Abstract
Combining pharmacological interventions with evidence-based behavioral interventions may help optimize treatment outcomes for alcohol use disorder (AUD). While several effective behavioral interventions for AUD have been developed, the vast majority target individual patients, despite evidence that behavioral interventions for couples have the ability to outperform individual treatments for AUD. Alcohol Behavioral Couples Therapy (ABCT) is an evidence-based behavioral intervention for couples that has been shown to significantly reduce AUD severity as well as improve relationship functioning. Accumulating evidence suggests that the neuropeptide oxytocin has the ability to reduce alcohol craving and consumption, symptoms of tolerance and withdrawal, and ameliorate neurobiological deficits associated with AUD. Furthermore, oxytocin has demonstrated the ability to increase prosocial behavior and cognition, and restore sensitivity to natural rewards such as interpersonal relationships. No study to date has examined the ability of oxytocin to enhance ABCT. Thus, the primary objective of this Phase II study is to examine the effects of oxytocin versus placebo in combination with ABCT in reducing AUD severity and improving relationship functioning. We also will utilize neuroimaging techniques before and after treatment to investigate the underlying pathophysiology of AUD among couples and identify prognostic indicators of treatment outcome. The findings from this study might provide critical new information to help inform clinical practice and accelerate research on the pharmacological treatment of AUD.
Keywords: couple therapy, alcohol, substance use disorder, oxytocin, functional magnetic resonance imaging
1. Introduction
1.1. Research Objectives and Hypotheses
Combining pharmacological interventions with evidence-based behavioral treatments may help maximize and sustain AUD treatment outcomes1–3. Alcohol Behavioral Couples Therapy (ABCT)4 is a manual-guided, evidence-based psychotherapy for the treatment of AUD that simultaneously targets relationship functioning, which is an important mechanism in the etiology, course, and treatment of AUD5–7. While adaptive relationship functioning facilitates successful treatment engagement and outcomes7–9, maladaptive relationship functioning interferes with AUD recovery10–13 and is a precipitant of relapse risk14,15. Thus, ABCT employs cognitive behavioral techniques to (1) reduce alcohol consumption, (2) enhance partners’ skills to facilitate recovery (e.g., communication, managing cravings), and (3) enhance relationship functioning. Although ABCT is an efficacious treatment, there is room for improvement, as more than half of ABCT patients report hazardous drinking during treatment and a similar proportion fail to achieve abstinence5,6,16,17.
Oxytocin is a promising candidate to enhance ABCT via neurobiological and behavioral pathways, including its potential to restore sensitivity to natural rewards such as interpersonal relationships18. Dysregulation of the corticolimbic circuitry involving the prefrontal cortex (PFC) and amygdala (AMY) (i.e., lack of “top down” control) likely makes it difficult to inhibit or modulate emotions, reward processing, and cognitions such as compulsive craving-related thoughts central to AUD19–22. Lower PFC-AMY connectivity is associated with increased drinking, drug use, and early relapse21,23–25. Similar to some existing findings, our team recently found that PFC-AMY connectivity is implicated in less adaptive responses to relationship conflict26,27. Importantly, oxytocin attenuates AMY reactivity and increases resting state connectivity between corticolimbic brain regions28–34, which are critical mediators of emotion regulation and other responses to social stress28–31,35. Collectively, these finding suggest that oxytocin is a promising candidate to help restore the neurobiological impairments underlying AUD.
While oxytocin’s pharmacokinetic mechanisms of action remain unclear, evidence also suggests that GABAergic transmission could underlie both prosocial and alcohol-relevant effects of oxytocin observed in neurobiological and behavioral measurements36. Behaviorally, human and animal studies indicate that oxytocin reduces alcohol withdrawal, tolerance, craving and self-administration37–42. However, emerging literature emphasizes individual and contextual differences moderate oxytocin’s effects on social behavior43–49. These nuanced findings may be explained by the social salience hypothesis50, which proposes that rather than selectively enhancing prosocial behavior, oxytocin might amplify an individual’s current social tendencies which, without corrective intervention, may be maladaptive11,15,51. ABCT has demonstrated the ability to insulate couples from maladaptive relationship behaviors that are proven antecedents to hazardous drinking and relapse by cultivating and implementing new adaptive skills that facilitate recovery52,53. Notably, treatment gains are greater among couples who begin ABCT with poorer relationship functioning and greater psychiatric comorbidity5, and within-session gains predict positive ABCT outcomes52. Thus, combining oxytocin with a behavioral intervention such as ABCT will ensure that oxytocin has an adaptive platform to enhance the positive gains made within ABCT sessions.
The primary objectives of the current study are to (1) compare the efficacy of ABCT with oxytocin vs. placebo in reducing alcohol consumption, (2) compare the efficacy of ABCT with oxytocin vs. placebo in improving relationship functioning, and (3) use neuroimaging techniques to determine the effects of treatment on corticolimbic connectivity in response to alcohol and relationship conflict cues. We hypothesize that compared to the ABCT + placebo group, the ABCT + oxytocin group will demonstrate significantly greater reduction in alcohol consumption and significantly greater improvement in relationship functioning from baseline to end of treatment. We also hypothesize that functional connectivity between the prefrontal cortex (PFC) and amygdala (AMY) after treatment will be stronger in participants who receive ABCT + oxytocin compared to those who receive ABCT + placebo. Furthermore, we hypothesize that baseline PFC-AMY functional connectivity in response to alcohol vs. neutral cues will predict the magnitude of change in alcohol use, and that baseline PFC-AMY functional connectivity in response to relationship conflict vs. neutral cues will predict amount of change in relationship functioning.
2. Materials and Methods
2.1. Research Design
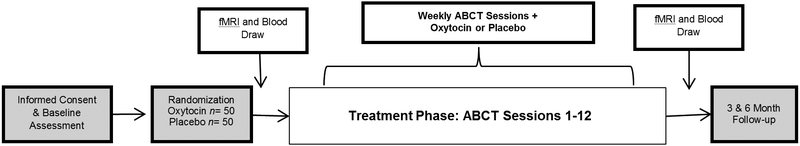
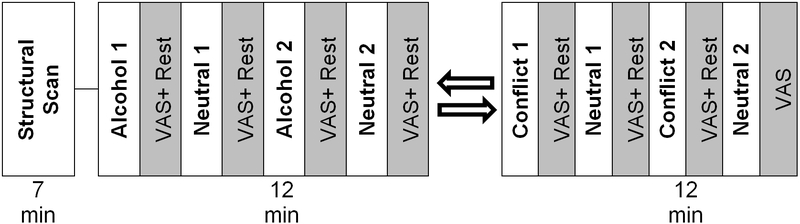
This is a 12-week, randomized, double-blind, placebo-controlled trial examining the efficacy of combining oxytocin (40 IU) with ABCT in the treatment of AUD. A repeated measures design with two intervention arms will be used: (1) ABCT + oxytocin compared to (2) ABCT + placebo. Participants will complete follow-up visits 3 months and 6 months following completion of the treatment phase (Figure 1). This study also will examine two validated AUD biomarkers and employ observational coding. Participants who meet eligibility criteria also will have the option to complete neuroimaging scans at baseline and at the end of the treatment phase to examine behavioral and functional-anatomic mechanisms of treatment response (Figure 2). The study will last for approximately five years.
