Abstract
The BCL-2 family plays important roles in acute myeloid leukemia (AML). Venetoclax, a selective BCL-2 inhibitor, has received FDA approval for the treatment of AML. However, drug resistance ensues after prolonged treatment, highlighting the need for a greater understanding of the underlying mechanisms. Using a genome-wide CRISPR/Cas9 screen in human AML, we identified genes whose inactivation sensitizes AML blasts to Venetoclax. Genes involved in mitochondrial organization and function were significantly depleted throughout our screen, including the mitochondrial chaperonin CLPB. We demonstrated that CLPB is upregulated in human AML, it is further induced upon acquisition of Venetoclax resistance and its ablation sensitizes AML to Venetoclax. Mechanistically, CLPB maintains the mitochondrial cristae structure via its interaction with the cristae-shaping protein OPA1, whereas its loss promotes apoptosis by inducing cristae remodeling and mitochondrial stress responses. Overall, our data suggest that targeting mitochondrial architecture may provide a promising approach to circumvent Venetoclax resistance.
Introduction
Acute Myeloid Leukemia (AML) is a hematopoietic neoplasm characterized by the proliferation and accumulation of aberrant immature myeloid progenitor cells. AML is associated with poor clinical outcome and high mortality, with an overall five-year survival rate of less than 15–30%. For AML patients, standard therapies often fail to achieve the complete remission, disease relapse is often fatal and salvage and bone marrow transplantation are only applicable to a few select AML patients, highlighting the need for novel targeted treatments. A number of such emerging treatments have targeted essential “hallmarks” of cancer, including the regulation of cancer cell survival by members of the BCL-2 protein family. Among these members, B-cell lymphoma 2 (BCL-2) is found upregulated in AML cells (1) and, specifically, in leukemic stem cells (LSC) (2). BCL-2 overexpression is a poor-risk factor in AML and is associated with poor response to standard cytotoxic therapy (1, 3).
Mechanistically, BCL-2 is the founding member of the BCL-2 protein family, and prevents programmed cell death by binding to proapoptotic members of the same family (4). Following a death signal, the proapoptotic factors are released from BCL-2 and induce the oligomerization of BAX and BAK on the outer mitochondrial membrane (OMM) (5). This is a crucial step for the release of cytochrome c from mitochondria to the cytosol where, together with dATP, APAF1 and caspase 9, it forms the apoptosome, a complex that subsequently activates the executioner caspases and thus apoptosis (6, 7).
In cancer cells, enhanced BCL-2 expression supports cell survival, as it leads to the suppression of mitochondrial-mediated apoptosis. Inhibiting BCL-2 via a selective BH3 mimetic such as Venetoclax has proven to be an efficient strategy to promote caspase-dependent cell death in AML (8). Venetoclax is an orally bioavailable drug that is approved for Chronic Lymphocytic Leukemia (CLL) and other hematological malignancies. Venetoclax recently received FDA approval for the treatment of newly-diagnosed elderly AML patients in combination with hypomethylating agents (9) (azacitidine or decitabine) as proposed by our group (10, 11) or with low dose cytarabine (LDAC). However, approximately 30% of patients do not respond upfront and many AML patients still develop resistance while on treatment (12). This highlights the need for a greater mechanistic understanding of Venetoclax resistance, both in combination and as monotherapy. To that end, multiple combinational therapies for Venetoclax have been proposed, including CDK (CDK9) and MCL-1 inhibition as we and others have suggested (13–15), with several of these combinations having entered clinical trials (16).
To shed light on the mechanisms of resistance to Venetoclax, and to propose novel Venetoclax treatment combinations, we performed a genome-wide CRISPR/Cas9 loss-of-function screen in human AML cells in the presence or absence of Venetoclax and identified CLPB as one of the top candidates whose ablation sensitizes AML cells to the drug treatment. We show that the mitochondrial protein CLPB is upregulated in AML patients and protects AML cells against caspase-dependent apoptosis and mitochondrial dysfunction. In particular, we demonstrate that CLPB is essential for sustaining the correct mitochondrial cristae morphology by its direct interaction with OPA1, the master regulator of mitochondrial dynamics. Moreover, CLPB deficiency leads to mitochondrial dysfunction which causes ATF4-mediated mitochondrial stress responses and alterations at the cell transcriptome and metabolome. Therefore, depleting CLPB sensitizes AML cells to Venetoclax-induced programmed cell death in vitro and in vivo. Our work suggests that targeting mitochondrial structure could be a promising strategy to overcome Venetoclax resistance in AML patients.
Results
CRISPR screen identifies synthetic lethal vulnerabilities for Venetoclax in AML
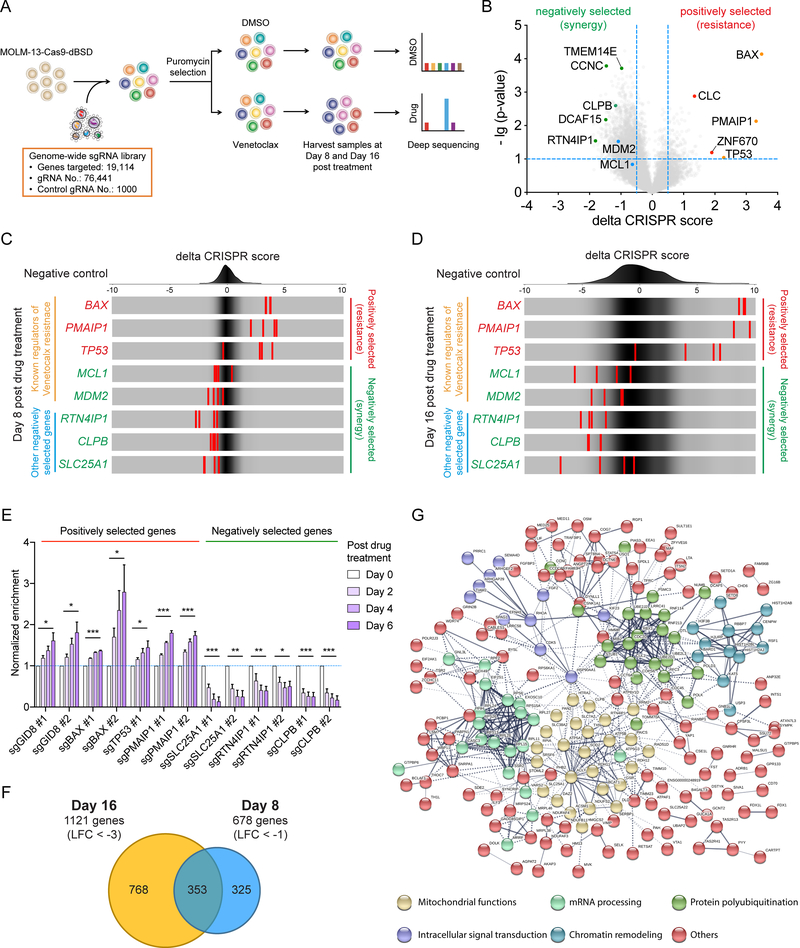
To systematically identify key factors that regulate Venetoclax resistance in human AML, we performed a genome-wide CRISPR/Cas9 loss-of-function screen (17). We transduced MOLM-13 AML cells with the Brunello sgRNA library (18), cultured them in the presence of Venetoclax or DMSO for 16 days, and sequenced the distribution of sgRNAs at day 8 and day 16 post-drug treatment (Fig. 1A; Supplementary Fig. S1A). Our strategy ensured that genes that confer resistance to or synergize with Venetoclax would be positively or negatively selected, respectively, following 8 and 16 days of drug treatment compared to DMSO treatment (Fig. 1B, C and D; Supplementary Fig. S1B and Supplementary Table 1). BAX and PMAIP1 were among the positively selected genes (genes that their loss confers resistance), a finding consistent with the mechanism of action of the drug (14, 19, 20). Interestingly, the tumor suppressor p53 (TP53) was also positively selected in the screen, in agreement with recent studies (15), as well as the accompanying CRISPR screen performed by Tyner and colleagues. MDM2, encoding an E3 ubiquitin ligase that targets p53 for degradation (15, 21) and MCL1, a primary mode of resistance to Venetoclax (13, 22) were amongst the negatively selected genes (genes that their loss synergizes with Venetoclax) at both day 8 and day 16 of drug treatment (Fig. 1B, C and D; Supplementary Fig. S1B). We further validated a number of positively and negatively selected genes (GID8, BAX, TP53, PMAIP1, SLC25A1, RTN4IP1, CLPB) throughout the screen by selecting the top sgRNAs for each gene and monitoring their ability to confer resistance to or synergize with Venetoclax in AML cells performing a competition-based assay (Supplementary Fig. S1C). Our results confirmed that all the sgRNAs targeting the positively selected genes demonstrated significant resistance to Venetoclax, while the sgRNAs targeting the negatively selected genes strongly sensitized MOLM-13 cells to the drug treatment (Fig. 1E and Supplementary Fig. S1D). Likewise, a significant increase of half maximal inhibitory concentration (IC50) in KASUMI-1 and MOLM-13 cells upon deletion of BAX and PMAIP1 validated the gain of resistance of Venetoclax in BAX- and PMAIP1-deficient AML cells. Notably, TP53 sgRNAs increased the Venetoclax IC50 in p53 wild-type MOLM-13 but not p53-mutant KASUMI-1, confirming the specificity of the guides used in this study (Supplementary Fig. S1E and F). Together, our genome-wide CRISPR/Cas9 loss-of-function screen successfully revealed potential modes of resistance to Venetoclax as well as synthetic lethal partners in AML.
Figure 1. Genome-wide CRISPR screen identifies genes controlling mitochondrial physiology as synthetic lethal with Venetoclax treatment in AML.
A. Schematic outline of the viability-based, genome wide CRISPR/Cas9 loss-of-function screen.
B. Volcano plot showing both positively and negatively selected genes in the CRISPR screen at day 8 post drug treatment. A number of positively and negatively selected genes are shown in red and green, respectively. Known regulators of Venetoclax resistance are shown in orange (positively selected) and blue (negatively selected), respectively.
C-D. Frequency histograms of the delta CRISPR score of the negative control guides (top), and selected genes at day 8 (C) and day 16 (D) post drug treatment.
E. Validation of selected genes in the CRISPR screen using a competition-based survival assay in MOLM-13. The normalized enrichment scores were calculated as shown in supplementary Fig. S1C. Data represent mean ± SEM (n=4 for each sgRNA).
F. Venn diagram of the negatively selected genes (“sensitizers”) in the CRISPR screen at day 8 (Log fold change < −1) and day 16 (Log fold change < −3) post drug treatment.
G. STRING protein–protein interaction network of the 353 common negatively selected genes as defined in (F). The minimum required interaction score was set to 0.5, and the disconnected dots were removed. k-means clustering was applied with the number of clusters set to 6.
