Abstract
During cancer progression, tumor cells undergo molecular and phenotypic changes collectively referred to as cellular plasticity. Such changes result from microenvironmental cues, stochastic genetic and epigenetic alterations, and/or treatment-imposed selective pressures, thereby contributing to tumor heterogeneity and therapy resistance. Epithelial-mesenchymal-plasticity (EMT) is the best-known case of tumor cell plasticity, but recent work has uncovered other examples, often with functional consequences. In this review, we explore the nature and role(s) of these diverse cellular plasticity programs in pre-malignant progression, tumor evolution, and adaptation to therapy, and consider ways in which targeting plasticity could lead to novel anti-cancer treatments.
INTRODUCTION
The ability of cells to adopt different identities along a phenotypic spectrum is a phenomenon broadly known as cellular plasticity. Although it is a signature feature of embryonic differentiation, cellular plasticity has also been widely observed in terminally-differentiated adult cells faced with chronic physiologic and pathologic stresses. Under such circumstances, cellular plasticity serves as a mechanism of tissue adaptation or regeneration, but it can also predispose tissues to cancerous transformation.
While loss of normal cell identity and function is intrinsic to the malignant process, cancer cells undergo further phenotypic changes during tumor progression and treatment. Tumor cells are exposed to diverse metabolic conditions, signaling molecules, stromal elements, and therapeutic agents, which collectively form a volatile microenvironment that can fuel changes in cellular phenotype. Such changes may involve genetic alterations, but they more commonly involve transcriptional or epigenetic fluctuations. The resulting pliability in cell state can facilitate multiple aspects of tumor progression, including tumor initiation and metastasis, immune evasion, and chemoresistance. Consequently, elucidating the mechanisms by which cancer cells exploit plasticity to cope with selective pressures may lead to novel therapeutic opportunities. In leukemia, for example, treatment regimens that target a tumor’s differentiation state are highly effective, providing a rationale for pursuing such “differentiation therapies” in solid tumors. Here, we review our current understanding of cellular plasticity in cancer initiation and progression and suggest ways in which mechanistic insights could have implications for therapy.
CELLULAR PLASTICITY IN PRE-MALIGNANCY: METAPLASIA
In several adult tissues, cells change their identity as part of a physiologic response to injury or inflammation [1, 2]. Such changes may occur at the level of individual cells, where the phenomenon is commonly referred to as “trans-differentiation,” or at the level of an entire tissue, where the transformation is referred to as “metaplasia.” Metaplasia is thought to serve a protective function in the face of chronic damage, either by replacing lost tissue or forming barriers better suited to withstand hostile conditions. But in multiple organs – particularly those comprising the GI tract and other endoderm-derived tissues – the phenomenon is associated with a predisposition to cancer (Table 1). Importantly, metaplasia and trans-differentiation are not synonymous; while metaplastic tissues may arise through the conversion of one terminally differentiated cell type into another (i.e. trans-differentiation), alternative mechanisms – e.g. selective proliferation, drop-out of certain cell types, or alterations in stem cell differentiation patterns – could also account for metaplastic tissue changes. While lineage tracing studies in mice have provided insight into the programs underlying some forms of metaplasia, little is known about the cellular and molecular mechanisms leading to metaplasia in humans.
Table 1.
Examples of metaplasia in cancer
| Tissue Affected | Associated States/Conditions | Cell of Origin | Cellular Conversion | Resultant Cancer Type | References |
|---|---|---|---|---|---|
| Esophagus | Gastroesophageal reflux disease (GERD), acid/bile | Unknown (squamous cells, gastric stem cells, basal cells) | Squamous esophageal → columnar intestinal | Esophageal adenocarcinoma | [14–16] |
| Stomach | Helicobacter pylori, smoking, alcohol, high salt intake | Unknown (chief cells, isthmus stem cells, crypt stem cells | Squamous gastric → SPEM/columnar intestinal | Gastric adenocarcinoma | [21, 22, 118] |
| Pancreas | Inflammation (pancreatitis) | Acinar cells | Acinar → ductal | Pancreatic ductal adenocarcinoma | [34–36, 38, 119] |
| Liver | Chronic injury (alcohol, fatty liver, viral hepatitis) | Hepatocytes | Hepatocyte → biliary | Intrahepatic cholangiocarcinoma | [27–30] |
| Lung | Cigarette smoke | Unknown (neuroendocrine cells, club cells, type II cells) | Cuboidal → stratified squamous | Non-small-cell lung cancer (squamous cell carcinoma) | [39] |
| Cervix | Low vaginal pH, human papilloma virus (HPV) | Endocervical cells | Columnar endocervix → squamous | Squamous cell carcinoma of the cervix | [41, 120–122] |
| Cervical → gastric | Gastric type adenocarcinoma |
Intestinal metaplasia of the esophagus and stomach
In Barrett’s esophagus (BE), the normal squamous epithelium of the esophagus is replaced by columnar cells that harbor features of the small intestine. Clinically, the precise cause of Barrett’s esophagus is unknown, although chronic gastroesophageal reflux disease (GERD) – in which the distal esophagus is exposed to gastric contents – is strongly associated with the condition [3]. Gastric acid and bile salts conspire to injure the epithelial cells lining the esophagus in GERD, resulting in inflammation and the production of reactive oxygen species (ROS). In response to these injuries, mucus-secreting columnar epithelial cells typical of the small intestine replace the normal squamous epithelium of the esophagus. While this new mucus-producing epithelium provides better protection against stomach acid, it can also serve as a precursor for esophageal adenocarcinoma [4, 5]. Barrett’s lesions rarely regress, suggesting that the cellular and molecular changes underlying the phenomenon are stable.
The precise cellular origins of BE remain controversial. Because the lesions almost always involve the area of transition between the glandular mucosa of the stomach and the squamous mucosa of the esophagus – the so-called “squamocolumnar junction” (SCJ) – the cells giving rise to BE are presumed to reside in or near this zone. One potential mechanism for metaplasia involves a phenomenon known as “trans-commitment,” in which a stem or progenitor cell is diverted from its normal differentiation path. In several studies in mice, stem or progenitor cells residing at or near the SCJ have been implicated as giving rise to BE-like lesions [6–8], although the extent to which these candidates for the “cell-of-origin” of BE represent distinct or overlapping cell populations is unclear. In addition, other sources of esophageal metaplasia have been invoked, including the squamous cells of the esophagus themselves, either through direct conversion of differentiated epithelial cells (trans-differentiation) or trans-commitment of stem cells residing at the basal layer of the esophageal mucosa [9]. The interpretation of BE studies in animals is confounded by substantial differences between mouse and human anatomy; consequently, the cellular origins of Barrett’s metaplasia in human patients remains entirely unknown.
Metaplasia is also associated with the development of gastric malignancy. Analogous to the esophagus, the normal foveolar and oxyntic epithelium of the stomach may be replaced by intestinal epithelium, a process referred to as spasmolytic polypeptide-expressing metaplasia (SPEM). Several factors, such as chronic Helicobacter pylori, hyperacidity, smoking, alcohol intake, and high salt intake, can lead to the loss of the acid secreting parietal cells and enzyme producing chief cells in the stomach (so-called “atrophic gastritis”) [10–12]. As these cells die, other cells that express spasmolytic polypeptide (SP) begin to emerge, giving rise to a metaplastic epithelium. The epithelium of the stomach can also undergo intestinal metaplasia – either directly or through a SPEM intermediate – which is also at risk for malignant transformation. Although the cellular origins of SPEM, intestinal metaplasia, and adenocarcinoma of the stomach remain controversial, gastric chief cells [13] and gastric isthmus stem cells [14] have emerged as the strongest candidates.
Ductal metaplasia in the liver
Liver injury is associated with dramatic changes in cellular identity. Two cell types – hepatocytes, which comprise the bulk of liver mass, and cholangiocytes (or biliary epithelial cells; BECs), which line the bile-transporting ducts of the liver – perform all the liver’s major functions. Following toxin-induced injury, hepatocytes undergo a trans-differentiation into BECs, likely as a way of regenerating lost bile ducts [15, 16]. This transition occurs in a stepwise fashion over a period of 2–3 weeks – involving a series of intermediate states – and is driven by signals from the Notch and/or Hippo pathway [15, 17, 18].
As is the case with the esophagus and stomach, chronic liver injury (and the ensuing cellular plasticity) predisposes to cancer. Cancers arising in the liver exhibit one of two histopathological forms: hepatocellular carcinoma (HCC) and cholangiocarcinoma (CC). Cirrhosis, or severe scarring of the liver, is a risk factor for both. It has been assumed, based on such histological classifications, that these distinct tumor types arise from their corresponding normal counterpart; in other words, HCCs have been presumed to arise from hepatocytes and CCs have been presumed to arise from cholangiocytes. Consequently, CC is often referred to as “bile duct cancer.”
However, several lines of evidence suggest that at least a subset of CCs arise from hepatocytes, not cholangiocytes. Specifically, the activation of various oncogenic mutations (Notch, Kras, Pten) in murine hepatocytes in vivo results in carcinomas with histological characteristics of CC [19–21], suggesting that biliary trans-differentiation precedes cancer initiation. Importantly, these results do not preclude a biliary origin for CC, as suggested by other studies [22, 23], but rather suggest that CC may arise from either hepatocytes or BECs. Collectively, these studies suggest that a given cell type (i.e. the hepatocyte) may give rise to tumors with vastly different histological characteristics as a result of lineage plasticity elicited by distinct oncogenic pressures. In the future, it will be important to distinguish human CCs that are hepatocyte-derived from those that are BEC-derived, as tumors with distinct cellular origins are likely to exhibit distinct biological features which could further translate into divergent therapeutic opportunities.
Ductal metaplasia in the pancreas
The exocrine pancreas is responsible for synthesizing digestive enzymes and delivering them to the intestine. It does so through two principal cellular components: acinar cells, which produce enzymes, and ductal cells, which line the pancreatic ducts and carry the enzyme-rich pancreatic juice to the intestine. Cancers of the exocrine pancreas generally fall into two corresponding histological categories – pancreatic acinar carcinoma and pancreatic ductal carcinoma (PDAC). Consequently, these two tumor types were long believed to arise from the corresponding normal cells (i.e. acinar carcinomas were presumed to arise from acinar cells and ductal carcinomas were presumed to arise from ductal cells). But as we have already seen in the liver, cellular plasticity can give rise to a false impression regarding a cancer’s cellular origins, and multiple studies in the mouse have called this simple lineage relationship into question.