Figure 1.
Overview of study design.
Figure 2.
Overview of fMRI session assessing response to alcohol, conflict, and neutral cues.
2.2. Participants
Participants are 100 couples (total N=200) aged 18–70 years comprised of the identified patient (IP) with current AUD and their romantic relationship partner. ABCT is equipped to treat couples if one or both partners meet diagnostic criteria for AUD. Thus, provided that at least one partner meets current AUD criteria, the couple is eligible to participate. Couples of any gender identity and sexual orientation are welcome to participate. This study will enroll an equal distribution of men and women IPs to account for sex differences in oxytocin response and maximize generalizability of findings. Additional inclusion criteria are: (1) English fluency and intellectual functioning sufficient to provide informed consent and accurately complete assessments and participate in treatment as assessed by a criterion of ≥ 26 on the Mini-Mental Status Exam 54, (2) more than one hazardous drinking episode (i.e., >7 drinks per week or > 3 drinks per occasion for women, or >14 drinks per week or >4 drinks per occasion for men) in the past 30 days by the IP, (3) married or cohabiting for ≥ 6 months, or in a committed relationship of at least 1-year duration, (4) maintenance of psychotropic medications on a stable dose for at least 4 weeks before study initiation, and 5) concurrent substance use disorders (e.g., marijuana) are acceptable provided that alcohol is the IP’s primary substance of choice. The inclusion of participants with other substance use disorders is essential because of the marked frequency of co-occurrence among patients with AUD. Exclusion criteria include: (1) meeting DSM-5 diagnostic criteria for a history of or current psychotic or bipolar affective disorders, (2) current suicidal or homicidal ideation and intent, (3) severe, unilateral intimate partner violence in the past 6 months as defined by the Revised Conflict Tactics Scale 55, (4) pregnancy or breastfeeding for women, (5) participants with clinically significant medical or psychiatric conditions that in the opinion of the investigators may adversely affect safety or study participation will be excluded and referred for treatment. Participants presenting with, (6) significant withdrawal symptoms as evidenced by a score of ≥10 on the revised Clinician Institute Withdrawal Assessment of Alcohol 56. Additional exclusions for the neuroimaging component of the study include claustrophobia; cardiac pacemaker; metal fragments in eye, skin, or body; heart valve replacement; brain clips; venous umbrella; history of aneurysm surgery; intracranial bypass, renal, or aortic clips; joint replacements; non-removable hearing aid, neurostimulator or insulin pump; shunts/stents; metal mesh/coil implants; metal plate/pin/screws/wires; or any other metal implants.
2.3. Procedures
This study was reviewed and approved by the Institutional Review Board (IRB) of the Medical University of South Carolina (MUSC). Following phone screening for preliminary eligibility, participants complete a face-to-face informed consent and baseline assessment. In a private room apart from their partner, participants are given a full description of the study procedures and asked to read and sign an IRB-approved informed consent form before any study procedures are conducted. During the baseline visit, participants complete a breathalyzer, urine pregnancy test (for women), ethyl glucuronide (EtG) testing, metal screening, a history and physical exam, urine drug screen, and a battery of standardized self-report and interview measures (see Table 1 for assessment measures and timeline). Ineligible individuals are referred clinically for treatment. Provided full eligibility criteria are met, participants are scheduled for a visit to complete a blood draw for phosphatidyl ethanol (PEth) and a functional magnetic resonance imaging (fMRI) session (described in the Neuroimaging Procedures section) prior to entering the treatment phase of the study.
Table 1.
Assessment instruments and timeline.
| Instrument | Purpose | BSL | Weekly | Wk 6 |
Wk 12 |
3M F/U |
6M F/U |
|---|---|---|---|---|---|---|---|
| Demographics Form | Characterize sample | X | |||||
| Mini Mental Status Exam 54 | Screen for cognitive deficits | X | |||||
| MINI International Neuropsychiatric Interview 110 | Assess DSM-5 psychiatric disorders | X | |||||
| Concomitant Medications Form | Assess concomitant medications | X | X | X | X | X | X |
| Time Line Follow-Back 64 | Primary outcome: AUD | X | X | X | X | X | X |
| Penn Alcohol Craving Scale 67 | Assess alcohol craving | X | X | X | X | X | X |
| Alcohol Use Disorders Identification Test 111 | Assess alcohol problems | X | X | X | X | X | |
| Alcohol Dependence Scale 112 | Assess domains affected by AUD | X | X | X | X | ||
| Readiness to Change Questionnaire 113) | Assess readiness to change AUD | X | X | X | X | X | |
| Clinical Institute Withdrawal Assessment of Alcohol-Revised 56 | Assess alcohol withdrawal | X | X | X | X | ||
| Traumatic Life Events Questionnaire 114 | Assess Trauma History | X | |||||
| PTSD Checklist 115 | Assess PTSD symptoms | X | X | X | X | ||
| Beck Depression Inventory-II 116 | Assess depression | X | X | X | X | X | X |
| Cognitive Emotion Regulation Questionnaire 117 | Assess emotion regulation | X | X | X | X | ||
| Dyadic Adjustment Scale-short form 65 | Primary outcome: Relationship functioning | X | X | X | X | X | X |
| Revised Conflict Tactics Scale 55 | Assess intimate partner violence | X | X | X | X | X | |
| Reasons for Violence Scale 118 | Assess reasons for partner violence | X | X | X | X | ||
| Treatment Services Review | Monitor service utilization | X | X | X | X | X | |
| Helping Alliance Questionnaire, Therapist and Client Version 119 | Asses therapeutic alliance | X | X | X | |||
| Treatment Adherence | Assess homework compliance | X | X | X | |||
| BSL=Baseline. Wk= Week. F/U = Follow-Up. | |||||||
2.4. Study Interventions
2.4.1. Study Medication, Dosage, and Administration.
Participants are randomized in a 1:1 manner to the oxytocin or placebo condition. Specifically, all participants and study staff including investigators, research assistants, assessors, clinicians, and supervisors will be blind to drug condition. In order to ensure that the treatment groups are balanced with respect to alcohol consumption (TLFB) and sex of the IP, a stratified randomization process will be used. Participants receive the same medication at each session, and partners within each couple are randomized to the same drug condition, meaning that both the IP and their partners will be taking their assigned medication (or placebo). A 40 IU dose of intranasal oxytocin or matching placebo (saline) is self-administered 30 minutes prior to the start of each weekly ABCT therapy session. The dose and timing of medication administration is based on past research in our group and others 47,57–60. A 40 IU dose has demonstrated extensive safety and efficacy, is within the normal dosing range, and one of the most common concentrations utilized in human research 61–63. The MUSC research pharmacy compounds and dispenses the oxytocin and matching placebo nasal sprays. Research staff instructs participants on the correct method of administration and observes participants’ self-administration. Randomization is carried out by a research pharmacist not involved in clinical management of participants in order to preserve the double-blind design.
