Abstract
For over a decade, first responders and the general public have been able to treat suspected opioid overdoses using an improvised nasal naloxone device (INND) constructed from a prefilled syringe containing 2 mg of naloxone (1 mg/mL) attached to a mucosal atomization device. In recent years, the U.S. Food and Drug Administration approved Ezvio®, an autoinjector that delivers 2 mg by intramuscular (IM) injection and Narcan® nasal spray (2-mg and 4-mg strengths; 0.1 mL/dose) for the emergency treatment of a known or suspected opioid overdose. The present study was conducted to compare the pharmacokinetics of naloxone using the FDA-approved devices (each administered once) and either 1 or 2 administrations using the INND. When naloxone was administered twice using the improvised device, the doses were separated by 2 minutes. The highest maximum plasma concentration was achieved using the 4-mg FDA-approved spray. The highest exposures at 5 minutes post-dose, based on AUC values, were after administration with the autoinjector and the 4-mg FDA-approved spray; at 10, 15, and 20 minutes post-dose, the latter yielded the greatest exposure. Even after 2 administrations, the INND failed to achieve naloxone plasma levels comparable to the FDA-approved devices at any timepoint. The ease of use and higher plasma concentrations achieved using the 4-mg FDA-approved spray, compared to the INND, should be considered when deciding which naloxone device to use.
Keywords: Naloxone, Intranasal, Pharmacokinetics, Opioid overdose
INTRODUCTION
Deaths due to opioid overdoses have increased substantially over the last 20 years. Of the 60,000 opioid-related deaths in 2017 in the United States, 14,958 were due to natural and semisynthetic opioids, 15,958 were due to heroin, and 29,406 were due to synthetic opioids, much of which was fentanyl.1 Fentanyl, which is approximately 50- to 100-fold more potent than morphine on the mu opioid receptor2,3, is being detected in an increasing percentage of overdose cases and found in other recreational drugs, such as methamphetamine and cocaine.
Naloxone (17-allyl-4,5α-epoxy-3,14-dihydroxymorphinan-6-one HCl) is a high-affinity opiate receptor antagonist that has been used by the parenteral route of administration to treat the symptoms of opioid overdose for over 40 years.4 An increasing number of government jurisdictions have endorsed the use of naloxone for intranasal (IN) administration by non-medical personnel, such as police and the general public population, to treat opioid overdoses,4,5 An improvised nasal naloxone device (INND), consisting of a prefilled naloxone syringe intended for parenteral use attached to a mucosal atomization device, was first described in 1994.6 Since then, it has often been prepared by pharmacists, dispensed to patients, and provided to first responders, either with an individual physician’s prescription or with a standing order authorizing dispensing by pharmacies. The INND has not been approved for use by the U.S. Food and Drug Administration (FDA).
In recent years, the FDA has approved 2 types of devices that can be used by non-medical personnel for the administration of naloxone. Evzio®, approved in April 2014, is an autoinjector (Autoinjector) for intramuscular (IM) injection of 2 mg naloxone that gives audible instructions for its use. The initial approval was for administration of 0.4 mg in 0.4 mL, but a subsequent approval was for a 2-mg dose in the same volume; the lower dose autoinjector was withdrawn from the market. Narcan® devices for nasal administration of 4 and 2 mg naloxone in 0.1 mL (hereafter referred to as FDANxSpray) were approved by the FDA in November 2015 and January 2017, respectively. As of the time of this writing, only the 4-mg product is available; the manufacturer has not marketed the 2-mg product and has no current plans to launch it (personal communication, Fintan Keegan, Adapt Pharma).
The present study was designed to directly compare the pharmacokinetics (PK) of the FDA-approved naloxone devices and the INND. This study was also designed to compare the PK of naloxone following 1 and 2 administrations of the INND, with repeated doses separated by the recommended time of 2–3 minutes. It was hypothesized that due to the large volume of fluid (2 mL) and incomplete absorption prior to the second dose, 2 administrations using the INND would not yield a substantial increase in the naloxone Cmax compared to a single administration.