Next, we aimed to identify key pathways and biological processes that are enriched in our screen. We performed Gene Ontology (GO) analysis focusing firstly on positively selected genes that were at least 8-fold (LFC > 3) enriched. The majority of these genes significantly clustered into key biological processes categories that regulate the intrinsic apoptotic signaling pathway, including cytochrome c release and mitochondrial outer membrane permeabilization (Supplementary Fig. S1G). STRING protein-protein interaction network analysis was then performed using the positively selected genes at both day 8 and day 16 of Venetoclax treatment (Supplementary Fig. S1H). One of the major clusters from this network was the p53-mediated apoptotic signaling pathway in mitochondria (Supplementary Fig. S1I). We were particularly interested in the negatively selected genes, as they are potential therapeutic targets for circumventing Venetoclax resistance. Gene Ontology analysis of the “sensitizer” genes revealed strong enrichment for mitochondrial processes, such as mitochondrial transport, organization and oxidative phosphorylation (Supplementary Fig. S1J). Moreover, when we combined the two time-points of our screen, we successfully identified 353 common negatively selected genes (Fig. 1F). Among these, 55 were genes that encode proteins functioning in mitochondria, highlighting the relevance of mitochondrial processes in the sensitization of AML cells to Venetoclax. Consistently, mapping this 353 gene-set on the STRING database generated a highly connected network consisting mainly of genes that regulate mitochondrial functions, as well as chromatin remodelers, ubiquitin ligases, signal transducers and components of mRNA processing (Fig. 1G). Therefore, our genome-wide CRISPR/Cas9 loss-of-function screen identified mitochondrial processes as key potential synthetic lethal vulnerabilities for Venetoclax in AML.
Venetoclax treatment leads to aberrant mitochondrial structure and depolarization in AML
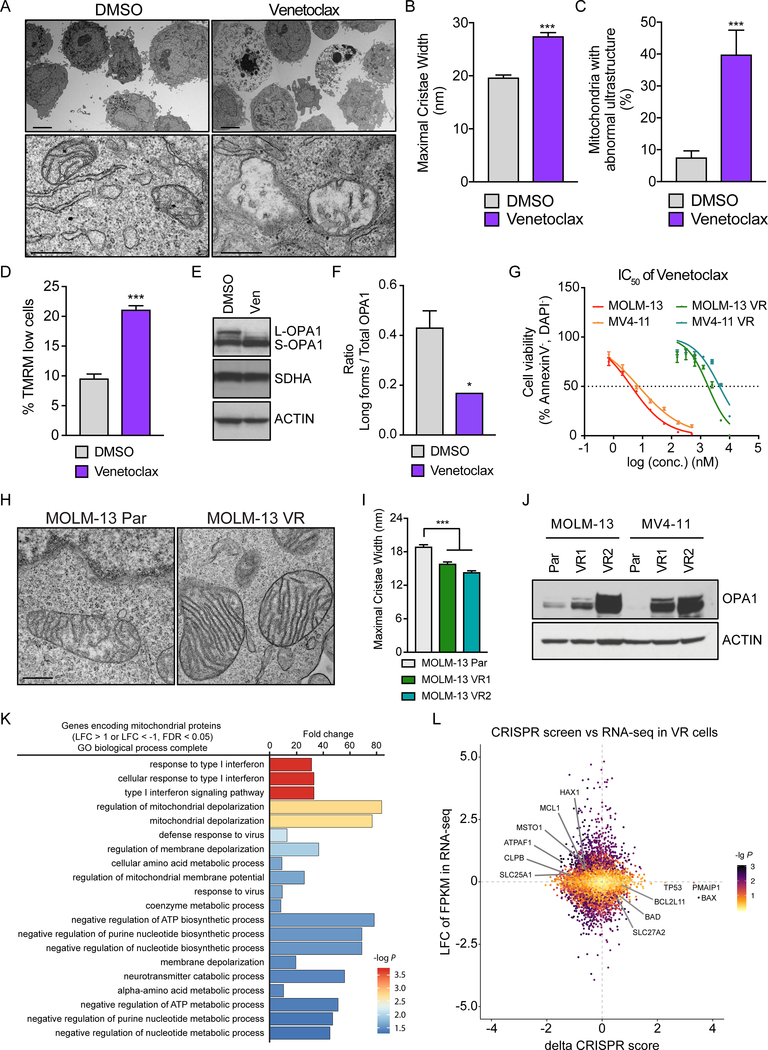
Given that Venetoclax action converges on mitochondria which actively participate in programmed cell death, our screen results intrigued us to further investigate how BCL-2 inhibition impacts mitochondrial structure and function in AML. Mitochondria change their shape early during the process of apoptosis; the individual inner membrane lamellae, named cristae, fuse and the opposing faces of the cristae membrane (cristae junctions) open, allowing the redistribution of cytochrome c from the cristae lumen to the intermembrane space (23, 24). To determine the mitochondrial structure alterations in AML, we performed electron microscopy-based morphometric analysis of treated AML cells (THP-1 and MOLM-13) with Venetoclax. Indeed, mitochondria exhibit abnormal ultrastructure after Venetoclax treatment, characterized by a lower number of cristae and increase of the cristae lumen width (Fig. 2A–C, Supplementary Fig. S2A–C). The normal mitochondrial structure is crucial for the maintenance of the mitochondrial membrane potential, which is the driving force behind ATP production and mitochondrial homeostasis. Early during apoptosis, the mitochondrial membrane potential collapses leading to the complete cytochrome c release from mitochondria to the cytosol (25). We therefore predicted that Venetoclax, as an inducer of apoptosis, causes loss of mitochondrial membrane potential in leukemic cells. Indeed, staining of AML cells with the cell-permeant, cationic fluorescent dye, tetramethylrhodamine methyl-ester (TMRM) revealed depolarization of mitochondria after treatment with the BCL-2 inhibitor (Fig. 2D, Supplementary Fig. S2D). To further investigate how Venetoclax treatment may be causing widening of the cristae, we turned to the mitochondrial protein Optic Atrophy 1 (OPA1), which functions as a molecular staple at cristae junctions to prevent cytochrome c mobilization and release (26). In physiological conditions, membrane-associated (long) and soluble, cleaved (short) forms of OPA1 coexist to form oligomers and complexes that contribute to cristae maintenance. Upon an intrinsic death stimulus, OPA1 proteolytical cleavage is enhanced allowing the “opening” of the cristae junctions and the cytochrome c redistribution (25). We observed a decrease in the long (L-OPA1) forms and accumulation of short (S-OPA1) forms of OPA1 in response to Venetoclax treatment, suggesting its proteolysis following BCL-2 inhibition (Fig. 2E and F). Venetoclax treatment also led to caspase activation, as indicated by caspase-3 cleavage (Supplementary Fig. S2E) and subsequently cell death, as demonstrated using Annexin-V staining (Supplementary Fig. S2F).
Figure 2. Mitochondrial response upon Venetoclax treatment and after acquisition of drug resistance.
A. Representative electron micrographs of THP-1 treated with 4 μM Venetoclax or DMSO for 16 hrs. Scale bars represent 5 μm (upper panel) and 0.5 μm (lower panel).
B. Quantification of the maximal cristae width in 60 randomly selected mitochondria from 15 cells in experiments as in (A) (n= 200 cristae per condition). Data represent mean ± SEM of 3 independent experiments.
C. Quantification of the percentage of mitochondria with abnormal ultrastructure in experiments as in (A) (n= 100 mitochondria per condition). Data represent mean ± SEM of 3 independent experiments.
D. Quantification of the membrane potential loss after staining with TMRM in THP-1 cells treated with 4 μM Venetoclax or DMSO for 16 hrs. Data represent mean ± SD (n=3).
E-F. THP-1 were treated with 4 μM Venetoclax for 16 hrs and equal amounts (30 μg) of cell lysates were separated by SDS-PAGE and immunoblotted using the indicated antibodies (E). L-OPA1, long forms of OPA1; S-OPA1, short forms of OPA1. The bar plot (F) shows the quantitative densitometric analysis of the ratio of long OPA1 forms to the total OPA1. Data represent mean ± SEM of 3 independent experiments.
G. IC50 curves of Venetoclax in parental or Venetoclax-resistant (VR) AML cell lines. Data represent mean ± SD (n=3 for each cell line).
H. Representative electron micrographs of mitochondria from parental (Par.) or Venetoclax-resistant (VR) MOLM-13. Scale bar represents 0.5 μm.
I. Quantification of the maximal cristae width in 60 randomly selected mitochondria from 15 cells in experiment as in (H) (n= 282 cristae per condition). Data represent mean ± SEM.
J. Western blot analysis in cell lysates from parental (Par.) or Venetoclax-resistant (VR) AML cell lines.
K. Gene Ontology analysis of differentially expressed genes involved in mitochondrial processes in Venetoclax-resistant cells (MOLM-13 VR1) compared to the parental cell line (Log fold change > 1 or < −1, FDR < 0.05).
L. Scatter plot presenting the delta CRISPR score (Fig. 1B) plotted against the Log fold change from MOLM-13 VR RNA-seq (supplementary Fig. S3A). Colors correspond to the common -log (p-value) which was generated using the geometric mean of the CRISPR screen p-value and the RNA-Seq FDR. Selected genes are highlighted.
Data with statistical significance are as indicated, *p< 0.05, ***p< 0.001.
Venetoclax resistance is coupled to OPA1 overexpression and tighter mitochondrial cristae
To further investigate the mechanisms of Venetoclax resistance, we generated four human AML clones highly resistant to the drug, by growing the parental MOLM-13 and MV4–11 cells in increasing doses of the drug for over eight weeks. IC50 analysis verified more than 100-fold increase of Venetoclax concentration required for the 50% cell death in the Venetoclax-resistant (VR) cell lines in respect to the original clones (Par.) (Fig. 2G). Driven by the role of BCL-2 inhibition in mitochondrial architecture, we aimed to closely examine the organelle’s structure in the Venetoclax-resistant AML cells. We discovered that Venetoclax-resistant AML cells exhibit tighter cristae (approximately 14–15 nm in diameter) and a higher number of cristae per mitochondrion than the parental sensitive clones (Fig. 2H and I, Supplementary Fig. S2G–I). This phenotype could be explained by the significant induction of OPA1 protein expression in the AML Venetoclax-resistant cell lines (Fig. 2J and Supplementary Fig. S2J). Notably, this is not simply the result of a general increase of mitochondrial biogenesis, as the mitochondrial protein TOM20 does not display similar expression changes (Supplementary Fig. S2K). These data suggested that mitochondria change their shape in Venetoclax-resistant AML cells, most likely to antagonize the cell intrinsic apoptotic signaling cascade initiated by BCL-2 inhibition.
Since MCL-1 upregulation has been previously implicated in the development of Venetoclax-resistance (12), we aimed to examine the protein levels of the major BCL-2 family members (MCL-1, BCL-2 and BCL-XL) in the generated Venetoclax-resistant cell lines. We observed a slight increase of MCL-1 and BCL-2 protein levels in MOLM-13-VR cells. Also, we found no increase of neither BCL-XL protein nor any of the examined BH3 anti-apoptotic proteins in the MV4–11-VR cell lines. These results suggest that BCL-2 and MCL-1 upregulation may serve as one of the potential mechanisms of cellular resistance to Venetoclax, however at least in AML, it appears to exist alternative modes of resistance acquisition. (Supplementary Fig. S2L). In agreement with that notion, and based on the generated resistant AML lines, OPA1 upregulation and mitochondrial structural adaptations appear to represent a more universal mechanism of Venetoclax resistance.