2.4.2. Psychosocial Intervention: Alcohol Behavioral Couple Therapy (ABCT)
All participants receive 12 weekly ABCT therapy sessions delivered by trained clinicians consistent with the published manual 4. The main goal of ABCT is to concurrently reduce AUD symptom severity and improve relationship functioning. Patients receive psychoeducation pertaining to the interconnectedness of AUD and relationship functioning. AUD-focused components of the treatment help patients identify and manage cravings, urges to drink, and thoughts about alcohol use; enhance individual problem solving and decision-making abilities; identify and plan for “high-risk” situations in which vulnerability to relapse is heightened; learn drink refusal skills; and cope with a potential relapse. ABCT also teaches couples to work together to enhance reciprocity and communication skills in the relationship, increase positive rewards of initiating and maintaining drinking reductions and abstinence, and implement ways partners can help minimize and manage alcohol use triggers, assist each other with drink refusal skills, and help prevent relapse.
2.5. Primary Outcome Measures
The primary outcomes are (1) alcohol consumption and (2) relationship functioning. Alcohol consumption (e.g., percent days abstinent, percent heavy drinking days) is measured by the Time Line Follow-Back TLFB; 64 60 days prior to study entry, weekly during the 12 weeks of treatment, and at follow-up. The TLFB uses a calendar to stimulate recall, yields consistently high test-retest correlations, and correlates well with other self-reports and collateral reports. Relationship functioning will be assessed using the 7-item version of the Dyadic Adjustment Scale DAS-7; 65. The DAS-7 is a self-report survey based on the original 32-item measure 66. It is used to assess four domains relationship functioning and has demonstrated strong psychometric properties 65.
2.6. Secondary Outcome Measures
Additional AUD outcomes include alcohol craving PACS; 67 and ethanol metabolites and traditional biomarkers (e.g., ethyl glucuronide [EtG] and Phosphatidylethanol [PEth]) to corroborate participant self-reports of abstinence and alcohol use 68–70. PEth is among the most specific biomarkers used to detect heavy drinking and monitor abstinence 71–73. The conjugated alcohol metabolite EtG remains positive in urine for several days following cessation and is a useful biomarker of recent drinking in outpatient settings 74. Additionally, treatment satisfaction, working alliance, and functioning in domains related to AUD (e.g., depression, intimate partner violence, emotion regulation) are assessed. In order to explore secondary outcomes such as the effects of treatment on within-session behaviors, we will employ observational coding to assess frequency of positive, negative, and alcohol change talk behaviors using the System for Coding Couple Interaction in Therapy-Alcohol SCCIT-A; 75 in ABCT sessions 1, 6, and 12.
2.7. Neuroimaging Procedures
All neuroimaging scans are conducted at the MUSC Center for Biomedical Imaging, which houses a Siemens 3T Prisma MRI scanner (Siemens Medical, Erlangen, Germany). Neuroimaging sessions (Figure 2) last approximately 60 minutes each and occur at baseline and week 12. At baseline, personalized imagery scripts are be developed for alcohol and neutral cues according to the manualized procedures described by Sinha and Tuit 76. Participants also will develop a relationship conflict cue consistent with the procedures described by Flanagan and colleagues 77. During initial scanner tuning, localizing, and structural scanning, participants are shown “relaxing” images (i.e., 20 scenic pictures, each displayed for 30 seconds). For co-registration and normalization of functional images, a high-resolution T1-weighted MPRAGE anatomical image is acquired with the following parameters: TR = 2300 ms, TE = 2.26 ms, flip angle = 8°, field of view = 256 mm, slice thickness 1.0 mm, 192 slices. The scanning planes are oriented parallel to the anterior commissure–posterior commissure line.
A block design consisting of two 12-minute scans is employed: an alcohol cue scan and a relationship conflict cue scan. The alcohol cue scan is divided into four, 2-minute blocks of alcohol cues and neutral cues separated by 30 seconds of rest plus 30 seconds in which to complete response ratings using a modified version of the Visual Analogue Scale VAS; 78. During the blocks of alcohol cue, participants listen to an audio-recorded script describing in detail their most salient recent use of alcohol. During the blocks of neutral cue, participants listen to an audio-recorded script describing a relaxing, non-stimulating scenario. The relationship conflict block is also divided into four, 2-minute blocks of relationship conflict cues and neutral cues separated by 30 seconds of rest. During the relationship conflict blocks, participants listen to an audio-recorded script of a conflict task completed in the laboratory with their partner. The same excerpt is used for each block and each visit. To minimize potential carry-over effects, the scans are counterbalanced so that half of the participants in each treatment arm (e.g., oxytocin or placebo) are exposed to the alcohol cue first and the remaining participants in each group are exposed to the relationship conflict cue first. This order is preserved from pre- to post-treatment scanning for each participant. T2*-weighted gradient-echo planar images (EPI) are acquired with the following parameters: TR = 1100 ms, TE = 30 ms, flip angle = 65º, matrix 64 × 64, field of view = 192 mm, slice thickness = 3 mm with no gap, multiband factor = 3, with 51 slices to cover the entire brain. A gradient field map with the same spatial resolution and slices as the EPI is collected to correct for geometric distortions cause by magnetic field inhomogeneity.
2.8. Data Analytic Plan
2.8.1. Power Analysis
This study is powered to detect moderate treatment group differences in percent days abstinent (PDA) and percent of days of heavy drinking (PDH), and relationship functioning as measured by the TLFB and DAS-7, respectively at end-of-treatment (weeks 9–12). Assuming 2-sided hypothesis testing and alpha levels of 0.05, we will have 80% power to detect treatment group differences with effect sizes of 0.6 in the presence of 30% attrition (n=70 couples) during the treatment phase. This approach is consistent with treatment group differences in PDA (d=0.59) and PDH improvements (d=0.79) in a prior ABCT trial (n=102)5, although we recognize that that trial compared individual therapy vs. ABCT (not ABCT ± medication). In that study, PDA increased from 34.98% ± 29.17% to 80.52% ± 27.75% at the end of treatment in the ABCT group, and PDH decreased from 56.83% ± 28.87% to 10.52% ± 22.16% in the ABCT group. Incorporating an arcsine or other appropriate transformation to account for non-normality of these metrics will enable us to discern whether oxytocin enhances end of treatment PDA by an additional absolute 9% or greater (i.e., to 80.5% in the ABCT+ placebo group vs. 89.5% in the ABCT + oxytocin group) and whether oxytocin further decreases PDH by an absolute 6% or greater (i.e., to 10.5% in the in the ABCT+ placebo group vs. 4.5% in the ABCT + oxytocin group). Another prior ABCT trial found that DAS-7 scores (21.1 ± 6.7) remain relatively constant throughout treatment6. We will have 80% power to detect treatment group differences of 4 units of improvement in DAS-7 scores.79
2.8.1. General
Baseline clinical and descriptive characteristics will be examined and compared between treatment groups using chi-square tests, Fisher’s exact tests, t-tests, or Wilcoxon rank sum tests, as appropriate. Baseline characteristics that are significantly different between treatment groups will be included as model covariates, and sex will be included as a primary demographic covariate in order to account for potential sex differences in primary and secondary outcomes. The primary analysis will focus on end-of-treatment (i.e., the final three weeks of the treatment phase) outcomes among IPs using an intent-to-treat framework. Participants who decline to continue in treatment prior to session 12 will be invited to complete all remaining study assessments.