METHODS
Study Participants
The study protocol was approved by the MidLands Independent Review Board (Overland Park, KS), and all participants gave written informed consent before participation. The study site was Vince & Associates Clinical Research (Overland Park, KS), and the study was conducted in accordance with the International Conference on Harmonization for Good Clinical Practices guidelines.7 This trial was registered as NCT03386591 (www.clinicaltrials.gov).
Healthy volunteers of both sexes, aged 18-55 years, with body mass index (BMI) 18-32 kg/m2, participated in this study. Participants were not taking either prescription or over-the-counter medications, and they were either nonsmokers or they smoked 20 or fewer cigarettes per day. Screening procedures conducted within 21 days of study initiation included the following: medical history, physical examination, evaluation for evidence of nasal irritation or nasal symptoms, 12-lead electrocardiogram, complete blood count, clinical chemistry, coagulation markers, hepatitis and human immunodeficiency screening, urinalysis, and urine drug screen. Female participants were tested for pregnancy at screening and admission to the clinic. Participants were excluded if they had either abnormal nasal anatomy or nasal symptoms, an upper respiratory tract infection, used opioid analgesics for pain relief within the previous 14 days, or in the judgment of the investigator, had significant acute or chronic medical conditions.
Study Design
The study was an inpatient, open-label, randomized, 5-period, 5-treatment, crossover study. Participants were randomly assigned to 1 of 5 sequences to ensure at least 6 participants in each sequence. On the day after clinic admission, participants were administered the study drug in randomized order with a 2-day washout period between doses. Participants remained in the clinic for 10 days until all 5 treatments were administered; they were contacted by telephone 3 to 5 days later as a follow-up. Participants were required to abstain from alcohol from admission to the end of the last blood draw of the study and from nicotine and caffeine-containing products for at least 1 hour prior to and 2 hours after dose administration. Participants fasted overnight from midnight the day prior to until 4 hours after dose administration.
On days of dosing, a participant’s vital signs were required to be within the normal range before receiving naloxone, defined as systolic blood pressure < 160 mmHg and diastolic blood pressure <100 mmHg. Each participant received each of the following treatments according to the randomization scheme:
Treatment A: 2 mL naloxone of 1 mg/mL solution (one 1-mL spray in each nostril) at 0 minutes using the INND
Treatment B: 2 mL naloxone of 1 mg/mL solution (one 1-mL spray in each nostril) at 0 and 2 minutes using the INND
Treatment C: 2 mg naloxone IN at 0 minutes using the 2-mg FDANxSpray (0.1 mL of a 20 mg/mL solution)
Treatment D: 4 mg naloxone IN at 0 minutes using the 4-mg FDANxSpray (0.1 mL of a 40 mg/mL solution)
Treatment E: 2 mg naloxone IM at 0 minutes using the Autoinjector (0.4 mL of a 5 mg/mL solution)
IN naloxone dosing was administered in the supine position, and participants remained in this position for approximately 1 hour after dosing. Participants were instructed not to breathe when the drug was administered to simulate an opioid overdose of a person in respiratory arrest. The nasal passage was examined by medical personnel for irritation using a 6-point scale at pre-dose and at 5 minutes and 0.5, 1, and 4 hours post-dose. Nasal irritation was scored as follows: 0 (normal appearing mucosa, no bleeding); 1 (inflamed mucosa, no bleeding); 2 (minor bleeding that stops within 1 minute); 3 (minor bleeding taking 1 to 5 minutes to stop); 4 (substantial bleeding for 4 to 60 minutes, does not require medical intervention); and 5 (ulcerated lesions, bleeding that requires medical intervention). The IM injection was into the anterolateral aspect of the thigh, as indicated in the package instructions. Twelve-lead electrocardiograms were performed pre-dose and at 1 and 8 hours post-dose. Venous blood samples were collected for the analyses of plasma naloxone concentrations pre-dose and at 3, 5, 10, 15, 20, 30, 45, and 60 minutes and 2, 3, 4, 6, 8, and 12 hours post-dose using Vacutainer® tubes containing sodium heparin. The plasma was stored at < −60°C until analyzed.