To further explore the molecular mechanisms underpinning Venetoclax resistance, RNA-sequencing (RNA-Seq) was performed. Indeed, the transcriptional landscape of the AML cells changed significantly upon acquisition of resistance to Venetoclax (Supplementary Fig. S3A–B). Interestingly, among the differentially expressed genes, there were many involved in various mitochondrial processes. We focused on such genes (“Genes encoding mitochondrial proteins”, using the MitoMiner dataset; Supplementary Fig. S3A–B) and performed GO analysis to dissect the functional changes in mitochondria upon acquisition of Venetoclax resistance. Strikingly, pathways that regulate mitochondrial membrane organization, potential and depolarization were strongly enriched in both MOLM-13-VR and MV4–11-VR, though strong variances were observed at global transcriptomic level in these cell lines (Fig. 2K, Supplementary Fig. S3C–D), which is consistent with our findings that mitochondrial structure adaptation is universally required for gain of Venetoclax resistance in AML cells. Besides, metabolic processes of key cellular components, like amino acids, coenzyme, ATP and nucleotides were also enriched within the top 20 enriched pathways, suggesting the metabolic adaptation is likewise important for Venetoclax resistance. Finally, we observed enhanced interferon responses in MOLM-13-VR cells, which might be a result of mtDNA release to the cytosol as a consequence of incomplete apoptosis and partial mitochondrial dysfunction during the persistent Venetoclax treatment (27) (Fig. 2K, Supplementary Fig. S3D). Overall, these data suggested that AML blasts require transcriptional and post-translational mitochondrial adaptation to acquire resistance to Venetoclax.
Targeting the mitochondrial protein CLPB overcomes Venetoclax resistance in AML
We next attempted to determine whether there is a correlation between genes differentially expressed in the resistant cell lines and genes-hits in our CRISPR screen. We initially focused on the downregulated genes in MOLM-13-VR cells compared to MOLM-13-Par cells, whose ablation confers resistance to Venetoclax (positively selected genes). We identified the validated genes TP53, BAX, PMAIP1, as well as the pro-apoptotic genes BAD and BCL2L11 (also known as BIM), and SLC27A2, a gene involved in fatty acid metabolism. Moreover, we also focused on the upregulated genes in MOLM-13-VR cells compared to MOLM-13-Par cells, whose ablation sensitizes cells to the drug (negatively selected genes). We identified genes encoding the mitochondrial proteins CLPB and SLC25A1, as well as anti-apoptotic genes like MCL-1 and HAX1. We also found ATPAF1 and MSTO1 whose products are involved in the maintenance of mitochondrial structure and functions (Fig. 2L).
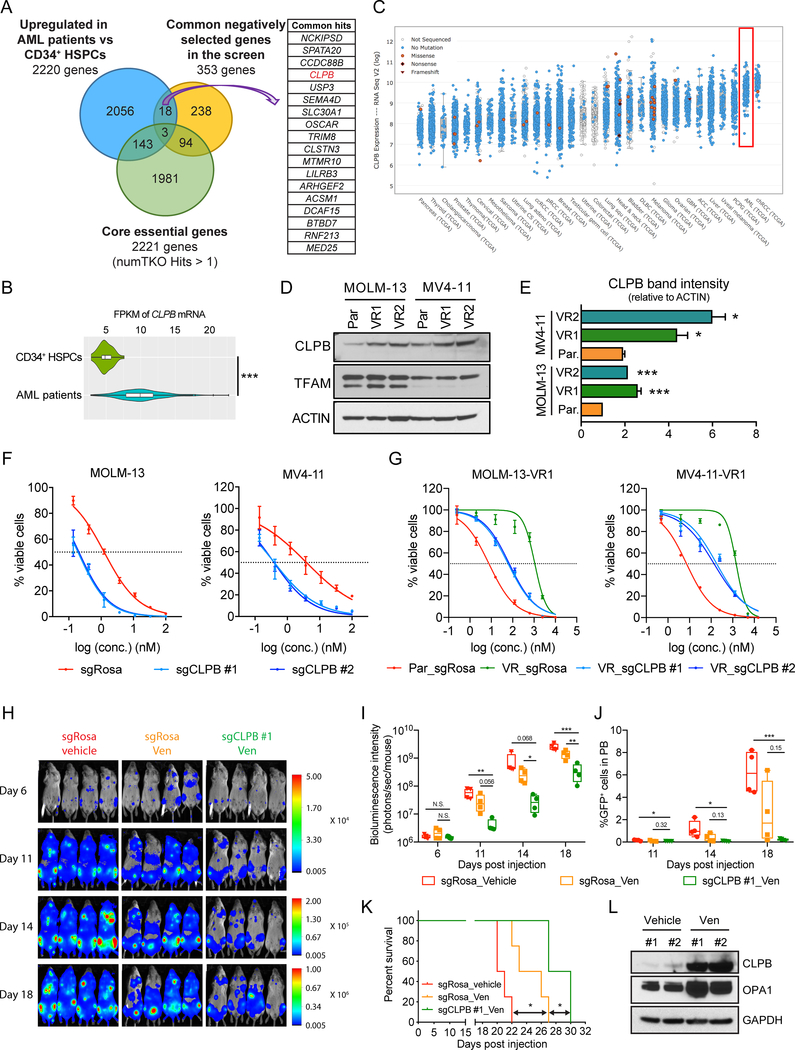
Driven by these findings we hypothesized that targeting proteins regulating mitochondrial cristae maintenance and function might open up potential synthetic lethal vulnerabilities for Venetoclax treatment. To further refine the candidate list from the screen, we excluded 97 core cellular fitness genes (17), genes generally required for all cell types and therefore may not serve as ideal future therapeutic targets. From the remaining 256 genes, we focused on genes that displayed higher mRNA expression levels in AML patient samples compared to healthy primary CD34+ hematopoietic stem and progenitor cells (HSPCs) according to The Cancer Genome Atlas (TCGA). After applying these criteria, we identified 18 candidates (Fig. 3A). One of the top-scoring candidates was CLPB, that encodes a mitochondrial AAA+ ATPase chaperonin. Our expression studies revealed that CLPB gene expression is significantly higher in AML patient samples when compared to normal CD34+ HSPCs (Fig. 3B). Moreover, CLPB median expression in AML was the second highest among all other cancer subtypes in the TCGA dataset and the third highest among all cancer cell lines in the Cancer Cell Line Encyclopedia (CCLE) database (Fig. 3C; Supplementary Fig. S4A).
Figure 3. Targeting the mitochondrial protein CLPB synergizes with Venetoclax in AML.
A. Venn diagram of the 353 common negatively selected genes (“sensitizers”) throughout the screen, the 2221 core essential genes defined by Hart et al. (17) as well as 2220 genes which are upregulated in AML patients compared to healthy CD34+ HSPCs. Among these, 18 genes (listed in the table) were found non-essential and upregulated in AML patients.
B. Violin plot of CLPB mRNA expression level (FPKM) from RNA-sequencing in TCGA AML patients (200 patients) and normal human CD34+ HSPCs (6 healthy donors).
C. CLPB mRNA expression levels across diverse cancers from TCGA (log2 FPKM). Sorted by median expression level.
D-E. Western blotting (D) and quantitative densitometric analysis (E) of CLPB protein levels of whole cell lysates from parental (Par.) and Venetoclax-resistant (VR) AML cell lines.
F-G. IC50 curves of Venetoclax in parental AML cells (F) and Venetoclax-resistant (VR) AML cells (G) transduced with CLPB sgRNAs or negative control (sgRosa). Transduced AML cells were selected with puromycin and then treated with Venetoclax for 48 hours. Viable cells were measured by Cell-TiterGlo. Data represent mean ± SD (n=3 for each group).
H. Bioluminescent images of mice transplanted with MOLM-13 cells transduced with sgRosa or sgCLPB #1. Mice were administrated with vehicle or Venetoclax (Ven) from day 6 to day 20 post transplantation as described in supplementary Fig. S5E. The same mice are depicted at each time-point (n=4 mice per group).
I. Quantification of bioluminescence emitted from the whole body of each mouse described in (H) at the indicated time points.
J. Flow cytometry analysis of GFP+ sgRNA-expressing leukemia cells in peripheral blood of MOLM-13 leukemia recipient mice described in (H) at the indicated time points.
K. Kaplan-Meier survival curves of the MOLM-13 leukemia recipient mice described in (H). The p-values were determined using Log rank Mantel-Cox test.
L. Western blotting in whole cell lysates from sorted GFP+ sgRosa-expressing leukemia cells in the bone marrow of MOLM-13 leukemia recipient mice treated with vehicle or Venetoclax (Ven), as described in (H). Animals were sacrificed when they showed signs of late stage leukemia.
Data with statistical significance are as indicated, *p< 0.05, **p< 0.01, ***p< 0.001, N.S., not significant.
As a further suggestion of a role in this process, we observed higher levels of CLPB protein in Venetoclax-resistant human AML cell lines when compared to the parental sensitive clones (Fig. 3D and E). Based on our RNA-Seq data (Supplementary Fig. S4C), CLPB transcripts are only slightly upregulated in Venetoclax-resistant cells, indicating that this overexpression is post-translational. Importantly, the protein levels of the mitochondrial transcription factor A (TFAM) were unchanged, indicating that the total mitochondrial mass is not altered. Furthermore, when we treated AML cells with Venetoclax we observed a significant increase in the CLPB protein, indicating that cells that survive Venetoclax treatment have higher CLPB levels (Supplementary Fig. S4D–E). In addition, we quantified the CLPB protein levels in 4 different AML cell lines (MOLM-13, MV4–11, OCI-AML3 and THP-1) and found that cell lines with high CLPB protein levels were more resistant to Venetoclax than those with low CLPB levels (Supplementary Fig. S4F, G and H); however, the small statistical sample of cell lines analyzed precludes us from suggesting a definite predictive role of CLPB protein levels to Venetoclax sensitivity.
To further evaluate the importance of CLPB function across different human AML cells, we depleted CLPB in multiple AML cell lines using CLPB-targeting sgRNAs (Supplementary Fig. S6A), and we subsequently measured the Venetoclax IC50. CLPB depletion significantly reduced the IC50 of Venetoclax in all the cell lines tested (Fig. 3F, Supplementary Fig. S5A). Additionally, CLPB-deficient AML cells were negatively selected upon Venetoclax addition in a much faster pace than the DMSO control groups in a competition-based viability assay (Supplementary Fig. S5B), confirming that CLPB ablation can sensitize AML cells to Venetoclax treatment. Considering that CLPB is stabilized in Venetoclax-resistant AML cells (Fig. 3D and E), we sought to validate the sensitization effect of CLPB depletion in our Venetoclax-resistant cells. Similar to the results observed in the parental clones (Fig. 3F), ablation of CLPB significantly re-sensitized VR cells to the drug treatment (Fig. 3G, Supplementary Fig. S5C–D). The synthetic lethal relationship between CLPB ablation and Venetoclax is specific to Venetoclax-mechanisms of action, since no synergistic effect was detected in AML cells treated with Cytarabine, Idarubicin or JQ-1 treatment (Supplementary Fig. S4I–J). Moreover, in contrast to Venetoclax, treatment of MOLM-13 with Idarubicin did not lead to increase of CLPB protein levels in the surviving cells (Supplementary Fig. S4K). Finally, to avoid potential side-effects of CRISPR-mediated gene deletion, silencing of CLPB using four distinct shRNAs with different efficacies further validated the dose dependent competitive disadvantage of CLPB loss in MOLM-13 treated with Venetoclax (Supplementary Fig. S6B, C and D). Altogether, our findings revealed a functional role of mitochondrial CLPB in the response of AML cells to Venetoclax and suggested that targeting CLPB can overcome Venetoclax resistance in AML.