Acinar-to-ductal metaplasia (ADM) [24] describes a process wherein normal pancreatic acinar cells assume a duct-like state. ADM is observed in the setting of chronic injury (pancreatitis) in both mice and humans. While resolution of pancreatitis leads to regression of ADM lesions in the normal pancreas, further histologic changes occur when oncogenic signals from the KRAS oncogene are present, resulting in precancerous pancreatic intraepithelial neoplasia (PanIN). Support for an ADM → PanIN → PDAC progression model is supported by several lines of evidence including mutational and clinical-pathological observations, in vitro trans-differentiation of acinar cells, and lineage tracing studies [25–29]. Hence, it appears likely that many if not most PDACs do not arise from duct cells at all, but instead trace their origins to acinar cells that have undergone trans-differentiation to a duct-like state.
Unlike intestinal metaplasia in the esophagus, ductal metaplasia in the pancreas appears to be reversible, indicating a high degree of plasticity associated with the ADM state. In murine models, withdrawal of an ADM-provoking stimulus – either inflammatory or genetic – allows rapid and complete resolution of histological abnormalities. Likewise, acute pancreatitis in mice and humans typically resolves without histological sequelae. The difference in plasticity exhibited by the esophagus and pancreas may be related to the rapidity of cellular transitions in different tissues. While ADM occurs rapidly (over days), Barrett’s esophagus develops over much longer periods of time (weeks to months – the exact timing is unknown). The degree of chronicity of metaplasia is also likely to influence cancer risk, as individuals with recurrent or chronic pancreatitis have a markedly increased risk of developing PDAC while those with one or two episodes of uncomplicated acute pancreatitis do not [30].
Squamous metaplasia in the lung and cervix
While Barrett’s metaplasia involves the replacement of esophageal squamous epithelium with intestinal epithelium, metaplasia in the lung involves conversions in the opposite direction. Specifically, the simple cuboidal or columnar epithelium of the airways and alveoli can be replaced by a stratified squamous epithelium. As with the instances of metaplasia discussed above, squamous metaplasia in the lung is thought to arise as a response to chronic injury, where it is thought to have a protective effect. But squamous metaplasia also predisposes these cells to malignant transformation, including cystic keratinizing epithelioma and squamous cell carcinoma [31]. Similarly, the epithelium of the female genitourinary tract – the uterine endometrium and the endocervix – can undergo premalignant squamous metaplasia. In the cervix, the resulting squamous epithelium can be infected with human papillomavirus (HPV), which can lead to dysplasia and squamous cell carcinoma [32]. Cellular plasticity in the context of cervical cancer is further exemplified by the entity of gastric metaplasia, wherein the cervical mucosa takes on the appearance of the foveolar and pyloric epithelium of the stomach. Gastric metaplasia of the cervix can progress to “gastric type adenocarcinoma,” which represents the most common form of non-HPV-associated cervical cancer [33]
Plasticity and cancer risk
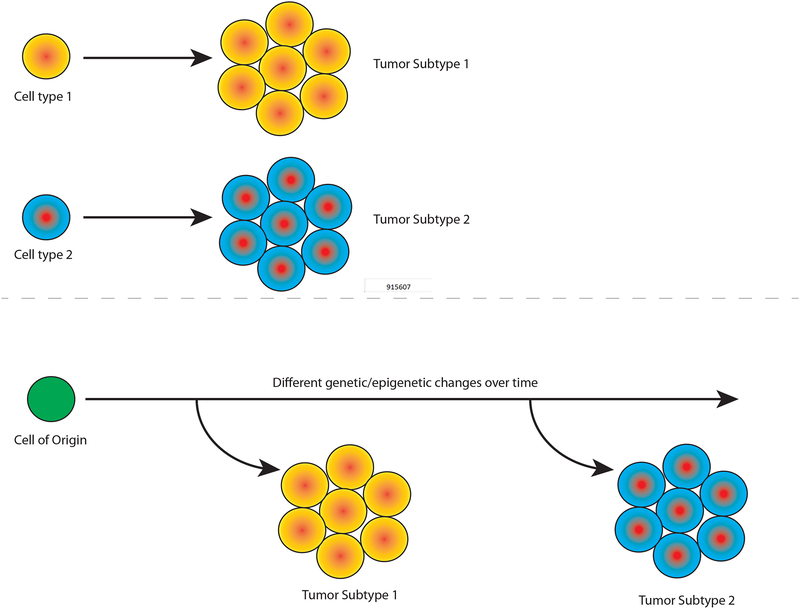
These observations raise provocative questions about the earliest stages of cancer initiation; particularly, why and how does tissue metaplasia confer an increased cancer risk? Classical models, based on straightforward histological correlations, held that a tumor’s final histopathological state indicated its cell of origin. As intuitive as this inference may seem, the strong association between metaplasia and malignancy suggest that this simple model is flawed, forcing consideration of more nuanced mechanisms (Fig. 1).
Figure 1. The contribution of lineage plasticity to tumor initiation.
Solid tumors are classified based on the organ in which they arise and their histological, molecular, and/or transcriptomic profiles. For example, primary tumors in the liver can be histologically classified as hepatocellular carcinoma (HCC) or cholangiocarcinoma (CC). While the cellular origins of divergent tumor types remain unclear, there are two general prevailing hypotheses. (A) One hypothesis proposes that different tumor types arise from different cells of origin. With respect to liver cancer, this would imply that HCC arises from hepatocytes, while CC’s are derived from cholangiocytes. (B) Alternatively, different tumor types may arise in a single organ through lineage plasticity, wherein distinct genetic or epigenetic events may induce a common cell-of-origin to acquire divergent malignant phenotypes. There is evidence for lineage plasticity in cancers of the esophagus (intestinal metaplasia), pancreas (acinar-to-ductal metaplasia), liver (biliary trans-differentiation), and lung and cervix (squamous metaplasia). See text for details.
One attractive hypothesis is that metaplasia sensitizes cells to the transforming effects of oncogenic stimuli to which they would otherwise be resistant. Metaplasia is associated with large alterations in chromatin landscapes, leading to dynamic changes in gene expression. These epigenetic and transcriptional changes allow tissues to cope with an acute injury, but they may simultaneously sow the seeds for malignant transformation by rendering tumor-promoting genes more “open” and/or tumor-suppressing genes more “closed.” Such structural changes in the epigenome, in the right cellular context, may therefore create favorable conditions for oncogenes to act.
Mutations in oncogenes and tumor suppressor genes have vastly different effects in different cells of origin. For example, pancreatic acinar cells are sensitive to the transforming effects of mutant KRAS and TRP53, while pancreatic ductal cells are relatively resistant [28, 34]. By contrast, pancreatic ductal cells are sensitive to the transforming effects of KRAS and loss of PTEN [35]. These observations, along with similar studies in the liver, indicate that the likelihood of a tumor forming – and the tumor’s eventual histological type (e.g. acinar vs. ductal carcinoma and CC vs. HCC) – depends on both the specific oncogenic drivers that are present and the cellular compartment in which they are expressed [19–23]. Consistent with this notion, loss of the tumor suppressor LKB1 in club cells and bronchioalveolar stem cells (BASCs) of the lung not only accelerates KRAS-driven lung adenocarcinoma, but also renders the resultant tumors more susceptible to a lineage switch to squamous cell carcinoma [36].
Taken together, these studies raise the possibility that the epigenetic and transcriptional rewiring accompanying metaplasia may itself serve as an oncogenic stimulus. For example, while the epigenetic state of a pancreatic duct cell may confer resistance to the oncogenic effects of mutant KRAS and TRP53 at baseline, its superimposition on a pre-existing acinar state (as would occur during ADM) may confer sensitivity to the same oncogenic signals. Further studies in animal models and human clinical specimens – including careful examination of the chromatin states associated with normal, metaplastic, and pre-malignant tissues – will help resolve these issues.
EPITHELIAL-MESENCHYMAL PLASTICITY
During embryonic development, when cells regularly shift identities to form differentiated tissues, cellular plasticity is common. The best-known example, first described by Elizabeth Hay as an “epithelial to mesenchymal transformation,” is now commonly referred to as the “epithelial-mesenchymal transition,” or EMT [37] (see Box 1 for other examples of epithelial and/or mesenchymal plasticity). The programs encompassing EMT – or the reverse process of mesenchymal-to-epithelial transition (MET) – span metazoan evolution and encompass multiple overlapping and distinct programs.
Box 1: Other shades of epithelial/mesenchymal plasticity.
Epithelial-to-endothelial transition (EET): Vasculogenic mimicry (VM), involving the EET, involves aggressive tumor cells acquiring the morphology, phenotypic markers, and function of endothelial cells. These EET-derived cells have been shown to integrate with true endothelium-lined vasculature, helping to form a fluid-conducting meshwork that forms independently of, or simultaneously with, angiogenesis. First described in melanoma, EET has since been observed in many other carcinomas, as well as during normal embryonic development, where fetal cytotrophoblasts undergo an EET as they invade into the maternal tissue to establish the placenta and its microcirculation (reviewed in [39] and [40]). Still, the true functional significance of EET, the molecular mechanisms driving this process, and its therapeutic implications, particularly with respect to angiogenesis inhibitors, remain unclear.
Endothelial-to-hematopoietic transition (EHT): In the developing embryo, hemogenic endothelial cells residing in the large arteries give rise to multi-lineage hematopoietic stem and progenitor cells (HSPCs) through a process referred to as EHT. During this process, key transcription factors, such as Runx1 and Ets and GATA factors drive the transition from an endothelial to hematopoietic fate. This transition involves phenotypic changes reminiscent of an EMT, whereby flat endothelial cells round up, lose cell-cell interactions, and undergo extensive cell shape rearrangements (reviewed in [41]).
Endothelial-to-mesenchymal transition (EndoMT): During embryonic cardiac development, a subset of endothelial cells acquires a mesenchymal identity to help form the valves and septa of the heart. Since endothelial cells are characterized by their barrier-forming adherens and tight junctions, this process is often thought to be analogous to EMT. Lineage tracing studies have also found that EndoMT contributes to the development of various cardiovascular diseases, including myocardial infarction, cardiac fibrosis, valve calcification, and atherosclerosis (reviewed in [42] and [43]). In tumors, EndoMT has been found to be a source of cancer-associated fibroblasts (CAFs) [44] and was recently shown to promote lung fibrosis and tumorigenesis [45], as well as pro-tumorigenic macrophage polarization [46].