Study Drugs
Naloxone HCl, USP for injection, 2 mg/mL, in Leuer-Jet™ Prefilled Syringes (International Medication Systems, Ltd, South El Monte, CA), LMA™ mucosal atomization devices (Teleflex, Morrisville, NC), 2-mg Autoinjectors (kaleo, Inc, Richmond, VA) and 4-mg FDANx Spry devices (Adapt Pharma, Radnor, PA) were purchased from commercial sources.The 2-mg FDANxSpray devices were generously provided by Adapt Pharma (Radnor, PA). Each INND was constructed by attaching a mucosal atomization device to a prefilled syringe.
Analytical Methods
Plasma naloxone concentrations were determined using a validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay as detailed in Krieter et al.8 The calibration curves (peak area ratios) were linear (r2 > 0.980) over the concentration range of 0.01 ng/mL to 10 ng/mL, and the lower limit of quantitation was 0.01 ng/mL. The inter-day precision of the calibration curves and quality control samples ranged from 2.21% to 4.66%, and the accuracy ranged between −3.88% and 1.50% during the analysis of the samples.
Data Analyses
The safety population included all participants who received at least 1 dose of naloxone; the PK population included all participants who completed all 5 dosing periods with sufficient data to calculate meaningful PK parameters. PK parameters were calculated using standard noncompartmental methods and a validated installation of WinNonlin® Phoenix, version 6.3 (Pharsight Corp., Mountain View, CA). Values of peak plasma concentrations (Cmax) and the time to reach Cmax (Tmax) were the observed values obtained directly from the concentration-time data. The terminal elimination half-life (t½) was estimated by linear regression analysis. The area under the concentration time curve from time zero to the last quantifiable concentration (AUC0-t) was determined by the linear up/log down trapezoidal method and extrapolated to infinity (AUC) by adding the value of the last quantifiable concentration divided by the terminal rate constant. The extrapolated percentage of AUC was less than 20% for all concentration profiles; therefore, only AUC is reported. The apparent total clearance (CL/F) was calculated as the dose (D) divided by AUC. Within an ANOVA framework, comparisons of ln-transformed dose-normalized PK parameters were performed using a mixed effects model where sequence, period, and treatment were the independent factors. The 90% confidence interval (CI) for the ratio of the geometric least squares means of Cmax and AUC were constructed for comparison of the 4 IN treatments to the IM formulation. The 90% CIs were obtained by exponentiation of the 90% CIs for the differences between the least squares means based upon an ln scale.
RESULTS
Participant Characteristics
Seventeen male and 13 female participants received at least 1 dose of naloxone (Table 1), and 27 of the 30 participants completed the study. One female participant discontinued for personal reasons after the second treatment period and another female participant was removed from the study after the first treatment period due to an episode of mild syncope during blood draws. A male participant was removed after the second period due to disruptive behavior.
Table 1.
Participant Demographics
| All | Female | Male | |
|---|---|---|---|
| N | 30 | 13 | 17 |
| Mean age, years (range) | 33.7 (19-55) | 30.3 (19-54) | 36.2 (19-55) |
| Race | |||
| White | 12 | 3 | 9 |
| Black/African American | 17 | 9 | 8 |
| Native American | 1 | 1 | 0 |
| Ethnicity | |||
| Hispanic or Latino | 2 | 2 | 0 |
| Not Hispanic or Latino | 28 | 11 | 17 |
| Mean weight, kg (range) | 77.7 (50.3-109.6) | 68.2 (50.3-79.7) | 85.0 (61.8-110) |
| Mean BMI,a kg/m2 (range) | 26.6 (19.7-31.9) | 26.0 (19.7-31.9) | 27.1 (21.5-31.4) |
BMI, body mass index
Pharmacokinetics
Cmax was highest after IN administration of 4 mg naloxone using the 4-mg FDANxSpray device (5.9 ng/mL; Table 2). It was similar when 2 mg naloxone was administered using the Autoinjector and the 2-mg FDANxSpray device (3.8 and 3.6 ng/mL, respectively) and lowest when 2 and 4 mg was given by 1 and 2 IN doses using the INND (1.4 and 2.3 ng/mL, respectively). The median Tmax value was 20 minutes for all of the IN doses and slightly longer after the IM dose. The elimination half-life of naloxone ranged between 1.4 and 2.2 hours.
