Abstract
Purpose:
Heavy ion and proton brain irradiations occur during space travel and in Hadron therapy for cancer. Heavy ions produce distinct patterns of energy deposition in neuron cells and brain tissues compared to X-rays leading to large uncertainties in risk estimates. We make a critical review of findings from research studies over the last 25 years for understanding risks at low dose.
Conclusions:
A large number of mouse and rat cognitive testing measures have been reported for a variety of particle species and energies for acute doses. However tissue reactions occur above dose thresholds and very few studies were performed at the heavy ion doses to be encountered on space missions (<0.04 Gy/y) or considered dose-rate effects, such that threshold doses are not known in rodent models. Investigations of possible mechanisms for cognitive changes have been limited by experimental design with largely group specific and not subject specific findings reported. Persistent oxidative stress and activated microglia cells are common mechanisms studied, while impairment of neurogenesis, detriments in neuron morphology, and changes to gene and protein expression were each found to be important in specific studies. Future research should focus on estimating threshold doses carried out with experimental designs aimed at understating causative mechanisms, which will be essential for extrapolating rodent findings to humans and chronic radiation scenarios, while establishing if mitigation are needed.
Keywords: Space radiation, Hadron therapy, high LET radiation, cognitive detriments, neuroinflammation
Introduction
Studies with rodents at particle accelerators generating heavy ion beams is the principle approach for elucidating potential cognitive and other central nervous system (CNS) risks from heavy ion irradiation during space travel (Cucinotta et al. 2014). These studies are of interest for understanding normal tissue damage to cancer patients treated with protons and carbon beams (Shulz-Ertner and Tsujii, 2007) for doses that occur near the periphery of the tumor volume. Studies in rodents have detailed significant cognitive changes at moderate doses of heavy ions (0.1 Gy or more), however with few studies at low doses (<0.1 Gy) or at low dose-rates. For medical radiation exposures, normal tissue risks adjacent to a tumor are a major consideration, while for the whole-body exposures in space missions performance detriments during a mission should be avoided and post-mission risks limited to acceptable values. Tissue reactions (also denoted as deterministic or non-cancer effects) occur above dose thresholds usually attributed to damage of a significant number of cells within a tissue, immune system depression, or other tissue effects (ICRP 2011, NCRP 2000). Tissue reaction severity is expected to increase with dose, including an inverse correlation between dose and latency. Dose limits for occupational exposures include limits for tissue reactions of the skin, blood forming system, and lens. Dose limits are intended to avoid all risk using estimated doses below a likely threshold for clinically significant effects.
Cognitive risks for low to moderate radiation doses likely involve distinct factors compared to damage to other tissues or high dose CNS effects (Tolifon and Fike 2000), including distinct modes of oxidative damage, neuroinflammation, changes to neuro-receptors and synapse plasticity, and possible disruption of the connectome. Defining clinical-significant effects for heavy ion exposures based on rodent studies has not been possible in the past due to issues in extrapolating results to humans along with the scarcity of dose threshold studies. For astronauts clinical significance during a mission may require a unique definition because of the high performance needed for mission operations. Risk assessment could consider relative biological effectiveness (RBE) factors and extrapolate rodent study findings using observations of cognitive changes in cancer patients following conventional radiation therapy, however it would be limited by the higher doses incurred in patients and confounding issues of their diseases. Furthermore most proton and heavy ion studies have not been made with a reference radiation as part of the study and therefore very little RBE information is available. Mechanisms of cognitive changes induced by heavy ions should be understood to elucidate whether or not qualitative differences occur or play a minor role compared to X-rays in order to validate an RBE approach. Progress in elucidating the mechanisms leading to cognitive changes in heavy ion experiments has been slow with most studies focusing on radiation quality dependences at higher heavy ion doses than occur in space (>0.05 Gy) using a variety of cognitive testing approaches. Few studies have studied possible mechanisms in detail with most studies not showing correlations in individual animals.
In this paper we make a critical review of research on cognitive detriments in rodent models made with proton and heavy ion beams at accelerators over the last ~25 years. A primary goal for research should be to address the determination of dose limits for early or late effects related to cognitive changes or whether other tissue reaction limits would likely prevent such risks from occurring for most exposure scenarios. We first review the doses and types of radiation encountered on space missions, which show that cumulative heavy ions doses for all charge groups will be less than 0.04 Gy per year. Experimental studies are then reviewed focusing on studies at lower doses (<0.2 Gy acute). Finally we make recommendations for new studies that would help establish threshold doses and lead to a recommendation of a dose limit.
Physics Considerations
The energy spectra and tissue doses of the GCR has been accurately established over the last few decades of research. Models of the GCR spectra consider the effects of solar modulation over the approximately 11-year solar cycle within our solar system. Solar cycle modulation can be predicted using empirical models using neutron monitor counts measured on the Earth’s surface or sunspot numbers (Badhwar et al 1994). The Voyager I spacecraft has determined the energy spectra of the major GCR elements up to kinetic energies of a few hundred MeV/u (Chadwick et al 2016), while numerous balloon and spacecraft experiments have determined the energy spectra at medium energy (George et al 2009, Adriana et al 2009) or above a few 1000 MeV/u where solar modulation is minimal. This allows models of high accuracy developed by the authors as shown in Figure 1 panels A and B for GCR protons and iron particles where fluxes with kinetic energies range from about 100 to 10,000 MeV/u are most numerous. The ranges of heavy ions in materials at the same velocity as a proton scales as A/Z2 where A and Z are the mass and charge numbers of the heavy ion. Because of their shorter ranges the median energy in brain tissues for heavy ions is ~1500 MeV/u, while protons and helium will have a lower median (<500 MeV/u) because of larger ranges along with secondary particle production in nuclear fragmentation (Kim et al 2015).
Figure 1.

Cosmic ray energy and charge composition, and absorbed doses in the brain. (A) Proton energy spectra comparisons of model to measurements near-Earth for several time periods and for the local inter-stellar (LIS) environments. (B) Iron particle energy spectra comparisons of model to measurements near-Earth for several time period and for the local inter-stellar environments. (C) The contribution of protons, helium and several heavy ion charge groups to annual brain doses for average spacecraft shielding (10 g/cm2 aluminum). Model calculations for different times in the solar cycle and for the LIS spectra are shown. (D) The attenuation of cosmic ray doses charge numbers Z=1,2 (protons and helium) and Z>2 (heavy ions) versus tissue depth. Results show that doses do not vary appreciably for different brain regions.
The space environment and charged particle transport have been adequately characterized in the past with predictive models of dose and dose equivalent having an overall agreement to measurements of about 10% (Cucinotta et al 2008; Zeitlin et al 2013; Kim et al 2014). Table 1 shows comparisons (Kim et al 2014) to the recent measurements from the Mar Surface Lander (MSL) detector (Zeitlin et al 2013, Hassler et al 2014 ). Transit times to and from Mars are about 6 months and heavy ion doses are significantly reduced on the Mars surface due to the 2π shielding of the planet and the Mars atmosphere which consists of about 18 g/cm2 of CO2 in the vertical direction (Kim et al 2014). Figure 1 panel C shows predictions of the charge distribution of absorbed doses at 10 cm depth brain tissue for a typical spacecraft shield of 10 g/cm2 of aluminum. Figure 1 panel Dshows results versus depth in tissue for 10 and 20 g/cm2 of aluminum. Results are shown for the local interstellar spectra (LIS) outside the influence of the solar wind and sun’s magnetic field, the recent deep solar minimum in 2009 with the highest recorded near-Earth particle fluxes, and for an average period in the 11-year solar cycle.
Table 1.
Comparison of NSCR-2012 Model (Kim et al 2015) to measurements for average dose-rate and dose equivalent rate on cruise phase to Mars (Zeitlin et al 2013) and on Martian surface (Hassler et al 2014).
| GCR DOSE RATE (mGy/d) |
GCR DOSE EQUIVALENT RATE (mSv/d)y) |
|
|---|---|---|
| Model Cruise to Mars | 0.445 | 1.80 |
| Model Mars surface | 0.20 | 0.72 |
| RAD Cruise to Mars | 0.481±0.08 | 1.84±0.33 |
| RAD Mars Surface | 0.205±0.05 | 0.70±0.17 |
Heavy ions traversing shielding materials or tissue lose energy from atomic/molecular collisions and undergo nuclear absorption processes with mean free paths of about 10–20 cm of tissue for iron particles at median GCR energies. There is a gradual loss of heavy ions in materials and tissue, while the fluence of lighter particles such as protons and helium ions may increase due to heavy ion absorption and fragmentation of target nuclei in nuclear interactions. Mesons, gamma-rays and electron-positron pairs are also produced which is a factor for space radiation exposures but play no role in Hadron therapy because of the lower beam energies (<400 MeV/u). Absorbed doses within the astronaut brain from GCR is dominated by proton and helium doses, however the heavy ion components (Z>2) are a major concern due to the possibility of large RBE’s or qualitative differences with X-rays or gamma-rays. The heavy ion doses for all particles are at most 0.04 Gy/y, with more than half the dose for particles with kinetic energies above 1500 MeV/u, while the Z>10 dose is less than 0.01 Gy/y. Brain doses at solar maximum would be about half of those shown for average solar cycle conditions. Heavy ion dose-rates on International Space Station missions are reduced several fold from the results of Figure 1 and Table 1 because of the Earth’s magnetic field and solid body (NCRP 2000; Cucinotta et al. 2008).