2.8.2. Clinical outcomes
We hypothesize that as compared to the ABCT + placebo group, the ABCT + oxytocin group will demonstrate (1) significantly greater reduction in alcohol consumption from baseline to end of treatment (PDA and PDH measured by TLFB), and (2) significantly greater improvement in relationship functioning (measured by DAS-7) from baseline to end of treatment. To test hypotheses 1 and 2, a generalized linear modeling (GLM) framework will be used with appropriate link functions, treating end-of-treatment (weeks 9–12) percent days abstinent (PDA), percent days hazardous drinking (PDH), and DAS-7 scores as dependent variables (in separate models), and treating treatment group (ABCT + oxytocin vs. ABCT + placebo) as the primary independent variable. Baseline values for PDA, PDH, and DAS-7 will be included as covariates, along with sex and other potentially significant baseline characteristics. Treatment group x sex interactions will be explored, to gain a sense of whether the treatment is more efficacious in men vs. women or vice versa. Since PDA and PDH may exhibit non-Gaussian distributional forms and/or zero-inflation, alternative modeling strategies (e.g., arcsine or Box-Cox transformations, two-part Hurdle models) may be explored. Model fit will be compared by examination of Likelihood Ratio chi-square values. If transformations are necessary, inverse-transformations will be used in conveying the model results. Generalized estimating equation models will be used in secondary analyses comparing time trends in the outcomes over the course of the study.
2.8.3. Neuroimaging outcomes
Pilot work conducted by our team compared the effects of a novel auditory relationship conflict cue versus a validated neutral cue on functional connectivity in corticolimbic brain regions. We also explored sex differences in neural correlates of relationship conflict. Participants demonstrated greater PFC-AMY functional connectivity during the relationship conflict cue compared to the neutral cue. Women, as compared to men, demonstrated stronger PFC-AMY connectivity to the relationship conflict cue compared to the neutral cue80. Thus, in the current study, we hypothesize that PFC-AMY functional connectivity after treatment will be greater in participants who receive ABCT+ oxytocin compared to those who receive ABCT + placebo. To test hypothesis 3, preprocessing and analysis of fMRI data will use FSL v 5.0 81. Preprocessing includes rigid-body head motion correction of EPI images within a run, high-pass temporal filtering (sigma = 150 seconds), geometric distortion correction, slice timing correction, spatial filtering (FWHM = 6 mm) and registration to the MNI standard brain template. ‘fsl_motion_outliers’ will be used to determine head motion outliers which will be used as a covariate of no interest in statistical analysis (together with the 6 rigid-body translation and rotation head motion parameters). The primary analysis of fMRI data will use psychophysiological interaction (PPI) modeling 82 with a seed region defined in the right AMY region from the Harvard–Oxford probabilistic structural atlas thresholded at 50%. Time series will be extracted from each participant’s right AMY using ‘fslmeants” after warping the AMY mask into each participant’s EPI space. This time series will serve as the physiological regressor for each run. The primary psychological regressors are based on the alcohol cue blocks in the alcohol run or the conflict cue blocks in the conflict run. The interaction between the primary psychological regressor for each run and the physiological regressor is the primary variable of interest.
To assess whether corticolimbic connectivity is modulated by oxytocin, the parameter estimate from the PPI interaction term will be extracted in the right and left inferior frontal cortex, opercular portion (IFC; −47, 18, 6) in each participant. The IFC regions-of-interest (ROI) are based on our preliminary study where the right AMY was functionally connected to the left IFC for the conflict cue, especially in women. In addition, the right IFC is strongly implicated in behavioral inhibition 83 and may not be activated to the same degree in participants with AUD 84. The parameter estimates from the left and right IFC regions in each IP at pre- and post-treatment will be used in statistical analyses testing hypotheses 1 and 2 (described above). Because the hypothesized ROI may not yield the most robust response, we will also conduct a whole-brain, voxel-wise PPI analysis to identify PFC regions that show the greatest change in right AMY connectivity as a function of treatment. The voxel-wise PPI analysis will be conducted separately for each run (alcohol, conflict) and session. Group-level analyses will be carried out using FLAME 1 (FMRIB’s Local Analysis of Mixed Effects) with session treated as a repeated measure and treatment group as a between-subjects factor. FLAME2 will generate z statistical images for each interaction term of interest. The final statistical maps will be voxel thresholded (at p = .05) and will indicate which regions show the greatest change in connectivity with the right AMY as a function of treatment and treatment group.
3. Discussion
This objective of this paper is to present the design and methodology for a Phase II randomized controlled trial examining the efficacy of intranasal oxytocin to enhance ABCT treatment outcomes. AUD is a prevalent, chronic, and debilitating condition for which few medications are approved. Several behavioral interventions, including ABCT, have garnered strong empirical support to reduce AUD symptoms, and some studies demonstrate that behavioral interventions for couples outperform individual approaches to treatment. However, a substantial number of patients with AUD do not complete treatment and continue to struggle with alcohol-related problems following treatment completion, suggesting that there is ample room to improve behavioral treatment approaches. Existing literature also demonstrates that combining behavioral intervention with medication is an effective approach to maximize treatment outcomes AUD 2,85. To our knowledge, this is the first to examine the efficacy of a medication-enhanced psychotherapy approach for couples with AUD, and one of very few adequately powered randomized controlled trials of intranasal oxytocin among patients with AUD.
Three medications are currently FDA-approved for the treatment of AUD and most target reduction in motivation to seek alcohol (e.g., intervening at the binge/intoxication stage of the 3-stage model of addiction 86. Given the heterogeneous nature of AUD etiology and course 87,88 and the limitations of currently available medications including non-compliance 89,90, developing new medications for AUD is a salient focus of ongoing research efforts. More recently, increased attention has been paid to medications that target brain stress systems and sensitize reward pathways to social stimuli that are commonly eroded in the course of addiction 91–95. As described more thoroughly in the introduction section, oxytocin is a medication that has demonstrated promise to achieve this goal 96,97. Additionally, examining the effects of medications such as oxytocin that have short (i.e., 3–4 hours) half-lives that have the specific potential to enhance within-session treatment gains is one approach that has not been examined extensively in the AUD field, although this approach has been examined more frequently for diagnoses such as posttraumatic stress disorder and anxiety disorders 60,98,99. Further, the combination of oxytocin with an evidence-based behavioral intervention such as ABCT may help maximize compliance, optimize treatment outcomes, and reduce alcohol consumption.
Despite extensive literature demonstrating that the combination of cognitive behavioral interventions with FDA-approved medication might be the most effective treatment approach for AUD 85, all previous studies have, to our knowledge, employed cognitive behavioral interventions for individuals, not couples-based interventions. Thus, no studies have yet to examine the comparative efficacy of medication only, couples-based cognitive behavioral therapy only, and combined medication and couple-based therapy in the treatment of AUD. Notably, recent studies have found that factors that might be enhanced by primary or adjunctive couples treatment, such as coping skills and alcohol use in patients’ social network, influence outcomes by treatment approach 100,101. Collectively, these findings suggest that the present study will make a substantial contribution to the existing literature by bridging the longstanding gap between medication-focused randomized controlled trials and those examining couples treatment for AUD. This study is equipped to measure critical factors in treatment safety and engagement including adverse events, the number of homework assignments completed during treatment, and relational factors such as psychological and physical intimate partner violence prior to and during treatment. This study is also the first to examine the effects of a medication versus placebo on within-session behaviors among couples and the extent to which those behaviors are associated with end of treatment outcomes.