In carcinomas, which arise from epithelial cells, the manifestation of an EMT program is reflected in a tumor’s grade. High-grade disease is aggressive and marked by an obliteration of normal tissue structure and architecture. Such tumors – often referred to as “poorly differentiated” – bear the histopathological and molecular hallmarks of EMT. By contrast, low-grade disease is characterized by a “moderately-to-well differentiated” histology that reflects the cancer cells’ retention of an epithelial phenotype. Across human cancer, high-grade (poorly differentiated) tumors carry a worse prognosis than low-grade (well-differentiated) tumors.
Importantly, such grading schemes describe the dominant cellular phenotype within a tumor and thus fail to capture the dynamic plasticity that exists in cancer. Rather than being wholly comprised of cancer cells with either a mesenchymal or an epithelial phenotype, most carcinomas are composites of the two phenotypes existing in equilibrium. In poorly-differentiated tumors, this equilibrium is shifted to the mesenchymal state, while in well-differentiated tumors it is shifted to the epithelial state. Thus, it is the relative abundance of cells in either state that indicates tumor grade. Although the determinants of “equilibrium constants” governing epithelial-mesenchymal plasticity in tumors are unknown, it is likely that genetic and epigenetic factors existing before or acquired during tumor progression are responsible. As we have already seen, the normal “cell-of-origin” from which a tumor arises plays an important role in shaping tumor histology, and it likely also influences the epithelial-mesenchymal “setpoint” of a tumor [38].
Mechanisms of epithelial-mesenchymal plasticity
Cells may switch between epithelial and mesenchymal states several times during development. One of the most striking examples of EMT is gastrulation, when the epithelial cells of the epiblast lose their epithelial features (apical-basal polarity and cell-cell junctions) and migrate through the primitive streak to form the three embryonic germ layers. In addition, epithelial-mesenchymal plasticity plays a critical role in the development of the neural crest (and its descendants in the thymus, heart, enteric nervous system, and melanocytes), the liver, the kidney, and other tissues. EMT can be induced by any of several pleiotropic and evolutionarily conserved signaling factors (e.g. TGFβ, EGF, HGF, NOTCH, FGF, and WNT ligands), which initiate a signaling cascade leading to the expression of one or more so-called “EMT transcription factors” (EMT-TFs). These EMT-TFs – which include SNAIL, TWIST, ZEB, and PRRX family members – function as transcriptional activators and repressors whose principal function is the repression of genes whose products are necessary for maintenance of the epithelial state (i.e. proteins comprising junctional complexes and epithelial intermediate filaments). Loss of E-cadherin, a key component of epithelial adherens junctions, is considered a hallmark of EMT. In parallel, EMT involves the induction of genes associated with the mesenchymal state, including the mesenchymal intermediate filament protein vimentin [47]. EMT is essential for normal development and its disruption leads to dramatic developmental defects including problems with gastrulation, neural crest migration, and other developmental abnormalities [37]. In other contexts, including kidney development, reversion from a mesenchymal state to an epithelial state (MET) is critical for proper organogenesis.
While most studies of EMT mechanisms have focused on transcriptional regulatory programs, post-transcriptional programs also regulate the epithelial phenotype. During development, the abundance or localization of E-cadherin – a critical component of epithelial adherens junctions – can be lost through one of several post-transcriptional mechanisms. These include p38-mediated regulation of E-cadherin protein levels during mouse gastrulation [48–50], EGF-mediated endocytosis of E-cadherin during zebrafish epiboly [51], and transcription-independent regulation of E-cadherin by the GATA factor Serpent during Drosophila endoderm development [52]. Similarly, post-transcriptional programs have recently been shown to mediate cancer-associated EMT, where a significant percentage of carcinoma cells lose their epithelial phenotype through internalization of epithelial proteins rather than transcriptional repression [53].
EMT is rarely observed in adult tissues under homeostatic conditions, but it can emerge upon injury or stress and is a common feature of malignancy. Many of the factors shown to induce EMT during embryogenesis or under in vitro settings are present in tumors, including a variety of soluble growth factors and matrix components, hypoxia, inflammation, and increased tissue stiffness [47]. The best studied of these inducers is TGFβ, which can induce EMT in a wide assortment of cultured carcinoma cells. TGFβ signaling induces the formation of an active SMAD complex, which partners with other DNA binding proteins to induce the transcription of EMT-TFs such as SNAIL, TWIST, and ZEB [54].
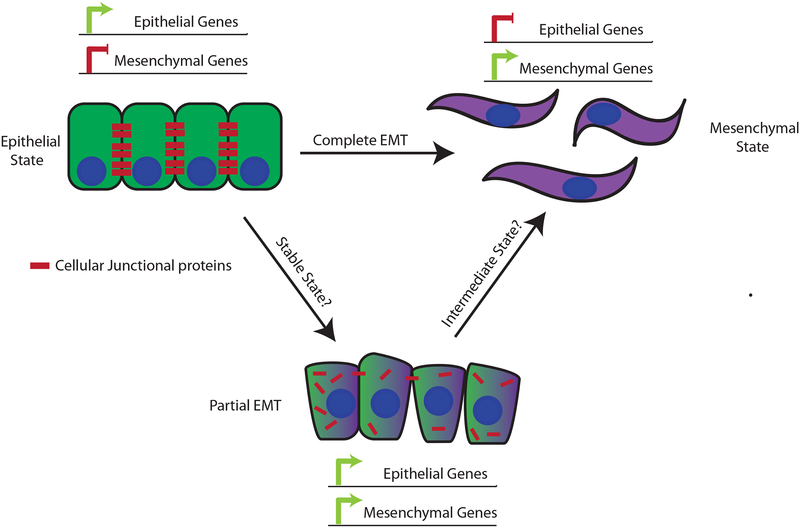
Importantly, EMT does not represent a single program. Rather, it encompasses a phenotypic spectrum characterized by different degrees of epithelial and mesenchymal features and involving a range of mechanisms (Fig. 2). Hence, definitions of EMT have evolved over time to accommodate a variety of phenotypic transitions that involve some measurable changes in the epithelial or mesenchymal features of a cell [37, 55]. This conceptual flexibility has also led to the recognition that cells having “intermediate” epithelial-mesenchymal phenotype – occupying a “partial EMT” (p-EMT) state – have properties distinguishing them from cells with a purely epithelial or mesenchymal phenotype. Cells residing in such p-EMT states may simultaneously express epithelial and mesenchymal features or may have lost their epithelial characteristics without acquiring mesenchymal traits. Still unresolved is whether these intermediate states are “metastable,” suggesting an incomplete or intermediate step as cells transition, or whether p- EMT represents a stable state of its own [56]. Such partial states may facilitate the collective cell migration of tumor cells [53, 57, 58], leading to the formation of highly metastatic circulating tumor cell clusters [59, 60]. For this reason, and the greater cellular plasticity possibly afforded by partial EMT, p-EMT states are thought to confer carcinoma cells with a higher degree of metastatic competence as compared to complete EMT programs [61].
Figure 2. Epithelial-mesenchymal plasticity.
Epithelial cells are characterized by intercellular connections comprised of junctional proteins such as E-cadherin. Over the course of EMT, these cells lose these junctions and instead acquire the functional and morphologic phenotypes reminiscent of fibroblasts. These changes are orchestrated by a transcriptional rewiring that results in the silencing or repression of epithelial genes and a concomitant upregulation of mesenchymal genes. While this process classically represents a “complete EMT,” there is increasing evidence of partial EMT states, which are frequently defined by dual expression of epithelial and mesenchymal genes at both the transcriptional and protein levels. It is unclear whether these observed partial EMT states represent stable states or are transient intermediates along an EMT spectrum. While the mechanisms underlying partial EMT are still mostly unknown, there is evidence that relocalization of the junctional proteins plays a role during this process.
Epithelial-mesenchymal plasticity in metastasis
Because most molecular studies of epithelial-mesenchymal plasticity in the last decade were conducted using cultured carcinoma cells, the mechanisms underlying EMT in tumors, and the functional role(s) of epithelial mesenchymal plasticity in vivo, have been hard to pin down. One challenge impeding in vivo studies was the difficulty in distinguishing carcinoma cells which have undergone an EMT (and hence exhibit a mesenchymal phenotype) from fibroblasts or other mesenchymal cells that normally populate the tumor stroma. In addition, most metastatic lesions were known to exhibit epithelial features, an observation that on face value seemed at odds with a role for EMT in metastasis (discussed below). Thus, despite longstanding evidence for epithelial-mesenchymal plasticity in tumors [62, 63], the importance of EMT in cancer biology – and even its very existence – has long been questioned [64]. With more recent advances in lineage tracing techniques and intravital imaging, however, it has become widely accepted that cancer cells acquire a variety of EMT-like phenotypes during tumor progression in both model systems and cancer patients [57, 65–67].
The debate over EMT in vivo has been particularly contentious regarding metastasis. When carcinomas spread, they lose contact with neighboring epithelial cells. The acquisition of invasive behavior thus requires (by definition) that carcinoma cells remodel the tight junctions, adherens junctions, and other complexes that mediate their intimate intercellular connections. Because EMT involves a loss of epithelial characteristics and/or an acquisition of mesenchymal characteristics, it has emerged as the most straightforward mechanism to account for cancer cell invasion. Indeed, gain-of-function approaches have clearly shown that EMT is sufficient to enhance invasion and metastasis. For example, overexpression of certain EMT-TFs (e.g. SNAIL1, TWIST1, ZEB1) in epithelial carcinoma cells promotes the loss of E-cadherin, acquisition of a spindle-like mesenchymal morphology, and enhanced migratory and invasive behavior in vitro [68–70]. By contrast, loss-of-function studies have been more difficult to interpret and suggest that tissue- and context-dependent differences dictate the molecular mechanisms underlying EMT in a given tumor [71]. For example, deleting either SNAIL1 or TWIST1 in a spontaneous mouse model of PDA had minimal effect on metastasis [72], while ZEB1 ablation in a similar model drastically reduced colonization, invasion, and metastasis [73]. Taken together, these results suggest that distinct and overlapping EMT inducers – including both transcriptional and post-transcriptional mechanisms – play distinct roles in metastatic spread.
When carcinomas metastasize, they commonly exhibit an epithelial appearance, which has complicated models emphasizing the importance of EMT in metastasis. Specifically, if EMT (and its associated loss of epithelial features) is important for invasion and metastasis, then why don’t metastatic lesions exhibit a more mesenchymal histology? Again, the answer to this apparent paradox appears to be plasticity – in this case involving the reversion to an epithelial state mediated by a mesenchymal-to-epithelial transition (MET). In other words, while the more motile phenotypes associated with EMT may facilitate spread, the greater cellular cohesiveness associated with MET may facilitate growth at the distant site (i.e. colonization).