Cognitive Changes in Rodents after Heavy Ion Exposure
A major focus of space CNS radiobiology has been studies employing cognitive tests in mice and rats. The extrapolation of these results to humans has not been established, while issues of use of higher doses and dose-rates compared to those that occur in space has not been adequately addressed. Studies are diverse utilizing a variety of cognitive tests, doses, particle types, sex, age at exposure and time after exposure. These studies indicate significant deficits will occur at doses of 0.5 Gy and higher of high charge and energy (HZE) particles, while both positive and negative results were reported at lower doses. Particle species most often used in experiments are Fe, H, O, Ti and Si. Rabin et al (2007; 2011) has been the only investigator that has detailed dose responses with 3 or more doses per cognitive test. Therefore information on dose thresholds in rodents has not been reliably established. No studies have been made for a chronic exposure or for the complex radiation field that occurs in space (Kim et al 2015). Cognitive detriments suggested by various studies suggest the striatum, hippocampus, and the pre-frontal cortex are impacted. Older studies by Joseph and collaborators (see eg Joseph et al 1992; Rabin et al. 1991; 2000) of sensorimotor deficits, conditioned taste aversion, and fixed operant responses were reviewed earlier by Cucinotta et al 2014 showing differential results for heavy ion doses below 1 Gy.
The relationship between radiation quality and possible dose thresholds was studied for the HZE particle-induced disruption for fixed-operant responses, which tests for a broad range of complex learning, in Sprague–Dawley rats by Rabin et al (2007; 2011). Lower dose thresholds were found for 16O of lower LET compared to heavier particles of higher LET (Rabin et al 2011). These results suggested that particle fluence and not dose or LET is a better predictor of the effectiveness of different particles for neurobehavioral dysfunction. A similar conclusion was found for novel object recognition (NOR) and spatial memory (Rabin et al 2011). Figure 2 shows results from Rabin et al (2007) for NOR, which tests for memory deficits (Sivakumaran et al 2018), in Sprague-Dawley rats. Figure 2 (Panel A) shows the dose response for the discrimination index for novel and familiar objects after doses of 12C (290 MeV/u) particles. Figure 2 (Panel B) shows Rabin et al (2011) estimates of threshold doses testing for a significant differences between control and irradiated animals. The threshold doses reported by Rabin et al (2007; 2011) are above the annual GCR doses in free space for some but not all particle types shown in Figure 1. Figure 2 (Panel A) is suggestive of an inverted U-shape. Here detriments in NOR occur at lower dose but return to baseline values for doses of a few Gy suggestive of a compensating response mechanism at higher doses. Of note is that studies with X-rays, gamma-rays and low LET protons of cognitive changes at doses of a few Gy often yield negative results (Rabin et al 2002; Rabin et al 2015; Haerich et al 2012), however if the true dose response is an inverted U-shape, cognitive detriments would occur at lower doses then tested.
Figure 2.
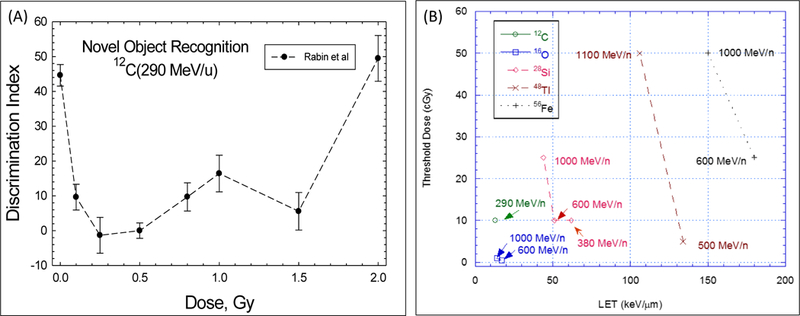
Dose response and threshold doses for novel object recognition (NOR) in Sprague-Dawley rats. (A) Results from Rabin et al. (2007; 2011) are re-plotted as a discrimination index illustrating the preference of control (unirradiated) animals to explore a novel object, while irradiated animals may show no preference for a novel versus familiar object. (B) Dose threshold estimates for detriments in NOR from Rabin et al (2007; 2011) for several particle types and energies.
Raber and co-workers have investigated various effects of radiation exposures on rodent models. They have studied the sex dependence effects of radiation exposure on C57BL/6J and B6D2F1 mouse models and have found that female mice were more sensitive to radiation exposure that they have attributed to higher levels of oxytocin in female then in male mice (Villasana et al 2010 and Raber et al 2016). Raber and coworkers also have studies where irradiation have resulted in enhancement rather than impairment of cognitive performance. Enhancement in cued fear conditioning (amygdala dependent and hippocampus-independent) was observed during16O irradiation of 6 month old B6D2F1 male and female mice, but contextual fear conditioning (hippocampus dependent) was unaffected (Raber et al 2015a). In addition, exposures of 2 month old C57BL/6 male mice to 28Si (Raber et al 2014) and 56Fe (Raber et al 2013) particle radiation resulted in enhanced long term potentiation (LTP) in CA1 and contextual fear conditioning in hippocampus, respectively. They have suggested that this “enhancement” in cognition due to irradiation might be due to injury in other brain regions and the subsequent compensation in the hippocampus. Moreover, radiation exposure effects depend on the cognitive test used and genetic factors involved. For instance, 56Fe exposure enhanced water maze performance in apoE3 mice, while reduced cognitive performance in apoE2 and apoE4 mice (Haley et al 2012). Exposures to 28Si and 48Ti radiation of 6 – 7 month old B6D2F1 male and female mice reduced contextual fear conditioning (Raber et al 2015b). These results highlight the importance of strains with distinct genetic backgrounds.
In other studies Raber and coworkers (Iancu et al 2018) have looked into the effects of environmental stressors in addition to radiation effects on brain function. They discussed the qualitative differences between environmental challenges (such as stress) and radiation where stress leads to actual shift in behavioral and cognitive performances but do not completely alter the genotype-phenotype correlations, while irradiation does not directly alter behavioral and cognitive performances but has great effects on genotype-phenotype correlations. Using genetically heterogeneous mouse models (HS/Npt), they evaluated the stability of genetic component of hippocampus-dependent contextual fear conditioning and amygdala-dependent/hippocampus-independent cued fear conditioning upon exposures to 56Fe and 28Si particle radiation and 137Cs γ rays. Their results suggests that hippocampus is particularly sensitive to long-term effects of environmental stressors that might affect radiation effects. In addition, different particles (Fe vs Si) lead to involvement of different biological mechanisms, pathways and ontologies.
Individual Variations
The studies of Britten (Britten et al 2014; 2017; 2018; Wyrobek and Britten 2016) have considered HZE particle irradiation tests in rats for neurocognitive tasks regulated by the prefrontal cortex and hippocampus, with a focus on the possibility of impairments of complex executive functions. In one set of studies male Wistar rats received either sham treatment or were irradiated and tested 3 months later for their ability to perform attentional set shifting (Britten et al 2014) or spatial memory learning (Wyrobek and Britten 2016). Compared to the controls, rats that received 0.2 Gy of 56Fe(1000 MeV/u) particles showed significant impairments in their ability to complete the attentional set-shifting test, with only 17% of irradiated rats completing all stages as opposed to 78% of the control rats (Britten et al 2014). Individual variations amongst control and irradiated rats were studied for spatial memory learning in 56Fe exposures of 0.05, 0.1, 0.15 and 0.2 Gy (Wyrobek and Britten 2016). Interestingly the authors were able to divide subjects into poor and good learners with a linear dose response found for poor learners and no risk at these doses for good learners. A similar grouping of poor and good responders were found in proton exposures by Davis et al (2014) studying attentional deficits and dopaminergic protein levels. Since astronauts are certainly pre-selected as good learners and the heavy ion doses on a Mars mission are less than values used in the experiments these results suggest low or perhaps no risk would occur on a mission to Mars. However a more complex picture is suggested by studies of Raber and co-workers (Iancu et al 2018). They utilized a genetically heterogeneous mouse model, dense genotype data, and behavior shifting challenges to explore and quantify the size and stability of the genetic component of fear learning and memory-related measures. Results suggested that exposure to ionizing radiation (Fe, Si and gamma-rays) and other external stressors altered genotype–phenotype correlations, although different behavioral and cognitive measures were affected to different extents.
Mechanisms of Radiation Induced Cognitive Detriments
We next consider several areas of research that have been made with heavy ion beams that have considered possible associative or correlative mechanisms leading to cognitive detriments. Figure 3 illustrates several such areas in a schematic representation. These include stress due to reactive oxygen species (ROS), neuroinflammation, impairment of hippocampal neurogenesis, and changes to neuron morphology. Neuroinflammation is a major factor associated with neurodegenerative diseases, such as AD and Parkinson’s disease (PD) (Perry 2010), while neuron morphological changes also play a role in many cognitive diseases and aging (Conrad et al 2017). A limitation in many of these studies is that the doses employed are often an order of magnitude larger than would occur on long-term space mission as described in Figure 1 and Table 1. Biochemical studies of changes to dopameric neurons (eg. Joseph et al. 1999; Rice 2009; Rice 2008; Davis et al 2014) and long-term potentiation (eg Rudobeck et al 2014) were reviewed previously by Cucinotta et al (2014).
Figure 3.

An illustration showing the possible neuronal damage mechanisms linked to cognitive detriments as caused by radiation exposures.