This study is also the first to use a pre-post treatment neuroimaging design to examine neural correlates of treatment outcome among couples with AUD. Neuroimaging is a valuable addition to treatment development efforts, particularly in the AUD field, to examine prognostic indicators of both pharmacological and behavioral treatment response, and to characterize and clarify treatment outcomes 102–105. In the current study, we are using a manual-guided, validated imagery script procedure to examine neural correlates of alcohol and neutral cues, and a novel adaptation of this procedure will be used to target neural responses to couple conflict directly. This is a critical advancement in the literature because couple conflict is known to influence treatment seeking for substance use disorders and engagement, and is commonly cited as a source of stress associated with alcohol use and relapse abstinence 106,107,108. Thus, this study examines subjective and neurobiological responses to all three cues at baseline, and how neural responses at baseline and end of treatment are related to alcohol and relationship outcomes. Accomplishing this goal is particularly applicable to pharmacological treatment development efforts and to the study of oxytocin specifically, as neural mechanisms of action have not yet been clearly established for this medication.
In summary, the current study employs a multimodal and interdisciplinary approach to examine the efficacy of combining intranasal oxytocin with ABCT in the treatment of AUD among couples. The primary goal is to examine whether a 40 IU dose of intranasal oxytocin, as compared to placebo, reduces alcohol use and associated problems, and improves relationship functioning during 12 weeks of ABCT therapy. The findings will inform a rapidly growing literature examining oxytocin in the treatment of various psychiatric diagnoses including substance use disorders. In addition to examining safety outcomes such as adverse events on a weekly basis, this study also examines neurobiological outcomes, changes in within-session behaviors using observational coding, and moderators of treatment outcome such as sex, which is a known correlate of oxytocin treatment outcomes in various populations 45,109. The findings from this study will inform future research on oxytocin in the treatment of AUD, neuroimaging methodology applied to couples, and the potential translation of oxytocin to treatment settings for patients with AUD.
Acknowledgments
Funding: This research was supported by the National Institute on Alcohol Abuse and Alcoholism (R01AA027212; K23AA023845), the National Center for Advancing Translational Sciences (UL1TR001450), and the National Institute on Drug Abuse (K02DA039229).
Role of the funding source: The funding sources have no involvement in the study design, the collection, analysis and interpretation of data, the writing of this manuscript, or the decision to submit this manuscript for publication.
Abbreviations:
- ABCT
Alcohol Behavioral Couple Therapy
- AMY
amygdala
- AUD
alcohol use disorder
- BOLD
blood oxygen level dependent
- DSM-5
Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition
- EPI
gradient-echo planar images
- EtG
ethyl glucuronide
- FDA
Food and Drug Administration
- fMRI
functional magnetic resonance imaging
- IRB
Institutional Review Board
- MPRAGE
magnetization-prepared rapid gradient-echo
- MRI
magnetic resonance imaging
- MUSC
Medical University of South Carolina
- PEth
phosphatidyl ethanol
- PFC
prefrontal cortex
- PPI
psychophysiological interaction
- TLFB
Timeline Follow Back
- VA
U.S. Department of Veterans Affairs
- U.S.
United States.
- VAS
Visual Analogue Scale
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest to declare.
References
- 1.Anton RF, O’Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295(17):2003–2017. [DOI] [PubMed] [Google Scholar]
- 2.Balldin J, Berglund M, Borg S, et al. A 6‐Month Controlled Naltrexone Study: Combined Effect With Cognitive Behavioral Therapy in Outpatient Treatment of Alcohol Dependence. Alcoholism: Clinical and Experimental Research. 2003;27(7):1142–1149. [DOI] [PubMed] [Google Scholar]
- 3.Donovan DM, Anton RF, Miller WR, Longabaugh R, Hosking JD, Youngblood M. Combined pharmacotherapies and behavioral interventions for alcohol dependence (The COMBINE Study): examination of posttreatment drinking outcomes. Journal of Studies on Alcohol and Drugs. 2008;69(1):5–13. [DOI] [PubMed] [Google Scholar]
- 4.McCrady BS, Epstein EE. Overcoming Alcohol Problems: A Couples-Focused Program. Oxford University Press; 2008. [Google Scholar]
- 5.McCrady BS, Epstein EE, Cook S, Jensen N, Hildebrandt T. A randomized trial of individual and couple behavioral alcohol treatment for women. Journal of Consulting and Clinical Psychology. 2009;77:243–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCrady BS, Epstein EE, Hallgren KA, Cook S, Jensen NK. Women with alcohol dependence: A randomized trial of couple versus individual plus couple therapy. Psychology of Addictive Behaviors. 2016;30(3):287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCrady BS, Hayaki J, Epstein EE, Hirsch LS. Testing hypothesized predictors of change in conjoint behavioral alcoholism treatment for men. Alcoholism: Clinical and Experimental Research. 2002;26(4):463–470. [PubMed] [Google Scholar]
- 8.Meis LA, Griffin JM, Greer N, et al. Couple and family involvement in adult mental health treatment: a systematic review. Clinical psychology review. 2013;33(2):275–286. [DOI] [PubMed] [Google Scholar]
- 9.Owens MD, Hallgren KA, Ladd BO, Rynes K, McCrady BS, Epstein E. Associations between Relationship Satisfaction and Drinking Urges for Women in Alcohol Behavioral Couples and Individual Therapy. Alcoholism treatment quarterly. 2013;31(4):415–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quigley BM, Crane CA, Testa M. Dyadic alcohol use as a moderator of the relationship between partner conflict and subsequent day relationship functioning: a daily diary analysis. Alcoholism: Clinical and Experimental Research. 2013;37:24A–24A. [Google Scholar]
- 11.Testa M, Crane CA, Quigley BM, Levitt A, Leonard KE. Effects of administered alcohol on intimate partner interactions in a conflict resolution paradigm. Journal of Studies on Alcohol and Drugs. 2014;75(2):249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez LM, Overup CS, Neighbors C. Perceptions of partners’ problematic alcohol use affect relationship outcomes beyond partner self-reported drinking: Alcohol use in committed romantic relationships. Psychology of addictive behaviors. 2013;27(3):627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cranford JA, Floyd FJ, Schulenberg JE, Zucker RA. Husbands’ and wives’ alcohol use disorders and marital interactions as longitudinal predictors of marital adjustment. Journal of abnormal psychology. 2011;120(1):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lemke S, Brennan PL, Schutte KK, Moos RH. Upward pressures on drinking: Exposure and reactivity in adulthood. Journal of studies on alcohol and drugs. 2007;68(3):437–445. [DOI] [PubMed] [Google Scholar]
