Abstract
We present a case of a patient with multiply-relapsed, refractory myeloma whose clinical course showed evidence of a synergistic abscopal-like response to CAR T-cell therapy and localized radiation therapy (XRT). Shortly after receiving B-cell maturation antigen (BCMA)–targeted CAR T-cell therapy, the patient required urgent high-dose steroids and XRT for spinal cord compression. Despite the steroids, the patient had a durable systemic response that could not be attributed to XRT alone. Post-XRT findings included a second wave of fever and increased CRP and IL6, beginning 21 days post-CAR T cells, which is late for cytokine-release syndrome from CAR T-cell therapy alone on this trial. Given this response, which resembled CRS, immediately following XRT, we investigated changes in the patient’s T-cell receptor (TCR) repertoire over 10 serial time points. Comparing T-cell diversity via Morisita’s Overlap Indices (CD), we discovered that, although the diversity was initially stable after CAR T-cell therapy compared with baseline (CD = 0.89–0.97, baseline vs 4 time points post-CAR T cells), T-cell diversity changed after the conclusion of XRT, with >30% newly expanded TCRs (CD = 0.56–0.69, baseline vs 4 time points after XRT). These findings suggest potential synergy between radiation and CAR T-cell therapies resulting in an abscopal-like response.
Keywords: Myeloma, Cellular immunotherapy, CAR, BCMA, Abscopal
Introduction
Most chimeric antigen receptors (CAR) in clinical use consist of a single chain variable fragment (scFv) for directing T-cell specificity in frame with a transmembrane domain and cytoplasmic domains that provide T cells with supra-physiologic signals for activation (CD3ζ) and co-stimulation (4–1BB or CD28) when binding antigen. Thereby, autologous CAR T cells provide antitumor immunity independent of any T-cell receptor (TCR)-major histocompatibility complex (MHC) interaction, although the endogenous TCR remains intact (1).
Radiation therapy (XRT) works locally though DNA damage of tumor cells and can induce a systemic abscopal effect; that is, it can provide an immune mediated antitumor response outside the radiation field. This response is facilitated by release of neo-antigens that generate and shape a systemic TCR-mediated antitumor immune response (2,3). Checkpoint blockade synergizes with concurrent XRT, likely because of this increased T-cell exposure to neo-antigens (4–8).
In contrast with the synergy between XRT and checkpoint blockade, to date there is little evidence demonstrating synergy between XRT and CAR T-cell therapy. Weiss and colleagues demonstrate that XRT may increase trafficking of CAR T cells to the site of an irradiated tumor and enhance local effector function (9,10). However, evidence for synergy between these two therapeutic modalities outside of the radiation field has yet to be reported.
CAR T-cell therapy targeting CD19 has shown efficacy for the treatment of relapsed/refractory adult and pediatric B-cell acute lymphoblastic leukemia (11,12) and large B-cell lymphomas (13). CAR T-cell therapy targeting B-cell maturation antigen (BCMA; TNFRSF17) is under clinical investigation at our center and elsewhere for the treatment of multiple myeloma (MM) (14). MM is a bone marrow–predominant plasma cell malignancy that, despite advances in medical management over the last decade, remains largely incurable. Late-stage manifestations can include multiple extra-osseous plasmacytomas, and responses to therapy become progressively shorter.
Here we report on a patient receiving CAR T-cell therapy for refractory MM who received urgent high-dose steroids and palliative XRT to the whole brain and thoracic spine days later. This case illustrates how BCMA-targeted CAR T-cell therapy can facilitate elimination of a large MM burden despite the early and continued administration of high-dose steroids. The patient’s clinical course suggests that local XRT in combination with CAR T-cell therapy may, through an as yet undefined mechanism, enhance a systemic antitumor effect.