Neuroinflammation
Neuroinflammation is characterized by activation of resident microglia and astrocytes and local expression of a wide range of inflammatory mediators. ROS are key signaling molecules in the inflammatory response. Neuroinflammation has been studied in the mouse brain following exposure to HZE particles (see review by Cucinotta et al 2014). Responses at low to moderate dose and early time points after irradiation have not been accurately determined. Neuroinflammation following low LET radiation responses is minimal at doses less than 10 Gy before 30 d post-irradiation (Moravan et al 2011). In mice HZE irradiation was about 3 times more effective compared to gamma-rays for induction of an acute neuroinflammatory response, and increased expression of the CCR2 receptor was observed in the subgranular zone 9 months after 1–3 Gy 12C or 56Fe irradiation (Rola et al., 2005). In vitro studies have shown that HZE particles produce significantly higher levels of ROS compared to low LET radiation (Limoli et al 2007), and a persistent inflammatory response at lower doses for post-irradiation times of 1-month or more (Parihar et al 2016, Cherry et al. 2013). However it is not clear if a significant induction of neuroinflammation occurs in the hippocampus at lower HZE particle doses (0.1 to 0.5 Gy) within the first 30 d post-irradiation (Rola et al, 2008) with less known for other brain regions. Therefore current data do not support a conclusion on whether cognitive changes associated with neuroinflammation would occur during a space mission impacting astronaut performance or following mission conclusion if at all. On the other hand for cancer patients undergoing Hadron therapy neuroinflammation plays a significant role based on current evidence.
Microglia are the primary resident immune cells of the CNS. Microglia makeup approximately 12% of the cells in the brain and are derived from myeloid cells in the periphery. The density of microglial cells varies by brain region in adult human (0.5 – 16.6%) (Block 2007) and their distribution, morphology and dynamic behavior changes with age (Wong 2013). Microglial cells play an important role during neuronal circuit and synaptic maturation during development (Weinhard et al 2018, Miyamoto et al 2016). Dynamic interactions between synapse and microglia that includes partial presynaptic phagocytosis (trogocytosis) and induction of spine head filopodia is observed in hippocampal cultures (Weinhard et al 2018). In the developing somatosensory cortex, microglia have induced formation of filopodia mediated by local increase in Ca2+ and actin accumulation (Miyamoto et al 2016). Disruptions in synapse development have been associated in neurodevelopmental disorders such as autism and schizophrenia (Miyamoto et al 2016).
Microglia monitor the brain environment and act as host defense in response to stimuli by releasing pro-inflammatory molecules during their activated state. However, other circumstances have caused over-activation of microglia that are highly detrimental to neurons and other brain cells (Block 2007). A variety of insults can lead to activation of microglia including reactive oxygen species (ROS), cells undergoing apoptosis, and damaged dendrites. Activated microglia cells can remove damage synapses and have been suggested to eliminate entire dendritic trees in damaged neurons (Kettenman et al 2013). Over-activated microglia cells (Kettenman et al 2013) release cytotoxic factors such as superoxide, nitric oxide (NO) and TNFα, which may lead to a damage cascade and a neuro-degenerative state. These changes in neuro-inflammatory response leading to self-perpetuating degenerative process have been linked to the development and progression of chronic neurodegenerative diseases, which include Alzheimer’s disease, Parkinson’s disease, age-related macular degeneration (AMD), Amyotropic lateral sclerosis (ALS) and prion disease (Wong 2013, Perry 2010).
Microglial activation has been experimentally monitored by changes in microglial morphology (ramified, amoeboid), measuring various biomarkers (CD68/ED-1, MH-II, OX-6) and expression of inflammatory cytokines and chemokines (IL-1β, TNFα, IL-6 and interferon-γ, COX-2, IL-18, IFNγ-inducible protein 10). Although the activation of microglia is a delayed response increasing over several weeks, it is a sustained response for several months post irradiation. Distinct magnitude and duration of microglial activation have been observed after exposure to different radiation quality which includes 137Cs γ-rays (Greene-Schloesser et al 2012, Moravan et al 2011, Jenrow et al 2013, Schindler et al 2008, Schnegg et al 2013, Ramanan et al 2009, Conner et al 2010, Greene-Schloesser et al 2014), X-rays (Mizumatsu et al 2003, Rola et al 2004, Monje et al 2002, Tada et al 2000, Cheng et al 2016, Kalm et al 2009, Son et al 2014, Hwang et al 2006, Archaya et al 2016), protons (Sweet et al 2014, Raber et al 2016b), carbon (Rola et al 2005), oxygen and titanium (Parihar, et al. 2016) and iron (Rola et al 2008). Inhibition of colony stimulating factor-1 receptor (CSF1R) reducing gene expression of microglial marker and eliminating microglia post-irradiation have been shown to improve radiation-induced cognitive dysfunction (Archaya et al 2016).
Radiation and Neuronal Mitochondria
Mitochondria are dynamic cell organelles that are essential regulators of intracellular calcium signaling, reactive oxygen production, cellular metabolism, survival, growth and death. To maintain functional homeostasis, mitochondria implement several processes including constant merging and diving apart (fusion and fission), moving throughout the cell (transport), selectively remove damage from network (mitophagy), generating new mitochondrial contents (biogenesis) and interacting with other organelles (Chen and Chan 2009; Labbe et al 2014). As neurons have high temporal and spatial metabolic needs, the dynamic balance among these processes is notably important for neuronal function and survival (Mattson et al 2008; Fischer, et al 2016). Altered mitochondrial fusion and fission and mitochondrial transport are associated with impaired neuronal viability (Rintoul and Reynolds 2010). Mitochondria contribute to energy requirements in both presynaptic and postsynaptic transmission (Mattson et al. 2008, Devine and Kittler 2018). Mitochondria are collocated with about 10% of spines or synapses (Li, et al. 2004). Other roles for mitochondria include local protein synthesis which is important for the plasticity of spines and synapses, pruning of dendrites and spines and for synaptic transmission (Devine and Kittler 2018).
Mitochondrial dysfunction is critical for normal neuronal activity and maybe crucial for cell survival. For instance, mitochondrial structural and functional damages can impair the ability of the organelle to adapt to the metabolic needs of the cellular environment and result in dysregulated calcium signaling and deficits in oxidative phosphorylation (Greenwood 2007). Mitochondrial size was found to decrease after a traumatic brain injury (TBI) with increased fission events caused by the increase in translocation of dynamin-related protein 1 (Drp1), which is a key regulator of mitochondrial fission (Fischer 2016). Disruption in the balance of mitochondrial fission and fusion has also been an area of intense investigation in the pathogenesis and progression of neurodegenerative diseases (Flippo 2017, Gao 2017). Disturbance of mitochondrial dynamic regulators (such as Drp1, pDrp1, SNO-Drp1, Mfn½, Fis1 and Miro 1) results in abnormalities in fusion, fission, trafficking and proper transport and localization of mitochondria in neurites and synapses that eventually leads to degeneration and neuronal death (Gao 2017).
Mitochondria are typically 1 to 2 μm in length and represent a similar size target for radiation track as individual spines. Perturbations in mitochondrial dynamics have been observed following exposures to ionizing radiation such as electron beam (Chien 2015), deuteron beam (Samorajski 1975), X-rays (Shimura 2017), 137Cs γ-rays (Kempf 2015) and protons (Parihar 2014). Elongation of mitochondria with parallel arrays of cristae was observed after irradiation with deuteron beams (Samorajski 1975). X-rays exposures of neural stem cells and differentiated neurons revealed enhanced mitochondrial oxidative phosphorylation in neuron cells but not in stem cells after long-term fractionated radiation and cellular senescence of stem cells after high doses of single acute radiation (Shimura 2017). Brain region specific changes in mitochondrial dynamics has also been observed. Increased expression of mitochondrial complexes I and III resulting in enhanced fusion was observed after 0.2 Gy irradiation of hippocampal neurons (Chien 2015) while reduced expression in mitochondrial complex I occurred after 0.1 Gy irradiation of neurons in the cortex (Kempf 2015). Meanwhile, increasing expression of mitochondrial catalase (MCAT) have shown to reduce mitochondrial oxidative stress caused by reactive oxygen species (ROS) generated upon exposure to proton radiation and prevents radiation-induced neurocognitive detriments (Parihar 2014).
Altered Neurogenesis
Adult neurogenesis occurs in the adult dentate gyrus subventricular zone (SVZ) and the subgranular zone (SGZ), where cognitive actions such as memory and learning are determined (Kemperman et al 2004). Studies of radiation impairment of neurogenesis show that it is not likely an issue for astronauts exposed to low doses of heavy ions, while an important issue in Hadron therapy with proton and carbon beam treatment of brain cancers, especially for younger patients. Radiation not only affects differentiated neural cells but also proliferation and differentiation of neuronal precursor cells (Mizumatsu, et al., 2003; Monje et al., 2002; Tofilon and Fike, 2000). Cucinotta et al (2014) reviewed studies of altered neurogenesis in the SGZ after radiation exposure using HZE nuclei. Of note is that Rola et al. (2008) quantified neurogenesis and activated microglial as an indicator or neurogenesis at two months post-IR using doses of 0.5–4.0 Gy of 56Fe (1000 MeV/u). However significant changes were not observed at 0.5 Gy, which is potentially an important result for understanding GCR risks to the CNS because heavy ion mission doses are not expected to exceed 0.04 Gy. Studies of fractionated radiation exposures with iron particles did not reveal a significant difference between acute (1 Gy) and fractionated exposures (0.2 Gy × 5 in 1 d intervals) (Riviera et al 2014). Mathematical models (Cacao and Cucinotta 2016) predict that impaired neurogenesis will play a minor role for heavy ion doses below 0.5 Gy, so although a concern for Hadron therapy, altered neurogenesis is not likely for astronauts during space travel. However the large variability observed in studies of various strains of rats exposed to photons (Cacao and Cucinotta 2018a) also should be considered since almost all heavy ion studies have used C57BL/6J mice.
Detriments to Neuron Morphology
Alterations in morphology for hippocampal and pre-frontal cortex neurons has been observed in recent experiments, including changes in neuronal branching and length, and dendritic spine morphology, and the shape, size and number of spines (Chakraborti et al 2012; Parihar and Limoli 2013, Parihar et al 2015; Allen et al 2015). It is well known that aging and neurological diseases such as Alzheimer’s disease and other dementia are related to morphology detriments and reduction in complexity (Terry et al 1991), which suggest radiation induced changes to morphology could play an important role in understanding cognitive changes.