Finally, we sought to examine the synergistic effects of CLPB deletion with Venetoclax in vivo by using pre-clinical animal models. Specifically, NOD SCID gamma (NSG) mice were transplanted with MOLM-13-Par cells or MOLM-13-VR cells transduced with sgRosa or sgCLPB. After transplantation, we treated the mice with Venetoclax or vehicle from day 6 to day 20. Bioluminescence imaging and examination of peripheral blood circulating leukemic cells (GFP+) indicated that CLPB deletion synergizes efficiently with Venetoclax in both Venetoclax-sensitive and resistant xenografts (Fig. 3H–K, Supplementary Fig. S5E–J). In the course of these in vivo experiments, we determined that mice bearing wild-type tumors and being treated with Venetoclax exhibit significantly elevated CLPB and OPA1 protein levels at late stages of tumor progression compared to the vehicle-treated mice (Fig. 3L), suggesting once more that mitochondrial structure adaptation occurs also in vivo as a mechanism of resistance to the drug.
CLPB ablation impairs mitochondrial structure in AML cells rendering them more susceptible to apoptosis
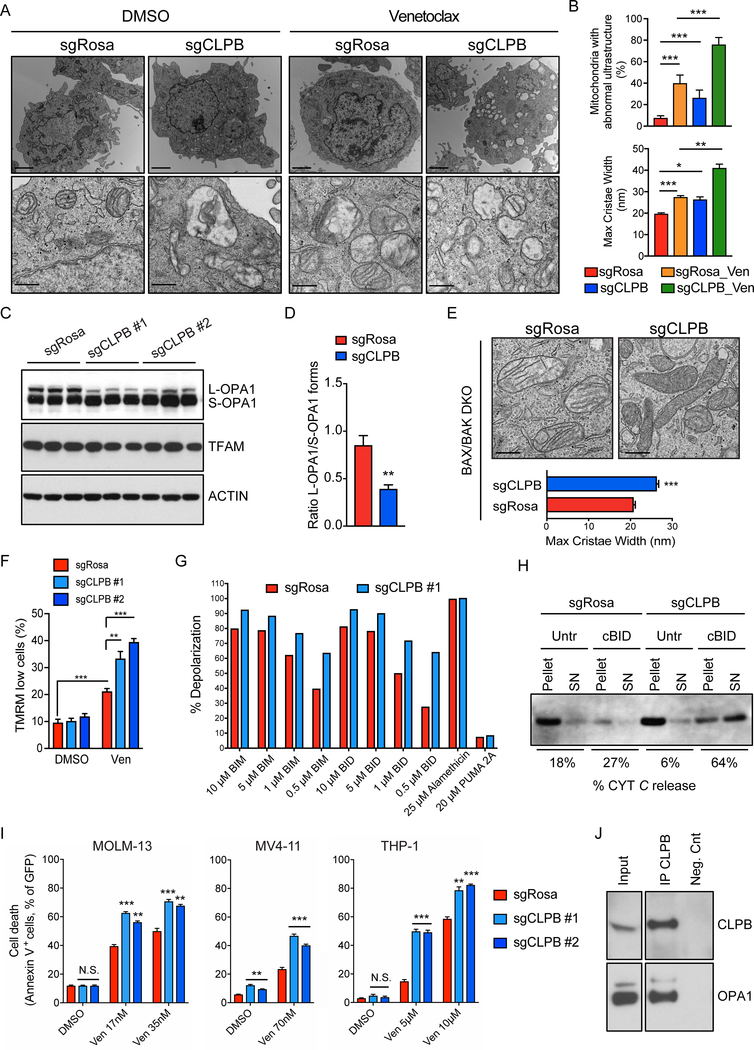
Next, we aimed to investigate how CLPB loss promotes Venetoclax-induced programmed cell death in AML cells. First, to confirm the subcellular localization of CLPB, we performed confocal imaging which indicated that endogenous CLPB co-localizes with the mitochondrial marker TOM20 in THP-1 and HeLa cells (Supplementary Fig. S7A). Notably, CLPB expression in patients with AML correlates with the transcription of genes whose products participate in the control of mitochondrial organization, such as OPA3 and ATAD3A (Supplementary Fig. S7B and C), suggesting a similar function of CLPB in leukemic cells. To test the hypothesis that CLPB participates in the organization of mitochondrial ultrastructure, we performed electron microscopy in CLPB-knockout AML cells. Mitochondria lacking CLPB exhibited an aberrant structure, characterized by wider cristae lumen (maximal cristae width – from a mean of 18 nm in the controls to 27 nm in the knockout). As expected, the mitochondrial structure abnormalities were exacerbated when the cells were treated overnight with Venetoclax and they were more significant in mitochondria lacking CLPB compared to wild-type (Fig. 4A–B, Supplementary Fig. S7D). The structural perturbations of CLPB-ablated mitochondria were accompanied by a prominent accumulation of short OPA1 forms, indicating excessive OPA1 processing, as demonstrated by western blotting (Fig. 4C and D). These phenomena were observed also in cells deficient for BAX and BAK, which do not respond to cell death stimuli, suggesting that the mitochondrial morphological defects are not a simple result of undergoing apoptosis (Fig. 4E). In addition, TMRM staining after Venetoclax treatment indicated an elevated percentage of cells with depolarized mitochondria in AML cells lacking CLPB, suggesting that CLPB-knockout cells are more prone to Venetoclax-induced mitochondrial depolarization (Fig. 4F and Supplementary Fig. S7E). To expand on these results, we performed a “BH3 profiling” in which permeabilized sgCLPB and sgRosa-expressing AML cells were treated with increasing doses of BH3 peptides and mitochondrial membrane potential was monitored over time using the JC-1 dye. As expected, CLPB-deficient AML cells undergo faster depolarization and are more primed for cell death than wild-type counterparts upon treatment with the BH3-only “activators”, BIM and BID (Fig. 4G, Supplementary Fig. S7F–G). Given the wider cristae and the higher sensitivity to mitochondrial membrane potential loss upon apoptosis stimulation, we speculated that CLPB-deficient mitochondria would readily release cytochrome c following a death stimulus. To address this hypothesis, we isolated functional mitochondria from wild-type and CLPB-knockout THP-1 cells, stimulated them with the recombinant proapoptotic caspase-8 cleaved BID (cBID) and centrifuged them to retrieve both the pellet (mitochondria) and supernatant (released factors) fractions. Western blot analysis indicated that CLPB-deficient mitochondria are more sensitive to cytochrome c release upon cBID stimulation compared to the wild-type organelles (Fig. 4H). All the above suggest an increased responsiveness of CLPB-ablated AML cells to programmed cell death. Indeed, immunoblotting against pro- and cleaved caspase-3 verified a prevalent caspase-3 activation upon BCL-2 inhibition in AML cells lacking CLPB relative to the wild-type (Supplementary Fig. S7H). Moreover, Annexin-V staining in puromycin-selected AML cells transduced with sgRNAs targeting CLPB confirmed hypersensitivity of these cells to Venetoclax compared to the negative controls (Fig. 4I). Taken together, these data suggest that CLPB is an important protein for the maintenance of physiological mitochondrial cristae, as well as the control of cytochrome c release and ultimately the execution of cell death.
Figure 4. CLPB loss induces mitochondrial ultrastructure defects sensitizing AML cells to mitochondria-mediated cell death.
A. Representative electron micrographs of THP-1 transduced with sgRNAs targeting CLPB or control (sgRosa) and treated with 4 μM Venetoclax or DMSO for 16 hrs. Scale bars represent 2 μm (upper panel) and 500 nm (lower panel).
B. Quantification of the percentage of mitochondria with abnormal ultrastructure in experiments as in (A) (n= 100 mitochondria per condition; upper panel). Quantification of the maximal cristae width in 60 randomly selected mitochondria from 15 cells in experiments as in (A) (n= 200 cristae per condition; lower panel).
C. Equal amounts (30 μg) of protein from THP-1 infected with sgRNAs targeting CLPB or control (sgRosa) were separated by SDS-PAGE and immunoblotted using the indicated antibodies.
D. Quantitative densitometric analysis of the ratio of OPA1 long (L-OPA1) versus short (S-OPA1) forms in experiments as in (C). Data represent mean ± SEM of 8 independent experiments.
E. Representative electron micrographs and morphometric analysis of BAX/BAK double-knockout MOLM-13 cells transduced with sgRNAs targeting CLPB or control (sgRosa). Scale bars represent 500 nm. Morphometric analysis was performed in 60 randomly selected mitochondria (n= 200 cristae per condition) in two independent experiments.
F. Quantification of the membrane potential loss after staining with TMRM in THP-1 infected with sgRNAs targeting CLPB or control (sgRosa) and treated with 4 μM Venetoclax or DMSO for 16 hours. Data represent mean ± SD (n=3 for each group).
G. BH3 profiling: Mitochondrial depolarization measured by JC-1 in permeabilized THP-1 transduced with sgRosa or sgCLPB upon stimulation with BIM and BID peptides. Depolarization (%) was calculated based on the area under the curve for each condition and normalized to CCCP positive control and 1% DMSO as negative control as previously described (48).
H. Isolated mitochondria from THP-1 transduced as indicated were treated with recombinant cBID for 30 min and centrifuged at 12.000 x g for 10 min. Pellet and supernatant (SN) of each sample were separated with SDS-PAGE and immunoblotted for cytochrome c (cyt c). % Cyt c release is calculated as the percentage of the supernatant to the total (pellet and supernatant) cytochrome c band intensity.
I. AML cells were transduced with sgRNAs targeting CLPB or control (Rosa), treated with Venetoclax or DMSO for 16 hrs and cell death was determined by flow cytometry using Annexin V. Data represent mean ± SD (n=3).
J. Liver mitochondrial lysates were immunoprecipitated with anti-CLPB coupled to magnetic Protein-G beads. Co-precipitated proteins were separated by SDS-PAGE and immunoblotted against CLPB and OPA1. Input was diluted 1:10.
Data with statistical significance are as indicated, *p< 0.05, **p< 0.01, ***p< 0.001, N.S., not significant.
CLPB interacts with the anti-apoptotic mitochondrial proteins HAX1 and OPA1
To investigate how CLPB participates in the preservation of the proper mitochondrial ultrastructure and the regulation of apoptosis, we sought to identify the interacting partners of this chaperonin in human AML cells. We performed immunoprecipitation (IP) of the endogenous human CLPB followed by mass spectrometry (MS) in whole THP-1 cell lysates (Supplementary Fig. S7I). Our analysis uncovered 64 mitochondrial proteins found exclusively in the CLPB pull-down and not in the IgG sample (negative control), indicating their potential association with CLPB. Among the top enriched CLPB interactors, we identified two proteins involved in the regulation of mitochondrial morphology and apoptosis, HAX1 and OPA1 (26, 28), ranked No. 1 and 3, respectively (Supplementary Table 2). Western blotting of the CLPB immunoprecipitation in THP-1 lysates and mouse liver mitochondria confirmed the physical interaction of CLPB with HAX1 and OPA1 (Fig. 4J, Supplementary Fig. S7J–K). Altogether these data suggest that CLPB regulates mitochondrial cristae morphology and apoptosis in AML cells, via its specific interactions with OPA1 and HAX1.