Materials and Methods
Clinical protocol:
The patient was treated on a trial evaluating autologous BCMA-targeted CAR T cells (Trial registration ID: NCT03070327; MSKCC IRB approval #17–025; trial conducted in accordance with US Common Rule; informed written consent obtained from patients). Gene modification is accomplished via a retrovirus encoding a CAR that consists of a human-derived anti-BCMA scFv, a CD8 transmembrane domain, the signaling domains of 4–1BB and CD3ζ fused to a “self-cleaving” P2A element, and a separate gene encoding a surrogate transduction marker (15). Under the trial protocol, patients undergo leukopheresis, and peripheral blood mononuclear cells (PBMCs) are frozen. At the appropriate time, PBMCs are thawed, selected and activated with CD3/CD28 beads, and expanded in the presence of IL2. The patient is admitted for cyclophosphamide and fludarabine (300 mg/m2 and 30 mg/m2, respectively, x3 consecutive days) for lymphodepletion prior to the administration of CAR T cells. This is a 3 × 3 dose escalation study, and the patient presented was treated on dose level 3, at a planned 4.50 × 108 dose of CAR+ viable T cells.
Radiation therapy:
External beam radiation therapy using 6MV photons was delivered using a linear accelerator (Varian Medical Systems, Palo Alto, CA). The target areas included the T1 to T8 vertebral bodies with a right-sided paraspinal tumor mass and the whole brain to the C2 vertebral body). Conventional opposed fields were used (AP/PA for the thorax and opposed laterals for the whole brain fields). The total dose was 2000cGy in 5 daily fractions to each site.
Cytokine measurement:
IL1β, IL6, IL10, and TNFα in peripheral serum were detected by 4-plex microfluidic sandwich immunoassays performed on an Ella (Protein Simple; San Jose, CA). CRP was detected by automated immunoturbidimetric assay performed on an Abbott Architect Chemistry Analyzer (Abbott Laboratories; Chicago, IL). Ferritin was detected by automated two-site immunoenzymatic assay on a Tosoh AIA 2000 (Tosoh Bioscience; Japan). D-dimer was detected by automated immunoturbidimetric assay on the Stago STA-R Max analyzer (Stago; France).
Flow cytometry:
To stain for CAR T cells we used anti-CD3 clone 7D-6 (Thermo-Fisher; Waltham, MA), anti-CD8 clone 3B5 (Thermo-Fisher; Waltham, MA), and cetuximab (Eli Lilly, Indianapolis, IN) conjugated with a lightning link labeling kit (Innova Biosciences, Cambridge, UK). Viability was ascertained with 7AAD exclusion (Thermo-Fisher; Waltham, MA). Flow cytometry was performed on a BD LSR II (BD biosciences; San Jose, CA), and analyzed with FlowJo (FlowJo LLC; Ashland, Oregon).
TCR clonotype tracking:
DNA was extracted via QIAmp Blood DNA mini kit (Qiagen; Hilden, GR) from PBMCs or bone marrow mononuclear cells. Clonotypes were monitored by TCR Vβ CDR3 sequencing via immunoSEQ assay (Adaptive Biotechnologies; Seattle, WA).
Results
Clinical evaluation
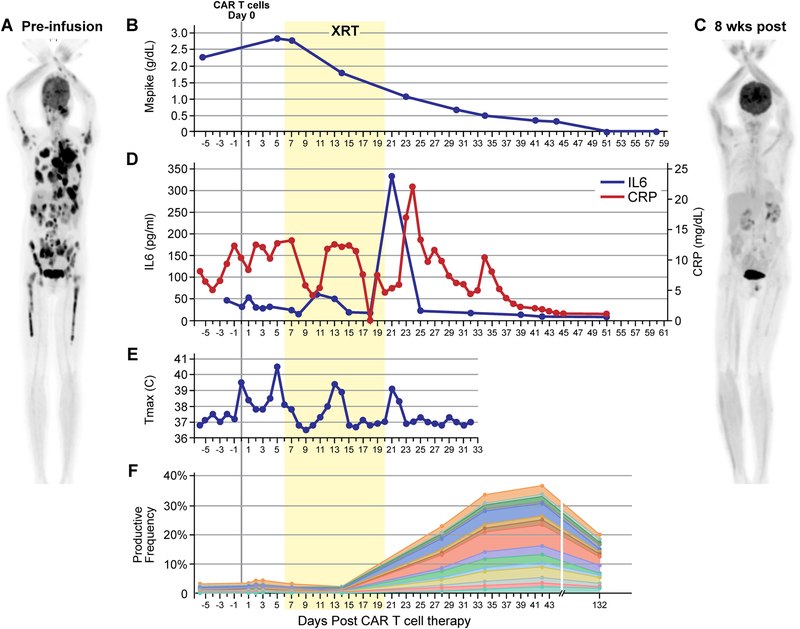
A 63-year-old African American woman was diagnosed with IgA λ multiple myeloma (MM) with high-risk cytogenetics (amplification 1q21) in 2010. She received 8 lines of therapy including combinations with lenalidomide, bortezomib, pomalidomide, carfilzomib, daratumumab, and autologous stem cell transplant, each time with progression. Most recently her disease was refractory to therapy with dexamethasone, cyclophosphamide, etoposide, and cisplatin (DCEP). She enrolled on a clinical trial of BCMA-targeted CAR T-cell therapy including a 4–1BB co-stimulatory domain (15) ( NCT03070327). Her clinical course is presented in Fig. 1A–F. Baseline findings indicated extensive disease, including monoclonal paraprotein (M-spike) of 2.26g/dL, a baseline bone marrow biopsy revealing 95% plasma cell infiltration, and a PET/CT scan demonstrating widespread bone-based and extra-osseous MM lesions, including extensive soft tissue and pleural-based masses (Fig. 1A).