Figure 4 from Cucinotta et al (2018) displays three types of neurons reconstructed from the public depository WWW.NEUROMORPHO.ORG (Ascoli 2006), which illustrates some of the variability of neuron morphology in different brain regions. The neuron cell body or soma containing the nucleus will have variable volume between different types of neurons. As noted by Cucinotta et al (2018) the soma diameter can be as small as 7 μm in cerebral granule cells that are the smallest and most numerous of neurons in the brain, 15 μm in dentate granule cells (Amaral et al 2007) in the hippocampus, and 20 μm or larger for pyramidal cells in the hippocampus and cortex (Larkman et al 1991). Dendrites have larger radius close to soma (~3 μm), become thinner as the path-length distance from the soma increases with diameters less than ~0.5 μm at the distal tips. A proximal dendrite gives rise to two daughters, distal dendrites at the dendritic bifurcation points. In considering the topology of a dendrite the number of branch points and branch order, including the determination of bifurcation points along a dendritic path, are additional factors in defining dendritic complexity.
Figure 4.
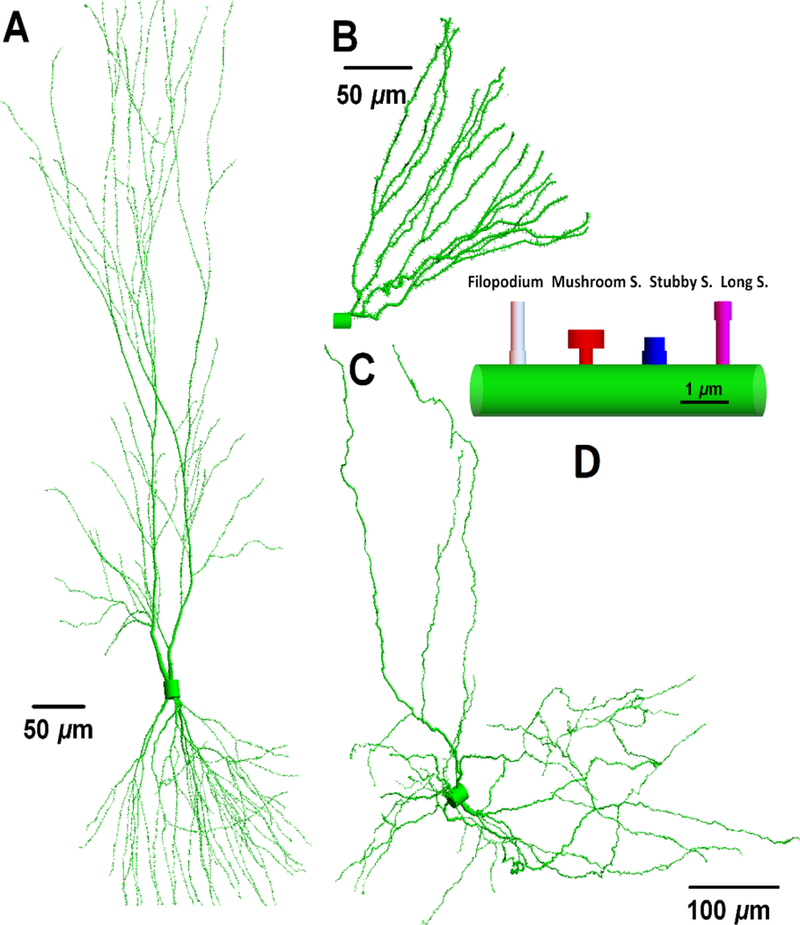
Neurons and neuronal compartments (reproduced from Cucinotta et al 2018). The neuronal morphology and arborization are distinct for pyramidal (A), dentate granule cell (B) and dopaminergic (C) neurons. However the underlying geometries are similar in different neuron types with filopodia and long, mushroom and stubby spines (D). Neuromorpho ID numbers (Ascoli 2006) corresponding to structures shown are A) NMO 00207, B) NMO 07642, and C) NMO 09579. Total dendritic lengths and number of branches: (14793, 2491, 10539) μm and (181, 29, 417), respectively.
Alp and Cucinotta (2018) note that single neuron observations can be achieved by neuro-tracers (Wearne et al 2005) or traditional Golgi staining (Valverde 1970) and with tissues prepared with green fluorescent protein (GFP). The latter approach considers a population of neurons on thin slices (~60 μm depth) of brain sections (Hama et al 2011). A short-coming of the use of brain slices is the dose dependence of cell loss, which includes apoptosis from either soma or dendrite damage, can modify the number of neurons in a slice (Alp and Cucinotta 2018). Here the number of cells lost by either mechanism is not likely known. In contrast the older Sholl analysis method (Sholl 1953) considers a single neuron. Sholl analysis evaluates the contribution of total dendritic length and branch points in concentric circles centered at the soma and provides a quantitative method to compare damaged and control neuron morphologies.
Experiments have shown differential effects on the radiation changes to various spine types which could depend on neuron type, brain region and possibly other factors. For example Parihar et al (2014) studying proton irradiation of 2-month old male transgenic mice (strain Tg(Thy1-EGFP) report significant reduction in immature spine densities in hippocampal granular cell neurons, such as filopodia and long spines, with no significant changes to more mature spines (stubby and mushroom), which have large head volume connected to a dendrite with thin spine neck due to the addition of pre- and post-synaptic proteins, or to spine volume. However in a more recent study (Dickstein et al 2018) changes to neurons of 6-month old male transgenic mice (strain Tg(Thy1-EGFP) irradiated with 4He, 16O or 28Si ions included reduction in spine density and volumes of all spine types in CA1 apical dendritic spines. Other variable results in spine density changes across neuron types were reported by Chakraborti et al (2012) and Allen et al (2015) following 56Fe particle irradiation of C57BL6/J male mice using Golgi staining methods.
Observations of reductions in dendritic length and spine density in the mouse pre-frontal cortex were reported by Parihar et al (2015) at 30 d post-irradiation for 16O and 48Ti exposures of 0.05 and 0.3 Gy. Figure 5 (from Parihar et al (2015)) shows their results for spine density changes (panel a). Parihar et al (2015) also report correlations in object discrimination after radiation and spine density changes in individual animals (Figure 5 panel b). These subject-specific data strengthen a conclusion on changes in morphology as a mechanism for cognitive change.
Figure 5.
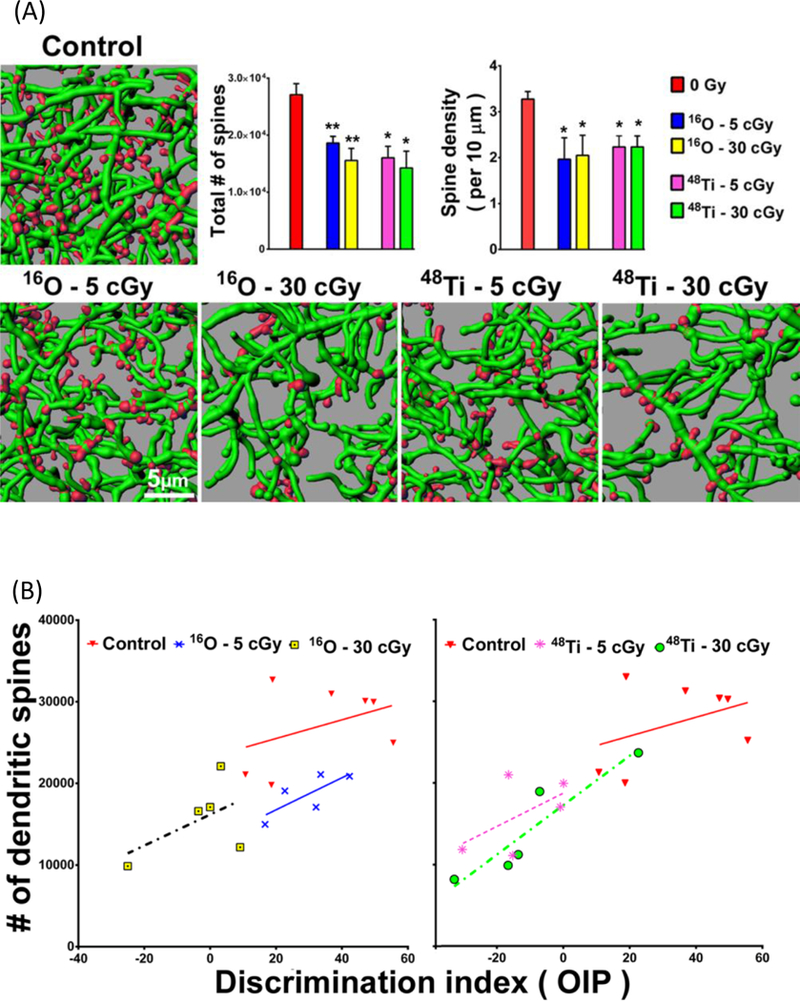
Reductions in dendritic spine density in the mPFC after heavy ion exposure and correlation of spine density changes with cognitive changes (reproduced from Parihar et al 2015). (A) Representative digital images of 3D reconstructed dendritic segments (green) containing spines (red) in unirradiated (controls) and irradiated mouse brains. Dendritic spine number and density are quantified in charged particle–exposed animals 8 weeks after exposure. *P = 0.05, **P = 0.01, ANOVA. (B) Dendritic spine density (per 1.3 mm2) is plotted against the corresponding performance of each animal on the object in place (OiP) task. (A and B) Reduction in spine number after irradiation is correlated with reduced discrimination index (DI) for novelty after exposure to 5 or 30 cGy of 16O or 48Ti charged particles. The correlation between spine density and DI is significant for the 30 cGy 48Ti data (green circles; P = 0.016).