Table 2.
Pharmacokinetics of Naloxone in Healthy Participants after Administration using the INND, the 2-mg and 4-mg FDANxSpray Devices, and the Autoinjector
| Parameter (Units)a | One Dose INND 2 mg IN (Trt A) | Two Doses INND 4 mg IN b (Trt B) | 2-mg FDANxSpray IN (Trt C) | 4-mg FDANxSpray IN (Trt D) | Autoinjector 2 mg IM (Trt E) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cmax (ng/mL) | 1.4 | (45.4) | 2.3 | (36.7) | 3.6 | (42.6) | 5.9 | (34.3) | 3.8 | (33.4) |
| Cmax/D (ng/mL/mg) | 0.8 | (45.4) | 0.6 | (36.7) | 2.0 | (42.6) | 1.6 | (34.3) | 2.1 | (33.4) |
| Tmax (h) | 0.3 | (0.1-0.8) | 0.3 | (0.2-0.8) | 0.3 | (0.2-0.8) | 0.3 | (0.2-1.0) | 0.5 | (0.1-3.0) |
| AUC (ng·min/mL) | 125 | (27.2) | 214c | (23.9) | 329d | (22.1) | 583 | (29.5) | 532 | (18.5) |
| AUC /D (ng·min/mL/mg) | 69.0 | (27.2) | 59.3c | (23.9) | 183d | (22.1) | 162 | (29.5) | 296 | (18.5) |
| AUC0-5m (ng·min/mL) | 0.7 | (133) | 0.8 | (67.9) | 1.2 | (131) | 2.0 | (111) | 2.6 | (84.4) |
| AUC0-10m (ng·min/mL) | 4.0 | (81.8) | 5.4 | (52.1) | 8.8 | (80.3) | 12.8 | (72.7) | 12.3 | (55.2) |
| AUC0-20m (ng·min/mL) | 16.0 | (55.3) | 23.5 | (44.4) | 37.3 | (60.4) | 55.4 | (49.3) | 43.0 | (41.4) |
| t½ (h) | 1.5 | (40.3) | 2.2c | (36.9) | 1.5d | (33.5) | 2.2 | (38.2) | 1.4 | (18.4) |
| CL/F (L/h) | 866 | (27.2) | 1010c | (23.9) | 328d | (22.1) | 370 | (29.5) | 203 | (18.5) |
N=27
%CV, percent coefficient of variation; AUC, area under the plasma concentration-time curve from time zero to infinity; AUC0-5m, AUC from time zero to 5 min; AUC0-10m, AUC from time zero to 10 min; AUC0-20m, AUC from time zero to 20 min; CL/F, apparent clearance; Cmax, maximum plasma concentration; t½, terminal half-life; Tmax, time to Cmax; relative BA, bioavailability relative to IM injection; INND, improvised nasal naloxone device; IN, intranasal; IM, intramuscular; Trt, treatment.
Geometric mean values (%CV) for all except Tmax which is median (minimum, maximum)
Second dose using INND administered 2 minutes after the first dose.
