Abstract
Aims
Prevalence of non-alcoholic fatty liver disease (NAFLD) is increasing in developing countries but its causes are not known. We aimed to ascertain the prevalence and determinants of NAFLD in a new largely unmedicated population-based cohort from the rapidly gentrifying region of Pinggu, China.
Materials and methods
We randomized cluster sampled 4002 Pinggu residents aged 26–76 years. Data from 1238 men and 1928 women without significant alcohol drinking or hepatitis virus B or C infection were analyzed. NAFLD was defined using a liver-spleen ratio (L/S ratio) ≤1.1 on unenhanced abdominal computed tomography(CT) scanning.
Results
26.5% of men and 20.1% of women had NAFLD. NAFLD prevalence was highest in younger men and older women. In multivariate logistic regression models, higher body mass index, waist circumference, serum triglyceride, alanine transaminase, and hemoglobin A1c independently increased the odds of NAFLD in both men and women separately. Higher annual household income and systolic blood pressure for men and, higher serum uric acid and red meat intake and lower physical activity levels for women also independently associated with higher odds of NAFLD. Individuals with L/S ratio ≤1.1 had linearly increasing rates of obesity, diabetes, and metabolic syndrome that paralleled fatty liver increase.
Conclusions
NAFLD is common in a gentrifying Chinese population particularly in younger men of high socioeconomic status and older women with sedentary behavior who eat red meat. Demographic factors add independent risk of NAFLD above traditional metabolic risk factors. A CT L/S ratio of ≤1.1 identifies individuals at high risk of metabolic disease.
Keywords: Non-alcoholic fatty liver disease, diabetes, obesity, physical activity, red meat
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a spectrum of disease that develops in the presence of more than 5% triglyceride content in the liver in the absence of excessive alcohol intake. NAFLD is a risk factor for both incident1,2 and prevalent3 cardiovascular disease independent of conventional risk factors. NAFLD also predisposes to cirrhosis4 and hepatocellular carcinoma5. NAFLD prevalence has increased over the last 30 years particularly in developed and now developing countries worldwide6,7; causes of this increase in prevalence are only now being elucidated. The prevalence of NAFLD in the population based on histology is not known as a biopsy cannot be justified in otherwise healthy individuals. NAFLD estimates in the population have been determined using non-invasive markers of the disease such as alanine aminotransferase (ALT) or imaging, most commonly ultrasound. Both of these methods however have been shown to be highly inaccurate for quantitating NAFLD. Magnetic resonance spectroscopy (MRS) and magnetic resonance imaging quantify liver fat well but are cumbersome and expensive to implement clinically in large populations. Computed Tomography (CT) allows for accurate quantification of liver fat while also being clinically available and cost effective to implement in large populations to estimate the prevalence of this disease. A CT liver spleen density ratio (L/S ratio) ≤ 1.1 corresponds to more than 30% liver steatosis found in a biopsy specimen among living donors for liver transplantation8. In small studies9–12, NAFLD has been associated with obesity and development of related metabolic diseases and some demographic and lifestyle factors, but whether those contribute to the disease independently of each other in large cohorts is not known. Further, the effects of rapid modernization associates with development of obesity and related metabolic disorders but what components of that gentrification contribute to disease is not known.
Here we use CT to measure liver fat in a largely unmedicated population-based cohort outside of Beijing in Pinggu, China. We chose this area as it has undergone gentrification and within a limited geographic area contains individuals with diverse exposures that may be able to give us insights into what determinants in developing regions contribute to higher levels of NAFLD. In this population with measured NAFLD we also measured many metabolic and demographic variables and examined the relationship of these to NAFLD.
Materials and methods
Study Population
We used two-stage cluster random sampling to recruit participants aged 25–74 years from 25 villages out of five towns and seven residents’ committees out of one street in Pinggu district located in Beijing China, from March 2012 to May 2013. 5004 residents were invited and 3350 initially participated13. In the second round, the 5004 originally selected residents were re-invited to participate and 1579 residents were additionally invited between September 2013 to July 2014 for a total of 6583 invited residents. A total of 4002 individuals aged 26–76 years were enrolled, which gave a response rate of 60.8%. We excluded individuals who were hepatitis B or C positive (n=139), had a history of hepatitis B infection (n=1), had significant alcohol consumption [men > 210 g/week or women > 140 g/week14] (n=656), were missing a CT scan [including 4 women who planned to conceive and 3 with positive β-human chorionic gonadotropin] (n=18) or glucose (n=5) or physical activity (PA) (n=17) data. We analyzed 3166 individuals with complete data.
The Pinggu metabolic disease study was approved by the ethics committees of Peking University Medical Center and University of Michigan. All participants gave written informed consent.
Non-laboratory assessments
Participants were interviewed by trained doctors and nurses in the local clinics. Height and weight were measured when participants standing without shoes and light clothing. Body mass index (BMI) was calculated as weight divided by height squared (kg/m2). Waist circumference was measured at the middle point level between lower rib margin and the iliac crest. Blood pressure was measured three times after 10min resting and the mean of the three measures was used. Education was divided into elementary school or lower, middle school, college or higher education. Annual household income was divided into <25000, ≥25000 and <75000, ≥75000 Chinese Yuan (CNY, 1000 CNY=155 USD) and response as “Don’t know”. Long-form International Physical Activity Questionnaire15 was administered to classify the PA level. Participants reported the frequency and duration of walking, moderate and vigorous activities during work, transportation, domestic/yard work, and leisure time in the previous seven days. PA scores expressed as metabolic equivalents (MET)-minutes/week were calculated for intensity-specific PAs. PA level was classified into low, moderate, and high16. Smoking status was divided into non-smoker, ex-smoker if smoking was stopped or averaged < 1 cigarette daily and current smoker if averaged ≥ 1 cigarette daily. An interviewer-administered 103-item food frequency questionnaire containing questions about meat and alcohol consumption was used. Participants reported the intake frequency and the amount of food during the past year on a daily, weekly, monthly or yearly basis. Red meat intake was defined as the total amount of pork, beef and mutton intake per week which was divided into low consumption and high consumption according to the median amount. Food frequency questionnaire (FFQ) was validated before the survey. There was a positive linear relationship between FFQ and 24hr food recall for meat intake (Spearman Correlation 0.18) which indicates good agreement (Guan et al unpublished). For assessing alcohol consumption (g/week), ethanol content was assumed to be 5.3% by volume for beer, 12.9% by volume for wine, 30.8% by weight for low-alcohol liquor and 50.1% by weight for high-alcohol liquor. Significant alcohol consumption was defined as > 210 g/week in men and > 140 g/week in women14. Individuals with significant alcohol consumption were excluded from the data analysis. Alcohol consumption was categorized into 0, > 0 and ≤ 140, > 140 and ≤ 210 g/week in men and 0, > 0 and ≤ 70, > 70 and ≤ 140 g/week in women.