Cucinotta et al (2018) note that in-vivo studies of neuron morphology at early time-points (minutes to a few days post-irradiation) have not been reported. In-vitro studies of neurite outgrowth assays using neuronal cell cultures show changes within hours after low to intermediate doses of x ray, carbon beam or neutron radiation (Al-Jahdari et al 2009; Pani et al 2016). These changes are consistent with UV-laser studies (Tao and Rolls 2011) where neurites were severed in Drosophila neurons demonstrating rapid clearance of damaged segments within 24-h. Severing of dendrites with femtosecond lasers in neurons of Caenorhabditis Elegans showed rapid fusion of ends or severing of the distal end of the neurons (Oren-Suissa et al 2017). In addition cisplatin, which induces ROS and DNA adducts, have been shown to cause early morphological changes (<1-d) in rat hippocampal neuron cell cultures (Andres et al 2014). In Drosophila (Williams et al 2005) branch retraction and local degeneration or fragmentation are cellular mechanisms of dendritic pruning that have been observed. Local degeneration appears to be the mechanism in proximal dendrites, while branch retraction the mechanism in distal branches and in proximal dendrites after fragmentation. Destabilization of microtubule cytoskeleton occurs after the severing event, and then microtubule thinning with phagocyte aided fragmentation and/or retraction (Oren-Suissa et al 2017).
Cucinotta et al (2018) note that energy deposition in neurons likely plays a role in the radiation quality dependence of morphological changes observed in the studies noted. However common mechanisms of chronic ROS and inflammatory responses from radiation and for other insults such as chronic stress, fatigue, and sleep deprivation which also lead to changes in morphology, suggests an indirect pathway through signaling and changes to neuro-transmitters or receptors induced by radiation could impact cognition leading to morphology detriments. Activated microglia cells also can degrade neuron morphology (Kettenmann et al 2013), however this is likely a delayed effect occurring over several weeks following radiation exposure at low to moderate doses (Cacao and Cucinotta 2016; 2018). Therefore low dose heavy ion studies are needed showing the time courses for neuron morphology changes from the first few hours post-irradiation to several months post-irradiation.
Computer models (Alp et al 2015; Alp and Cucinotta 2017; Alp and Cucinotta 2018; Cacao et al 2018b) of neurons can be developed from morphological data repositories such as Neuromorpho (Ascoli 2006) with data for different animal species and neuron types. Neuromorpho collects reconstructed neurons in mice, rats and primates, and provide a depository for utilization of structures for various applications. In contrast to this approach Cacao et al (2018) have used a Monte-Carlo approach to develop in-silico neurons based on reported averages and variances of neuron morphology including branch lengths and numbers, and spine density.
The track structure of heavy ions is distinct from that of photons. The primary particle track includes a core of high energy deposition from direction ionization and excitation and low energy electrons (<5 keV). The penumbra of energy deposition can extend for 100’s of microns from the particle path at median GCR due to the production of energetic secondary electrons, denoted as δ-rays (Cucinotta et al 2000; Plante and Cucinotta 2008). The energies of δ-rays can be as high as 1 MeV at the median HZE particle energy (~1500 MeV/u). The complex energy deposition events from the traversal of diverse species of heavy ions of brain regions, such as the hippocampus, is illustrated in computer modeling results shown in Figure 6, which were made using the methods described in Alp and Cucinotta (2018). The use of track structure models and possible biological targets in the neuron soma and dendritic arbors have not been considered until recently. The large radial and longitudinal extensions of a particles track due to δ-rays relative to the spatial extent of a neurons dentate arbor (1000’s of microns depending on neuron types and animal species) is an interesting computational challenge that has been considered in recent work (Alp et al 2017; Alp and Cucinotta 2018). The radial extension from δ-rays can exceed the size of neurons with correlated events possible within individual dendrite arbors and between axons and dendrites over multiple neurons. Because the energies and mixture of GCR (Figure 1) exceed current particle accelerator capabilities, validated models of morphological changes could serve a useful predictive role in the future.
Figure 6.
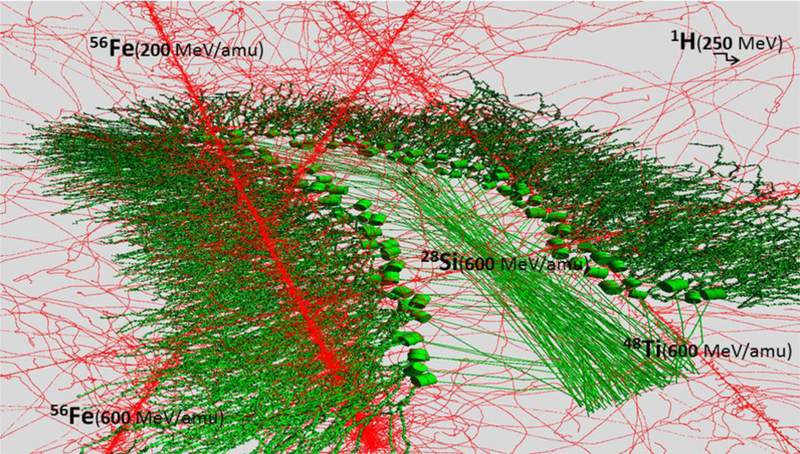
Computer model simulations of ionization patterns (red dots) from passage of several particle types (proton, 28Si, 48Ti and 56Fe) through the mouse hippocampus. Stochastic patterns of particle track core intersecting multiple dendrites, axons or soma with large radial extension of δ–rays events are shown.
Activity-Regulated Cytoskeleton (Arc) in Heavy ion Irradiation
Arc is an activity induced gene whose transcription is correlated temporally and spatially with a stimulus. Arc mRNA localizes to dendrites and activated synapses, and regulates spine morphology by increasing the proportion of thin spines (Peebles et al 2010). As noted in the review of Cucinotta et al (2014) studies in C57Bl/6J mice irradiated with X rays (Rosi et al. 2008) or heavy ions (Raber et al. 2013) suggests Arc protein is decreased in hippocampal neurons by 10-d post irradiation and both Arc protein and mRNA are decreased several months post irradiation with these changes modifying the behavioral activity of animals. Optimal Arc levels are necessary for memory processing, while both increases and decreases of Arc can impair the synaptic plasticity related to memory formation. Arc changes have been correlated with increased activation of microglia (Vyas et al, 2002; Rosi 2011).
In the hippocampus the dentate gyrus is characterized by the presence of a curved cell layer, densely packed with granule cells. Adjacent to granule cell layer is the CA1, is referred to as the enclosed blade while the opposite portion of the granule cell layer is called the free blade. Raber et al. (2013) noted that alterations to the dentate gyrus observed after HZE particle exposure is related to expression levels of Arc. For irradiation with 56Fe (600 MeV/u) at doses of 0.5 and 1.0 Gy a significant correlation between deficits in tests for contextual freezing and the percentage of Arc-positive cells in the enclosed and free-blade of the dentate gyrus 3-months post-IR were observed (Raber et al 2013). This suggested that the enclosed blade is more important for contextual freezing and the free blade for cued freezing.
Raber and coworkers (Impey et al 2016a, 2016b, 2017) have also investigated the effects of radiation on epigenetics. Their results have indicated that Arc is not a direct target of epigenetic regulation for acute doses and that DNA methylation, more specifically 5hmC (a DNA pyrimidine nitrogen base thought to function in DNA methylation), plays an important role in long-term response (20 weeks post irradiation) of brain function following irradiation and this effect is not specific to radiation quality as both were also observed in 56Fe (Impey et al 2016) and proton irradiation (Impey et al 2016a, Impey et al 2017). At 2 weeks post irradiation of 56Fe, impaired object recognition and network stability (via Arc mRNA localization) was observed at 0.1 and 0.4 Gy doses, but not with 0.2 Gy dose, while no impairments were observed at 20 weeks post irradiation indicating that impairments are transient (Impey et al 2016). They explained that 0.1 Gy dose caused sufficient cognitive damage but not enough to induce synapse remodeling or repair that would have mitigated the cognitive injury. However, they speculate that higher level of radiation injury at 0.2 Gy dose induced a level of synapse remodeling/repair sufficient to mitigate the damage, while at 0.4 Gy dose, the damage was severe that repair is not sufficient for recovery.
Conclusions
Studies of possible cognitive detriments from space radiation exposures have used a wide variety of cognitive tests, doses and particle types. To date very few studies have been performed in the range of heavy ion doses to be encountered on a Mars mission (<0.05 Gy) or other long duration space travels. Studies on dose-rate effects over several weeks with low cumulative doses (<0.1 Gy) are needed to improve simulations of the space environment. Obviously such research along with insights on the extrapolation from rodent to humans is needed to perform an adequate risk assessment and to understand if reducing brain doses through shielding or limiting mission length or to solar maximum time periods would provide sufficient protection. However space CNS radiobiology studies have used doses of interest for elucidating possible changes to patients treated with proton and carbon beams. Mechanistic studies have made progress in identifying likely causative factors, including activation of microglial cells and neuro-inflammation, and detriments to neuron morphology. Additional studies are needed that report on correlations of molecular or cellular endpoints in individual animals to their cognitive performance. The inverted U-shape response observed by several investigators suggests that a compensatory mechanism may be active that will complicate the understanding of dose response models. Mechanistic studies of the origin of such a dose response are needed for both space radiation risk assessments and for understanding cognitive detriments in Hadron therapy.
Acknowledgement
Supported by the National Institute of Health-National Cancer Institute (NIH-NCI) Grant 1RO1CA208526-01.