N = 21
N = 26
Values of AUC were approximately the same when 2 mg of naloxone was given by the Autoinjector and when 4 mg naloxone was administered by the 4-mg FDANxSpray device. Values of AUC were lowest after 2 or 4 mg naloxone was delivered by the INND. When the dose of naloxone was considered, both Cmax/D and AUC/D were highest for the IM dose (2.1 ng/mL/mg and 296 ng·min/mL/mg) and lowest for the 1 and 2 doses using the INND. The relative bioavailability of IN naloxone, compared to the IM dose, was 54-62% for FDANxSpray spray devices and 19-23% using the INND (Table 3).
Table 3.
Statistical Summary of Naloxone Treatment Comparisons (Intranasal versus Intramuscular Administration)
| Parameter | IN Administration (Test) | Comparison (E as Reference) | Ratio (Test/Reference) of Adjusted Meansa | 90% CI for Ratio |
|---|---|---|---|---|
| Cmax/Dose | INND 2-mg × 1 IN (Trt A) | A vs E | 37.2 | 32.1-43.0 |
| INND 2-mg × 2 IN (Trt B) | B vs E | 29.6 | 25.5-34.2 | |
| 2-mg FDANxSpray IN (Trt C) | C vs E | 93.2 | 80.6-108 | |
| 4-mg FDANxSpray IN (Trt D) | D vs E | 76.4 | 66.0-88.4 | |
| AUC /Dose | INND 2-mg IN (Trt A) | A vs E | 23.3 | 21.2-25.7 |
| INND 2-mg × 2 IN (Trt B) | B vs E | 19.6 | 17.7-21.8 | |
| 2-mg FDANxSpray IN (Trt C) | C vs E | 61.7 | 56.0-68.0 | |
| 4-mg FDANxSpray IN (Trt D) | D vs E | 54.6 | 49.7-60.1 | |
AUC/Dose, AUC per mg naloxone administered; Cmax/Dose, Cmax per mg naloxone administered; CI, confidence interval; INND, improvised nasal naloxone device; IN, Intranasal; Trt, Treatment.
Geometric least-squares mean ratio between treatments, expressed as a percentage of Reference (Treatment E, 2 mg IM using IM 2-mg autoinjector device)
Because quick absorption of naloxone is important in reversing respiratory depression in persons who have overdosed on an opioid, Table 2 includes the AUC during the first few minutes after naloxone administration. Exposures during the first 5, 10, and 20 minutes are approximately 2- to 3-fold higher using either the 2-mg and 4-mg FDANxSpray devices or the Autoinjector compared to exposures following use of the INND. Higher mean concentrations were apparent after only 5 minutes when naloxone was administered using any of the 3 FDA-approved devices compared to either 1 or 2 doses using the INND (Figure 1).