Figure 1. BCMA-targeted CAR T-cell therapy followed by radiation therapy (XRT) led to clinical response, expansion of TCR clonality, and CRS-like findings after XRT.
The patient received conditioning therapy with cyclophosphamide and fludarabine followed by CAR T cells on day 0. Radiation therapy (XRT) took place over 10 fractions between day 6 and day 20 (yellow box). (A) Pre-treatment PET/CT scan showing extensive intra-osseous and extra-osseous FDG-avid disease including soft tissue and pleural-based masses. (B) Decrease in M-spike commencing during XRT. (C) PET/CT 8 weeks post-therapy demonstrating resolution of MM lesions. (D) Prodiuction of IL6 and CRP (pro-inflammatory markers associated with active CAR T-cell function) peaked after the conclusion of XRT. (E) Daily maximum temperature curve revealed a fever at the time of peak IL6 and CRP. (F) TCR clonality analysis demonstrating expansion of novel TCR clones. The subset of TCRs comprising newly expanding clones are shown over time.
The patient received cyclophosphamide/fludarabine lymphodepleting chemotherapy followed by a single infusion of CAR T cells. Shortly thereafter, she exhibited signs of lower extremity weakness and confusion. The patient remained awake and alert, however, mental status changes in addition to confusion manifested as intermittent aphasia, decreased short-term recall (could recall 0/3 objects), and attention/calculation (could not spell WORLD backwards). To evaluate these neurologic findings an MRI spine and brain were obtained which revealed spinal cord compression and multiple epidural masses causing a cerebral mid-line shift (Supplementary Fig. S1A). On day 5 post-CAR T cells, M-spike had increased from baseline to 2.83g/dL. Although her neurologic symptoms resolved by this point, given the lesion compressing her thoracic spinal cord on imaging and increasing MM serologic markers, there was concern for progression of her cord compression. The patient therefore received high-dose steroids (dexamethasone taper starting at 10mg every 12 hours for a total of 13 days) and palliative XRT to the thoracic spine (T1 to T8) followed by the whole brain to C2; delivered between day 6–20 post-CAR T cells (2000cGy in 5 fractions to each site, in series; Fig. 1; Supplementary Fig. S1B). Her MRI at 4 weeks showed near-resolution of disease at the sites of radiation therapy (Supplementary Fig. S1A).
In addition to this response at the site of radiation therapy, a favorable systemic response was subsequently observed. Seven weeks post-CAR T cells, her M-spike was undetectable (Fig. 1B). PET/CT at 8 weeks post-CAR T cells showed complete radiographic resolution of disease including innumerable sites outside the radiation field (Fig. 1C). The patient’s clinical response persisted through 9 months post-therapy.