Footnotes
Disclosure Statement
The authors declare no conflicts of interest.
References
- Adriani O, et al. 2013. Time dependence of the proton flux measured by PAMELA during the 2006 July-2009 December solar minimum, Astrophys. J., 765: 91, doi: 10.1088/0004-637X/765/2/91. [DOI] [Google Scholar]
- Al-Jahdari WS, Suzuki Y, Yukari Y, Hamada N, et al. 2009. The radiobiological effectiveness of carbon-ion beams on growing neurons. Int J Radiat Biol. 85:70–79. [DOI] [PubMed] [Google Scholar]
- Allen A, Raber J, Chakraborti A, Sharma S, Fike JR. 2015. 56Fe irradiation alters spine density and dendritic complexity in the mouse hippocampus. Radiat Res. 184:586–594. [DOI] [PubMed] [Google Scholar]
- Alp M, Parihar VK, Limoli CL, Cucinotta FA. 2015. Irradiation of neurons with high-energy charged particles: an in silico modeling approach. PLoS Comput. Biol. 11(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alp M, Cucinotta FA. 2017. Track structure model of microscopic energy deposition by protons and heavy ions in segments of neuronal cell dendrites represented by cylinders or spheres. Life Sci Space Res 13:27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alp M, Cucinotta FA. 2018. Biophysics model of heavy ion degradation of neuron morphology in mouse hippocampal granular cell layer neurons. Radiat Res. 189:312–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, Scharfman HE, Lavenex P. 2007. The dentate gyrus: fundamental neuroanatomical organization (dentate gyrus for dummies). Prog Brain Res. 163:3–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres AL, Gong X, Di K, Bota DA. 2014. Low-doses of cisplatin injure hippocampal synapses: A mechanism for ‘chemo’ brain? Expt Neur. 255:137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archaya MM, et al. 2016. Elimination of microglia improves cognitive function following cranial irradiation. Sci. Rep. 6:31545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascoli GA. 2006. Mobilizing the base of neuroscience data: the case of neuronal morphologies. Nat Rev Neurosci 7:318–324. [DOI] [PubMed] [Google Scholar]
- Badhwar GD, Cucinotta FA, O’Neill PM. 1994. An analysis of interplanetary space radiation exposure for various solar cycles. Radiat Res. 138:201–208. [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS. 2007. Microglia-mediated neurotoxicity: uncovering the molecular mechanism. Nature Rev Neurosci. 8:57–69. [DOI] [PubMed] [Google Scholar]
- Britten RA, Davis LK, Jewell JS, Miller VD, Hadley MM, et al. 2014. Exposure to mission relevant doses of 1 GeV/nucleon 56Fe particles leads to impairment of attentional set-shifting performance in socially mature rats. Radiat Res. 182:292–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten RA, Jewell JS, Duncan VD, Hadley MM, Macadat E, et al. 2017. Spatial memory performance of socially mature Wistar rats is impaired after exposure to low (5cGy) doses of 1 GeV/n 48Ti particles. Radiat Res. 187:60–65. [DOI] [PubMed] [Google Scholar]
- Britten RA, Jewell JS, Duncan VD, Hadley MM, Macadat E, et al. 2018. Impaired attentional set-shifting performance after exposure to 5 cGy of 600 MeV/u 28Si particles. Radiat Res. 189:273–282. [DOI] [PubMed] [Google Scholar]
- Cacao E, Cucinotta FA. 2016. Modeling Heavy-Ion Impairment of Hippocampal Neurogenesis after Acute and Fractionated Irradiation. Radiat Res. 186: 624–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacao E, Cucinotta FA, 2018a. Modeling reveals the dependence of hippocampal neurogenesis radiosensitivity on age and strain of rats. Front Neurosci| Neurogen. 2018:00980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacao E, Parihar VP, Limoli CL, Cucinotta FA. 2018b. Stochastic modeling of radiation-induced dendritic damage on in silico mouse hippocampal neurons. Sci Rep. 8:5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborti A, Allen A, Allen B, Rosi S, Fike JR. 2012. Cranial irradiation alters dendritic spine density and morphology in the hippocampus. PLoS One 7:e40844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Chan DC. 2009. Mitochondrial dynamics-fusion, fission, movement, and mitophagy-in neurodegenerative diseases. Hum Mol Genet. 18:169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z, Li YQ, Wong CS. 2016. Effects of aging on hippocampal neurogenesis after irradiation. Int J Radiat Oncol Biol Phys. 94(5):1181–1189. [DOI] [PubMed] [Google Scholar]
- Cherry JD, Liu B, Frost JL, Lemere CA, Williams JP, et al. , 2012. Galactic cosmic radiation leads to cognitive impairment and increased Aβ plaque accumulation in a mouse model of Alzheimer’s disease. PLoS ONE 7 (12): e53275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien L, et al. 2015. Low-dose ionizing radiation induces mitochondrial fusion and increases expression of mitochondrial complexes I and III in hippocampal neurons. Oncotarget 6 (31):30628–30639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner KR, Payne VS, Forbes E, Robbins ME, Riddle DR. 2010. Effects of the AT1 receptor antagonist L-158,809 on microglia and neurogenesis after fractionated whole-brain irradiation. Radiat Res. 173: 49 –56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucinotta FA, Nikjoo H, Goodhead DT. 2000. Model for radial dependence of frequency distributions for energy imparted in nanometer volumes from HZE particles. Radiat Res. 153: 459–468. [DOI] [PubMed] [Google Scholar]
- Cucinotta FA, Kim MH, Willingham V, George KA. 2008. Physical and biological organ dosimetry analysis for International Space Station Astronauts. Radiat Res. 170:127–138, 2008. [DOI] [PubMed] [Google Scholar]
- Cucinotta FA, Alp M, Sulzman FM, Wang M. 2014. Space radiation risks to the central nervous system. Life Sci Space Res. 2:54–69. [Google Scholar]
- Cucinotta FA, Alp M, Cacao E. 2018. Detriments in neuron morphology following heavy ion irradiation- what’s the target? Radiat Protect Dosim. 10.1093/rpd/ncy265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings AC, Stone EC, Heikkila BC, Lai N, Webber WR, Johannesson G, et al. 2016. Galactic cosmic rays in the local interstellar medium: Voyager 1 observations and model results. Astrophys J. 831:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CM, DeCicco-Skinner KL, Roma PG, Hienz RD. 2014. Individual differences in attentional deficits and dopaminergic protein levels following exposure to proton radiation. Radiat Res. 181:258–271. [DOI] [PubMed] [Google Scholar]
- Devine MJ, Kittler JT. 2018. Mitochondria at the neuronal presynapse in health and disease. Nat. Rev. Neurosci. 19:63–80. [DOI] [PubMed] [Google Scholar]
- Dickstein DL, Talty R, Breshahan E, Varghese M, Perry B, et al. 2018. Alterations in synaptic density and myelination in response to exposure to high-energy charged particles. J Comp Neurol. 2018;1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer TD, Hylin MJ, Zhao J, Moore AN, Waxham MN, Dash PK. 2016. Altered mitochondrial dynamics and TBI pathophysiology. Front Sys Neurosci. 10:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flippo KH and Strack S. 2017. Mitochondrial dynamics in neuronal injury, development and plasticity. J Cell Sci. 130:671–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Wang L, Liu J, Xie F, Su B, Wang X. 2017. Abnormalities of mitochondrial dynamics in neurodegenerative diseases. Antioxidants 6:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George JS, Lave KA, Wiedenbeck ME, Binns WR, Cummings AC, Davis AJ, et al. 2009. Elemental compositions and energy spectra of galactic cosmic rays during solar cycle 23. Astrophys J 698:1666–1681. [Google Scholar]
- Greene-Schloesser D, Robbins ME, Peiffer AM, Shaw EG, Wheeler KT, and Chan MD. 2012. Radiation-induced brain injury: A review. Front Oncol. 2:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene-Schloesser DM, Kooshki M, Payne V, D’Agostino RB Jr, Wheeler KT, Metheny-Barlow LJ, Robbins ME. 2014a. Cellular response of the rat brain to single doses of 137Cs γ-rays does not predict its response to prolonged ‘biologically equivalent’ fractionated doses. Int J Radiat Biol. 90:790–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene-Schloesser D, Payne V, Peiffer AM, Hsu FC, Riddle DR, Zhao W, Chan MD, Metheny-Barlow L, Robins ME. 2014b. The peroximal proliferator-activator receptor (PPAR) α agonit, fenofibrate, prevents fractionated whole-brain irradiation-induced cognitive impairment. Radiat Res. 181:33– 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood SM, Mizielinska SM, Frenguelli BG, Harvey J. and Connolly CN. 2007. Mitochondrial dysfunction and dendritic beading during neuronal toxicity. J Biol Chem. 282: 26235–26244. [DOI] [PubMed] [Google Scholar]
- Haerich P, Eggers C, Pecaut MJ, 2012. Investigation of the effects of head irradiation with gamma rays and protons on startle and pre-pulse inhibition behavior in mice. Radiat Res 177: 685–692. [DOI] [PubMed] [Google Scholar]