- 15.Testa M, Derrick JL. A Daily Process Examination of the Temporal Association Between Alcohol Use and Verbal and Physical Aggression in Community Couples. Psychology of Addictive Behaviors. 2013;28(1):127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magill M, Kiluk BD, McCrady BS, Tonigan JS, Longabaugh R. Active ingredients of treatment and client mechanisms of change in behavioral treatments for alcohol use disorders: Progress 10 years later. Alcoholism: Clinical and Experimental Research. 2015;39(10):1852–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCrady BS, Wilson AD, Muñoz RE, Fink BC, Fokas K, Borders AZ. Alcohol‐Focused Behavioral Couple Therapy. Family Process. 2016;55(3):443–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGregor IS, Bowen MT. Breaking the loop: Oxytocin as a potential treatment for drug addiction. Hormones and Behavior. 2012;61(3):331–339. [DOI] [PubMed] [Google Scholar]
- 19.Wilcox CE, Pommy JM, Adinoff B. Neural circuitry of impaired emotion regulation in substance use disorders. American Journal of Psychiatry. 2016;173(4):344–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koob GF. Brain stress systems in the amygdala and addiction. Brain Research. 2009;1293:61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nature Reviews Neuroscience. 2011;12(11):652–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park SQ, Kahnt T, Beck A, et al. Prefrontal cortex fails to learn from reward prediction errors in alcohol dependence. Journal of Neuroscience. 2010;30:7749–7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gu H, Salmeron BJ, Ross TJ, et al. Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. Neuroimage. 2010;53(2):593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McHugh MJ, Demers CH, Salmeron BJ, Devous MD Sr., Stein EA, Adinoff B. Cortico-amygdala coupling as a marker of early relapse risk in cocaine-addicted individuals. Front Psychiatry. 2014;5:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3(8):760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koban L, Pichon S, Vuilleumier P. Responses of medial and ventrolateral prefrontal cortex to interpersonal conflict for resources. Social Cognitive and Affective Neuroscience. 2013;9(5):561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hooker CI, Gyurak A, Verosky SC, Miyakawa A, Ayduk Ö. Neural activity to a partner’s facial expression predicts self-regulation after conflict. Biological Psychiatry. 2010;67(5):406–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomas B, Markus H, Aline V, Urs F, Ernst F. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron. 2008;58:639–650. [DOI] [PubMed] [Google Scholar]
- 29.Domes G, Heinrichs M, Glascher J, Buchel C, Braus DF, Herpertz SC. Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biological Psychiatry. 2007;62:1187–1190. [DOI] [PubMed] [Google Scholar]
- 30.Petrovic P, Kalisch R, Singer T, Dolan RJ. Oxytocin attenuates affective evaluations of conditioned faces and amygdala activity. J Neurosci. 2008;28(26):6607–6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dodhia S, Hosanagar A, Fitzgerald DA, et al. Modulation of Resting-State Amygdala-Frontal Functional Connectivity by Oxytocin in Generalized Social Anxiety Disorder. Neuropsychopharmacology. 2014;39:2061–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bethlehem R, van Honk J, Auyeung B, Baron-Cohen S. Oxytocin, brain physiology, and functional connectivity: a review of intranasal oxytocin fMRI studies. Psychoneuroendocrinology. 2013;38(7):962–974. [DOI] [PubMed] [Google Scholar]
- 33.Sripada CS, Phan KL, Labuschagne I, Welsh RC, Nathan PJ, Wood AG. Oxytocin enhances resting-state connectivity between amygdala and medial frontal cortex. The International Journal of Neuropsychopharmacology. 2013;16(2):255–260. [DOI] [PubMed] [Google Scholar]
- 34.Kumar J, Völlm B, Palaniyappan L. Oxytocin affects the connectivity of the precuneus and the amygdala: a randomized, double-blinded, placebo-controlled neuroimaging trial. International Journal of Neuropsychopharmacology. 2015;18(5):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sripada CS, Phan KL, Labuschagne I, Welsh R, Nathan PJ, Wood AG. Oxytocin enhances resting-state connectivity between amygdala and medial frontal cortex. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2013;16(2):255–260. [DOI] [PubMed] [Google Scholar]
- 36.Mitchell IJ, Gillespie SM, Abu-Akel A. Similar effects of intranasal oxytocin administration and acute alcohol consumption on socio-cognitions, emotions and behaviour: Implications for the mechanisms of action. Neuroscience & Biobehavioral Reviews. 2015;55:98–106. [DOI] [PubMed] [Google Scholar]
- 37.Becker HC, King CE, Griffin WC. Oxytocin reduces alcohol self-administration and stress-induced alcohol relapse behavior in mice. Alcohol. 2017;60:218. [Google Scholar]
- 38.King CE, McGinty JF, Becker HC. Effects of oxytocin on stress-induced reinstatement of alcohol-seeking in mice with and without a history of stress. Alcohol. 2017;60:231–232. [Google Scholar]
- 39.Pedersen CA, Smedley KL, Leserman J, et al. Intranasal oxytocin blocks alcohol withdrawal in human subjects. Alcoholism: Clinical and Experimental Research. 2013;37(3):484–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitchell JM, Arcuni PA, Weinstein D, Woolley JD. Intranasal oxytocin selectively modulates social perception, craving, and approach behavior in subjects with alcohol use disorder. Journal of addiction medicine. 2016;10(3):182–189. [DOI] [PubMed] [Google Scholar]
- 41.Mitchell J, Weinstein D, Arcuni P, Laxamana J, Woolley J. The effects of intranasal oxytocin on social cognition, implicit preferences and craving in alcoholics. Drug and Alcohol Dependence. 2015;146:e42. [Google Scholar]
- 42.Stevenson JR, Wenner SM, Freestone DM, et al. Oxytocin reduces alcohol consumption in prairie voles. Physiology & Behavior. 2017;179:411–421. [DOI] [PubMed] [Google Scholar]
- 43.Hurlemann R Oxytocin-augmented psychotherapy: Beware of context. Neuropsychopharmacology. 2016;42(1):188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bartels A Oxytocin and the social brain: beware the complexity. Neuropsychopharmacology. 2012;37(8):1795–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ditzen B, Nater UM, Schaer M, et al. Sex-specific effects of intranasal oxytocin on autonomic nervous system and emotional responses to couple conflict. Social Cognitive and Affective Neuroscience. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ditzen B, Schaer M, Gabriel B, Bodenmann G, Ehlert U, Heinrichs M. Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biological Psychiatry. 2009;65(9):728–731. [DOI] [PubMed] [Google Scholar]
- 47.Flanagan JC, Baker NL, McRae AL, Brady KT, Moran‐Santa Maria M. Effects of Adverse Childhood Experiences on the Association between Intranasal Oxytocin and Social Stress Reactivity among Individuals with Cocaine Dependence. Psychiatry Research 2015;229(1–2):94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mah BL, Bakermans‐Kranenburg MJ, IJzendoorn MH, Smith R. Oxytocin promotes protective behavior in depressed mothers: a pilot study with the enthusiastic stranger paradigm. Depression and Anxiety. 2015;32(2):76–81. [DOI] [PubMed] [Google Scholar]