Laboratory measurements
Blood samples were drawn in the morning after a 10–12 hours fast. Participants without known diabetes underwent a 75g 2-h oral glucose tolerance test and those with known diabetes had fasting plasma glucose measured. Plasma glucose, serum total cholesterol, triglycerides, high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), ALT, aspartate aminotransferase (AST) and uric acid were measured using an automated clinical chemistry analyser (UnicelDxC 800; Beckman Coulter, Miami, FL, USA). Hemoglobin A1c (HbA1c) was measured using cation-exchange high pressure liquid chromatography (Adams A1c HA-8160; Arkray, Kyoto, Japan) which conform to the Diabetes Control and Complications Trial standards. Serum hepatitis B virus surface antigen and hepatitis C antibodies were tested using an enhanced chemiluminescence assays (Ortho-Clinical Diagnostics, NJ, USA).
Definition of diseases or conditions
Known diabetes was defined by a self-reported history of diabetes diagnosed by a doctor and/or on glucose lowering treatment. Undiagnosed diabetes was defined as fasting plasma glucose (FPG) ≥ 7.0mmol/l and/or 2-h plasma glucose (2-hPG) ≥ 11.1mmol/l17. Impaired glucose tolerance (IGT) was defined as FPG < 7.0 mmol/l and 7.8 ≤ 2-hPG < 11.1 mmol/l. Impaired fasting glucose (IFG) was defined as 6.1 ≤ FPG < 7.0mmol/l and 2-hPG < 7.8 mmol/l. Normal glucose tolerance was defined as FPG < 6.1 mmol/l and 2-hPG < 7.8mmol/l17.
Overweight was defined as BMI between 23.0 and 27.49 kg/m2, and obesity as BMI ≥ 27.5 kg/m2 according to the World Health Organization Asian-specific BMI cut points18. According to the International Diabetes Federation criteria in 200519, central obesity was defined by waist circumference ≥ 90cm for men or ≥ 80cm for women19. Metabolic syndrome was defined if central obesity plus any two of the following four components were present: (1) Triglycerides ≥ 1.7 mmol/l, (2) HDL-C < 1.03 mmol/l in men or < 1.29 mmol/l in women, (3) Systolic blood pressure ≥ 130 mmHg or diastolic blood pressure ≥ 85 mmHg or treatment of previously diagnosed hypertension, (4) Fasting plasma glucose ≥ 5.6 mmol/l or previously diagnosed Type 2 diabetes19.
Abnormal CT scan and definition of NAFLD
Individuals underwent unenhanced abdominal CT scan using a 64-slice multidetector scanner (LightSpeed VCT, General Electric Healthcare, Milwaukee, WI, USA). Continuous 5mm thick slices (120kVp, 120–150mA) from the lung base to the pubic symphysis were acquired in the supine position. The Hounsfield Units (HU) of three 1cm2 areas from liver avoiding vessels and two areas in spleen were measured similar to what was done in the Framingham Heart Study20,21. The mean of the liver and spleen measurements was used to calculate the L/S ratios. NAFLD was defined as L/S ratio ≤ 1.18.
Statistical analysis
Statistical analyses were performed using SPSS for Windows, version22 (SPSS, Inc., Chicago, IL). Continuous variables with normal distribution were presented as means ± SD and compared using t-tests. Variables with skewed distribution were presented as median (25th, 75th percentage) and compared using Mann-Whitney U tests. Categorical data were presented as number and percentage and compared using Chi squared tests. A multivariable logistic regression model was used to estimate the odds ratio and its 95% confidence interval [OR (95%CI)] of candidate factors for prevalent NAFLD. Interaction between annual house income and alcohol consumption, PA level or red meat intake in men and interaction between post-menopausal status and PA level or red meat intake in women were examined. The difference was considered significant if the p-value of interaction was P< 0.05.
Results
NAFLD prevalence and associated factors
The prevalence of NAFLD was 26.5% (95% CI 24.1, 29.0), 20.1% (95% CI 18.4, 22.0) and 22.6% (95% CI 21.2, 24.1) in men, women and men and women together in the overall study population, respectively. Individuals with NAFLD were more obese, hypertensive, hyperglycemic, hyperuricemic and had a higher proportion of individuals with hypertriglycemia and metabolic syndrome and a lower proportion of individuals with a high PA level. They had higher ALT and AST levels and more individuals with NAFLD consumed red meat above the median level. A higher proportion of men with NAFLD had an annual household income ≥ 75000CNY. Women with NAFLD had a higher concentration of total cholesterol, higher proportion of post-menopausal status and lower proportion of them had received a college or higher education (Table1). Only 16.9% individuals with NAFLD (23.5% men and 11.3% women) had an abnormal ALT > 40 U/l.
Table1.
