google Scholar]
- Nguyen K, Devidas M, Cheng SC, La M, Raetz EA, Carroll WL, Winick NJ, Hunger SP, Gaynon PS & Loh ML (2008) Factors influencing survival after relapse from acute lymphoblastic leukemia: A Children’s Oncology Group study. Leukemia 2008. 22: 2142–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewerth D, Kaspers GJ, Jansen G, van MJ, Zweegman S, Jenkins G, Whitlock JA, Hunger SP, Lu X, Alonzo TA, van de Ven PM, Horton TM, & Cloos J (2016) Proteasome subunit expression analysis and chemosensitivity in relapsed paediatric acute leukaemia patients receiving bortezomib-containing chemotherapy. J.Hematol.Oncol, 9, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyvold C, Madsen HO, Ryder LP, Seyfarth J, Svejgaard A, Clausen N, Wesenberg F, Jonsson OG, Forestier E, & Schmiegelow K (2002) Precise quantification of minimal residual disease at day 29 allows identification of children with acute lymphoblastic leukemia and an excellent outcome. Blood, 99, 1253–1258. [DOI] [PubMed] [Google Scholar]
- Oskarsson T, Soderhall S, Arvidson J, Forestier E, Montgomery S, Bottai M, Lausen B, Carlsen N, Hellebostad M, Lahteenmaki P, Saarinen-Pihkala UM, Jonsson OG, & Heyman M (2016) Relapsed childhood acute lymphoblastic leukemia in the Nordic countries: prognostic factors, treatment and outcome. Haematologica, 101, 68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paganin M, Zecca M, Fabbri G, Polato K, Biondi A, Rizzari C, Locatelli F, & Basso G (2008) Minimal residual disease is an important predictive factor of outcome in children with relapsed ‘high-risk’ acute lymphoblastic leukemia. Leukemia, 22, 2193–2200. [DOI] [PubMed] [Google Scholar]
- Peto R & Peto J (1972) Asymptotically efficient rank invariant test procedures. Journal of the Royal Statistical Society, Series A, 135, 185–207. [Google Scholar]
- Pui CH, Carroll WL, Meshinchi S, & Arceci RJ (2011) Biology, Risk Stratification, and Therapy of Pediatric Acute Leukemias: An Update. J Clin Oncol, 29, 551–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pui C-H, Pei D, Raimondi SC, Coustan-Smith E, Jeha S, Cheng C, Bowman WP, Sandlund JT, Ribeiro RC, Rubnitz JE, Inaba H, Gruber TA, Leung WH, Yang JJ, Downing JR, Evans WE, Relling MV, Campana D. (2017) Clinical impact of minimal residual disease in children with different subtypes of acute lymphoblastic leukemia treated with Response-Adapted therapy. Leukemia, 31, 333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz EA, Borowitz MJ, Devidas M, Linda SB, Hunger SP, Winick NJ, Camitta BM, Gaynon PS, & Carroll WL (2008a) Reinduction platform for children with first marrow relapse of acute lymphoblastic Leukemia: A Children’s Oncology Group Study[corrected]. J.Clin.Oncol, 26, 3971–3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz EA, Cairo MS, Borowitz MJ, Blaney SM, Krailo MD, Leil TA, Reid JM, Goldenberg DM, Wegener WA, Carroll WL, & Adamson PC (2008b) Chemoimmunotherapy reinduction with epratuzumab in children with acute lymphoblastic leukemia in marrow relapse: a Children’s Oncology Group Pilot Study. J.Clin.Oncol, 26, 3756–3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson PG, Hideshima T, Mitsiades C, & Anderson K (2004) Proteasome inhibition in hematologic malignancies. Ann.Med, 36, 304–314. [DOI] [PubMed] [Google Scholar]
- Teachey DT & Hunger SP (2013) Predicting relapse risk in childhood acute lymphoblastic leukaemia. Br.J.Haematol, 162, 606–620. [DOI] [PubMed] [Google Scholar]
- Zugmaier GG (2015) Long-term survival and T-cell kinetics in relapsed/refractory ALL patients who achieved MRD response after blinatumomab treatment. Blood, 126, 2578–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