CLPB loss suppresses AML cell growth in vitro and in vivo
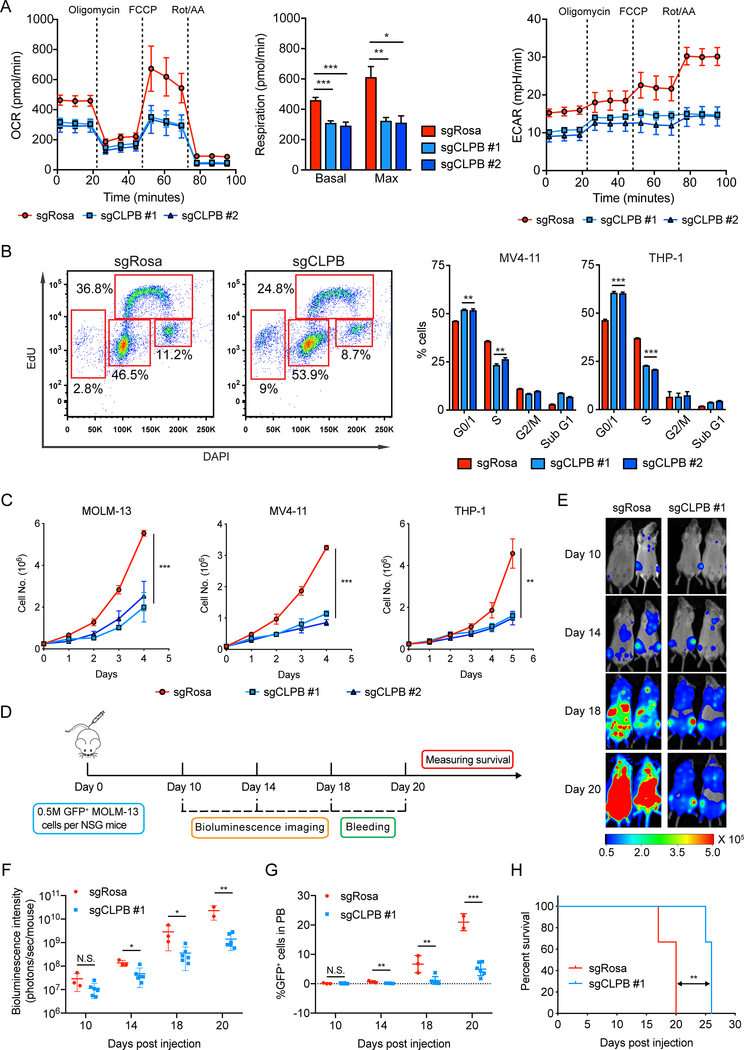
Given the mitochondrial structure defects, we hypothesized that CLPB ablation directly affects mitochondrial functions. Indeed, using the live cell metabolic assay platform Seahorse XF, we were able to show impaired basal and maximal mitochondrial respiration, as well as defective glycolysis in CLPB deleted AML, suggesting a more quiescent metabolic state compared to the highly energetic wild-type AML cells (Fig. 5A). We then asked whether such bioenergetic defects have an impact on cell proliferation. EdU incorporation assays suggested a suppression of cell replication in AML cells deficient of CLPB (Fig. 5B). We verified these cell growth defects measuring cell doublings across time (Fig. 5C). More importantly, suppression of cell growth was not seen or was less pronounced in a number of non-AML cancer cell lines, including T-ALL, B-ALL, CML and melanoma (Supplementary Fig. S6E), highlighting once more the specificity of CLPB dependency in AML cells (Supplementary Fig. S4B).
Figure 5. CLPB ablation leads to cell growth suppression in AML in vitro and in vivo.
A. Oxygen consumption rate (OCR), respiration (bar plot) and extracellular acidification rate (ECAR) of wild-type and CLPB-knockout MOLM-13 determined by Seahorse Extracellular Flux Analysis. Data represent mean ± SEM (n=5).
B. Representative flow cytometry plots showing EdU cell cycle analysis of wild-type and CLPB knockout MV4–11 (left panel). Bar charts depict the mean percentage of cell populations ± SD (n=3) in MV4–11 or THP-1 AML cells (right panel).
C. Cell growth analysis of AML cells transduced with sgRNAs targeting CLPB or control (sgRosa) (mean ± SD, n=3).
D. Schematic outline of the mouse model of CLPB dependency in AML.
E. Bioluminescent images of mice transplanted with MOLM-13 cells transduced with sgRosa (n=3) or sgCLPB #1 (n=6). Representative images of two mice per sgRNA construct are shown. The same mice are depicted at each time-point.
F. Quantification of bioluminescence emitted from the whole body of each mouse transduced with sgRosa or sgCLPB #1 construct at the indicated time points.
G. Flow cytometry analysis of GFP+ sgRNA-expressing leukemia cells in peripheral blood of MOLM-13 leukemia recipient mice at indicated time points.
H. Kaplan-Meier survival curves of recipient mice transduced with sgRosa and sgCLPB #1 are plotted. The p-values were determined using Log rank Mantel-Cox test.
Data with statistical significance are as indicated, *p< 0.05, **p< 0.01, ***p< 0.001, N.S., not significant.
Finally, we examined the in vivo significance of CLPB in AML progression using a pre-clinical mouse model. We labeled the Cas9-competent MOLM-13 cells with luciferase (Luci) and transduced them with sgRNA targeting CLPB or negative control (sgRosa). sgRNA-infected MOLM-13 (GFP+) were then sorted by FACS and injected intravenously into NSG mice (Fig. 5D). We observed a significant delay in leukemia progression in mice receiving CLPB-deficient MOLM-13 cells, as determined by bioluminescent imaging (Fig. 5E and F). FACS analysis of peripheral blood from recipient mice detected a significant lower percentage of circulating leukemia cells harboring CLPB sgRNAs compared to the negative control (Fig. 5G), which correlated with the significant prolonged survival seen in mice harboring CLPB-deficient MOLM-13 (Fig. 5H). These data suggested that CLPB is required for AML maintenance, which is consistent with the physiological function of CLPB in regulating key mitochondrial biological processes, including cristae structure, membrane potential and oxidative phosphorylation.
Loss of CLPB induces mitochondrial stress response
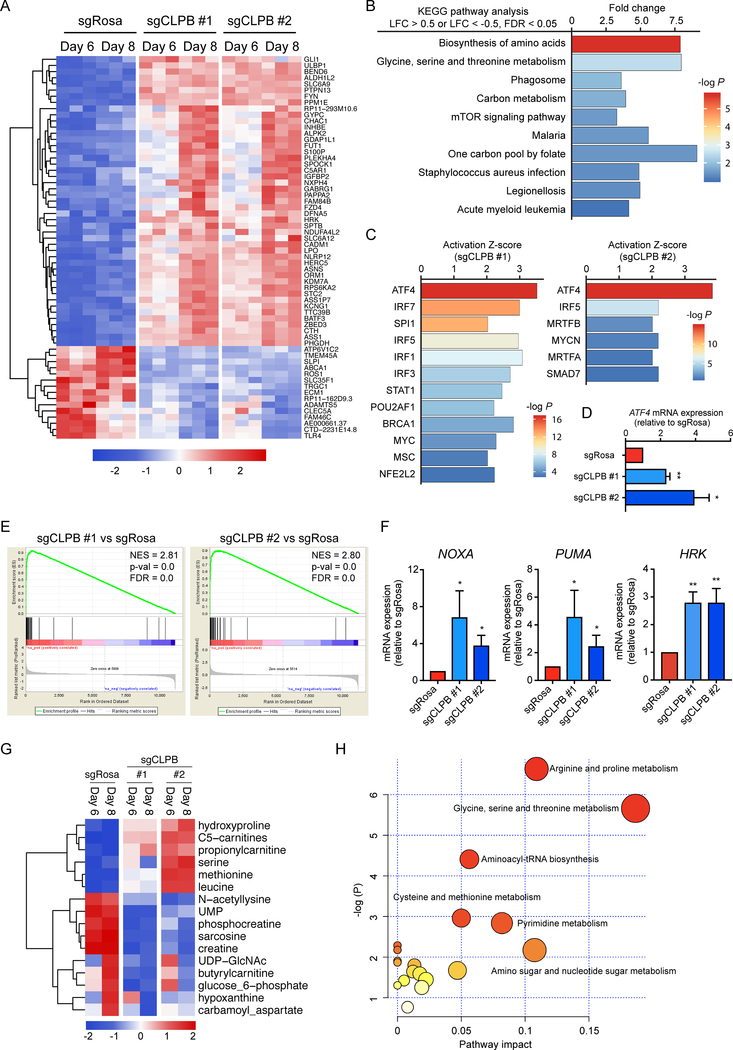
To further focus on the underlying mechanisms of CLPB function, we performed RNA-Sequencing in AML cells upon CLPB deletion. We used two CLPB-targeting sgRNAs and profiled cells at days 6 and 8 after CLPB depletion (Fig. 6A). KEGG pathway analysis of the differentially expressed genes in both CLPB-deficient MOLM-13 cells (sgCLPB #1 and #2) showed strong enrichment on metabolic pathways, like biosynthesis of amino acids and one carbon metabolism (Fig. 6B), all consistent with the functions of CLPB in regulating mitochondrial processes (Fig. 5). Ingenuity Pathway Analysis (IPA) was performed to reveal the upstream transcriptional regulators that are responsible for the transcriptional landscape changes upon CLPB ablation. Interestingly, ATF4, a key transcription factor induced by the integrative stress response (29), ranked first in both CLPB-targeting sgRNAs transduced MOLM-13 cells (Fig. 6C). Indeed, qPCR analysis verified the upregulation of ATF4 and its downstream effector CHOP in AML cells lacking CLPB (Fig. 6D, Supplementary Fig. S8D, E and F). Considering our previous results and given that ATF4 has been identified as a key regulator of the mitochondrial stress response in mammals (30), we speculated that CLPB-deficient AML cells are under mitochondrial stress. To this end, we performed Gene-Set Enrichment Analysis (GSEA) using a combined mitochondrial stress expression gene signature extracted from the RNA-Seq studies described by Quirós et al (30). Such analysis revealed that genes associated with mitochondrial stress response were indeed strongly enriched in CLPB-deficient MOLM-13 cells at both time-points (Fig. 6E; Supplementary Fig. S8A). To functionally confirm the mitochondrial stress in CLPB-deficient AML cells, we measured mitochondrial reactive oxygen species (ROS) upon treatment with the complex III inhibitor, Antimycin A, using MitoSOX Red. CLPB deficiency in MOLM-13 led to a significant elevation of the mean MitoSOX fluorescent intensity after Antimycin A addition relative to the steady-state levels, indicating greater ROS accumulation inside CLPB ablated mitochondria upon challenge (Supplementary Fig. S8B), thus, demonstrating that AML cells are under mitochondrial stress upon CLPB depletion. Given that proapoptotic BH3-only proteins can be induced by ATF4 (31), we wondered if these pro-death sensitizers were upregulated in response to CLPB depletion. Indeed, NOXA, PUMA and HRK gene expression was significantly upregulated in CLPB-deficient AML cells (Fig. 6F, Supplementary Fig. S8G). In line with this finding, BH3-profiling revealed increased sensitivity of CLPB-ablated cells to the peptide “sensitizers” PUMA and BMF-γ, as well as to the MCL-1 inhibitor (MS-1) (Supplementary Fig. S8H). Such findings were also consistent with our previous results showing that CLPB ablated AML cells are more primed to apoptosis (Fig. 4G–H).
Figure 6. CLPB deficiency amplifies proapoptotic signals by inducing mitochondrial stress response.
A. Heatmap showing the differentially expressed genes in MOLM-13 transduced with sgRNAs targeting CLPB or control (sgRosa) at day 6 and day 8 post transduction (Log fold change > 1 or < −1, False Discovery Rate < 0.05 in all samples). Common genes are shown in the heatmap.
B. KEGG pathway enrichment analysis of the differentially expressed genes in MOLM-13 transduced with sgRNAs targeting CLPB or control (sgRosa) at day 6 post transduction.