Characteristics of men and women according to with or without non-alcoholic fatty liver disease
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| Total | Non-NAFLD | NAFLD | P value | Total | Non-NAFLD | NAFLD | P value | |
| N (%) | 1238 | 910 (73.5) | 328 (26.5) | 1928 | 1540 (79.9) | 388 (20.1) | ||
| Age (year) | 49.1±12.4 | 49.6±12.3 | 47.9±12.5 | 0.034 | 50.0±11.6 | 49.5±11.6 | 51.8±11.4 | < 0.001 |
| BMI (kg/m2) | 26.1±3.8 | 25.6±3.5 | 27.5±4.1 | < 0.001 | 26.1±4.0 | 25.6±3.7 | 28.0±4.2 | < 0.001 |
| BMI (kg/m2) category | < 0.001 | < 0.001 | ||||||
| <23.0 | 254 (20.5) | 211 (23.2) | 43 (13.1) | 426 (22.1) | 381 (24.7) | 45 (11.6) | ||
| 23.0–27.49 | 564 (45.6) | 442 (48.6) | 122 (37.2) | 0.122 | 856 (44.4) | 722 (46.9) | 134 (34.5) | 0.014 |
| ≥ 27.5 | 420 (33.9) | 257 (28.2) | 163 (49.7) | < 0.001 | 646 (33.5) | 437 (28.4) | 209 (53.9) | < 0.001 |
| Waist circumference(cm) | 89.1±10.3 | 87.9±9.8 | 92.7±10.7 | < 0.001 | 84.3±10.8 | 82.9±10.1 | 90.0±11.5 | < 0.001 |
| ≥ 90cm in men or ≥ 80cm in women | 607 (49.0) | 395 (43.4) | 212 (64.6) | < 0.001 | 1241 (64.4) | 928 (60.3) | 313 (80.7) | < 0.001 |
| Systolic blood pressure (mmHg) | 130±16 | 129±16 | 133±17 | < 0.001 | 128±19 | 127±19 | 133±19 | < 0.001 |
| Diastolic blood pressure (mmHg) | 80±11 | 79±11 | 82±12 | 0.001 | 76±11 | 76±10 | 79±12 | < 0.001 |
| Glucose tolerance | < 0.001 | < 0.001 | ||||||
| Normal | 673 (54.4) | 546 (60.0) | 127 (38.7) | 1107 (57.4) | 980 (63.6) | 127 (32.7) | ||
| IGT/IFG | 301 (24.3) | 206 (22.6) | 95 (29.0) | < 0.001 | 507 (26.3) | 370 (24.0) | 137 (35.3) | < 0.001 |
| Previously undiagnosed diabetes | 117 (9.5) | 65 (7.1) | 52 (15.9) | < 0.001 | 138 (7.2) | 76 (4.9) | 62 (16.0) | < 0.001 |
| Known diabetes | 147 (11.9) | 93 (10.2) | 54 (16.5) | < 0.001 | 176 (9.1) | 114 (7.4) | 62 (16.0) | < 0.001 |
| FPG (mmol/l) | 6.3±1.9 | 6.2±1.8 | 6.6±2.0 | < 0.001 | 5.9±1.5 | 5.8±1.3 | 6.5±1.9 | < 0.001 |
| HbA1c (%) | 5.9±1.0 | 5.8±1.0 | 6.1±1.1 | < 0.001 | 5.8±0.9 | 5.7±0.8 | 6.2±1.1 | < 0.001 |
| Total cholesterol (mmol/l) | 4.8±0.9 | 4.8±0.9 | 4.8±1.1 | 0.368 | 5.0±1.0 | 4.9±1.0 | 5.2±1.0 | < 0.001 |
| Triglycerides ≥ 1.7 mmol/l | 410 (33.1) | 254 (27.9) | 156 (47.6) | < 0.001 | 488 (25.3) | 308 (20.0) | 180 (46.4) | < 0.001 |
| HDL-C (mmol/l) | 1.05±0.28 | 1.08±0.28 | 0.99±0.26 | < 0.001 | 1.21±0.29 | 1.23±0.28 | 1.13±0.30 | < 0.001 |
| LDL-C (mmol/l) | 2.83±0.81 | 2.83±0.80 | 2.83±0.83 | 0.994 | 2.92±0.82 | 2.90±0.81 | 3.01±0.85 | 0.017 |
| ALT (U/l) | 23 (17,31) | 22 (17,28) | 28 (20,40) | < 0.001 | 17 (14,23) | 17 (14,21) | 23 (16,31) | < 0.001 |
| ALT (U/l) by quartile | < 0.001 | < 0.001 | ||||||
| < 17 (men) or < 14 (women) | 242 (19.5) | 201 (22.1) | 41 (12.5) | 383 (19.9) | 348 (22.6) | 35 (9.0) | ||
| 17–22 (men) or 14–16 (women) | 356 (28.8) | 294 (32.3) | 62 (18.9) | 0.880 | 473 (24.5) | 408 (26.5) | 65 (16.8) | 0.038 |
| 23–30 (men) or 17–22 (women) | 324 (26.2) | 233 (25.6) | 91 (27.7) | 0.002 | 583 (30.2) | 489 (31.8) | 94 (24.2) | 0.002 |
| ≥ 31 (men) or ≥ 23 (women) | 316 (25.5) | 182 (20.0) | 134 (40.9) | < 0.001 | 489 (25.4) | 295 (19.2) | 194 (50.0) | < 0.001 |
| AST (U/l) | 22 (19,25) | 21 (18,25) | 23 (19,28) | < 0.001 | 20 (18,24) | 20 (18,23) | 22 (19,27) | < 0.001 |
| Uric acid (umol/l) | 322±78 | 317±76 | 337±83 | < 0.001 | 249±62 | 241±59 | 278±65 | < 0.001 |
| Metabolic syndrome | 503 (40.6) | 314 (34.5) | 189 (57.6) | < 0.001 | 925 (48.0) | 650 (42.2) | 275 (70.9) | < 0.001 |
| Post-menopausal status | - | - | - | - | 977 (50.7) | 745 (48.4) | 232 (59.8) | < 0.001 |
| Annual household income (CNY) | 0.001 | 0.926 | ||||||
| < 25000 | 178 (14.4) | 145 (15.9) | 33 (10.1) | 429 (22.3) | 341 (22.1) | 88 (22.7) | ||
| 25000–74999 | 688 (55.6) | 514 (56.5) | 174 (53.0) | 0.061 | 1061 (55.0) | 853 (55.4) | 208 (53.6) | 0.691 |