Inflammatory response
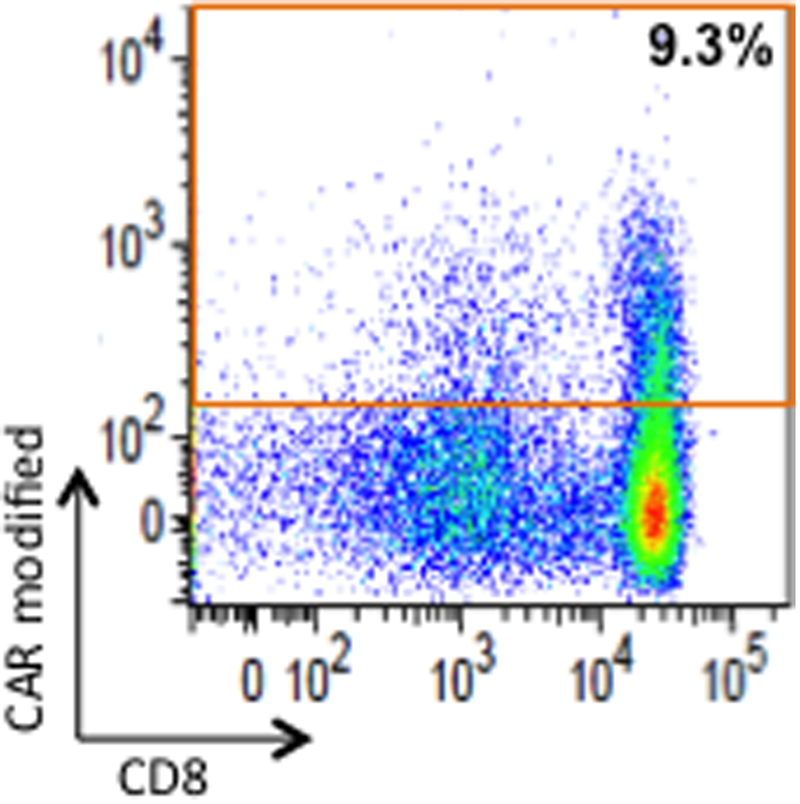
We prospectively monitored changes in serum inflammatory markers CRP, Ferritin, and D-dimer, as well as cytokines IL6, IL10, TNFα, and IL1β. Inflammatory markers associated with CAR T-cell activity (16) increased shortly after the conclusion of radiation therapy. These included markers characteristic of CAR T cell mediated cytokine-release syndrome (CRS) (17): IL6 (to 333 pg/mL from 32 pg/mL; Fig. 1D) and CRP (to 22.11 mg/dL from 6.43 mg/dL; Fig. 1D), as well as more general markers of inflammation that are often seen elevated in CRS: Ferritin (to 13,197 ng/mL from 2,921 ng/mL Supplementary Fig. S2A) and D-Dimer (to 915 ng/mL from <150 ng/mL; Supplementary Fig. S2B). Serum IL10 had a bi-modal peak. The first peak occurred early after the administration of CAR T cells, corresponding to timing of peak serum IL10 concentrations after CAR T cells reported by others (18) and the timing of CRS seen in other patients on this study (19). A later second peak occurred during radiation therapy (Supplementary Fig. S2C). TNFα and IL1β remained stable and within normal limits throughout the clinical course (Supplementary Fig. S2C). Around the conclusion of radiation therapy, the patient developed a fever to >39 °C with no clinical or laboratory evidence of infection (Fig. 1E). At this time, she became tachycardic to 110s beats/min (from recent baseline low 60s beats/min) and systolic blood pressure decreased to 90s mmHg (from recent baseline 110–130s mmHg); heart rate and blood pressure returned to baseline after 3 days of tachycardia and relative hypotension. She did not require vasopressors or supplemental oxygen, therefore this would be considered grade 1 CRS. A delayed CRS-like response was only seen in this patient and not in other patients on the trial (n=13). Despite 13 days of systemic steroids, CAR T cells expanded and persisted (Supplementary Fig. S3), representing 9.3% of CD3+ cells in the blood by flow cytometry around the time of inflammatory response (Fig. 2). Given the temporal relationship of these CRS-like findings immediately after radiation therapy, we investigated, using stored samples, the timing of changes in her TCR repertoire.
Figure 2. Persistence of CAR T cells around the time of expansion of TCR clonality and CRS-like findings after XRT.
CAR T cells in the patient’s blood were assessed at 5 weeks after CAR T-cell therapy by flow cytometry with a fluorophore-labeled antibody recognizing the surrogate transduction marker that is additionally expressed by the CAR vector. Flow cytometry of peripheral blood mononuclear cells was gated on viable CD3+ cells. The red box outlines cells positive for the transduction marker (9.3% of circulating T cells). Note that these persistent gene-modified T cells were predominantly CD8+.