C. Ingenuity Pathway Analysis of the differentially expressed genes (Log fold change > 1 or < −1, False Discovery Rate < 0.05 in all samples) in MOLM-13 transduced with two independent sgRNAs targeting CLPB (left panel, sgCLPB #1; right panel, sgCLPB #2) or control (sgRosa) at day 6 post transduction revealing activation of the ATF4 upstream pathway.
D. qPCR analysis of ATF4 transcripts in MOLM-13 transduced with sgRNAs targeting CLPB or control (sgRosa) (mean ± SEM, n=3).
E. Enrichment score plots from Gene-set enrichment analysis (GSEA) using the mitochondrial stress expression signature defined by Quirós et al (30) and the RNA-Seq data of MOLM-13 transduced with sgRNAs targeting CLPB or control (sgRosa) at day 6 post transduction. FDR, false discovery rate; NES, normalized enrichment score.
F. qPCR analysis of the relative expression levels of NOXA (PMAIP1) mRNA, PUMA (BBC3) mRNA and HRK mRNA in MOLM-13 transduced with CLPB-targeting sgRNAs or control (sgRosa) at day 6 post transduction (mean ± SD, n=3).
G. Heatmap showing the significantly differentially detected metabolites in MOLM-13 transduced with two independent sgRNAs targeting CLPB or control (sgRosa) at day 6 or day 8 post transduction (p < 0.05). Average intensity of each metabolite was shown.
H. Metabolome pathway enrichment analysis of top altered metabolites in (G).
Data with statistical significance are as indicated, *p< 0.05, **p< 0.01.
Considering that CLPB-deficient AML cells are under mitochondrial stress and harbor dysfunction in the biosynthesis of several metabolites (Fig. 6B), a more detailed metabolic profiling was performed using mass spectroscopy. Among the 150 metabolites tested, the intensity of 16 substances was significantly changed in CLPB-deficient MOLM-13 cells (Fig. 6G, Supplementary Table 3). Pathway analysis of the metabolomics data revealed significant enrichment in amino acid related pathways (glycine, serine and threonine metabolism, arginine and proline metabolism, aminoacyl-tRNA biosynthesis) (Fig. 6H). Indeed, the glycine, serine and threonine biosynthesis is one of the most altered metabolic pathways in cells under mitochondrial stress (30). Notably, the majority of the altered metabolites upon CLPB depletion were strongly correlated with the differentially expressed genes shown in Fig. 6A, highlighting the consistency of our RNA-Seq and metabolomics data (Supplementary Fig. S8C). These findings suggest that CLPB deficiency amplifies proapoptotic signals through the induction of the ATF4-mediated mitochondrial stress response.
CLPB targeting overcomes p53-mediated Venetoclax resistance and sensitizes AML cells to combined Venetoclax and Azacitidine treatment
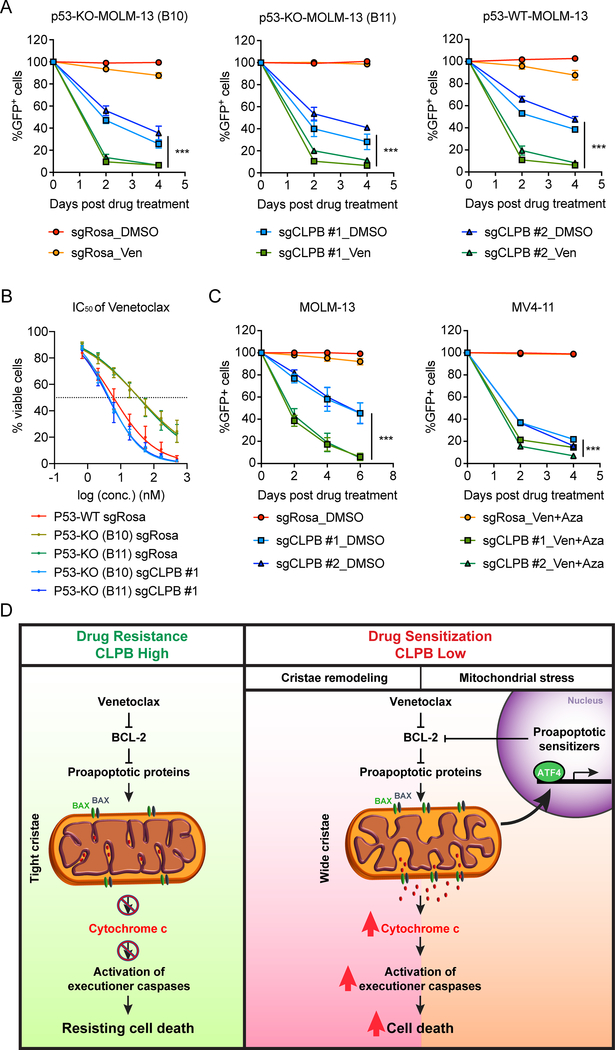
Stabilization of the p53 protein synergizes with Venetoclax in AML (15). Based on data included here and in the accompanying manuscript by Tyner and colleagues, p53 deletion can confer resistance to the drug in AML cells (Fig. 1B–E, Supplementary Fig. S1B and S1D–F). Therefore, the key question of obvious clinical significance is whether CLPB deletion can sensitize p53-deficient AML cells to Venetoclax treatment. To this end, we deleted p53 in MOLM-13 using two independent sgRNAs targeting TP53 (Supplementary Fig. S9A). Our studies showed that CLPB-deficient AML cells were more sensitive to Venetoclax treatment, even in a TP53-knockout (p53-KO) background (Fig. 7A). More importantly, CLPB depletion was able to fully re-sensitize p53-deficient MOLM-13 to Venetoclax treatment by inducing the ATF4-mediated upregulation of proapoptotic sensitizers (Fig. 7B, Supplementary Fig. S9D–E). Likewise, CLPB ablation led to a synergistic effect with Venetoclax also in an AML cell line with naturally occurring TP53 mutation (KASUMI-1) (Supplementary Fig. S9B–C). Collectively, CLPB-mediated re-sensitization of Venetoclax-resistant AML cells is p53-independent.
Figure 7. CLPB targeting overcomes p53-mediated Venetoclax resistance and sensitizes AML cells to combined Venetoclax and Azacitidine treatment.
A. Synergistic effect of CLPB depletion and BCL-2 inhibition in TP53-knockout (KO) MOLM-13 cell lines (two clones, B10 and B11) generated as shown in Supplementary Fig. S9. Plotted are GFP+ percentages measured during 4 days in culture and normalized to Day 0 of drug treatment. Negative control (sgRosa) and two independent sgRNAs targeting CLPB are shown in the graphs. Data represent mean ± SD (n=4).
B. IC50 curves of Venetoclax in p53 wild-type (WT) and p53-deficient (KO) (two clones, B10 and B11) MOLM-13 cells transduced with sgCLPB #1 or sgRosa. Transduced AML cells were selected with puromycin and then treated with Venetoclax for 48 hours. Viable cells were measured by Cell-TiterGlo. Data represent mean ± SD (n=6 for each group).
C. Validation of the synergistic effect of CLPB depletion and Venetoclax + Azacitidine combined treatment using a competition-based survival assay in MOLM-13 (left) and MV4–11 (right) cells. Plotted are GFP+ percentages measured during 6 days (for MOLM-13) or 4 days (for MV4–11) in culture and normalized to Day 0 of drug treatment. Negative control (sgRosa) and two independent sgRNAs targeting CLPB are shown in the graphs. Data represent mean ± SD (n=6 for MOLM-13 and n=3 for MV4–11).
D. Schematic outline of this study. Targeting the mitochondrial CLPB sensitizes AML cells to Venetoclax treatment by 1) promoting apoptotic cristae remodeling and 2) inducing mitochondrial stress response which will amplify the programmed cell death pathway.
Data with statistical significance are as indicated, ***p< 0.001.
Since resistance to Venetoclax monotherapy rapidly ensues in AML (14, 32), multiple combinational therapies for Venetoclax have been proposed and tested, some of which are in clinical trials (https://www.cancer.gov/about-cancer/treatment/clinical-trials/intervention/Venetoclax). Among these, the combination of Venetoclax with hypomethylating agents (HMA), like azacitidine, is currently in clinics due to its favorable responses in the clinical trials (33). To investigate if CLPB inhibition can enhance the efficacy of Venetoclax and Azacitidine combined treatment, we tested the possibility that CLPB depletion can further sensitize AML cells to the combined treatment. Indeed, CLPB-deficient AML cells were negatively selected out upon combined treatment in a much faster pace than the DMSO control groups (Fig. 7C), confirming that CLPB ablation can sensitize AML cells to combined Venetoclax/Azacitidine treatment. Altogether, our findings highlight that loss of the mitochondrial protein CLPB leads to structural and functional defects of mitochondria, hence sensitizing AML cells to apoptosis. Therefore, targeting CLPB synergizes with Venetoclax and Venetoclax/Azacitidine combination in AML in a p53-independent manner (Fig. 7D).
Discussion
The recent FDA approval for Venetoclax introduces an exciting and novel strategy to target AML. In the initial single-agent Venetoclax studies in AML, response rates were modest and rather short-lived (12). Our genome-wide CRISPR/Cas9 screen identified both possible mechanisms of resistance and synthetic lethal combinations with Venetoclax in AML, including the targeting of a number of proteins regulating mitochondrial structure. These proteins can control the architecture of both the outer mitochondrial membrane (OMM) and the mitochondrial cristae. Interestingly, both these structures are essential for BCL-2 family function (5), cytochrome c release and cell death (34). For the complete release of the cristae-endowed cytochrome c stores, the inner mitochondrial membrane needs to be remodeled and the cristae widened by the proteolytic cleavage of OPA1 and the disruption of its complexes, which normally keep two opposing cristae membranes tight (26, 35). Both OMM rupture and cristae remodeling is what we observed by electron microscopy upon Venetoclax treatment in AML cells. The changes in mitochondrial architecture could, at least partially, explain the reported impairment in oxidative phosphorylation following BCL-2 inhibition in the leukemic stem cells (LSC) derived from AML patients (2). On the opposite side, Venetoclax-resistant AML cells display mitochondria with narrow cristae lumen and increased protein levels of the cristae “staple” OPA1, possibly rendering them more resistant to the cytochrome c release upon stimulation.
One of the top-ranked genes revealed by our screen to act synergistically with Venetoclax is the mitochondrial chaperonin CLPB. Although a plethora of studies in bacteria and yeast have extensively characterized the homologue of CLPB as a protein chaperone which disaggregates misfolded proteins (36–38), the exact function of the mammalian CLPB has not been investigated thoroughly. Of note, the amino-acid sequence of the human CLPB is only about 20% identical to the orthologue in Escherichia coli (38). CLPB belongs to the large AAA+ ATPase superfamily, whose members act both as general protein chaperones and as targeted proteases that degrade specific substrates. Other mitochondrial AAA+ ATPases, such as YME1L, AFG3L2, ATAD3, m-AAA protease, participate in the organelle’s protein quality control, mitochondrial protein synthesis and maintenance of mitochondrial architecture (39–43). Wortmann and colleagues, who studied the mutations in CLPB in patients with an autosomal recessive metabolic syndrome, hypothesized a possible role of CLPB in apoptosis, due to its in situ predicted interaction with HAX1 (44). Our study provides the biochemical evidence of the association of CLPB with HAX1 and uncovers its function in the cell protection from intrinsic mitochondria-mediated apoptosis. Furthermore, our work reveals that CLPB physically interacts with OPA1 - possibly as a means to protect it from its proteolytic cleavage - and, thus, demonstrates a role of CLPB in the maintenance of the physiological mitochondrial structure. Loss of CLPB results in abnormal mitochondria with wider cristae than the normal; hence such organelles are more prone to release their cytochrome c following a death signal, such as Venetoclax treatment. Our work proposes a specific CLPB dependency of AML cells. Its depletion leads to cell cycle suppression, possibly due to the defects in both oxidative phosphorylation and glycolysis. As a consequence of the mitochondrial structural and functional impairment, cells lacking CLPB are more sensitive to oxidative stress. As a response, the CLPB-deficient AML cells alter their transcriptional program and reprogram their metabolism in an ATF4-dependent manner, thus leading to cell cycle arrest and enhancement of apoptotic pathways. Overall, we show that CLPB is most likely required for AML progression and, importantly, can synergize with Venetoclax to induce rapid cell death both in vivo and in vitro.