| ≥ 75000 | 349 (28.2) | 232 (25.5) | 117 (35.7) | < 0.001 | 388 (20.1) | 306 (19.9) | 82 (21.1) | 0.827 |
| Didn’t report | 23 (1.9) | 19 (2.1) | 4 (1.2) | 0.894 | 50 (2.6) | 40 (2.6) | 10 (2.6) | 0.932 |
| Education | 0.119 | 0.003 | ||||||
| Elementary school or lower | 153 (12.4) | 118 (13.0) | 35 (10.7) | 475 (24.6) | 360 (23.4) | 115 (29.6) | ||
| middle school | 864 (69.8) | 641 (70.4) | 223 (68.0) | 0.442 | 1197 (62.1) | 959 (62.3) | 238 (61.3) | 0.051 |
| College or higher | 221 (17.9) | 151 (16.6) | 70 (21.3) | 0.064 | 256 (13.3) | 221 (14.4) | 35 (9.0) | 0.001 |
| Physical activity level | 0.021 | 0.064 | ||||||
| Low | 176 (14.2) | 119 (13.1) | 57 (17.4) | 106 (5.5) | 86 (5.6) | 20 (5.2) | ||
| Moderate | 484 (39.1) | 346 (38.0) | 138 (42.1) | 0.335 | 735 (38.1) | 567 (36.8) | 168 (43.3) | 0.358 |
| High | 578 (46.7) | 445 (48.9) | 133 (40.5) | 0.013 | 1087 (56.4) | 887 (57.6) | 200 (51.5) | 0.905 |
| Smoking† | 0.317 | - | ||||||
| Non-smoker | 306 (24.7) | 221 (24.3) | 85 (25.9) | 1909 (99.0) | 1521 (98.8) | 388 (100.0) | ||
| Ex-smoker | 237 (19.1) | 167 (18.4) | 70 (21.3) | 0.653 | 3 (0.2) | 3 (0.2) | 0 (0.0) | - |
| Current-smoker | 695 (56.1) | 522 (57.4) | 173 (52.7) | 0.336 | 16 (0.8) | 16 (1.0) | 0 (0.0) | - |
| Red meat intake by median (g/week) | 0.062 | 0.037 | ||||||
| ≥ 325 (men) or ≥ 200 (women) | 621 (50.2) | 442 (48.6) | 179 (54.6) | 1022 (53.0) | 798 (51.8) | 224 (57.7) | ||
| Alcohol drinking (g/week) | 0.775 | 0.379 | ||||||
| 0 | 412 (33.3) | 308 (33.8) | 104 (31.7) | 1741 (90.3) | 1386 (90.0) | 355 (91.5) | ||
| 0.1–140 for men or 0.1–70 for women | 695 (56.1) | 506 (55.6) | 189 (57.6) | 0.477 | 164 (8.5) | 137 (8.9) | 27 (7.0) | 0.231 |
| 140.1–210 for men or 70.1–140 for women | 131 (10.6) | 96 (10.5) | 35 (10.7) | 0.736 | 23 (1.2) | 17 (1.1) | 6 (1.5) | 0.503 |
Data were expressed as means ± SD for continuous data with normal distribution, median (25th, 75th percentage) for continuous data with skewed distribution and n (%) for categorical data.
NAFLD, non-alcoholic fatty liver disease; BMI, body mass index; IGT/IFG, impaired glucose tolerance and/or impaired fasting glucose; FPG, fasting plasma glucose; HbA1c, hemoglobin A1c; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CNY, China Yuan.
P value was for the difference between the non-NAFLD and NAFLD using t test for normal distributed data, Mann-Whitney U test for skewed distributed data and Chi square test for categorical data. For multicategorical variables p-value taken from univariate logistic regression models comparing the categories to the lowest category in each variable.
Comparing between categories was not performed in women due to the small number of women smokers.
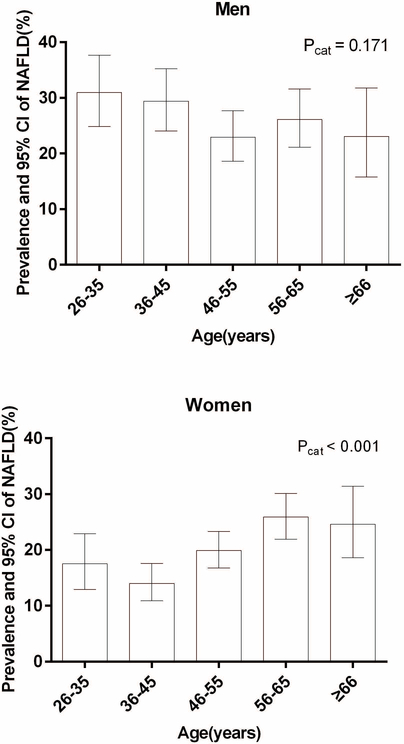
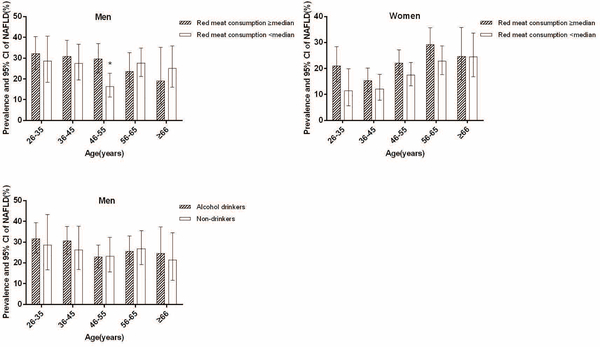
The prevalence of NAFLD in men aged 26–35, 36–45, 46–55, 56–65 and ≥ 66yrs was 31.0%, 29.4%, 22.9%, 26.1% and 23.1% [P for the category (Pcat) was 0.171], respectively, and in women it was 17.5%, 14.0%, 19.9%, 25.9% and 24.6 (Pcat < 0.001), respectively (Figure1). Younger men aged ≤ 45 yrs have a higher prevalence than older men aged > 45 yrs (30.1% vs. 24.2%, P=0.021) whereas younger women aged ≤ 45 yrs have a lower prevalence of NAFLD compared to older women aged > 45yrs (15.2% vs. 22.8%, P < 0.001). Men who consumed more red meat had a higher prevalence of NAFLD only in 46–55 yrs age group (P=0.003). Women who consumed more or less red meat had a similar prevalence of NAFLD in each age group. Alcohol drinkers and non-drinkers had a similar prevalence of NAFLD in each age group in men (Figure2). The prevalence of NAFLD between women alcohol drinkers and non-drinkers in each age group was not compared due to the small number of women drinkers in some categories.