TCR clonotype serial analysis
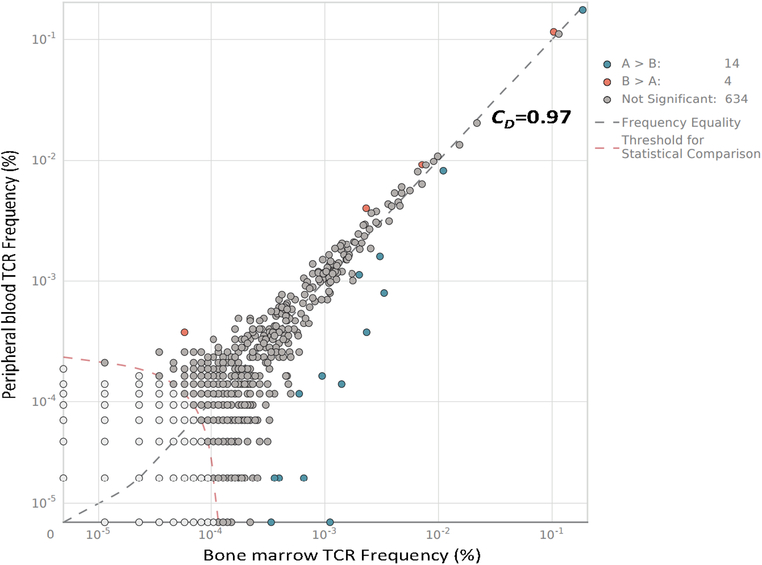
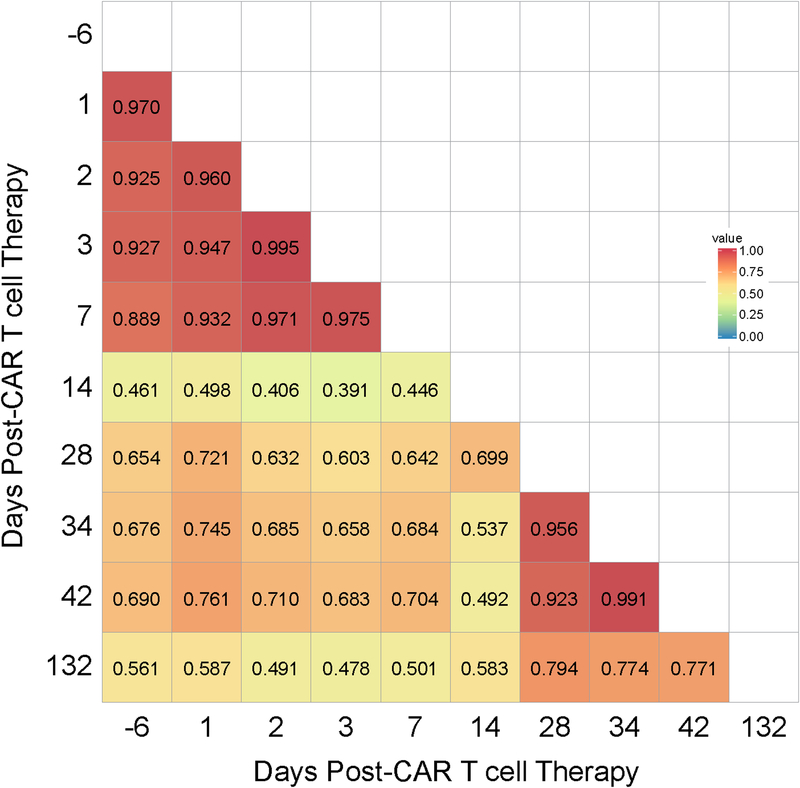
Because the post-radiation findings were suggestive of clinical and laboratory findings often correlating with CRS, we investigated the timing of changes in the patient’s TCR repertoire using stored samples. We found that the patient’s baseline peripheral blood TCR diversity mirrored that in her baseline bone marrow (Fig. 3; TCR diversity compared by Morisita’s Overlap Index; CD=0.97). Given this high degree of overlap between peripheral blood and bone marrow, we concluded that the peripheral blood may be a suitable surrogate to investigate changes in TCR diversity over time in this patient with intra- and extra-osseous MM. Serial analysis of the patient’s peripheral blood TCR repertoire demonstrated TCR diversity was stable at four time points across the first week after CAR T-cell infusion. Values for CD compared to baseline were 0.89–0.97 throughout this time (Fig. 4). A spike in new TCR clone expansion was noted upon initial evaluation, day eight after the conclusion of radiation therapy (CD=0.56–0.69, 4–19 weeks post-CAR T-cell therapy vs baseline). These newly expanded clones made up >30% of the T-cell repertoire at 4 weeks. Many persisted through at least 19 weeks post CAR T-cell infusion, the most recent time point assessed (Fig. 1F). No TCR clone expansion was seen in any clones highly expressed at baseline (Supplementary Fig. S4).