Recently, Jordan and colleagues demonstrated that the FDA approved combined treatment of Venetoclax with Azacitidine disrupts the tricarboxylic acid (TCA) cycle in LSCs of AML patients and that relapsed AML patients remodel their metabolism (45, 46). These studies support the notion that targeting mitochondrial functions concomitantly with Venetoclax and Azacitidine could have a clinical relevance for AML patients. In line with this idea, we show that depleting CLPB which impacts on the cellular metabolic status of the AML cells sensitizes them to the combined treatment in a p53-independent way. Interestingly, a bacterial CLPB inhibitor has been developed and proposed to be utilized as an antimicrobial agent (47). Since this inhibitor targets the conserved domain of the bacteria and the human orthologues (44) and displays moderate toxicity in human cell lines (47), it could synergize with Venetoclax treatment in AML cells. In general, targeting mitochondrial proteins that would promote apoptotic cristae remodeling should be investigated as a potential way to overcome Venetoclax resistance in AML, in addition to ongoing trials targeting other BCL-2 family members, such as MCL-1 (R. Tibes, personal communication), either directly with novel MCL-1 inhibitors, or indirectly via cyclin dependent kinase inhibition affecting MCL-1 transcript expression, as we and other have proposed (11, 16). In conclusion, targeting CLPB and possibly its interactome in combination with Venetoclax treatment directly promotes the apoptotic cristae remodeling and indirectly induces mitochondrial stress responses, which will both amplify the programmed cell death pathway and suppress the growth of AML cells. Our data open a potential avenue for novel Venetoclax drug combinations in AML.
Methods
Cell Lines and Cell Culture
All human leukemia cells (MOLM-13, THP-1, MV4–11, CuTLL-1, Nalm-6, K562) were cultured in RPMI medium supplemented with 10% FBS and 1% penicillin/streptomycin. The adherent cell lines, HeLa and HEK293T cells were grown in DMEM medium with 10% FBS and 1% penicillin streptomycin. SK-MEL-239 were cultured in RPMI, 10% FBS and 1% penicillin/streptomycin. Cas9-expressing cell lines transduced with retroviral Cas9–2A-blast (Addgene, plasmid no. 73310) were selected with blasticidin (InvivoGen) 48 hours after transduction. All transfections were performed in HEK293T cells using Polyethylenimine (PEI) reagent at 4:2:3 ratios of sgRNA or shRNA construct (Supplementary Table 4): pVSVG: pPax2 in OPTI-MEM solution. Viral supernatant was collected 36 hrs and 48 hrs post-transfection. Spin infections were performed at room temperature at 1,500 x g for 30 mins with polybrene reagent (1:2000; Fisher Scientific).
For the generation of the Venetoclax-resistant cell lines, MOLM-13 and MV4–11 were cultured in media containing increasing doses of Venetoclax (from 5 nM to 1000 nM) for more than 8 weeks to achieve complete resistance to the drug.
Cell lines information is as follows:
MOLM-13, DSMZ, ACC554
THP-1, ATCC, TIB-202
MV4–11, ATCC, CRL-9591
CuTLL-1, Adolfo Ferrando Lab
Nalm-6, ATCC, CRL-3273
K562, ATCC, CCL-243
HEK293T, ATCC, CRL-1573
SK-MEL-239, Eva Hernando Lab
HeLa, ATCC, CCL-2.
All the cell lines were determined negative for mycoplasma, as indicated by the latest Mycoplasma test (December 2018) using the LookOut Mycoplasma PCR Detection Kit (Sigma). Cells were used for experiments within 15 to 20 passages from thawing.
CRISPR Screen
Cas9-expressing MOLM-13 were transduced with the Brunello sgRNA library (18) virus at a low MOI (~0.3). On day 2 post-transduction, GFP+ percentage was assessed to determine infection efficiency and sgRNA coverage (~1000X). Then, puromycin (1 μg/ml) was added for 5 days to select infected (GFP+) cells. After selection, viable infected cells were isolated by Histopaque 1077 (Sigma Aldrich), and grown without antibiotics for 2 days. After recovery, 100 X 106 infected cells were cultured with 10 nM Venetoclax (Selleckchem) or DMSO. Another 100 X 106 cells were used for genomic DNA (gDNA) extraction and served as an initial reference (day 0 of drug treatment). The concentration of Venetoclax was increased during the screen (from 10 nM to 2 μM) to avoid spontaneous gain of drug resistance. For each passage, 100 X 106 cells were placed back into culture until 16 days post drug treatment. gDNA of cells containing ~1000X coverage was harvested on days 8 and 16 post drug treatment using Qiagen DNA kit according to manufacturer’s protocol. Further details on library construction and data analysis can be found in Supplementary data.
Drug Treatment and IC50 Measurements
Cells were plated in 96-well plates and exposed to Venetoclax at concentrations ranging from 0.4 nM to 300 nM (for MOLM-13 and MV4–11), 150 nM to 10 μM (for Venetoclax-resistant cell lines and THP-1) with a minimum of three technical replicates per concentration per cell line. Cell viability was measured with the CellTiter-Glo reagent (Promega) according to manufacturer’s instructions. Absolute viability values were converted to percentage viability versus DMSO control treatment, and then non-linear fit of log(inhibitor) versus response (three parameters) was performed in GraphPad Prism v7.0 to obtain the IC50 values. For Venetoclax and Azacitidine combined treatment, two drugs were mixed at the fixed ratio (Ven:Aza = 1:4) before diluting to certain concentrations.
Transmission Electron Microscopy
Cultured cells were fixed in 0.1M sodium cacodylate buffer (pH 7.4) containing 2.5% glutaraldehyde and 2% paraformaldehyde for 2 hrs and post-fixed with 1% osmium tetroxide and 1% potassium ferrocyanide for one hour at 4°C. Then block was stained in 0.25% aqueous uranyl acetate, processed in a standard manner and embedded in EMbed 812 (Electron Microscopy Sciences, Hatfield, PA). Ultrathin sections (60 nm) were cut, mounted on copper grids and stained with uranyl acetate and lead citrate. Stained grids were examined under Philips CM-12 electron microscope and photographed with a Gatan (4k x2.7k) digital camera. For morphometric analysis, maximal cristae width was measured using Image J (NIH) as shown in Supplementary Fig. S2A in at least 50 randomly selected mitochondria from a minimum of 15 cells/experiment.
Mitochondrial Respiration
Oxygen consumption rate (OCR, pmol/min) was determined using the XFe24 Extracellular Flux Analyzer and the XF Cell Mito Stress Test kit (Agilent). Details can be found in Supplementary information.
Mitochondrial Membrane Potential
Mitochondrial membrane potential was monitored cytofluorimetrically using tetramethylrhodamine methyl ester (TMRM). Cells were loaded with 10 nM TMRM in presence of 2 μM Cyclosporin H in Hank’s Balanced Salt Solution (HBSS) supplemented with 10 mM HEPES, 30 min at 37°C. When indicated, the experiments were performed in cells pre-treated with Venetoclax for 16 hours at the indicated concentrations.
Metabolomics
7×105 cells were flash frozen as pellets and processed using the LC-MS/MS with the hybrid metabolomics method. Details can be found in the Supplementary information.
Animal experiments
For in vivo experiments, Cas9 and luciferase-expressing parental and Venetoclax-resistant MOLM-13 cells were transduced with sgRosa or sgCLPB #1 constructs. At day 2 post-transduction, sgRNA positive cells (GFP+) were sorted by FACS. 0.5 million sgRNA-expressing leukemia cells were intravenously injected into recipient NSG mice. For experiments that required drug administration, recipient mice were treated with vehicle or Venetoclax (100 mg/kg, Selleckchem) daily by oral gavage from day 6 to day 20. Venetoclax was prepared in 10% ethanol, 30% polyethyleneglycol-400 (Sigma), and 60% phosal 50 propylene glycol (Lipoid). Whole-body bioluminescent imaging was performed at indicated time points by intraperitoneally injection of Luciferin (Goldbio) at a 50 mg/kg concentration and imaging was performed after 5 mins using an IVIS imager. Bioluminescent signals (radiance) were quantified using Living Image software with standard regions of interests (ROI) rectangles. Peripheral blood of recipient NSG mice was collected at indicated time points after transplantation, and sgRNA positive cells (GFP+) were analyzed by flow cytometry. All animal experiments were performed in accordance with protocols approved by the New York University Institutional Animal Care and Use Committee (IACUC, ID: IA16–00008_TR1).
Data and Software Availability
Gene Expression Omnibus: all newly generated RNA-Sequencing data were deposited under accession number GSE125403.
Supplementary Material
Statement of Significance.
Genome-wide CRISPR/Cas9 screen reveals genes involved in mitochondrial biological processes participate in the acquisition of Venetoclax resistance. Loss of mitochondrial protein CLPB leads to structural and functional defects of mitochondria, hence sensitizing AML cells to apoptosis. Targeting CLPB synergizes with Venetoclax and Venetoclax/Azacitidine combination in AML in a p53-independent manner.
ACKNOWLEDGEMENTS
We would like to thank all members of the I.A. lab for useful discussions and comments on the manuscript; A. Heguy and the NYU Genome Technology Center (supported in part by National Institutes of Health (NIH)/National Cancer Institute (NCI) grant P30CA016087–30) for expertise with sequencing experiments; M. Cammer and the NYU Langone Microscopy Laboratory (grant NCRR S10 RR023704–01A1) for assistance with confocal microscopy; A. Liang, C. Petzold and K. Dancel-Manning and the NYU Langone Health DART Microscopy Lab for their assistance with TEM work; NYU Langone Health’s Metabolomics Laboratory for the help in acquiring and analyzing the data presented; NYU High Throughput Biology Laboratory which is partially supported by Laura and Isaac Perlmutter Cancer Center Support Grant, (NIH/NCI P30CA16087) and NYSTEM Contract C026719. The proteomics studies were supported in part by NYU School of Medicine and the Laura and Isaac Perlmutter Cancer Center Support grant P30CA016087 from the NCI. The Orbitrap Fusion Lumos mass spectrometer used in this study was purchased with a shared instrumentation grant (1S10OD010582–01A1) from the NIH. Also, we thank C. Quirin and L. Scorrano laboratory for the recombinant cBID. Finally, we thank L. Pernas and M.E. Soriano for critical reading of the manuscript.
I.A. was supported by the NIH and the NCI (R01CA173636, R01CA216421, R01CA133379, R01CA169784), the Leukemia & Lymphoma Society (TRP#6340–11 and LLS#6373–13), the Chemotherapy Foundation, the Taub Foundation, and The Alex’s Lemonade Stand Foundation for Childhood Cancer. The work was also supported by the New York State Department of Health (#CO030132, C32587GG, C32563GG). R.T. was supported by Leukemia and Lymphoma Society (CDA#2312–15 and TRP#6080–12) and NCI grant R01-CA17897.