Figure1.
Prevalence of non-alcoholic fatty liver disease (NAFLD) and 95%CI according to age in men and women
Pcat was for the difference among age groups using a Chi squared test.
Figure2.
Prevalence of NAFLD by red meat consumption and alcohol drinking according to age and sex
Red meat consumption ≥ median indicates ≥ 325g/w for men and ≥ 200g/w for women; * P=0.003 for the difference between red meat consumption ≥ median and < median in men ages 45–55.
Cardiovascular risk factors across the spectrum of NAFLD
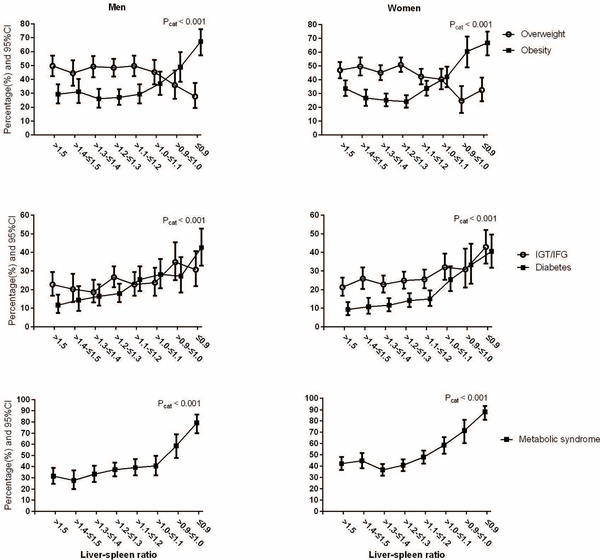
Men and women were categorized into groups according to L/S ratio. There were 181 (14.6%), 119 (9.6%), 177 (14.3%), 252 (20.4%), 181 (14.6%), 135 (10.9%), 92 (7.4%) and 101 (8.2%) men with L/S ratio > 1.5, > 1.4 - ≤ 1.5, > 1.3 - ≤1.4, > 1.2 - ≤ 1.3, > 1.1 - ≤ 1.2, > 1.0 - ≤ 1.1, > 0.9 - ≤ 1.0 and ≤ 0.9, respectively, and for women, they were 286 (14.8%), 232 (12.0%), 346 (17.9%), 370 (19.2%), 306 (15.9%), 181 (9.4%), 81 (4.2%) and 126 (6.5%), respectively.
The prevalence of metabolic complications of obesity, diabetes and metabolic syndrome began to increase at a cutoff of L/S ratio ≤ 1.1 in both men and women (Figure3).
Figure3.
Percentage of metabolic disorders in men and women according to liver spleen ratio
Overweight indicates BMI 23.0–27.49 kg/m2, and obesity indicates BMI ≥27.5 kg/m2; IGT/IFG, impaired glucose tolerance and/or impaired fasting glucose; Pcat was for the difference among liver-spleen ratio groups using a Chi squared test.
Factors independently associated with NAFLD
Increased age was associated with a decreased risk of having NAFLD in men [OR 0.87 (95%CI 0.77–0.99), model1 in Table2], and an increased risk in women [1.22 (1.09–1.36), model1 in Table2]. However, the association was no longer significant after adjustment for BMI or waist circumference, systolic blood pressure HbA1c, total cholesterol, hypertriglycerides and uric acid (model2 in Table2, model1 in Table S1). In the full model (model3 in Table2, model2 in Table S1) which was fitted with post-menopausal status (for women), annual household income, education background, PA level, smoking status (for men), red meat intake and alcohol drinking, increased BMI, waist circumference, HbA1c, elevated serum triglycerides and ALT were independently associated with the presence of NAFLD in both men and women. Increased systolic blood pressure and annual household income ≥ 75000 CNY were independently associated with the presence of NAFLD in men, whereas, increased uric acid, low PA level and red meat intake ≥ 200g/week were independently associated with the presence of NAFLD in women. Low/moderate alcohol consumption did not statistically significantly increase or decrease the odds of NAFLD in this population (Table 2).
Table2.