Figure 1. Mean (SD) Plasma Concentrations of Naloxone in Healthy Participants Using the INND, 2-mg and 4-mg FDANxSpray Devices, and the Autoinjector.
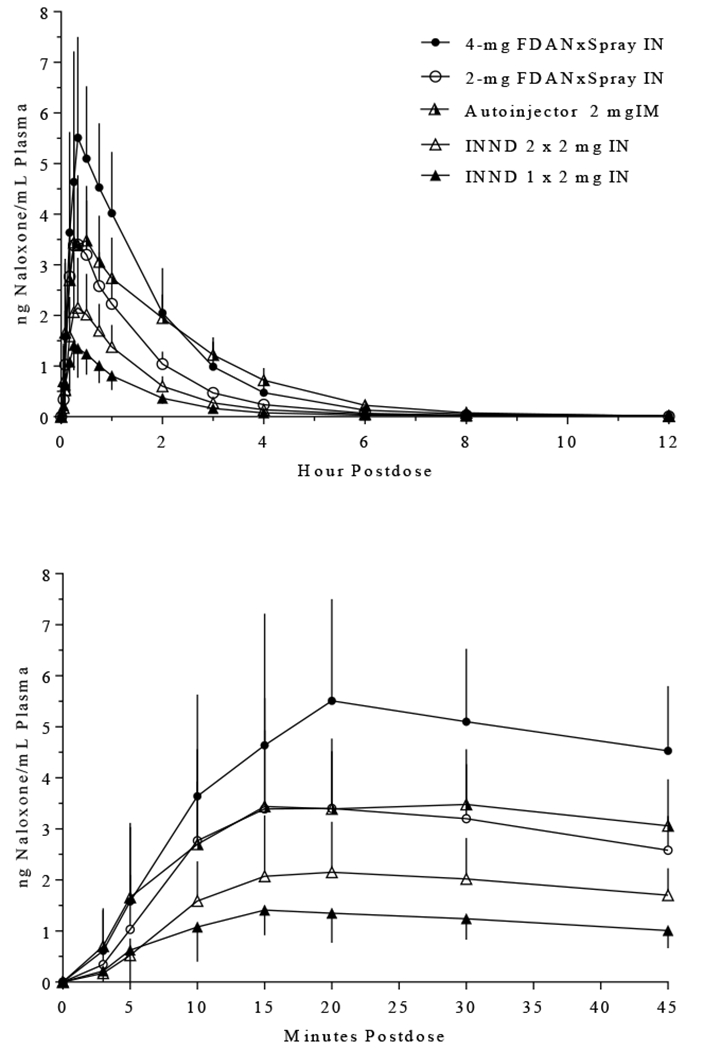
Naloxone was administered intranasally either once (at time zero) or twice (at zero and 2 minutes) using the INND. Naloxone was administered only once (at time zero) using the nasal spray devices and IM Autoinjector. The bottom panel displays the mean plasma concentrations during the first 45 minutes after administration.
There were no clinically relevant differences in the pharmacokinetic parameters of naloxone due to sex (Table 4).
Table 4.
Pharmacokinetics of Naloxone in Female and Male Participants after Administration using the INND, Nasal Spray 2-mg and Nasal Spray 4-mg Nasal Spray Devices, and IM 2-mg Autoinjector
| Parameter (Units)a | INND 1 × 2 mg IN (Trt A) | INND 2 × 2 mg IN (Trt B) | 2-mg FDANxSpray IN (Trt C) | 4-mg FDANxSpray IN (Trt D) | Autoinjector 2 mg IM (Trt E) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | |||||||||||
| Cmax (ng/mL) | 1.5 | (30.2) | 1.4 | (55.0) | 2.4 | (19.8) | 2.2 | (45.9) | 3.7 | (44.5) | 3.6 | (42.7) | 5.0 | (24.0) | 6.6 | (35.8) | 4.7 | (27.0) | 3.3 | (29.7) |
| Tmax (h) | 0.3 (0.2-0.5) | 0.3 (0.1-0.8) | 0.3 (0.3-0.5) | 0.3 (0.2-0.8) | 0.3 (0.2-0.8) | 0.3 (0.2-0.8) | 0.5 (0.3-0.8) | 0.3 (0.2-1.0) | 0.5 (0.1-0.8) | 0.5 (0.1-3.0) | ||||||||||
| AUC (ng·min/mL) | 130 | (19.8) | 122 | (31.8) | 209 | (16.6) | 217 | (28.1) | 343 | (21.8) | 320 | (22.7) | 551 | (36.9) | 606 | (23.8) | 606 | (14.3) | 487 | (15.7) |
| t½ (h) | 1.3 | (16.5) | 1.7 | (48.1) | 2.0 | (37.9) | 2.3 | (36.1) | 1.4 | (27.4) | 1.6 | (37.6) | 2.0 | (39.3) | 2.2 | (38.3) | 1.4 | (10.4) | 1.5 | (22.3) |
| CL/F (L/h) | 834 | (19.8) | 889 | (31.8) | 1040 | (16.6) | 997 | (28.1) | 315 | (21.8) | 338 | (22.7) | 392 | (36.9) | 356 | (23.8) | 179 | (14.3) | 222 | (15.7) |
N = 16 male and 11 female participants
%CV, percent coefficient of variation; AUC, area under the plasma concentration-time curve from time zero to infinity; CL/F, apparent clearance; Cmax, maximum plasma concentration; t½, terminal half-life; Tmax, time to Cmax; INND, improvised nasal naloxone device; IN, intranasal; IM, intramuscular; relative BA, bioavailability relative to IM injection; Trt, treatment.