Figure 3. TCR repertoire of bone marrow mirrored that of peripheral blood.
The TCR repertoire of bone marrow and peripheral blood mononuclear cells from the same time point was evaluated. TCR diversity was compared by Morisita’s overlap index (CD).
Figure 4. Addition of radiation therapy to CAR T-cell therapy increased TCR repertoire diversity over time.
Heatmap of Morisita’s overlap indices (CD) displays the degree of similarity between TCR repertoire from peripheral blood samples over time. The index ranges from 0 (no overlap of TCR clonotypes) to 1 (all TCR clonotypes occur in the same proportions in both samples).
Discussion
Here, we report that BCMA-targeted CAR T-cell therapy could mediate eradication of a large burden of MM, including substantial extra-osseous disease. High-dose steroid administration did not mitigate the efficacy of CAR T-cell therapy. Furthermore, the timing, unique to this patient, of CRS-like clinical signs and inflammatory markers coincided with the expansion of new TCR clones after radiation therapy, supporting the hypothesis of synergy between XRT and CAR T-cell therapy.
For frequent, serial assessment of TCR clonality, we relied on peripheral blood mononuclear cells. Despite the fact that MM is a bone marrow based malignancy, and although marrow is accessible, bone marrow cannot be sampled as frequently as can peripheral blood. We found that, when assessed at the same time point, the patient’s bone marrow mononuclear cells and PBMCs had a high degree of overlap in TCR repertoires (CD=0.97). Therefore, we concluded that TCR clonality in the peripheral blood is relevant in this patient with widespread MM.
Serial analysis of TCR repertoires revealed expansion of new TCR clones coinciding with clinical and laboratory signs of CRS. The confluence of these events shortly after radiation therapy in this previously CAR T-cell treated patient supports the likelihood of synergy between radiation and CAR T-cell therapy.
One limitation of this study is that the stored material was not of sufficient quantity to determine the antigen targets driving the specific TCR clone expansion. However, newly expanded TCR clones comprised >30% of the T-cell population after XRT at a time when CAR+ T cells constituted ~10% of CD3 cells, indicating that at least some T-cell expansion was driven by non-CAR modified T cells.
Radiation therapy, alone or in combination with checkpoint blockade, is known to “shape” the TCR repertoire of expanded clones (2,20). However, given that CARs result in HLA-independent T-cell activation, it is not necessarily intuitive that local radiation therapy would buoy systemic responses by CAR T cells in the way that it can buoy checkpoint blockade, i.e. through increased neo-antigen exposure through an abscopal effect (6).
Synergy of CAR T-cell and radiation therapy may occur through several possible mechanisms: (1) Synergy could be explained by the cytokines secreted by CAR T-cells, increasing the likelihood of endogenous T-cells mounting an abscopal-like response. (2) As has been shown pre-clinically, radiation therapy may enhance effector functions and migration of CAR T-cells (9,21). (3) Increased signaling through the TCR of CAR T-cells may enhance clonal CAR T-cell expansion. Combinations of these mechanisms or unknown mechanisms are also possible explanations.
A similar phenomenon may have been at work in a CAR T cell–treated patient with CNS lymphoma for whom, after relapse, biopsy of a mass precipitated a clinical remission (22). These observations support further investigation of CAR T-cell therapy combined with radiation therapy.
Supplementary Material
Acknowledgments:
We would like to thank Elizabeth Halton NP, Claudia Diamonte RN, and John Pineda RN, for caring for our cellular therapy patients; Pavan Anant, Analisa Wills, and Sarah Yoo for administrative and clinical research assistance; Janet Novak (MSKCC Editorial and Grant Services) for editorial support; and Susan Weil (MSKCC Medical Graphics) for assistance with presentation of data.