Odds ratio (95% CI) for non-alcoholic fatty liver disease of candidate factors corresponding to a one standard deviation increase in continuous variables and categorical data as indicated using logistic regression analysis
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Model1 | Model2 | Model3 | Model1 | Model2 | Model3 | |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Age (years) | 0.87 (0.77, 0.99) | 1.00 (0.86, 1.17) | 1.02 (0.86, 1.21) | 1.22 (1.09, 1.36) | 0.88 (0.76, 1.03) | 0.84 (0.66, 1.06) |
| BMI (kg/m2) | 1.26 (1.08, 1.47) | 1.23 (1.05, 1.44) | 1.30 (1.13, 1.48) | 1.27 (1.11, 1.45) | ||
| Systolic blood pressure (mmHg) | 1.18 (1.03, 1.36) | 1.22 (1.05, 1.41) | 1.05 (0.91, 1.21) | 1.07 (0.92, 1.24) | ||
| HbA1c (%) | 1.26 (1.11, 1.44) | 1.26 (1.11, 1.44) | 1.28 (1.15, 1.44) | 1.29 (1.15, 1.44) | ||
| Total cholesterol (mmol/l) | 0.89 (0.78, 1.02) | 0.90 (0.78, 1.03) | 1.00 (0.88, 1.14) | 1.00 (0.88, 1.13) | ||
| Triglycerides ≥ 1.7 vs. <1.7 mmol/l | 1.60 (1.19, 2.15) | 1.58 (1.17, 2.13) | 2.15 (1.65, 2.81) | 2.13 (1.62, 2.79) | ||
| ALT (U/l) | ||||||
| <17 (men) or <14 (women) | 1.00 | 1.00 | 1.00 | 1.00 | ||
| 17–22 (men) or 14–16 (women) | 0.86 (0.55, 1.35) | 0.85 (0.54, 1.33) | 1.28 (0.81, 2.02) | 1.28 (0.81, 2.02) | ||
| 23–30 (men) or 17–22 (women) | 1.37 (0.88, 2.12) | 1.38 (0.89, 2.14) | 1.17 (0.75, 1.81) | 1.16 (0.75, 1.81) | ||
| ≥ 31 (men) or ≥ 23 (women) | 2.29 (1.46, 3.58) | 2.30 (1.46, 3.62) | 3.56 (2.34, 5.42) | 3.57 (2.33, 5.47) | ||
| Uric acid (umol/l) | 1.14 (0.99, 1.31) | 1.13 (0.98, 1.31) | 1.44 (1.27, 1.63) | 1.44 (1.27, 1.64) | ||
| Post-menopausal status yes vs. no | - | 1.06 (0.70, 1.58) | ||||
| Annual household income (CNY) n (%) | ||||||
| <25000 | 1.00 | 1.00 | ||||
| 25000–74999 | 1.36 (0.85, 2.18) | 1.05 (0.76, 1.46) | ||||
| ≥ 75000 | 1.96 (1.15, 3.34) | 1.41 (0.91, 2.18) | ||||
| Didn’t report | 0.70 (0.20, 2.39) | 0.92 (0.40, 2.10) | ||||
| Education | ||||||
| Elementary school or lower | 1.00 | 1.00 | ||||
| Middle school | 0.73 (0.45, 1.19) | 0.86 (0.61, 1.21) | ||||
| College or higher | 0.79 (0.43, 1.46) | 0.58 (0.32, 1.06) | ||||
| Physical activity level | ||||||
| High | 1.00 | 1.00 | ||||
| Moderate | 1.30 (0.86, 1.96) | 1.23 (0.70, 2.18) | ||||
| Low | 1.05 (0.78, 1.43) | 1.35 (1.04, 1.75) | ||||
| Smoking | ||||||
| Non-smoker | 1.00 | - | ||||
| Ex-smoker | 1.04 (0.70, 1.56) | - | ||||
| Current-smoker | 0.92 (0.66, 1.27) | - | ||||
| Red meat intake ≥ 325 vs. < 325 (men) or ≥ 200 vs. <200 (women) g/week | 1.00 (0.76, 1.33) | 1.41 (1.09, 1.82) | ||||
| Alcohol drinking (g/week) | ||||||
| 0 | 1.00 | 1.00 | ||||
| 0.1–140 for men or 0.1–70 for women | 0.93 (0.69, 1.27) | 0.91 (0.57, 1.46) | ||||
| 140.1–210 for men or 70.1–140 for women | 0.98 (0.60, 1.59) | 1.33 (0.45, 3.86) |
BMI, body mass index; HbA1c, hemoglobin A1c; ALT, alanine aminotransferase; CNY, China Yuan.
Model1 fitted with Age
Model2 fitted with Model1+BMI, Systolic blood pressure, HbA1c, total cholesterol, triglycerides, ALT and uric acid
Model3 fitted with Model2 + post-menopausal status (for women), annual house income, education level, physical activity level, smoking status (for men), red meat intake and alcohol consumption
We tested whether annual house income and alcohol consumption (P=0.804), PA level (P=0.425) or red meat intake (P=0.550) in men and post-menopausal status and PA level (P=0.449) or red meat intake (P=0.096) in women non-linearly increased the prevalence of NAFLD using interaction modeling, but none were statistically significant.
There were 10 women and 5 men who had other data except for CT scan. As compared with the 3166 participants included in the study, participants who did not perform CT scan were younger (40.5 vs. 49.7 yrs, P=0.003). No significant differences were found in BMI (25.2 vs. 26.1 kg/m2, P=0.344), systolic blood pressure (122 vs. 129 mmHg, P=0.122) and fasting blood glucose (5.9 vs. 6.1 mmol/l, P=0.662).
Discussion
Our study showed 26.5% men and 20.1% women had NAFLD in Pinggu China. Men aged ≤ 45yrs or women aged > 45yrs were more likely to have NAFLD than their older/younger counterparts respectively. Obesity, hyperglycemia, hypertriglycemia and high ALT concentration were all independently associated with the presence of NAFLD in both men and women. High annual household income and systolic blood pressure for men and low physical activity level and high serum uric acid and consumption of red meat for women were also independent risk factors for the presence of NAFLD.