There is significant experimental support for this model, including the observation that mesenchymal carcinoma cell lines efficiently escape from primary tumors but are poor colonizers, while epithelial carcinoma lines have the opposite properties [74]. Similarly, direct comparison of differently-sized lesions in a model of metastatic pancreas cancer suggests that metastatic lesions become more epithelial as they grow [75]. Functional studies confirm the importance of MET in metastatic colonization. In a spontaneous model of squamous cell carcinoma, for example, ectopic expression of the TWIST1 EMT-TF promoted EMT and invasion but inhibited colonization; only when TWIST1 was repressed could metastatic outgrowth occur [76]. Likewise, knockdown of the PRRX1 EMT-TF in a model of breast cancer metastasis resulted in MET and efficient lung colonization after tail vein injection [77]. Taken together, these studies indicate that epithelial-mesenchymal plasticity plays a central role in multiple phases of metastasis – at the primary site, mesenchymal phenotypes foster invasive behavior, while at metastatic sites, epithelial phenotypes foster outgrowth.
Epithelial-mesenchymal plasticity as a therapy resistance mechanism
Epithelial-mesenchymal plasticity also appears to influence sensitivity to various chemotherapeutic drugs and targeted agents. In general, resistance to therapy is more commonly associated with a mesenchymal state than an epithelial state. For example, the expression of an EMT-related gene signature in tumors has been associated with resistance to neoadjuvant therapy in breast cancer and resistance to treatment with inhibitors of the epidermal growth factor receptor (EGFR) and/or PI3 kinase (PI3K) in non-small cell lung cancer (NSCLC) [78–81]. These clinical studies are in line with cell culture experiments suggesting that well-differentiated tumor cell lines (exhibiting an epithelial phenotype) are more sensitive to EGFR inhibitors than poorly differentiated tumors (exhibiting a mesenchymal phenotype) [82]. Not surprisingly, EMT-TFs have been directly implicated as mediators of EMT-associated resistance through a variety of postulated mechanisms, including regulation of drug transporters [72, 83] or the activity of EMT-associated intermediate filament protein vimentin [80].
Epithelial-mesenchymal plasticity has also been associated with resistance to immunotherapy [84]. In murine melanoma cells, for example, SNAIL is necessary and sufficient for resistance to dendritic cell and cytotoxic T cell-mediated killing via induction of regulatory T cells [85]. Likewise, melanomas that are innately resistant to anti-PD1 treatment display a transcriptional signature reminiscent of EMT-related processes, including the downregulation of E-cadherin and the concomitant upregulation of mesenchymal factors involved in extracellular matrix remodeling, angiogenesis, and wound healing [86].
While these and other studies establish a clear link between therapeutic resistance and EMT, several important caveats need to be made. First, the extent to which epithelial-mesenchymal plasticity is associated with innate versus acquired resistance remains to be determined. In clinical samples, resistance/relapse is often thought to be driven by the outgrowth of rare, intrinsically-resistant clones (Fig. 3A). Alternatively, resistant clones may emerge de novo through plasticity-associated programs as a result of therapy-induced selective pressures (Fig. 3B). Although these two scenarios can be difficult to distinguish, emerging single cell methodologies are likely to enable an interrogation of the genetic and epigenetic events underlying resistance [87]. Second, the mechanisms underlying EMT-associated therapy resistance are unclear. Is the epithelial-mesenchymal state of a cell itself responsible for increased drug tolerance? Or do much narrower mechanisms (e.g. the expression of a drug transporter) account for resistance, making any role for EMT more indirect? Finally, it is important to remember that epithelial-mesenchymal plasticity involves considerable changes in gene expression and protein composition. Thus, while much of the field has focused on EMT-associated resistance, such global cellular changes are likely to give rise to new vulnerabilities. In other words, as cells shift from an epithelial state to a mesenchymal state, they are likely to become resistant to some drugs and sensitive to others. Indeed, cultured carcinoma cells with a mesenchymal phenotype exhibit resistance to an EGFR inhibitor but sensitivity to the genotoxic drug gemcitabine relative to cells with an epithelial phenotype [82], a relationship that holds true in vivo [75]. A comprehensive analysis of drug sensitivity when cells reside in either an epithelial or a mesenchymal state will likely provide valuable information about plasticity-associated vulnerabilities, knowledge that could be used to guide therapy for well-differentiated versus poorly-differentiated tumors.
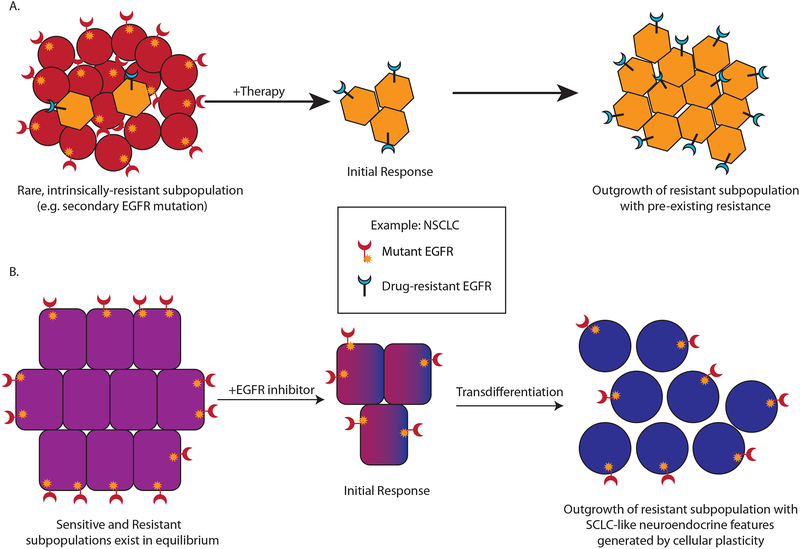
Figure 3. Cellular plasticity and therapy resistance.
Acquired resistance to therapy is associated with a variety of histologic, molecular, and/or transcriptomic changes. (A) In some cases, resistance is mediated by a pre-existing subpopulation of tumor cells that are already intrinsically (and stably) resistant at the onset of therapy. Under these circumstances, therapy induces an initial response, but survival and eventual outgrowth of the resistant subpopulation results in tumor recurrence. Such resistance can occur through genetic or epigenetic means (i.e. mutations or epigenetic silencing of the target of therapy). (B) Alternatively, cancer cells may switch back and forth between drug-sensitive and drug-resistant state as a result of cellular plasticity programs. Under these circumstances, treatment would result in killing of cells in the sensitive state. Resistant cells returning to the sensitive state would also be killed, but any cells capable of stabilizing or “locking-in” the resistant state would have a selective advantage leading to recurrence. These two paradigms are exemplified by NSCLC’s that are driven by mutations in EGFR. In tumors with a pre-existing subpopulation of resistant cells (e.g. cells with a secondary EGFR mutation), initial response to EGFR inhibition (EGFRi) is followed by relapse, driven by the resistant subpopulation. In tumors where cancer cells cycle between a sensitive state and a resistant state, therapy will result in the outgrowth of tumor cells that manage to stably adapt the resistant state. As shown in the figure, this includes NSCLC’s that acquire a SCLC-like identity with neuroendocrine features after treatment. While such resistant tumors typically harbor the same EGFR mutation as the original (pre-treatment) tumor, they no longer depend on it for survival. This paradigm also likely applies to tumors that utilize epithelial-mesenchymal plasticity as a treatment escape mechanism.
OTHER PLASTICITY MECHANISMS CONFERRING THERAPY RESISTANCE
While many of the relationships between EMT and chemosensitivity remain to be worked out, it is clear that epithelial-mesenchymal plasticity influences therapy response. Resistance may also arise from other plasticity mechanisms, with an important example coming from studies of NSCLC. Most lung cancer patients with EGFR-activating mutations exhibit good initial responses to EGFR inhibitors, but these are typically followed by relapses mediated by mutations in downstream targets, secondary mutations in EGFR itself, or epithelial-mesenchymal plasticity (as discussed above). In some cases, however, tumors undergo a dramatic change in lineage identity resulting in the conversion from an epithelial phenotype to a neuroendocrine-like phenotype reminiscent of small-cell lung cancer (SCLC) [79, 88]. Such tumors typically harbor the same EGFR mutation as the original (pre-treatment) tumor, indicating an evolutionary process rather than the existence of a secondary tumor or a traditional (mutation-driven) means of resistance (Fig. 3).
The mechanisms driving neuroendocrine plasticity and therapy resistance are not known. It is likely that EGFR inhibition suppresses lineage-directing or lineage-maintaining pathways, creating a degree of plasticity that allows for trans-differentiation into a cellular state that no longer depends on EGFR signaling for survival. This notion is consistent with the observation that SCLCs do not normally express EGFR or rely on its activity for growth and survival [89]. Instead, SCLCs utilize mutations in the Retinoblastoma (RB) tumor suppressor gene for survival [90, 91], as loss of RB occurs in virtually 100% of SCLC primary tumor samples and cell lines derived from TKI-resistant EGFR-patients that have adopted a SCLC phenotype [92]. Although these results suggest that RB loss is necessary for lineage plasticity and TKI-resistance, depletion of RB is not sufficient on its own to induce these phenotypes. This implies that either: (i) RB loss simply renders the cells permissive to other lineage programs, and a genomic or epigenomic “second hit” is required for cell state switching; or (ii) in this setting, RB loss is merely a marker, and not a regulator of SCLC identity.
Similarly, prostate cancers resistant to androgen receptor (AR) inhibition also adopt neuroendocrine features [93]. As in lung cancer, lineage plasticity in prostate carcinoma is mediated by loss of RB (and TRP53) function through repressive transcriptional and/or epigenetic activity of SOX2 or EZH2 [93–95]. Thus, in addition to their growth-inhibitory properties, tumor suppressor genes function to maintain lineage fidelity in some cancers. Accordingly, losing such genes – particularly RB – initiates a cellular plasticity program whose end-state (a neuroendocrine phenotype) enables tumor cells to escape the toxic effects of chemotherapy.