Mean values (%CV) for all except Tmax which is median (minimum, maximum)
Safety
A total of 8 treatment-related adverse events were reported by the participants; all were mild and resolved quickly. There were 2 instances each of dizziness and headache.
DISCUSSION
In order to reverse an opioid overdose, the plasma concentration of naloxone needs to achieve an adequate concentration quickly after administration. The dose of naloxone necessary for a reversal is due to a number of variables, such as duration of effect, the specific potency and type of opioid consumed, the administration route, any other ingested drugs, and the patient’s underlying opioid tolerance.9
While an intravenous (IV) dose is the fastest means of achieving a high plasma concentration, the general public and many first responders, such as police, are not trained or equipped to administer naloxone IV. Loimer et al. were the first to show that nasal administration of naloxone can be effective.6 It can buy time while waiting for the arrival of trained medical personnel. IN administration, which does not involve needles, is an advantage in the view of many individuals.4 The prevalence in the last few years of fentanyl and other potent synthetic opioids, though, may require multiple administrations of naloxone to achieve reversal of an overdose.10
In Massachusetts during the first half of 2016, 74% of overdose opioid overdose deaths involved fentanyl.11 Of 64 persons who were trained by the Massachusetts Department of Public Health to use the INND, 75% reported witnessing, giving, or receiving a naloxone administration to successfully reverse an opioid or fentanyl overdose between October 2015 and April 2016. Of these events, 83% reported that 2 or more doses of naloxone using the INND per suspected fentanyl overdose were used before the person responded. In a retrospective study of 2166 patients treated by paramedics in New Jersey from 2014 to 2016 for a suspected opioid overdose, 91% experienced complete resolution of symptoms with a single dose of naloxone using an INND and 9% needed a second dose, generally by the IV route.12
Training is needed to understand how to assemble and use the INND, and even with training, there is a 45% failure rate in its use by the public.13 A portion of the naloxone solution delivered using the device may be lost dripping down the nasopharynx or externally from the nose due to the introduction of 1 mL solution into each naris. The optimum volume for nasal delivery is approximately 0.10 to 0.15 mL.14 Approximately 90% of subjects in human use studies could use correctly either the Autoinjector or the FDANxSpray device without any training.8,13
Previous data on the PK of naloxone following a single use of the INND were reported in a patent but study details were minimal.15 The results in the present study show that even with a second administration using the INND, maximum plasma concentrations were 60% less compared to the 4-mg FDANxSpray device. Previous PK data for the Autoinjector can be found in the product label.16 The current study was designed as a direct, within-subject comparison of the FDA-approved devices and the INND.
The comparatively low plasma levels of naloxone observed following multiple administrations of the INND are a cause for concern. The use of fentanyl and its analogs, whether intended or unintentional17, necessitates rapid attainment of higher concentrations after it is determined that the person may have overdosed on an opioid. Since fentanyl has a fast onset,11 the need to act in an expeditious manner has become more urgent. The ease of use8 and higher plasma concentrations using the 4-mg FDANxSpray device compared to the INND should be considered when deciding which naloxone device to use. The likelihood that extreme overdoses with fentanyl, carfentanil, and related compounds may require even higher plasma concentrations of naloxone for reversal suggest there is merit in developing new products with similar ease of use that deliver higher and/or multiple doses of naloxone.
DATA SHARING
For data sharing, please contact the corresponding author.
Acknowledgments
The study was supported with funds from the NIH Office of the Director through the following NIDA contracts: N01-DA-17-8935 with Vince & Associates (clinical site); N01-DA-14-8914 with Technical Resources International (data management); and N01-DA-13-8920 with XenoBiotic Laboratories (bioanalytical services).