Our finding that NAFLD is prevalent in an Asian population and associates with metabolic diseases is consistent with other reported studies21–23. In a Caucasian population, the Framingham Offspring and the Third Generation study, the prevalence of fatty liver detected by CT was 19%, 15% and 17% in men, women and the whole study population, respectively21. Individuals with NAFLD in Pinggu had a lower BMI on average than individuals in Framingham but were about the same average age21. This result is consistent with observations that individuals of Asian ancestry develop metabolic disease at lower BMIs than individuals of European ancestry24. We found that obesity, hyperglycemia, high blood pressure and hypertriglycerides were all associated with NAFLD as also noted in Caucasian21,25 and Asian23,26 populations. Our study, however, also identified demographic and dietary factors as contributing to the disease independently of metabolic factors. In particular, we found that high intake of red meat was independently associated with NAFLD in women. Two small sample-sized studies reported a higher intake of meat11 or red meat27 increased the risk of the presence of NAFLD. However, these two studies did not take gender into consideration when observing the association between red meat intake and NAFLD.
To our knowledge, this is the first large population-based study in Chinese people using unenhanced abdominal CT scan to detect NAFLD. Our findings that fatty liver is prevalent and associates with metabolic disease support that CT can reliably measure NAFLD in an Asian population. CT based quantitation of liver fat is correlated (r=0.92) with histologic based fatty infiltration28. It is less expensive and clinically more widely available than proton MRS currently and less operator dependent and accurate than ultrasound29. The L/S ratio provided a more accurate evaluation for liver fat content than ultrasound. Further, we show that only 16.9% of those with NAFLD had abnormally elevated ALT (> 40 U/l) suggesting that ALT is not a good marker of the disease despite its widespread use.
From a public health perspective, our results suggest screening, prevention and intervention strategies for NAFLD should be focused on young and middle-aged men as well as elder women who were at a higher risk of NAFLD in this cohort. Our data demonstrate that an L/S ratio≤1.1 is an appropriate cutoff point for discriminating a population with a higher prevalence of metabolic disorders in both men and women. Thus, screening for metabolic disorders in individuals found to have a low L/S ratio on abdominal CT should be considered.
Moreover, our study provides current information on the prevalence of NAFLD in men and women from a largely unmedicated general population in mainland China. In this study, although men and women had similar prevalence of obesity defined by BMI, NAFLD is more prevalent in men than in women, consistent with one previous report22. Such a result is consistent with the fact that a higher proportion of men than women had metabolic complications of obesity, specifically hypertriglycemia and diabetes. In several studies26,30,31, the prevalence of NAFLD increased as age increased in women and only one study among the above31 showed the same result in men. The other two studies26,30 including ours showed the peak prevalence of NAFLD in young or middle-aged men. The reason why the peak prevalence of NAFLD differs between men and women is not clear. In our study, the peak in NAFLD prevalence in men corresponded with increased sedentary behavior, increased alcohol consumption, increased eating of red meat, and high household income. Only high household income, however independently associated with NAFLD in men in a multivariate model. This variable although clearly not pathophysiologically plausible, may best capture behaviors such as alcohol consumption, increased sedentary behavior, and eating red meat that could be more directly contributing to NAFLD pathophysiology in men. Although we identified distinct unique demographic determinants that independently correlated with NAFLD in men versus women, we cannot rule out that biological variables also contributed to the differences between men and women and can be studied through future work on the genetics of NAFLD in this population.
Strength of the current study includes that it is the largest non-medicated population-based study using CT scanning to measure NAFLD in Asia. The study is also contemporary and focuses on a region undergoing rapid gentrification which allowed us to capture a wide diversity of exposures to be able to identify demographic and dietary factors that also associate with NAFLD. Limitations include that this region may not be representative of all of China and that based on cross-sectional data we cannot infer causality between associated factors and NAFLD. Although we excluded individuals with excess alcohol drinking from the analysis and controlled for any residual alcohol consumption using regression, we cannot rule out that there was under reporting of drinking which may have contributed to fatty liver on CT scanning. We do not see a protection from development of fatty liver with low levels of alcohol consumption as some have reported32.
In conclusion, we found that 26.5% men and 20.1% women had NAFLD in Pinggu, China. NAFLD was independently associated with obesity, hyperglycemia, hypertriglycemia and elevated ALT in both men and women. Higher annual household income and blood pressure for men and higher serum uric acid, lower physical activity level and higher red meat intake for women were also independently associated with NAFLD in multivariate models. An L/S ratio≤1.1 indicated a high risk of metabolic disorders in general populations and individuals with such findings on CT scanning may want to be screened for concomitant metabolic disease.
Supplementary Material
Acknowledgments
This work is supported in part by the grants from Ministry of Science and Technology of the People’s Republic of China for the Study of the Major Chronic Non-communicable Disease Prevention and Control Research, National Key R&D Program of China (2016YFC1305600, 2016YFC1305603, 2016YFC1304900, 2016YFC1304901). EKS is supported by NIH grants K23 DK080145, RO1 DK106621, RO1 DK107904, The Doris Duke Medical Foundation, The University of Michigan Biological Sciences Scholars Program, and The University of Michigan Department of Internal Medicine. YP was supported by the NIH T32 HG000040. YYG was supported by a summer fellowship from the Epidemiology Department, University of Michigan.
Footnotes
Conflict of interest
The authors have declared no conflict of interests.