Finally, another feature of plasticity with therapeutic implications concerns the acquisition of a “cancer stem cell” (CSC)-like state. CSC’s, also referred to as tumor-initiating cells (TICs), are hypothesized to comprise a stable tumor subpopulation with enhanced self-renewal properties [96]. Although there has been disagreement over the generalizability of the CSC model, researchers have aggressively pursued drugs with the potential to specifically target CSCs with the hope that such agents might target the cells most crucial for tumor growth. Regrettably, emerging data suggest that the CSC state is not as stable as once thought, as several recent studies have demonstrated that CSCs can emerge from non-CSCs (reviewed in [97, 98]), thus dampening the prospects for CSC-directed therapy. In some cases, this plasticity may involve the engagement of EMT programs, which are associated with the acquisition of CSC-like states [99]. Regardless of the precise mechanism (EMT-dependent or -independent), cellular plasticity seems to provide a renewable source of tumor cells with tumor-initiation and self-renewal properties, imposing a further challenge to therapeutic approaches that seek to target specific tumor subpopulations [100].
NON-CELL AUTONOMOUS CONSEQUENCES OF CELLULAR PLASTICITY
Cancer cells exist within a complex tumor microenvironment (TME) comprised of fibroblasts, endothelial cells, leukocytes, and extracellular matrix. While these TME components are known to exert a powerful influence on the phenotype and function of cancer cells, reciprocal signaling from the cancer cells can also have potent effects on the TME. Consequently, a change in cancer cell phenotypes (as a result of cellular plasticity) can have a marked influence on surrounding non-cancer cells (Fig. 4).
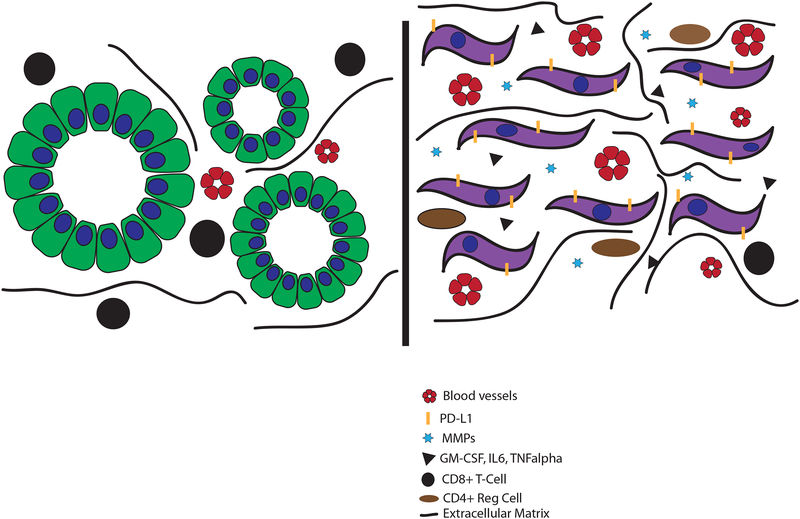
Figure 4. Epithelial-mesenchymal plasticity results in changes to the tumor microenvironment (TME).
As cells undergo EMT, their secretory patterns change, resulting in differences in components of the extracellular matrix (ECM) and ECM-modifying factors such as matrix metalloproteinases (MMPs). Cells that have undergone EMT also secrete higher levels of pro-angiogenic and pro-inflammatory cytokines like GM-CSF, IL6, and TNFα. These factors recruit immunosuppressive leukocyte populations into the tumor, which results in the exclusion of CD8+ T cells. Collectively, these TME-remodeling factors facilitate tumor cell invasion, metastasis, and immune evasion.
These non-cell autonomous consequences of cellular plasticity have been best-studied in the context of EMT, where the mesenchymal state is associated with an altered “secretome” compared to the epithelial state. For example, EMT in MDCK cells results in enhanced secretion of extracellular proteases including matrix metalloproteinases (MMPs) and kallikreins, as well as extracellular matrix (ECM) constituents including collagens, fibulins, and SPARC [101]. Such secreted factors, in turn, have effects on stromal cells in the TME, affecting the migration of fibroblasts and the branching of blood vessels. Likewise, the secretomes of head and neck squamous cell carcinoma (HNSCC) cells are markedly different when cells with high E-cadherin expression (epithelial) are compared to those with low E-cadherin expression (mesenchymal); many of these secreted factors are enriched in fibroblast-conditioned medium [102]. Cancer cells that have undergone EMT may also package secreted factors into exosomes – extracellular vesicles containing a diverse assortment of protein and nucleic acid cargo – enabling long-distance modification of the TME or other tumor cells.
A growing body of literature suggests that the cancer cell secretome following EMT is associated with significant changes in the immune microenvironment. Specifically, poorly-differentiated carcinomas whose cancer cells have a predominantly mesenchymal histology tend to be associated with increased vascularity and a pro-inflammatory/immunosuppressive immune infiltrate [103, 104]. This, in turn, may enable tumor cells to evade immune attack, rendering them resistant to the effects of immune checkpoint blockade, as discussed above [84]. Many of these secreted factors released by cancer cells upon EMT are themselves targets of the EMT-TFs SNAIL, SLUG, TWIST1/2, and ZEB1/2. In ovarian cancer cells, for example, TWIST1 drives the expression of discoidin domain receptor 2 (DDR2), which promotes mesothelial cell clearance and paves the way for increased tumor cell invasion [105]. In breast cancer cells, SNAIL-induced EMT promotes a pro-tumor inflammatory microenvironment by upregulating the production of cytokines including GM-CSF, IL1α, IL-6, and TNFα [106]. Likewise, ZEB1-mediated EMT in NSCLC cells results in increased tumor programmed cell death 1 ligand 1 (PD-L1) expression, thus reducing the total number and activity of tumor-infiltrating CD8+ lymphocytes [107]. Thus, while epithelial-mesenchymal plasticity is most often associated with pro-invasive, pro-migratory phenotypes, the associated effects on the TME add another dimension to EMT-mediated disease progression. The precise mechanisms by which EMT mediates such processes – most importantly innate or acquired resistance to immunotherapy [86] – represents an area ripe for future investigation.
THERAPY: HARNESSING CELLULAR PLASTICITY FOR TREATMENT
A promising avenue for anti-cancer therapy involves targeting cell plasticity itself – harnessing the molecular programs that prompt cells to adopt a new identity, one that is not associated with malignant properties. The most dramatic example of such “differentiation therapy” is acute promyelocytic leukemia (APL), in which all tumor cells carry a translocation involving the retinoic acid receptor α (RARα), resulting in an RARα fusion gene. Administration of all-trans retinoic acid (ATRA) – which binds to and activates the product of this fusion gene – prompts terminal granulocytic differentiation of the leukemia cells and is associated with high response rates and cures [108].
While this approach has had less success in solid tumors, there have been some promising developments. One area of focus is tumors with mutations in isocitrate dehydrogenase 1 and 2 (IDH1 and IDH2), which are found in subsets of patients with glioblastoma, cholangiocarcinoma, and AML. IDH mutations lead to the formation of an oncometabolite (2-hydroxyglutarate; 2HG), which in turn results in DNA hypermethylation through the inhibition of α-ketoglutarate (αKG)-dependent dioxygenases. Encouragingly, treatment of IDH1 mutant glioma cells with either an inhibitor of IDH1 or an inhibitor of DNA methyltransferase (DNMT) reversed DNA hypermethylation and induced the re-expression of genes associated with gliogenic differentiation [109, 110].
Although tumors with RARα fusion genes or IDH1/2 mutations are rare, emerging studies suggest that differentiation therapy could have much broader applications, including tumors that lack an obvious genetic target. For example, in non-APL tumors (which lack the RARα fusion gene), inhibition of the histone demethylase LSD1 re-activates the ATRA differentiation pathway through a process of epigenetic reprogramming, making the cells vulnerable to ATRA treatment [111]. Likewise, alternative methods to promote melanocyte differentiation in melanoma [112], or to leverage cellular plasticity programs to expose new vulnerabilities in pancreatic cancer [113, 114] have shown promise in preclinical models. An intriguing recent development involves the use of cellular plasticity to engineer new cell states that are intrinsically anti-proliferative/anti-metastatic [115]. Thus, while translating these recent advances to the clinic would involve complex therapeutic regimens, there are already examples of promising treatment paradigms based on strategically maintaining cells in a particular state [116, 117]. Collectively, these studies provide a framework for considering that pathways that maintain cancer cells in an undifferentiated state as future targets for cancer therapy.
CONCLUDING REMARKS
The phenotypic adaptability embodied by cellular plasticity underlies normal development and tissue regeneration, but in tumors, cancer cells exploit this malleability to achieve a selective advantage. Cancers may use cellular plasticity programs to adjust to an unfavorable metabolic environment, evade immune attack, spread from a primary to metastatic site, and escape the toxic effects of anti-cancer drugs. Thus, plasticity programs embody many of the barriers hindering advances in cancer treatment.
Plasticity occurs in diverse contexts and rarely involves stable genetic alterations, which has made it difficult to understand the process in molecular terms. Consequently, mechanistic studies of cellular plasticity have focused on in vitro approaches, making their applicability to tumor cell plasticity in vivo uncertain. In addition, plasticity typically involves a continuum of cellular phenotypes, which has led to confusion over definitions of cellular state. Nevertheless, the last few years have seen a greater emphasis on in vivo studies of cellular plasticity, fueled in part by results from the clinic – including the observation that tumor initiation is often associated with plasticity (metaplasia) and that therapeutic resistance can emerge through plasticity. It is also now clear that more than one type of cell in a normal tissue is competent to give rise to carcinoma, suggesting that the programs giving rise to cellular plasticity are themselves plastic, allowing for multiple types of cellular conversions in the setting of injury or genetic perturbation.
These insights provide a rationale for further probing the mechanisms underlying cellular plasticity, which may include genetic, epigenetic, transcriptional, and post-transcriptional programs (Box 2). The timing is good, as emerging technologies in epigenetics and cell biology can yield molecular insights that would have been unimaginable a few years ago. Once we have a better understanding of how cancer cells accomplish such global changes in cellular phenotype, we will be able to consider ways to target plasticity in a rational manner, opening new therapeutic approaches to what are likely to be the most recalcitrant features of human tumors.
Box 2: Key Concepts and Outstanding Questions.
Cellular plasticity has a protective effect in normal tissues exposed to chronic injury, but chronic injury resulting in metaplasia can predispose to cancer. In what cellular/molecular settings does metaplasia also facilitate tumor initiation? In the context of cancers associated with metaplasia, what is the cell of origin and how does a change in cellular identity facilitate malignant transformation?
Epithelial-mesenchymal plasticity is bi-directional manner, leading cells to either adopt a more mesenchymal state (via EMT) or a more epithelial state (via MET). What determines the “equilibrium constants” governing transitions between these states, thereby giving rise to poorly-differentiated or well-differentiated tumors?