Financial support: E.L.S. is a Special Fellow of The Leukemia & Lymphoma Society and an American Society of Hematology Scholar, additional support: Technology Development Grant, MSKCC; the Multiple Myeloma Research Foundation; the Lymphoma Research Foundation; and the Society of Immunotherapy for Cancer. O.L. reports grant support: NIH, FDA, MMRF, IMF, LLS, Perelman Family Foundation, Rising Tides Foundation, Amgen, Celgene, Janssen, Takeda, Glenmark, Seattle Genetics, Karyopharm; R.B.J. reports support from NIH (R01 CA138738–05, P01 CA059350, PO1 CA190174–01), The Annual Terry Fox Run for Cancer Research (New York, NY) organized by the Canada Club of New York, Kate’s Team, Carson Family Charitable Trust, William Lawrence and Blanche Hughes Foundation, Emerald Foundation, the Experimental Therapeutics Center of Memorial Sloan-Kettering Cancer Center. All MSK investigators acknowledge MSK Core Facilities Grants (P30 CA008748; U54 OD020355–01).
Footnotes
Conflict of interest: E.L.S. and R.J.B., licensed intellectual property, Juno Therapeutics, a Celgene Company. E.L.S., S.M, I.R, and R.J.B., research funding, Juno Therapeutics, a Celgene Company; E.L.S., B.D.S., O.L., and R.J.B., consultancy, Juno Therapeutics, a Celgene Company; E.L.S., consultancy Fate Therapeutics, Precision Biosciences; S.M. research funding, Takeda Oncology, Janssen Oncology; M.B.G, honoraria, Dava Oncology; A.D.G., research funding, AROG, Pfizer, ADC Therapeutics; consultancy, Abbvie, Celgene, Daiichi-Sankyo; honoraria, Dava Oncology; B.D.S. consultancy Kite Pharma/Gilead, and Novartis; A. R., research funding, Varian Medical Systems, Boehringer Ingelheim, Pfizer, AstraZeneca; consultancy, Astrazeneca, Merck; O.L. funding, Amgen, Celgene, Janssen, Takeda, Glenmark, Seattle Genetics, Karyopharm; consultancy, Adaptive, Amgen, Binding Site, BMS, Celgene, Cellectis, Glenmark, Janssen, Pfizer; Independent Data Monitoring Committee (IDMC): Takeda, Merck, Janssen, Theradex; I.R., research funding, Fate Therapeutics. All other authors declare that they have no competing interests. A patent has been filed on data presented in this paper.
References
- 1.Khalil DN, Smith EL, Brentjens RJ, Wolchok JD. The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat Rev Clin Oncol [Internet]. 2016. [cited 2016 Aug 1];13:394 Available from: http://www.ncbi.nlm.nih.gov/pubmed/27118494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sridharan V, Margalit DN, Lynch SA, Severgnini M, Zhou J, Chau NG, et al. Definitive chemoradiation alters the immunologic landscape and immune checkpoints in head and neck cancer. Br J Cancer [Internet]. 2016. [cited 2019 Jan 14];115:252–60. Available from: http://www.nature.com/articles/bjc2016166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang C, Wang X, Soh H, Seyedin S, Cortez MA, Krishnan S, et al. Combining Radiation and Immunotherapy: A New Systemic Therapy for Solid Tumors? Cancer Immunol Res [Internet]. 2014. [cited 2018 Jun 13];2:831–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25187273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Golden EB, Demaria S, Schiff PB, Chachoua A, Formenti SC. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol Res [Internet]. 2013. [cited 2018 Jun 13];1:365–72. Available from: http://cancerimmunolres.aacrjournals.org/cgi/doi/10.1158/2326-6066.CIR-13-0115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grimaldi AM, Simeone E, Giannarelli D, Muto P, Falivene S, Borzillo V, et al. Abscopal effects of radiotherapy on advanced melanoma patients who progressed after ipilimumab immunotherapy. Oncoimmunology [Internet]. 2014. [cited 2018 Jun 13];3:e28780 Available from: http://www.tandfonline.com/doi/abs/10.4161/onci.28780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park SS, Dong H, Liu X, Harrington SM, Krco CJ, Grams MP, et al. PD-1 Restrains Radiotherapy-Induced Abscopal Effect. Cancer Immunol Res [Internet]. 2015. [cited 2018 Jun 13];3:610–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25701325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Victor CT-S, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. Nature Publishing Group, a division of Macmillan Publishers Limited. All Rights Reserved.; 2015;520:373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiss T, Schneider H, Silginer M, Steinle A, Pruschy M, Polić B, et al. NKG2D-Dependent Antitumor Effects of Chemotherapy and Radiotherapy against Glioblastoma. Clin Cancer Res [Internet]. 2018. [cited 2018 Jun 13];24:882–95. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29162646 [DOI] [PubMed] [Google Scholar]