References
- 1.Hamaguchi M, Kojima T, Takeda N, et al. Nonalcoholic fatty liver disease is a novel predictor of cardiovascular disease. World J Gastroenterol. 2007;13(10):1579–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pisto P, Santaniemi M, Bloigu R, Ukkola O, Kesaniemi YA. Fatty liver predicts the risk for cardiovascular events in middle-aged population: a population-based cohort study. BMJ Open. 2014;4(3):e004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mellinger JL, Pencina KM, Massaro JM, et al. Hepatic steatosis and cardiovascular disease outcomes: An analysis of the Framingham Heart Study. J Hepatol. 2015;63(2):470–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001–2013. Gastroenterology. 2015;149(6):1471–1482. [DOI] [PubMed] [Google Scholar]
- 5.Younossi ZM, Otgonsuren M, Henry L, et al. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology. 2015;62(6):1723–1730. [DOI] [PubMed] [Google Scholar]
- 6.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. [DOI] [PubMed] [Google Scholar]
- 7.Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313(22):2263–2273. [DOI] [PubMed] [Google Scholar]
- 8.Iwasaki M, Takada Y, Hayashi M, et al. Noninvasive evaluation of graft steatosis in living donor liver transplantation. Transplantation. 2004;78(10):1501–1505. [DOI] [PubMed] [Google Scholar]
- 9.Zelber-Sagi S, Nitzan-Kaluski D, Goldsmith R, et al. Role of leisure-time physical activity in nonalcoholic fatty liver disease: a population-based study. Hepatology. 2008;48(6):1791–1798. [DOI] [PubMed] [Google Scholar]
- 10.Perseghin G, Lattuada G, De Cobelli F, et al. Habitual physical activity is associated with intrahepatic fat content in humans. Diabetes Care. 2007;30(3):683–688. [DOI] [PubMed] [Google Scholar]
- 11.Zelber-Sagi S, Nitzan-Kaluski D, Goldsmith R, et al. Long term nutritional intake and the risk for non-alcoholic fatty liver disease (NAFLD): a population based study. J Hepatol. 2007;47(5):711–717. [DOI] [PubMed] [Google Scholar]
- 12.Shi L, Liu ZW, Li Y, et al. The prevalence of nonalcoholic fatty liver disease and its association with lifestyle/dietary habits among university faculty and staff in Chengdu. Biomed Environ Sci. 2012;25(4):383–391. [DOI] [PubMed] [Google Scholar]
- 13.Zou X, Li Y, Cai X, et al. Decreased Glycemic Difference Between Diabetes and Nondiabetes in the Elderly Leads to the Reduced Diagnostic Accuracy of Hemoglobin A1c for Diabetes Screening in an Aged Chinese Population. Diabetes Technol Ther. 2016;18(4):226–232. [DOI] [PubMed] [Google Scholar]
- 14.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142(7):1592–1609. [DOI] [PubMed] [Google Scholar]
- 15.IPAQ. International Physical Activity Questionnaire (October 2002) long last 7 days self-administered format. [Internet]. 2002; https://sites.google.com/site/theipaq/questionnaire_links. Accessed July 9, 2018.
- 16.IPAQ. Guidelines for Data Processing and Analysis of the International Physical Activity Questionnaire (IPAQ)-Short and Long Forms. [Internet]. 2005; https://sites.google.com/site/theipaq/scoring-protocol. Accessed July 9, 2018.
- 17.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–553. [DOI] [PubMed] [Google Scholar]
- 18.Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–163. [DOI] [PubMed] [Google Scholar]
- 19.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23(5):469–480. [DOI] [PubMed] [Google Scholar]
- 20.Speliotes EK, Massaro JM, Hoffmann U, et al. Liver fat is reproducibly measured using computed tomography in the Framingham Heart Study. J Gastroenterol Hepatol. 2008;23(6):894–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Speliotes EK, Massaro JM, Hoffmann U, et al. Fatty liver is associated with dyslipidemia and dysglycemia independent of visceral fat: the Framingham Heart Study. Hepatology. 2010;51(6):1979–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu JZ, Dai YN, Wang YM, Zhou QY, Yu CH, Li YM. Prevalence of Nonalcoholic Fatty Liver Disease and Economy. Dig Dis Sci. 2015;60(11):3194–3202. [DOI] [PubMed] [Google Scholar]
- 23.Wong VW, Chu WC, Wong GL, et al. Prevalence of non-alcoholic fatty liver disease and advanced fibrosis in Hong Kong Chinese: a population study using proton-magnetic resonance spectroscopy and transient elastography. Gut. 2012;61(3):409–415. [DOI] [PubMed] [Google Scholar]
- 24.Yoon KH, Lee JH, Kim JW, et al. Epidemic obesity and type 2 diabetes in Asia. Lancet. 2006;368(9548):1681–1688. [DOI] [PubMed] [Google Scholar]
- 25.DeFilippis AP, Blaha MJ, Martin SS, et al. Nonalcoholic fatty liver disease and serum lipoproteins: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2013;227(2):429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eguchi Y, Hyogo H, Ono M, et al. Prevalence and associated metabolic factors of nonalcoholic fatty liver disease in the general population from 2009 to 2010 in Japan: a multicenter large retrospective study. J Gastroenterol. 2012;47(5):586–595. [DOI] [PubMed] [Google Scholar]
- 27.Georgoulis M, Kontogianni MD, Margariti A, et al. Associations between dietary intake and the presence of the metabolic syndrome in patients with non-alcoholic fatty liver disease. Journal of human nutrition and dietetics : the official journal of the British Dietetic Association. 2015;28(4):409–415. [DOI] [PubMed] [Google Scholar]
- 28.Bydder GM, Chapman RW, Harry D, Bassan L, Sherlock S, Kreel L. Computed tomography attenuation values in fatty liver. The Journal of computed tomography. 1981;5(1):33–35. [DOI] [PubMed] [Google Scholar]
- 29.Lee SS, Park SH. Radiologic evaluation of nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20(23):7392–7402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fan JG, Zhu J, Li XJ, et al. Prevalence of and risk factors for fatty liver in a general population of Shanghai, China. J Hepatol. 2005;43(3):508–514. [DOI] [PubMed] [Google Scholar]
- 31.Lazo M, Hernaez R, Eberhardt MS, et al. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988–1994. Am J Epidemiol. 2013;178(1):38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ajmera VH, Terrault NA, Harrison SA. Is moderate alcohol use in nonalcoholic fatty liver disease good or bad? A critical review. Hepatology. 2017;65(6):2090–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.