- 49.Flanagan JC, Fischer MS, Nietert PJ, et al. Effects of oxytocin on cortisol reactivity and conflict resolution behaviors among couples with substance misuse. Psychiatry Research. 2018;260:346–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shamay-Tsoory SG, Abu-Akel A. The social salience hypothesis of oxytocin. Biological Psychiatry. 2016;79(3):194–202. [DOI] [PubMed] [Google Scholar]
- 51.Leonard KE, Eiden RD. Marital and family processes in the context of alcohol use and alcohol disorders. Annual Review of Clinical Psychology. 2007;3:285–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hallgren KA, McCrady BS. We‐Language and Sustained Reductions in Drinking in Couple‐Based Treatment for Alcohol Use Disorders. Family Process. 2016;55(1):62–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hallgren KA, Owens MD, Brovko JM, Ladd BO, McCrady BS, Epstein EE. Trajectories of drinking urges during individual-and couple-based cognitive-behavioral treatment for alcohol use disorders. Alcoholism Treatment Quarterly. 2015;33(2):161–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 55.Straus MA, Hamby SL, Boney-McCoy S, Sugarman DB. The Revised Conflict Tactics Scales (CTS2). Journal of Family Issues. 1996;17(3):283–316. [Google Scholar]
- 56.Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of Alcohol Withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA‐Ar). British Journal of Addiction. 1989;84(11):1353–1357. [DOI] [PubMed] [Google Scholar]
- 57.McRae-Clark AL, Baker NL, Moran-Santa Maria M, Brady KT. Effect of oxytocin on craving and stress response in marijuana-dependent individuals: a pilot study. Psychopharmacology. 2013;228(4):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cardoso C, Ellenbogen MA, Orlando MA, Bacon SL, Joober R. Intranasal oxytocin attenuates the cortisol response to physical stress: a dose–response study. Psychoneuroendocrinology. 2012. [DOI] [PubMed] [Google Scholar]
- 59.Guastella AJ, Hickie IB, McGuinness MM, et al. Recommendations for the standardisation of oxytocin nasal administration and guidelines for its reporting in human research. Psychoneuroendocrinology. 2013;38(5):612–625. [DOI] [PubMed] [Google Scholar]
- 60.Flanagan JC, Sippel LM, Wahlquist A, Moran-Santa Maria MM, Back SE. Augmenting Prolonged Exposure Therapy for PTSD with Intranasal Oxytocin: A Randomized, Placebo-Controlled Pilot Trial. Journal of Psychiatric Research. 2017;98:64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.MacDonald E, Dadds MR, Brennan JL, Williams K, Levy F, Cauchi AJ. A review of safety, side-effects and subjective reactions to intranasal oxytocin in human research. Psychoneuroendocrinology. 2011;36(8):1114–1126. [DOI] [PubMed] [Google Scholar]
- 62.Cardoso C, Ellenbogen MA, Orlando MA, Bacon SL, Joober R. Intranasal oxytocin attenuates the cortisol response to physical stress: A dose–response study. Psychoneuroendocrinology. 2013;38(3):399–407. [DOI] [PubMed] [Google Scholar]
- 63.Frijling JL, van Zuiden M, Koch S, et al. Efficacy of oxytocin administration early after psychotrauma in preventing the development of PTSD: study protocol of a randomized controlled trial. BMC psychiatry. 2014;14(1):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. Totowa, NJ: Humana Press; 1992. [Google Scholar]
- 65.Hunsley J, Best M, Lefebvre M, Vito D. The Seven-Item Short Form of the Dyadic Adjustment Scale: Further Evidence for Construct Validity. The American Journal of Family Therapy. 2001;29(4):325–335. [Google Scholar]
- 66.Spanier GB. Measuring Dyadic Adjustment: New Scales for Assessing the Quality of Marriage and Similar Dyads. Journal of Marriage and Family. 1976;38(1):15–28. [Google Scholar]
- 67.Flannery BA, Volpicelli JR, Pettinati HM. Psychometric properties of the Penn alcohol craving scale. Alcoholism: Clinical and Experimental Research. 1999;23(8):1289–1295. [PubMed] [Google Scholar]
- 68.Anton RF, Lieber C, Tabakoff B. Carbohydrate‐Deficient Transferrin and γ‐Glutamyltransferase for the Detection and Monitoring of Alcohol Use: Results From a Multisite Study. Alcoholism: Clinical and Experimental Research. 2002;26(8):1215–1222. [DOI] [PubMed] [Google Scholar]
- 69.Litten RZ, Bradley AM, Moss HB. Alcohol biomarkers in applied settings: recent advances and future research opportunities. Alcoholism: Clinical and Experimental Research. 2010;34(6):955–967. [DOI] [PubMed] [Google Scholar]
- 70.Allen JP, Litten RZ. Recommendations on use of biomarkers in alcoholism treatment trials. Alcoholism: Clinical and Experimental Research. 2003;27(10):1667–1670. [DOI] [PubMed] [Google Scholar]
- 71.Bertholet N, Winter MR, Cheng DM, Samet JH, Saitz R. How Accurate Are Blood (or Breath) Tests for Identifying Self-Reported Heavy Drinking Among People with Alcohol Dependence? Alcohol and Alcoholism. 2014;49(4):423–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Walther L, Bejczy A, Löf E, et al. Phosphatidylethanol is Superior to Carbohydrate‐Deficient Transferrin and γ‐Glutamyltransferase as an Alcohol Marker and is a Reliable Estimate of Alcohol Consumption Level. Alcoholism: Clinical and Experimental Research. 2015;39(11):2200–2208. [DOI] [PubMed] [Google Scholar]
- 73.Kechagias S, Dernroth DN, Blomgren A, et al. Phosphatidylethanol compared with other blood tests as a biomarker of moderate alcohol consumption in healthy volunteers: a prospective randomized study. Alcohol and Alcoholism. 2015;50(4):399–406. [DOI] [PubMed] [Google Scholar]
- 74.Dahl H, Carlsson AV, Hillgren K, Helander A. Urinary ethyl glucuronide and ethyl sulfate testing for detection of recent drinking in an outpatient treatment program for alcohol and drug dependence. Alcohol and Alcoholism. 2011;46(3):278–282. [DOI] [PubMed] [Google Scholar]
- 75.Owens MD, McCrady BS, Borders AZ, Brovko JM, Pearson MR. Psychometric properties of the System for Coding Couples’ Interactions in Therapy–-Alcohol. Psychology of Addictive Behaviors. 2014;28(4):1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sinha R, Tuit K. Imagery script development procedures manual. Charleston, SC: CreateSpace; 2012. [Google Scholar]
- 77.Flanagan JC, Yonce S, Calhoun CD, Back SE, Brady KT, Joseph JE. Preliminary Development of a Neuroimaging Paradigm to Examine Neural Correlates of Relationship Conflict. Psychiatry Research: Neuroimaging. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Childress AR, McLellan AT, O’Brien CP. Abstinent opiate abusers exhibit conditioned craving, conditioned withdrawal and reductions in both through extinction. British Journal of Addiction. 1986;81(5):655–660. [DOI] [PubMed] [Google Scholar]
- 79.Back SE, Flanagan JC, Jones JL, et al. Doxazosin for the treatment of co-occurring PTSD and alcohol use disorder: Design and methodology of a randomized controlled trial in military veterans. Contemporary Clinical Trials. 2018;73:8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Flanagan JC, Yonce S, Calhoun CD, Back SE, Brady KT, Joseph JE. Preliminary development of a neuroimaging paradigm to examine neural correlates of relationship conflict. Psychiatry Research: Neuroimaging. 2019;283:125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. NeuroImage. 2012;62:782–790. [DOI] [PubMed] [Google Scholar]
- 82.Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6(3):218–229. [DOI] [PubMed] [Google Scholar]