- Haley GE, Yeiser L, Olsen RH, Davis MJ, Johnson LA, Raber J. 2013. Early effects of whole-body 56Fe irradiation on hippocampal function in C57BL/6J mice. Radiat Res. 179:590–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama H, Kurokawa H, Kawano H, Ando R, Shimogori T, Noda H, et al. 2011. Scale: a chemical approach for fluorescence imaging and reconstruction of transparent mouse brain. Nat Neurosci. 14:1481–1488. [DOI] [PubMed] [Google Scholar]
- Hassler DM, Zeitlin C, Wimmer-Schweingruber RF, Ehresmann B, Rafkin S, Eigenbrode J et al. 2014. The radiation environment on the surface of Mars measured on the Mars science Laboratory’s curiosity rover. Science 343:6169–6172. [DOI] [PubMed] [Google Scholar]
- Hwang SY, Jung JS, Kim TH, Lim SJ, Oh ES, Kim JY, Ji KA, Joe EH, Cho KH, Han IO. 2006. Ionizing radiation induces astrocyte gliosis through microglia activation. Neurobiol Disease 21:457–467. [DOI] [PubMed] [Google Scholar]
- Iancu OD, Boutros SW, Olsen RHJ, Davis MJ, Stewart B, et al. 2018. Space radiation alters genotype–phenotype correlations in fear learning and memory tests. Front Genetics 9:404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICRP, 2012. ICRP Statement on Tissue Reactions / Early and Late Effects of Radiation in Normal Tissues and Organs–Threshold Doses for Tissue Reactions in a Radiation Protection Context. ICRP Publication 118. Ann. ICRP 41(½). [DOI] [PubMed] [Google Scholar]
- Impey S, Pelz C, Tafessu A, Marzulla T, Turker M, et al. 2016a. Proton irradiation induces persistent and tissue-specific DNA methylation changes in the left ventricle and hippocampus. BMC Genomics, 17: 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impey S, Jopson T, Pelz C, Tafessu A, Fareh F, et al. 2016b. Short- and long-term effects of 56Fe irradiation on cognition and hippocampal DNA methylation and gene expression. BMC Genomics, 17:825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impey S, Jopson T, Pelz C, Tafessu A, Fareh F, et al. 2017. Bi-directional and shared epigenomic signatues following proton and 56Fe irradiation. Scientific Reports, 7:10227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenrow KA, Brown SL, Lapanowski K, Naei H, Kolozsvary A, Kim JH. 2013. Selective inhibition of microglia-mediated neuroinflammation mitigates radiation-induced cognitive impairment. Radiat Res. 179:549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jim HS, et al. 2009. Cognitive functioning in breast cancer survivors: a controlled comparison. Cancer 115:1776–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph JA, Hunt WA, Rabin BM, Dalton TK, 1992. Possible “accelerated striatal aging” induced by 56Fe heavy-particle irradiation: implications for manned space flights. Radiat. Res. 130:88–93. [PubMed] [Google Scholar]
- Kalm M, Lannering B, Bjork-Eriksson T, Blomgren K. 2009. Irradiation-induced loss of microglia in the young brain. J Neuroimmunol 206, 70–75. [DOI] [PubMed] [Google Scholar]
- Kempf SJ, et al. 2015. Low-dose ionizing radiation rapidly affects mitochondrial and synaptic signaling pathways in murine hippocampus and cortex. Journal of Proteome 14, 2055–2064. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Wiskott L, Gage FH. 2004. Functional significance of adult neurogenesis. Curr Opin Neurobiol. 14:186–191. [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Kirchoff F, Verkhratsky A. 2013. Microglia: new roles for the synaptic stripper. Neuron 77, 10–17. [DOI] [PubMed] [Google Scholar]
- Kim MY, Cucinotta FA, et al. 2014. Comparison of Martian surface ionizing radiation measurements from MSL-RAD with Badhwar-O’Neill 2011/HZETRN model calculations. J. Geophys Res 119:1311–1321. [Google Scholar]
- Kim MY, Rusek A, Cucinotta FA. 2015. Issues in ground-based GCR simulation for space radiobiology. Front. Radiat Onc 2015.00122. [Google Scholar]
- Labbé K, Murley A, Nunnari J. 2014. Determinants and functions of mitochondrial behavior. Annu. Rev. Cell Dev. Biol. 30:357–391. [DOI] [PubMed] [Google Scholar]
- Larkman AU. 1991. Dendritic morphology of pyramidal neurons of the visual cortex of the rat: III. Spine distributions. J Comp Neurol. 306: 332–343. [DOI] [PubMed] [Google Scholar]
- Li Z, Okamato K, Hayashi Y, Sheng M. 2004. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell 119, 873–887. [DOI] [PubMed] [Google Scholar]
- Limoli CL, Giedzinski E, Baure J, Rola R, Fike JR. 2007. Redox changes induced in hippocampal precursor cells by heavy ion irradiation. Radiat Environ Biophys., 46:167–172.6 (2) (2007), pp. 167–172 [DOI] [PubMed] [Google Scholar]
- Mattson MP, Gleichmann M, Cheng A. 2008. Mitochondria in neuroplasticity and neurological disorders. Neuron 60:748–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumatsu S, Monje ML, Morhardt DR, Rola R, Palmer TD, Fike JR. 2003. Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res. 63:4021–4027. [PubMed] [Google Scholar]
- Miyamoto A, et al. 2016. Microglia contact induces synapse formation in developing somatosensory cortex. Nature Comm. 7:12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje ML, Mizumatsu S, Fike JR, Palmer TD. 2002. Irradiation induces neural precursor-cell dysfunction. Nature Med. 8:955–962. [DOI] [PubMed] [Google Scholar]
- Monje ML, Vogel H, Masek M, Ligon KL, Fischer PG, Palmer TD. 2007. Impaired human hippocampal neurogenesis after treatment for central nervous system malignancies. Ann. Neurology 62:515–520. [DOI] [PubMed] [Google Scholar]
- Moravan MJ, Olschowka JA, Williams JP and O’Banion MK. 2011. Cranial irradiation leads to acute and persistent neuroinflammation with delayed increases in T-cell infiltration and CD11c expression in C57BL/6 mouse brain. Radiat Res. 176:459–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren-Suissa M, Gattengo T, Kravtsov V, Podbilewicz B. 2017. Extrinsic repair of injured dendrites as a paradigm for regeneration by fusion in Caenorhabditis elegans. Genetics 206: 215–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pani G, Verslegers M, Quintens R, Samari N, de Saint Georges L, van Oostveldt P., et al. 2016. Combined exposure to simulated microgravity and acute or chronic radiation reduces neuronal network integrity and survival. PLoS One 0155260, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parihar VK, Limoli CL. 2013. Cranial irradiation compromises neuronal architecture in the hippocampus. Proc Natl Acad Sci USA 110:12822–12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parihar VK, Allen B, Tran KK, Macaraeg TG, Chu EM, Kwok SF, et al. 2015. What happens to your brain on the way to Mars? Sci. Adv. 1:e1400256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parihar VK, Allen BD, Caressi C, Kwok S, Chu E, et al. 2016. Cosmic radiation exposure and persistent cognitive dysfunction. Sci Rep. 6:34773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecaut MJ, Haerich P, Miller CN, Smith AL, Zendejas ED, Nelson GA. 2004. The effects of low-dose, high-LET radiation exposure on three models of behavior in C57BL/6 mice. Radiat Res. 162:148–156. [DOI] [PubMed] [Google Scholar]
- Peebles CL, Yoo J, Thwin MT, Palop JJ, Noebels JL, Finkbeiner S. 2010. Arc regulates spine morphology and maintains network stability in vivo. Proc Natl Acad Sci. USA 107: 18173–18178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, Nicoll JAR, Holmes C. 2010. Microglia in neurodegenerative disease. Nature Rev Neurol. 6:193–201. [DOI] [PubMed] [Google Scholar]
- Plante I, Cucinotta FA. 2008. Ionization and excitation cross sections for the interaction of HZE particles in liquid water and application to Monte-Carlo simulation of radiation tracks. New J Phys. 10:1–15. [Google Scholar]
- Raber J, Allen AR, Rosi S, Sharma S, Dayger C, Davis MJ, Fike JR. 2013. Effects of whole body 56Fe radiation on contextual freezing and Arc-positive cells in the dentate gyrus. Behav Brain Res. 246:162–167. [DOI] [PubMed] [Google Scholar]
- Raber J, Rudobeck E, Camplbell-Beachler M, Allen AR, Allen, B, et al. 2014. 28Silicon radiation-induced enhancement of synaptic plasticity in the hippocampus of naïve and cognitively tested mice. Radiat Res. 181:362–368. [DOI] [PubMed] [Google Scholar]
- Raber J, Marzulla T, Kronenberg A, Turker MS. 2015a. 16Oxygen irradiation enhances cued fear memory in B6D2F1 mice. Life Sci Space Res. 7: 61–65. [DOI] [PubMed] [Google Scholar]
- Raber J, Marzulla T, Stewart B, Kronenberg A, Turker MS. 2015b. 28Silicon irradiation impairs contextual fear memory in B6D2F1 mice. Radiat Res. 186:708–712. [DOI] [PubMed] [Google Scholar]
- Raber J, Allen AR, Sharma S, Allen B, Rosi S, Olsen RHJ, Davis MJ, Eiwaz M, Fike JR, Nelson GA. 2016a. Effects of proton and combined 56Fe radiation on the hippocampus. Radiat Res. 185:20–30. [DOI] [PubMed] [Google Scholar]
- Raber J, Weber SJ, Kronenberg A, Turker MS. 2016b. Sex- and dose-dependent effects of calcium ion irradiation on behavioral performance of B6D2F1 mice during contextual fear conditioning training. Life Sci Space Res. 9:56–61. [DOI] [PubMed] [Google Scholar]
- Rabin BM, Hunt WA, Joseph JA, Dalton TK, Kandasamy SB, 1991. Relationship between linear energy transfer and behavioral toxicity in rats following exposure to protons and heavy particles. Radiat Res. 128:216–221. [PubMed] [Google Scholar]