Epithelial-mesenchymal plasticity involves a diverse set of molecular programs. How should we define “partial EMT” versus “complete EMT”? Is co-expression or epithelial and mesenchymal qualities a key feature, or does the definition also include cells that have lost epithelial features but not yet gained mesenchymal features? Can these classifications be based solely on the expression of markers, or should a functional definition be applied? Are partial EMT states intermediates along a (linear) path of epithelial-mesenchymal plasticity, or do they represent distinct end-states of their own? How many partial EMT states are there, by what mechanism does each arise, and are they associated with distinct functional contributions?
Epithelial-mesenchymal plasticity is associated with a shift in the sensitivity of carcinoma cells to various therapies (including immunotherapy). By what molecular mechanisms does epithelial-mesenchymal plasticity shift the sensitivity profile? What underlies the variation in EMT-TF activity in different contexts? Do different mechanisms of plasticity confer different sensitivity/resistance profiles?
Differentiation therapy is highly effective for certain leukemias. Do similar opportunities exist for carcinomas, whereby targeting tumor drivers can promote differentiation and slow malignant growth? Alternatively, can cellular plasticity be exploited to drive cells to state that renders them more sensitive to certain agents? What are the therapeutic combinations that would lead to such synthetic lethality?
SIGNIFICANCE.
Changes in cell identity, or cellular plasticity, are common at different stages of tumor progression, and it has become clear that cellular plasticity can be a potent mediator of tumor progression and chemoresistance. Understanding the mechanisms underlying the various forms of cell plasticity may deliver new strategies for targeting the most lethal aspects of cancer: metastasis and resistance to therapy.
ACKNOWLEDGEMENTS
We apologize to the numerous colleagues whose contributions were not included in this review due to space considerations. This work was supported by the Abramson Family Cancer Research Institute and grants from the National Cancer Institute (CA224970 to S.Y. and CA229803 to B.Z.S.) and the V Foundation (T2017–009).
Footnotes
Disclosure of Potential Conflicts of Interests
The authors declare no potential conflicts of interest.
REFERENCES:
- 1.Merrell AJ, and Stanger BZ (2016). Adult cell plasticity in vivo: de-differentiation and transdifferentiation are back in style. Nature reviews 17, 413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tata PR, and Rajagopal J (2016). Cellular plasticity: 1712 to the present day. Current opinion in cell biology 43, 46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spechler SJ, and Souza RF (2014). Barrett’s esophagus. The New England journal of medicine 371, 836–845. [DOI] [PubMed] [Google Scholar]
- 4.Bhat S, Coleman HG, Yousef F, Johnston BT, McManus DT, Gavin AT, and Murray LJ (2011). Risk of malignant progression in Barrett’s esophagus patients: results from a large population-based study. Journal of the National Cancer Institute 103, 1049–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hvid-Jensen F, Pedersen L, Drewes AM, Sorensen HT, and Funch-Jensen P (2011). Incidence of adenocarcinoma among patients with Barrett’s esophagus. The New England journal of medicine 365, 1375–1383. [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Ouyang H, Yamamoto Y, Kumar PA, Wei TS, Dagher R, Vincent M, Lu X, Bellizzi AM, Ho KY, et al. (2011). Residual embryonic cells as precursors of a Barrett’s-like metaplasia. Cell 145, 1023–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quante M, Bhagat G, Abrams JA, Marache F, Good P, Lee MD, Lee Y, Friedman R, Asfaha S, Dubeykovskaya Z, et al. (2012). Bile acid and inflammation activate gastric cardia stem cells in a mouse model of Barrett-like metaplasia. Cancer cell 21, 36–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang M, Li H, Zhang Y, Yang Y, Lu R, Liu K, Lin S, Lan X, Wang H, Wu H, et al. (2017). Transitional basal cells at the squamous-columnar junction generate Barrett’s oesophagus. Nature 550, 529–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rhee H, and Wang DH (2018). Cellular origins of Barrett’s esophagus: the search continues. Curr Gastroenterol Rep 20, 51. [DOI] [PubMed] [Google Scholar]
- 10.Amieva M, and Peek RM Jr. (2016). Pathobiology of Helicobacter pylori-Induced Gastric Cancer. Gastroenterology 150, 64–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giroux V, and Rustgi AK (2017). Metaplasia: tissue injury adaptation and a precursor to the dysplasia-cancer sequence. Nature reviews. Cancer 17, 594–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Correa P, Piazuelo MB, and Wilson KT (2010). Pathology of gastric intestinal metaplasia: clinical implications. The American journal of gastroenterology 105, 493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mills JC, and Goldenring JR (2017). Metaplasia in the Stomach Arises From Gastric Chief Cells. Cellular and molecular gastroenterology and hepatology 4, 85–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayakawa Y, Fox JG, and Wang TC (2017). Isthmus Stem Cells Are the Origins of Metaplasia in the Gastric Corpus. Cellular and molecular gastroenterology and hepatology 4, 89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yanger K, Zong Y, Maggs LR, Shapira SN, Maddipati R, Aiello NM, Thung SN, Wells RG, Greenbaum LE, and Stanger BZ (2013). Robust cellular reprogramming occurs spontaneously during liver regeneration. Genes & development 27, 719–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tarlow BD, Pelz C, Naugler WE, Wakefield L, Wilson EM, Finegold MJ, and Grompe M (2014). Bipotential adult liver progenitors are derived from chronically injured mature hepatocytes. Cell stem cell 15, 605–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yimlamai D, Christodoulou C, Galli GG, Yanger K, Pepe-Mooney B, Gurung B, Shrestha K, Cahan P, Stanger BZ, and Camargo FD (2014). Hippo pathway activity influences liver cell fate. Cell 157, 1324–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schaub JR, Huppert KA, Kurial SNT, Hsu BY, Cast AE, Donnelly B, Karns RA, Chen F, Rezvani M, Luu HY, et al. (2018). De novo formation of the biliary system by TGFbeta-mediated hepatocyte transdifferentiation. Nature 557, 247–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan B, Malato Y, Calvisi DF, Naqvi S, Razumilava N, Ribback S, Gores GJ, Dombrowski F, Evert M, Chen X, et al. (2012). Cholangiocarcinomas can originate from hepatocytes in mice. The Journal of clinical investigation 122, 2911–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sekiya S, and Suzuki A (2012). Intrahepatic cholangiocarcinoma can arise from Notch-mediated conversion of hepatocytes. The Journal of clinical investigation 122, 3914–3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ikenoue T, Terakado Y, Nakagawa H, Hikiba Y, Fujii T, Matsubara D, Noguchi R, Zhu C, Yamamoto K, Kudo Y, et al. (2016). A novel mouse model of intrahepatic cholangiocarcinoma induced by liver-specific Kras activation and Pten deletion. Scientific reports 6, 23899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guest RV, Boulter L, Kendall TJ, Minnis-Lyons SE, Walker R, Wigmore SJ, Sansom OJ, and Forbes SJ (2014). Cell lineage tracing reveals a biliary origin of intrahepatic cholangiocarcinoma. Cancer research 74, 1005–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hill MA, Alexander WB, Guo B, Kato Y, Patra K, O’Dell MR, McCall MN, Whitney-Miller CL, Bardeesy N, and Hezel AF (2018). Kras and Tp53 Mutations Cause Cholangiocyte- and Hepatocyte-Derived Cholangiocarcinoma. Cancer research 78, 4445–4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reichert M, and Rustgi AK (2011). Pancreatic ductal cells in development, regeneration, and neoplasia. The Journal of clinical investigation 121, 4572–4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grippo PJ, Nowlin PS, Demeure MJ, Longnecker DS, and Sandgren EP (2003). Preinvasive pancreatic neoplasia of ductal phenotype induced by acinar cell targeting of mutant Kras in transgenic mice. Cancer research 63, 2016–2019. [PubMed] [Google Scholar]
- 26.Strobel O, Dor Y, Alsina J, Stirman A, Lauwers G, Trainor A, Castillo CF, Warshaw AL, and Thayer SP (2007). In vivo lineage tracing defines the role of acinar-to-ductal transdifferentiation in inflammatory ductal metaplasia. Gastroenterology 133, 1999–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De La OJ, Emerson LL, Goodman JL, Froebe SC, Illum BE, Curtis AB, and Murtaugh LC (2008). Notch and Kras reprogram pancreatic acinar cells to ductal intraepithelial neoplasia. Proceedings of the National Academy of Sciences of the United States of America 105, 18907–18912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kopp JL, von Figura G, Mayes E, Liu FF, Dubois CL, Morris J.P.t., Pan FC, Akiyama H, Wright CV, Jensen K, et al. (2012). Identification of Sox9-dependent acinar-to-ductal reprogramming as the principal mechanism for initiation of pancreatic ductal adenocarcinoma. Cancer cell 22, 737–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamaguchi J, Yokoyama Y, Kokuryo T, Ebata T, and Nagino M (2018). Cells of origin of pancreatic neoplasms. Surgery today 48, 9–17. [DOI] [PubMed] [Google Scholar]
- 30.Rijkers AP, Bakker OJ, Ahmed Ali U, Hagenaars J, van Santvoort HC, Besselink MG, Bollen TL, and van Eijck CH (2017). Risk of Pancreatic Cancer After a Primary Episode of Acute Pancreatitis. Pancreas 46, 1018–1022. [DOI] [PubMed] [Google Scholar]
- 31.Dotto GP, and Rustgi AK (2016). Squamous Cell Cancers: A Unified Perspective on Biology and Genetics. Cancer cell 29, 622–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zsemlye M (2008). High-grade cervical dysplasia: pathophysiology, diagnosis, and treatment. Obstetrics and gynecology clinics of North America 35, 615–621; ix. [DOI] [PubMed] [Google Scholar]
- 33.Talia KL, and McCluggage WG (2018). The developing spectrum of gastric-type cervical glandular lesions. Pathology 50, 122–133. [DOI] [PubMed] [Google Scholar]
- 34.Bailey JM, Hendley AM, Lafaro KJ, Pruski MA, Jones NC, Alsina J, Younes M, Maitra A, McAllister F, Iacobuzio-Donahue CA, et al. (2016). p53 mutations cooperate with oncogenic Kras to promote adenocarcinoma from pancreatic ductal cells. Oncogene 35, 4282–4288. [DOI] [PubMed] [Google Scholar]