The authors did not use the assistance of a professional medical writing company for manuscript preparation.
Footnotes
P.K., S.G., and D.M. are employees of the National Institutes of Health; C.N.C. is a former employee of the National Institutes of Health (NIH).
None of the authors have any disclosures.
REFERENCES
- 1.National Institute on Drug Abuse. Overdose death rates, Revised, 2018. https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates
- 2.Gardocki JF, Yelnosky J. A study of some of the pharmacologic actions of fentanyl citrate. Tox Appl Pharm 1964; 6:48–62. [DOI] [PubMed] [Google Scholar]
- 3.Armenian P, Vo KT, Barr-Walker J, Lynch KL. Fentanyl, fentanyl analogs and novel synthetic opioids: A comprehensive review. Neuropharmacology. 2018; 134(Part A):121–132. [DOI] [PubMed] [Google Scholar]
- 4.Wermeling DP. A response to the opioid epidemic: naloxone nasal spray. Drug Deliv Transl Res 2013; 3:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryan SA, Dunne RB. Pharmacokinetic properties of intranasal and injectable formulations of naloxone for community use: a systemic review. Pain Manag 2018; 8:231–245. [DOI] [PubMed] [Google Scholar]
- 6.Loimer N, Hofmann P, Chaudhry HR. Nasal administration of naloxone is as effective as the intravenous route in opiate addicts. Int J Add 1994; 29:819–927. [DOI] [PubMed] [Google Scholar]
- 7.International Conference on Harmonisation (ICH). Consolidated Good Clinical Practice: Guidelines (E6). 1996. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf.
- 8.Krieter P, Chiang N, Gyaw S, et al. Pharmacokinetic properties and human use characteristics of an FDA-approved intranasal naloxone product for the treatment of opioid overdose. J Clin Pharm 2016; 56:1243–53. [DOI] [PubMed] [Google Scholar]
- 9.Li K, Marmenian P, Mason J, Grock A. Narcan or Nar-can’t: tips and tricks to safely reversing opioid toxicity. Ann Emerg Med 2018; 72:9–11. [DOI] [PubMed] [Google Scholar]
- 10.Faul M, Lurie P, Kinsman JM, Dailey MW, Crabaugh C, Sasser SM. Multiple naloxone administrations among emergency medical service providers is increasing. Prehosp Emerg Care. 2017; 21:411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Somerville NJ, O’Donnell J, Gladden RM, et al. , 2017. Characteristics of fentanyl overdose – Massachusetts, 2014-2016, MMWR Morb. Mortal. Wkly. Rep 66 (14):382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klebacher R, Harris MI, Ariyaprakai N, et al. Incidence of naloxone redosing in the age of the new opioid epidemic. Prehosp Emerg Care. 2017; 21:682–687. [DOI] [PubMed] [Google Scholar]
- 13.Edwards ET, Edwards ES, Davis E, Mulcare M. Wiklund M, Kelley G. Comparative usability study of a novel auto-injector and an intranasal system for naloxone delivery. Pain Ther 2015; 4:89–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Constantino HR, Illum L, Brandt G, Johnson PH, Quay SC. Intranasal delivery: physicochemical and therapeutic aspects. Intl J Pharmaceutics. 2007; 337:1–24. [DOI] [PubMed] [Google Scholar]
- 15.Wyse J, DeHart MP, inventors; AntiOP, Inc., assignee, Intranasal naloxone compositions and methods of making and using same. United States patent US 9192570 B2 2015. November 25.
- 16.Evzio® (naloxone hydrochloride injection) Auto-injector for intramuscular or subcutaneous use. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/209862lbl.pdf
- 17.Sutter ME, Gerona RR, Davis MT, et al. Fatal fentanyl: one pill can kill. Acad Emerg Med 2017; 24:106–113. [DOI] [PubMed] [Google Scholar]