- 83.Swick D, Ashley V, Turken U. Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. Neuroimage. 2011;56(3):1655–1665. [DOI] [PubMed] [Google Scholar]
- 84.Campanella S, Absil J, Sinde CC, et al. Neural correlates of correct and failed response inhibition in heavy versus light social drinkers: an fMRI study during a go/no-go task by healthy participants. Brain Imaging and Behavior. 2017;11(6):1796–1811. [DOI] [PubMed] [Google Scholar]
- 85.Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. Journal of the American Medical Association. 2006;295(17):2003–2017. [DOI] [PubMed] [Google Scholar]
- 86.Koob GF. Addiction is a reward deficit and stress surfeit disorder. Frontiers in Psychiatry. 2013;4:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Johnson BA. Medication treatment of different types of alcoholism. The American Journal of Psychiatry. 2014;167(6):630–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72(8):757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Swift R, Oslin DW, Alexander M, Forman R. Adherence monitoring in naltrexone pharmacotherapy trials: a systematic review. Journal of Studies on Alcohol and Drugs 2011;72:1012–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pettinati HM, Weiss RD, Miller WR, Donovan D, Ernst DB, Rounsaville BJ. Medical Management Treatment Manual: A Clinical Research Guide for Medically Trained Clinicians Providing Pharmacotherapy as Part of the Treatment for Alcohol Dependence. Bethesda, MD: NIAAA;2004. [Google Scholar]
- 91.Berglund M, Thelander S, Salaspuro M, Franck J, Andréasson S, Öjehagen A. Treatment of Alcohol Abuse: An Evidence‐Based Review. Alcoholism: Clinical and Experimental Research. 2003;27(10):1645–1656. [DOI] [PubMed] [Google Scholar]
- 92.Chick J, Lehert P, Landron F. Does acamprosate improve reduction of drinking as well as aiding abstinence? Journal of Psychopharmacology. 2003;17(4):397–402. [DOI] [PubMed] [Google Scholar]
- 93.Maisel NC, Blodgett JC, Wilbourne PL, Humphreys K, Finney JW. Meta‐analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108(2):275–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014;311(18):1889–1900. [DOI] [PubMed] [Google Scholar]
- 95.Mason BJ. Emerging pharmacotherapies for alcohol use disorder. Neuropharmacology. 2017;122:244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59(1):11–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pedersen CA. Oxytocin, Tolerance, and the Dark Side of Addiction. International Review of Neurobiology. Vol 1362017:239–274. [DOI] [PubMed] [Google Scholar]
- 98.Hetrick SE, Purcell R, Garner B, Parslow R. Combined pharmacotherapy and psychological therapies for post traumatic stress disorder (PTSD). Cochrane Database Syst Rev. 2010;7(7). [DOI] [PubMed] [Google Scholar]
- 99.Guastella AJ, Howard AL, Dadds MR, Mitchell P, Carson DS. A randomized controlled trial of intranasal oxytocin as an adjunct to exposure therapy for social anxiety disorder. Psychoneuroendocrinology. 2009;34(6):917–923. [DOI] [PubMed] [Google Scholar]
- 100.Witkiewitz K, Roos CR, Tofighi D, Van Horn ML. Broad coping repertoire mediates the effect of the combined behavioral intervention on alcohol outcomes in the COMBINE Study: An application of latent class mediation. Journal of studies on alcohol and drugs. 2018;79(2):199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Worley MJ, Witkiewitz K, Brown SA, Kivlahan DR, Longabaugh R. Social network moderators of naltrexone and behavioral treatment effects on heavy drinking in the COMBINE study. Alcoholism: Clinical and Experimental Research. 2015;39(1):93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jasinska AJ, Stein EA, Kaiser J, Naumer MJ, Yalachkov Y. Factors modulating neural reactivity to drug cues in addiction: a survey of human neuroimaging studies. Neurosci Biobehav Rev. 2014;38:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schacht JP, Anton RF, Myrick H. Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta‐analysis and systematic review. Addiction Biology. 2013;18(1):121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Volkow ND, Baler RD. Brain imaging biomarkers to predict relapse in alcohol addiction. JAMA Psychiatry. 2013;70(7):661–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cabrera EA, Wiers CE, Lindgren E, Miller G, Volkow ND, Wang GJ. Neuroimaging the Effectiveness of Substance Use Disorder Treatments. J Neuroimmune Pharmacol. 2016;11(3):408–433. [DOI] [PubMed] [Google Scholar]
- 106.Hunter-Reel D, McCrady BS, Hildebrandt T, Epstein EE. Indirect effect of social support for drinking on drinking outcomes: The role of motivation. Journal of Studies on Alcohol and Drugs. 2010;71(6):930–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chermack S, Grogan-Kaylor A, Perron BE, Murray RL, De Chavez P, Walton MA. Violence among men and women in substance use disorder treatment: A multi-level event-based analysis. Drug and Alcohol Dependence. 2010;112(3):194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Grosso JA, Epstein EE, McCrady BS, et al. Women’s motivators for seeking treatment for alcohol use disorders. Addictive behaviors. 2013;38(6):2236–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rilling JK, DeMarco AC, Hackett PD, et al. Sex differences in the neural and behavioral response to intranasal oxytocin and vasopressin during human social interaction. Psychoneuroendocrinology. 2014;39:237–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- 111.Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG. The Alcohol Use Disorders Identification Test. Geneva, Switzerland: World Health Organization; 2001. [Google Scholar]
- 112.Skinner HA, Allen BA. Alcohol dependence syndrome: measurement and validation. Journal of Abnormal Psychology. 1982;91(3):199. [DOI] [PubMed] [Google Scholar]
- 113.Miller WR, Tonigan JS. Assessing drinkers’ motivation for change: the Stages of Change Readiness and Treatment Eagerness Scale (SOCRATES). Psychology of Addictive Behaviors. 1996;10(2):81. [Google Scholar]
- 114.Kubany ES, Leisen MB, Kaplan AS, et al. Development and preliminary validation of a brief broad-spectrum measure of trauma exposure: the Traumatic Life Events Questionnaire. Psychological Assessment. 2000;12(2):210. [DOI] [PubMed] [Google Scholar]
- 115.Weathers FW, Litz BT, Herman D, Huska J, Keane T. The PTSD checklist—civilian version (PCL-C). Boston, MA: National Center for PTSD; 1994. [Google Scholar]
- 116.Beck A, Steer R, Brown G. Manual for the BDI-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 117.Garnefski N, Kraaij V. Cognitive emotion regulation questionnaire – development of a short 18-item version (CERQ-short). Personality and Individual Differences. 2006;41(6):1045–1053. [Google Scholar]
- 118.Stuart GL, Moore TM, Gordon KC, Hellmuth JC, Ramsey SE, Kahler CW. Reasons for intimate partner violence perpetration among arrested women. Violence Against Women. 2006;12(7):609–621. [DOI] [PubMed] [Google Scholar]
- 119.Luborsky L, Barber JP, Siqueland L, et al. The revised Helping Alliance questionnaire (HAq-II): psychometric properties. The Journal of Psychotherapy Practice and Research. 1996;5(3):260. [PMC free article] [PubMed] [Google Scholar]