- Rabin BM, Joseph JA, Shukitt-Hale B, McEwen J. 2000. Effects of exposure to heavy particles on a behavior mediated by the dopaminergic system. Adv Space Res. 25:2065–2074. [DOI] [PubMed] [Google Scholar]
- Rabin BM, Buhler LL, Joseph JA, Shukitt-Hale B, Jenkins DG. 2002. Effects of exposure to 56Fe particles or protons on fixed-ratio operant responding in rats. J Radiat Res. 43 (Suppl.): S225–S228. [DOI] [PubMed] [Google Scholar]
- Rabin BM, Shukitt-Hale B, Joseph JA, Carrihill-Knoll KL, Carey AN, Cheng V. 2007. Relative effectiveness of different particles and energies in disrupting behavioral performance. Radiat Environ Biophys. 46(2):173–177. [DOI] [PubMed] [Google Scholar]
- Rabin BM, Carrihill-Knoll K, Hinchman M, Shukitt-Hale B, Joseph JA, Foster BC, 2009. Effects of heavy particle irradiation and diet on object recognition memory in rats. Adv Space Res. 43(8):1193–1199. [Google Scholar]
- Rabin BM, Carrihill-Knoll KL, Shukitt-Hale B, 2011. Operant responding following exposure to HZE particles and its relationship to particle energy and linear energy transfer. Adv Space Res.48 (2):370–377. [Google Scholar]
- Rabin BM, Carrihill-Knoll KL, Shukitt-Hale B. 2015. Comparison of the effectiveness of exposure to low-LET helium particles (4He) and gamma rays (137Cs) on the disruption of cognitive performance. Radiat Res. 184:266–272. [DOI] [PubMed] [Google Scholar]
- Ramanan S, Kooshki M, Zhao W, Hsu FC, Riddle DR, Robbins ME. 2009. The PPARα agonist, fenofibrate, preserves hippocampal neurogenesis and inhibits microglial activation following whole brain irradiation. Int. J. Radiat Oncol Biol Phys 75 (3): 870–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice O, Saintvictor S, Michalaelidas M, Panayotis P, Gatley SJ, 2006. MicroPET investigation of chronic long-term neurotoxicity from heavy ions irradiation. AAPS J. 8:E508–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice OV, Grande AV, Dehktyar N, Bruneus M, Robinson JK, Gatley SJ. 2009. Long-term effects of irradiation with iron-56 particles on the nigrostriatal dopamine function. Radiat Env Biophys. 48:215–225. [DOI] [PubMed] [Google Scholar]
- Rintoul GL, Reynolds IJ. 2010. Mitochondrial trafficking and morphology in neuronal injury. Biochimica Biophysica Acta 1802: 143–150. [DOI] [PubMed] [Google Scholar]
- Riviera PD, Shih H, LeBlanc JA, Cole MG, Amaral AZ, Mukherjee S, et al. , 2014. Acute and fractionated exposure to high-let 56Fe HZE-particle radiation both result in similar long-term deficits in adult hippocampal neurogenesis. Radiat Res. 180:658–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rola R, Raber J, Rizk A, Otsuka S, VandenBerg SR, Morhardt DR, Fike JR. 2004. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Expt Neurol. 188: 316–330. [DOI] [PubMed] [Google Scholar]
- Rola R, Otsuka S, Obenaus A, Nelson GA, Limoli CL, VandenBerg SR, Fike JR. 2004. Indicators of hippocampal neurogenesis are altered by 56Fe-particle irradiation in a dose-dependent manner. Radiat Res. 162:442–446. [DOI] [PubMed] [Google Scholar]
- Rola R, Sarkissian V, Obenaus A, Nelson GA, Otsuka S, et al. 2005. High-LET radiation induces inflammation and persistent changes in markers of hippocampal neurogenesis. Radiat Res. 164: 556–560. [DOI] [PubMed] [Google Scholar]
- Rola R, Fishman K, Baure J, Rosi S, et al. 2008. Hippocampal neurogenesis and neuroinflammation after cranial irradiation with (56)Fe particles. Radiat Res. 169:626–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosi S, Andres-Mach M, Fishman KM, Levy W, Ferguson RA, Fike JR. 2008. Cranial irradiation alters the behaviorally induced immediate-early gene Arc (Activity-Regulated Cytoskeleton-Associated Protein). Cancer Res. 68:9763–9770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosi S 2011. Neuroinflammation and the plasticity-related immediate-early gene Arc. Brain Behav. Immun. 25 (Suppl 1): S39–S49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosi S, Belarbi K, Ferguson RA, Fishman K, Obenaus A, Raber J, Fike J, 2012. Trauma-induced alterations in cognition and Arc expression are reduced by previous exposure to (56)Fe irradiation. Hippocampus. 22:544–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudobeck E, Nelson GA, Sokolova IV, Vlkolinsky R, 2014. 28Si radiation impairs neuronal output in CA1 neurons of mouse ventral hippocampus without altering dendritic excitability. Radiat Res. 181: 407–415. [DOI] [PubMed] [Google Scholar]
- Samorajski T 1975. Late untrastructural changes in neuronal mitochondria after ionizing radiation of the brain. J Comp Neur. 161: 255–268. [DOI] [PubMed] [Google Scholar]
- Schindler MK, Elizabeth Forber M, Robbins ME, Riddle DR. 2008. Aging-Dependent Changes in the Radiation Response of the Adult Rat Brain. Int J Radiat Oncol Biol Phys. 70:826–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnegg CI, Greene-Schloesser, Kooshki M, Payne VS, Hsu FC, Robbins ME. 2013. The PPARδ agonist, GW0742, inhibits nuroinflammation, but does not restore neurogenesis or prevent early delayed hippocampal-dependent cognitive impairment after whole-brain irradiation. Free Radic Biol Med. 0:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimura T, et al. 2017. A comparison of radiation-induced mitochondrial damage between neural progenitor stem cells and differentiated cells. Cell Cycle 16:565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sholl DA. 1953. Dendritic organization in the neurons of the visual and motor cortices of the cat. J. Anatomy 87, 387–406. [PMC free article] [PubMed] [Google Scholar]
- Shulz-Ertner D, Tsujii H. 2007. Particle radiation therapy using proton and heavier ion beams. J Clinic Oncol. 25:953–964. [DOI] [PubMed] [Google Scholar]
- Sivakumaran MH, Mackenzie AK, Callan IR, Ainge JA, O’Connor AR. 2018. The discrimination ratio derived from novel object recognition tasks as a measure of recognition memory sensitivity, not bias. Scientific Rep. 8:11579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son Y, Yang M, Kim JS, Kim J, Kim SH, Kim JC, Shin T, Wang H, Jo SK, Jung U, Moon C. 2014. Hippocampal dysfunction during the chronic phase following a single exposure to cranial irradiation. Expt Neurol. 254:134–144. [DOI] [PubMed] [Google Scholar]
- Sweet TB, Panda N, Hein Am, Das SL, Hurley SD, Olschowka JA, Williams JP, O’Banion MK. 2014. Central nervous system effects of whole-body proton irradiation. Radiat Res. 182:18–34. [DOI] [PubMed] [Google Scholar]
- Tada E, Parent JM, Lowenstein DH, Fike JR. 2000. X-irradiation causes a prolonged reduction in cell proliferation in the dentate gyrus of adult rats. Neurosci. 99:33–41. [DOI] [PubMed] [Google Scholar]
- Tao J, Rolls MM. 2011. Dendrites have a rapid program of injury-induced degeneration that is molecularly distinct from developmental pruning. J. Neurosci. 31:5398–5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry RD, et al. (1991) Physical basis of cognitive alterations in Alzheimer’s disease: Synapse loss is the major correlate of cognitive impairment. Ann Neurol 30:572–580. [DOI] [PubMed] [Google Scholar]
- Tolifon PJ, Fike JR. 2000. The radioresponse of the central nervous system: a dynamic process. Radiat Res. 153:357–370. [DOI] [PubMed] [Google Scholar]
- Valverde F 1970. The Golgi method. A tool for comparative structural analyses In: Contemporary Research Methods in Neuroanatomy. Ed. Nauta WJH and Ebbeson SOE (Berlin: Springer; ); pp 12–13. [Google Scholar]
- Villasana L, Rosensberg J, Raber J. 2010. Sex-dependent effects of 56Fe irradiation on contextual fear conditioning in C57BL/6J mice. Hippocampus, 20: 19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wearne SL, Rodriguez A, Ehlenberger DB, Rocher AB, Henderson SC, Hof PR. 2005. New techniques for imaging, digitization and analysis of three-dimensional neural morphology on multiple scales. Neurosci. 136: 661–680. [DOI] [PubMed] [Google Scholar]
- Weinhard L, et al. 2018. Microglia remodel synapses by presynaptic trogocytosis and spine head filopodia induction. Nature Comm. 9:1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DW, Truman JW. 2005. Cellular mechanisms of dendrite pruning in Drosophila: insights from in vivo time-lapse of remodeling dendritic arborizing sensory neurons. Development 132:3631–3642. [DOI] [PubMed] [Google Scholar]
- Wong WT. 2013. Microglial aging in the healthy CNS: phenotypes, drivers and rejuvenation. Front Cellular Neurosci. 7:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyrobek AJ, Britten RA. 2016. Individual variations in dose response to spatial memory learning among outbred Wistar rats exposed from 5 to 20 cGy of 56Fe particles. Environ Mol Mut. 57:331–340. [DOI] [PubMed] [Google Scholar]
- Zeitlin C, Hassler DM, Cucinotta FA, Ehresmann B, Wimmer-Schweingruber RF, Brinza DE, et al. 2013. Measurements of the energetic particle radiation environment in transit to Mars on the Mars Science laboratory. Science 340:1080–1084. [DOI] [PubMed] [Google Scholar]