- 35.Kopp JL, Dubois CL, Schaeffer DF, Samani A, Taghizadeh F, Cowan RW, Rhim AD, Stiles BL, Valasek M, and Sander M (2018). Loss of Pten and Activation of Kras Synergistically Induce Formation of Intraductal Papillary Mucinous Neoplasia From Pancreatic Ductal Cells in Mice. Gastroenterology 154, 1509–1523 e1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang H, Fillmore Brainson C, Koyama S, Redig AJ, Chen T, Li S, Gupta M, Garcia-de-Alba C, Paschini M, Herter-Sprie GS, et al. (2017). Lkb1 inactivation drives lung cancer lineage switching governed by Polycomb Repressive Complex 2. Nature communications 8, 14922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nieto MA, Huang RY, Jackson RA, and Thiery JP (2016). Emt: 2016. Cell 166, 21–45. [DOI] [PubMed] [Google Scholar]
- 38.Latil M, Nassar D, Beck B, Boumahdi S, Wang L, Brisebarre A, Dubois C, Nkusi E, Lenglez S, Checinska A, et al. (2017). Cell-Type-Specific Chromatin States Differentially Prime Squamous Cell Carcinoma Tumor-Initiating Cells for Epithelial to Mesenchymal Transition. Cell stem cell 20, 191–204 e195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hendrix MJ, Seftor EA, Hess AR, and Seftor RE (2003). Vasculogenic mimicry and tumour-cell plasticity: lessons from melanoma. Nature reviews. Cancer 3, 411–421. [DOI] [PubMed] [Google Scholar]
- 40.Sun B, Zhang D, Zhao N, and Zhao X (2017). Epithelial-to-endothelial transition and cancer stem cells: two cornerstones of vasculogenic mimicry in malignant tumors. Oncotarget 8, 30502–30510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dejana E, Hirschi KK, and Simons M (2017). The molecular basis of endothelial cell plasticity. Nature communications 8, 14361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kovacic JC, Mercader N, Torres M, Boehm M, and Fuster V (2012). Epithelial-to-mesenchymal and endothelial-to-mesenchymal transition: from cardiovascular development to disease. Circulation 125, 1795–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Y, Lui KO, and Zhou B (2018). Reassessing endothelial-to-mesenchymal transition in cardiovascular diseases. Nature reviews. Cardiology 15, 445–456. [DOI] [PubMed] [Google Scholar]
- 44.Zeisberg EM, Potenta S, Xie L, Zeisberg M, and Kalluri R (2007). Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer research 67, 10123–10128. [DOI] [PubMed] [Google Scholar]
- 45.Choi SH, Nam JK, Kim BY, Jang J, Jin YB, Lee HJ, Park S, Ji YH, Cho J, and Lee YJ (2016). HSPB1 Inhibits the Endothelial-to-Mesenchymal Transition to Suppress Pulmonary Fibrosis and Lung Tumorigenesis. Cancer research 76, 1019–1030. [DOI] [PubMed] [Google Scholar]
- 46.Choi SH, Kim AR, Nam JK, Kim JM, Kim JY, Seo HR, Lee HJ, Cho J, and Lee YJ (2018). Tumour-vasculature development via endothelial-to-mesenchymal transition after radiotherapy controls CD44v6(+) cancer cell and macrophage polarization. Nature communications 9, 5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lamouille S, Xu J, and Derynck R (2014). Molecular mechanisms of epithelial-mesenchymal transition. Nature reviews 15, 178–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zohn IE, Li Y, Skolnik EY, Anderson KV, Han J, and Niswander L (2006). p38 and a p38-interacting protein are critical for downregulation of E-cadherin during mouse gastrulation. Cell 125, 957–969. [DOI] [PubMed] [Google Scholar]
- 49.Hirano M, Hashimoto S, Yonemura S, Sabe H, and Aizawa S (2008). EPB41L5 functions to post-transcriptionally regulate cadherin and integrin during epithelial-mesenchymal transition. The Journal of cell biology 182, 1217–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee JD, Silva-Gagliardi NF, Tepass U, McGlade CJ, and Anderson KV (2007). The FERM protein Epb4.1l5 is required for organization of the neural plate and for the epithelial-mesenchymal transition at the primitive streak of the mouse embryo. Development 134, 2007–2016. [DOI] [PubMed] [Google Scholar]
- 51.Song S, Eckerle S, Onichtchouk D, Marrs JA, Nitschke R, and Driever W (2013). Pou5f1-dependent EGF expression controls E-cadherin endocytosis, cell adhesion, and zebrafish epiboly movements. Developmental cell 24, 486–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Campbell K, Whissell G, Franch-Marro X, Batlle E, and Casanova J (2011). Specific GATA factors act as conserved inducers of an endodermal-EMT. Developmental cell 21, 1051–1061. [DOI] [PubMed] [Google Scholar]
- 53.Aiello NM, Maddipati R, Norgard RJ, Balli D, Li J, Yuan S, Yamazoe T, Black T, Sahmoud A, Furth EE, et al. (2018). EMT Subtype Influences Epithelial Plasticity and Mode of Cell Migration. Developmental cell 45, 681–695 e684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu J, Lamouille S, and Derynck R (2009). TGF-beta-induced epithelial to mesenchymal transition. Cell research 19, 156–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Klymkowsky MW, and Savagner P (2009). Epithelial-mesenchymal transition: a cancer researcher’s conceptual friend and foe. The American journal of pathology 174, 1588–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jolly MK, Tripathi SC, Jia D, Mooney SM, Celiktas M, Hanash SM, Mani SA, Pienta KJ, Ben-Jacob E, and Levine H (2016). Stability of the hybrid epithelial/mesenchymal phenotype. Oncotarget 7, 27067–27084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lecharpentier A, Vielh P, Perez-Moreno P, Planchard D, Soria JC, and Farace F (2011). Detection of circulating tumour cells with a hybrid (epithelial/mesenchymal) phenotype in patients with metastatic non-small cell lung cancer. British journal of cancer 105, 1338–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Armstrong AJ, Marengo MS, Oltean S, Kemeny G, Bitting RL, Turnbull JD, Herold CI, Marcom PK, George DJ, and Garcia-Blanco MA (2011). Circulating tumor cells from patients with advanced prostate and breast cancer display both epithelial and mesenchymal markers. Molecular cancer research: MCR 9, 997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aceto N, Bardia A, Miyamoto DT, Donaldson MC, Wittner BS, Spencer JA, Yu M, Pely A, Engstrom A, Zhu H, et al. (2014). Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 158, 1110–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maddipati R, and Stanger BZ (2015). Pancreatic Cancer Metastases Harbor Evidence of Polyclonality. Cancer discovery 5, 1086–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bierie B, Pierce SE, Kroeger C, Stover DG, Pattabiraman DR, Thiru P, Liu Donaher J, Reinhardt F, Chaffer CL, Keckesova Z, et al. (2017). Integrin-beta4 identifies cancer stem cell-enriched populations of partially mesenchymal carcinoma cells. Proceedings of the National Academy of Sciences of the United States of America 114, E2337–E2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brabletz T, Jung A, Reu S, Porzner M, Hlubek F, Kunz-Schughart LA, Knuechel R, and Kirchner T (2001). Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proceedings of the National Academy of Sciences of the United States of America 98, 10356–10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Savagner P (2001). Leaving the neighborhood: molecular mechanisms involved during epithelial-mesenchymal transition. BioEssays: news and reviews in molecular, cellular and developmental biology 23, 912–923. [DOI] [PubMed] [Google Scholar]
- 64.Tarin D, Thompson EW, and Newgreen DF (2005). The fallacy of epithelial mesenchymal transition in neoplasia. Cancer research 65, 5996–6000; discussion 6000–5991. [DOI] [PubMed] [Google Scholar]
- 65.Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, Reichert M, Beatty GL, Rustgi AK, Vonderheide RH, et al. (2012). EMT and dissemination precede pancreatic tumor formation. Cell 148, 349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, Isakoff SJ, Ciciliano JC, Wells MN, Shah AM, et al. (2013). Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 339, 580–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dongre A, and Weinberg RA (2019). New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nature reviews 20, 69–84. [DOI] [PubMed] [Google Scholar]
- 68.Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, and Nieto MA (2000). The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nature cell biology 2, 76–83. [DOI] [PubMed] [Google Scholar]
- 69.Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, and Garcia De Herreros A (2000). The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nature cell biology 2, 84–89. [DOI] [PubMed] [Google Scholar]
- 70.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, and Weinberg RA (2004). Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 117, 927–939. [DOI] [PubMed] [Google Scholar]
- 71.Ye X, Tam WL, Shibue T, Kaygusuz Y, Reinhardt F, Ng Eaton E, and Weinberg RA (2015). Distinct EMT programs control normal mammary stem cells and tumour-initiating cells. Nature 525, 256–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zheng X, Carstens JL, Kim J, Scheible M, Kaye J, Sugimoto H, Wu CC, LeBleu VS, and Kalluri R (2015). Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 527, 525–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Krebs AM, Mitschke J, Lasierra Losada M, Schmalhofer O, Boerries M, Busch H, Boettcher M, Mougiakakos D, Reichardt W, Bronsert P, et al. (2017). The EMT-activator Zeb1 is a key factor for cell plasticity and promotes metastasis in pancreatic cancer. Nature cell biology 19, 518–529. [DOI] [PubMed] [Google Scholar]
- 74.Chaffer CL, Brennan JP, Slavin JL, Blick T, Thompson EW, and Williams ED (2006). Mesenchymal-to-epithelial transition facilitates bladder cancer metastasis: role of fibroblast growth factor receptor-2. Cancer research 66, 11271–11278. [DOI] [PubMed] [Google Scholar]
- 75.Aiello NM, Bajor DL, Norgard RJ, Sahmoud A, Bhagwat N, Pham MN, Cornish TC, Iacobuzio-Donahue CA, Vonderheide RH, and Stanger BZ (2016). Metastatic progression is associated with dynamic changes in the local microenvironment. Nature communications 7, 12819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tsai JH, Donaher JL, Murphy DA, Chau S, and Yang J (2012). Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer cell 22, 725–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ocana OH, Corcoles R, Fabra A, Moreno-Bueno G, Acloque H, Vega S, Barrallo-Gimeno A, Cano A, and Nieto MA (2012). Metastatic colonization requires the repression of the epithelial-mesenchymal transition inducer Prrx1. Cancer cell 22, 709–724. [DOI] [PubMed] [Google Scholar]
- 78.Farmer P, Bonnefoi H, Anderle P, Cameron D, Wirapati P, Becette V, Andre S, Piccart M, Campone M, Brain E, et al. (2009). A stroma-related gene signature predicts resistance to neoadjuvant chemotherapy in breast cancer. Nature medicine 15, 68–74. [DOI] [PubMed] [Google Scholar]