Footnotes
Conflicts of interest
R.T. was an advisory board member for AbbVie. No potential conflicts of interest are disclosed by other authors.
References
- 1.Campos L, Rouault JP, Sabido O, Oriol P, Roubi N, Vasselon C, et al. High expression of bcl-2 protein in acute myeloid leukemia cells is associated with poor response to chemotherapy. Blood. 1993;81:3091–6. [PubMed] [Google Scholar]
- 2.Lagadinou ED, Sach A, Callahan K, Rossi RM, Neering SJ, Minhajuddin M, et al. BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. Cell Stem Cell. 2013;12:329–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tzifi F, Economopoulou C, Gourgiotis D, Ardavanis A, Papageorgiou S, and Scorilas A. The Role of BCL2 Family of Apoptosis Regulator Proteins in Acute and Chronic Leukemias. Adv Hematol. 2012;2012:524308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chao DT and Korsmeyer SJ. BCL-2 family: regulators of cell death. Annu Rev Immunol. 1998;16:395–419. [DOI] [PubMed] [Google Scholar]
- 5.Antignani A and Youle RJ. How do Bax and Bak lead to permeabilization of the outer mitochondrial membrane? Curr Opin Cell Biol. 2006;18:685–9. [DOI] [PubMed] [Google Scholar]
- 6.Zou H, Li Y, Liu X, and Wang X. An APAF-1.cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem. 1999;274:11549–56. [DOI] [PubMed] [Google Scholar]
- 7.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–89. [DOI] [PubMed] [Google Scholar]
- 8.Pan R, Hogdal LJ, Benito JM, Bucci D, Han L, Borthakur G, et al. Selective BCL-2 inhibition by ABT-199 causes on-target cell death in acute myeloid leukemia. Cancer Discov. 2014;4:362–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DiNardo CD, Pratz KW, Letai A, Jonas BA, Wei AH, Thirman M, et al. Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: a non-randomised, open-label, phase 1b study. Lancet Oncol. 2018;19:216–228. [DOI] [PubMed] [Google Scholar]
- 10.Bogenberger JM, Delman D, Hansen N, Valdez R, Fauble V, Mesa RA, et al. Ex vivo activity of BCL-2 family inhibitors ABT-199 and ABT-737 combined with 5-azacytidine in myeloid malignancies. Leuk Lymphoma. 2015;56:226–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bogenberger JM, Kornblau SM, Pierceall WE, Lena R, Chow D, Shi CX, et al. BCL-2 family proteins as 5-Azacytidine-sensitizing targets and determinants of response in myeloid malignancies. Leukemia. 2014;28:1657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Konopleva M, Pollyea DA, Potluri J, Chyla B, Hogdal L, Busman T, et al. Efficacy and Biological Correlates of Response in a Phase II Study of Venetoclax Monotherapy in Patients with Acute Myelogenous Leukemia. Cancer Discov. 2016;6:1106–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramsey HE, Fischer MA, Lee T, Gorska AE, Arrate MP, Fuller L, et al. A Novel MCL1 Inhibitor Combined with Venetoclax Rescues Venetoclax-Resistant Acute Myelogenous Leukemia. Cancer Discov. 2018;8:1566–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tahir SK, Smith ML, Hessler P, Rapp LR, Idler KB, Park CH, et al. Potential mechanisms of resistance to venetoclax and strategies to circumvent it. BMC Cancer. 2017;17:399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan R, Ruvolo V, Mu H, Leverson JD, Nichols G, Reed JC, et al. Synthetic Lethality of Combined Bcl-2 Inhibition and p53 Activation in AML: Mechanisms and Superior Antileukemic Efficacy. Cancer Cell. 2017;32:748–760 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bogenberger J, Whatcott C, Hansen N, Delman D, Shi CX, Kim W, et al. Combined venetoclax and alvocidib in acute myeloid leukemia. Oncotarget. 2017;8:107206–107222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hart T, Chandrashekhar M, Aregger M, Steinhart Z, Brown KR, MacLeod G, et al. High-Resolution CRISPR Screens Reveal Fitness Genes and Genotype-Specific Cancer Liabilities. Cell. 2015;163:1515–26. [DOI] [PubMed] [Google Scholar]
- 18.Doench JG, Fusi N, Sullender M, Hegde M, Vaimberg EW, Donovan KF, et al. Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR-Cas9. Nat Biotechnol. 2016;34:184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karim CB, Espinoza-Fonseca LM, James ZM, Hanse EA, Gaynes JS, Thomas DD, et al. Structural Mechanism for Regulation of Bcl-2 protein Noxa by phosphorylation. Sci Rep. 2015;5:14557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reyna DE, Garner TP, Lopez A, Kopp F, Choudhary GS, Sridharan A, et al. Direct Activation of BAX by BTSA1 Overcomes Apoptosis Resistance in Acute Myeloid Leukemia. Cancer Cell. 2017;32:490–505 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nag S, Qin J, Srivenugopal KS, Wang M, and Zhang R. The MDM2-p53 pathway revisited. J Biomed Res. 2013;27:254–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teh TC, Nguyen NY, Moujalled DM, Segal D, Pomilio G, Rijal S, et al. Enhancing venetoclax activity in acute myeloid leukemia by co-targeting MCL1. Leukemia. 2018;32:303–312. [DOI] [PubMed] [Google Scholar]
- 23.Wasilewski M and Scorrano L. The changing shape of mitochondrial apoptosis. Trends Endocrinol Metab. 2009;20:287–94. [DOI] [PubMed] [Google Scholar]
- 24.Pernas L and Scorrano L. Mito-Morphosis: Mitochondrial Fusion, Fission, and Cristae Remodeling as Key Mediators of Cellular Function. Annu Rev Physiol. 2016;78:505–31. [DOI] [PubMed] [Google Scholar]
- 25.Burke PJ Mitochondria, Bioenergetics and Apoptosis in Cancer. Trends Cancer. 2017;3:857–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frezza C, Cipolat S, Martins de Brito O, Micaroni M, Beznoussenko GV, Rudka T, et al. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell. 2006;126:177–89. [DOI] [PubMed] [Google Scholar]
- 27.West AP, Khoury-Hanold W, Staron M, Tal MC, Pineda CM, Lang SM, et al. Mitochondrial DNA stress primes the antiviral innate immune response. Nature. 2015;520:553–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan J, Ma C, Cheng J, Li Z, and Liu C. HAX-1 inhibits apoptosis in prostate cancer through the suppression of caspase-9 activation. Oncol Rep. 2015;34:2776–81. [DOI] [PubMed] [Google Scholar]
- 29.Wortel IMN, van der Meer LT, Kilberg MS, and van Leeuwen FN. Surviving Stress: Modulation of ATF4-Mediated Stress Responses in Normal and Malignant Cells. Trends Endocrinol Metab. 2017;28:794–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quiros PM, Prado MA, Zamboni N, D’Amico D, Williams RW, Finley D, et al. Multi-omics analysis identifies ATF4 as a key regulator of the mitochondrial stress response in mammals. J Cell Biol. 2017;216:2027–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pike LR, Phadwal K, Simon AK, and Harris AL. ATF4 orchestrates a program of BH3-only protein expression in severe hypoxia. Mol Biol Rep. 2012;39:10811–22. [DOI] [PubMed] [Google Scholar]
- 32.Bose P, Gandhi V, and Konopleva M. Pathways and mechanisms of venetoclax resistance. Leuk Lymphoma. 2017;58:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DiNardo CD, Pratz K, Pullarkat V, Jonas BA, Arellano M, Becker PS, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133:7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scorrano L and Korsmeyer SJ. Mechanisms of cytochrome c release by proapoptotic BCL-2 family members. Biochem Biophys Res Commun. 2003;304:437–44. [DOI] [PubMed] [Google Scholar]
- 35.Scorrano L, Ashiya M, Buttle K, Weiler S, Oakes SA, Mannella CA, et al. A distinct pathway remodels mitochondrial cristae and mobilizes cytochrome c during apoptosis. Dev Cell. 2002;2:55–67. [DOI] [PubMed] [Google Scholar]
- 36.Lee S, Sowa ME, Watanabe YH, Sigler PB, Chiu W, Yoshida M, et al. The structure of ClpB: a molecular chaperone that rescues proteins from an aggregated state. Cell. 2003;115:229–40. [DOI] [PubMed] [Google Scholar]
- 37.Rottgers K, Zufall N, Guiard B, and Voos W. The ClpB homolog Hsp78 is required for the efficient degradation of proteins in the mitochondrial matrix. J Biol Chem. 2002;277:45829–37. [DOI] [PubMed] [Google Scholar]
- 38.Abrahao J, Mokry DZ, and Ramos CHI. Hsp78 (78 kDa Heat Shock Protein), a Representative AAA Family Member Found in the Mitochondrial Matrix of Saccharomyces cerevisiae. Front Mol Biosci. 2017;4:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gerdes F, Tatsuta T, and Langer T. Mitochondrial AAA proteases--towards a molecular understanding of membrane-bound proteolytic machines. Biochim Biophys Acta. 2012;1823:49–55. [DOI] [PubMed] [Google Scholar]
- 40.Glynn SE Multifunctional Mitochondrial AAA Proteases. Front Mol Biosci. 2017;4:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gilquin B, Taillebourg E, Cherradi N, Hubstenberger A, Gay O, Merle N, et al. The AAA+ ATPase ATAD3A controls mitochondrial dynamics at the interface of the inner and outer membranes. Mol Cell Biol. 2010;30:1984–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anand R, Wai T, Baker MJ, Kladt N, Schauss AC, Rugarli E, et al. The i-AAA protease YME1L and OMA1 cleave OPA1 to balance mitochondrial fusion and fission. J Cell Biol. 2014;204:919–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Almajan ER, Richter R, Paeger L, Martinelli P, Barth E, Decker T, et al. AFG3L2 supports mitochondrial protein synthesis and Purkinje cell survival. J Clin Invest. 2012;122:4048–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wortmann SB, Zietkiewicz S, Kousi M, Szklarczyk R, Haack TB, Gersting SW, et al. CLPB mutations cause 3-methylglutaconic aciduria, progressive brain atrophy, intellectual disability, congenital neutropenia, cataracts, movement disorder. Am J Hum Genet. 2015;96:245–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pollyea DA, Stevens BM, Jones CL, Winters A, Pei S, Minhajuddin M, et al. Venetoclax with azacitidine disrupts energy metabolism and targets leukemia stem cells in patients with acute myeloid leukemia. Nat Med. 2018;24:1859–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jones CL, Stevens BM, D’Alessandro A, Reisz JA, Culp-Hill R, Nemkov T, et al. Inhibition of Amino Acid Metabolism Selectively Targets Human Leukemia Stem Cells. Cancer Cell. 2018;34:724–740 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martin I, Underhaug J, Celaya G, Moro F, Teigen K, Martinez A, et al. Screening and evaluation of small organic molecules as ClpB inhibitors and potential antimicrobials. J Med Chem. 2013;56:7177–89. [DOI] [PubMed] [Google Scholar]
- 48.Ryan J and Letai A. BH3 profiling in whole cells by fluorimeter or FACS. Methods. 2013;61:156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Gene Expression Omnibus: all newly generated RNA-Sequencing data were deposited under accession number GSE125403.