- 10.Weiss T, Weller M, Guckenberger M, Sentman CL, Roth P. NKG2D-Based CAR T Cells and Radiotherapy Exert Synergistic Efficacy in Glioblastoma. Cancer Res [Internet]. 2018. [cited 2018 Jun 13];78:1031–43. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29222400 [DOI] [PubMed] [Google Scholar]
- 11.Park JH, Rivière I, Gonen M, Wang X, Sénéchal B, Curran KJ, et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N Engl J Med [Internet]. 2018. [cited 2018 Feb 1];378:449–59. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29385376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med [Internet]. Massachusetts Medical Society; 2018. [cited 2018 Feb 1];378:439–48. Available from: http://www.nejm.org/doi/10.1056/NEJMoa1709866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med [Internet]. 2017. [cited 2018 Feb 24];377:2531–44. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29226797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brudno JN, Maric I, Hartman SD, Rose JJ, Wang M, Lam N, et al. T Cells Genetically Modified to Express an Anti-B-Cell Maturation Antigen Chimeric Antigen Receptor Cause Remissions of Poor-Prognosis Relapsed Multiple Myeloma. J Clin Oncol [Internet]. 2018. [cited 2018 Jun 8];JCO2018778084 Available from: http://ascopubs.org/doi/10.1200/JCO.2018.77.8084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith EL, Staehr M, Masakayan R, Tatake IJ, Purdon TJ, Wang X, et al. Development and Evaluation of an Optimal Human Single-Chain Variable Fragment-Derived BCMA-Targeted CAR T Cell Vector. Mol Ther [Internet]. 2018. [cited 2018 Apr 22]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/29678657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, et al. Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med [Internet]. 2014. [cited 2015 Mar 8];6:224ra25 Available from: http://stm.sciencemag.org/content/6/224/224ra25.full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, et al. Efficacy and Toxicity Management of 19–28z CAR T Cell Therapy in B Cell Acute Lymphoblastic Leukemia. Sci Transl Med. 2014;6:224ra25–224ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kochenderfer JN, Somerville RPT, Lu T, Shi V, Bot A, Rossi J, et al. Lymphoma Remissions Caused by Anti-CD19 Chimeric Antigen Receptor T Cells Are Associated With High Serum Interleukin-15 Levels. J Clin Oncol [Internet]. 2017. [cited 2018 Jun 30];35:1803–13. Available from: http://ascopubs.org/doi/10.1200/JCO.2016.71.3024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mailankody S, Ghosh A, Staehr M, Purdon TJ, Roshal M, Halton E, et al. Clinical Responses and Pharmacokinetics of MCARH171, a Human-Derived Bcma Targeted CAR T Cell Therapy in Relapsed/Refractory Multiple Myeloma: Final Results of a Phase I Clinical Trial. Blood [Internet]. American Society of Hematology; 2018. [cited 2019 Feb 1];132:959–959. Available from: http://www.bloodjournal.org/content/132/Suppl_1/959 [Google Scholar]
- 20.Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature [Internet]. 2015. [cited 2019 Jan 14];520:373–7. Available from: http://www.nature.com/articles/nature14292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeSelm C, Palomba ML, Yahalom J, Hamieh M, Eyquem J, Rajasekhar VK, et al. Low-Dose Radiation Conditioning Enables CAR T Cells to Mitigate Antigen Escape. Mol Ther [Internet]. 2018. [cited 2019 Jan 14];26:2542–52. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30415658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abramson JS, McGree B, Noyes S, Plummer S, Wong C, Chen Y-B, et al. Anti-CD19 CAR T Cells in CNS Diffuse Large-B-Cell Lymphoma. N Engl J Med [Internet]. 2017. [cited 2018 May 15];377:783–4. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28834486 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.