- 79.Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, Bergethon K, Shaw AT, Gettinger S, Cosper AK, et al. (2011). Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Science translational medicine 3, 75ra26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang Z, Lee JC, Lin L, Olivas V, Au V, LaFramboise T, Abdel-Rahman M, Wang X, Levine AD, Rho JK, et al. (2012). Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nature genetics 44, 852–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Byers LA, Diao L, Wang J, Saintigny P, Girard L, Peyton M, Shen L, Fan Y, Giri U, Tumula PK, et al. (2013). An epithelial-mesenchymal transition gene signature predicts resistance to EGFR and PI3K inhibitors and identifies Axl as a therapeutic target for overcoming EGFR inhibitor resistance. Clinical cancer research: an official journal of the American Association for Cancer Research 19, 279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Collisson EA, Sadanandam A, Olson P, Gibb WJ, Truitt M, Gu S, Cooc J, Weinkle J, Kim GE, Jakkula L, et al. (2011). Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nature medicine 17, 500–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Saxena M, Stephens MA, Pathak H, and Rangarajan A (2011). Transcription factors that mediate epithelial-mesenchymal transition lead to multidrug resistance by upregulating ABC transporters. Cell death & disease 2, e179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Terry S, Savagner P, Ortiz-Cuaran S, Mahjoubi L, Saintigny P, Thiery JP, and Chouaib S (2017). New insights into the role of EMT in tumor immune escape. Molecular oncology 11, 824–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kudo-Saito C, Shirako H, Takeuchi T, and Kawakami Y (2009). Cancer metastasis is accelerated through immunosuppression during Snail-induced EMT of cancer cells. Cancer cell 15, 195–206. [DOI] [PubMed] [Google Scholar]
- 86.Hugo W, Zaretsky JM, Sun L, Song C, Moreno BH, Hu-Lieskovan S, Berent-Maoz B, Pang J, Chmielowski B, Cherry G, et al. (2016). Genomic and Transcriptomic Features of Response to Anti-PD-1 Therapy in Metastatic Melanoma. Cell 165, 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shaffer SM, Dunagin MC, Torborg SR, Torre EA, Emert B, Krepler C, Beqiri M, Sproesser K, Brafford PA, Xiao M, et al. (2017). Rare cell variability and drug-induced reprogramming as a mode of cancer drug resistance. Nature 546, 431–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yu HA, Arcila ME, Rekhtman N, Sima CS, Zakowski MF, Pao W, Kris MG, Miller VA, Ladanyi M, and Riely GJ (2013). Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clinical cancer research: an official journal of the American Association for Cancer Research 19, 2240–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Byers LA, Wang J, Nilsson MB, Fujimoto J, Saintigny P, Yordy J, Giri U, Peyton M, Fan YH, Diao L, et al. (2012). Proteomic profiling identifies dysregulated pathways in small cell lung cancer and novel therapeutic targets including PARP1. Cancer discovery 2, 798–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Peifer M, Fernandez-Cuesta L, Sos ML, George J, Seidel D, Kasper LH, Plenker D, Leenders F, Sun R, Zander T, et al. (2012). Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nature genetics 44, 1104–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rudin CM, Durinck S, Stawiski EW, Poirier JT, Modrusan Z, Shames DS, Bergbower EA, Guan Y, Shin J, Guillory J, et al. (2012). Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer. Nature genetics 44, 1111–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Niederst MJ, Sequist LV, Poirier JT, Mermel CH, Lockerman EL, Garcia AR, Katayama R, Costa C, Ross KN, Moran T, et al. (2015). RB loss in resistant EGFR mutant lung adenocarcinomas that transform to small-cell lung cancer. Nature communications 6, 6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sharma A, Yeow WS, Ertel A, Coleman I, Clegg N, Thangavel C, Morrissey C, Zhang X, Comstock CE, Witkiewicz AK, et al. (2010). The retinoblastoma tumor suppressor controls androgen signaling and human prostate cancer progression. The Journal of clinical investigation 120, 4478–4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mu P, Zhang Z, Benelli M, Karthaus WR, Hoover E, Chen CC, Wongvipat J, Ku SY, Gao D, Cao Z, et al. (2017). SOX2 promotes lineage plasticity and antiandrogen resistance in TP53- and RB1-deficient prostate cancer. Science 355, 84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ku SY, Rosario S, Wang Y, Mu P, Seshadri M, Goodrich ZW, Goodrich MM, Labbe DP, Gomez EC, Wang J, et al. (2017). Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. Science 355, 78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Meacham CE, and Morrison SJ (2013). Tumour heterogeneity and cancer cell plasticity. Nature 501, 328–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nassar D, and Blanpain C (2016). Cancer Stem Cells: Basic Concepts and Therapeutic Implications. Annual review of pathology 11, 47–76. [DOI] [PubMed] [Google Scholar]
- 98.Batlle E, and Clevers H (2017). Cancer stem cells revisited. Nature medicine 23, 1124–1134. [DOI] [PubMed] [Google Scholar]
- 99.Shibue T, and Weinberg RA (2017). EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nature reviews. Clinical oncology 14, 611–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.de Sousa EMF, and de Sauvage FJ (2019). Cellular Plasticity in Intestinal Homeostasis and Disease. Cell stem cell 24, 54–64. [DOI] [PubMed] [Google Scholar]
- 101.Mathias RA, Chen YS, Wang B, Ji H, Kapp EA, Moritz RL, Zhu HJ, and Simpson RJ (2010). Extracellular remodelling during oncogenic Ras-induced epithelial-mesenchymal transition facilitates MDCK cell migration. Journal of proteome research 9, 1007–1019. [DOI] [PubMed] [Google Scholar]
- 102.Rasanen K, Sriswasdi S, Valiga A, Tang HY, Zhang G, Perego M, Somasundaram R, Li L, Speicher K, Klein-Szanto AJ, et al. (2013). Comparative secretome analysis of epithelial and mesenchymal subpopulations of head and neck squamous cell carcinoma identifies S100A4 as a potential therapeutic target. Molecular & cellular proteomics: MCP 12, 3778–3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chockley PJ, and Keshamouni VG (2016). Immunological Consequences of Epithelial-Mesenchymal Transition in Tumor Progression. J Immunol 197, 691–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lou Y, Diao L, Cuentas ER, Denning WL, Chen L, Fan YH, Byers LA, Wang J, Papadimitrakopoulou VA, Behrens C, et al. (2016). Epithelial-Mesenchymal Transition Is Associated with a Distinct Tumor Microenvironment Including Elevation of Inflammatory Signals and Multiple Immune Checkpoints in Lung Adenocarcinoma. Clinical cancer research: an official journal of the American Association for Cancer Research 22, 3630–3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Grither WR, Divine LM, Meller EH, Wilke DJ, Desai RA, Loza AJ, Zhao P, Lohrey A, Longmore GD, and Fuh KC (2018). TWIST1 induces expression of discoidin domain receptor 2 to promote ovarian cancer metastasis. Oncogene 37, 1714–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Brenot A, Knolhoff BL, DeNardo DG, and Longmore GD (2018). SNAIL1 action in tumor cells influences macrophage polarization and metastasis in breast cancer through altered GM-CSF secretion. Oncogenesis 7, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen L, Gibbons DL, Goswami S, Cortez MA, Ahn YH, Byers LA, Zhang X, Yi X, Dwyer D, Lin W, et al. (2014). Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nature communications 5, 5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Degos L, Dombret H, Chomienne C, Daniel MT, Miclea JM, Chastang C, Castaigne S, and Fenaux P (1995). All-trans-retinoic acid as a differentiating agent in the treatment of acute promyelocytic leukemia. Blood 85, 2643–2653. [PubMed] [Google Scholar]
- 109.Turcan S, Fabius AW, Borodovsky A, Pedraza A, Brennan C, Huse J, Viale A, Riggins GJ, and Chan TA (2013). Efficient induction of differentiation and growth inhibition in IDH1 mutant glioma cells by the DNMT Inhibitor Decitabine. Oncotarget 4, 1729–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rohle D, Popovici-Muller J, Palaskas N, Turcan S, Grommes C, Campos C, Tsoi J, Clark O, Oldrini B, Komisopoulou E, et al. (2013). An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science 340, 626–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schenk T, Chen WC, Gollner S, Howell L, Jin L, Hebestreit K, Klein HU, Popescu AC, Burnett A, Mills K, et al. (2012). Inhibition of the LSD1 (KDM1A) demethylase reactivates the all-trans-retinoic acid differentiation pathway in acute myeloid leukemia. Nature medicine 18, 605–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Natale CA, Li J, Zhang J, Dahal A, Dentchev T, Stanger BZ, and Ridky TW (2018). Activation of G protein-coupled estrogen receptor signaling inhibits melanoma and improves response to immune checkpoint blockade. eLife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Genovese G, Carugo A, Tepper J, Robinson FS, Li L, Svelto M, Nezi L, Corti D, Minelli R, Pettazzoni P, et al. (2017). Synthetic vulnerabilities of mesenchymal subpopulations in pancreatic cancer. Nature 542, 362–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Andricovich J, Perkail S, Kai Y, Casasanta N, Peng W, and Tzatsos A (2018). Loss of KDM6A Activates Super-Enhancers to Induce Gender-Specific Squamous-like Pancreatic Cancer and Confers Sensitivity to BET Inhibitors. Cancer cell 33, 512–526 e518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ishay-Ronen D, Diepenbruck M, Kalathur RKR, Sugiyama N, Tiede S, Ivanek R, Bantug G, Morini MF, Wang J, Hess C, et al. (2019). Gain Fat-Lose Metastasis: Converting Invasive Breast Cancer Cells into Adipocytes Inhibits Cancer Metastasis. Cancer cell 35, 17–32 e16. [DOI] [PubMed] [Google Scholar]
- 116.Zhang J, Cunningham JJ, Brown JS, and Gatenby RA (2017). Integrating evolutionary dynamics into treatment of metastatic castrate-resistant prostate cancer. Nature communications 8, 1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gallaher JA, Enriquez-Navas PM, Luddy KA, Gatenby RA, and Anderson ARA (2018). Spatial Heterogeneity and Evolutionary Dynamics Modulate Time to Recurrence in Continuous and Adaptive Cancer Therapies. Cancer research 78, 2127–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]